

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020643
ARTICLE
Effect of Paclobutrazol Application on Plant Growth and Flower Quality in Herbaceous Peony
1Joint International Research Laboratory of Agriculture and Agri-Product Safety, The Ministry of Education of China, Institutes of Agricultural Science and Technology Development, Yangzhou University, Yangzhou, 225009, China
2Jiangsu Key Laboratory of Crop Genetics and Physiology, College of Horticulture and Plant Protection, Yangzhou University, Yangzhou, 225009, China
*Corresponding Author: Jun Tao. Email: taojun@yzu.edu.cn
Received: 04 December 2021; Accepted: 12 January 2022
Abstract: Herbaceous peony (Paeonia lactiflora Pall.) is an important ornamental plant worldwide. In its natural state, P. lactiflora often manifests traits like rapidly elongating internodal growth, loose plant types, and soft inflorescence stems. However, very little has been known about the measures for controlling these traits. This study investigated the effect of applying paclobutrazol (PBZ) on the plant growth and flower quality in P. lactiflora. The results indicated that PBZ application reduced the plant height (8.05%), plant crown width (14.72%), and leaf area (10.90%), but increased the leaf thickness (18.18%) and stem diameter (over 11%) in P. lactiflora. Meanwhile, PBZ application was also found to increase the chlorophyll (Chl) a (29.63%), Chl b (33.33%), Chl a+b (30.56%), SPAD (27.32%), relative water content (0.47%), soluble sugar (5.09%) and activities of three antioxidant enzymes (superoxide dismutase 169.66%, peroxidase 3.59%, catalase 319.30%), but decreased the relative electrical conductivity (18.52%). Additionally, the application of PBZ was found to affect the flowering quality of P. lactiflora, increasing the flower diameter and fresh weight only in the flower-bud stage. This initiates the bloom stage, where there was a decrease in the total content of the aromatic compounds except for the flower-bud stage, and faded the flower color by reducing the content of anthocyanin. These results demonstrated that the application of PBZ can regulate the P. lactiflora plant types with no significant decrease in its ornamental values. This might provide a theoretical basis for further applying PBZ in P. lactiflora for use in urban landscape spaces.
Keywords: Paeonia lactiflora; growth bioregulator; flower color; aromatic compounds
Herbaceous peony (Paeonia lactiflora Pall.) is a perennial root and herbaceous flower which belongs to the Paeoniaceae family. It is not only a traditionally famous flower in China but also a world-famous flower having more than 4000 years of cultivation history [1]. The evolution of the market, as well as the economic development, has gradually favored the demand for P. lactiflora worldwide because of its huge flowers with bright hues and beautiful flower types with intense fragrances. As a result, this flower has been deemed as “the minister of flowers” in China and has been crowned “the queen of flowers” abroad. The features like strong adaptability and requirement for minimal care have made P. lactiflora the new favorite of the urban landscape greening with the evolution of the flower industry with applications in perennial borders, flower beds, and specialized gardens display [2]. However, P. lactiflora in its natural state, often manifests traits like the faster elongation growth of the internodes, the higher stems, the loose plant types, the soft inflorescence stems, and show increased susceptibility to lodging, which seriously affect the overall modeling and ornamental values [3]. Therefore, to adjust their plant types for obtaining excellent ornamental effects, these features need to be controlled by adopting certain measures. However, to our knowledge, most of the studies on P. lactiflora have been focused on the tissue culture [4], cut flower quality [5–8], abiotic stress [9,10]. Therefore, there is a scarcity of information on controlling the plant types and ornamental effects by improvising the cultivation techniques.
Plant growth regulators are extracts from microorganisms or artificially synthesized whose physiological functions are similar to those of the plant hormones. They may be classified into growth promoters, growth inhibitors, and growth retardants depending upon their effects on plant growth [11]. Among them, the use of plant growth retardant is one of the commonly used methods for regulating plant growth. In this process, a small dose can check the plants from achieving the anticipated growth, such that the plant growth is not completely stopped since the cell number is not reduced when dwarfing. Hence, this method is extremely suitable for extensive field applications. Paclobutrazol (PBZ), an important plant growth retardant in horticultural, has been widely used in inhibiting the vegetative growth in plants [12–14], promoting reproductive growth of plants [15–17], and improving plant resistance [18–20]. In ornamental plants, studies have been performed on Rosa damascena [21], Lilium oriental hybrids ‘Sorbonne’ [22], Stevia rebaudiana [23,24], Helianthus annuus [25], Dahlia spp [26] focusing on the effects of PBZ on the growth, physical and chemical properties in ornamental plants. In P. lactiflora, only Wu et al. [2] has investigated the effect of PBZ on the height, crown width, and stem diameter of five different cultivars. However, not much has been reported on the effect of PBZ on the physiological activity and flower quality of P. lactiflora.
To clarify the effect of applying PBZ on the plant growth, physiological activity, and flower quality in P. lactiflora, a main P. lactiflora cv. ‘Zifengyu’ having higher stems and loose plant types were selected for analysis. First, the morphological parameters of the plant were measured, as well as the physiological indices and ultrastructure were observed. Next, the flowering quality was studied, focusing on the flower color and aromatic compounds. The results, therefore, laid a theoretical foundation for further application of PBZ in P. lactiflora of urban landscape spaces.
P. lactiflora cv. ‘Zifengyu’ was used in this study, which was collected from the National Herbaceous Peony Germplasm Repository of Yangzhou University, Jiangsu Province, China (32°39′N, 119°42′E). Under field conditions, after their buds were exposed to the ground in March 2016, 100 mg·mL-1 PBZ (Beijing Solarbio Science & Technology, China) was used as the foliar-spraying once a week until the flowers withered, whereas the control was treated with deionized water. The flowers of four different developmental stages (S1, flower-bud stage; S2, initiating bloom; S3, bloom stage; and S4, wither stage) were taken and used to study flower quality, and in S3, the plant morphological indices were measured and the leaves were used for determining the physiological indices and observing the microstructures. All the samples were immediately frozen in liquid nitrogen and stored at –80°C until further analysis.
2.2 Morphological Indices Measurement
The plant height and plant crown width were measured using a meter stick, and the stem diameter was measured using a micrometer scale. The leaf area was determined according to a paper weighing method, and the leaf thickness was determined under an optical microscope (OLYMPUS CX31, Japan).
2.3 Physiological Indices Determination
The chlorophyll (Chl) a, Chl b, and Chl a+b, relative water content (RWC), relative electrical conductivity (REC), soluble protein, and soluble sugar contents were all determined according to the method reported by Zou [27]. The anthocyanin content was analyzed using the method reported by Meng et al. [28], and the SPAD value was detected using the SPAD chlorophyll meter (SPAD-502 PLUS, Konica Minolta, Japan).
2.4 Protective Enzyme Activities Measurement
Firstly, the extracts which was from 0.5 g leaf powders extracting by ice-cold 50 mM phosphate buffer (pH 7.8) were centrifuged at 4°C and 10,000 × g for 15 min, this isolated supernatants called crude extracts could be used for enzyme activities assay. Thereafter, superoxide dismutase (SOD, EC 1.15.1.1), peroxidase (POD, EC 1.11.1.7) and catalase (CAT, EC 1.11.1.6) activities were evaluated using the method reported by Zou [27].
2.5 Ultrastructure Observation
Firstly, fifteen-minute wash were performed 3 times for fixed leaves using 0.1 mol·L-1 phosphate buffer, and post-fixed with 1% osmium tetroxide for 4 h at room temperature. After 3 times fifteen-minute wash again, the leaves were dehydrated using 50%, 70%, 85%, 95% and 100% gradient ethanol for 15 min each. Moreover, they were treated with 100% acetone solution (15 min) and acetone solution containing anhydrous sodium sulfate (15 min), infiltrated in Spurr resin and then hardened at 70°C for 24 h. seventy-nm-thick sections were cut using a Leica EM UC6 ultramicrotome (Leica Co., Austria) with a diamond knife and stained using 1% uranyl acetate in 70% methanol, and 1% lead citrate before examination. After these, the samples were observed and imaged with a Tecnai 12 transmission electron microscope (Philips Co., Holland).
2.6 Flower Quality and Color Indices Measurement
The diameter and fresh weight of the flowers were measured using a micrometer scale and balance, respectively. The flower color indices were measured on a TC-P2A chroma meter (Beijing Optical Instrument Factory, China) using three color parameters including L*, a*, and b* values.
2.7 Analysis of Aromatic Compounds
Before sampling, the solid-phase microextraction fiber (75 μm CAR/PDMS, SUPELCO, USA) was aged for 20 min in the injection port of gas phase chromatography (Trace GC, Thermo, USA), the aged temperature was 250°C. The whole flower was put in the sample bottle under the condition of 40°C water bath, after 40 min adsorption, the aged microextraction fiber was inserted the injection port of gas chromatography/mass spectrometry (Trace DSQ II, Thermo, USA) and desorbed 2 min under 250°C, and then started the instrument to collect data. The flow rate of the helium carrier gas on Supelcowax 10 capillary chromatographic column with 30 m length, 0.25 mm inner diameter and 0.25 μm film was 0.8 mL·min-1. The injector temperature was 250°C, the column temperature was programmed as follows: the initial temperature was maintained at 40°C for 4 min, and then increased from 40°C to 90°C at 5 °C·min-1, and finally increased to 230°C at a rate of 8 °C·min-1, which was maintained for 4 min. The mass spectral ionization temperature was set to 200°C. The electron energy was 70 eV. Mass spectra were obtained by automatic scanning at m/z 33–450 amu. And the data were analyzed using the Xcalibur software.
Qualitative analysis was performed as follows: spectrometric data were compared with those obtained from the NIST library and Wiley library, combined with the manual resolution of mass spectra. Quantitative analysis was done with caprylic aldehyde as the internal standard with 0.082 g·L-1 concentration. The selected ion monitoring (SIM) technique was used for quantitative analysis of aromatic compounds, and the calculation formula was referenced to the method of Tian et al. [29].
Content of each compound (μg·g-1) = [Peak area of each compound/Peak area of internal standard × Concentration of internal standard (μg·μL-1) × Volume of internal standard (μL)] / Sample weight (g).
All data were means of three replicates at least with standard deviations. The results were analyzed for variance using the SAS/STAT statistical analysis package (version 6.12, SAS Institute, Cary, NC, USA). And the figures were completed by SigmaPlot 10.0 (SPSS Inc., USA).
PBZ application significantly affected the morphological indices of P. lactiflora (Table 1). Upon applying PBZ, the plant height and plant crown width were found to be significantly lower than those in the control, and the levels were reduced by 8.05% and 14.72%, respectively; meanwhile, the leaf area was also reduced by 10.90%, but there were no significant differences observed between them. Instead, upon PBZ application, the leaf thickness was significantly higher than that in the control with 18.18%; when the stem diameter was considered, the top stem diameter was found to remain unaffected, while the middle and bottom stem diameter all presented significant increase by more than 11% compared to the control.

The physiological indices were found to be a direct reflection of the current physiological state of the plant. Upon applying PBZ, the leaves of P. lactiflora were affected the most as evident from the transformation of the leaves from green into dark green, which was closely related to the chlorophyll content in the body. Analyzing the chlorophyll content revealed that applying PBZ enhances the contents of Chl a, Chl b, and Chl a+b as well as Chl a/b, and Chl b with the maximal increase of 33.33%, consistent with the value of SPAD. Additionally, in contrast with the control, REC was found to decrease significantly by 18.52% and 13.81%, respectively; whereas the RWC and soluble sugar content, were found to increase (Table 2).

3.3 Activities of the Protection Enzymes
The activities of three protection enzymes including SOD, CAT and POD in P. lactiflora were shown in Fig. 1. These antioxidant enzymes activities presented the same tendency and were all higher upon PBZ application than those in the control. Moreover, the SOD and CAT activities were found to have a significant difference between the two, and the CAT activity was found to show the highest increase being approximately 320%. When the antioxidant enzymes activities were assessed, the SOD showed the highest activity being about 98.19 times the lowest CAT activity.

Figure 1: Effect of PBZ application on the antioxidant enzymes activities of P. lactiflora. SOD, superoxide dismutase; POD, peroxidase; CAT, catalase. The values represent the mean ± standard deviation (n = 3), and different letters indicate significant differences (P < 0.05)
Observing the cell walls, vacuoles, and chloroplasts in the mesophyll cells during the bloom stage of P. lactiflora revealed the vacuoles to occupy the major space of the whole cells, where most of the organelles including the chloroplasts were pushed into the cellular edges, and closed to the cell walls. Additionally, the observation reported white starch grains in the chloroplasts and their grana thylakoids had curled, swollen and irregular arrangements (Fig. 2A). Upon applying PBZ, the mesophyll cells were found to be covered with the chloroplasts, with regularly arranged grana lamellae, and some white starch grains. Moreover, the number of chloroplasts in the unit area and their areas were all found to be significantly higher than those of the control (Fig. 2B).

Figure 2: Effect of applying PBZ on the cell ultrastructure of P. lactiflora. CH, chloroplast; CW, cell wall; SG, starch grain; V, vacuole
3.5 Flower Diameter and Fresh Weight
The development of P. lactiflora witnessed a gradual increase in the flower diameter and fresh weight upon PBZ application as well as in the control, and all of them were found to attain the maximum value in S4 (Fig. 3). After PBZ treatment, the flower diameter and fresh weight of P. lactiflora were all found to be slightly higher than those of the control in S1 and S2, whereas, in the S3 and S4, the values of flower diameter and fresh weights were significantly lowered than those of the control.

Figure 3: Effect of PBZ application on the flower diameter and fresh weight of P. lactiflora. S1, flower-bud stage; S2, initiating bloom stage; S3, bloom stage; S4, withering stage. The values represent the mean ± standard deviation (n = 3), and the different letters indicate the significant differences (P < 0.05)
The differences in the flower color were observed upon applying PBZ as well as in the control group and evaluated by the a*/b* value. As shown in Fig. 4, the a*/b* values between the group treated with PBZ as well as the control showed gradual reduction during the development of P. lactiflora, and the former was found to be significantly lower than that of the latter. These results revealed a gradual fading of the flower color during the development of P. lactiflora, and the application of PBZ was found to further lighten the flower color.

Figure 4: Effect of PBZ application in changing the flower color of P. lactiflora. S1, flower-bud stage; S2, initiating bloom stage; S3, bloom stage; S4, withering stage. The values represent the mean ± standard deviation (n = 3), and the different letters indicate the significant differences (P < 0.05)
The shade of the violet series petals in P. lactiflora mainly depends on the content of anthocyanins [30]. During the development of P. lactiflora, there was also a reduction in the anthocyanin contents upon treatment with PBZ as well as in the control. Compared to the control, the content of anthocyanins was always found to be lower upon PBZ application, and the maximum decrease observed in S4 was found to be 12.5% (Fig. 5).
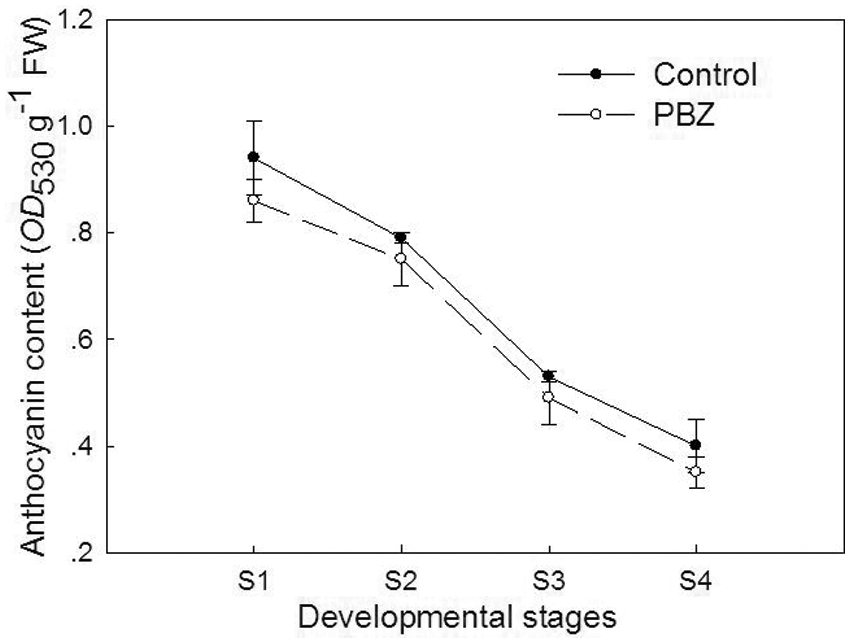
Figure 5: Effect of PBZ application on the anthocyanin content of P. lactiflora. S1, flower-bud stage; S2, initiating bloom stage; S3, bloom stage; S4, withering stage. The values represent the mean ± standard deviation (n = 3)
The GC/MS total ionic chromatogram of the aromatic compounds of P. lactiflora flowers upon PBZ application and in the control has been represented in Fig. 6. Each component was compared with those obtained from the NIST and Wiley libraries, and the retrieved aromatic compounds were listed in Supplementary Table 1. Both in the group treated with PBZ as well as the control group demonstrated, a total of 46 aromatic compounds (the content was greater than 0.1 μg·g-1), namely 17 alkanes, 13 alcohols, 9 esters, 4 aldehydes, and 3 ketones.
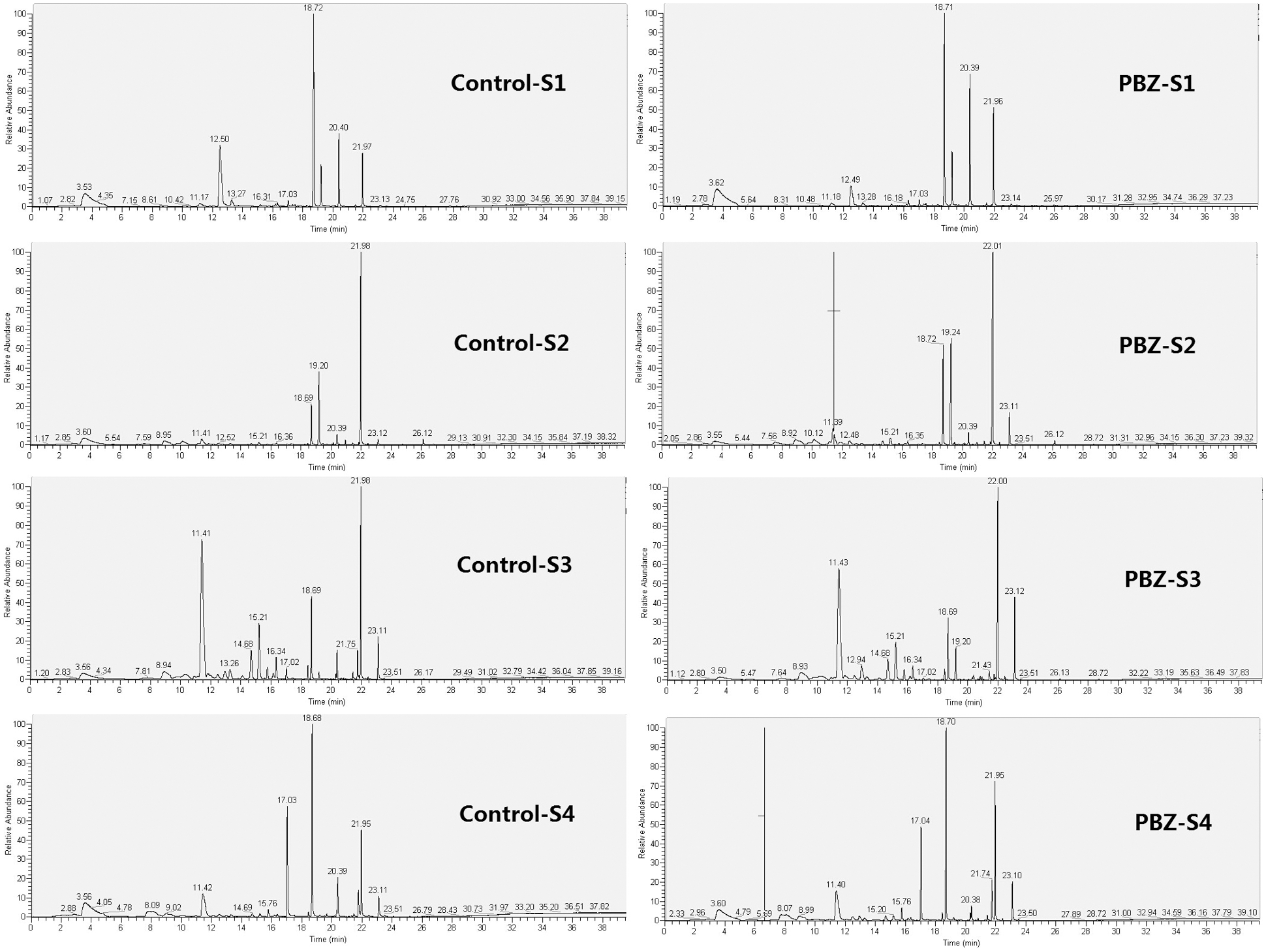
Figure 6: The total ionic chromatogram of the aromatic compounds of P. lactiflora flowers. S1, flower-bud stage; S2, initiating bloom stage; S3, bloom stage; S4, withering stage
A total of 9 aromatic compounds was identified in S1, which included 4 and 8 components under the control conditions as well as upon PBZ application, respectively. In the control, the alcohols were found to have the highest content, and their relative content was found to reach 46.98%, the most abundant components being 1-octanol (1.05 μg·g-1). Meanwhile, the PBZ application was found to increase the levels of alkanes the most, with a relative content of 42.16%, and 1-octanol (3.37 μg·g-1) together with α- caryophyllene (2.54 μg·g-1) was found to constitute the maximum content. Additionally, 1-hexadecanol, octyl acetate, cis-3-hexenyl acetate, 1-dodecane, and α-caryophyllene were the unique components identified after applying PBZ.
A total of 36 aromatic compounds were identified in S2, which included 30 components in the control and 27 components upon the PBZ application, respectively. In the control, the alcohols were the most abundant with a relative content of 59.68%, and the major components comprised D-citronellol (58.89 μg·g-1), caryophyllene (26.28 μg·g-1) as well as 1-octanol (11.34 μg·g-1). Nevertheless, the alcohols were the most abundant with a relative content of 56.85% under the PBZ application, and D-citronellol (29.49 μg·g-1), caryophyllene (16.43 μg·g-1) as well as 1-octanol (10.93 μg·g-1) were found to have the maximum contents. Moreover, the application of PBZ was found to detect leaf alcohol, (E)-2-hexenal, ethyl octanoate, 1-dodecane, 2-ethenyl-1,1-dimethyl-3-methylene-cyclohexane and tricyclo[4,2,2,0(1,5)]dec-7-ene.
In S3, a total of 31 aromatic compounds was identified which included 28 components for the control group and 27 components for the PBZ-treated group. The alkanes were found to be the highest in the control group relative content reaching to about 67.45%, the most abundant components were (Z)-3,7-dimethyl-1,3,6-octatriene (127.49 μg·g-1), D-citronellol (38.32 μg·g-1), (E,Z)-2,6-Dimethyl-2,4,6-octatriene (26.54 μg·g-1), 1-octanol (16.90 μg·g-1), (2E,4E,6E)-3,4-dimethyl-2,4,6-octatriene (15.59 μg·g-1), myrcene (14.63 μg·g-1). Meanwhile, upon treatment with PBZ, the alcohols levels were found to be the highest with a relative content of 63.34%, and (Z)-3,7-dimethyl-1,3,6-octatriene (46.21 μg·g-1), D-citronellol (29.49 μg·g-1) together with D-citronellol (23.00 μg·g-1) were found to have the maximum contents. Additionally, 2-octen-1-ol, rhodinal and1-hexadecanol were the unique components found upon PBZ application.
In S4, a total of 15 aromatic compounds were identified which included 14 components for the control group and 12 components for the PBZ-treated group. In the control group, the alcohols were found to be the most abundant at a relative content of 40.75%, and the major components were 1-octanol (4.68 μg·g-1), octyl acetate (3.42 μg·g-1), (Z)-3,7-dimethyl-1,3,6-octatriene (2.99 μg·g-1), D-citronellol (2.02 μg·g-1), ethylbenzene (1.88 μg·g-1). Nevertheless, the application of PBZ, the alcohols were found to show the most abundant relative content of 45.68%, and 1-octanol (2.07 μg·g-1), D-citronellol (1.22 μg·g-1), octyl acetate (1.10 μg·g-1) as well as (Z)-3,7-dimethyl-1,3,6-octatriene (1.43 μg·g-1) was found to have the maximal contents. Moreover, PBZ application was found to detect 2-ethenyl-1,1-dimethyl-3-methylene-cyclohexane.
The assorted statistics of the main aromatic compounds have been listed in Supplementary Table 2. Firstly, the major aromatic compounds were not found to be the same throughout the development of P. lactiflora. Generally, the alcohols were found to be the major aromatic compounds found in P. lactiflora in S1, S2, and S4, but in S3, the alkanes were found to be the major aromatic compounds. When the total content of the aromatic compounds was assessed, their contents were first found to increase both under the application of PBZ as well as in the control and then decrease in S4. The highest contents in S3 were found to be 124.38 μg·g-1 and 312.54 μg·g-1, respectively. When PBZ was considered, the content of the aromatic compounds was found to be higher than those of the control only in S1 with an increase of about 331.47%.
The plant growth retardant, PBZ is a member of the triazole family, affecting the plant metabolism by interfering with the ent-kaurene oxidation pathway and finally inhibiting the biosynthesis of gibberellins (GAs) [31]. Therefore, PBZ is primarily used for controlling the size and growth of plants yielding more desirable compact plants. A study by Lenzi et al. [32] reported the possibility of reducing the height of the plants by treating PBZ in Dianthus barbatus × chinensis, and PBZ was found to be effective in controlling the plant height without inducing any toxicity symptoms. In Borrichia frutescens, the application of PBZ was found to reduce the shoot mass, root mass, leaf number, plant height, and internode extension by 52.9%, 48.5%, 56.7%, 54.9%, and 50.1% [33]. A study in P. lactiflora, by Wang et al. [3] found 100 mg·mL-1 PBZ to have the best-integrated effect on reducing the plant height and plant crown breadth as well as increasing the stem diameter. In this study, the results were in good agreement with the report by Wang et al. [3]. Applying 100 mg·mL-1 PBZ was found to reduce the P. lactiflora plant height, plant crown width, and leaf area by 8.05%, 14.72%, and 10.90%, respectively, whereas the leaf thickness and stem diameter were all found to increase. This was mainly because PBZ might slow down the rate of plant growth by inhibiting the longitudinal division of the terminal cells of the plants such that more nutrients are instead used for the plant horizontal growth, resulting in the lower plant height, crown breadth, and leaf area together with the increased stem diameter and leaf thickness.
REC was the important metric under conditions of external environmental stress of the plants and is found to increase significantly under adverse conditions. In this study, PBZ application was found to significantly reduce the REC of P. lactiflora by 18.52%. This result was found to be consistent with the reports by Stevia rebaudiana [23], revealing that PBZ can protect the structure and function of the plant cell membranes from damage. At the same time, there was an enhanced activity of the protective enzymes like the SOD, POD, and CAT when P. lactiflora was treated with PBZ, suggesting that PBZ could reduce the degree of membrane lipid peroxidation triggered by the adverse situation and scavenge the reactive oxygen species (ROS) in P. lactiflora, consistent with the studies in Dahlia pinnata [34] and Stevia rebaudiana [24]. Chloroplast is an organelle specialized for photosynthesis in plants, and chlorophyll is the important pigment in photosynthesis in plant chloroplasts, which are all influenced by PBZ. Zheng et al. [22] found that PBZ can enhance the chlorophyll contents in Lilium oriental hybrids ‘Sorbonne’. At the same time, Feng et al. [35] showed that the contents of chlorophyll were increased in Dahlia pinnata, and the chloroplast structure was relatively completed. These results were also reflected in this study, laying the foundation for maintaining strong photosynthesis, with the huge accumulation of soluble sugar in P. lactiflora.
Plant growth retardants greatly affect the flowering quality. In flower diameter, the study by Newton et al. [36] found that applying PBZ did not affect the diameter of the first flower, but when Tagetes erecta was treated with PBZ, its flower diameter was reduced in S3 but was enhanced in S4 [37]. The result of our study was partially according to the latter, the application of PBZ was found to reduce the P. lactiflora flower diameter in S3 and S4. When the flower color was considered, treatment of P. lactiflora with PBZ was found to yield lighter violet flowers than that of the control, not consistent with Consolida orientalis [38]. However, the loss of flower color was observed in the other plant growth retardants, for example, spraying Chrysanthemum morifolium with daminozide exhibited a significant loss of color [39], and applying prohexadione-Ca was found to significantly decrease the petal coloration of Rosa hybrida [40], associated with the reduction in anthocyanin. This tendency to reduce the anthocyanin content was also found in P. lactiflora upon treatment with PBZ. Although the aromatic compound is an important index in flowering quality, there was no report on the aromatic compounds of the ornamental plant flowers upon PBZ application. In this study, a total of 46 aromatic compounds were identified which included 17 alkanes, 13 alcohols, 9 esters, 4 aldehydes, and 3 ketones upon treatment with PBZ as well as in the control, and their contents were found to vary during the development of P. lactiflora. When the total content was concerned, the application of PBZ application was found to decrease the total content of the aromatic compounds except in S1. But in general, these results demonstrated that PBZ can be applied for inhibiting the excessive plant growth of P. lactiflora in the urban landscape space.
In conclusion, the present study demonstrated that applying PBZ can regulate the P. lactiflora plant types but did not significantly decrease its ornamental values. Application of PBZ was found to decrease the plant height, plant crown width, leaf area, REC, and aromatic compounds in P. lactiflora. On the other hand, PBZ was found to increase the leaf thickness, stem diameter, chlorophyll, RWC, soluble sugar, and antioxidant enzymes activities. These results, thus unraveled the critical role of PBZ in regulating the plant types of P. lactiflora.
Author Contributions: JT and DZ planned and designed the experiments; YW and DZ performed the experiments; YW and JL analyzed the data and wrote the manuscript. All authors carefully read and approved the final manuscript.
Funding Statement: This work was supported by the National Natural Science Funds (32102411), the Natural Science Foundation of Jiangsu Province of China (BK20200924), the Natural Science Foundation of Jiangsu Higher Education Institutions of China (20KJB210005), Jiangsu Association for Science and Technology young Scientific and technological Talents Project-Supported by Yanqing Wu, the Agricultural Science & Technology Independent Innovation Fund of Jiangsu Province (CX [20]3021), the Graduate Innovation Program of Jiangsu Province (XKYCX19_119) and the Excellent Doctoral Dissertation Fund of Yangzhou University.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Yu, X. N. (2019). Herbaceous peony (in Chinese). Beijing: China Forestry Publishing House. [Google Scholar]
2. Wu, Y., Wei, M., Zhao, D., Tao, J. (2016). Flavonoid contents and expression analysis of its biosynthetic genes in a chimera of herbaceous peony (Paeonia lactiflora Pall.). Journal of Integrative Agriculture, 15(9), 2023–2031. DOI 10.1016/S2095-3119(15)61318-1. [Google Scholar] [CrossRef]
3. Wang, N., Han, Y., Zhang, W. (2014). Effects of paclobutrazol of different concentration on growth of Paeonia lactiflora. Forestry Science and Technology Information, 46(1), 16–17. DOI 10.3969/j.issn.1009-3303.2014.01.006. [Google Scholar] [CrossRef]
4. Zhao, D. Q., Xue, Y. F., Shi, M., Tao, J. (2017). Rescue and in vitro culture of herbaceous peony immature embryos by organogenesis. Scientia Horticulturae, 217(43), 123–129. DOI 10.1016/j.scienta.2017.01.040. [Google Scholar] [CrossRef]
5. Zhao, D. Q., Xu, C., Luan, Y. T., Shi, W. B., Tang, Y. H. et al. (2021). Silicon enhances stem strength by promoting lignin accumulation in herbaceous peony (Paeonia lactiflora Pall.). International Journal of Biological Macromolecules, 190, 769–779. DOI 10.1016/j.ijbiomac.2021.09.016. [Google Scholar] [CrossRef]
6. Zhao, D. Q., Luan, Y. T., Xia, X., Shi, W. B., Tang, Y. H. et al. (2020). Lignin provides mechanical support to herbaceous peony (Paeonia lactiflora Pall.) stems. Horticulture Research, 7(1), 213. DOI 10.1038/s41438-020-00451-5. [Google Scholar] [CrossRef]
7. Zhao, D. Q., Shi, W. B., Xia, X., Tang, Y. H., Tao, J. (2020). Microstructural and lignin characteristics in herbaceous peony cultivars with different stem strengths. Postharvest Biology and Technology, 159, 111043. DOI 10.1016/j.postharvbio.2019.111043. [Google Scholar] [CrossRef]
8. Zhao, D. Q., Tang, Y. H., Xia, X., Sun, J., Meng, J. S. et al. (2019). Integration of transcriptome, proteome, and metabolome provides insights into how calcium enhances the mechanical strength of herbaceous peony inflorescence stems. Cells, 8(2), 102. DOI 10.3390/cells8020102. [Google Scholar] [CrossRef]
9. Zhao, D. Q., Zhang, X. Y., Wang, R., Liu, D., Sun, J. et al. (2019). Herbaceous peony tryptophan decarboxylase confers drought and salt stresses tolerance. Environmental and Experimental Botany, 162(3), 345–356. DOI 10.1016/j.envexpbot.2019.03.013. [Google Scholar] [CrossRef]
10. Zhao, D. Q., Xia, X., Su, J. H., Wei, M. R., Tao, J. (2019). Overexpression of herbaceous peony HSP70 confers high temperature tolerance. BMC Genomics, 20, 70. DOI 10.1186/s12864-019-5448-0.2019. [Google Scholar] [CrossRef]
11. Martínez-Damián, M. T., Cano-Hernández, R., Moreno-Pérez, E. C., Sánchez-del Castillo, F., Cruz-Álvarez, O. (2019). Effect of preharvest growth bioregulators on physicochemical quality of saladette tomato. Revista Chapingo Serie Horticultura, 25(1), 29–43. DOI 10.5154/r.rchsh.2018.06.013. [Google Scholar] [CrossRef]
12. Ghosh, A., Chikara, J., Chaudhary, D. R., Prakash, A. R., Boricha, G. et al. (2010). Paclobutrazol arrests vegetative growth and unveils unexpressed yield potential of Jatropha curcas. Journal of Plant Growth Regulation, 29(3), 307–315. DOI 10.1007/s00344-010-9137-0. [Google Scholar] [CrossRef]
13. Sun, Y., Chen, J., Chang, W., Teng, M., Wu, F. (2010). Irrigation with 5 degrees C water and paclobutrazol promotes strong seedling growth in tomato (Solanum lycopersicon). Journal of Horticultural Science & Biotechnology, 85(4), 305–311. DOI 10.1080/14620316.2010.11512672. [Google Scholar] [CrossRef]
14. Orozco-Meléndez, L. R., Hernández-Rodríguez, O. A., Cruz-Álvarez, O., Robles-Hernández, L., Ávila-Quezada, G. D. et al. (2022). Paclobutrazol and its use in fruit production: A review. Phyton-International Journal of Experimental Botany, 91(1), 1–12. DOI 10.32604/phyton.2022.016908. [Google Scholar] [CrossRef]
15. Esmaielpour, B., Hokmalipour, S., Jalilvand, P., Salimi, G. (2011). The investigation of paclobutrazol effects on growth and yield of two potato (Solanum tuberosum) cultivars under different plant density. Journal Food Agriculture & Environment, 9(3–4), 289–294. [Google Scholar]
16. Xu, G., Luo, R., Yao, Y. (2013). Paclobutrazol improved the reproductive growth and the quality of seed oil of Jatropha curcas. Journal of Plant Growth Regulation, 32(4), 875–883. DOI 10.1007/s00344-013-9353-5. [Google Scholar] [CrossRef]
17. Haldankar, P. M., Thorat, V., Mayekar, A. J., Khirsagar, P. J., Korake, G. N. et al. (2014). Effect of paclobutrazol and post flowering foliar sprays of nutrients for accelerating harvesting of jackfruit. Indian Journal of Horticulture, 71, 476–480. [Google Scholar]
18. Srivastav, M., Kishor, A., Dahuja, A., Sharma, R. R. (2010). Effect of paclobutrazol and salinity on ion leakage, proline content, and activities of antioxidant enzymes in mango (Mangifera indica L.). Scientia Horticulturae, 125(4), 785–788. DOI 10.1016/j.scienta.2010.05.023. [Google Scholar] [CrossRef]
19. Baninasab, B., Ghobadi, C. (2011). Influence of paclobutrazol and application methods on high-temperature stress injury in cucumber seedlings. Journal of Plant Growth Regulation, 30(2), 213–219. DOI 10.1007/s00344-010-9188-2. [Google Scholar] [CrossRef]
20. Zhou, Z., Ma, H., Liang, K., Huang, G., Pinyopusarerk, K. (2012). Improved tolerance of teak (Tectona grandis L.f.) seedlings to low-temperature stress by the combined effect of arbuscular mycorrhiza and paclobutrazol. Journal of Plant Growth Regulation, 31(3), 427–435. DOI 10.1007/s00344-011-9252-6. [Google Scholar] [CrossRef]
21. Misra, A., Srivastava, N. K., Kumar, R., Khan, A. (2005). Effect of palcobutrazol (PP333) on flower quality and quantity of Rosa damascene. Communications in Soil Science and Plant Analysis, 36(4–6), 477–486. DOI 10.1081/CSS-200043219. [Google Scholar] [CrossRef]
22. Zheng, R., Wu, Y., Xia, Y. (2012). Chlorocholine chloride and paclobutrazol treatments promote carbohydrate accumulation in bulbs of Lilium oriental hybrids ‘Sorbonne’. Journal of Zhejiang University-SCIENCE B, 13(2), 136–144. DOI 10.1631/jzus.B1000425. [Google Scholar] [CrossRef]
23. Hajihashemi, S., Ehsanpour, A. A. (2013). Influence of exogenously applied paclobutrazol on some physiological traits and growth of Stevia rebaudiana under in vitro drought stress. Biologia, 68(3), 414–420. DOI 10.2478/s11756-013-0165-7. [Google Scholar] [CrossRef]
24. Hajihashemi, S., Ehsanpour, A. A. (2014). Antioxidant response of Stevia rebaudiana B. to polyethylene glycol and paclobutrazol treatments under in vitro culture. Applied Biochemistry and Biotechnology, 172(8), 4038–4052. DOI 10.1007/s12010-014-0791-8. [Google Scholar] [CrossRef]
25. Koutroubas, S. D., Damalas, C. A. (2015). Sunflower response to repeated foliar applications of paclobutrazol. Planta Daninha, 33(1), 129–135. DOI 10.1590/S0100-83582015000100015. [Google Scholar] [CrossRef]
26. Rivera-Espejel, E. A., Cruz-Alvarez, O., Mejía-Muñoz, J. M., García-Mateos, M. R., Colinas-León, M. T. et al. (2019). Physicochemical quality, antioxidant capacity and nutritional value of edible flowers of some wild dahlia species. Folia Horticulturae, 31(2), 331–342. DOI 10.2478/fhort-2019-0026. [Google Scholar] [CrossRef]
27. Zou, Q. (2000). Plant physiology experimental guidance (in Chinese). Beijing: China Agricultural Press. [Google Scholar]
28. Meng, X. C., Wang, X. J. (2004). Regulation of flower development and anthocyanin accumulation in Gerbera hybrida. Journal of Horticultural Science & Biotechnology, 79(1), 131–137. DOI 10.1080/14620316.2004.11511725. [Google Scholar] [CrossRef]
29. Tian, C., Wei, J., Liu, X., Wang, N., Wang, H. et al. (2009). GC-MS analysis of fruit aromatic components of pear cultivars originated from different species of Pyrus. Journal of Fruit Science, 26(3), 294–299 (in Chinese). [Google Scholar]
30. Zhao, D., Tao, J., Han, C., Ge, J. (2012). Flower color diversity revealed by differential expression of flavonoid biosynthetic genes and flavonoid accumulation in herbaceous peony (Paeonia lactiflora Pall.). Molecular Biology Reports, 39(12), 11263–11275. DOI 10.1007/s11033-012-2036-7. [Google Scholar] [CrossRef]
31. Graebe, J. E. (1987). Gibberellin biosynthesis and control. Annual Reviews Plant Physiology, 38(1), 419–465. DOI 10.1146/annurev.pp.38.060187.002223. [Google Scholar] [CrossRef]
32. Lenzi, A., Nannicini, M., Mazzeo, P., Baldi, A. (2015). Effect of paclobutrazol in potted plants of four cultivars of Dianthus barbatus × chinensis. European Journal of Horticultural Science, 80(2), 87–93. DOI 10.17660/eJHS.2015/80.2.7. [Google Scholar] [CrossRef]
33. Carver, S. T., Arnold, M. A., Byrne, D. H., Armitage, A. R., Lineberger, R. D. et al. (2014). Growth and flowering responses of sea marigold to daminozide, paclobutrazol, or uniconazole applied as drenches or sprays. Journal of Plant Growth Regulation, 33(3), 626–631. DOI 10.1007/s00344-014-9411-7. [Google Scholar] [CrossRef]
34. Zhen, H., Yuan, Z., Feng, L., Ding, X., Wang, X. et al. (2012). Effects of PP333 on the physiological characteristics in Dahlia pinnata. Chinese Agriculture Science Bulletin, 28(1), 153–157. DOI 10.3969/j.issn.1000-6850.2012.01.029. [Google Scholar] [CrossRef]
35. Feng, L., Yuan, Z., Yin, Y., Zhao, X. (2014). Effects of paclobutrazol on the photosynthetic characteristics and ultrastructure of Dahlia pinnata leaves. Acta Prataculturae Sinica, 23(4), 114–121. DOI 10.11686/cyxb20140414. [Google Scholar] [CrossRef]
36. Newton, L. A., Runkle, E. S. (2010). Effects of paclobutrazol sprays on inflorescences of three potted moth orchid clones. Horttechnology, 20(5), 892–895. DOI 10.21273/HORTTECH.20.5.892. [Google Scholar] [CrossRef]
37. Yang, S., Jiang, W. (2005). The influence of paclobutrazol on physiological activities and ornamental characteristics of Tagetes erecta L. Shandong Agriculture Science, 2, 45–47. DOI 10.3969/j.issn.1001-4942.2005.02.017. [Google Scholar] [CrossRef]
38. Mansuroglu, S., Karaguzel, O., Ortacesme, V., Sayan, M. S. (2009). Effect of paclobutrazol on flowering, leaf, and flower colour of Consolida orientalis. Pakistan Journal of Botany, 41(10), 2323–2332. DOI 10.1094/MPMI-22-10-1312. [Google Scholar] [CrossRef]
39. Roepke, J., Jean, T., Perkel, K. J., Blom, T., Bozzo, G. G. (2013). Daminozide alters anthocyanin metabolism in ray florets of bronze chrysanthemum (Chrysanthemum morifolium Ramat.). Journal of Plant Growth Regulation, 32(3), 453–460. DOI 10.1007/s00344-012-9315-3. [Google Scholar] [CrossRef]
40. Schmitzer, V., Veberic, R., Stampar, F. (2012). Prohexadione-Ca application modifies flavonoid composition and color characteristics of rose (Rosa hybrida L.) flowers. Scientia Horticulturae, 146, 14–20. DOI 10.1016/j.scienta.2012.07.035. [Google Scholar] [CrossRef]

 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |