

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.021556
REVIEW
Crop Improvement and Abiotic Stress Tolerance Promoted by Moringa Leaf Extract
1Department of Genetics and Plant Breeding, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
2Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, AB T6G 2R3, Canada
3Chemistry Research Unit, United States Department of Agriculture-Agricultural Research Service, Gainesville, 32608, USA
4Department of Biochemistry & Molecular Biology, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
5Department of Crop Botany, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
6Department of Agronomy and Haor Agriculture, Sylhet Agricultural University, Sylhet, 3100, Bangladesh
7Botany and Microbiology Department, Faculty of Science, Assiut University, Assiut, 71516, Egypt
8Botany and Microbiology Department, Faculty of Science, South Valley University, Qena, 83523, Egypt
9Department of Botany and Plant Physiology, Czech University of Life Sciences Prague, Prague, 165 00, Czech Republic
10Institute of Plant and Environmental Sciences, Faculty of Agrobiology and Food Resources, Slovak University of Agriculture, Nitra, 94976, Slovakia
*Corresponding Authors: Mona F. A. Dawood. Email: mo_fa87@aun.edu.eg; Md. Tahjib-UI-Arif. Email: tahjib@bau.edu.bd
Received: 20 January 2022; Accepted: 04 March 2022
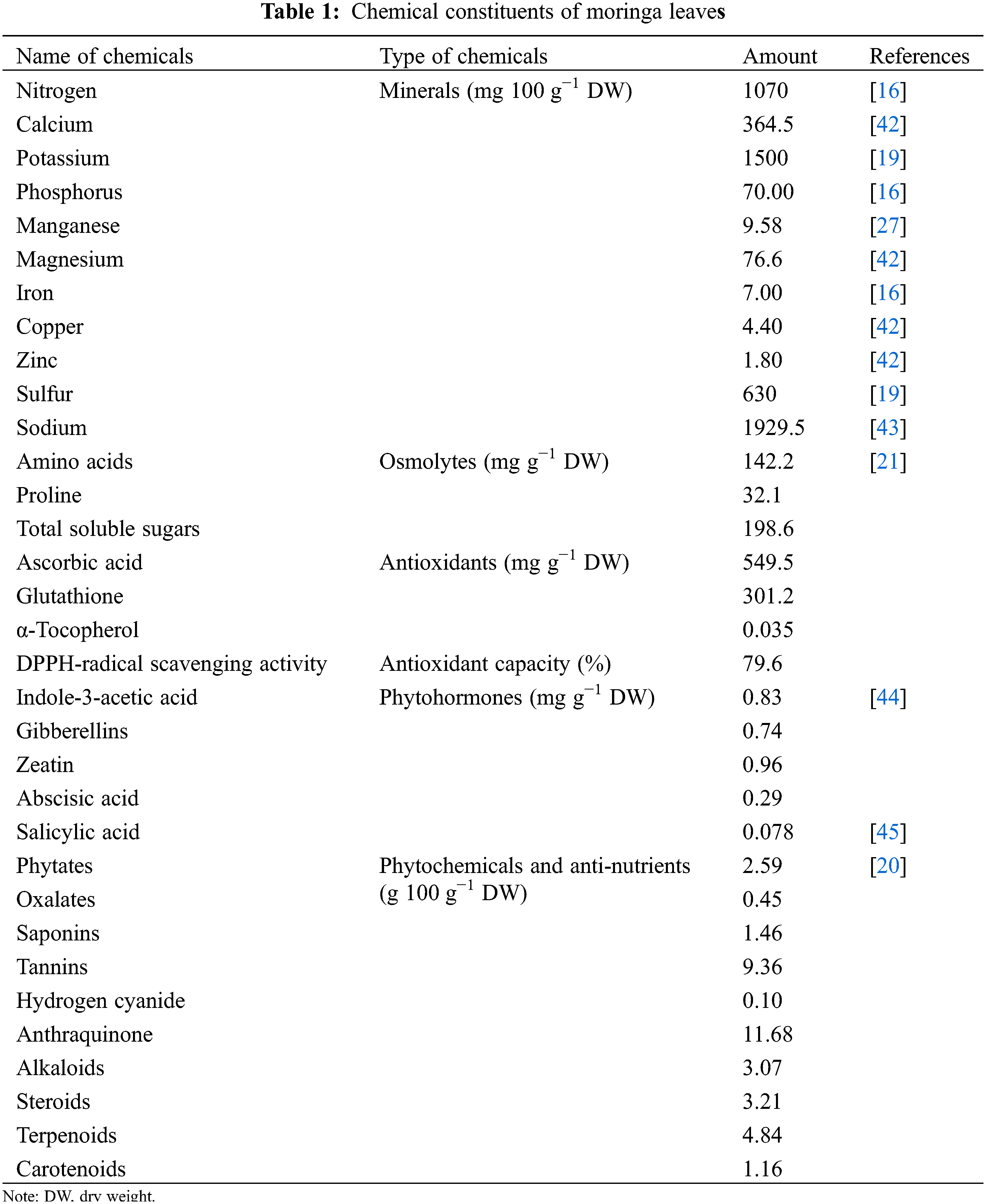
Abstract: Moringa leaf extract (MLE) has been shown to promote beneficial outcomes in animals and plants. It is rich in amino acids, antioxidants, phytohormones, minerals, and many other bioactive compounds with nutritional and growth-promoting potential. Recent reports indicated that MLE improved abiotic stress tolerance in plants. Our understanding of the mechanisms underlying MLE-mediated abiotic stress tolerance remains limited. This review summarizes the existing literature on the role of MLE in promoting plant abiotic stress acclimation processes. MLE is applied to plants in a variety of ways, including foliar spray, rooting media, and seed priming. Exogenous application of MLE promoted crop plant growth, photosynthesis, and yield under both nonstress and abiotic stress conditions. MLE treatment reduced the severity of osmotic and oxidative stress in plants by regulating osmolyte accumulation, antioxidant synthesis, and secondary metabolites. MLE also improves mineral homeostasis in the presence of abiotic stress. Overall, this review describes the potential mechanisms underpinning MLE-mediated stress tolerance.
Keywords: Abiotic stress; antioxidants; biostimulant; plant growth; moringa extract; osmotic stress; oxidative stress
Plant growth is hampered by abiotic stresses such as drought, extreme temperatures, flooding, salinity, ozone, ultraviolet radiation, and heavy metals that together cause crop yield losses estimated to be up to 50% worldwide [1]. Abiotic stresses disrupt normal growth, development, metabolism, and productivity. They impact plants throughout development, from seed germination to maturity, disrupting a multitude of physiological, biochemical, and molecular processes [2–6]. Drought- and saline-affected lands are becoming more common across the world, a trend that is expected to continue [7], and agricultural lands near urban centers continue to be polluted with heavy metals [8]. Approximately 21% of the agricultural land area is affected by salinity stress [9]. Some predict that 30% of arable land will be made ill-suited for agriculture by salinization by the end of 2028, and 50% by the middle of the twenty-first century [9]. Global temperature is expected to increase by approximately 3°C with CO2 concentrations reaching approximately 500–1000 ppm by 2100 [10]. During abiotic stress, which is expected to be more common with changing climates, plants accumulate reactive oxygen species (ROS) that cause physiological harm [11,12]. For instance, salinity and drought [13,14], heavy metals [15] and cold stress [4] inhibit photosynthesis and disrupt plant water relations and metabolic homeostasis.
Moringa oleifera L. (drumstick) is a cultivated species that belongs to the Moringaceae family [16]. It originated in the sub-Himalayan region of India, Pakistan, Bangladesh, Afghanistan, and Egypt, but is now found in many of the world’s tropical and subtropical regions [17]. Due to its exceptional nutritional and medicinal properties, moringa has been used in agriculture as a yield enhancer and in medicine as a nutritional supplement [18]. Extensive research into its chemical composition and medical applications has been conducted, but the use of moringa in crop treatment for abiotic stress tolerance is a relatively new research area. Moringa leaf extract (MLE) represents an organic and sustainable source of plant growth-promoting compounds, growth regulators, osmoprotectants, antioxidants, secondary metabolites, and mineral nutrients that promote plant resiliency to stress [19–21].
This review aims to discuss the use of MLE in protecting plants from environmental stress, summarizing recent results that have investigated the mitigating effects of MLE on abiotic stress. MLE-induced plant improvement under nonstressed conditions is also discussed. Finally, we present a mechanistic view of MLE-induced crop defense. The following paragraphs of this review address the benefits of MLE on osmolyte balance, antioxidant status, oxidative stress mitigation, mineral absorption, and phytohormone control in plants.
2 Moringa Leaf Extract: Chemical Composition
Moringa leaf extract contains high levels of plant growth hormones, antioxidants, vitamins, secondary metabolites, and minerals (Table 1) [22–24]. Growth hormones such as gibberellins, indole-3-acetic acid (IAA), abscisic acid (ABA), salicylic acid (SA), and cytokinins, minerals such as sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), zinc (Zn2+), iron (Fe3+), and manganese (Mn2+), more than 40 natural antioxidants such as ascorbic acid (ASC), glutathione (GSH), β-carotene, tocopherols, vitamins A, B, C, D, and K, and many secondary metabolites occur at high levels in MLE [16,20,25–34]. Of particular note, plant growth-regulating cytokinins are present in the forms of zeatin, dihydrozeatin and isopentyladenine [35,36]. Among these, zeatin contents remain at very high concentrations between 5 and 200 μg g−1 [37,38]. Additionally, there are high levels of several allelochemicals, including isothiocyanates and nitriles [39,40]. Of course, the chemical composition of MLE can vary with developmental stage, tissue, and growing conditions [41].
3 Exogenous Application of MLE to Alleviate Abiotic Stress
Abiotic stresses such as salinity, drought, flooding, heat, cold and heavy metals inhibit the growth and development of plants and reduce crop yield [46–48]. One possible solution to offset yield loss is the application of organic biostimulants such as MLE, which is considered a more ecofriendly and sustainable approach than chemicaclly synthesized fertilizers and protectants [49]. MLE can improve seedling emergence, plant growth, development and yield during periods of abiotic and biotic stresses [49]. In recent years, several studies have examined the mitigation of abiotic stress via exogenous application of MLE, the results from which are summarized in Table 2. In the following sections, we will discuss what is known regarding the impact of MLE on plants under various abiotic stresses.
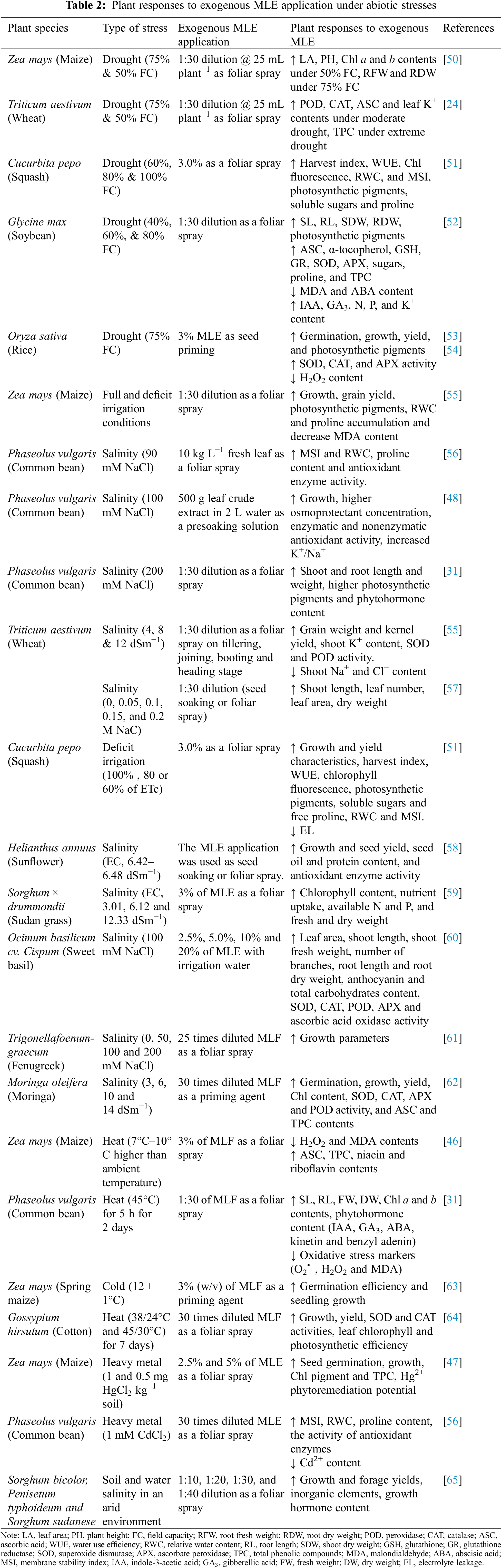
Water accounts for 80%–95% of the fresh biomass of plants and plays a vital role in physiological processes, including plant growth, development, and metabolism [66]. Thus, water scarcity or osmotic stress is considered the main environmental constraint for crops that could destabilize world food security [67]. Drought stress typically leads to a reduction in leaf size, stem elongation, root growth, and water use efficiency (WUE) [50,55,68]. Other effects of drought include the reduction of photosynthetically active radiation, a curtailed harvest index (HI) [69], metabolic disruptions [70], the inhibition of certain enzymatic activities [24], the reduction of soil water potential, ionic imbalance and disturbances in solute accumulation [71,72]. MLE has been shown to be an effective plant growth modulator during drought stress events [73]. Foliar or root application of MLE led to the enhancement of leaf area, plant height (PH), biomass production, RWC, WUE, MSI, and chlorophyll content in maize (Zea mays L.) [50,55], Glycine max (soybean) [52] and Cucurbita pepo (Squash) [51] under drought stress. MLE application increased the accumulation of osmoprotectants and enzymatic and nonenzymatic antioxidants such as peroxidase (POD), catalase (CAT), ascorbate (ASC) and leaf K+ contents in Triticum aestivum (wheat) under drought stress [24]. Moreover, MLE application increased total phenolic compounds (TPCs) in wheat plants under extreme drought [24]. Electrolyte leakage (EL) along with morphophysiological trait improvement was also observed after MLE application to drought-stressed squash plants [51]. Finally, exogenous MLE application enhanced the yield of maize under drought stress [55].
Soil salinity can negatively impact crop yield by affecting growth parameters [74,75]. Salinity affects plant growth by disrupting physiological and biochemical processes, particularly water relations and nutrient balance [76]. Salinity can have major impacts on germination by altering seed imbibition due to the lower osmotic potential of soil [77], changing nucleic acid metabolism and transcriptome profiles [78,79], altering protein metabolism [80], and disturbing hormonal balance [81].
To help alleviate the harmful effects of soil salinity on crops, several growth regulators, osmoprotectants and fertilizers have been successfully used [82], including MLE [83]. Previous research revealed that moringa leaves contain high levels of essential plant nutrients, hormones, and antioxidants [84]. Therefore, MLE application improved salt stress tolerance and grain yield in wheat by enhancing seed germination, protein synthesis, and antioxidant activities under salinity stress [28]. Foliar application of MLE to wheat modulated antioxidants, proteins, and essential mineral content in a way that helped ameliorate the negative effects of salinity stress [55]. Exogenous MLE application to salt stressed Phaseolus vulgaris (common bean) led to increased shoot and root length and weight, a response associated with higher photosynthetic pigments, membrane stability index (MSI), relative water content (RWC) and phytohormone content [56,61]. Enhanced fresh weight, dry weight, mineral uptake such as nitrogen (N) and phosphorus (P) uptake, and protection against photooxidative damage in chlorophylls under salt stress conditions were also found in MLE applied to salt stressed Sorghum × drummondii (Sudan grass) plants [59]. Salinity stress can trigger metabolic disruptions and arrest protein synthesis and these effects are prevented by exogenous MLE, and that can play a key role in the signaling of plant adaptive responses to salinity [61].
Seed priming with MLE improved salt tolerance in common bean by enhancing osmolyte accumulation, chlorophyll pigments, enzymatic and nonenzymatic antioxidants, and K+ content [48]. Furthermore, pretreatment of Moringa oleifera seeds with MLE improved seedling emergence and growth characteristics, nutrient homeostasis, and superoxide dismutase (SOD) and catalase (CAT) activities under salt stress [62]. Both foliar application and seed presoaking with MLE led to increased growth, yield and changes in stem anatomy, including stem section diameter, average number of xylem vessels, average thickness of xylem vessels, and average diameter of xylem vessels, in salt-stressed Helianthus annus (sunflower) [58]. MLE-treated, salt-stressed sunflower plants showed higher antioxidant enzyme activity, proline and soluble sugar accumulation, and N, P, and K+ contents than non-MLE-treated, salt-stressed plants [58]. Enhanced anthocyanin, total carbohydrate, and antioxidant potentials such as SOD, CAT, POD, ascorbate peroxidase (APX) and ASC oxidase were also observed in MLE-treated Ocimum basilicum cv. Cispum (sweet basil) plants under salt stress [60].
Global warming is posing a major concern for humanity by changing climate patterns and increasing temperature. Heat stress severely impacts plant growth and development, threatening crop production and food security [85]. Application of MLE has been shown to combat heat stress in maize plants by reducing oxidative damage markers (hydrogen peroxide, H2O2 and lipid peroxidation products, MDA) and enhancing antioxidant potentials such as ASC, TPCs, and niacin and riboflavin contents [46]. Additionally, MLE treatment enhanced growth and yield in heat-stressed Gossypium hirsutum (cotton) plants by improving photosynthetic efficiency, causing higher chlorophyll content, and promoting higher SOD and CAT activities [64]. MLE application also mitigated the growth inhibitory effects of heat stress in common bean by enhancing the levels of IAA, GA3, ABA, kinetin and benzyl adenine and reducing oxidative stress markers [31]. Finally, MLE has been shown to improve cold stress tolerance in spring maize by improving the germination rate and growth [62].
Heavy metals in excessive concentrations can disturb plant growth, development, metabolism, and senescence [86]. Exogenous MLE has been found to increase the tolerance of plants to heavy metal stress. Howladar [56] showed that foliar application of MLE treatment improved cadmium stress tolerance; increased photosynthetic pigments, RWC, proline content, MSI and WUE; and decreased electrolyte leakage (EL) in common bean [56]. Moreover, MLE application enhanced antioxidant enzyme activities and reduced lipid peroxidation in cadmium-stressed common bean plants [56]. Bibi et al. [47] demonstrated that MLE improved the germination, growth and chlorophyll content of maize seedlings under mercury stress.
4 Possible Mechanisms of MLE-Mediated Abiotic Stress Tolerance
To explore the mechanisms underlying MLE-mediated abiotic stress tolerance, the following sections summarize recent reports on the interaction of MLE with major osmolytes, mineral nutrients, secondary metabolites, phytohormones, ROS signaling, and the modulation of antioxidants.
4.1 Influence of MLE on Osmolytes
The synthesis and accumulation of osmolytes, compounds that counterbalance osmotic pressure, are among the first responses of host plants to osmotic stress caused by environmental challenges [87]. The accumulation of solutes in plant cells undergoing stress conditions causes the osmotic potential of the cells to become highly negative and leads to endosmosis of water to maintain cell turgor. This osmotic adjustment is controlled by the accumulation of solutes/osmolytes [88] and is an important factor for combatting drought [89,90] salinity [91], osmotic [92], heavy metal [93], temperature [94], light, and pesticide stress [95] (Fig. 1). Upon perception of abiotic stress, signaling pathways induce transcription factors that upregulate stress responsive genes related to biosynthesis and accumulation of osmolytes, including free amino acids and their derivatives, carbohydrates and soluble sugars, polyols, polyamines, free amines, and other secondary metabolites [87].
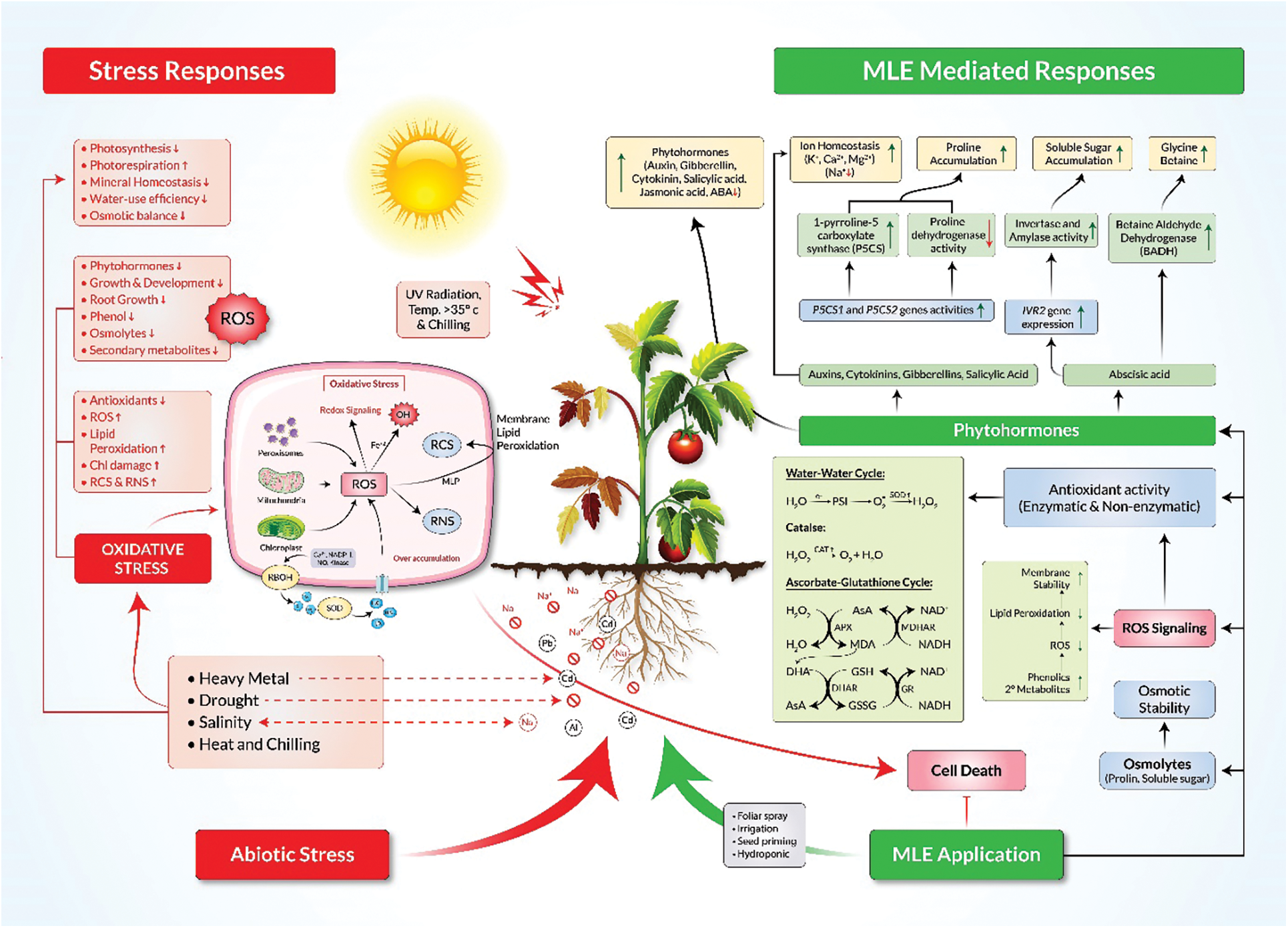
Figure 1: Potential mechanisms of MLE-mediated abiotic stress tolerance in plants. MLE consists of a complex blend of phytohormones, minerals, antioxidants and secondary metabolites that promote enhanced phytohormone production, osmolyte accumulation, ion homeostasis and scavenging of reactive oxygen species (ROS). MLE mediates the detoxification of ROS by triggering the water-water cycle and the ascorbate-glutathione cycle and by promoting the accumulation of secondary metabolites in cells. It also protects plants from overaccumulation of reactive carbonyl species (RCS) and reactive nitrogen species (RNS). MLE, Moringa leaf extract; ABA, abscisic acid; AsA, ascorbic acid; GSH, reduced glutathione; GSSG, oxidized glutathione; P5CS, Δ1-pyrroline-5-carboxylate synthetase; BADH, betaine aldehyde dehydrogenase
A range of osmotically active molecules accumulate under drought stress. Among these, proline helps to adjust the cellular osmotic balance, protect biological membranes, and stabilize enzymes and proteins by detoxifying excess ROS [96]. Proline accumulation under stress conditions results from increasing synthesis and degradation of proteins [97,98]. Numerous reports show that exogenous application of MLE increases the abundance of proline and other osmolytes under various abiotic stresses (Table 3). Treatment of sunflower with MLE via seed soaking or foliar spray led to increased total soluble sugar and proline contents and resulted in improved sunflower growth, seed yield and oil content under salt stress [58]. Similarly, MLE application improved osmolyte status in salt stressed Trigonellafoenum-graecum (Fenugreek) [48], common bean [61], and Sudan grass [59], resulting in improved growth and development of plants. In addition, the application of MLE to drought-stressed Zea mays enhanced proline content [55]. MLE also induced proline and total soluble sugar contents in drought-stressed Glycine max (Soybean) [52] and Cucurbita pepo (Squash) [51] leading to improved growth and development. Moreover, Zea mays subjected to chilling stress and treated with MLE showed an increase in proline content [63]. The increase in proline could be due to enhanced gene expression of biosynthetic genes that may be induced by MLE responsive phytohormones such as auxins, gibberellins, cytokinins, and abscisic acid (Table 1; Fig. 1) all of which have been shown to promote osmolyte accumulation [96]. The proline biosynthetic genes P5CS1 and P5CS2 are up-regulated by auxins, while cytokinin downregulates P5CS1 but upregulates P5CS2 in Arabidopsis [99–102] (Fig. 1). A gibberellic acid (GA)-responsive element, GARE, is present upstream of SbP5CS. Proline biosynthesis is also modulated by ABA-dependent pathways [100] (Fig. 1).
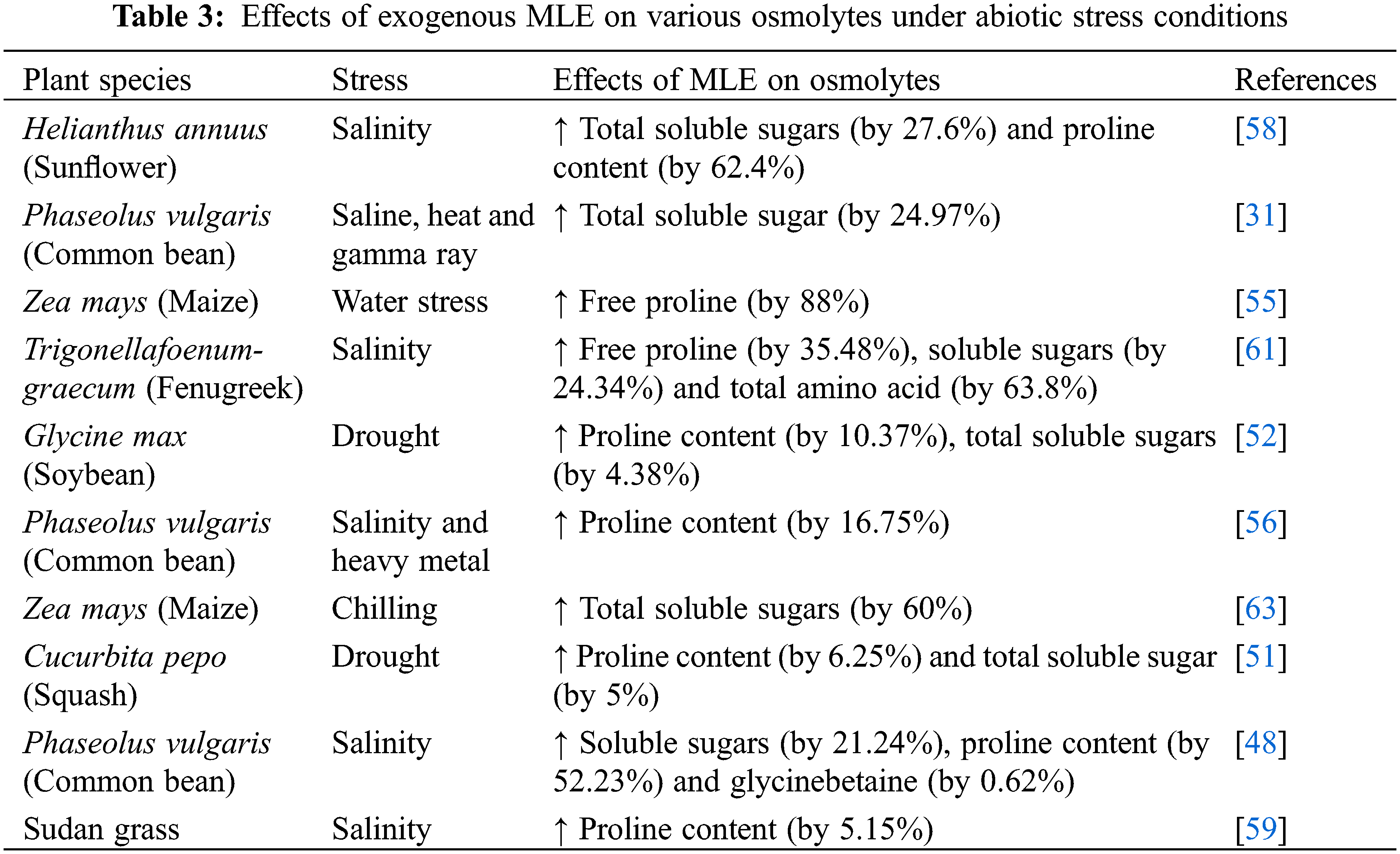
Application of MLE also promotes the accumulation of glycinebetaine, another important osmolyte [103]. Glycinebetaine is synthesized from choline in a two-step oxidation by a ferredoxin (Fd)-dependent choline monooxygenase (CMO) and a betaine aldehyde dehydrogenase (BADH) with a strong preference for nicotinamide adenine dinucleotide (NAD+), typically via the unstable intermediate betaine [87]. Glycinebetaine biosynthesis is induced under abiotic stress after the application of the MLE component ABA, which activates the GB biosynthetic enzyme BADH [104,105].
4.2 Influence of MLE on Mineral Nutrients
Treatment of plants with MLE can help support mineral homeostasis, which is critical for plants to tolerate abiotic stresses [106]. Salinity stress is associated with the reduction of chlorophyll content caused by excessive Na+ accumulation in leaves, which leads to reduced Mg2+ and downregulation of chlorophyll biosynthesis [107]. Mg2+ deficiency can also disrupt the vascular system, transportation of carbohydrates, and protein synthesis [108–110]. Moreover, salt stress can interrupt K+ and Ca2+ uptake and transportation [111] and cause salt-sensitive plants to have lower K+/Na+ and Ca2+/Na+ under salinity conditions [112]. The K+/Na+ ratio is an important factor for estimating plant growth rates, and increasing the K+/Na+ and Ca2+/Na+ ratios leads to the activation of plant defenses [113–117]. However, antagonistic relationships between Na+ and ions such as K+, Ca2+ and Mg2+ have been observed in salt-tolerant crops [61,113,117,118]. These antagonisms were amplified in crops such as lettuce, wheat, okra, fenugreek and Brassica juncea after the application of MLE [24,111,115]. This amplification resulted from increased K+, Ca2+, Mg2+ and better maintenance of the K+/Na+ and Ca2+/Na+ ratios, which served to protect photosynthetic pigments [31]. It is possible that components of MLE, such as hormones like IAA, GAs, SA, and ABA, function to maintain ion homeostasis [21,119]. In plants under salinity stress, exogenous application of auxins, ABA and SA have all been shown to enhance Ca2+ and K+ [120–122], while application of GA and IAA enhance Mg2+ [120,121]. Additionally, MLE contains high levels of Mg2+, Ca2+ and K+, which provides plants with greater exposure to these nutrients and promotes tolerance to abiotic stresses [19,42].
4.3 Influence of MLE on ROS Signaling and Antioxidants
Redox homeostasis is fundamental to cellular function and integrity, and its regulation includes control of ROS and modulation of the cellular redox state [123]. The equilibrium between the production and scavenging of ROS such as singlet oxygen (1O2), hydrogen peroxide (H2O2), superoxide (O2•ˉ), and hydroxyl radicals (•OH) is controlled by enzymatic and nonenzymatic antioxidants [123,124]. The enzymatic antioxidants responsible for scavenging ROS are SOD, CAT, the ASC-GSH cycle enzymes [APX, monodehydroascorbate reductases (MDHAR), dehydroascorbate reductases (DHAR), glutathione reductase (GR)], peroxiredoxins (PRX), glutathione peroxidase (GPX), and glutathione-S-transferase (GST), whereas the nonenzymatic antioxidants include more diverse compounds such as ASC, GSH, phenolic compounds, alkaloids, nonprotein amino acids, and α-tocopherols [123,125–128]. Upregulation of antioxidant enzymes occurs when plants are exposed to oxidative stress. This upregulation serves as a proactive acclimation response that results in lower ROS levels and higher tolerance to conditions that cause oxidative stress [123], and promoting this process can improve a plant’s tolerance and adaptive capacity to abiotic stresses [129–131]. The primary mechanism by which plants balance ROS is the ASC-GSH pathway [128,132], which involves the successive oxidation and reduction of ascorbate, glutathione, and NADPH. The redox reactions are catalyzed enzymatically by APX, MDHAR, DHAR, and GR and nonenzymatically by tocopherol, carotenoids, and phenolic compounds [128,132–134] (Fig. 1).
Exogenous application of MLE to plants under abiotic stress can supplement antioxidants such as ASC and GSH (Table 4). It is possible that MLE application directly supplements ASC and GSH and thereby helps to improve abiotic stress tolerance. In various salt-stressed plant species, MLE promoted the activities of SOD, CAT, APX, GR, and POD and led to higher ASC and GSH contents (Table 4). The enhanced activity of the abovementioned enzymes resulted in a decline in oxidative damage to cells and growth improvement, highlighting the direct involvement of MLE in stress mitigation [61]. The improved antioxidant system in MLE-treated plants helps lower oxidative stress and peroxidation of lipids [136], enhances biosynthesis of cysteine and GSH to maintain the GSH/GSSG ratio [137–139], increases the accumulation of osmolytes such as proline and glycinebetaine [137] and α-tocopherol [140], all of which help plants withstand abiotic stress.
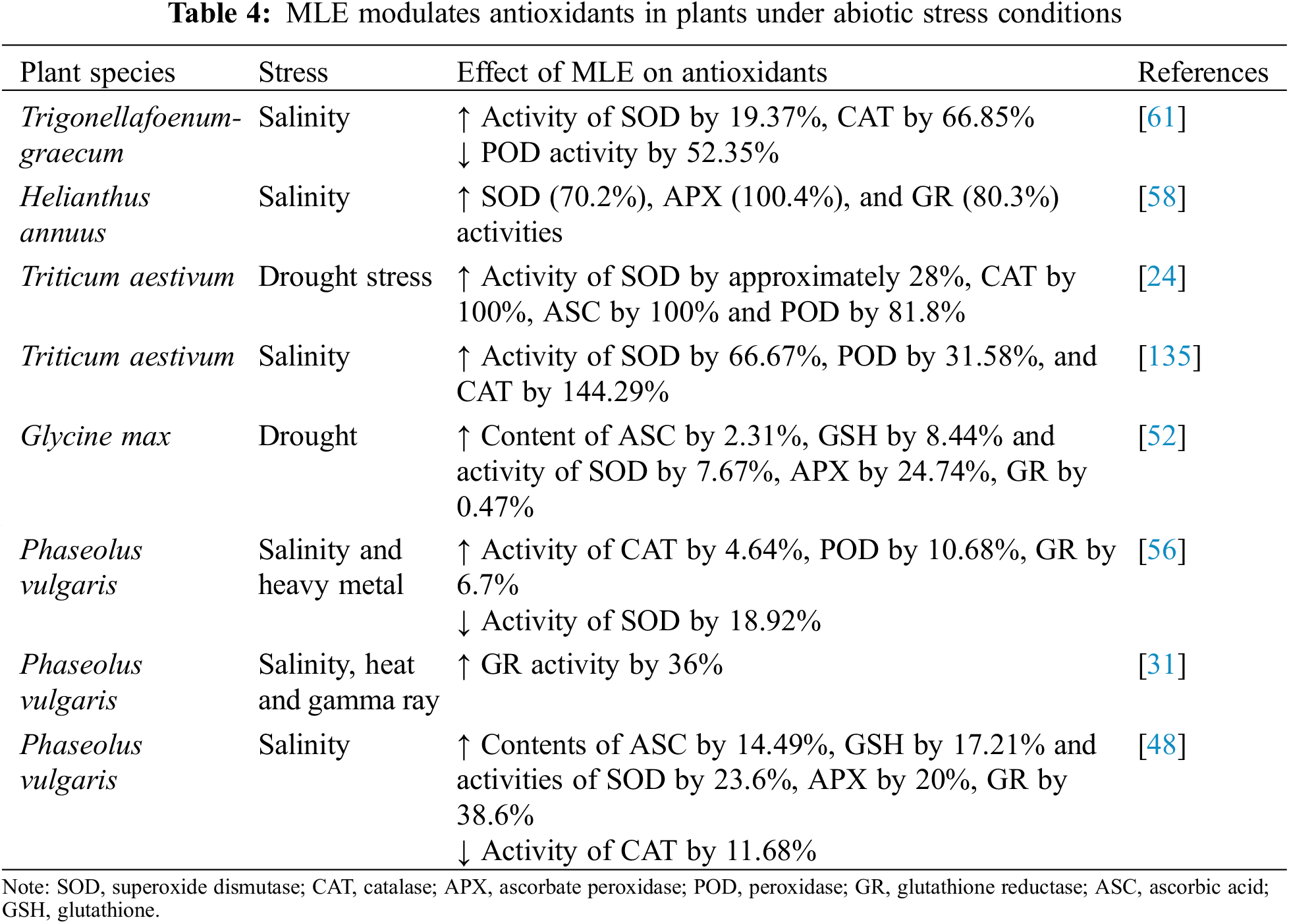
The antioxidant α-tocopherol is a primary component of MLE (Table 1). Exogenous application of α-tocopherol to plants under drought and salt stress promotes stress tolerance, enhances tocopherol content, and decreases lipid peroxidation [141,142]. The upregulation of proline is also associated with H2O2 accumulation and the activity of antioxidant enzymes such as SOD, POD, APX and CAT under abiotic stress [143]. Taken together, MLE application supplements the plants with antioxidants present in MLE itself and increases endogenous antioxidant activity and production that ultimately helps plants withstand abiotic stresses (Fig. 1).
4.4 Influence of MLE on Major Secondary Metabolites
Plants produce and accumulate high levels of secondary metabolites such as phenylpropanoids, flavonoids, tannins, coumarins, and lignin precursors, a group of metabolites collectively known as phenolics that are involved in scavenging free radicals and enhancing membrane stability under stress conditions [98,144–146]. There are large quantities of phenolics in MLE (Table 1), and these have been suggested to be responsible for the prevention of membrane leakage and lipid peroxidation observed in MLE-treated, salt-stressed Phaseolus vulgaris plants [56]. MLE-treated Phaseolus vulgaris had higher levels of phenolics, which enhanced salt tolerance and membrane stability by ameliorating ROS [135] (Fig. 1). MLE application also enhanced carotenoids, which help protect proteins, DNA, and RNA from damage by quenching free radicals produced during photosynthesis [12,147,148]. Anthocyanin, another phenolic compound found in MLE, acts as an antioxidant under stress conditions [149–153]. Therefore, plants supplemented with MLE receive a wide range of secondary metabolites that may directly protect plants against abiotic stress-induced oxidative damage and thus enhance stress tolerance.
4.5 Influence of MLE on Phytohormones
Exogenous application of MLE can modulate phytohormone contents in plants. Supplementation with MLE increased auxins, gibberellins, and cytokinins but decreased ABA in common bean plants under salinity, heat and gamma ray stress conditions [31]. Similarly, fertilization of rocket plants with MLE enhanced auxin, gibberellin and cytokinin contents and reduced ABA content under nonstress conditions [154]. Spraying common bean with MLE increased the contents of benzoic acid, trans-cinnamic acid, SA, trans-jasmonic acid, IAA, indole-3-propionic acid, indole-3-butyric acid, trans-zeatin, trans-zeatin riboside, gibberellic acid (GA3), gibberellin A4 (GA4), gibberellin A7 (GA7), and decreased ABA content [155]. MLE contains high levels of phytohormones such as zeatin, dihydrozeatin and isopentyladenine [35–38], auxins, gibberellins and salicylates [154,155]. Hormones present in MLE may contribute to the improvement in abiotic stress tolerance and growth observed in MLE-treated plants (Fig. 1).
5 Role of MLE in Crop Improvement under Nonstress Conditions
Along with mitigating abiotic stresses, exogenous MLE can also provide benefits under nonstress conditions by improving plant growth, development, and agronomic characteristics (Table 5). For instance, seed priming with MLE can promote germination indices under nonstressed conditions in a wide range of plant species, including pea [156], wheat [135], okra [157], maize [158] and pepper [159]. Seed pretreatment with MLE solutions improved the rate of seed emergence, vigor of seedlings, and overall growth of wheat plants [135]. Moreover, seed priming with MLE enhanced germination, plant growth, α-amylase activity, and total soluble sugars in pea seedlings under nonstress conditions [156]. Numerous studies have reported that exogenous application of MLE improved the vegetative growth of plants and economic yield performance of several plant species, including snap bean [160], okra [157], Freesia hybrida [161], Cyperous rotandous [162], wheat [163,164], tomato [165,166], maize [167], soybean [168], pepper [169], sweet pepper [170], lettuce [171], sunflower [172], and gladiolus [173]. Both vegetative growth parameters such as PH, SL, SFW, SDW, and leaf number as well as yield components such as cob length, cob diameter, grains per cob, 100-grain weight, and grain weight per plant were improved after foliar application of MLE to maize [167]. Moreover, Prunus salicina trees sprayed with MLE exhibited higher fruit setting, total yield, fruit weight, firmness, color, TSS value, titrable acidity ratio, ascorbic acid content, anthocyanin content, antioxidant activity, reduced titrable acidity and less fruit drop compared to untreated plants [174].

Application of exogenous MLE can also boost nutrient content in a variety of plant species (as summarized in Table 3). Foliar spray of MLE enhanced N, P, K+, Ca2+, Mg2+, and Zn2+ contents in leaves of Kinnow’ mandarin [181]. Similarly, higher contents of N, P, K+, Ca2+, Mg2+, and Fe2+ were observed in the rocket (Eruca vesicaria subsp. sativa) plants when sprayed with MLE [154]. Additionally, exogenous application of MLE can improve photosynthetic efficiency under nonstress conditions [154]. For instance, exogenous MLE application on rocket plants increased the photosynthetic rate, stomatal conductance, chl a and chl b, and carotenoid contents compared with untreated plants [154].
6 Conclusion and Future Prospects
Application of MLE has been shown to be an effective and eco-friendly approach to protect plants against abiotic stressors. The complex blend of antioxidants, metabolites, phytohormones, and minerals present in MLE appears to help protect plants by influencing many aspects of plant physiology, metabolism, hormone signaling, cellular homeostasis, redox potential, and developmental processes. Additional investigations into the precise nature of the protection offered by MLE are needed and may provide information important for crop plant protection and crop productivity, helping ensure food security. Future studies should aim to identify the particular MLE bioactive molecules that confer stress tolerance in plants and the underlying mechanisms.
Authors Contribution: Conceptualization: MT-U-A; writing original draft: MAUI and JAN; editing and revision: MT-U-A, CTH, MSH, AS, AAMS, MB, MFAD, and AAHAL. All authors approved the final version of the manuscript.
Acknowledgement: The use of trade name, commercial product or corporation in this publication is for the information and convenience of the reader and does not imply an official recommendation, endorsement or approval by the USDA or the Agricultural Research Service for any product or service to the exclusion of others that may be suitable. USDA is an equal opportunity provider and employer.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Yadav, S., Modi, P., Dave, A., Vijapura, A., Patel, D. et al. (2020). Effect of abiotic stress on crops. In: Hasanuzzaman, M., Filho, M. C. M. T., Fujita, M., Nogueira, T. A. R. (Eds.Sustainable crop production. IntechOpen Publishing, London. DOI 10.5772/intechopen.88434. [Google Scholar] [CrossRef]
2. Nupur, J. A., Hannan, A., Islam, M. A. U., Sagor, G., Robin, A. H. K. (2020). Root development and anti-oxidative response of rice genotypes under polyethylene glycol induced osmotic stress. Plant Breeding and Biotechnology, 8(2), 151–162. DOI 10.9787/PBB.2020.8.2.151. [Google Scholar] [CrossRef]
3. Sharma, A., Kumar, V., Shahzad, B., Ramakrishnan, M., Sidhu, G. P. S. et al. (2019). Photosynthetic response of plants under different abiotic stresses: A review. Journal of Plant Growth Regulation, 39, 509–531. DOI 10.1007/s00344-019-10018-x. [Google Scholar] [CrossRef]
4. Sohag, A. A. M., Tahjib-Ul-Arif, M., Afrin, S., Khan, M. K., Hannan, M. A. et al. (2020). Insights into nitric oxide-mediated water balance, antioxidant defence and mineral homeostasis in rice (Oryza sativa L.) under chilling stress. Nitric Oxide, 100, 7–16. DOI 10.1016/j.niox.2020.04.001. [Google Scholar] [CrossRef]
5. Tahjib-UI-Arif, M., Sohag, A. A. M., Afrin, S., Bashar, K. K., Afrin, T. et al. (2019). Differential response of sugar beet to long-term mild to severe salinity in a soil–pot culture. Agriculture, 9(10), 223. DOI 10.3390/agriculture9100223. [Google Scholar] [CrossRef]
6. Tahjib-Ul-Arif, M., Afrin, S., Polash, M. A. S., Akter, T., Ray, S. R. et al. (2019). Role of exogenous signaling molecules in alleviating salt-induced oxidative stress in rice (Oryza sativa L.A comparative study. Acta Physiologiae Plantarum, 41(5), 1–14. DOI 10.1007/s11738-019-2861-6. [Google Scholar] [CrossRef]
7. Burke, M., Emerick, K. (2016). Adaptation to climate change: Evidence from US agriculture. American Economic Journal: Economic Policy, 8(3), 106–40. DOI 10.1257/pol.20130025. [Google Scholar] [CrossRef]
8. Li, X., Zhao, Z., Yuan, Y., Wang, X., Li, X. (2018). Heavy metal accumulation and its spatial distribution in agricultural soils: Evidence from Hunan Province, China. RSC Advances, 8(19), 10665–10672. DOI 10.1039/C7RA12435J. [Google Scholar] [CrossRef]
9. Shrivastava, P., Kumar, R. (2015). Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi Journal of Biological Sciences, 22(2), 123–131. DOI 10.1016/j.sjbs.2014.12.001. [Google Scholar] [CrossRef]
10. Taub, D. (2010). Effects of rising atmospheric concentrations of carbon dioxide on plants. Nature Education Knowledge, 3(10), 21. [Google Scholar]
11. Chan, Z., Yokawa, K., Kim, W. Y., Song, C. P. (2016). ROS regulation during plant abiotic stress responses. Frontiers in Plant Science, 7, 1536. DOI 10.3389/fpls.2016.01536. [Google Scholar] [CrossRef]
12. Choudhury, F. K., Rivero, R. M., Blumwald, E., Mittler, R. (2017). Reactive oxygen species, abiotic stress and stress combination. The Plant Journal, 90(5), 856–867. DOI 10.1111/tpj.13299. [Google Scholar] [CrossRef]
13. Tahjib-Ul-Arif, M., Siddiqui, M. N., Sohag, A. A. M., Sakil, M. A., Rahman, M. M. et al. (2018). Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. Journal of Plant Growth Regulation, 37(4), 1318–1330. DOI 10.1007/s00344-018-9867-y. [Google Scholar] [CrossRef]
14. Zornoza, P., Vázquez, S., Esteban, E., Fernández-Pascual, M., Carpena, R. (2002). Cadmium-stress in nodulated white lupin: Strategies to avoid toxicity. Plant Physiology and Biochemistry, 40(12), 1003–1009. DOI 10.1016/S0981-9428(02)01464-X. [Google Scholar] [CrossRef]
15. Sohag, A. A. M., Tahjib-Ul-Arif, M., Brestic, M., Afrin, S., Sakil, M. A. et al. (2020). Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant, Soil and Environment, 66(1), 7–13. DOI 10.17221/PSE. [Google Scholar] [CrossRef]
16. Fuglie, L. J. (1999). The miracle tree: Moringa oleifera, natural nutrition for the tropics. USA: Church World Service. [Google Scholar]
17. Domenico, M., Lina, C., Francesca, B. (2019). Sustainable crops for food security: Moringa (Moringa oleifera Lam.). In: Reference module in food science. Amsterdam: Elsevier. [Google Scholar]
18. Padayachee, B., Baijnath, H. (2012). An overview of the medicinal importance of moringaceae. Journal of Medicinal Plants Research, 6(48), 5831–5839. DOI 10.5897/JMPR12.1187. [Google Scholar] [CrossRef]
19. Moyo, B., Masika, P. J., Hugo, A., Muchenje, V. (2011). Nutritional characterization of moringa (Moringa oleifera Lam.) leaves. African Journal of Biotechnology, 10(60), 12925–12933. DOI 10.5897/AJB. [Google Scholar] [CrossRef]
20. Nweze, N. O., Nwafor, F. I. (2014). Phytochemical, proximate and mineral composition of leaf extracts of Moringa oleifera Lam. from nsukka, South-Eastern Nigeria. IOSR Journal of Pharmacy and Biological Sciences, 9(1), 99–103. DOI 10.9790/3008. [Google Scholar] [CrossRef]
21. Rady, M. M., Kuşvuran, A., Alharby, H. F., Alzahrani, Y., Kuşvuran, S. (2019). Pretreatment with proline or an organic bio-stimulant induces salt tolerance in wheat plants by improving antioxidant redox state and enzymatic activities and reducing the oxidative stress. Journal of Plant Growth Regulation, 38(2), 449–462. DOI 10.1007/s00344-018-9860-5. [Google Scholar] [CrossRef]
22. Bakhtavar, M. A., Afzal, I., Basra, S. M. A., Ahmad, A. H., Noor, M. A. (2015). Physiological strategies to improve the performance of spring maize (Zea mays L.) planted under early and optimum sowing conditions. PLoS One, 10(4), e0124441. DOI 10.1371/journal.pone.0124441. [Google Scholar] [CrossRef]
23. Shindano, J., Kasase, C. (2009). Moringa (Moringa oleiferaA source of food and nutrition, medicine and industrial products. In: Juliani, H. R., Simon, J. E., Ho, C. T. (Eds.African natural plant products: New discoveries and challenges in chemistry and quality, pp. 421–467. Oxford University Press USA. [Google Scholar]
24. Yasmeen, A., Basra, S. M. A., Wahid, A., Farooq, M., Nouman, W. et al. (2013). Improving drought resistance in wheat (Triticum aestivum) by exogenous application of growth enhancers. International Journal of Agriculture and Biology, 15(6), 6. [Google Scholar]
25. Anwar, F., Ashraf, M., Bhanger, M. I. (2005). Interprovenance variation in the composition of Moringa oleifera oilseeds from Pakistan. Journal of the American Oil Chemists’ Society, 82(1), 45–51. DOI 10.1007/s11746-005-1041-1. [Google Scholar] [CrossRef]
26. Anwar, F., Latif, S., Ashraf, M., Gilani, A. H. (2007). Moringa oleifera: A food plant with multiple medicinal uses. Phytotherapy Research, 21(1), 17–25. DOI 10.1002/(ISSN)1099-1573. [Google Scholar] [CrossRef]
27. Aslam, M., Anwar, F., Nadeem, R., Rashid, U., Kazi, T. et al. (2005). Mineral composition of Moringa oleifera leaves and pods from different regions of Punjab, Pakistan. Asian Journal of Plant Sciences, 4(4417–421. DOI 10.3923/ajps.2005.417.421. [Google Scholar] [CrossRef]
28. Basra, S., Iftikhar, M., Afzal, I. (2011). Potential of moringa (Moringa oleifera) leaf extract as priming agent for hybrid maize seeds. International Journal of Agriculture and Biology, 13(6), 1006–1010. [Google Scholar]
29. Fakir, M., Islam, M., Sagar, A., Kashem, M., Rahim, M. (2015). Farmers’ knowledge, attitude and practices of moringa as nutritional and medicinal food in mymensingh region of Bangladesh. Presented at the International Symposium on Moringa, 365–372. [Google Scholar]
30. Imran, S., Afzal, I., Basra, S., Saqib, M. (2013). Integrated seed priming with growth promoting substances enhances germination and seedling vigour of spring maize at low temperature. International Journal of Agriculture and Biology, 15(6). [Google Scholar]
31. Latif, H. H., Mohamed, H. I. (2016). Exogenous applications of moringa leaf extract effect on retrotransposon, ultrastructural and biochemical contents of common bean plants under environmental stresses. South African Journal of Botany, 106, 221–231. DOI 10.1016/j.sajb.2016.07.010. [Google Scholar] [CrossRef]
32. Mahmood, K. T., Mugal, T., Haq, I. U. (2010). Moringa oleifera: A natural gift–A review. Journal of Pharmaceutical Sciences and Research, 2(11), 775. [Google Scholar]
33. Rady, M. M., Mohamed, G. F. (2015). Modulation of salt stress effects on the growth, physio-chemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Scientia Horticulturae, 193, 105–113. DOI 10.1016/j.scienta.2015.07.003. [Google Scholar] [CrossRef]
34. Saini, R. K., Sivanesan, I., Keum, Y. S. (2016). Phytochemicals of moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3Biotech, 6(2), 1–14. DOI 10.1007/s13205-016-0526-3. [Google Scholar] [CrossRef]
35. Andrews, D. (2006). Nutraceutical moringa composition. https://patents.google.com/patent/US20090098230A1/en. [Google Scholar]
36. Price, M. L. (2007). The moringa tree. ECHO Technical Note, 17391, 1–19. [Google Scholar]
37. Makkar, H. A. Becker, K. (1996). Nutrional value and antinutritional components of whole and ethanol extracted Moringa oleifera leaves. Animal Feed Science and Technology, 63(1–4), 211–228. DOI 10.1016/S0377-8401(96)01023-1. [Google Scholar] [CrossRef]
38. Fuglie, L. (2000). The miracle tree: Moringa oleifera: Natural nutrition for the tropics. The multiple attributes of moringa. International Journal of Advance Research, Ideas and Innovations in Technology, 3, 172. [Google Scholar]
39. Faizi, S., Siddiqui, B. S., Saleem, R., Siddiqui, S., Aftab, K. (1995). Fully acetylated carbamate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochemistry, 38(4), 957–963. DOI 10.1016/0031-9422(94)00729-D. [Google Scholar] [CrossRef]
40. Faizi, S., Siddiqui, B. S., Saleem, R., Noor, F., Husnain, S. (1997). Isolation and structure elucidation of a novel glycoside niazidin from the pods of Moringa oleifera. Journal of Natural Products, 60(12), 1317–1321. DOI 10.1021/np970038y. [Google Scholar] [CrossRef]
41. Brockman, H. (2016). Renewable chemicals and bioproducts: A potential for agricultural diversification and economic development. Bulletin 4875. Western Australia, Perth: Department of Agriculture and Food. [Google Scholar]
42. Anjorin, T. S., Ikokoh, P., Okolo, S. (2010). Mineral composition of Moringa oleifera leaves, pods and seeds from two regions in Abuja, Nigeria. International Journal of Agriculture and Biology, 12(3), 431–434. [Google Scholar]
43. Ogbe, A., Affiku, J. P. (2021). Proximate study, mineral and anti-nutrient composition of Moringa oleifera leaves harvested from lafia, Nigeria: Potential benefits in poultry nutrition and health. Journal of Microbiology, Biotechnology and Food Sciences, 2021, 296–308. [Google Scholar]
44. Rehman, H., Nawaz, Q., Basra, S. M. A., Afzal, I., Yasmeen, A. (2014). Seed priming influence on early crop growth, phenological development and yield performance of linola (Linum usitatissimum L.). Journal of Integrative Agriculture, 13(5), 990–996. DOI 10.1016/S2095-3119(13)60521-3. [Google Scholar] [CrossRef]
45. Desoky, E. S. M., Merwad, A. R. M., Rady, M. M. (2018). Natural biostimulants improve saline soil characteristics and salt stressed-sorghum performance. Communications in Soil Science and Plant Analysis, 49(8), 967–983. DOI 10.1080/00103624.2018.1448861. [Google Scholar] [CrossRef]
46. Batool, A., Wahid, A., Farooq, M. (2016). Evaluation of aqueous extracts of moringa leaf and flower applied through medium supplementation for reducing heat stress induced oxidative damage in maize. International Journal of Agriculture and Biology, 18(4), 757–764. DOI 10.17957/IJAB. [Google Scholar] [CrossRef]
47. Bibi, A., Ullah, F., Mehmood, S., Bibi, K., Khan, S. U. et al. (2016). Moringa oleifera lam. leaf extract as bioregulator for improving growth of maize under mercuric chloride stress. Acta Agriculturae Scandinavica, Section B—Soil & Plant Science, 66(6), 469–475. DOI 10.1080/09064710.2016.1173225. [Google Scholar] [CrossRef]
48. Rady, M. M., Varma, C., Howladar, B., M, S. (2013). Common bean (Phaseolus vulgaris L.) seedlings overcome NaCl stress as a result of presoaking in Moringa oleifera leaf extract. Scientia Horticulturae, 162, 63–70. DOI 10.1016/j.scienta.2013.07.046. [Google Scholar] [CrossRef]
49. Zulfiqar, F., Casadesús, A., Brockman, H., Munné-Bosch, S. (2020). An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Science, 295, 110194. DOI 10.1016/j.plantsci.2019.110194. [Google Scholar] [CrossRef]
50. Ali, Z., Basra, S. M. A., Munir, H., Mahmood, A. (2011). Mitigation of drought stress in maize by natural and synthetic growth promoters. Journal of Agriculture and Social Sciences, 7(2), 8. [Google Scholar]
51. El-Mageed, A., Semida, T. A., Rady, M. M. (2017). Moringa leaf extract as biostimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agricultural Water Management, 193, 46–54. DOI 10.1016/j.agwat.2017.08.004. [Google Scholar] [CrossRef]
52. Hanafy, R. S. (2017). Using Moringa olifera leaf extract as a bio-fertilizer for drought stress mitigation of Glycine max L. plants. Egyptian Journal of Botany, 57(2), 281–292. DOI 10.21608/ejbo.2017.596.1027. [Google Scholar] [CrossRef]
53. Khan, S., Ibrar, D., Bashir, S., Rashid, N., Hasnain, Z. et al. (2022). Application of Moringa leaf extract as a seed priming agent enhances growth and physiological attributes of rice seedlings cultivated under water deficit regime. Plants, 11(3), 261. DOI 10.3390/plants11030261. [Google Scholar] [CrossRef]
54. Khan, S., Basit, A., Hafeez, M. B., Irshad, S., Bashir, S. et al. (2021). Moringa leaf extract improves biochemical attributes, yield and grain quality of rice (Oryza sativa L.) under drought stress. PLos One, 16(7), e0254452. DOI 10.1371/journal.pone.0254452. [Google Scholar] [CrossRef]
55. Maswada, H. F., Abd El-Razek, U. A., El-Sheshtawy, A. N. A., Elzaawely, A. A. (2018). Morpho-physiological and yield responses to exogenous moringa leaf extract and salicylic acid in maize ( Zea mays L.) under water stress. Archives of Agronomy and Soil Science, 64(7), 994–1010. DOI 10.1080/03650340.2017.1406079. [Google Scholar] [CrossRef]
56. Howladar, S. M. (2014). A novel Moringa oleifera leaf extract can mitigate the stress effects of salinity and cadmium in bean (Phaseolus vulgaris L.) plants. Ecotoxicology and Environmental Safety, 100, 69–75. DOI 10.1016/j.ecoenv.2013.11.022. [Google Scholar] [CrossRef]
57. Ahmed, T., Abou Elezz, A., Khalid, M. F. (2021). Hydropriming with moringa leaf extract mitigates salt stress in Wheat seedlings. Agriculture, 11, 1254. DOI 10.3390/agriculture11121254. [Google Scholar] [CrossRef]
58. Rady, M. M., Mohamed, G. F., Abdalla, A. M. (2015). Integrated application of salicylic acid and Moringa oleifera leaf extract alleviates the salt-induced adverse effects in common bean plants. International Journal of Agricultural Technology, 11, 20. [Google Scholar]
59. Taha, R. (2016). Improving salt tolerance of Helianthus annuus (L.) plants by moringa oleifera leaf extract. Egyptial Journal of Agronomy, 38(1), 117–140. DOI 10.21608/agro.2016.301. [Google Scholar] [CrossRef]
60. Merwad, A. R. M. (2017). Effect of humic and fulvic substances and moringa leaf extract on Sudan grass plants grown under saline conditions. Canadian Journal of Soil Science, 97(4), 703–716. DOI 10.1139/CJSS-2017-0050. [Google Scholar] [CrossRef]
61. Hassanein, R. A., Abdelkader, A. F., Faramawy, H. M. (2019). Moringa leaf extracts as biostimulants-inducing salinity tolerance in the sweet basil plant. Egyptian Journal of Botany, 59(2), 303–318. DOI 10.21608/ejbo.2019.5989.1242. [Google Scholar] [CrossRef]
62. Latef, A. A. A., Alhmad, M. F. A., Hammad, S. A. (2017). Foliar application of fresh moringa leaf extract overcomes salt stress in fenugreek (Trigonellafoenum-graecum) plants. Egyptian Journal of Botany, 57(1), 23. [Google Scholar]
63. Nouman, W., Basra, S. M. A., Yasmeen, A., Gull, T., Hussain, S. B. et al. (2014). Seed priming improves the emergence potential, growth and antioxidant system of Moringa oleifera under saline conditions. Plant Growth Regulation, 73(3), 267–278. DOI 10.1007/s10725-014-9887-y. [Google Scholar] [CrossRef]
64. Afzal, I., Hussain, B., Basra, S. M. A., Rehman, H. (2012). Priming with moringa leaf extract reduces imbibitional chilling injury in spring maize. Seed Science and Technology, 40(2), 271–276. DOI 10.15258/sst. [Google Scholar] [CrossRef]
65. Sarwar, M., Saleem, M. F., Ullah, N., Rizwan, M., Ali, S. et al. (2018). Exogenously applied growth regulators protect the cotton crop from heat-induced injury by modulating plant defense mechanism. Scientific Reports, 8(1), 1–15. DOI 10.1038/s41598-018-35420-5. [Google Scholar] [CrossRef]
66. Abusuwar, A. O., Abohassan, R. A. (2017). Effect of Moringa olifera leaf extract on growth and productivity of three cereal forages. Journal of Agricultural Science, 9(7), 236. DOI 10.5539/jas.v9n7p236. [Google Scholar] [CrossRef]
67. Seleiman, M. F., Al-Suhaibani, N., Ali, N., Akmal, M., Alotaibi, M. et al. (2021). Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants, 10(2), 259. DOI 10.3390/plants10020259. [Google Scholar] [CrossRef]
68. Praba, M. L., Cairns, J., Babu, R., Lafitte, H. (2009). Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. Journal of Agronomy and Crop Science, 195(1), 30–46. DOI 10.1111/j.1439-037X.2008.00341.x. [Google Scholar] [CrossRef]
69. Earl, H. J., Davis, R. F. (2003). Effect of drought stress on leaf and whole canopy radiation use efficiency and yield of maize. Agronomy Journal, 95(3), 688–696. DOI 10.2134/agronj2003.6880. [Google Scholar] [CrossRef]
70. Lawlor, D. W., Cornic, G. (2002). Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant, Cell & Environment, 25(2), 275–294. DOI 10.1046/j.0016-8025.2001.00814.x. [Google Scholar] [CrossRef]
71. Kamínek, M., Motyka, V., Vaňková, R. (1997). Regulation of cytokinin content in plant cells. Physiologia Plantarum, 101(4), 689–700. DOI 10.1111/j.1399-3054.1997.tb01053.x. [Google Scholar] [CrossRef]
72. Pospíšilová, J., Synková, H., Rulcová, J. (2000). Cytokinins and water stress. Biologia Plantarum, 43(3), 321–328. DOI 10.1023/A:1026754404857. [Google Scholar] [CrossRef]
73. Yasmeen, A., Basra, S. M. A., Ahmad, R., Wahid, A. (2012). Performance of late sown wheat in response to foliar application of Moringa oleifera Lam. leaf extract. Chilean Journal of Agricultural Research, 72(1), 92. DOI 10.4067/S0718-58392012000100015. [Google Scholar] [CrossRef]
74. Munns, R., Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59, 651–681. DOI 10.1146/annurev.arplant.59.032607.092911. [Google Scholar] [CrossRef]
75. Wu, G. Q., Jiao, Q., Shui, Q. Z. (2015). Effect of salinity on seed germination, seedling growth, and inorganic and organic solutes accumulation in sunflower (Helianthus annuus L.) Plant, Soil and Environment, 61(5), 220–226. DOI 10.17221/22/2015-PSE. [Google Scholar] [CrossRef]
76. Van Zelm, E., Zhang, Y., Testerink, C. (2020). Salt tolerance mechanisms of plants. Annual Review of Plant Biology, 71, 403–433. DOI 10.1146/annurev-arplant-050718-100005. [Google Scholar] [CrossRef]
77. Khan, M. A., Ḵẖān, M. A., Weber, D. J. (2006). Ecophysiology of high salinity tolerant plants, vol. 40, pp. 1–397. Netherlands: Springer Science & Business Media, Dordrecht. [Google Scholar]
78. Heidari, M. (2010). Nucleic acid metabolism, proline concentration and antioxidants enzyme activity in canola (Brassica nupus L.) under salinity stress. Agricultural Sciences in China, 9(4), 504–511. DOI 10.1016/S1671-2927(09)60123-1. [Google Scholar] [CrossRef]
79. Zhang, H., Zhao, X., Sun, Q., Yan, C., Wang, J. et al. (2020). Comparative transcriptome analysis reveals molecular defensive mechanism of Arachis hypogaea in response to salt stress. International Journal of Genomics, 2020, 6524093. DOI 10.1155/2020/6524093. [Google Scholar] [CrossRef]
80. Kosová, K., Prášil, I. T., Vítámvás, P. (2013). Protein contribution to plant salinity response and tolerance acquisition. International Journal of Molecular Sciences, 14(4), 6757–6789. DOI 10.3390/ijms14046757. [Google Scholar] [CrossRef]
81. Yu, Z., Duan, X., Luo, L., Dai, S., Ding, Z. et al. (2020). How plant hormones mediate salt stress responses. Trends in Plant Science, 25(11), 1117–1130. DOI 10.1016/j.tplants.2020.06.008. [Google Scholar] [CrossRef]
82. Ashraf, M., Athar, H., Harris, P., Kwon, T. (2008). Some prospective strategies for improving crop salt tolerance. Advances in Agronomy, 97, 45–110. DOI 10.1016/S0065-2113(07)00002-8. [Google Scholar] [CrossRef]
83. Foidl, N., Makkar, H., Becker, K. (2001). The potential of Moringa oleifera for agricultural and industrial uses. In: What development potential for moringa products. [Google Scholar]
84. Yang, X., Chen, X., Ge, Q., Li, B., Tong, Y. et al. (2006). Tolerance of photosynthesis to photoinhibition, high temperature and drought stress in flag leaves of wheat: A comparison between a hybridization line and its parents grown under field conditions. Plant Science, 171(3), 389–397. DOI 10.1016/j.plantsci.2006.04.010. [Google Scholar] [CrossRef]
85. Hassan, M. U., Chattha, M. U., Khan, I., Chattha, M. B., Barbanti, L. et al. (2020). Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosystems–An International Journal Dealing with all Aspects of Plant Biology, 155, 211–234. DOI 10.1080/11263504.2020.1727987. [Google Scholar] [CrossRef]
86. Ghori, N. H., Ghori, T., Hayat, M., Imadi, S., Gul, A. et al. (2019). Heavy metal stress and responses in plants. International Journal of Environmental Science and Technology, 16(3), 1807–1828. DOI 10.1007/s13762-019-02215-8. [Google Scholar] [CrossRef]
87. Jogawat, A. (2019). Osmolytes and their role in abiotic stress tolerance in plants. Molecular Plant Abiotic Stress: Biology and Biotechnology, 91–104. DOI 10.1002/9781119463665. [Google Scholar] [CrossRef]
88. Sharma, A., Shahzad, B., Kumar, V., Kohli, S. K., Sidhu, G. P. S. et al. (2019). Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules, 9(7), 285. DOI 10.3390/biom9070285. [Google Scholar] [CrossRef]
89. Ajithkumar, I. P., Panneerselvam, R. (2014). ROS scavenging system, osmotic maintenance, pigment and growth status of Panicum sumatrense roth. under drought stress. Cell Biochemistry and Biophysics, 68(3), 587–595. DOI 10.1007/s12013-013-9746-x. [Google Scholar] [CrossRef]
90. Anjum, S. A., Tanveer, M., Hussain, S., Shahzad, B., Ashraf, U. et al. (2016). Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environmental Science and Pollution Research, 23(12), 11864–11875. DOI 10.1007/s11356-016-6382-1. [Google Scholar] [CrossRef]
91. Wang, X., Yuan, B., Chen, Y., Li, X., Ren, Y. (2014). Integration of micro-filtration into osmotic membrane bioreactors to prevent salinity build-up. Bioresource Technology, 167, 116–123. DOI 10.1016/j.biortech.2014.05.121. [Google Scholar] [CrossRef]
92. Conde, A., Silva, P., Agasse, A., Conde, C., Gerós, H. (2011). Mannitol transport and mannitol dehydrogenase activities are coordinated in Olea europaea under salt and osmotic stresses. Plant and Cell Physiology, 52(10), 1766–1775. DOI 10.1093/pcp/pcr121. [Google Scholar] [CrossRef]
93. Sharma, S. S., Dietz, K. J. (2006). The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. Journal of Experimental Botany, 57(4), 711–726. DOI 10.1093/jxb/erj073. [Google Scholar] [CrossRef]
94. Hayashi, H., Sakamoto, A., Murata, N. (1998). Enhancement of the tolerance of arabidopsis to high temperatures by genetic engineering of the synthesis of glycinebetaine. The Plant Journal, 16(2), 155–161. DOI 10.1046/j.1365-313x.1998.00284.x. [Google Scholar] [CrossRef]
95. Ningthoujam, M., Habib, K., Bano, F., Zutshi, S., Fatma, T. (2013). Exogenous osmolytes suppresses the toxic effects of malathion on Anabaena variabilis. Ecotoxicology and Environmental Safety, 94, 21–27. DOI 10.1016/j.ecoenv.2013.04.022. [Google Scholar] [CrossRef]
96. Iqbal, N., Umar, S., Khan, N. A., Khan, M. I. R. (2014). A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environmental and Experimental Botany, 100, 34–42. DOI 10.1016/j.envexpbot.2013.12.006. [Google Scholar] [CrossRef]
97. Kishor, P. K., Sangam, S., Amrutha, R., Laxmi, P. S., Naidu, K. et al. (2005). Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Current Science, 88, 424–438. [Google Scholar]
98. Sharma, A., Shahzad, B., Rehman, A., Bhardwaj, R., Landi, M. et al. (2019). Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules, 24(13), 2452. DOI 10.3390/molecules24132452. [Google Scholar] [CrossRef]
99. Abrahám, E., Rigó, G., Székely, G., Nagy, R., Koncz, C. et al. (2003). Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in arabidopsis. Plant Molecular Biology, 51(3), 363–372. DOI 10.1023/A:1022043000516. [Google Scholar] [CrossRef]
100. Hare, P., Cress, W., van Staden, J. (1999). Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. Journal of Experimental Botany, 50(333), 413–434. DOI 10.1093/jxb/50.333.413. [Google Scholar] [CrossRef]
101. Strizhov, N., Ábrahám, E., Ökrész, L., Blickling, S., Zilberstein, A. et al. (1997). Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in arabidopsis. The Plant Journal, 12(3), 557–569. DOI 10.1046/j.1365-313x.1997.00557.x. [Google Scholar] [CrossRef]
102. Yoshiba, Y., Kiyosue, T., Katagiri, T., Ueda, H., Mizoguchi, T. et al. (1995). Correlation between the induction of a gene for Δ1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. The Plant Journal, 7(5), 751–760. DOI 10.1046/j.1365-313X.1995.07050751.x. [Google Scholar] [CrossRef]
103. Batool, A., Wahid, A., Abbas, G., Akhtar, M. N., Hasnain, Z. et al. (2021). Physiological implication of moringa extracts applications for osmolytes production in maize crop under heat stress. Pakistan Journal of Botany, 53(5), 1593–1604. DOI 10.30848/PAK.J.BOT. [Google Scholar] [CrossRef]
104. Yang, C., Zhou, Y., Fan, J., Fu, Y., Shen, L. et al. (2015). SpBADH of the halophyte Sesuvium portulacastrum strongly confers drought tolerance through ROS scavenging in transgenic arabidopsis. Plant Physiology and Biochemistry, 96, 377–387. DOI 10.1016/j.plaphy.2015.08.010. [Google Scholar] [CrossRef]
105. Zhang, L., Gao, M., Hu, J., Zhang, X., Wang, K. et al. (2012). Modulation role of abscisic acid (ABA) on growth, water relations and glycinebetaine metabolism in two maize (Zea mays L.) cultivars under drought stress. International Journal of Molecular Sciences, 13(3), 3189–3202. DOI 10.3390/ijms13033189. [Google Scholar] [CrossRef]
106. Nazar, R., Umar, S., Khan, N. A. (2015). Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signaling & Behavior, 10(3), e1003751. DOI 10.1080/15592324.2014.1003751. [Google Scholar] [CrossRef]
107. Lim, S. D., Kim, J. H., Lee, J., Hwang, S. G., Jang, C. S. (2020). A rice sitl1 mutant induced by gamma-ray irradiation shows enhanced insensitivity to salinity via reduced accumulation in Na+ and Mg2+. Authorea Preprints, DOI 10.22541/au.158809507.72091318. [Google Scholar] [CrossRef]
108. Mastrototaro, L. (2017). SLC41A1, SLC41A3 and CNNM2: Magnesium responsive genes with potential involvement in human ailments. DOI 10.17169/refubium-9056. [Google Scholar] [CrossRef]
109. Sabino, M. A., Sereno, O., Dantas, F. L. (2018). Morphology study of alginate micro/nano particles for the encapsulation of divalents Mg2+ and Zn2+ ions. International Journal of Advances in Medical Biotechnology, 1(1), 22–30. DOI 10.25061/2595-3931/IJAMB/2018.v1i1.12. [Google Scholar] [CrossRef]
110. Sun, O. J., Payn, T. W. (1999). Magnesium nutrition and photosynthesis in Pinus radiata: Clonal variation and influence of potassium. Tree Physiology, 19(8), 535–540. DOI 10.1093/treephys/19.8.535. [Google Scholar] [CrossRef]
111. Iqbal, N., Umar, S., Khan, N. A. (2015). Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). Journal of Plant Physiology, 178, 84–91. DOI 10.1016/j.jplph.2015.02.006. [Google Scholar] [CrossRef]
112. Roy, R. C., Sagar, A., Tajkia, J. E., Razzak, M. A., Hossain, A. Z. (2018). Effect of salt stress on growth of sorghum germplasms at vegetative stage. Journal of the Bangladesh Agricultural University, 16(1), 67–72. DOI 10.3329/jbau.v16i1.36483. [Google Scholar] [CrossRef]
113. Ahanger, M. A., Agarwal, R., Tomar, N. S., Shrivastava, M. (2015). Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L cultivar kent). Journal of Plant Interactions, 10(1), 211–223. DOI 10.1080/17429145.2015.1056260. [Google Scholar] [CrossRef]
114. Azooz, M., Shaddad, M., Abdel-Latef, A. (2004). The accumulation and compartmentation of proline in relation to salt tolerance of three sorghum cultivars. Indian Journal of Plant Physiology, 9, 1–8. [Google Scholar]
115. Azooz, M. M., Metwally, A., Abou-Elhamd, M. F. (2015). Jasmonate-induced tolerance of hassawi okra seedlings to salinity in brackish water. Acta Physiologiae Plantarum, 37(4), 77. DOI 10.1007/s11738-015-1828-5. [Google Scholar] [CrossRef]
116. Jatav, K. S., Agarwal, R., Tomar, N. S., Tyagi, S. (2014). Nitrogen metabolism, growth and yield responses of wheat (Triticum aestivum L.) to restricted water supply and varying potassium treatments. The Journal of Indian Botanical Society, 93(3&4), 177–189. [Google Scholar]
117. Tomar, N. S., Agarwal, R. (2013). Influence of treatment of Jatropha curcas L. leachates and potassium on growth and phytochemical constituents of wheat (Triticum aestivum L.). American Journal of Plant Sciences, 4(5), 1134–1150. DOI 10.4236/ajps.2013.45140. [Google Scholar] [CrossRef]
118. Ahmad, P., Ashraf, M., Hakeem, K. R., Azooz, M., Rasool, S. et al. (2014). Potassium starvation-induced oxidative stress and antioxidant defense responses in Brassica juncea. Journal of Plant Interactions, 9(1), 1–9. DOI 10.1080/17429145.2012.747629. [Google Scholar] [CrossRef]
119. Rubio, V., Bustos, R., Irigoyen, M. L., Cardona-López, X., Rojas-Triana, M. et al. (2009). Plant hormones and nutrient signaling. Plant Molecular Biology, 69(4), 361–373. DOI 10.1007/s11103-008-9380-y. [Google Scholar] [CrossRef]
120. Latef, A., Tahjib-Ul-Arif, A. A. H., Rhaman, M., S, M. (2021). Exogenous auxin-mediated salt stress alleviation in faba bean (Vicia faba L.). Agronomy, 11(3), 547. DOI 10.3390/agronomy11030547. [Google Scholar] [CrossRef]
121. Fahad, S., Hussain, S., Matloob, A., Khan, F. A., Khaliq, A. et al. (2015). Phytohormones and plant responses to salinity stress: A review. Plant Growth Regulation, 75(2), 391–404. DOI 10.1007/s10725-014-0013-y. [Google Scholar] [CrossRef]
122. Shaki, F., Maboud, H. E., Niknam, V. (2019). Effects of salicylic acid on hormonal cross talk, fatty acids profile, and ions homeostasis from salt-stressed safflower. Journal of Plant Interactions, 14(1), 340–346. DOI 10.1080/17429145.2019.1635660. [Google Scholar] [CrossRef]
123. Hossain, M. S., Dietz, K. J. (2016). Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Frontiers in Plant Science, 7, 548. DOI 10.3389/fpls.2016.00548. [Google Scholar] [CrossRef]
124. Hossain, M. S., Persicke, M., ElSayed, A. I., Kalinowski, J., Dietz, K. J. (2017). Metabolite profiling at the cellular and subcellular level reveals metabolites associated with salinity tolerance in sugar beet. Journal of Experimental Botany, 68(21–22), 5961–5976. DOI 10.1093/jxb/erx388. [Google Scholar] [CrossRef]
125. Gupta, M., Sharma, P., Sarin, N. B., Sinha, A. K. (2009). Differential response of arsenic stress in two varieties of Brassica juncea L. Chemosphere, 74(9), 1201–1208. DOI 10.1016/j.chemosphere.2008.11.023. [Google Scholar] [CrossRef]
126. Hasanuzzaman, M., Fujita, M. (2012). Heavy metals in the environment: Current status, toxic effects on plants and possible phytoremediation. In: Phytotechnologies: Remediation of environmental contaminants, pp. 7–73.Boca Raton: CRC Press. [Google Scholar]
127. Hasanuzzaman, M., Gill, S. S., Fujita, M. (2013). Physiological role of nitric oxide in plants grown under adverse environmental conditions. In: Plant acclimation to environmental stress, pp. 269–322. New York: Springer. [Google Scholar]
128. Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7(9), 405–410. DOI 10.1016/S1360-1385(02)02312-9. [Google Scholar] [CrossRef]
129. de Carvalho, M. H. C. (2008). Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signaling & Behavior, 3(3), 156–165. DOI 10.4161/psb.3.3.5536. [Google Scholar] [CrossRef]
130. Gill, S. S., Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48(12), 909–930. DOI 10.1016/j.plaphy.2010.08.016. [Google Scholar] [CrossRef]
131. Sharma, P., Dubey, R. S. (2005). Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regulation, 46(3), 209–221. DOI 10.1007/s10725-005-0002-2. [Google Scholar] [CrossRef]
132. Mittler, R., Vanderauwera, S., Gollery, M., van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends in Plant Science, 9(10), 490–498. DOI 10.1016/j.tplants.2004.08.009. [Google Scholar] [CrossRef]
133. Gratão, P. L., Polle, A., Lea, P. J., Azevedo, R. A. (2005). Making the life of heavy metal-stressed plants a little easier. Functional Plant Biology, 32(6), 481–494. DOI 10.1071/FP05016. [Google Scholar] [CrossRef]
134. Scandalios, J. (2005). Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Brazilian Journal of Medical and Biological Research, 38(7), 995–1014. DOI 10.1590/S0100-879X2005000700003. [Google Scholar] [CrossRef]
135. Yasmeen, A., Basra, S. M. A., Farooq, M., ur Rehman, H., Hussain, N. (2013). Exogenous application of moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regulation, 69(3), 225–233. DOI 10.1007/s10725-012-9764-5. [Google Scholar] [CrossRef]
136. Hamada, A., Al-Hakimi, A. (2009). Exogenous ascorbic acid or thiamine increases the resistance of sunflower and maize plants to salt stress. Acta Agronomica Hungarica, 57(3), 335–347. DOI 10.1556/AAgr.57.2009.3.8. [Google Scholar] [CrossRef]
137. Hossain, M. A., Hasanuzzaman, M., Fujita, M. (2011). Coordinate induction of antioxidant defense and glyoxalase system by exogenous proline and glycinebetaine is correlated with salt tolerance in mung bean. Frontiers of Agriculture in China, 5(1), 1–14. DOI 10.1007/s11703-010-1070-2. [Google Scholar] [CrossRef]
138. Hussain, T. M., Hazara, M., Sultan, Z., Saleh, B. K., Gopal, G. R. (2008). Recent advances in salt stress biology–a review. Biotechnology and Molecular Biology Reviews, 3(1), 8–13. [Google Scholar]
139. Ruiz, J., Blumwald, E. (2002). Salinity-induced glutathione synthesis in brassica napus. Planta, 214(6), 965–969. DOI 10.1007/s00425-002-0748-y. [Google Scholar] [CrossRef]
140. Munné-Bosch, S., Falara, V., Pateraki, I., López-Carbonell, M., Cela, J. et al. (2009). Physiological and molecular responses of the isoprenoid biosynthetic pathway in a drought-resistant Mediterranean shrub, Cistus creticus exposed to water deficit. Journal of Plant Physiology, 166(2), 136–145. DOI 10.1016/j.jplph.2008.02.011. [Google Scholar] [CrossRef]
141. Ali, Q., Tariq Javed, M., Haider, M. Z., Habib, N., Rizwan, M. et al. (2020). Α-Tocopherol foliar spray and translocation mediates growth, photosynthetic pigments, nutrient uptake, and oxidative defense in maize (Zea mays L.) under drought stress. Agronomy, 10(9), 1235. DOI 10.3390/agronomy10091235. [Google Scholar] [CrossRef]
142. Semida, W. M., Abd El-Mageed, T. A., Howladar, S. M., Rady, M. M. (2016). Foliar-applied α-tocopherol enhances salt-tolerance in onion plants by improving antioxidant defence system. Australian Journal of Crop Science, 10, 1030–1039. DOI 10.21475/ajcs. [Google Scholar] [CrossRef]
143. Nounjan, N., Nghia, P. T., Theerakulpisut, P. (2012). Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. Journal of Plant Physiology, 169(6), 596–604. DOI 10.1016/j.jplph.2012.01.004. [Google Scholar] [CrossRef]
144. Arora, A., Byrem, T. M., Nair, M. G., Strasburg, G. M. (2000). Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Archives of Biochemistry and Biophysics, 373(1), 102–109. DOI 10.1006/abbi.1999.1525. [Google Scholar] [CrossRef]
145. Michalak, A. (2006). Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish Journal of Environmental Studies, 15(4). [Google Scholar]
146. Verstraeten, S. V., Keen, C. L., Schmitz, H. H., Fraga, C. G., Oteiza, P. I. (2003). Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Radical Biology and Medicine, 34(1), 84–92. DOI 10.1016/S0891-5849(02)01185-1. [Google Scholar] [CrossRef]
147. Ahmad, P., Hashem, A., Abd-Allah, E. F., Alqarawi, A., John, R. et al. (2015). Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Frontiers in Plant Science, 6, 868. DOI 10.3389/fpls.2015.00868. [Google Scholar] [CrossRef]
148. Telfer, A., Frolov, D., Barber, J., Robert, B., Pascal, A. (2003). Oxidation of the two β-carotene molecules in the photosystem II reaction center. Biochemistry, 42(4), 1008–1015. DOI 10.1021/bi026206p. [Google Scholar] [CrossRef]
149. Chalker-Scott, L. (1999). Environmental significance of anthocyanins in plant stress responses. Photochemistry and Photobiology, 70(1), 1–9. DOI 10.1111/j.1751-1097.1999.tb01944.x. [Google Scholar] [CrossRef]
150. Ilyas, M., Arshad, M., Saeed, F., Iqbal, M. (2015). Antioxidant potential and nutritional comparison of moringa leaf and seed powders and their tea infusions. Journal of Animal and Plant Science, 25(1), 226–233. [Google Scholar]
151. Manguro, L. O. A., Lemmen, P. (2007). Phenolics of Moringa oleifera leaves. Natural Product Research, 21(1), 56–68. DOI 10.1080/14786410601035811. [Google Scholar] [CrossRef]
152. Siddhuraju, P., Becker, K. (2003). Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. Journal of Agricultural and Food Chemistry, 51(8), 2144–2155. DOI 10.1021/jf020444+. [Google Scholar] [CrossRef]
153. Wahid, A. (2007). Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. Journal of Plant Research, 120(2), 219–228. DOI 10.1007/s10265-006-0040-5. [Google Scholar] [CrossRef]
154. Abdalla, M. M. (2013). The potential of Moringa oleifera extract as a biostimulant in enhancing the growth, biochemical and hormonal contents in rocket (Eruca vesicaria subsp. sativa) plants. International Journal of Plant Physiology and Biochemistry, 5(3), 42–49. DOI 10.5897/IJPPB. [Google Scholar] [CrossRef]
155. Elzaawely, A. A., Ahmed, M. E., Maswada, H. F., Xuan, T. D. (2017). Enhancing growth, yield, biochemical, and hormonal contents of snap bean (Phaseolus vulgaris L.) sprayed with moringa leaf extract. Archives of Agronomy and Soil Science, 63(5), 687–699. DOI 10.1080/03650340.2016.1234042. [Google Scholar] [CrossRef]
156. Noor, M. A., Ahmad, W., Afzal, I., Salamh, A., Afzal, M. et al. (2016). Pea seed invigoration by priming with magnetized water and moringa leaf extract. Philippine Agricultural Scientist, 99, 171–175. [Google Scholar]
157. Kanchani, A. M. K. D. M., Harris, K. D. (2019). Effect of foliar application of moringa (Moringa oleifera) leaf extract with recommended fertilizer on growth and yield of okra (Abelmoschus esculentus). AGRIEAST: Journal of Agricultural Sciences, 13(2), 38. DOI 10.4038/agrieast.v13i2.73. [Google Scholar] [CrossRef]
158. Mahboob, W., Rehman, ur, H., Ahmad Basra, S. M., Afzal, I., Asad Abbas, M. et al. (2015). Seed priming improves the performance of late sown spring maize (Zea mays) through better crop stand and physiological attributes. International Journal of Agriculture and Biology, 17(3), 491–498. DOI 10.17957/IJAB. [Google Scholar] [CrossRef]
159. Hala, H., Nabila, A. E. (2017). Effect of Moringa oleifera leaf extract (MLE) on pepper seed germination, seedlings improvement, growth, fruit yield and its quality. Middle East Journal of Agriculture Research, 6, 448–463. [Google Scholar]
160. Emongor, V. E. (2015). Effects of moringa (Moringa oleifera) leaf extract on growth, yield and yield components of snap beans (Phaseolus vulgaris). Current Journal of Applied Science and Technology, 114–122. DOI 10.9734/BJAST/2015/14795. [Google Scholar] [CrossRef]
161. Ahmad, I., Tanveer, M. U., Liaqat, M., Dole, J. M. (2019). Comparison of corm soaks with preharvest foliar application of moringa leaf extract for improving growth and yield of cut Freesia hybrida. Scientia Horticulturae, 254, 21–25. DOI 10.1016/j.scienta.2019.04.074. [Google Scholar] [CrossRef]
162. Ali, A., Abbas, N., Maqbool, M., Haq, T., Ahmad, M. et al. (2015). Influence of soil applied moringa leaf extract on vegetative growth of Cyperus Rotundus. Asian Journal of Agriculture and Biology, 3, 79–82. [Google Scholar]
163. Anjum, K., Cheema, S. A., Farooq, M., ur Rehman, H., Haider, F. U. (2019). Exploring the potential of selenium (Se) and moringa (Moringa oleifera L.) leaf extract on the production and performance of triticum aestivum L. Journal of Research in Ecology, 7(1), 2390–2402. [Google Scholar]
164. Brockman, H. G., Brennan, R. F. (2017). The effect of foliar application of moringa leaf extract on biomass, grain yield of wheat and applied nutrient efficiency. Journal of Plant Nutrition, 40(19), 2728–2736. DOI 10.1080/01904167.2017.1381723. [Google Scholar] [CrossRef]
165. Bashir, K., Bawa, J., Mohammed, I. (2014). Efficacy of leaf extract of drumstick tree (Moringa Oleifera Lam.) on the growth of local tomato (Lycopersicon esculentum). IOSR Journal of Pharmacy and Biological Sciences, 9(4), 74–79. DOI 10.9790/3008. [Google Scholar] [CrossRef]
166. Mvumi, C., Tagwira, F., Chiteka, A. Z. (2013). Effect of moringa extract on growth and yield of maize and common beans. Greener Journal of Agricultural Sciences, 3(1), 55–62. DOI 10.15580/GJAS. [Google Scholar] [CrossRef]
167. Biswas, A., Hoque, T., Abedin, M. (2016). Effects of moringa leaf extract on growth and yield of maize. Progressive Agriculture, 27(2), 136–143. DOI 10.3329/pa.v27i2.29322. [Google Scholar] [CrossRef]
168. Ogbuehi, H., Emeribe, E., Asagwara, J. (2017). Soil application of moringa leaf extract on root development and root exudates of soybean (Glycine max L.). International Journal of Agriculture Innovations and Research, 6(2), 325–330. [Google Scholar]
169. Matthew, A. (2016). Moringa leaf extract on the growth and yield of pepper (Capsicum annuum L.). ARPN Journal of Agricultural and Biological Science, 11(3), 107–109. [Google Scholar]
170. Dunsin, O., Odeghe, T. O. (2015). Response of sweet bell pepper to moringa leaf extract and organo-bio degradable fertilizer. Asian Journal of Agriculture and Biology, 3(4), 132–138. [Google Scholar]
171. Elbagory, M. (2019). Effectiveness of organic fertigation and moringa leaf extract spray as an alternative to chemical fertigation for improving yield and quality of lettuce under soilless condition. Environment, Biodiversity and Soil Security, 2(1), 175–182. DOI 10.21608/jenvbs.2019.6817.1047. [Google Scholar] [CrossRef]
172. Iqbal, M. A. (2014). Improving the growth and yield of canola (Brassica napus L.) with seed treatment and foliar sprays of brassica (Brassica naups L.) and moringa (Moringa olifera L.) leaf extracts. American-Eurasian Journal of Agricultural and Environmental Sciences, 14(10), 1067–1073. DOI 10.5829/idosi.aejaes.2014.14.10.12429. [Google Scholar] [CrossRef]
173. Younis, A., Akhtar, M. S., Riaz, A., Zulfiqar, F., Qasim, M. et al. (2018). Improved cut flower and corm production by exogenous moringa leaf extract application on gladiolus cultivars. Acta Scientiarum Polonorum Hortorum Cultus, 17(4), 25–38. DOI 10.24326/asphc. [Google Scholar] [CrossRef]
174. ShM, T., Ne, K., Ms, A., Am, A. (2017). Influence of foliar application with moringa (Moringa oleifera L.) leaf extract on yield and fruit quality of hollywood plum cultivar. Journal of Horticulture, 4(1), 193. DOI 10.4172/2376-0354. [Google Scholar] [CrossRef]
175. Khan, S., Basra, S. M. A., Nawaz, M., Hussain, I., Foidl, N. (2020). Combined application of moringa leaf extract and chemical growth-promoters enhances the plant growth and productivity of wheat crop (Triticum aestivum L.). South African Journal of Botany, 129, 74–81. DOI 10.1016/j.sajb.2019.01.007. [Google Scholar] [CrossRef]
176. Basra, S. M., Lovatt, C. J. (2016). Exogenous applications of moringa leaf extract and cytokinins improve plant growth, yield, and fruit quality of cherry tomato. HortTechnology, 26(3), 327–337. DOI 10.21273/HORTTECH.26.3.327. [Google Scholar] [CrossRef]
177. Khan, S. (2017). Screening of moringa landraces for leaf extract as biostimulant in wheat. International Journal of Agriculture and Biology, 19(5), 999–1006. DOI 10.17957/IJAB. [Google Scholar] [CrossRef]
178. El-Serafy, R. S., El-Sheshtawy, A. A. (2020). Effect of nitrogen fixing bacteria and moringa leaf extract on fruit yield, estragole content and total phenols of organic fennel. Scientia Horticulturae, 265, 109209. DOI 10.1016/j.scienta.2020.109209. [Google Scholar] [CrossRef]
179. Abdel-Rahman, S. S. A., Abdel-Kader, A. A. S. (2020). Response of fennel (Foeniculum vulgare, Mill) plants to foliar application of moringa leaf extract and benzyladenine (BA). South African Journal of Botany, 129, 113–122. DOI 10.1016/j.sajb.2019.01.037. [Google Scholar] [CrossRef]
180. Abbas, S., Zaglool, M., El-Ghadban, E., Abd El-Kareem, S., Waly, A. (2016). Effect of moringa leaf extract spray on sage (Salvia officinalis L.) plant under sandy soil conditions. Hortscience Journal of Suez Canal University, 5(1), 15–21. DOI 10.21608/hjsc.2016.6402. [Google Scholar] [CrossRef]
181. Nasir, M. (2016). Foliar application of moringa leaf extract, potassium and zinc influence yield and fruit quality of ‘Kinnow’ mandarin. Scientia Horticulturae, 210, 227–235. DOI 10.1016/j.scienta.2016.07.032. [Google Scholar] [CrossRef]
182. Mohamed, Y. A., El-Ghamriny, E., Bardisi, A., Nawar, D. A. (2019). Growth and productivity of garlic crop under different fertilizers type and some extracts. Life Science Journal, 16(3), 79–89. DOI 10.1186/s42269-020-0267-7. [Google Scholar] [CrossRef]
183. Rashid, N., Khan, S., Wahid, A., Ibrar, D., Hasnain, Z. et al. (2021). Exogenous application of biostimulants and synthetic growth promoters improved the productivity and grain quality of quinoa linked with enhanced photosynthetic pigments and metabolomics. Agronomy, 11(11), 2302. DOI 10.3390/agronomy11112302. [Google Scholar] [CrossRef]
184. Iqbal, J., Irshad, J., Bashir, S., Khan, S., Yousaf, M. et al. (2020). Comparative study of water extracts of moringa leaves and roots to improve the growth and yield of sunflower. South African Journal of Botany, 129, 221–224. DOI 10.1016/j.sajb.2019.06.032. [Google Scholar] [CrossRef]
185. Khan, S., Basra, S. M. A., Afzal, I., Nawaz, M., Rehman, H. U. (2017). Growth promoting potential of fresh and stored Moringa oleifera leaf extracts in improving seedling vigor, growth and productivity of wheat crop. Environmental Science and Pollution Research, 24(35), 27601–27612. DOI 10.1007/s11356-017-0336-0. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |