

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.021076
ARTICLE
Land Consolidation with Seedling Cultivation Could Decrease Soil Microbial PLFA Diversity
1Zhejiang Provincial Key Laboratory of Carbon Cycling in Forest Ecosystems and Carbon Sequestration, Zhejiang A&F University, Hangzhou, 311300, China
2The East Institute of Inventory and Planning, The State Forestry and Grassland Administration, Hangzhou, 311300, China
*Corresponding Author: Juan Liu. Email: ljuan1978@126.com
Received: 26 December 2021; Accepted: 24 January 2022
Abstract: The impact of land consolidation on the soil microbial PLFA diversity is of great importance for understanding the effective arable land usage, improving agricultural ecological conditions and environment. In this study, we collected the soil samples (0–20 cm) in experimental plots with 0 (Z0), 1 (Z1a) and 4 (Z4a) years of land consolidation in the forest station of Ningbo City, Zhejiang Province, southeastern China. The results were analyzed using ANOVA for randomized block design. Compared with control (Z0), the soil pH value under Z1a treatment increased by 14.6%, soil organic carbon (SOC) content decreased by 65.4%, so did the PLFA contents and relative abundance of all the microbial PLFA diversity (P < 0.05), respectively. Meanwhile, for the Z1a treatment, the ratio of fungi to bacteria (F/B) significantly decreased by 35.9% (P < 0.05), while the ratio of Gram-positive bacteria to Gram-negative bacteria (G+/G−) signific antly increased by 56.1%. This was strongly related to the increased soil pH values and the decrease of SOC. The Shannon index (H) and evenness index (E) of soil microbial PLFA diversity were significantly decreased after land consolidation (P < 0.05). Compared to the Z1 treatment, the microbial PLFA diversity was improved slightly. Therefore, the land consolidation could significantly affect the composition of soil microbial PLFA diversity, and decrease the soil ecosystem stability.
Keywords: Cultivated land balance; land use change; Pinus taiwanensis forest; PLFA; soil degradation; soil fertility variation
Sustainable land use is of importance to the economic development of human society [1,2], especially for China, which is a developing country with a large population, but few cultivated land per capita. From 1961 to 2015, the arable land per capita of China dropped from 0.16 ha to 0.09 ha, which was consistently lower than the average level of the world (0.37 ha in 1961 and 0.19 ha in 2015) [3,4]. Since 1980s, due to the rapid socio-economic development and urbanization, a large amount of arable land was occupied [5]. China’s arable land area decreased from 123 million ha to 121 million ha during the decade between 2000 and 2010 [6]. The decreasing arable land has seriously threatened the food production. By 2030, the total amount of arable land required is expected to be 146 million ha to ensure national food security [7]. Currently, urbanization is the main reason for the decrease of arable land [8]. During 1950 to 2015, the urbanization rate increased rapidly from 11.18% to 56.1%, leading to the shrinkage of arable land resources [7,9]. In addition, inefficient use of construction land, scattered and fragmented farmland, serious soil pollution and land degradation have caused serious food security and ecological issues. In order to deal with the increasing demand for arable lands, land consolidation is regarded as an effective approach.
Land consolidation adopts various measures to promote the quality of arable land, increase the effective arable land area, and improve agricultural ecological conditions and environment [2,10–13]. At present, land consolidation has been widely used [14,15]. From 2001 to 2015, China has replenished arable lands of 4.52 million ha through land consolidation [16].
Due to the different natural condition, land consolidation measures and management methods, the results obtained are quite different or opposite. After land consolidation, soil available phosphorus (AP), soil available potassium (AK) and soil organic carbon (SOC) decreased significantly, while pH value increased significantly [17]. The soil bulk density increased by 1.34 times after land consolidation [18]. Since the shrub coverage in woodlands has a significant positive correlation with soil organic carbon density (SOCD) [19], the cultivated land deriving from natural forest could decrease both the SOC and soil total nitrogen (TN) [20,21]. After land consolidation, the total value of ecological services in different regions decreased by 10.5%~33.1% [22], the fragmentation of arable land landscape increased [23], and the biological fertility index (BFI) decreased [21]. While other study revealed that land consolidation improved the soil quality [24]. Meanwhile, previous researches focused on the soil physical and chemical properties, the impact of land consolidation on soil microorganisms was rarely reported.
Soil microbial community is one of the most important functional components in soil biota, and it is a sensitive indicator of soil ecosystem changes, indicating changes in soil quality [25]. In general, different soil microorganisms can control the decomposition of multiple substances and the circulation of different nutrients in the soils [26]. Changes in soil quality caused by land consolidation are not only reflected in the negative impact on the content of SOC and labile carbon pools [27], but also reflected the changes in the amount of soil microorganisms [28–30]. However, there are few studies on the change of soil microbial community after land consolidation from forest land to arable land. At present, phospholipid fatty acid (PLFA) analysis is widely used in the study of soil microbial community diversity [31]. By analyzing soil microbial PLFA, microbial biomass, the total biomass and microbial community structure of different groups can be quantitatively reflected [32]. The objective of this study was to: investigate the variation of soil microbial PLFA diversity under different land consolidation years, in order to reveal the significant effect on soil microbial PLFA diversity caused by land consolidation and its impact on soil quality.
2.1 Study Site and Sample Collection
The study was carried out at the forest station of Ningbo City, Zhejiang Province, southeastern China (29°38′–29°48′ N,121°13′–121°58′ E). This area has a typical subtropical monsoon climate. The annual precipitation is approximate 2000 mm. The annual daylight hours and frost-free days are 1813 h and 240 d, respectively. The soil is classified as Ferralsols in the Food and Agriculture Organization (FAO) soil classification system.
In this study, land consolidation referred to the lands which were derived from the originally natural forests and further used as arable lands for seedling cultivation in southern China, due to lacking arable lands in this region. The experimental plots with 1 (Z1a) and 4 (Z4a) years of land consolidation period and reference plot (Z0) were selected from four watersheds of 20, 25, 26, 18 ha. Z0 treatment stands for the Pinus taiwanensis forest as reference plots. During 2013 and 2016, the Pinus taiwanensis forest land was used for arable land. The above hillside fields were transformed into terraced fields (not shown), with strong soil disturbance. The Z1a plots were set up in 2016 after land consolidation and no plants were in these plots. The Z4a plots (for seedling cultivation) had a land consolidation history since 2013, and arbor seedlings (Taxus wallichiana var. mairei, Chamaecyparis obtusa, etc.), has been planted, with an average seedling height of 0.8 m, an average ground diameter of 2.0 cm and a density of 30,000 plants per ha. The annual application of compound fertilizer (N:P2O5:K2O = 15:15:15) was 600 kg ha–1 for Z4a plots. Altogether, 12 plots were selected in the watersheds that served as 4 blocks. The slope, gradient and soil type of the plots in the same block were similar.
Soil samples (0–20 cm) were taken from the study plots in October, 2017. Six sub-samples from the same plot were mixed thoroughly to form a composite sample. The soil samples were preserved on ice for 3 hours before being shipped to the laboratory [33]. Soil samples were passed through a 2-mm nylon mesh. Part of the samples were used for the analysis of microbial community structure. Another half of samples were air-dried and used for determination of physical and chemical properties of the soil [33].
Soil pH was measured with a pH meter using an aqueous suspension (soil-water ratio of 1:2, W:V). The soil organic carbon content was determined using H2SO4 and K2Cr2O7, and titrating with Fe (NH4)2·(SO4)2·6H2O solution. Alkali-hydrolyzable nitrogen was analyzed using alkali-diffusion method. Available phosphorus and potassium were determined by the NaHCO3 extraction-colorimetry and NH4OAc extraction-flame photometry methods, respectively [34].
The extraction and analysis of PLFAs were carried out as described by Frostegård et al. [35,36]. In this study, the relative abundance of the PLFA was expressed in mol %. The communities of Gram+ bacteria were indicated by PLFA i14:0, i15:0, a15:0, i16:0, i17:0 and a17:0. Gram− bacteria were represented by 16:1ω7c, cy17:0, 18:1ω7c and cy19:0 [37]. Bacteria were characterized by i14:0, i15:0, a15:0, 15:0, i16:0, 16:1ω7c, 17:0, i17:0, a17:0, cy17:0, 18:1ω7c, cy19:0 [38]. Fungi were characterized by 18:1ω9c and 18:2ω6c [39]. Arbuscular mycorrhizal fungi were represented by 16:1ω5c [39]. Actinomycetes were characterized by Me16:0, Me17:0 and Me18:0. Protozoans were characterized by 20:4w6, 9, 12 and 15c [40].
The analysis of variance (ANOVA) for randomized block design was applied to test the land consolidation effects on pH, SOC, AN, AP, AK, PLFA, relative abundance of PLFA, the ratio of fungi/bacteria and G+/G−. A multiple comparison using Duncan’s multiple range test method was conducted when a significant effect was detected (α = 0.05). If the data did not meet the assumptions of normality, values were log transformed prior to ANOVA analysis. Statistical analyses were carried out using SPSS® for windows (version 18.0). Graphing was achieved with Origin 2019 software.
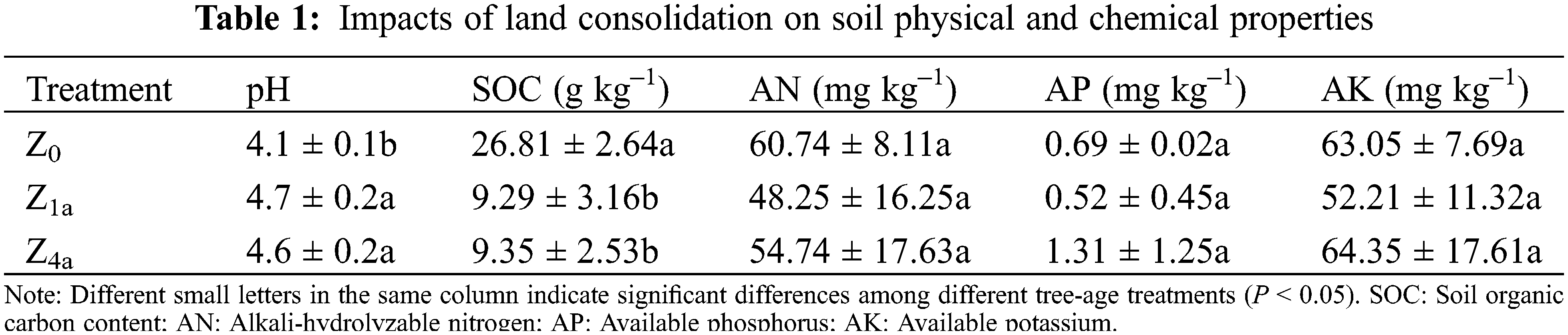
After land consolidation, the soil pH values increased significantly (P < 0.05), while the SOC contents decreased significantly (P < 0.05) (Table 1). Meanwhile, the changes of AN, AP and AK were not significant during the land consolidation process (Table 1).
3.2 Soil Microbial PLFAs Components
Soil bacteria PLFAs, fungi PLFAs, actinobacteria PLFAs, arbuscular mycorrhizal fungi (AMF) PLFAs and protozoan PLFAs ranked as follows: Z0 > Z4a > Z1a (Fig. 1). The total PLFA concentration, as an indicator of active soil microbial biomass, was highest in the Z0 soil samples (not shown). Compared with Z0 treatment, the bacteria PLFAs, fungi PLFAs, actinomycetes PLFAs, AMF PLFAs and protozoa PLFAs of Z1a and Z4a decreased significantly (P < 0.05), respectively (Fig. 1). While compared with Z1a treatment, the PLFAs of all components of soil microbial biomass was slightly increased after 4 years of land consolidation Z4a, but the differences were not significant (P > 0.05) (Fig. 1).
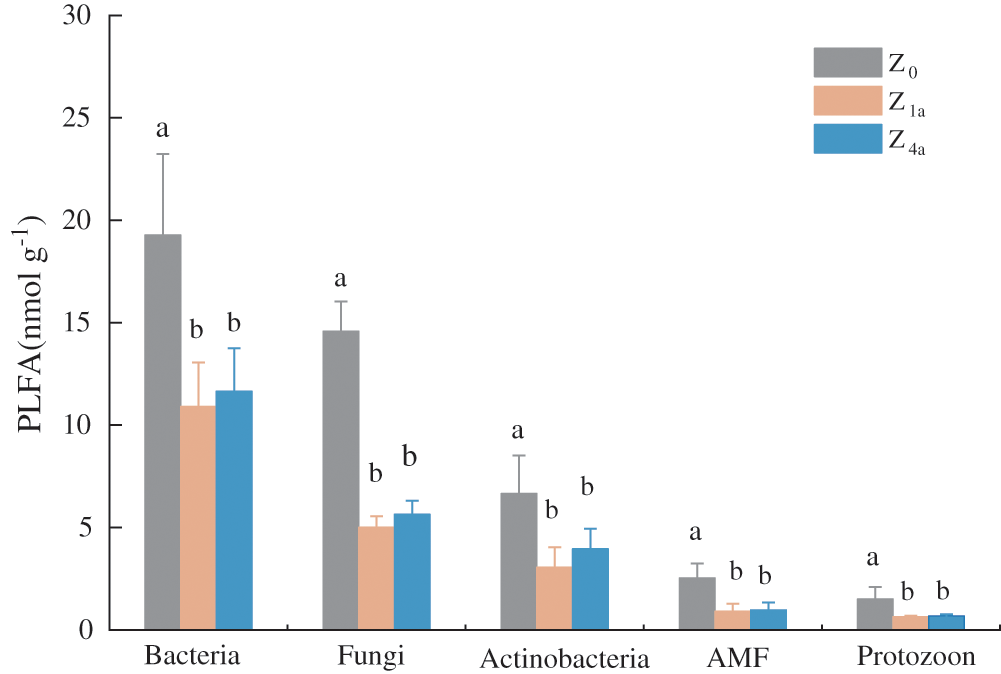
Figure 1: Soil phospholipid fatty acid contents on treatments with different consolidation history. Different letters indicate differences at P < 0.05 level. AMF = Arbuscular mycorrhizal fungi
The soil microbial community significantly changed after land consolidation (Figs. 2 and 3). The relative abundance analysis of PLFA showed that bacteria dominated in the soils, while the second most abundant component was the fungus and actinobacteria. Arbuscular mycorrhizal fungi and protozoans were less abundant in soil samples. Both the relative abundance of soil G− and G+ bacteria biomass ranked as follows: Z0 > Z4a > Z1a. Compared with Z0, after 1 year of land consolidation, the relative abundance of PLFA in all groups was significantly decreased (P < 0.05) (Fig. 2). while compared with Z1a, after land consolidation for 4 years, the relative abundance of PLFA in all groups slightly increased, but the difference was still not significant (P > 0.05) (Fig. 2).
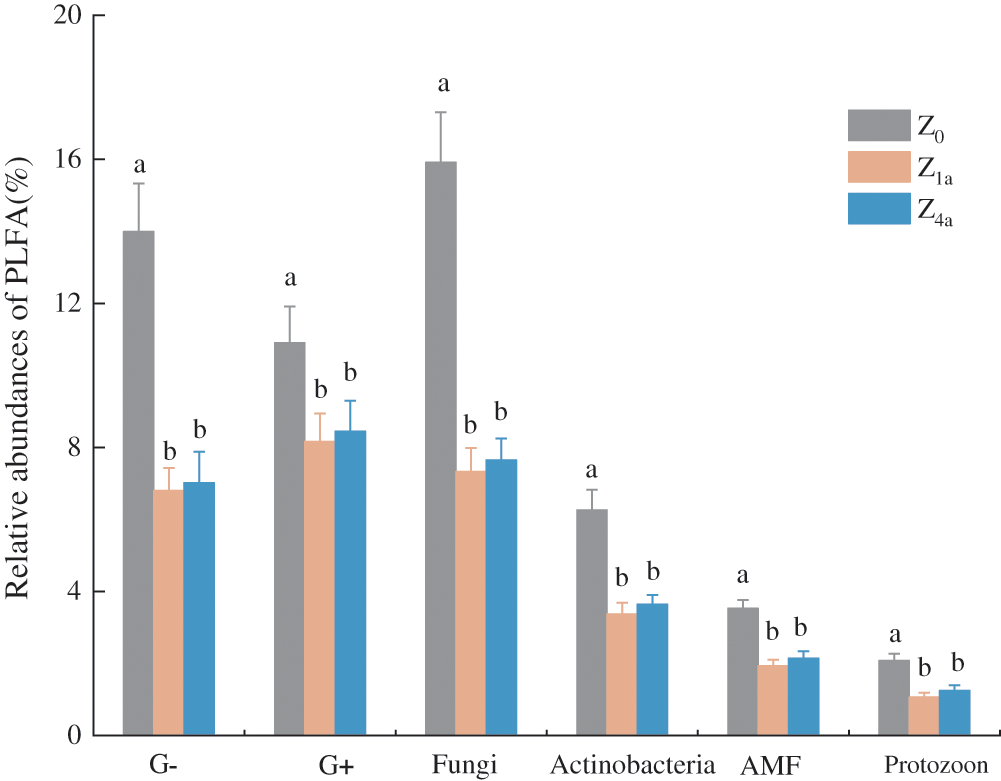
Figure 2: Relative abundance of phospholipid fatty acids of microbial groups in different consolidation history. (Different letters indicate differences at P < 0.05 level. G− = Gram-negative bacteria; G+ = Gram-positive bacteria; AMF = Arbuscular mycorrhizal fungi)
Compared with Z0, after 1 year of land consolidation, the soil fungal/bacteria ratio (F/B) decreased significantly (P < 0.05), and G+ bacteria to G− bacteria significantly increased(P < 0.05) (Fig. 2). Compared with Z1a, after land consolidation with 4 years, F/B increased, while G+/G− decreased slightly, but the difference was not significant (P > 0.05) (Fig. 3).
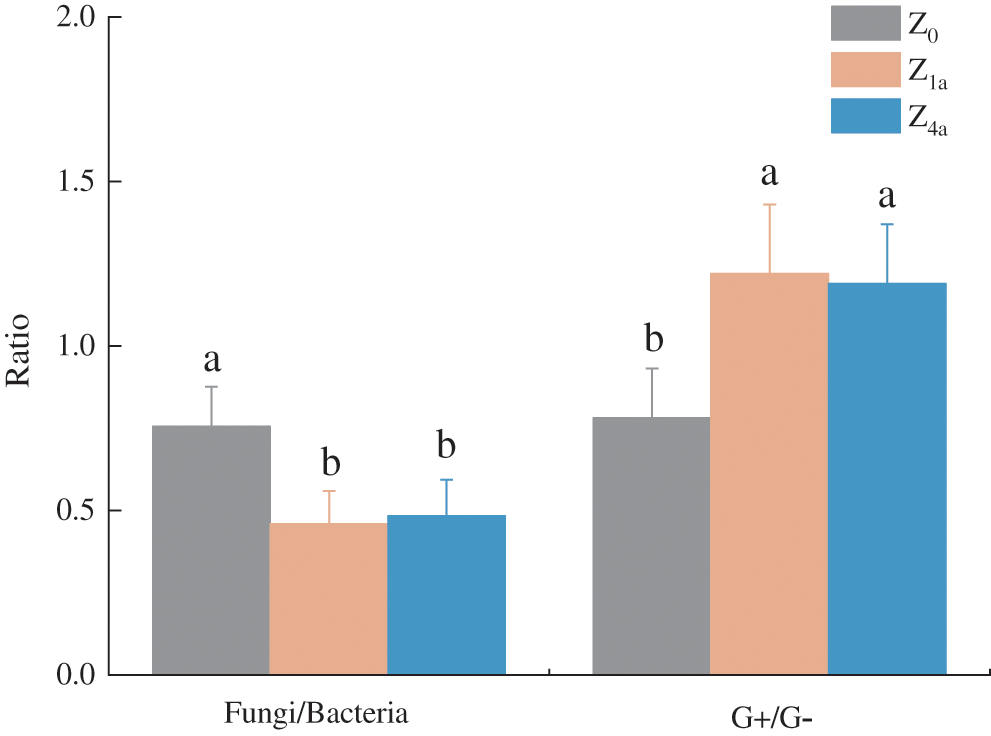
Figure 3: Ratio of Fungi/Bacteria and G+/G− within three treatments. Different letters indicate differences at P < 0.05 level
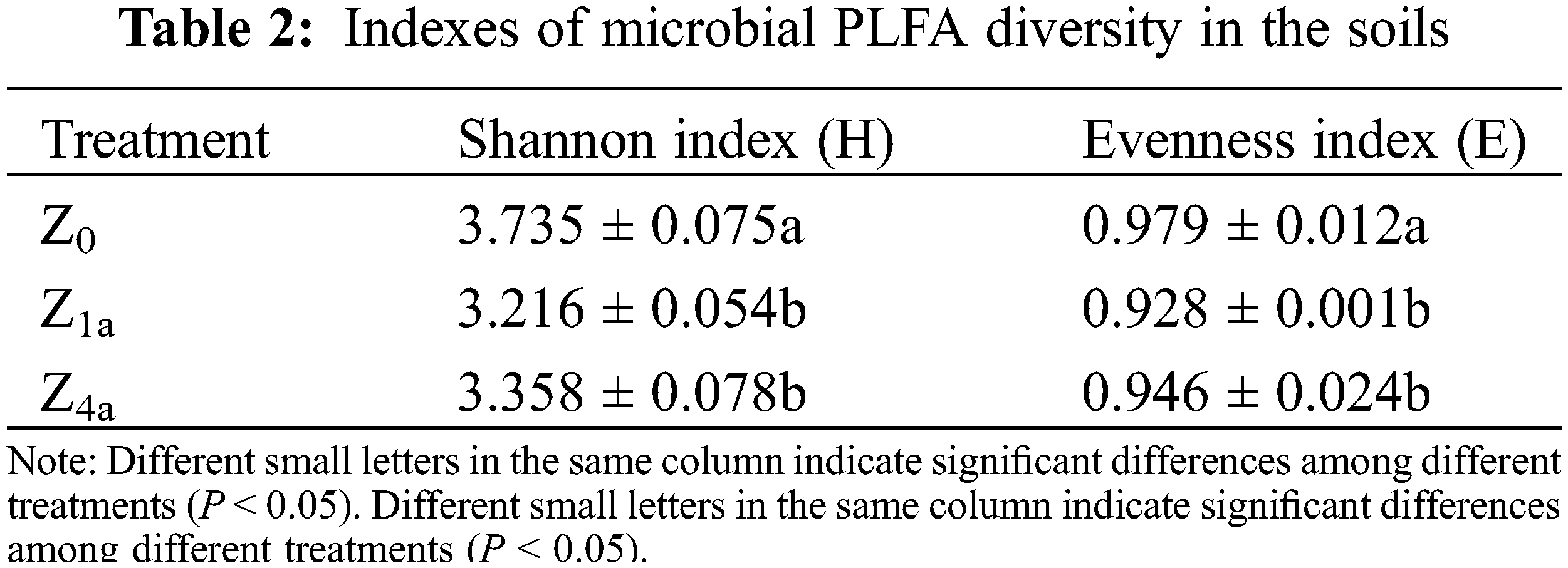
3.3 Soil Microbial Diversity Index
Both the microbial diversity index (Shannon index, H) and evenness index (E) for the different treatments were in the following order: Z0 > Z4a > Z1a. The H and E in Z0 treatment was much greater than in the Z4a and Z1a treatments (P < 0.05), but there were no significant differences in H and E between the Z4a and Z1a treatments (Table 2).
3.4 Correlation between Soil Chemical Properties and Microbial PLFA Diversity
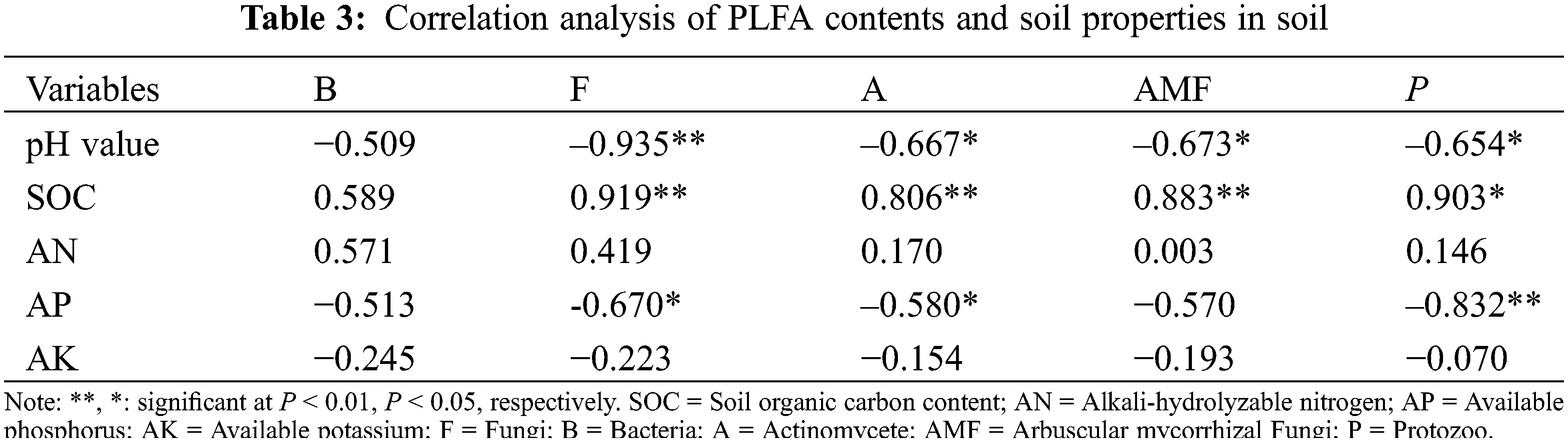
Soil protozoon, actinomycetes, arbuscular mycorrhizal fungiprotozoa and fungi were negatively and significantly correlated with soil pH (P < 0.05 or P < 0.01) (Table 3). Soil protozoa, fungi, actinomycetes and arbuscular mycorrhizal fungi were positively and significantly correlated with SOC (P < 0.05 or P < 0.01) (Table 3). Soil fungi, actinomycetes and protozoa was negatively and significantly correlated with soil available phosphorus (P < 0.05 or P < 0.01) (Table 3).
4.1 The Effect of Land Consolidation Change on SOC and pH
Land consolidation changed the underground ecosystems, resulting in destruction of biodiversity and reduction of soil organic carbon [41,42]. When manure is applied during land consolidation process, the microbial community diversity in soils are significantly higher than those areas with only chemical fertilizer application [43,44], as the application of manure into the soil can provide the carbon-containing substances and increase the soil organic matter (SOM) [45,46]. In this study, the SOC decreased significantly after land consolidation. The microbial PLFA diversity after land consolidation was closely related to the decrease of available nutrient content (SOC). This result is consistent with the previous findings that SOC is one of the main soil properties related to the changes in soil microbial community structure [47]. Meanwhile, the PLFAs of fungi, actinomycetes, arbuscular mycorrhizal hyphae (AMH) and protozoa were all positively and significantly correlated with SOC (Table 3), especially the differences in soil organic carbon often lead to the variation in microbial community composition [48]. Correlation analysis also showed that the PLFAs of different soil microorganisms were positively correlated with the available nutrients in soils [49].
In this study, the topsoil (0–15 cm) which generally had relatively high organic carbon content, was stripped during the land consolidation process, the bottom soil was exposed, and the SOC content decreased significantly (Table 1), which led to a decrease in the PLFAs components (Fig. 1). This was consistent with the finding of the study that the difference in microbial composition was related to the available carbon content in soil [50]. At the same time, soil pH increased significantly after land consolidation (Table 3), and pH was also one of the main soil characteristics that caused microbial community structure changes [47,51]. At a low pH, the content of fungi PLFAs is more abundant [52]. In our study, a negative and significant correlation between the content of fungi PLFAs and soil pH was found. Meanwhile, the contents of PLFAs of actinomycetes, arbuscular mycorrhizal hyphae (AMH), and protozoa had also a negative and significant correlation with soil pH (Table 3), which indicated that in a certain pH range, the stronger the acidity of the soil, the richer in the microbial community [53]. Therefore, changes in SOC and pH after land consolidation are the main influencing factors for changes in PLFAs of soil microbial community.
4.2 The Effect of Land Consolidation Change on Soil Microbial PLFA Diversity
Soil microbes are considered to dominate SOM decomposition and nutrient cycling in soils [26], while bacteria and fungi, account for about 90% of soil microbial biomass. Abundant SOC is a desired environment for fungal growth [54]. The composition of the soil microbial PLFA diversity changed significantly after land consolidation (Figs. 1 and 2). Since the location and site condition were almost the same in this research, land consolidation was considered to be the main factor that caused the significant differences in microbial PLFA diversity [55]. Arbuscular mycorrhizal fungi (AMF) are also an important part of the rhizosphere microbial community, and they can form mycorrhizal symbiotes with the roots of most terrestrial plants, accounting for more than 10% of the total soil microbial biomass [56,57]. AMF has a positive promotion effect on plant growth. Therefore, exploring which soil factors affect AMF is important to further reveal the changes of AMF after land consolidation. Studies have found that there is a negative correlation between soil pH and AMF abundance diversity [58]. This is consistent with the finding of this study. However, other study has found a positive correlation between soil pH and AMF diversity [59]. Soil pH is considered to be a key driver of AMF abundance diversity, but its positive or negative effect need further to be determined based on specific study area. The results of this study indicated that there was a significantly positive correlation between the content of AMF PLFAs and SOC content, which was contrary to other studies that considered SOC as the main negative driving factor for AMF development [60–62].
F/B ratio can reflect the relative content of fungi and bacteria, and the relative abundance of the two populations. Their ratio reflects the quality of SOM in the soil system. In farmland ecosystems, the higher the F/B ratio, the more stable the ecosystem [63]. After land consolidation, F/B decreased significantly (Fig. 3), indicating that land consolidation reduced the stability of the soil ecosystem. Whether before or after land consolidation, the F/B ratio was always less than 1 (Fig. 3), which did not mean that the contribution of fungi was less important than that of bacteria. In general, some substances in the soil must be decomposed by fungi before being used by bacteria. Therefore, fungi and bacteria are interdependent, and both are indispensable in the decomposition process. The PLFA method may underestimate the fungal biomass and lead to an F/B biomass ratio <1.0 [64].
Soil bacteria can be divided into two major groups, G+ and G−. G+ (such as Arthrobacter) is more tolerant to environmental stress (for example, hunger) than G− (Pseudomonas) [65]. G+ such as Arthrobacter grows faster in an oligotrophic environment [66]. Studies have found that with the removal of existing trees and root tissues in the soil, the G+/G− ratio increases, and ecosystem productivity decreases [67]. Consistent with the finding that the G+/G− ratio increased significantly after land consolidation in this study. A high proportion of G+ is considered to be a transition from eutrophication to oligotrophic soil environment, and G+/G− reflects changes in bacterial community structure [68]. G+/G− elevation is closely related to SOC quality decline [69]. And G− bacteria uses more plant-derived C sources, while G+ bacteria uses more SOM-derived carbon sources [70]. When comparing natural ecosystems, the G+/G− ratio can be used as a rough indicator of the relative C utilization of the bacterial community [59]. After land consolidation, G+/G− increased significantly due to the amount of SOC loss (Fig. 3). With the extension of land consolidation period and human management, various soil nutrients have increased. After 4 years of consolidation, G+/G− decreased slightly. After land consolidation of 4a, the ratio of G+/G− was slightly lower than that of land consolidation of 1a, probably because the planted crops increased the C source of plants, and the soil ecosystem and biodiversity were restored to a certain extent.
The content and relative abundance of PLFAs in soil significantly decreased after land consolidation, which was closely related to the significant decrease of SOC. F/B decreased significantly, while G+/G− increased significantly, both related to the decrease of SOC and the increase of pH value. After land consolidation, both Shannon index and evenness index of soil microbial diversity decreased significantly. Land consolidation strongly effected soil microbial PLFA diversity and its composition. This study demonstrated that land consolidation significantly affected the composition of soil microbial PLFA diversity and reduced the stability of soil ecosystems. As the land consolidation period extended, the soil microbial quality would improve.
Acknowledgement: We gratefully acknowledge Mr. X.B. Qian for the field work.
Authors’ Contributions: The authors confirm contribution to the paper as follows: study conception and design: Wu JS, Liu J; data collection: Jin J, Yan BJ; analysis and interpretation of results: Yan BJ, Jian YQ, Liang C.; draft manuscript preparation: Zhang S. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This study was supported by the Key Research and Development Plan of Zhejiang Province (No. 2019C02008-03) and Natural Science Foundation of Zhejiang Province (LY20C160004).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Li, Y. R., Liu, Y. S., Long, H. L., Cui, W. G. (2014). Community-based rural residential land consolidation and allocation can help to revitalize hollowed villages in traditional agricultural areas of China: Evidence from Dancheng County, Henan Province. Land Use Policy, 39(1), 188–198. DOI 10.1016/j.landusepol.2014.02.016. [Google Scholar] [CrossRef]
2. Liu, Y. S. (2018). Introduction to land use and rural sustainability in China. Land Use Policy, 74(7499), 1–4. DOI 10.1016/j.landusepol.2018.01.032. [Google Scholar] [CrossRef]
3. Du, X. D., Zhang, X. K., Jin, X. B. (2018). Assessing the effectiveness of land consolidation for improving agricultural productivity in China. Land Use Policy, 7(3), 360–367. DOI 10.1016/j.landusepol.2017.10.051. [Google Scholar] [CrossRef]
4. Yin, G. Y., Lin, Z. L., Jiang, X. L., Yan, H. W., Wang, X. M. (2019). Spatiotemporal differentiations of arable land use intensity—A comparative study of two typical grain producing regions in northern and southern China. Journal of Cleaner Production, 208(1), 1159–1170. DOI 10.1016/j.jclepro.2018.10.143. [Google Scholar] [CrossRef]
5. Zhou, Y., Guo, Y. Z., Liu, Y. S., Wu, W. X., Li, Y. R. (2018). Targeted poverty alleviation and land policy innovation: Some practice and policy implications from China. Land Use Policy, 74(11), 53–65. DOI 10.1016/j.landusepol.2017.04.037. [Google Scholar] [CrossRef]
6. Xu, L. L. Li, B., L., Yuan, Y. C., Gao, X. Z., Liu, H. J. et al. (2015). Changes in China’s cultivated land and the evaluation of land requisition-compensation balance policy from 2000 to 2010. Resources Science, 37(8), 1543–1551 (in Chinese). [Google Scholar]
7. He, G. H., Zhao, Y., Wang, L. Z., Jiang, S., Zhu, Y. G. (2019). China’s food security challenge: Effects of food habit changes on requirements for arable land and water. Journal of Cleaner Production, 229, 739–750. DOI 10.1016/j.jclepro.2019.05.053. [Google Scholar] [CrossRef]
8. Huang, Z. G., Du, X. J., Castillo, C. S. Z. (2019). How does urbanization affect farmland protection? Evidence from China. Resources, Conservation and Recycling, 145(1), 139–147. DOI 10.1016/j.resconrec.2018.12.023. [Google Scholar] [CrossRef]
9. Bai, X. M., Shi, P. J., Liu, Y. S. (2014). Realizing China’s urban dream. Nature, 509(7499), 158–160. DOI 10.1038/509158a. [Google Scholar] [CrossRef]
10. Coelho, J. C., Portela, J., Pinto, P. A. (1996). A social approach to land consolidation schemes: A Portuguese case study: The Valença project. Land Use Policy, 13(2), 129–147. DOI 10.1016/0264-8377(95)00037-2. [Google Scholar] [CrossRef]
11. Fu, W. J., Dong, J. Q., Ding, L., Yang, H., Ye, Z. et al. (2022). Spatial correlation of nutrients in a typical soil-hickory system of southeastern China and its implication for site-specific fertilizer application. Soil & Tillage Research, 217(2), 105265. DOI 10.1016/j.still.2021.105265. [Google Scholar] [CrossRef]
12. Jin, J., Wang, L. Q., Müller, K., Wu, J., Wang, H. et al. (2021). A 10-year monitoring of soil properties dynamics and soil fertility evaluation in Chinese hickory plantation regions of southeastern China. Scientific Reports, 11(1), 23531. DOI 10.1038/s41598-021-02947-z. [Google Scholar] [CrossRef]
13. Zhang, B. B., Niu, W. H., Ma, L. Y., Zuo, X. Y., Kong, X. B. et al. (2019). A company-dominated pattern of land consolidation to solve land fragmentation problem and its effectiveness evaluation: A case study in a hilly region of Guangxi Autonomous Region, Southwest China. Land Use Policy, 88(1), 104115. DOI 10.1016/j.landusepol.2019.104115. [Google Scholar] [CrossRef]
14. Luo, M., Zhang, H. Y. (2002). Land consolidation and its ecological and environmental impacts. Resources Science, 24, 60–63 (in Chinese). DOI 10.3321/j.issn:1007-7588.2002.02.012. [Google Scholar] [CrossRef]
15. Zhang, Q. Q., Luo, H. B., Yan, J. M. (2012). Integrating biodiversity conservation into land consolidation in hilly areas—A case study in southwest China. Acta Ecologica Sinica, 32(6), 274–278. DOI 10.1016/j.chnaes.2012.06.002. [Google Scholar] [CrossRef]
16. Ministry of Land and Resources of the People’s Republic of China. National Land Remediation Plan (2016–2020). (2017). Gazette of the State Council of the People’s Republic of China. Beijing, China (in Chinese). [Google Scholar]
17. Ye, Y. M., Wu, C. F. (2002). Influence of land consolidation on soil characteristics and the technology of soil reconstruction. Journal of Zhejiang University. Agriculture and Life Sciences, 28(3), 34–38 (in Chinese). DOI 10.3321/j.issn:1008-9209.2002.03.008. [Google Scholar] [CrossRef]
18. Meng, H., Jing, W., Guo, J., Zhang, X., Yan, Y. et al. (2009). Preliminary study on physical properties of soil compact in land consolidation in Loess Plateau. Chinese Agricultural Science Bulletin, 25(24), 549–552 (in Chinese). [Google Scholar]
19. Dai, W., Zhao, K. L., Fu, W. J., Jiang, P. K., Li, Y. et al. (2018). Spatial variation of organic carbon density in top soils of a typical subtropical forest, southeastern China. Catena, 167(2), 181–189. DOI 10.1016/j.catena.2018.04.040. [Google Scholar] [CrossRef]
20. Lu, X. Q., Toda, H., Ding, F., Fang, S., Yang, W. et al. (2014). Effect of vegetation types on chemical and biological properties of soils of karst ecosystems. European Journal Soil Biology, 61, 49–57. DOI 10.1016/j.ejsobi.2013.12.007. [Google Scholar] [CrossRef]
21. Dong, J., Zhou, K., Jiang, P., Wu, J., Fu, W. (2021). Revealing vertical and horizontal variation of soil organic carbon, soil total nitrogen and C:N ratio in subtropical forests of southeastern China. Journal of Environmental Management, 289(8), 112483. DOI 10.1016/j.jenvman.2021.112483. [Google Scholar] [CrossRef]
22. Wang, J., Yan, S. C., Yu, L., Zhang, Y. N. (2014). Evaluation of ecosystem service value and strategies for ecological design in land consolidation: A case of land consolidation project in Da’an City, Jilin Province China. Chinese Journal Applied Ecology, 25(4), 1093–1099 (in Chinese). DOI 10.13287/j.1001-9332.2014.0116. [Google Scholar] [CrossRef]
23. Deng, J. S., Wang, K., Li, J., Xu, J. F., Shen, Z. Q. et al. (2006). Impacts of farmland consolidation on farmland landscape. Chinese Journal of Applied Ecology, 17(1), 41–44 (in Chinese). [Google Scholar]
24. Xu, C., Ming, G., Xie, D., Tao, J., Sha, L. et al. (2009). Effect of land consolidation history on soil quality of purple hilly region. Transactions of the CSAE, 25(8), 242–248 (in Chinese). DOI 10.3969/j.issn.1002-6819.2009.08.044. [Google Scholar] [CrossRef]
25. Wang, M., Qu, L. Y., Ma, K. M., Li, G. L., Yang, X. D. (2014). Response of soil microbial community composition to vegetation types. Acta Ecologica Sinica, 34(22), 6640–6654 (in Chinese). DOI 10.5846/stxb201302200278. [Google Scholar] [CrossRef]
26. Harris, J. (2009). Soil microbial communities and restoration ecology: Facilitators or followers? Science, 325(5940), 573–574. DOI 10.1126/science.1172975. [Google Scholar] [CrossRef]
27. Wang, H. B., Jin, J., Fu, W., Morrison, L., Lin, H. P. et al. (2020). Converting evergreen broad-leaved forests into tea and Moso bamboo plantations affects labile carbon pools and the chemical composition of soil organic carbon. Science of the Total Environment, 711, 135225. DOI 10.1016/j.scitotenv.2019.135225. [Google Scholar] [CrossRef]
28. Vitali, F., Mastromei, G., Senatore, G., Caroppo, C., Casalone, E. (2016). Long lasting effects of the conversion from natural forest to poplar plantation on soil microbial communities. Microbiology Research, 182(8), 89–98. DOI 10.1016/j.micres.2015.10.002. [Google Scholar] [CrossRef]
29. Zhou, K., Zhang, Y., Ye, Z., Dou, C., Ye, Z. et al. (2021). Fertilizer application could improve the Zn and Cd accumulation of Sedum alfredii Hance. Phyton-International Journal of Experimental Botany, 90(4), 1217–1232. DOI 10.32604/phyton.2021.014951. [Google Scholar] [CrossRef]
30. Zhao, F. Z., Ren, C., Han, X., Yang, G., Wang, J. et al. (2019). Trends in soil microbial communities in afforestation ecosystem modulated by aggradation phase. Forest Ecology and Management, 441(3), 167–175. DOI 10.1016/j.foreco.2019.03.036. [Google Scholar] [CrossRef]
31. Zhao, J., Zeng, Z. X., He, X. Y., Chen, H. S., Wang, K. L. (2015). Effects of monoculture and mixed culture of grass and legume forage species on soil microbial community structure under different levels of nitrogen fertilization. European Journal Soil Biology, 68, 61–68. DOI 10.1016/j.ejsobi.2015.03.008. [Google Scholar] [CrossRef]
32. Wang, W. X., Shi, Z. M., Luo, D., Liu, D., Lu, H. L. et al. (2013). Characteristics of soil microbial biomass and community composition in three types of plantations in southern subtropical area of China. Chinese Journal Applied Ecology, 24(7), 1784–1792 (in Chinese). [Google Scholar]
33. Wu, W. F., Lin, H. P., Fu, W. J., Penttinen, P., Li, Y. F. et al. (2019). Soil organic carbon content and microbial functional diversity were lower in monospecific Chinese hickory stands than in natural Chinese hickory-broad-leaved mixed forests. Forests, 10(4), 357. DOI 10.3390/f10040357. [Google Scholar] [CrossRef]
34. Lu, R. K. (2000). Methods of analysis of soil and agro-chemistry. Beijing, China: China Agricultural Science and Technology Press (in Chinese). [Google Scholar]
35. Frostegård, A., Bååth, E., Tunlio, A. (1993). Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biology and Biochemistry, 25(6), 723–730. DOI 10.1016/0038-0717(93)90113-P. [Google Scholar] [CrossRef]
36. Zogg, G. P., Zak, D. R., Ringelberg, D. B., White, D. C., MacDonald, N. W. et al. (1997). Compositional and functional shifts in microbial communities due to soil warming. Soil Science Society of America Journal, 61(2), 475–481. DOI 10.2136/sssaj1997.03615995006100020015x. [Google Scholar] [CrossRef]
37. Frostegård, A., Bååth, E. (1996). The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biology and Fertility Soils, 22(1–2), 59–65. DOI 10.1007/BF00384433. [Google Scholar] [CrossRef]
38. Ibekwe, A. M., Kennedy, A. C. (1998). Phospholipid fatty acid profiles and carbon utilization patterns for analysis of microbial community structure under field and greenhouse conditions. FEMS Microbiology Ecology, 26(2), 151–163. DOI 10.1111/j.1574-6941.1998.tb00501.x. [Google Scholar] [CrossRef]
39. Olsson, P. A. (1999). Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiology Ecology, 29(4), 303–310. DOI 10.1111/j.1574-6941.1999.tb00621.x. [Google Scholar] [CrossRef]
40. Yu, S., He, Z. L., Chen, G. C., Huang, C. Y. (2003). Soil chemical characteristics and their impacts on soil microflora in the root layer of tea plants with different cultivating ages. Acta pedologica sinica, 40(3), 433–439(in Chinese). [Google Scholar]
41. Doran, J. W., Zeiss, M. R. (2000). Soil health and sustainability: Managing the biotic component of soil quality. Applied Soil Ecology, 15(1), 3–11. DOI 10.1016/S0929-1393(00)00067-6. [Google Scholar] [CrossRef]
42. Fang, X. H., Zhang, J., Meng, M., Guo, X., Wu, Y. et al. (2017). Forest-type shift and subsequent intensive management affected soil organic carbon and microbial community in southeastern China. European Journal of Forest Research, 136(4), 689–697. DOI 10.1007/s10342-017-1065-0. [Google Scholar] [CrossRef]
43. Zhong, W. H., Gu, T., Wang, W., Zhang, B., Lin, X. et al. (2010). The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant and Soil, 326(1–2), 511–522. DOI 10.1007/s11104-009-9988-y. [Google Scholar] [CrossRef]
44. Lin, Y. B., Ye, Y. M., Yang, J. H., Hu, Y. M., Shi, H. K. (2019). The effect of land consolidation on soil microbial diversity. Acta Scientiae Circumstantiae, 39(8), 2644–2653 (in Chinese). DOI 10.1016/j.catena.2021.105424. [Google Scholar] [CrossRef]
45. Haynes, R. J., Naidu, R. (1998). Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A review. Nutrient Cycling in Agroecosystems, 51(2), 123–137. DOI 10.1023/A:1009738307837. [Google Scholar] [CrossRef]
46. Zhang, J. Z., Bei, S., Li, B., Zhang, J., Christie, P. et al. (2019). Organic fertilizer, but not heavy liming, enhances banana biomass, increases soil organic carbon and modifies soil microbiota. Applied Soil Ecology, 136, 67–79. DOI 10.1016/j.apsoil.2018.12.017. [Google Scholar] [CrossRef]
47. Ji, L. F., Wu, Z., You, Z., Yi, X., Ni, K. et al. (2018). Effects of organic substitution for synthetic N fertilizer on soil bacterial diversity and community composition: A 10-year field trial in a tea plantation. Agriculture, Ecosystems & Environment, 268, 124–132. DOI 10.1016/j.agee.2018.09.008. [Google Scholar] [CrossRef]
48. Harrison, K. A., Bardgett, R. D. (2010). Influence of plant species and soil conditions on plant-soil feedback in mixed grassland communities. Journal of Ecology, 98(2), 384–395. DOI 10.1111/j.1365-2745.2009.01614.x. [Google Scholar] [CrossRef]
49. Steinweg, J. M., Plante, A. F., Conant, R. T., Paul, E. A., Tanaka, D. L. (2008). Patterns of substrate utilization during long-term incubations at different temperatures. Soil Biology and Biochemistry, 40(11), 2722–2728. DOI 10.1016/j.soilbio.2008.07.002. [Google Scholar] [CrossRef]
50. Ekelund, F., Rønn, R., Christensen, S. (2001). Distribution with depth of protozoa, bacteria and fungi in soil profiles from three Danish forest sites. Soil Biology and Biochemistry, 33(4–5), 475–481. DOI 10.1016/S0038-0717(00)00188-7. [Google Scholar] [CrossRef]
51. Bang-Andreasen, T., Nielsen, J., Voriskova, J., Heise, J., Rønn, R. et al. (2017). Wood ash induced pH changes strongly affect soil bacterial numbers and community composition. Frontiers in Microbiology, 8, 1400. DOI 10.3389/fmicb.2017.01400. [Google Scholar] [CrossRef]
52. Aciego Pietri, J. C., Brookes, P. C. (2009). Substrate inputs and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biology and Biochemistry, 41(7), 1396–1405. DOI 10.1016/j.soilbio.2009.03.017. [Google Scholar] [CrossRef]
53. Wan, W. J., Tan, J., Wang, Y., Qin, Y., He, H. et al. (2020). Responses of the rhizosphere bacterial community in acidic crop soil to pH: Changes in diversity, composition, interaction, and function. Science of the Total Environment, 700, 134–418. DOI 10.1016/j.scitotenv.2019.134418. [Google Scholar] [CrossRef]
54. Holtkamp, R., Kardol, P., Wal, A., Dekker, S., Putten, W. et al. (2008). Soil food web structure duringecosystem development after land abandonment. Applied Soil Ecology, 39(1), 23–34. DOI 10.1016/j.apsoil.2007.11.002. [Google Scholar] [CrossRef]
55. Rousk, J., Bååth, E., Brookes, P. C. (2010). Soil bacterial and fungal communities across a pH gradient in an arable soil. The ISME Journal, 4(10), 1340–1351. DOI 10.1038/ismej.2010.58. [Google Scholar] [CrossRef]
56. Fitter, A. H., Helgason, T., Hodge, A. (2011). Nutritional exchanges in the arbuscular mycorrhizal symbiosis: Implications for sustainable agriculture. Fungal Biology Reviews, 25(1), 68–72. DOI 10.1016/j.fbr.2011.01.002. [Google Scholar] [CrossRef]
57. Soka, G. E., Ritchie, M. E. (2016). Contributions of AM fungi and soil organic matter to plant productivity in tropical savanna soils under different land uses. Rhizosphere, 1, 45–52. DOI 10.1016/j.rhisph.2016.06.004. [Google Scholar] [CrossRef]
58. Xu, T. L., Veresoglou, S. D., Chen, Y. L., Rillig, M. C., Xiang, D. et al. (2016). Plant community, geographic distance and abiotic factors play different roles in predicting AMF biogeography at the regional scale in northern China. Environmental Microbiology Reports, 8(6), 1048–1057. DOI 10.1111/1758-2229.12485. [Google Scholar] [CrossRef]
59. Yang, W. Y., Sun, L. Y., Song, F. B., Yang, X. Q., Zhang, M. J. et al. (2019). Research advances in species diversity of arbuscular mycorrhizal fungi in terrestrial agro-ecosystem. Chinese Journal of Applied Ecology, 30(11), 3971–3979. DOI 10.13287/j.1001-9332.201911.036. [Google Scholar] [CrossRef]
60. Dumbrell, A. J., Nelson, M., Helgason, T., Dytham, C., Fitter, A. H. (2010). Relative roles of niche and neutral processes in structuring a soil microbial community. The ISME Journal, 4(3), 337–345. DOI 10.1038/ismej.2009.122. [Google Scholar] [CrossRef]
61. Fu, W., Fu, Z., Zhao, K., Tunney, H., Zhang, C. (2014). Variation of soil P and other nutrients in a long-term grazed grassland P experiment field. Archives of Agronomy and Soil Science, 60(10), 1459–1466. DOI 10.1080/03650340.2014.891018. [Google Scholar] [CrossRef]
62. Bainard, L. D., Chagnon, P. L., Cade-Menun, B. J., Lamb, E. G., LaForg, E. G. et al. (2017). Plant communities and soil properties mediate agricultural land use impacts on arbuscular mycorrhizal fungi in the Mixed Prairie ecoregion of the North American great plains. Agriculture. Ecosystems and Environment, 249(1), 187–195. DOI 10.1016/j.agee.2017.08.010. [Google Scholar] [CrossRef]
63. Vries, F. T., Ellis, H., Nick, V. E., Lijbert, B., Jaap, B. (2006). Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biology and Biochemistry, 38(8), 2092–2103. DOI 10.1016/j.soilbio.2006.01.008. [Google Scholar] [CrossRef]
64. Wang, X. L., Zhang, W., Shao, Y., Zhao, J., Zhou, L. et al. (2019). Fungi to bacteria ratio: Historical misinterpretations and potential implications. Acta Oecologica, 95(7), 1–11. DOI 10.1016/j.actao.2018.10.003. [Google Scholar] [CrossRef]
65. Djukic, I., Zehetner, F., Watzinger, A., Horacek, M., Gerzabek, M. H. (2013). In situ carbon turnover dynamics and the role of soilmicroorganisms therein: A climate warming study in an Alpine ecosystem. FEMS Microbiology Ecology, 83(1), 112–124. DOI 10.1111/j.1574-6941.2012.01449.x. [Google Scholar] [CrossRef]
66. Vries, F. T., Shade, A. (2013). Controls on soil microbial community stability under climate change. Frontiers in Microbiology, 4, 265. DOI 10.3389/fmicb.2013.00265. [Google Scholar] [CrossRef]
67. Fanin, N., Kardol, P., Farrell, M., Nilsson, M. C., Gundale, M. J. et al. (2019). The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biology and Biochemistry, 128, 111–114. DOI 10.1016/j.soilbio.2018.10.010. [Google Scholar] [CrossRef]
68. Saetre, P., Bååth, E. (2000). Spatial variation and patterns of soil microbial community structure in a mixed spruce-birch stand. Soil Biology and Biochemistry, 32(7), 909–917. DOI 10.1016/S0038-0717(99)00215-1. [Google Scholar] [CrossRef]
69. Kourtev, P. S., Ehrenfeld, J. G., Häggblomb, M. (2003). Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities. Soil Biology and Biochemistry, 35(7), 895–905. DOI 10.1016/S0038-0717(03)00120-2. [Google Scholar] [CrossRef]
70. Kramer, C., Gleixner, G. (2008). Soil organic matter in soil depth profiles: Distinct carbon preferences of microbial groups during carbon transformation. Soil Biology and Biochemistry, 40(2), 425–433. DOI 10.1016/j.soilbio.2007.09.016. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |