

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.019957
ARTICLE
Effect of Cadmium Stress on the Growth, Physiology, Stress Markers, Antioxidants and Stomatal Behaviour of Two Genotypes of Chickpea (Cicer arietinum L.)
1Environmental Physiology Laboratory, Department of Botany, Aligarh Muslim University, Aligarh, 202002, India
2Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
3Princess Dr NajlaBint Saud Al-Saud Center for Excellence Research in Biotechnology, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
4Department of Public Health, Daffodil International University, Dhaka, 1341, Bangladesh
*Corresponding Author: Khalid Rehman Hakeem. Email: kur.hakeem@gmail.com
Received: 26 October 2021; Accepted: 07 February 2022
Abstract: The current work was performed to know the impact of cadmium (Cd) toxicity on two different genotypes of chickpea (Cicer arietinum L.) namely Pusa-BG1053 and Pusa-BG372. Cadmium was applied in the form of cadmium chloride (CdCl2), in varying levels, 0, 25, 50, 75, and 100 mg Cd kg-1 soil. Plant growth as well as physiological attributes were decreased with increasing concentration of Cd. Both genotypes showed the maximum and significant reduction at the maximum dose of Cd (100 mg Cd kg-1 soil). Results of this study proved that the genotype Pusa-BG1053 was more tolerant and showed a lower decline in growth, photosynthetic and biochemical attributes than Pusa-BG372. This later genotype showed the maximum reduction and was sensitive to Cd stress. A better activity of antioxidants protected Pusa-BG1053 from Cd toxicity; on the other hand, the activity of antioxidants was much lower in Pusa-BG372. Scanning electron microscopic studies showed differences in both genotypes. In Pusa-BG1053, stomatal quantity was higher and stomata were slightly close to the characteristic guard cells. In Pusa-BG372 stomata were lower, slightly open and with highly affected guard cells. Root cell mortality due to the harsh effects of Cd appeared to be more evident in Pusa-BG372 than Pusa-BG1053, which was visible under a confocal microscope. As a result of this study, Pusa-BG1053 was a more tolerant genotype, and exhibited a minimum reduction in terms of all studied parameters than Pusa-BG372, which was a sensitive genotype to Cd toxicity.
Keywords: Antioxidants; cadmium; chickpea; genotypes; oxidative stress
Legumes are important in the food and nutritional security of millions of people worldwide, particularly of low-income families. Besides being a vital source of protein, starch, fibre, oil, and nutrients essential for human health, they also contain plenty of iron and zinc. It is an asset for the dietary management of protein for vegetarians as well as the poor populations. Besides containing a good amount of proteins, they also contain fats and carbohydrates [1]. Among various legumes, chickpea is considered, one of the essential grown legumes worldwide. It provides the second main source of proteins after Glycine max (soybean) and is also used as green manure, playing a pivotal role in crop rotation, hence, enhancing soil fertility. It is also used as fodder for animals.
Plants are exposed to many environmental situations that negatively affect plant growth and development. These factors include cold, drought, flooding, freezing, heat, salinity, or heavy metal stress [2,3]. Heavy metal toxicity, the most known abiotic environmental issue that causes hazardous effects in plants particularly legumes, change their physiological as well as metabolic activities, among various heavy metals (HMs), cadmium (Cd) is one of the major ones its toxicity reduces the general plant growth [3–6], and the symptoms of Cd injury in plants can be in the form of a slight injury to lethality resulting in crop failure. This is because Cd causes complex changes at biochemical, physiological, and genetic levels [7,8]. A high level of Cd alters the carbohydrate, proline, and protein contents [8–11], and fluster the absorption of nutrients like Nitrogen (N), Phosphorus (P), Potassium (K), Calcium (Ca), Magnesium (Mg), Manganese (Mn), Nickel (Ni), Copper (Cu), Iron (Fe), and Zinc (Zn) [6]. Fe deficiency causes chlorosis or yellowing of young leaves, affects root growth, decreased yield, and it also alters photosynthesis by reducing the chlorophyll and carotenoids contents. This is because it affects the photosynthetic apparatus and hence affects stomatal opening, size and density in the plant leaves [6,12]. Moreover, Cd can uplift the reactive oxygen species (ROS) levels [13,14]. The ROS causes oxidative damage by reacting with lipids, proteins, pigments as well as nucleic acids [6]. The peroxidation of lipids is normally recorded by an enhanced concentration of the malondialdehyde (MDA) content [10,14,15]. Plants have developed various strategies to deal with Cd toxicity, consisting of enzymatic and non-enzymatic systems [6,13,14,16–18], regulating Cd influx, accelerating Cd efflux, Cd chelation, Cd sequestration and remobilization, and scavenging of Cd-induced ROS [19,20]. Antioxidative enzymes like superoxide dismutase (SOD), catalase (CAT), and peroxidase (POX), can diminish the concentrations of ROS in plants [14]. High levels of Cd affect the cellularity of roots, due to a rapid reduction in vacuolization and osmotic disbalance [6].
The current study was aimed to elucidate the effect of Cd stress on the two chickpea genotypes based on their growth, chlorophyll and carotenoid content, nitrate reductase activity, carbonic anhydrase activity, proline content, lipid peroxidation, antioxidants, and scanning electron microscopic (SEM) and confocal microscopic studies.
2.1 Cd Treatment and Plant Material
Seeds of two genotypes of chickpea (Cicer arietinum L.), Pusa-BG372, and Pusa-BG1053 were collected from IARI Pusa, New Delhi. Seeds were treated for disinfection with 0.01% of mercuric chloride, then rinsed three times with distilled water, and then were sown in pots of 25 × 25 cm size, having a mixture of compost and soil in a 1:3 ratio. After 10 days of seed germination, thinning of seedlings was done to three per pot. The plants were treated with varying concentrations of Cadmium Chloride solutions (25, 50, 75, and 100 mg Cd kg-1 soil), and the control pots were supplied with tap water only and all the plants were irrigated on every alternate days. After 45 days of sowing, sampling was done and the following parameters were taken.
Plant (1) length, (2) dry weight, (3) leaf area, and (4) nodules number were determined, after 45 DAS. Plant length was taken with a centimetre scale. Dry weight of the plant was determined using an electronic balance (CY204, Scalteo Ins., Germany) after drying the plant material in an oven for 72 h at 80°C. Leaf area was measured on a leaf area meter (LA211, Systronics, Ahmedabad, India). The number of nodules per root system was counted on fresh roots.
2.3 Physiological and Biochemical Attributes
2.3.1 Total Chlorophyll and Carotenoid Concentrations
The method of Lichtenthaler et al. [21] was used to measure the total chlorophyll and carotenoid concentrations, by crushing 100 mg fresh leaves in 10 ml of 80% acetone. Absorbance for chlorophyll was measured at 663 nm and 645 nm and for carotenoid, at 480 nm and 510 nm on a UV-Visible spectrophotometer (Spectronic 20D; Milton Roy, USA).
2.3.2 Nitrate Reductase (NR) Activity
The activity of nitrate reductase (NR) was calculated according to the Jaworski protocol [22]. Small fragments of leaves were collected in separate vials, and potassium nitrate, phosphate buffer, and isopropanol were added, Vials were then incubated at 30°C for 2 h. Four ml of the mixture were taken out and sulphanilamide and N-1-naphthyl ethylenediamine hydrochlorides were added. Pink colour was developed after some time, and the absorbance was read at 540 nm on a spectrophotometer (Spectronic 20D; Milton Roy, USA).
2.3.3 Carbonic Anhydrase (CA) Activity
CA activity was calculated by the method of Dwivedi et al. [23]. Leaves were cut into smaller sections, and kept in a test tube containing cysteine hydrochloride. They were incubated in the mixture for about 20 min. The incubated mixture was treated with phosphate buffer, bromothymol blue indicator, and sodium bicarbonate, and then was again incubated. After that, some drops of methyl red were added as an indicator.
The estimation of leaf proline concentration was done following Bates et al. [24]. Sulphosalicylic acid was used for the extraction of samples. An equal amount of a solution of ninhydrin and glacial acetic acid was added to the extract. The reaction mixture was heated at 100oC; 5 ml of toluene were then added to the reaction mixture after cooling in an ice bath. Thereafter, the uppermost layer of the mixture was taken out, and the absorbance was measured at 528 nm on a spectrophotometer (Spectronic 20D; Milton Roy, USA).
2.3.5 Malondialdehyde (MDA) Concentration
Peroxidation of lipids in terms of MDA in fresh leaves was measured by the method of Cakmak et al. [25]. Homogenates of fresh leaves were made with 0.1% trichloroacetic acid (TCA). The supernatant was collected after centrifugation of samples, followed by 0.5% thiobarbituric acid addition. The mixture was heated at 100oC for about 30 min, and centrifuged again after cooling in an ice bath. The supernatant was collected and the absorbance was recorded at 523 and 600 nm.
2.4 Analysis of Enzymatic Antioxidants
For the estimation of enzymes, 1 g of fresh leaves was homogenized in 5 ml of 50 mM potassium phosphate buffer (pH 7.0), 1 mM phenyl methane sulfonylfluoride, 1 mM EDTA, 2% polyvinyl pyrrolidone, and 0.5% Triton X-100. After that, the homogenate was centrifuged for 10 min at 5°C at 15,000 rpm. The supernatant was collected and used for the estimation of the antioxidant enzymes.
The activity of Superoxide Dismutase (SOD) was calculated following the Beyer et al. [26] method, by recording the absorbance at 560 nm for 2 min at 25°C.
The activity of Peroxidase (POD) was calculated by using the method of Sanchez et al. [27]: the change in absorbance was measured at 436 nm on a spectrophotometer (Spectronic 20D; Milton Roy, USA) at 25°C for 1 min.
The activity of Catalase (CAT) was measured following Aebi [28], by observing the decrease in optical density at 240 nm at 25°C for one min with an interval of 30 s.
2.5 Estimation of Reactive Oxygen Species
Estimation of the superoxide radical O2•− was determined according to the method proposed by Kaur et al. [29], by immersing the leaf in a solution of 6 mM nitrozolium blue tetrachloride, which was prepared in sodium citrate buffer, incubated for 8 hours at room temperature. The leaf material was taken out from the solution and immersed in 100% ethanol followed by boiling at 100oC until chlorophyll removal; samples were transferredto 20% glycerol after cooling thenimages were captured with NIKON digital camera (COOLPIX110).
2.6 Scanning Electron Microscopy
Scanning electron microscopy (JEOL JSM-JSM 6510) was used to know the effect of Cd stress on stomata. Fresh leaf samples were fixed in 2.5% glutaraldehyde buffer (pH 7.3) for 2 h, and then fixed by 1% osmium oxide followed the critical point drying protocol. Fixed samples were dehydrated with ethanol (50, 70, 80, 90, and 100%), and they were coated with gold-palladium and observed at a magnification of 250X and 3000X under a scanning electron microscopy.
2.7 Confocal Microscopic Study for Cell Viability
Carefully uproot the plant, and separate the roots from the shoot. Roots were washed properly under tap water to remove the soil. Then, roots were cut into thin sections by using a sharp razor and collected in propidium iodine dye (5 µM) for 30–35 min. After staining the roots, they were placed on fresh glass slides and observed under a confocal microscope.
Three biological replicates were used for each treatment. Data were analyzed by one-way ANOVA, using a SPSS software (17.0 Version).
3.1 Effect of Cadmium Stress on Morphological Characteristics
Morphological characters of both chickpea cultivars showed a clear and significant reduction when the Cd stress conditions were increased (Figs. 1, 2a–2d). Among the concentrations of Cd, the lowest level (25 mg Cd kg-1 soil) caused a minimum reduction, sometimes statistically significant (e.g., plant height and dry weight on Pusa-BG372; Figs. 2a and 2b). The highest decline in morphological parameters in both varieties of C. arietinum was recorded at 100 mg of CdCl2 as compared to their respective controls. The highest level (100 mg Cd kg-1 soil) caused a maximum percent reduction of 56.32%, 61.99%, 49.47%, and 71.05% in plant length, plant dry biomass, leaf area, and nodule numbers, respectively, in Pusa-BG372 (Figs. 2a and 2d). These reductions were, respectively, of 31.01%, 38.72%, 27.02%, and 46.29% in Pusa-BG1053 at the highest Cd concentrations. Hence, more damage was recorded in Pusa-BG372 than Pusa-BG1053.

Figure 1: Effect of 100 mg Cd kg-1 soil on two varieties of C. aerietinum, PUSA-BG1053 and PUSA-BG372
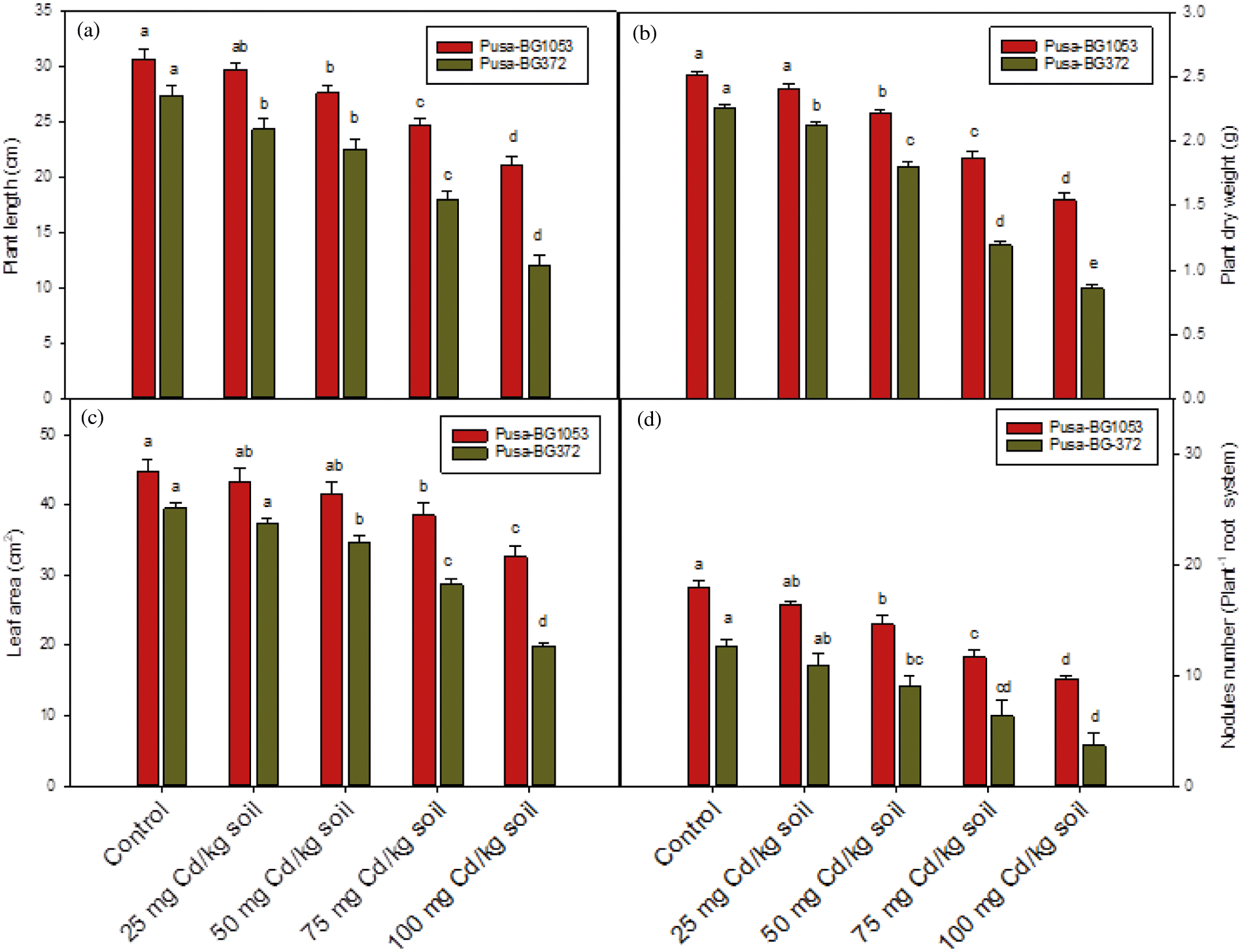
Figure 2: Effect of Cd on (a) Plant length, (b) Plant dry weight, (c) Leaf area, and (d) Nodule number per plant on two varieties of C. aerietinum, Pusa-BG1053 and Pusa-BG372. Different letters above the histograms on each variety of C. aerietinum indicate significant differences (P < 0.05) among the various Cd concentrations. Each histogram is the mean of n = 3. Vertical bars indicate S.E. of the means
3.2 Effect of Cadmium Stress on Physiological and Biochemical Attributes
3.2.1 Effect of Cadmium Treatment on Total Chlorophyll and Carotenoid Concentration
Total chlorophyll and carotenoids concentration were showed significant and maximum percent reduction under higher Cd levels (100 mg Cd kg-1 soil), in both the genotypes. Pusa-BG1053 showed a maximum and significant percent reduction of 29.29% and 32.23% in total chlorophyll and carotenoid respectively, while the same level of Cd caused severe and significant damage in Pusa-BG372, and reduced the total chlorophyll and carotenoid content by 65.8% 73.56% respectively than their respective control (Figs. 3a and 3b).
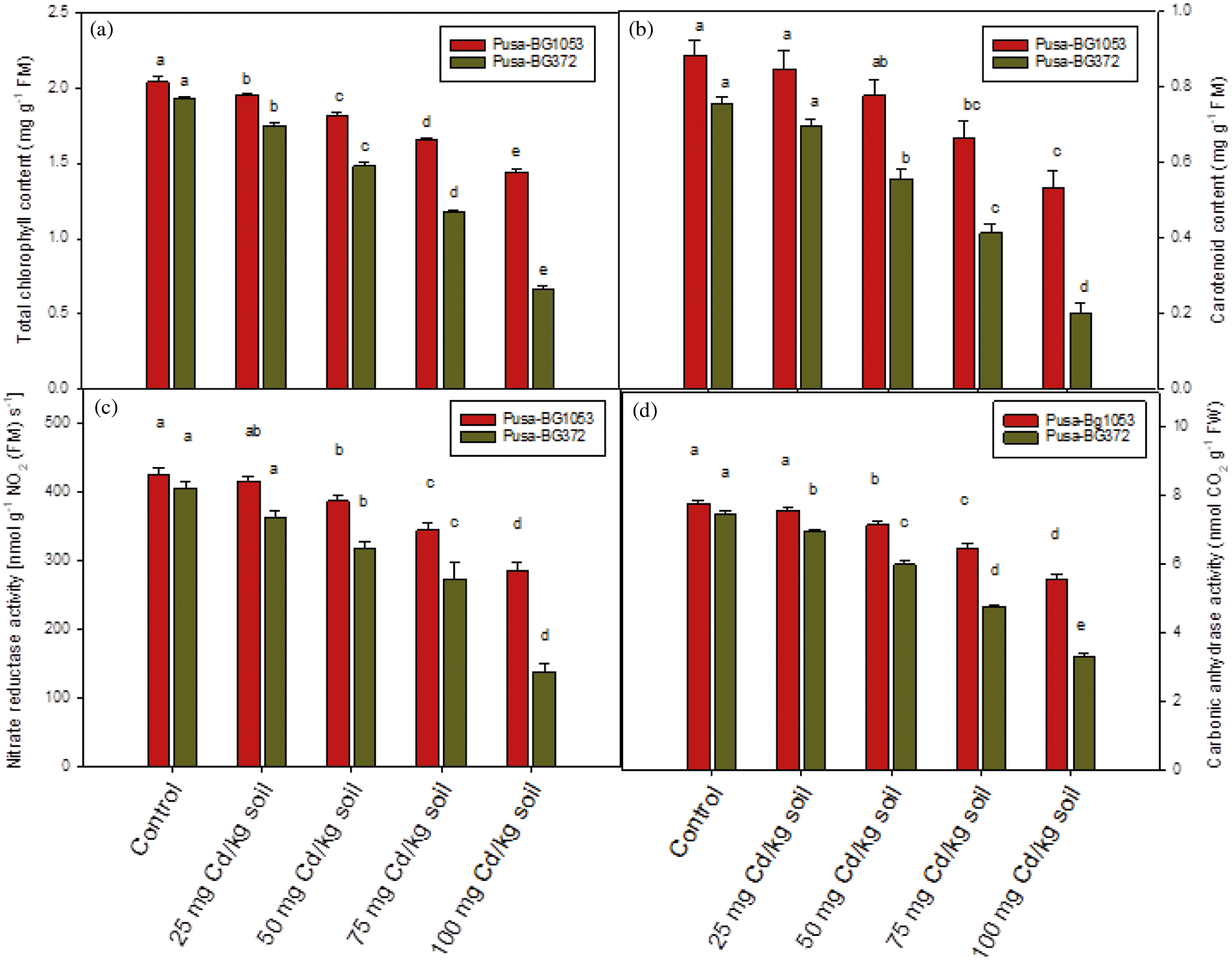
Figure 3: Effect of Cd on (a) Total chlorophyll concentration, (b) Carotenoid concentration, (c) Nitrate reductase activity, and (d) Carbonic anhydrase activity on two varieties of C. aerietinum, Pusa-BG1053 and Pusa-BG372. Different letters above the histograms on each variety of C. aerietinum indicate significant differences (P < 0.05) among the various Cd concentrations. Each histogram is the mean of n = 3. Vertical bars indicate S.E. of the means
3.2.2 Effect of Cadmium on NR Activity
The activity of the NR enzyme also showed a reduction under Cd treatment. The highest Cd level proved most toxic and caused a maximum and significant percent reduction of 65.83% in Pusa-BG372 than their respective control while the lowest level (25 mg Cd kg-1 soil) caused minimum and non-significant reduction. However, Pusa-BG1053 proved less sensitive as compared to Pusa-BG372 which is highly sensitive to Cd stress. Pusa-BG1053 exhibited a significant percent reduction of 33.16% in NR activity at 100 mg Cd kg-1 soil (Fig. 3c).
3.2.3 Effect of Cadmium on Carbonic Anhydrase Activity
The activity of Carbonic anhydrase was altered in response to different Cd concentrations (0, 25, 50, 75, and 100 mg Cd kg-1 soil) at 45 DAS in both the chickpea varieties, sometimes statistically non-significant (in Pusa-BG1053 at 25 mg Cd kg-1 soil; Fig. 3d). 100 mg of CdCl2 caused a maximum and significant reduction of 55.64% in Pusa-BG372 and 28.7% in Pusa-BG1053 as concerning their control (Fig. 3d).
Under Cd stressed condition, there is higher proline concentration was found in leaves of both the chickpea verities (Fig. 4a). The proline concentration was increased with increasing Cd concentration and was found maximum and statistically significant at the highest dose of Cd (100 mg Cd kg-1 soil) and maximum percent enhancement of 60.43% and 26.17% were recorded in Pusa-BG1053 and Pusa-BG372, respectively, in concern to their controls.
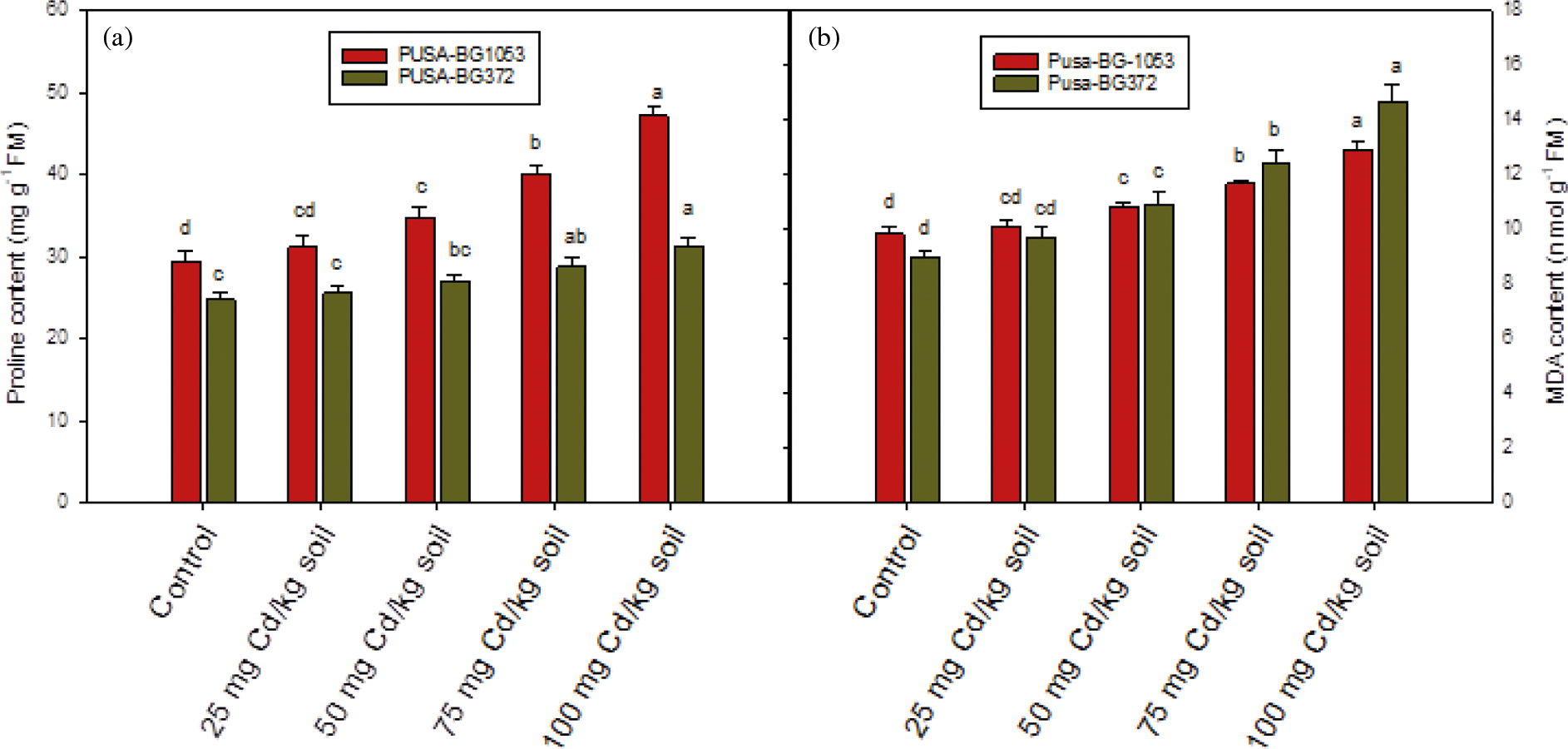
Figure 4: Effect of Cd on (a) proline concentration and (b) MDA concentration on two varieties of C. aerietinum, Pusa-BG1053 and Pusa-BG372. Different letters above the histograms on each variety of C. aerietinum indicate significant differences (P < 0.05) among the various Cd concentrations. Each histogram is the mean of n = 3. Vertical bars indicate S.E. of the means
3.2.5 Effect of Cadmium Treatment on MDA Concentration
MDA content which reflects the lipid peroxidation showed enhancement on increasing Cd concentration and was found maximum at 100 mg Cd kg-1 soil level. Both the varieties of chickpea exhibited enhanced MDA concentration and the maximum and statistically significant percent increase of 63.94% was found in Pusa-BG372, while Pusa-BG1053 showed a percent increase of 31.19% in MDA concentration (Fig. 4b). Hence it was proved that Pusa-BG372 exhibited more oxidative stress as compared to Pusa-BG1053.
3.3 Antioxidant Enzyme Activities
Antioxidant enzymes followed a similar trend of increase as of proline. Results showed that the activity of antioxidant enzyme enhances in response to Cd stress, and was maximum and statistically significant (statistically non-significant in case of Pusa-BG1053 at 25 mg Cd kg-1 soil) at the highest dose of Cd (100 mg Cd kg-1 soil). The activities of SOD, POD and CAT enzymes exhibited a maximum percent increase of 56.61%, 61.56%, and 56.91% in Pusa-BG1053 and 25.38%, 37.17%, and 25.65% in Pusa-BG372, respectively as compared to their respective control (Figs. 5a–5c).
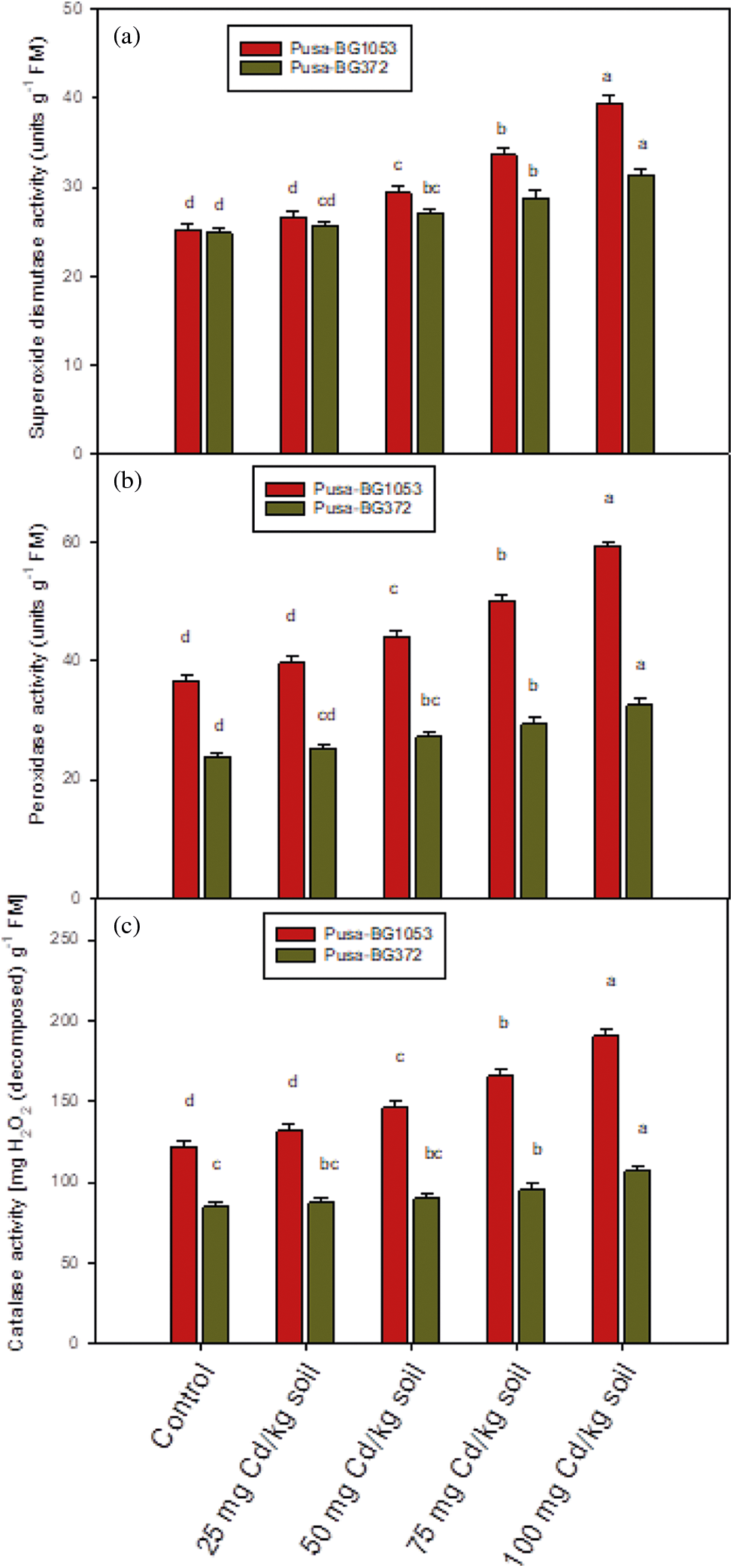
Figure 5: The effect of Cd on (a) SOD activity, (b) POD activity, and (c) CAT activity on two varieties of C. aerietinum, Pusa-BG1053 and Pusa-BG372, Different letters above the histograms on each variety of C. aerietinum indicate significant differences (P < 0.05) among the various Cd concentrations. Each histogram is the mean of n = 3. Vertical bars indicate S.E. of the means
3.4 Production of Superoxide Radicals (O2•−)
45-day old leaves of both the verities of chickpea showed O2•− generation in the form of blue spots on leaves under Cd stressed conditions. Pusa-BG372 showed a large number of spots as compared to Pusa-BG1053 because of the excessive accumulation of superoxide radicals (Fig. 6). This generation of spots gets increased on increasing Cd concentration and was found maximum at 100 mg Cd kg-1 soil, as compared to their control.

Figure 6: Effect of excessive accumulation of superoxide radicals due to 100 mg Cd Kg-1 soil concentrations of Cd, which leads to blue spots on leaves of two varieties of C. aerietinum, Pusa-BG1053 and Pusa-BG372, images represent (a) Control of BG-1053 (b) 100 mg Cd/ Kg soil of BG-1053 (c) Control of BG-372 and (d) 100 mg Cd/ Kg soil of BG-372
45-day old leaves of Pusa-BG1053 and Pusa-BG372 genotypes of chickpea showed a reduced number of stomata and affect stomatal behaviour, under Cd treatment. The highest Cd level (100 mg Cd kg-1 soil) presents a reduction in stomatal aperture as well as their number as compared to their control. Leaves of Pusa-BG372 as compared to Pusa-BG1053 exhibited less number of stomata, and reduced stomatal apertures (Fig. 7).
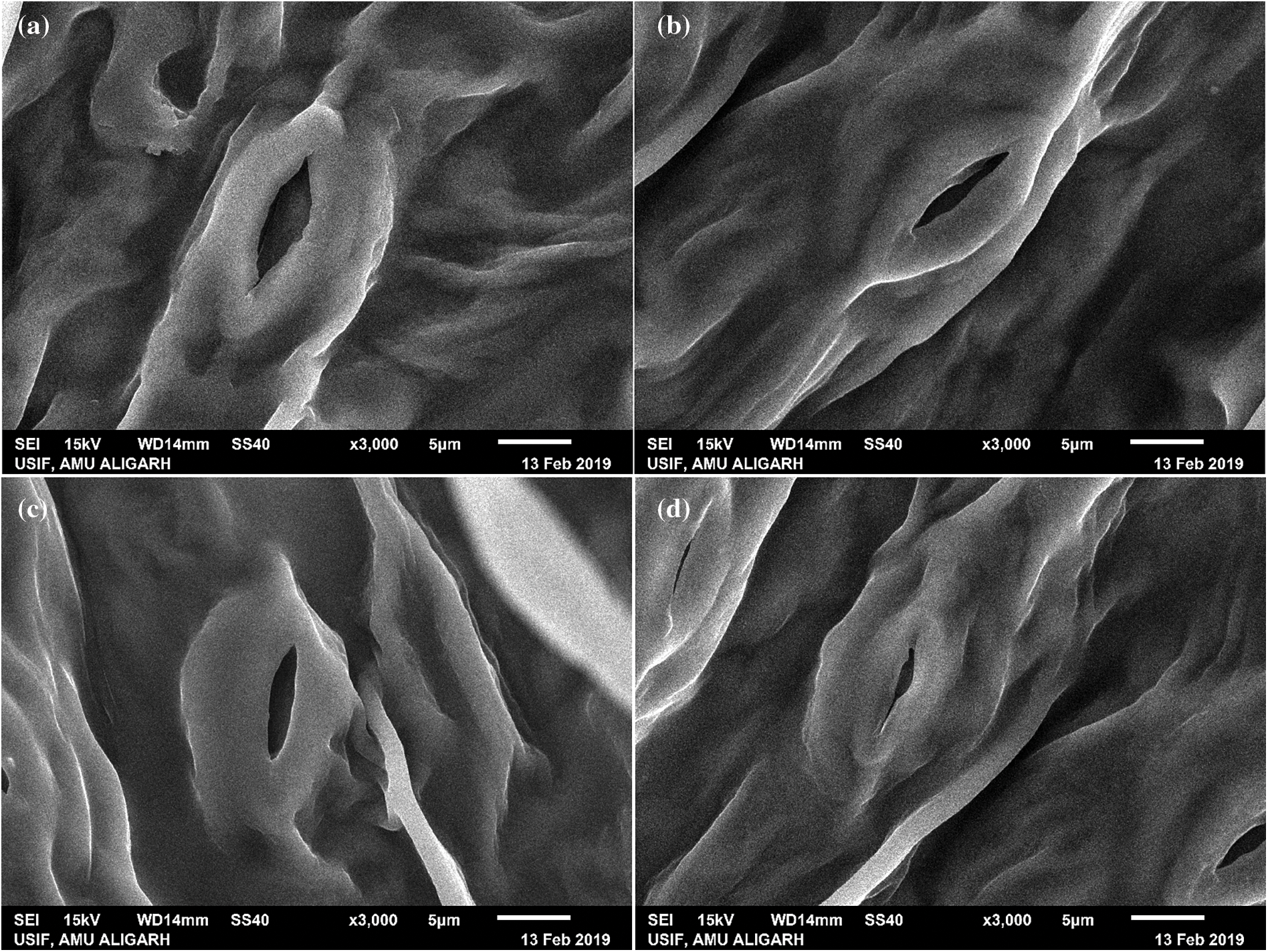
Figure 7: Effect of 100 mg Cd kg-1soil concentrations on stomatal opening in two varieties of C. aerietinum, Pusa-BG1053 and Pusa-BG372, images represent (a) Control of Pusa-BG1053 (b) 100 mg Cd Kg-1 soil of Pusa-BG1053 (c) Control of Pusa-BG372 and (d) 100 mg Cd kg-1 soil of Pusa-BG732
3.6 Confocal Studies Showed Cell Viability of Roots
The viability of cells gets decreased with an increase in Cd concentration (0, 25, 50, 75, and 100 mg). In the current work, both the varieties of chickpea showed the maximum number of dead cells as red spots at 100 mg Cd kg-1 soil, concentration but Variety Pusa-BG1053 presents lesser spots as compared to Pusa-BG372 (Fig. 8).
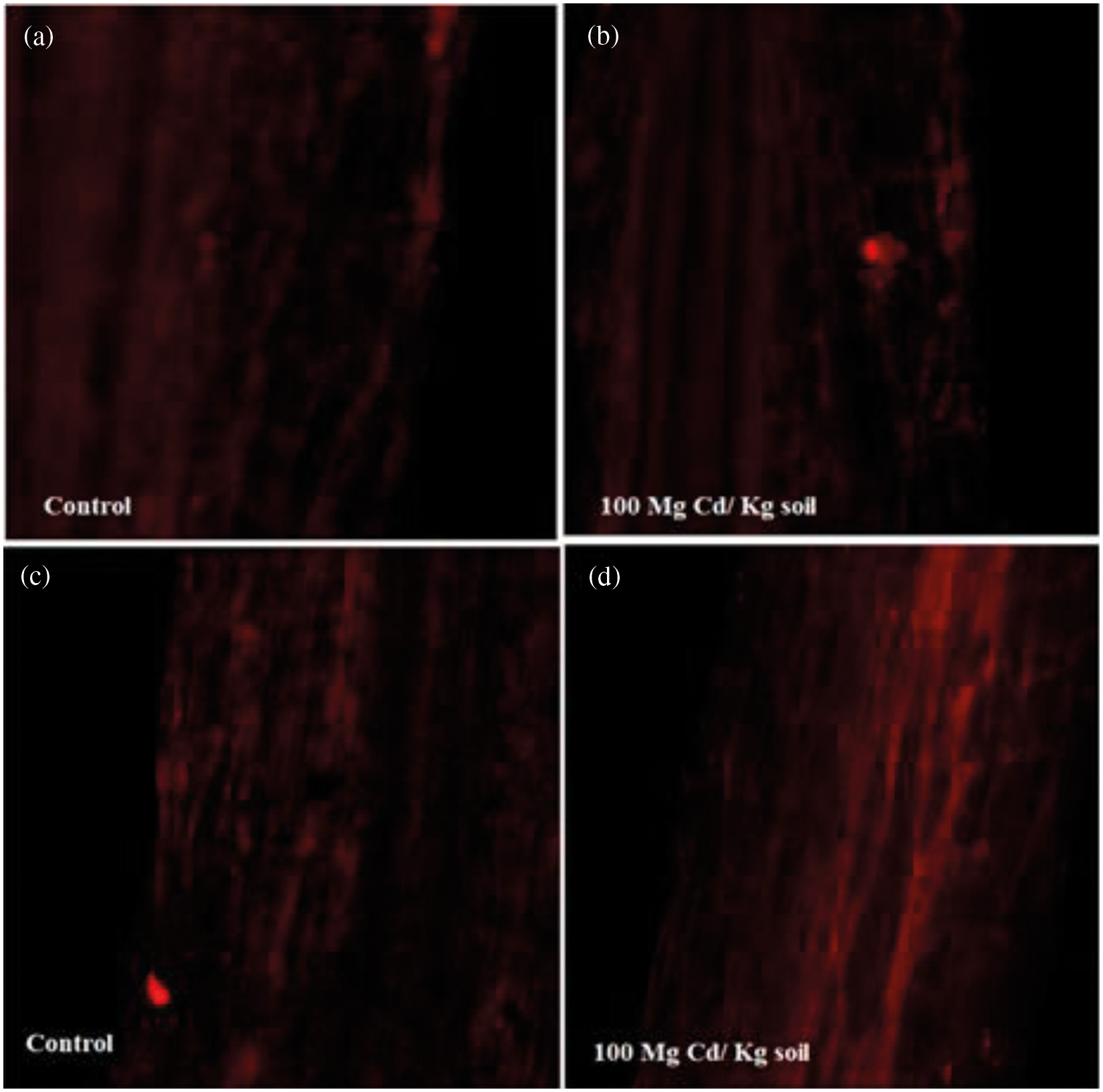
Figure 8: Effect of 100 mg Cd kg-1 soil concentrations of Cd on the cellular organization of roots in two varieties of C. aerietinum, Pusa-BG1053 and Pusa-BG372, images represent (a) Control of Pusa-BG1053 (b) 100 mg Cd kg-1 soil of Pusa-BG1053 (c) Control of Pusa-BG372 and (d) 100 mg Cd kg-1 soil of Pusa-BG-372
Environmental stress adversely affects the soil quality and crop productivity, which further leads to yield loss, under various agriculture production systems [30]. The contribution of environmental stress to global losses in crop production associated with abiotic stress becomes increasingly important and thus decreasing the yield by 70% [31]. Among various abiotic stresses, heavy metal contamination is most dangerous towards crop productivity and also affects human health [6,32]. Use of pesticides and chemical fertilizers and waste water irrigation contaminate the soil and have toxic levels of heavy metals which have adverse biological effects [33]. Cadmium (Cd) has become one of the most toxic pollutants due to the development of modern industry, its uptake and accumulation in plants pose potential risks to agriculture, the environment, and the health of human beings [34,35]. Cadmium is a non-essential element for plants that induces various toxic effects in plants [6] such as stunted growth of the plant, leaf chlorosis and necrosis, alteration in activities of various key enzymes that are involved in different metabolic pathways, inhibitions of nutrient uptake, homeostasis, and disturbance of cellular redox environment causing oxidative stress are the most common toxic effects of Cd stress [36–38]. It was previously revealed that plant genotypes performed differently under Cd stressed conditions [37–39], therefore, in the present study, two chickpea genotypes were tested for their different potential under different Cd concentrations by appraising the studied parameters.
The reduction in growth parameters in Cd-treated plants has been described [38,40] due to low water potential, higher Cd accumulation in different plant parts [6], restriction in water and nutritional uptake by the plant roots [11,41], structural alterations in plants [42], eruption in photosynthesis [38], devaluation of component of cell wall and reversal in the metabolism of carbohydrates [10,43]. Cd limits plant growth because it can interfere with the photosynthesis and translocation of photosynthetic products and essential nutrients [38,39,44], In the present study, it was observed that increasing concentration of Cadmium (0, 25, 50, 75, 100 mg Cd Kg−1 soil) had reduced growth traits in both tested genotypes of chickpea, which is as a decrease in plant length, dry weight of the plant, leaf area and nodules number per plant. The findings of this work are supported by the results of Zhou et al. [45]. The reason behind the reduction in seedling’s growth in Cd-treated plants could be due to the reduction in meristematic cells, activities of hydrolytic enzymes present in the cotyledons as well as endosperm, and hindrance in the movement of food to the radical and plumule [46]. Cd-stress also reduced the number of green leaves and may deleteriously affect the younger leaves [47], thus, the decrease in growth attributes could be due to the amassed effect of all these factors. At the highest Cd level, (100 mg Cd kg-1 soil) maximum and significant reductions were recorded in growth characteristics. Among the genotypes of chickpea, BG-1053 exhibited the lowest reduction, while in all the studied growth parameters, BG-372 showed the highest reduction. While the highest Cd level (100 mg Cd kg-1 of soil) proved to be more dangerous and declines the growth parameters in both the genotypes of chickpea than their respective control, to the large extent. Our findings match with the previous researches in references as in, wheat (Triticum aestivum) [12,48,49], rice (Oryza sativa) [50], maize (Zea maize) [51], mustard (Brassica napus) [42,52], tomato (Solanum lycopersicum) [53], and bean plants [44]. To detect the metal-induced damage in roots, degradation of chlorophyll and carotenoid content was used as visible symptoms of metal toxicity [54]. In the current study, an increase in Cd concentration caused the reduction in total chlorophyll and carotenoid concentration decreased at all the growth stages in both the chickpea plants and the extent of reduction was maximum in BG-372 and minimum in BG-1053 in the total chlorophyll & carotenoids concentration. The decline in chlorophyll concentration could be due to disturbed uptake and accumulation of some essential nutrients in plant-like Fe, Ca, Mg, K due to cadmium stress [6]. Kupper et al. [55] and de maria et al. [56] put forth that an increased concentration of Cd can replace the Mg from pigment chlorophyll molecule, thus reducing the photosynthetic ability in plants. Another possible reason for the decrease of pigment content may be because the higher accumulation of Cd can inhibit the uptake of other essential elements via roots [6]. Findings of this work were also in agreement with the results reported earlier in wheat (Triticum aestivum) [57], in barley (Hordeum vulgare) [58], in mungbean (Vigna radiata L.) [59], in cotton (Gossypium spp.) [3], in lettuce (Lactuca sativa) [60].
Nitrate Reductase (NR) is an important enzyme of plants, and its activity in both varieties gets decreased with an increased concentration of Cd. However, the maximum increase was observed in chickpea genotype BG-372 and minimum in BG-1053, respectively, concerning their control. Cd reduces the absorption and transportation of nitrate via roots in plants, which is the essential substrate of NR and thus eventually reduces its activity [61]. The results obtained in this study were also reported previously in maize (Zea maize) [61], chickpea (C. arietinum) [37], tomato (Lycopersicon esculentum) [62,63], soybean (Glycine max) [64]. Increasing the concentration of Cd causes a noticeable reduction in Carbonic Anhydrase (CA) activity [65], this reduction was may be due to the inhibitory impact of Cd on stomatal opening which in turn reduces the availability of CO2, ultimately leading to a reduction of CA activity [65,66]. Cd can also alter the structure of CA hence reducing its activity [67]. In the presented study, the maximum reduction in CA activity was recorded in BG-372 and the minimum was in BG-1053. Similar results were found earlier in chickpea (C. arietinum) [38,65], in French bean (Phaseolus vulgaris) [67]. Proline is a multifunctional metabolite [68], which provides a defence to the plants against Cd stress [9,69]. It is a non-enzymatic antioxidant [35], acting as a chelating agent [70], cytosolic osmoprotectant, and balances the plant water potential under stressed conditions [7]. In the current work, the level of proline concentration was increased significantly with an increased level of Cd. Among, two genotypes of chickpea, BG-1053 shows higher concentration of proline as compared to BG-372, over their respective control. An increase in proline concentration under enhanced dose of Cd was also recorded in chickpea genotypes (C. arietinum) [37], in mustard (Brassica juncea L.) [39,71], in pigeon pea (Cajanus cajan L.) [72], mung bean (Vigna radiata L.) [73]. MDA is the end product of membrane lipid peroxidation, its concentration is customarily considered as a general yardstick of peroxidation of lipid and stress level [14,15]. MDA concentration increases with the increasing level of Cd, due to excessive ROS generation, oxidation of membrane protein-like ion channels, transporters, enzymes, protein regulators, and phospholipids, due to oxidative stress [74]. Membrane permeability is also lost due to an increased level of MDA which was reported by Abdel Latef [10], in Capsicum annuum and Hussain et al. [75] in Zea maiz seedlings. In this study, an increase was recorded in both the genotypes, but a maximum increase was recorded in BG-372 as compared to BG-1053, at the highest level of Cd. The findings of this experiment are supported by the findings of Li et al. [76], who reported that oxidative stress is generated due to high levels of H2O2 and MDA levels in Arabidopsis under Cd stress, in pea (Pisum sativum) [77], in mungbean (Vigna radiata L.) [73]. Some cell organelles of plants like mitochondria, chloroplast, and peroxisomes exploit defence systems in terms of antioxidants [39], which cope with oxidative stress and provide a defence to the plant [78]. SOD, POD, and CAT are the important enzymatic antioxidantsthat prevent oxidative damage [76]. SOD catalyzes the dismutation of O2•− radicals to H2O2 and O2 and provides the first line of defence [79,80]. H2O2 being toxic product, whose decomposition is also important, which is carried out by CAT, which is a heme-containing tetrameric enzyme, localize in peroxisomes, convert it into H2O and O2 [14], and by POD which can scavenge H2O2, which is a major product of SOD [81]. H2O2 production mainly takes place in peroxisomes because of photorespiration, β-oxidation of fatty acids, purine catabolism, and oxidative damage [82]. The chances of OH• production is removed by SOD with the Haber-Weiss reaction [83], Under Cd stressed conditions, the activity of SOD is up-regulated [84]. In the present work, Cd toxicity enhanced the antioxidant enzymes activities (SOD, POD, and CAT). This enhancement in the antioxidant enzymes activities could be due to the over production of H2O2, which leads to oxidative stress in Cd-treated plants [84]. There were differences in the activities of these enzymes that showed, C. arietinum genotypes could acclimate Cd stress with the development of an antioxidant defence system, but BG-1053 performs better as compared to BG-372.
Cd ions severely affects the stomatal structure and opening at their highest level. That can be, due to the disturbance in turgor pressure of the guard cells, and damage of the guard subsidiary cells which control the stomatal structure and functioning. This abnormal stomatal closure can be due to the damage of guard subsidiary cells and loss in the turgor of the guard cells [6]. The results of confocal microscopic studies show red fluorescence, which surrounds the dead cells due to Cd. Cd affects the plant at the cellular level, the highest dose of Cd (100 mg Cd kg-1 soil) shows maximum dead cells. BG-372 shows more dead cells as compared to BG-1053 as compared to their respective control, similar results are found in Arabidopsis thaliana confocal studies, who show the destruction of root cells [85].
A perceptible difference was noticed in the tested genotypes. The variation in the studied parameters was observed could be due to variation in the genetic constitution and environmental interactions. All the growth parameters showed significant reduction with increasing concentration of Cd, while the MDA, proline antioxidant content, increases with an increase in Cd level. Among the different concentrations of Cd, the 100 mg Cd kg-1 of soil proved most toxic to the overall growth of chickpea crop and showed affected stomatal structure and dead root cells. Among the studied two genotypes of chickpea, the genotype BG-1053 proved the best and can face the adverse effects of Cd stress while BG-372 showed sensitiveness towards Cd stress.
Acknowledgement: The first author was deeply thankful to the Chairman, Department of Botany, Prof. Nafees Ahmad Khan, A.M.U., Aligarh, for providing necessary facilities and assistance for carrying out this research and to University Grant Commission, New Delhi India, for providing UGC Non-NET Fellowship.
Ethical Approval: In this research work, there is no experiment or study work has done on animals or humans.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Rasool, S., Ahmad, A., Siddiqi, T. O., Ahmad, P. (2013). Changes in growth, lipid peroxidation, and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiologiae Plantarum, 35(4), 1039–1050. DOI 10.1007/s11738-012-1142-4. [Google Scholar] [CrossRef]
2. Adrees, M., Ali, S., Rizwan, M., Ibrahim, M., Abbas, F. et al. (2015a). The effect of excess copper on growth and physiology of important food crops: A review. Environmental Sciences and Pollution Research, 2(11), 8148–8162. DOI 10.1007/s11356-015-4496-5. [Google Scholar] [CrossRef]
3. Rizwan, M., Hameed, A., Farooq, M. A., Farid, M., Bharwana., S. A. et al. (2016). Cadmium stress in cotton seedlings: Physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. South African Journal of Botany, 104, 61–68. DOI 10.1016/j.sajb.2015.11.006. [Google Scholar] [CrossRef]
4. Gallego, S. M., Pena, L. B., Barcia, R. A. (2012). Unraveling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environmental and Experimental Botany, 83(7), 33–46. DOI 10.1016/j.envexpbot.2012.04.006. [Google Scholar] [CrossRef]
5. Asgher, M., Khan, M. I. R., Anjum, N. A., Khan, N. A. (2015). Minimizing toxicity of cadmium in plants–role of plant growth regulators. Protoplasma, 252(2), 399–413. DOI 10.1007/s00709-014-0710-4. [Google Scholar] [CrossRef]
6. Haider, F. U., Liqun, C., Coulter, J. A., Cheema, S. A., Wu, J. et al. (2021). Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicology and Environmental Safety, 211(193), 111887. DOI 10.1016/j.ecoenv.2020.111887. [Google Scholar] [CrossRef]
7. Mushtaq, Z., Faizan, S., Gulzar, B., Mushtaq, H., Bushra, S. et al. (2021). Changes in growth, photosynthetic pigments, cell viability, lipid peroxidation and antioxidant defense system in two varieties of chickpea (Cicer arietinum L.) subjected to salinity stress. Phyton-International Journal of Experimental Botany, 91(1), 149–168. DOI 10.32604/phyton.2022.016231. [Google Scholar] [CrossRef]
8. El-Beltagi, H. S., Mohamed, H. I. (2013). Alleviation of cadmium toxicity in Pisum sativum L. seedlings by calcium chloride. Notulae Botanicae Horti Agrobotanicae Cluj-Napoca, 41(1), 157–168. DOI 10.15835/nbha4118910. [Google Scholar] [CrossRef]
9. Siddique, M. H., Al-Whaibi, M. H., Sakran, A. H., Basalah, M. O., Ali, H. M. (2012). Effect of calcium and potassium on antioxidant system of Vicia faba L. under cadmium stress. International Journal of Molecular Sciences, 13(6), 6604–6619. DOI 10.3390/ijms13066604. [Google Scholar] [CrossRef]
10. Abdel Latef, A. A. (2018). Growth and some physiological activities of pepper (Capsicum annuum L.) in response to cadmium stress and mycorrhizal symbiosis. Journal of Agriculture, Science and Technology, 15(7), 1437–1448. [Google Scholar]
11. Mondal, N. K., Das, C., Roy, S., Datta, J. K., Banerjee, A. (2013). Effect of varying cadmium stress on chickpea (Cicer arietinum L.) seedlings: An ultrastructural study. Annals of Environmental Science, 7, 59–70. [Google Scholar]
12. Rizwan, M., Meunier, J. D., Davidian, J. C., Pokrovsky, O. S., Bovet, N. et al. (2015a). Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environmental Science and Pollution Research, 23(2), 1414–1427. DOI 10.1007/s11356-015-5351-4. [Google Scholar] [CrossRef]
13. Sakouhi, L., Kharbech, O., Massoud, M. B., Munemasa, S., Murata, Y. et al. (2021). Oxalic acid mitigates cadmium toxicity in cicer arietinum L. germinating seeds by maintaining the cellular redox homeostasis. Journal of Plant Growth Regulation, 44(12), 1–13. DOI 10.1007/s00344-021-10334-1. [Google Scholar] [CrossRef]
14. Ahmad, P., Sarwat, M., Bhat, N. A., Wani, M. R., Kazi, A. G. et al. (2015). Alleviation of cadmium toxicity in, Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLos One, 10(1), e0114571. DOI 10.1371/journal.pone.0114571. [Google Scholar] [CrossRef]
15. Anjum, N. A., Sofo, A., Scopa, A., Roychoudhury, A., Gill, S. S. et al. (2015). Lipids and proteins-major targets of oxidative modifications in abiotic stressed plants. Environmental Science and Pollution Research, 22(6), 4099–4121. DOI 10.1007/s11356-014-3917-1. [Google Scholar] [CrossRef]
16. Ahmad, P., Abd Allah, E. F., Hashem, A., Sarwat, M., Gucel, S. (2016a). Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L. (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. Journal of Plant Growth Regulation, 35(4), 936–950. DOI 10.1007/s00344-016-9592-3. [Google Scholar] [CrossRef]
17. Hasanuzzaman, M., Fujita, M. (2011). Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biological Trace Element Research, 143(3), 1758–1776. DOI 10.1007/s12011-011-8998-9. [Google Scholar] [CrossRef]
18. Hasanuzzaman, M., Fujita, M. (2013). Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology, 22, 584–596. DOI 10.1007/s10646-013-1050-4. [Google Scholar] [CrossRef]
19. Lin, Y. F., Aarts, M. G. (2012). The molecular mechanism of zinc and cadmium stress response in plants. Cellular and Molecular Life Sciences, 69(19), 3187–3206. DOI 10.1007/s00018-012-1089-z. [Google Scholar] [CrossRef]
20. Shi, Y. Z., Zhu, X. F., Wan, J. X., Li, G. X., Zheng, S. J. (2015). Glucose alleviates cadmium toxicity by increasing cadmium fixation in root cell wall and sequestration into vacuole in Arabidopsis. Journal of Integrative Plant Biology, 57(10), 830–837. DOI 10.1111/jipb.12312. [Google Scholar] [CrossRef]
21. Lichtenthaler, H. K., Buschmann, C. (2001). Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Current Protocols in Food Analytical Chemistry, 1(1), F4–3. DOI 10.1002/0471142913.faf0403s01. [Google Scholar] [CrossRef]
22. Jaworski, E. G. (1971). Nitrate reductase assay in intact plant tissues. Biochemical and Biophysical Research Communication, 43(6), 1274–1279. DOI 10.1016/S0006-291X(71)80010-4. [Google Scholar] [CrossRef]
23. Dwivedi, R. S., Randhawa, N. S. (1974). Evaluation of a rapid test for the hidden hunger of zinc in plants. Plant Soil, 40(2), 445–451. DOI 10.1007/BF00011531. [Google Scholar] [CrossRef]
24. Bates, L. S., Waldren, R. P., Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205–207. DOI 10.1007/BF00018060. [Google Scholar] [CrossRef]
25. Cakmak, I., Horst, W. J. (1991). Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiologia Plantarum, 83(3), 463–468. DOI 10.1111/j.1399-3054.1991.tb00121.x. [Google Scholar] [CrossRef]
26. Beyer Jr, W. F., Fridovich, I. (1987). Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Analytical Biochemistry, 161(2), 559–566. DOI 10.1016/0003-2697(87)90489-1. [Google Scholar] [CrossRef]
27. Sanchez, M., Revilla, G., Zarra, I. (1995). Changes in peroxidase activity associated with cell walls during pine hypocotyl growth. Annals of Botany, 75(4), 415–419. DOI 10.1006/anbo.1995.1039. [Google Scholar] [CrossRef]
28. Aebi, H. (1984). Catalase in vitro. In: Methods in enzymology, vol. 105, pp. 121–126. Academic Press. DOI 10.1016/S0076-6879(84)05016-3. [Google Scholar] [CrossRef]
29. Kaur, N., Sharma, I., Kirat, K., Pati, P. K. (2016). Detection of reactive oxygen species in Oryza sativa L. (rice). Bio-Protocol, 6(24), e2061. DOI 10.21769/BioProtoc.2061. [Google Scholar] [CrossRef]
30. Smiri, M., Chaoui, A., Rouhier, N., Gelhaye, E., Jacquot, J. P. et al. (2011). Cadmium affects the glutathione/glutaredoxin system in germinating pea seeds. Biological Trace Element Research, 142(1), 93–105. DOI 10.1007/s12011-010-8749-3. [Google Scholar] [CrossRef]
31. Kang, J. S., Singh, H., Singh, G., Kang, H., Kalra, V. P. et al. (2017). Abiotic stress and its amelioration in cereals and pulses: A review. International Journal of Current Microbiology and Applied Sciences, 6(3), 1019–1045. DOI 10.20546/ijcmas.2017.603.120. [Google Scholar] [CrossRef]
32. Ullah, S., Khan, J., Hayat, K., Abdelfattah Elateeq, A., Salam, U. et al. (2020). Comparative study of growth, cadmium accumulation and tolerance of three chickpea (Cicer arietinum L.) cultivars. Plants, 9(3), 310. DOI 10.3390/plants9030310. [Google Scholar] [CrossRef]
33. Sidhu, G. S. (2016). Heavy metal toxicity in soils: Sources, remediation technologies and challenges. Advances in Plants & Agriculture Research, 5(1), 445–446. DOI 10.15406/APAR.2016.05.00166. [Google Scholar] [CrossRef]
34. Parmar, P., Kumari, N., Sharma, V. (2013). Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Botanical Studies, 54(1), 1–6. DOI 10.1186/1999-3110-54-45. [Google Scholar] [CrossRef]
35. Zhao, Y. (2011). Cadmium accumulation and antioxidative defences in leaves of Triticum aestivum L. and Zea mays L. African Journal of Biotechnology, 10(15), 2936–2943. DOI 10.5897/AJB10.1230. [Google Scholar] [CrossRef]
36. Anjum, S. A., Xie, X. Y., Wang, L. C., Saleem, M. F., Man, C. et al. (2011). Morphological, physiological and biochemical responses of plants to drought stress. African Journal of Agricultural Research, 6(9), 2026–2032. DOI 10.5897/AJAR10.027. [Google Scholar] [CrossRef]
37. Faizan, S., Kausar, S., Perveen, R. (2011). Varietal differences for cadmium-induced seedling mortality, foliar toxicity symptoms, plant growth, proline and nitrate reductase activity in chickpea (Cicer arietinum L). Biology and Medicine, 3, 196–206. [Google Scholar]
38. Faizan, S., Kausar, S., Perveen, R. (2012). Variation in growth, physiology and yield of four chickpea cultivars exposed to cadmium chloride. Journal of Environmental Biology, 33(6), 1137–1142. [Google Scholar]
39. Gill, S. S., Khan, N. A., Tuteja, N. (2011). Differential cadmium stress tolerance in five Indian mustard (Brassica juncea L.) cultivars: An evaluation of the role of antioxidant machinery. Plant Signaling & Behavior, 6(2), 293–300. DOI 10.4161/psb.6.2.15049. [Google Scholar] [CrossRef]
40. Farid, M., Shakoor, M. B., Ehsan, S., Ali, S., Zubair, M. et al. (2013). Morphological, physiological and biochemical responses of different plant species to Cd stress. International Journal of Chemical and Biochemical Sciences, 3(2013), 53–60. [Google Scholar]
41. Irfan, M., Ahmad, A., Hayat, S. (2014). Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi Journal of Biological Sciences, 21(2), 125–131. DOI 10.1016/j.sjbs.2013.08.001. [Google Scholar] [CrossRef]
42. Ali, B., Qian, P., Jin, R., Ali, S., Khan, M. et al. (2014). Physiological and ultra-structural changes in Brassica napus seedlings induced by cadmium stress. Biologia Plantarum, 58(1), 131–138. DOI 10.1007/s10535-013-0358-5. [Google Scholar] [CrossRef]
43. Hussain, M., Park, H. W., Farooq, M., Jabran, K., Lee, D. J. (2013). Morphological and physiological basis of salt resistance in different rice genotypes. International Journal of Agricultural Biology, 15(1), 970–974. [Google Scholar]
44. Saidi, I., Ayouni, M., Dhieb, A., Chtourou, Y., Chaibi, W. et al. (2013). Oxidative damages induced by short-term exposure to cadmium in bean plants: Protective role of salicylic acid. South African Journal of Botany, 85, 32–38. DOI 10.1016/j.sajb.2012.12.002. [Google Scholar] [CrossRef]
45. Zhou, Q., Guo, J. J., He, C. T., Shen, C., Huang, Y. Y. et al. (2016). Comparative transcriptome analysis between low-and high-cadmium-accumulating genotypes of pakchoi (Brassica chinensis L.) in response to cadmium stress. Environmental Science and Technology, 50(12), 6485–6494. DOI 10.1021/acs.est.5b06326. [Google Scholar] [CrossRef]
46. Houshmandfar, A., Moraghebi, F. (2011). Evaluation of heavy metal tolerance at different clover plant growth stages. Iranian Journal of Plant Physiology, 1, 95–99. DOI 10.3934/microbiol.2017.2.279. [Google Scholar] [CrossRef]
47. Eissa, M. A., Abeed, A. H. (2019). Growth and biochemical changes in quail bush (Atriplex lentiformis (Torr.) S. Wats) under Cd stress. Environmental Science and Pollution Research, 26(1), 628–635. DOI 10.1007/s11356-018-3627-1. [Google Scholar] [CrossRef]
48. Rizwan, M., Meunier, J. D., Miche, H., Keller, C. (2012). Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. Journal of Hazardous Material, 209, 326–334. DOI 10.1016/j.jhazmat.2012.01.033. [Google Scholar] [CrossRef]
49. Rehman, M. Z. U., Rizwan, M., Ghafoor, A., Naeem, A., Ali, S. et al. (2015). Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environmental Science and Pollution Research, 22(21), 16897–16906. DOI 10.1007/s11356-015-4883-y. [Google Scholar] [CrossRef]
50. Nahakpam, S., Shah, K. (2011). Expression of key antioxidant enzymes under combined effect of heat and cadmium toxicity in growing rice seedlings. Plant Growth Regulation, 63(1), 23–35. DOI 10.1007/s10725-010-9508-3. [Google Scholar] [CrossRef]
51. Vaculik, M., Pavlovic, A., Lux, A. (2015). Silicon alleviates cadmium toxicity by enhanced photosynthetic rate and modified bundle sheath’s cell chloroplasts ultrastructure in maize. Ecotoxicology and Environmental Safety, 120, 66–73. DOI 10.1016/j.ecoenv.2015.05.026. [Google Scholar] [CrossRef]
52. Ehsan, S., Ali, S., Noureen, S., Mahmood, K., Farid, M. et al. (2014). Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicology and Environmental Safety, 106(6), 164–172. DOI 10.1016/j.ecoenv.2014.03.007. [Google Scholar] [CrossRef]
53. Hediji, H., Djebali, W., Belkadhi, A., Cabasson, C., Moing, A. et al. (2015). Impact of long-term cadmium exposure on mineral con-tent of Solanum lycopersicum plants: Consequences on fruit production. South African Journal of Botany, 97, 176–181. DOI 10.1016/j.sajb.2015.01.010. [Google Scholar] [CrossRef]
54. Martins, L. L., Mourato, M. P., Cardoso, A. I., Pinto, A. P., Mota, A. M. et al. (2011). Oxidative stress induced by cadmium in Nicotiana tabacum L.: Effects on growth parameters, oxidative damage and antioxidant responses in different plant parts. Acta Physiologiae Plantarum, 33(4), 1375–1383. DOI 10.1007/s11738-010-0671-y. [Google Scholar] [CrossRef]
55. Kupper, H., Kupper, F., Spiller, M. (1998). In situ detection of heavy metal substituted chlorophylls in water plants. Photosynthesis Research, 58(2), 123–133. DOI 10.1023/A:1006132608181. [Google Scholar] [CrossRef]
56. de Maria, S., Puschenreiter, M., Rivelli, A. R. (2013). Cadmium accumulation and physiological response of sunflower plants to Cd during the vegetative growing cycle. Plant, Soil and Environment, 59(6), 254–261. DOI 10.17221/788/2012-PSE. [Google Scholar] [CrossRef]
57. Zhang, H., Xu, Z., Guo, K., Huo, Y., He, G. et al. (2020). Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicology and Environmental Safety, 202(1), 110856. DOI 10.1016/j.ecoenv.2020.110856. [Google Scholar] [CrossRef]
58. Chandra, R., Kang, H. (2016). Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. Forest Science and Technology, 12(2), 55–61. DOI 10.1080/21580103.2015.1044024. [Google Scholar] [CrossRef]
59. Chen, H. C., Zhang, S. L., Wu, K. J., Li, R., He, X. R. et al. (2020). The effects of exogenous organic acids on the growth, photosynthesis and cellular ultrastructure of Salix variegata Franch. under Cd stress. Ecotoxicology and Environmental Safety, 187(1), 109790. DOI 10.1016/j.ecoenv.2019.109790. [Google Scholar] [CrossRef]
60. Dias, M. C., Monteiro, C., Moutinho-Pereira, J., Correia, C., Gonçalves, B. et al. (2013). Cadmium toxicity affects photosynthesis and plant growth at different levels. Acta Physiolgiae Plantarum, 35(4), 1281–1289. DOI 10.1007/s11738-012-1167-8. [Google Scholar] [CrossRef]
61. Singh, P., Singh, I., Shah, K. (2019). Reduced activity of nitrate reductase under heavy metal cadmium stress in rice: An in silico answer. Frontiers in Plant Science, 9, 1948. DOI 10.3389/fpls.2018.01948. [Google Scholar] [CrossRef]
62. Hayat, S., Hasan, S. A., Ahmad, A. (2011). Growth, nitrate reductase activity and antioxidant system in cadmium stressed tomato (Lycopersicon esculentum Mill.) cultivars. Biotechnologie Agronomie Société Et Environnement, 15(3), 401–414. [Google Scholar]
63. Koc, E., Ustun, A. S., Celik, N. (2013). Effect of exogenously applied salicylic acid on cadmium chloride-induced oxidative stress and nitrogen metabolism in tomato (Lycopersicon esculentum L.). Turkish Journal of Biology, 37(3), 361–369. DOI 10.3906/biy-1211-13. [Google Scholar] [CrossRef]
64. Srivastava, R., Khan, R., Nasim, S. A., Manzoor, N. (2011). Cadmium treatment alters phytochemical and biochemical activity in Glycine max L. International Journal of Botany, 7(4), 305–309. DOI 10.3923/ijb.2011.305.309. [Google Scholar] [CrossRef]
65. Haneef, I., Faizan, S., Perveen, R., Kausar, S. (2014). Impact of bio-fertilizers and different levels of cadmium on the growth, biochemical contents and lipid peroxidation of Plantago ovata Forsk. Saudi Journal of Biological Sciences, 21(4), 305–310. DOI 10.1016/j.sjbs.2013.12.005. [Google Scholar] [CrossRef]
66. Faraz, A., Faizan, M., Sami, F., Siddiqui, H., Hayat, S. (2020). Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica juncea. Journal of Plant Growth Regulation, 39(2), 641–655. DOI 10.1007/s00344-019-10007-0. [Google Scholar] [CrossRef]
67. Siedlecka, A., Krupa, Z., Samuelsson, G., Oquist, G., Gardestrom, P. (1997). Primary carbon metabolism in Phaseolus vulgaris plants under Cd/Fe interaction. Plant Physiology and Biochemistry, 35(12), 951–957. [Google Scholar]
68. Szepesi, A., Szollosi, R. (2018). Mechanism of proline biosynthesis and role of proline metabolism enzymes under environmental stress in plants. In: Plant metabolites and regulation under environmental stress, pp. 337–353. USA: Academic Press. DOI 10.1016/B978-0-12-812689-9.00017-0. [Google Scholar] [CrossRef]
69. Abdel Latef, A. A., Tran, L. S. P. (2016). Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Frontiers in Plant Science, 7, 243. DOI 10.3389/fpls.2016.00243. [Google Scholar] [CrossRef]
70. Dar, M. I., Naikoo, M. I., Rehman, F., Naushin, F., Khan, F. A. (2016). Proline accumulation in plants: Roles in stress tolerance and plant development. In: Osmolytes and plants acclimation to changing environment: Emerging omics technologies, pp. 155–166. New Delhi: Springer.10.1007/978-81-322-2616-1_9. [Google Scholar] [CrossRef]
71. Hayat, S., Khalique, G., Wani, A. S., Alyemeni, M. N., Ahmad, A. (2014). Protection of growth in response to 28-homobrassinolide under the stress of cadmium and salinity in wheat. International Journal of Biological Macromolecules, 64, 130–136. DOI 10.1016/j.ijbiomac.2013.11.021. [Google Scholar] [CrossRef]
72. Hayat, K., Khan, J., Khan, A., Ullah, S., Ali, S. et al. (2021). Ameliorative effects of exogenous proline on photosynthetic attributes, nutrients uptake, and oxidative stresses under cadmium in pigeon pea (Cajanus cajan L.). Plants, 10(4), 796. DOI 10.3390/plants10040796. [Google Scholar] [CrossRef]
73. Yusuf, M., Fariduddin, Q., Ahmad, A. (2012). 24-Epibrassinolide modulates growth, nodulation, antioxidant system, and osmolyte in tolerant and sensitive varieties of Vigna radiata under different levels of nickel: A shotgun approach. Plant Physiology and Biochemistry, 57, 143–153. DOI 10.1016/j.plaphy.2012.05.004. [Google Scholar] [CrossRef]
74. Hall, J. A. (2002). Cellular mechanisms for heavy metal detoxification and tolerance. Journal of Experimental Botany, 53(366), 1–11. DOI 10.1093/jexbot/53.366.1. [Google Scholar] [CrossRef]
75. Hussain, A., Abbas, N., Arshad, F., Akram, M., Khan, Z. I. et al. (2013). Effects of diverse doses of Lead (Pb) on different growth attributes of Zea mays L. Agricultural Sciences, 4, 262–265. DOI 10.4236/as.2013.45037. [Google Scholar] [CrossRef]
76. Li, S., Chen, J., Islam, E., Wang, Y., Wu, J. et al. (2016). Cadmium-induced oxidative stress, response of antioxidants and detection of intracellular cadmium in organs of moso bamboo (Phyllostachys pubescens) seedlings. Chemosphere, 153, 107–114. DOI 10.1016/j.chemosphere.2016.02.062. [Google Scholar] [CrossRef]
77. Bavi, K., Kholdebarin, B., Moradshahi, A. (2011). Effect of cadmium on growth, protein content and peroxidase activity in pea plants. Pakistan Journal of Botany, 43(3), 1467–1470. DOI 10.32404/rean.v6i3.3322. [Google Scholar] [CrossRef]
78. Foyer, C. H., Shigeoka, S. (2011). Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiology, 155(1), 93–100. DOI 10.1104/pp.110.166181. [Google Scholar] [CrossRef]
79. Alscher, R. G., Erturk, N., Heath, L. S. (2002). Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. Journal of Experimental Botany, 53(372), 1331–1341. DOI 10.1093/jexbot/53.372.1331. [Google Scholar] [CrossRef]
80. Mourato, M., Reis, R., Martins, L. L. (2012). Characterization of plant antioxidative system in response to abiotic stresses: A focus on heavy metal toxicity. Advances in Selected Plant Physiology Aspects, 12, 1–17. DOI 10.5772/34557. [Google Scholar] [CrossRef]
81. Li, F. T., Qi, J. M., Zhang, G. Y., Lin, L. H., Fang, P. P. et al. (2013). Effect of cadmium stress on the growth, antioxidative enzymes and lipid peroxidation in two kenaf (Hibiscus cannabinus L.) plant seedlings. Journal of Integrative Agriculture, 12(4), 610–620. DOI 10.1016/S2095-3119(13)60279-8. [Google Scholar] [CrossRef]
82. Del Rio, L. A., Lopez-Huertas, E. (2016). ROS generation in peroxisomes and its role in cell signaling. Plant and Cell Physiology, 57(7), 1364–1376. DOI 10.1093/pcp/pcw076. [Google Scholar] [CrossRef]
83. Das, K., Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science, 2, 53. DOI 10.3389/fenvs.2014.00053. [Google Scholar] [CrossRef]
84. Boguszewska, D., Grudkowska, M., Zagdańska, B. (2010). Drought-responsive antioxidant enzymes in potato (Solanum tuberosum L.). Potato Research, 53(4), 373–382. DOI 10.1007/s11540-010-9178-6. [Google Scholar] [CrossRef]
85. Pollastri, S., Azzarello, E., Masi, E., Pandolfi, C., Mugnai, S. et al. (2012). Applications of confocal microscopy in the study of root apparatus. In: Measuring roots, pp. 93–108. Berlin, Heidelberg: Springer.DOI 10.1007/978-3-642-22067-8_6. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |