

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.021412
ARTICLE
How Physical Disturbance and Nitrogen Addition Affect the Soil Carbon Decomposition?
1Institute of Wetland Ecology & Clone Ecology/Zhejiang Provincial Key Laboratory of Plant Evolutionary Ecology and Conservation, Taizhou University, Taizhou, 318000, China
2College of the Environment & Safety Engineering, Jiangsu University, Zhenjiang, 212013, China
*Corresponding Author: Xiuwei Zhang. Email: xiuwei8689@163.com
Received: 13 January 2022; Accepted: 04 March 2022
Abstract: The decomposition of soil organic carbon (SOC) plays a critical role in regulating atmospheric CO2 concentrations and climate dynamics. However, the mechanisms and factors controlling SOC decomposition are still not fully understood. Here, we conducted a 60 days incubation experiment to test the effects of physical disturbance and nitrogen (N) addition on SOC decomposition. N addition increased the concentration of NO3− by 51% in the soil, but had little effect on the concentration of NH4+. N addition inhibited SOC decomposition, but such an effect differed between disturbed and undisturbed soils. In disturbed and undisturbed soils, application of N decreased SOC decomposition by 37% and 15%, respectively. One possible explanation is that extra N input suppressed microbial N mining and/or increased the stability of soil organic matter by promoting the formation of soil aggregates and incorporating part of the inorganic N into organic matter, and consequently decreased microbial mineralization of soil organic matter. Physical disturbance intensified the inhibition of N on SOC decomposition, likely because physical disturbance allowed the added N to be better exposed to soil microbes and consequently increased the availability of added N. We conclude that physical disturbance and N play important roles in modulating the stability of SOC.
Keywords: Soil organic matter; physical disturbance; microbial N mining; microbial biomass carbon; N availability
Soil organic matter (SOM) is one of the most important terrestrial carbon (C) pools, and CO2 from SOC decomposition is a key source of atmospheric CO2 and a major component of the global C cycle [1,2]. Therefore, SOC decomposition plays an important role in regulating atmospheric CO2 concentrations and climate change [1]. Understanding SOC decomposition is pivotal for predicting the SOM storage in the terrestrial ecosystem and climate dynamics in the future. Numerous studies have reported the response of SOC decomposition to environment change [3–5], but the mechanisms and factors influencing SOC decomposition are still not fully understood.
Physical disturbance and nitrogen (N) fertilization are two factors affecting SOC decomposition [6,7]. Physical disturbance is a major cause of SOM depletion and reduction in the number and stability of soil aggregates [8]. For example, physical disturbance could fragment the large macro-aggregates into small macro-aggregates, micro-aggregates and free silt and clay-sized fractions, resulting in the release of labile SOC pools initially occluded within the large macro-aggregates [6,9,10]. Moreover, soil aggregates affect oxygen diffusion [11] and microbial community composition and activities [12]. Physical disturbance may improve oxygen diffusion and change the composition and function of microbial communities that benefit from microbial activities due to soil aggregates destruction [13]. In previous studies, crushing was performed on bulk soils and isolated aggregates to test the effect of physical disturbance on SOC decomposition [14–16]. In most studies, SOC decomposition was increased by physical disruption during the initial few weeks of incubation [14,15]. However, there were also contrasting results showing that crushing aggregates did not affect soil respiration, which was often observed in soils with frequent management practices [16,17]. Even in well-structured soils without frequent management practices, the response of SOM to physical disruption is much dependent on the characteristics of SOM in different soil types [15,18].
At the same time, anthropogenic input of N, primarily through fertilizer application, fossil fuel combustion and legume cultivation, provides around 45% of the annual terrestrial N input, which is 2-fold of the natural rate of N input on Earth [19,20]. N deposition has been one of the major contributors to climate change and has altered the amount of plant input to soil [21]. It has been suggested that SOC decomposition was influenced by biotic and abiotic factors, such as plant input, microbial metabolism, N availability as well as temperature and moisture [22]. Therefore, changes in N and plant input may influence SOC dynamics, and the response of SOC dynamics to N addition and plant input may affect CO2 flux into the atmosphere [23]. Despite considerable research efforts, our knowledge of the responses of SOC decomposition to N change is still fragmented and often with contradictory results [5,23,24]. For example, some studies showed that SOC decomposition decreased with increasing N supply [25–28]. Others indicated that the effect of N addition on SOC sequestration was neutral or negative [29–31]. The ‘stoichiometric decomposition’ theory and ‘microbial nitrogen mining’ hypothesis also predict opposing effects of N addition on SOC decomposition [5,7]. Further, soil disturbance and N input may have an interactive effect on soil respiration. This was because soil aggregates may limit substrates or N exposure to microorganisms [32]. Therefore, a physical disturbance may improve the affinity of microorganisms to additional N input through the destruction of soil aggregates. To our knowledge, few studies have investigated the interactive effect of physical disturbance and N on the decomposition of SOC.
Here we performed a laboratory soil disturbance and N addition experiment to investigate the response of SOC decomposition to physical disturbance and N addition. The objective of this study was to investigate the main and interactive effects of physical disturbance and N addition on SOC decomposition. The mineral N concentration and microbial biomass carbon (MBC) as well as the dynamics of microbial metabolic quotient (qCO2) were also detected to explore the mechanisms regulating the response of SOC decomposition to physical disturbance and N addition.
2.1 Site Description and Soil Sampling
Soil was sampled from the plow layer (0∼20 cm) of a field continuously planted with maize at the experimental station of Heilongjiang Academy of Agricultural Sciences located near Harbin (45°75´N, 126°23´E, 128 m.a.s.l.), Heilongjiang Province, Northeast China. The station is in a typical continental monsoon zone with a mean annual temperature of 3°C–5°C, mean annual precipitation of 529 mm (ca 70% occurs between June and August). The soil in this study site is classified as an Aquic Mollisol (according to World Reference Base for Soil Resources, 2014). Soils were taken from four points randomly selected. Samples were air-dried in the field, passed through a 2 mm sieve, thoroughly homogenized and then brought to the laboratory. Visible roots and stones were carefully removed before the incubation experiment. The soil had a pH of 6.7, a total C concentration of 17.0 g kg−1 and a total N concentration of 1.4 g kg−1. The sand, silt, and clay concentrations were 43%, 22% and 35%, respectively.
2.2 Experimental Design and Soil Incubation
The experiment had four treatments: (1) control (without physical disturbance and non-N addition); (2) disturbance treatment (only physical disturbance applied); (3) +N treatment (only mineral N added) and (4) disturbance + N (simultaneously disturbance and N addition applied). Each treatment was replicated five times. A total of 20 columns with soil were incubated in this study. For each treatment, 200 g of air-dried soil was weighed and placed in an individual polypropylene column (5.2 cm in diameter, 20 cm in length). Both ends of the soil column were closed with one-hole rubber stopper connected to ventilation tubing. A continuous aerobic condition in each plastic container was maintained via automatic timer-controlled aeration with fresh air for one hour in each four-hour interval during the entire incubation experiment. Ammonium sulfate [(NH4)2SO4] was dissolved in deionized water and added at a rate of 100 mg N kg−1 soil in N-added treatments. The same amount of deionized water was added to the non-N treatments. Subsequently, all soil columns were pre-incubated for 60 days at a constant temperature (to mitigate the effects of soil disturbance caused by sample preparation, to stabilize the microenvironment, and to recover microbial activity). Soil moisture was adjusted to 65% water-holding capacity (WHC) by adding deionized water. Physical disturbance treatment was applied after the 60-day pre-incubation. For this, soil samples were roughly stirred and thoroughly re-homogenized using a glass stirring rod in disturbance treatments. Afterward, these soils were put back in their original containers. All soil columns were incubated for 60 days at 20°C. During incubation, soil moisture was monitored by weighing and frequently adjusted to reach 65% WHC by adding deionized water.
2.3 Soil Respiration Measurement
A dynamic CO2 trapping system was used to measure the rate of soil respiration [33]. The CO2 trapping was applied at day 0, 1, 3, 6, 12, 24, 36, 48 and 60, respectively. Briefly, we used an air pump to force ambient air through a soda-lime column to produce CO2-free air. Then, each soil column was aerated with CO2-free air to remove the CO2 that had previously existed in the container. After an equilibrating period of 2 h, the subsequent CO2 produced inside the soil columns for 24 h was trapped by a CO2 trapping bottle containing 12 mL 0.5 M NaOH solution. Blanks were also trapped to correct for inorganic C from the NaOH stock solution and sample handling. After CO2 trapping, the NaOH solution was directly analyzed for total inorganic carbon using a multi N/C® 2000 TOC analyzer (Analytik Jena, Germany).
Immediately after the CO2 trapping on day-60, all soil columns were destructively sampled and homogenized. The fresh soil samples were used to measure soil moisture concentration, mineral N and microbial biomass C (MBC).
Soil mineral N (NH4+ and NO3−) was measured by extracting 15 g of fresh soil with 30 mL 2 M KCl solution [34]. The concentration of NH4+ and NO3− was measured using a continuous flow injection analyzer (AA3, Bran + Luebbe, Norderstedt, Germany).
MBC was measured using the chloroform fumigation-extraction method [35]. Briefly, two subsamples of 15 g fresh soil were prepared for MBC measurement. One subsample was fumigated with ethanol-free chloroform in a vacuum desiccator in the dark for 24 h. The fumigated and non-fumigated subsamples were then extracted with a 30 mL 0.05 M K2SO4 solution. The concentration of total organic C was measured using a multi N/C® 2000 TOC analyzer. MBC was calculated as the difference in total organic C between fumigated and non-fumigated extracts with a conversion factor of 0.38. Microbial metabolic quotient (qCO2) was calculated as the soil microbial respiration rate per MBC (mg CO2-C g−1 MBC day−1).
We used repeated-measures ANOVA to test for the effects of physical disturbance, N addition, and their interaction on soil respiration rate and qCO2. We used two-way ANOVA to examine the effects of physical disturbance, N, and their interaction on MBC and soil mineral N. We also used the Tukey HSD test to compare differences among means. All statistical analyses were performed by SPSS Statistic 20.0 (IBM, Inc., Armonk, NY, USA) and the significance level was set at P < 0.05.
N addition substantially influenced the instantaneous respiration rates for both physically disturbed and undisturbed soils, each showing a lower respiration rate in N amended treatments (Fig. 1, F = 21.6, P = 0.006). However, such a negative effect of N addition was intensified by physical disturbance, as indicated by the significant disturbance × nitrogen effect (F = 8.1, P = 0.036). In general, the respiration rates of all soils declined during the initial 12 days and then increased to a peak value from 12 to 36 days. Afterward, the respiration rate of non-N treated soils decreased after day 36 until the end of incubation, while the respiration rate of N-treated soils decreased slowly or fluctuated (Fig. 1).
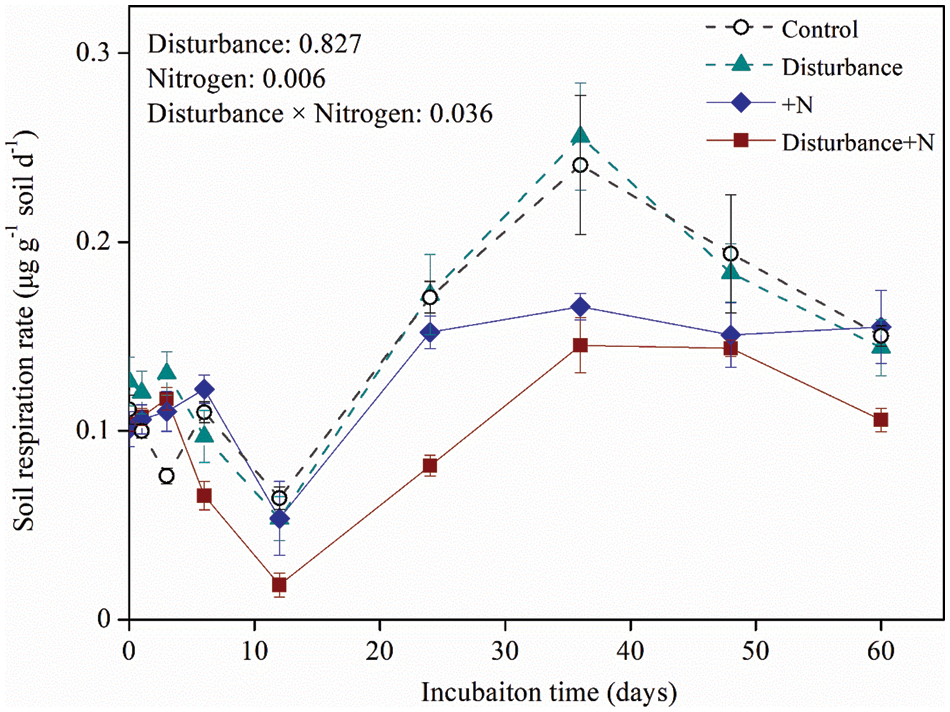
Figure 1: Dynamics of soil respiration over the incubation under physical disturbance (without disturbance and disturbance) and N addition (without N and +N) treatments. Error bars indicate standard errors of the mean (n = 5)
The cumulative C mineralized was significantly decreased by N addition (Fig. 2). The effect of physical disturbance on total C mineralized depended on N application (significant disturbance × nitrogen effect: F = 11.6, P = 0.019). In non-N added soil, the total C mineralized was similar in disturbed and undisturbed treatments, while in N amended soil, the total C mineralized was significantly lower in disturbed than undisturbed treatments. At the end of the incubation, the decrease of total C mineralized by N addition was about 37% and 15% in physically disturbed and undisturbed treatments, respectively.
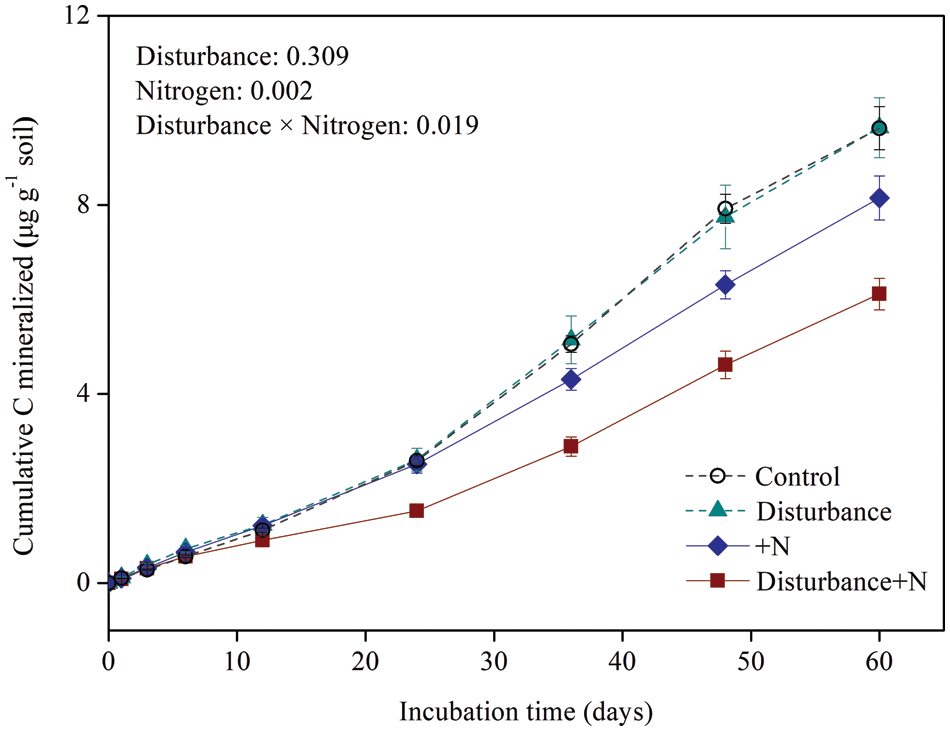
Figure 2: Cumulative C mineralized over the incubation under physical disturbance (without disturbance and disturbance) and N addition (without N and +N) treatments. Error bars indicate standard errors of the mean (n = 5)
3.2 Microbial Biomass C and Microbial Metabolic Quotient
Neither physical disturbance nor N amendment significantly affected MBC (P > 0.05, Fig. 3). Moreover, repeated-measures ANOVA showed that the microbial metabolic quotient (qCO2) significantly responded to neither physical disturbance nor N addition (P > 0.05, Fig. 4).
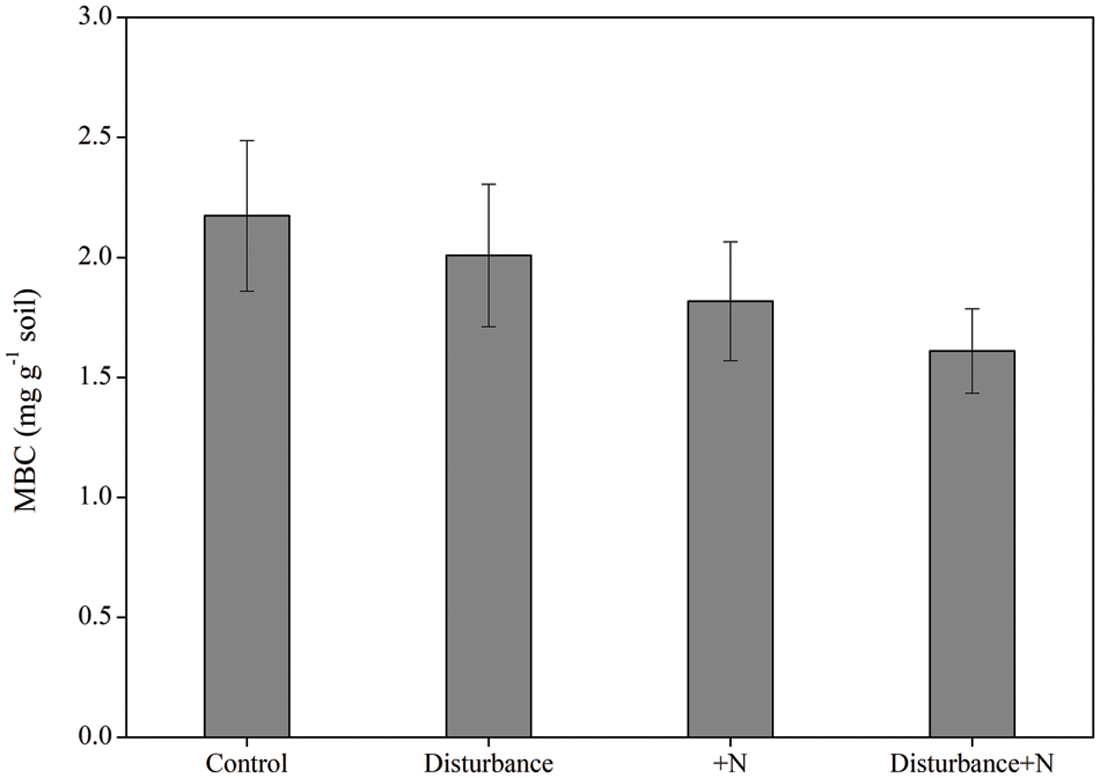
Figure 3: Microbial biomass C at the end of incubation under physical disturbance (without disturbance and disturbance) and N addition (without N and +N) treatments. Error bars indicate standard errors of the mean (n = 5)
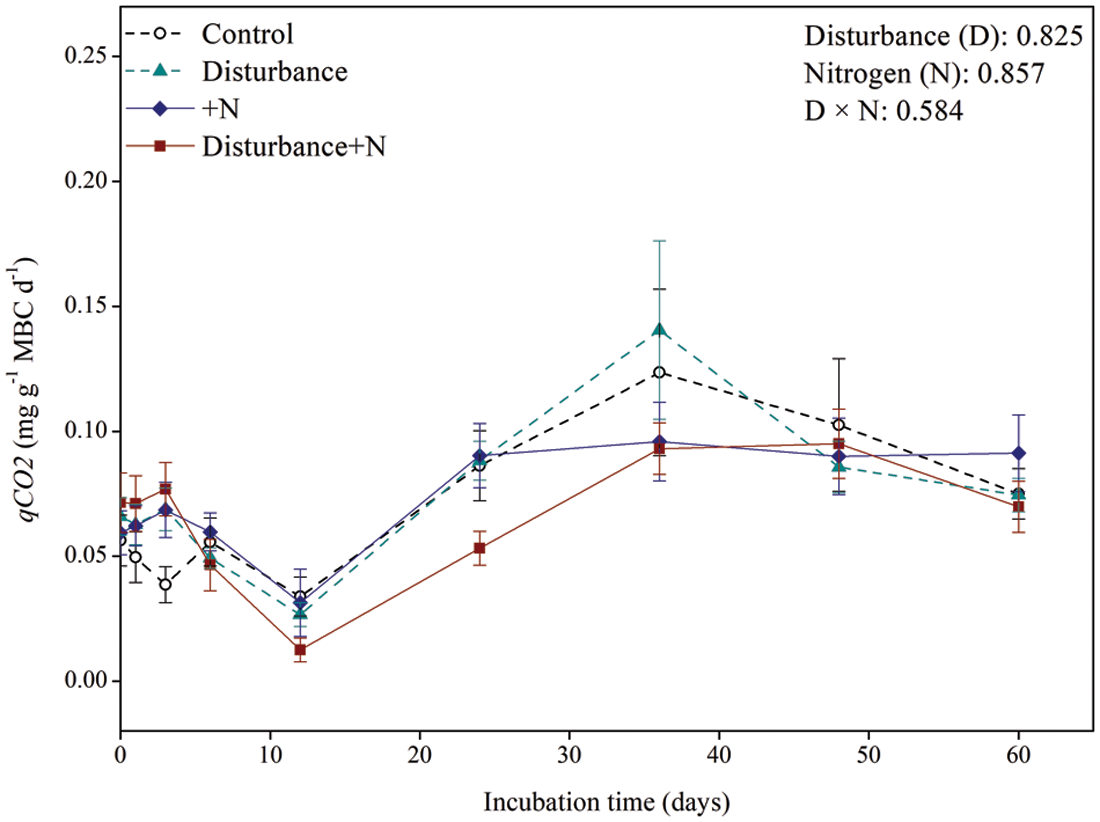
Figure 4: Dynamics of microbial metabolic quotient under physical disturbance (without disturbance and disturbance) and N addition (without N and +N) treatments. Error bars indicate standard errors of the mean (n = 5)
N addition significantly affected the mineral N concentration in both physically disturbed and undisturbed soils. In general, N addition significantly increased nitrate (NO3−; P < 0.001; 58% on average) and total mineral N (P < 0.001; 59% on average) in soils, but did not affect ammonium (NH4+) in soils (Fig. 5). Moreover, the physical disturbance had no significant effect on soil mineral N (P > 0.05).
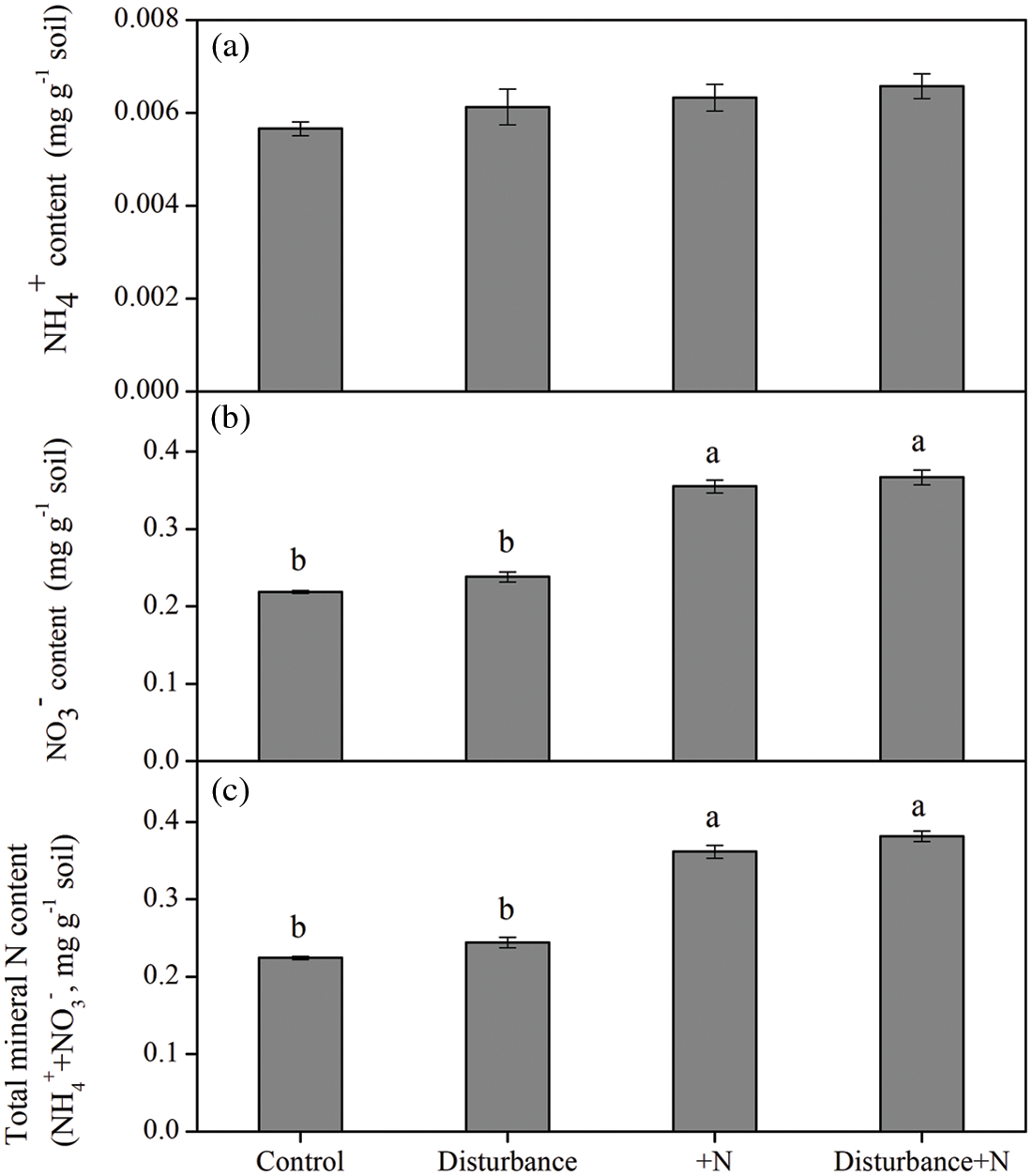
Figure 5: NH4+ (a), NO3− (b) and total mineral N (NH4+ plus NO3−) (c) concentration in the soil at the end of incubation under physical disturbance (without disturbance and disturbance) and N addition (without N and +N) treatments. Error bars indicate standard errors of the mean (n = 5)
Although N availability was an important factor influencing SOC decomposition, there remain large uncertainties and controversies associated with the nature of N regulation to the decomposition process [36]. Our result showed that N addition significantly reduced soil respiration rate (Fig. 1), which is consistent with many other studies [23,25,26,37,38]. Soil CO2 emission is mainly from two sources: root respiration (rhizospheric respiration) and microbial decomposition of SOM (heterotrophic respiration). The SOM-derived CO2 emissions account for 75% of the total soil CO2 emission and are considered basal respiration [23,39]. The N-induced reduction in soil respiration suggests that microbial decomposition of SOM was inhibited by N input.
On the one hand, low N availability can increase SOM decomposition as microbes used labile substrates to acquire N from N-rich organic matter [40]. Inorganic N addition could suppress microbial N mining and, in consequence decrease SOM decomposition [41]. Moreover, soil microbial communities have been shown to be C-limited [42]. N input may accentuate C limitation on microbial activity, leading to a decrease in SOC decomposition [43]. On the other hand, N addition may slow the decomposition of SOM by increasing soil aggregation and C occlusion in macro-aggregates [41]. Moreover, increased N input may enhance the resistance of SOM to microbial decomposition by incorporating part of the inorganic N into SOM, forming some recalcitrant compounds, such as indoles and pyrroles [44]. Berg et al. [45] also proposed that N may stabilize SOM because N-containing compounds can polymerize with aromatic substances in the soil to form recalcitrant organic matter.
Furthermore, under N-limited conditions, N addition allows more C to be allocated to microbial growth, rather than being lost through extracellular enzymes and respiration, resulting in decreased respiration and increased microbial growth efficiency [41]. In contrast, excessive soil N inputs may also result in N saturation in the soil, which in turn, change the composition of microbial communities or inhibit microbial growth and activities, and ultimately reduce soil respiration [25,46–48]. There is convincing evidence that soil CO2 efflux declines following N addition, accompanied by a decrease in microbial biomass [48]. Allison et al. [46] found that N fertilization reduced microbial diversity and altered the microbial community structure of active fungi in two boreal forest ecosystems. Zhao et al. [49] suggested that N input stimulated G- and actinomycetes. However, in our study, MBC and the metabolic quotient (qCO2) did not significantly respond to additional N input (Figs. 3 and 4). Therefore, the decomposition rate of SOM in our study may be predicted to decline mainly due to the suppression of the microbial N mining process and/or the enhanced stabilization of SOM.
Unexpectedly, we found that physical disturbance did not increase the respiration of the soil not fertilized with N (Figs. 1 and 2). SOM decomposition studies of physical disturbed vs. undisturbed soils have indicated that aggregate-protected pools are less decomposable than unprotected pools because protected pools are less exposed to microbial decay [50]. Our observation is inconsistent with many previous studies that reported an increase in readily decomposable C after physical disturbance [6,9,11]. Similar to our result, Tian et al. [16] did not find significant effects of soil crushing on CO2 production, and Drury et al. [17] also reported that aggregate crushing did not result in an increase of CO2 emissions from soil under continuous corn cropping. These conflicts may be attributed to the different characteristics of SOM and soil aggregates depending on soil management practices and ecosystems [16]. In cultivated and continuous cropping soils, physical disturbance often has no evident effect on CO2 emission. This may be interpreted as the exhaustion of the readily decomposable SOC pool due to the constant disruption of the most unstable soil structure before incubation [16]. In our study, although 60 days of pre-incubation was performed to mitigate the effects of soil disturbance, the processes of soil sieving and homogenization before pre-incubation may affect the properties and responses of SOM to physical disturbance.
Also contrary to the expectation, we found that in the N-treated soil physical disturbance reduced the respiration rate (Fig. 1) and the total C mineralized (Fig. 2). We are uncertain of the mechanism for this reduction. One possible interpretation is that physical disturbance increased the affinity of microbes to the added N in the soil. Soil aggregates may limit substrates or N exposure to microorganisms [32]. On the one hand, physical disturbance did not increase the respiration of the soil without N addition possibly due to the exhaustion of readily decomposable C pools before incubation. On the other hand, in mineral N amended soil, the physical disturbance may allow the added N to be better exposed to soil microbes due to aggregate disruption. Thus, we speculate that in N-treated soil the increase of N availability induced by physical disturbance may further inhibit microbial N mining and/or accentuate C limitation on microbial activity, leading to a decrease in SOC decomposition.
Our results highlight that mineral N input significantly reduced SOC decomposition mainly due to suppressed microbial N mining and/or enhanced stabilization of SOM rather than decreased soil microbial biomass or activity. Surprisingly, physical disturbance failed to increase the soil respiration in this study, and the underlying mechanisms are unclear and need to be explored in future studies. Overall, our results indicate that both physical disturbance and N input play important roles in modulating the stability of SOC. Future studies are required to integrate the changes of the microbial N mining process and the physicochemical stability of SOM after N addition and physical disturbance.
Authorship: The authors confirm contribution to the paper as follows: Study conception and design: Xiuwei Zhang; data collection: Muhammad Junaid Nazir; analysis and interpretation of results: Xiuwei Zhang; Muhammad Junaid Nazir; draft manuscript preparation: Muhammad Junaid Nazir; manuscript review & editing: Feihai Yu; Xiuwei Zhang; Daolin Du. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This work was supported by the Natural Science Foundation of China (32101385), the Natural Science Foundation of Zhejiang Province (LQ20D030001) and the Ten Thousand Talent Program of Zhejiang Province (2018R52016).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Carvalhais, N., Forkel, M., Khomik, M., Bellarby, J., Jung, M. et al. (2014). Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature, 514, 213–217. DOI 10.1038/nature13731. [Google Scholar] [CrossRef]
2. Shao, P., Zeng, X., Moore, D. J. P., Zeng, X. (2013). Soil microbial respiration from observations and earth system models. Environmental Research Letters, 8, 034034. DOI 10.1088/1748-9326/8/3/034034. [Google Scholar] [CrossRef]
3. Lehmann, J., Kleber, M. (2015). The contentious nature of soil organic matter. Nature, 528, 60–68. DOI 10.1038/nature16069. [Google Scholar] [CrossRef]
4. Luo, Z., Feng, W., Luo, Y., Baldock, J., Wang, E. (2017). Soil organic carbon dynamics jointly controlled by climate, carbon inputs, soil properties and soil carbon fractions. Global Change Biology, 23, 4430–4439. DOI 10.1111/gcb.13767. [Google Scholar] [CrossRef]
5. Meyer, N., Welp, G., Rodionov, A., Borchard, N., Martius, C. et al. (2018). Nitrogen and phosphorus supply controls soil organic carbon mineralization in tropical topsoil and subsoil. Soil Biology and Biochemistry, 119, 152–161. DOI 10.1016/j.soilbio.2018.01.024. [Google Scholar] [CrossRef]
6. Zhang, X., Yu, F. (2020). Physical disturbance accelerates carbon loss through increasing labile carbon release. Plant Soil and Environment, 66, 584–589. DOI 10.17221/257/2020-PSE. [Google Scholar] [CrossRef]
7. Chen, R., Senbayram, M., Blagodatsky, S., Myachina, O., Dittert, K. et al. (2013). Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Global Change Biology, 20, 2356–2367. DOI 10.1111/gcb.12475. [Google Scholar] [CrossRef]
8. Six, J., Paustian, K., Elliott, E. T., Combrink, C. (2000). Soil structure and organic matter I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Science Society of America Journal, 64, 681–689. DOI 10.2136/sssaj2000.642681x. [Google Scholar] [CrossRef]
9. Zakharova, A., Midwood, A. J., Hunt, J. E., Graham, S. L., Artz, R. R. E. et al. (2014). Loss of labile carbon following soil disturbance determined by measurement of respired δ13CO2. Soil Biology and Biochemistry, 68, 125–132. DOI 10.1016/j.soilbio.2013.10.001. [Google Scholar] [CrossRef]
10. Elliott, E. T. (1986). Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Science Society of America Journal, 56, 627–633. DOI 10.2136/sssaj1986.03615995005000030017x. [Google Scholar] [CrossRef]
11. Zakharova, A., Beare, M. H., Cieraad, E., Curtin, D. (2015). Turnbull MH, millard P. factors controlling labile soil organic matter vulnerability to loss following disturbance as assessed by measurement of soil-respired δ13CO2. European Journal of Soil Science, 66, 135–144. DOI 10.1111/ejss.12209. [Google Scholar] [CrossRef]
12. Mummey, D., Holben, W., Six, J., Stahl, P. (2006). Spatial stratification of soil bacterial populations in aggregates of diverse soils. Microbial Ecology, 51, 404–411. DOI 10.1007/s00248-006-9020-5. [Google Scholar] [CrossRef]
13. Wang, H., Liu, S., Kuzyakov, Y., Zhan, P., Wang, Q. et al. (2020). Differentiating microbial taxonomic and functional responses to physical disturbance in bulk and rhizosphere soils. Land Degradation & Development, 31, 2858–2871. DOI 10.1002/ldr.3679. [Google Scholar] [CrossRef]
14. Gregorich, E. G., Kachanoski, R. G., Voroney, R. P. (1989). Carbon mineralization in soil size fractions after various amounts of aggregate disruption. Journal of Soil Science, 40(3), 649–659. DOI 10.1111/j.1365-2389.1989.tb01306.x. [Google Scholar] [CrossRef]
15. Hassink, J. (1992). Effects of soil texture and structure on carbon and nitrogen mineralization in grassland soils. Biology and Fertility of Soils, 14(2), 126–134. DOI 10.1007/BF00336262. [Google Scholar] [CrossRef]
16. Tian, J., Pausch, J., Yu, G., Blagodatskaya, E., Gao, Y. et al. (2015). Aggregate size and their disruption affect 14C-labeled glucose mineralization and priming effect. Applied Soil Ecology, 90, 1–10. DOI 10.1016/j.apsoil.2015.01.014. [Google Scholar] [CrossRef]
17. Drury, C. F., Yang, X. M., Reynolds, W. D., Tan, C. S. (2004). Influence of crop rotation and aggregate size on carbon dioxide production and denitrification. Soil and Tillage Research, 79(1), 87–100. DOI 10.1016/j.still.2004.03.020. [Google Scholar] [CrossRef]
18. Adu, J. K., Oades, J. M. (1978). Physical factors influencing decomposition of organic materials in soil aggregates. Soil Biology and Biochemistry, 10(2), 109–115. DOI 10.1016/0038-0717(78)90080-9. [Google Scholar] [CrossRef]
19. Canfield, D. E., Glazer, A. N., Falkowski, P. G. (2010). The evolution and future of earth’s nitrogen cycle. Science, 330(6001), 192–196. DOI 10.1126/science.1186120. [Google Scholar] [CrossRef]
20. Galloway, J. N., Townsend, A. R., Erisman, J. W., Bekunda, M., Cai, Z. et al. (2008). Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science, 320(5878), 889–892. DOI 10.1126/science.1136674. [Google Scholar] [CrossRef]
21. Field, C. D., Evans, C. D., Dise, N. B., Hall, J. R., Caporn, S. J. (2017). Long-term nitrogen deposition increases heathland carbon sequestration. Science of the Total Environment, 592, 426–435. DOI 10.1016/j.scitotenv.2017.03.059. [Google Scholar] [CrossRef]
22. Paul, E. A. (2016). The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biology and Biochemistry, 98, 109–126. DOI 10.1016/j.soilbio.2016.04.001. [Google Scholar] [CrossRef]
23. Peng, Y., Song, S. Y., Li, Z. Y., Li, S., Chen, G. T. et al. (2020). Influences of nitrogen addition and aboveground litter-input manipulations on soil respiration and biochemical properties in a subtropical forest. Soil Biology and Biochemistry, 142, 107694. DOI 10.1016/j.soilbio.2019.107694. [Google Scholar] [CrossRef]
24. Liu, L., Greaver, T. L. (2010). A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecology Letters, 13(7), 819–828. DOI 10.1111/j.1461-0248.2010.01482.x. [Google Scholar] [CrossRef]
25. Zhou, S., Xiang, Y., Tie, L., Han, B., Huang, C. (2018). Simulated nitrogen deposition significantly reduces soil respiration in an evergreen broadleaf forest in western China. PLoS One, 13(9), e0204661. DOI 10.1371/journal.pone.0204661. [Google Scholar] [CrossRef]
26. Peng, Y., Chen, G. T., Li, S., Hu, H. L., Hu, T. X. et al. (2018). Nitrogen additions reduce rhizospheric and heterotrophic respiration in a subtropical evergreen broad-leaved forest. Plant and Soil, 431(1), 449–463. DOI 10.1007/s11104-018-3751-1. [Google Scholar] [CrossRef]
27. Janssens, I. A., Dieleman, W., Luyssaert, S., Subke, J. A., Reichstein, M. et al. (2010). Reduction of forest soil respiration in response to nitrogen deposition. Nature Geoscience, 3(5), 315–322. DOI 10.1038/ngeo844. [Google Scholar] [CrossRef]
28. Fan, H., Wu, J., Liu, W., Yuan, Y., Huang, R. et al. (2014). Nitrogen deposition promotes ecosystem carbon accumulation by reducing soil carbon emission in a subtropical forest. Plant and Soil, 379(1), 361–371. DOI 10.1007/s11104-014-2076-y. [Google Scholar] [CrossRef]
29. Forstner, S. J., Wechselberger, V., Müller, S., Keibinger, K. M., Díaz-Pinés, E. et al. (2019). Vertical redistribution of soil organic carbon pools after twenty years of nitrogen addition in two temperate coniferous forests. Ecosystems, 22(2), 379–400. DOI 10.1007/s10021-018-0275-8. [Google Scholar] [CrossRef]
30. Reid, J. P., Adair, E. C., Hobbie, S. E., Reich, P. B. (2012). Biodiversity, nitrogen deposition and CO2 affect grassland soil carbon cycling but not storage. Ecosystems, 15(4), 580–590. DOI 10.1007/s10021-012-9532-4. [Google Scholar] [CrossRef]
31. Lu, M., Zhou, X., Luo, Y., Yang, Y., Fang, C. et al. (2011). Minor stimulation of soil carbon storage by nitrogen addition: A meta-analysis. Agriculture, Ecosystems & Environment, 140(1–2), 234–244. DOI 10.1016/j.agee.2010.12.010. [Google Scholar] [CrossRef]
32. Kinyangi, J., Solomon, D., Liang, B., Lerotic, M., Wirick, S. et al. (2006). Nanoscale biogeocomplexity of the organomineral assemblage in soil: Application of STXM microscopy and C 1 s-NEXAFS spectroscopy. Soil Science Society of America Journal, 70(5), 1708–1718. DOI 10.2136/sssaj2005.0351. [Google Scholar] [CrossRef]
33. Tian, Q., Wang, X., Wang, D., Wang, M., Liao, C. et al. (2017). Decoupled linkage between soil carbon and nitrogen mineralization among soil depths in a subtropical mixed forest. Soil Biology and Biochemistry, 109, 135–144. DOI 10.1016/j.soilbio.2017.02.009. [Google Scholar] [CrossRef]
34. Lu, J., Dijkstra, F. A., Wang, P., Cheng, W. (2018). Rhizosphere priming of grassland species under different water and nitrogen conditions: A mechanistic hypothesis of C-N interactions. Plant and Soil, 429(1), 303–319. DOI 10.1007/s11104-018-3699-1. [Google Scholar] [CrossRef]
35. Vance, E. D., Brookes, P. C., Jenkinson, D. S. (1987). An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry, 19(6), 703–707. DOI 10.1016/0038-0717(87)90052-6. [Google Scholar] [CrossRef]
36. Hobbie, S. E., Vitousek, P. M. (2000). Nutrient limitation of decomposition in hawaiian forests. Ecology, 81(7), 1867–1877. DOI 10.1890/0012-9658(2000)081[1867:NLODIH]2.0.CO;2. [Google Scholar] [CrossRef]
37. Maaroufi, N. I., Nordin, A., Hasselquist, N. J., Bach, L. H., Palmqvist, K. et al. (2015). Anthropogenic nitrogen deposition enhances carbon sequestration in boreal soils. Global Change Biology, 21(8), 3169–3180. DOI 10.1111/gcb.12904. [Google Scholar] [CrossRef]
38. Mo, J., Zhang, W., Zhu, W., Gundersen, P., Fang, Y. et al. (2008). Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Global Change Biology, 14(2), 403–412. DOI 10.1111/j.1365-2486.2007.01503.x. [Google Scholar] [CrossRef]
39. Kuzyakov, Y. (2006). Sources of CO2 efflux from soil and review of partitioning methods. Soil Biology and Biochemistry, 38(3), 425–448. DOI 10.1016/j.soilbio.2005.08.020. [Google Scholar] [CrossRef]
40. Craine, J. M., Morrow, C., Fierer, N. (2007). Microbial nitrogen limitation increases decomposition. Ecology, 88(8), 2105–2113. DOI 10.1890/06-1847.1. [Google Scholar] [CrossRef]
41. Riggs, C. E., Hobbie, S. E., Bach, E. M., Hofmockel, K. S., Kazanski, C. E. (2015). Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry, 125(2), 203–219. DOI 10.1007/s10533-015-0123-2. [Google Scholar] [CrossRef]
42. Cleveland, C. C., Nemergut, D. R., Schmidt, S. K., Townsend, A. R. (2007). Increases in soil respiration following labile carbon additions linked to rapid shifts in soil microbial community composition. Biogeochemistry, 82(3), 229–240. DOI 10.1007/s10533-006-9065-z. [Google Scholar] [CrossRef]
43. Tao, B., Song, C. (2013). Effects of nitrogen addition, temperature and physical protection on first-phase decomposition of soil organic carbon in marshland. Fresenius Environmental Bulletin, 22(1), 1579–1584. [Google Scholar]
44. Thorn, K. A., Mikita, M. A. (1992). Ammonia fixation by humic substances: A nitrogen-15 and carbon-13 NMR study. Science of the Total Environment, 113(1–2), 67–87. DOI 10.1016/0048-9697(92)90017-M. [Google Scholar] [CrossRef]
45. Berg, B., Matzner, E. (1997). Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environmental Reviews, 5(1), 1–25. DOI 10.1139/a96-017. [Google Scholar] [CrossRef]
46. Allison, S. D., Hanson, C. A., Treseder, K. K. (2007). Nitrogen fertilization reduces diversity and alters community structure of active fungi in boreal ecosystems. Soil Biology and Biochemistry, 39(8), 1878–1887. DOI 10.1016/j.soilbio.2007.02.001. [Google Scholar] [CrossRef]
47. Bowden, R. D., Davidson, E., Savage, K., Arabia, C., Steudler, P. (2004). Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the harvard forest. Forest Ecology and Management, 196(1), 43–56. DOI 10.1016/j.foreco.2004.03.011. [Google Scholar] [CrossRef]
48. Peng, Y., Chen, G., Chen, G., Li, S., Peng, T. et al. (2017). Soil biochemical responses to nitrogen addition in a secondary evergreen broad-leaved forest ecosystem. Scientific Reports, 7, 2783. DOI 10.1038/s41598-017-03044-w. [Google Scholar] [CrossRef]
49. Zhao, Z., Ge, T., Gunina, A., Li, Y., Zhu, Z. et al. (2019). Carbon and nitrogen availability in paddy soil affects rice photosynthate allocation, microbial community composition, and priming: Combining continuous 13C labeling with PLFA analysis. Plant and Soil, 445(1), 137–152. DOI 10.1007/s11104-018-3873-5. [Google Scholar] [CrossRef]
50. Cambardella, C. A., Elliott, E. T. (1994). Carbon and nitrogen dynamics of soil organic matter fractions from cultivated grassland soils. Soil Science Society of America Journal, 58(1), 123–130. DOI 10.2136/sssaj1994.03615995005800010017x. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |