DOI:10.32604/biocell.2021.015261

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.015261 |  www.techscience.com/journal/biocell |
| Article |
Nicotine and menthol independently exert neuroprotective effects against cisplatin- or amyloid- toxicity by upregulating Bcl-xl via JNK activation in SH-SY5Y cells
1Technology Center, China Tobacco Guizhou Industrial Co., Ltd., Guiyang, 550003, China
2Department of Basic Medical Sciences, School of Medicine, Xiamen University, Xiamen, 361102, China
*Address correspondence to: Jian Liu, jliuemail@163.com; Fengguang Gao, gfengguang@xmu.edu.cn
Received: 04 December 2020; Accepted: 08 February 2021
Abstract: Nicotine and menthol, agonists of nicotinic acetylcholine receptor (nAChR) and transient receptor potential melastatin type 8 (TRPM8), serve important roles in the prevention of cell death-involved neurodegenerative diseases. However, the potential synergistic effects of nicotine and menthol on anti-apoptotic ability are still uncertain. In the present study, the potential synergistic effects of nicotine and menthol on cisplatin or amyloid β1-42 induced cell model of the neurodegenerative diseases were explored by assessing cell viability, TNF-α expression, caspase-3 activation, and the collapse of mitochondrial membrane potential in human SH-SY5Y neuroblastoma cells. Statistical significance was tested using Student’s t-test or one-way ANOVA with post hoc Newman-Keuls test. The results showed that: Firstly, SH-SY5Y cell viability was obviously increased by the treatments with nicotine and menthol. Secondly, nicotine and menthol independently alleviated cisplatin or amyloid β1-42 induced TNF-α up-regulation. Thirdly, nicotine and menthol abrogated the effect of cisplatin and amyloid β25-35 on caspase-3 activation. Interestingly, the effect of cisplatin and amyloid β1-42 on the collapse of mitochondrial membrane potential was efficiently attenuated by nicotine and menthol treatments. Most importantly, the inhibition of c-jun kinase (JNK) activation abolished the effect of cisplatin, and amyloid β1-42 stimulated Bcl-xl expression. All these findings indicate that nicotine and menthol independently exert neuroprotective effects by upregulating Bcl-xl via JNK activation. Nicotine and menthol augmented Bcl-xl expression and JNK phosphorylation, and thus they are potential therapeutic targets for altering the progress of neurodegenerative diseases.
Keywords: Nicotine; Menthol; Apoptosis; Mitochondrial membrane potential; Tumor necrosis factor-alpha
Apoptosis, which initiates either by the intrinsic pathway or the extrinsic pathway, is responsible for the physiological deletion of cells and appears to be intrinsically programmed (Pistritto et al., 2016). While intrinsic cell stress is sensed by the intrinsic pathway, signals from other cells such as tumor necrosis factor-alpha (TNF-α) and Fas signals are sensed by the extrinsic pathway (van Opdenbosch and Lamkanfi, 2019). After the signals of cell death are sensed, cysteinyl aspartate specific proteinases (caspases) such as caspase-3 and caspase-6 are activated, and the degradations of the proteins and chromatin occur (van Opdenbosch and Lamkanfi, 2019).
Alzheimer’s disease, which is characterized by memory loss and language deterioration, exhibits neurofibrillary tangle accumulation and β-amyloid protein-containing plaques (Rosenberg and Lambracht-Washington, 2013). β-amyloid induced neuron apoptosis, which is typically observed in the brains of the patients, features chromatin condensation, deoxyribonucleic acid (DNA) fragmentation, and caspase activation (Nalivaeva and Turner, 2013). Interestingly, there is evidence that nicotinic acetylcholine receptors (nAChR) provide neurotrophic support to neurons (Bono et al., 2020). These findings indicate that nAChR might be involved in β-amyloid inducing neuronal loss, and it serves an important role in the development of the disease.
Nicotine, an agonist of nAChR (Islam et al., 2013), prevents oxidative stress-induced hippocampal neuronal injury through α7-nAChR/Erk1/2 signaling pathway (Dong et al., 2020). Although the treatment with menthol increases nicotine addiction (Nonnemaker et al., 2019), menthol, an agonist of transient receptor potential melastatin Type 8 (TRPM8), was documented to ameliorate the pathological abnormalities in hepatic and pancreatic islets (Muruganathan et al., 2017; Rozza et al., 2014). Hence, as the agonists of the nicotinic acetylcholine receptors, nicotine and menthol play important roles in activating c-Jun kinase (JNK) activation and altering the progress of neurodegenerative diseases (Go et al., 2020; Lombardo and Maskos, 2015). In our previous studies, amyloid β and cisplatin were found to induce cell apoptosis in a concentration-dependent manner (Xue et al., 2014; Yan et al., 2014). By inhibiting JNK with SP600125, nicotine also exhibited an anti-apoptotic effect and facilitated the cross-presentation in dendritic cells (Gao and Gu, 2007; Xue et al., 2014). But the potential synergistic effects of nicotine and menthol on anti-apoptotic ability are still uncertain.
In the present study, we aimed to investigate the potential synergistic effects of nAChR and TRPM8 agonist on cell viability by using human SH-SY5Y neuroblastoma cells as a cell model of neurodegeneration (Bono et al., 2020). We demonstrated that as agonists of nAChR and TRPM8, nicotine and menthol exert neuroprotective abilities, which was supported by the following observations. Firstly, the treatments with nicotine and menthol efficiently increased cell viability. Secondly, cisplatin and amyloid β1-42 augmented TNF-α up-regulation was abolished by the pretreatments with nicotine and menthol. Thirdly, the effects of cisplatin and amyloid β25-35 on caspase 3 activation were abrogated by nicotine and menthol treatments. Interestingly, cisplatin and amyloid β1-42 induced collapse of mitochondrial membrane potential was alleviated by nicotine and menthol pretreatments. Most importantly, the inhibition of JNK activation abrogated the effects of nicotine and menthol on Bcl-xl up-regulation. All these findings indicate that the upregulating Bcl-xl via JNK activation contributes to nicotine and menthol independently exert a neuroprotective effect. Bcl-xl and JNK phosphorylation are potential therapeutic targets for dealing with nAChR and TRPM8 associated with the progress of neurodegenerative diseases.
Nicotine (-), amyloid β1-42, amyloid β25-35 and Rhodamine123 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Nicotine and menthol were provided by China Tobacco Guizhou Industrial Co., Ltd., Guiyang, China. Cisplatin was purchased from Calbiochem, Inc (Merck KGaA, Darmstadt, Germany). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies, Inc., Shanghai, China. SP600125 was from Cayman Chemical Company (Ann Arbor, MI, USA). Antibodies to β-actin (Mouse, #3700), myeloid cell leukemia-1 (Mcl-1) (Rabbit, #94296), Bcl-xl (Rabbit, #2764), phosphorylated JNK (Rabbit, #4668), TNF-α (Rabbit, #6945), cleaved caspase 3 (Rabbit, #9661) were purchased from Cell Signaling Technology, Inc., Beverly, MA, USA. Dulbecco’s modified eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from HyClone, GE Healthcare Life Sciences (Logan, UT, USA). The stock solution of amyloid β and cisplatin was prepared by dissolving amyloid β and cisplatin in phosphate buffer saline (PBS) and dimethyl sulfoxide (DMSO), respectively.
Human SH-SY5Y neuro-blastoma cells were obtained from the Shanghai Cell Bank and authenticated by STR profiling using fluorescence-labeled amplification product length polymorphism analysis. Mycoplasma testing was performed for this cell line. Cells were cultured in DMEM with 10% FBS at 37°C in 5% CO2. The cells were rinsed with 0.25% trypsin and passaged according to the subcultivation ratio of 1:20, and every 4 to 7 days, the medium was replaced. The concentrations of amyloid β1-42, amyloid β25-35, nicotine were based on previous studies (Xue et al., 2014).
Plate scan and optical microscope observation
Optical microscope observation was performed according to a previous description (Jiang et al., 2019). Briefly, a total of 5 × 104 SH-SY5Y cells were firstly seeded in 24-well plates. Then, synchronization was performed by serum starvation for at least 12 h to reduce the effect of cell proliferation. The cells were further pretreated with nicotine (1 µM), menthol (1 µM), nicotine and menthol used together (1 µM, respectively) for 16 h prior to 5 µg/mL cisplatin or 20 µM amyloid β1-42 stimulation. In the end, the cells were cultured for a further 48 h–72 h, and the effects of nicotine, menthol, nicotine, and menthol used together on cell morphology and viability were assessed by plate scan with crystal violet staining, cell count using an optical microscope with 100× magnification factor, respectively.
Cell proliferation assay was determined by CCK-8 assay according to a previous description (Wang et al., 2014). Based on the detection of the dehydrogenase activity in the viable cells, WST-8 products a water-soluble formazan dye upon reduction in the presence of an electron mediator. Briefly, a total of 3 × 104 SH-SY5Y cells were firstly inoculated in a 96-well plate in 100 μL/well medium. Then, the cells were subsequently treated with indicated amyloid β1-42 and cisplatin. CCK-8 solution (10 µL per well) was added to each well. After 2 h incubation, the OD450 value was determined at the wavelength of 450 nm.
Mitochondrial membrane potential assay
Mitochondrial membrane potential was determined using flow cytometry according to a previous description (Ke et al., 2013). Briefly, a total of 5 × 104 SH-SY5Y cells were seeded in 24-well plates. Then, the cells were pretreated with nicotine (1 µM), menthol (1 µM), nicotine and menthol used together (1 µM, respectively) for 16 h prior to 5 µg/mL cisplatin or 20 µM amyloid β1-42 stimulation. After that, the cells were removed by trypsinization, rinsed with PBS. The cells were re-suspended in binding buffer containing Rhodamine123 (1 µM) for 20 min at room temperature. After a thorough wash, the mitochondrial membrane potential of the cell was determined using flow cytometric analyses on FACSCalibur. Data were analyzed using CellQuest software.
Western blot analyses were performed according to the methods previously described (Xue et al., 2014). For TNF-α and cleaved caspase-3, SH-SY5Y cells were pretreated with DMSO/PBS, nicotine (1 µM), menthol (1 µM) or nicotine and menthol used together (1 µM, respectively) for 16 h prior to 5 µg/mL cisplatin or 20 µM amyloid β25-35 10 h stimulation. For Bcl-xl expression, the cells were pretreated with DMSO or SP600125 prior to the stimulations of nicotine, menthol, nicotine, and menthol used together. Left-handed nicotine, which has a powerful ability for cell proliferation (Wang et al., 2014), was used as the positive control. The cellular proteins were extracted and loaded onto 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After 120 min electrophoresis with 80 V, the proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane. Blocking was performed by incubation with 5% fat-free milk in TBST. The membrane was incubated with primary antibody at 4°C overnight with 1:1000 dilution. After washed six times with TBST (for 10 min each), the membrane was further incubated with corresponding horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature. The bound secondary antibody was visualized using enhanced chemiluminescence ECL. β-actin was used as a loading control. DMSO was employed as solvent control of cisplatin.
Each experiment was repeated at least 3 times to confirm data repeatability. Cell viabilities and Mean Fluorescent Index of Rhodamine123 in SH-SY5Y cells were expressed as the mean ± standard error. Statistical significance was tested using a Student’s t-test or one-way ANOVA with a post hoc Newman-Keuls test. P < 0.05 was considered to indicate a statistically significant difference.
The treatments with nicotine and menthol augment cell viability in SH-SY5Y cells
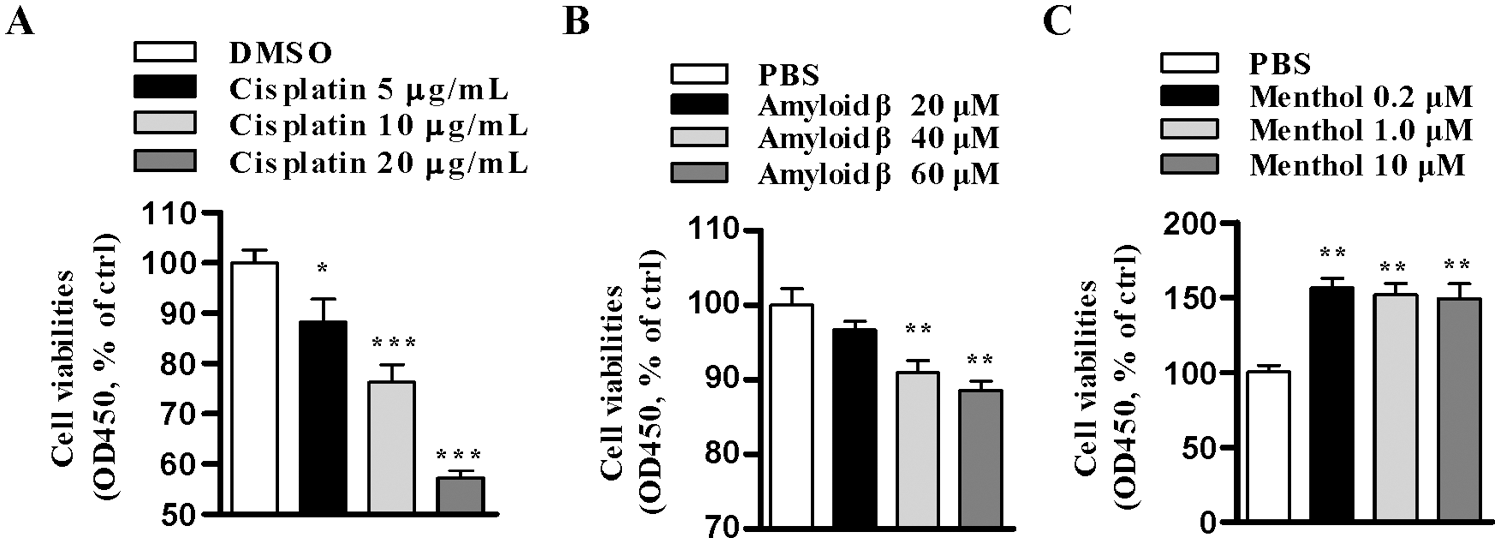
Figure 1: Cisplatin and amyloid β concentration-dependent decrease cell viability in SH-SY5Y cells.
In our previous studies, we have documented that amyloid β and cisplatin induce cell apoptosis in a concentration-dependent manner (Xue et al., 2014; Yan et al., 2014). Whereas as an agonist of nAChR, nicotine exhibits a positive effect in neurodegenerative disease and facilitates the cross-presentation in dendritic cells (Gao and Gu, 2007; Xue et al., 2014), menthol, an agonist of TRPM8, was also demonstrated to promote cell proliferation (Muruganathan et al., 2017; Rozza et al., 2014). As cell viability is the comprehensive effects of cell apoptosis and cell proliferation, CCK-8 assay determined cell viability revealed that amyloid β1-42 and cisplatin decrease SH-SY5Y cell viability in a concentration-dependent manner (Figs. 1A and 1B). Whereas the selection of the concentration of nicotine was documented (Xue et al., 2014), the selected concentration of menthol achieved a similar effect on cell proliferation to 0.2 and 10 µM menthol (Fig. 1C). We further treated SH-SY5Y cells with nicotine, menthol, or nicotine and menthol used together and observed the potential synergistic effect on cell viability. The treatment with cisplatin increased 50% cell death (Fig. 1C), but pretreatments with nicotine, menthol, or nicotine and menthol used together efficiently increased cell viability in both plate scan and optical microscope observation assays (Figs. 2A and 2B). Meanwhile, similar phenomena can be observed in amyloid β1-42 treated condition (Figs. 2C and 2D). Cell proliferation assay revealed that whereas the treatments with nicotine or menthol increased 89.6% and 91.4% cell viability, nicotine and menthol used together achieved about 88.2% increase in cell viability in cisplatin-treated condition (Fig. 2E). Similar results can also be derived in amyloid-β1-42 treated conditions (Fig. 2F). Although the plate scan and microscopic images indicated the synergic effect of menthol and nicotine, the cell count test did not confirm any significant statistical differences among these treatments (Figs. 2E and 2F). All these findings indicate that as agonists of nAChR and TRPM8, nicotine and menthol exhibit an independent proliferation effect in SH-SY5Y cells.
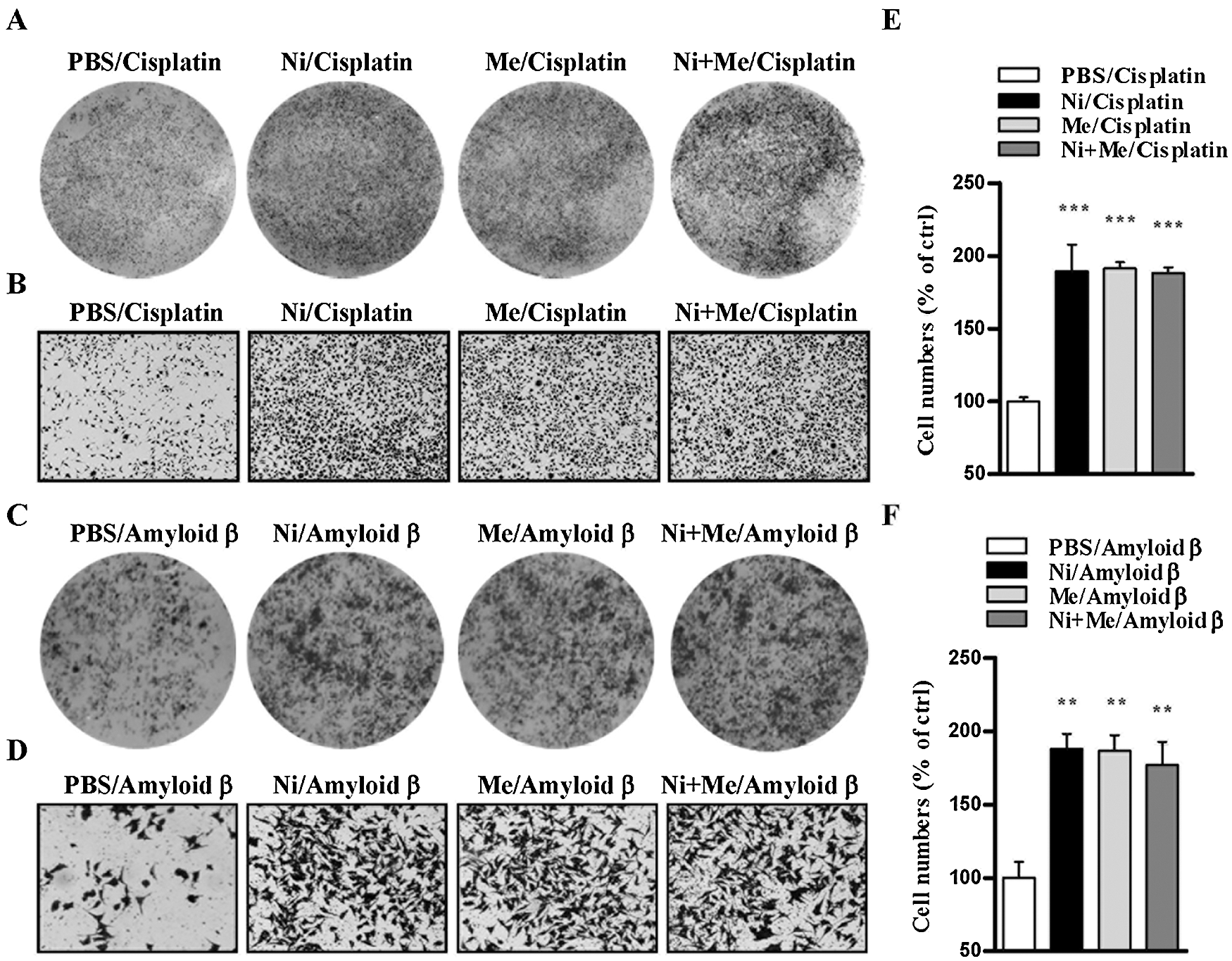
Figure 2: Nicotine and menthol augment cell viability in SH-SY5Y cells.
The treatments with nicotine and menthol attenuate cisplatin and amyloid β1-42 induced TNF-α expression in SH-SY5Y cells
Inflammations, especially indicated by the high level of TNF-α, play an important role in the progress of neurodegenerative diseases (Lombardo and Maskos, 2015). SH-SY5Y cells were incubated with nicotine, menthol, or nicotine and menthol used together prior to cisplatin and amyloid β1-42 stimulation. The level of TNF-α in the cells was subsequently assessed by Western blot analyses. The treatments with cisplatin and amyloid β1-42 significantly (P < 0.05) augmented TNF-α expression (Figs. 3A and 3B). However, pretreatments with nicotine, menthol, or nicotine and menthol used together achieved similar inhibitory effects of cisplatin and amyloid β1-42 on TNF-α expression (Figs. 3C and 3D). These findings indicate that as agonists of nAChR and TRPM8, respectively, nicotine exhibited no synergistic effect with menthol on cisplatin and amyloid β-induced cell inflammation.
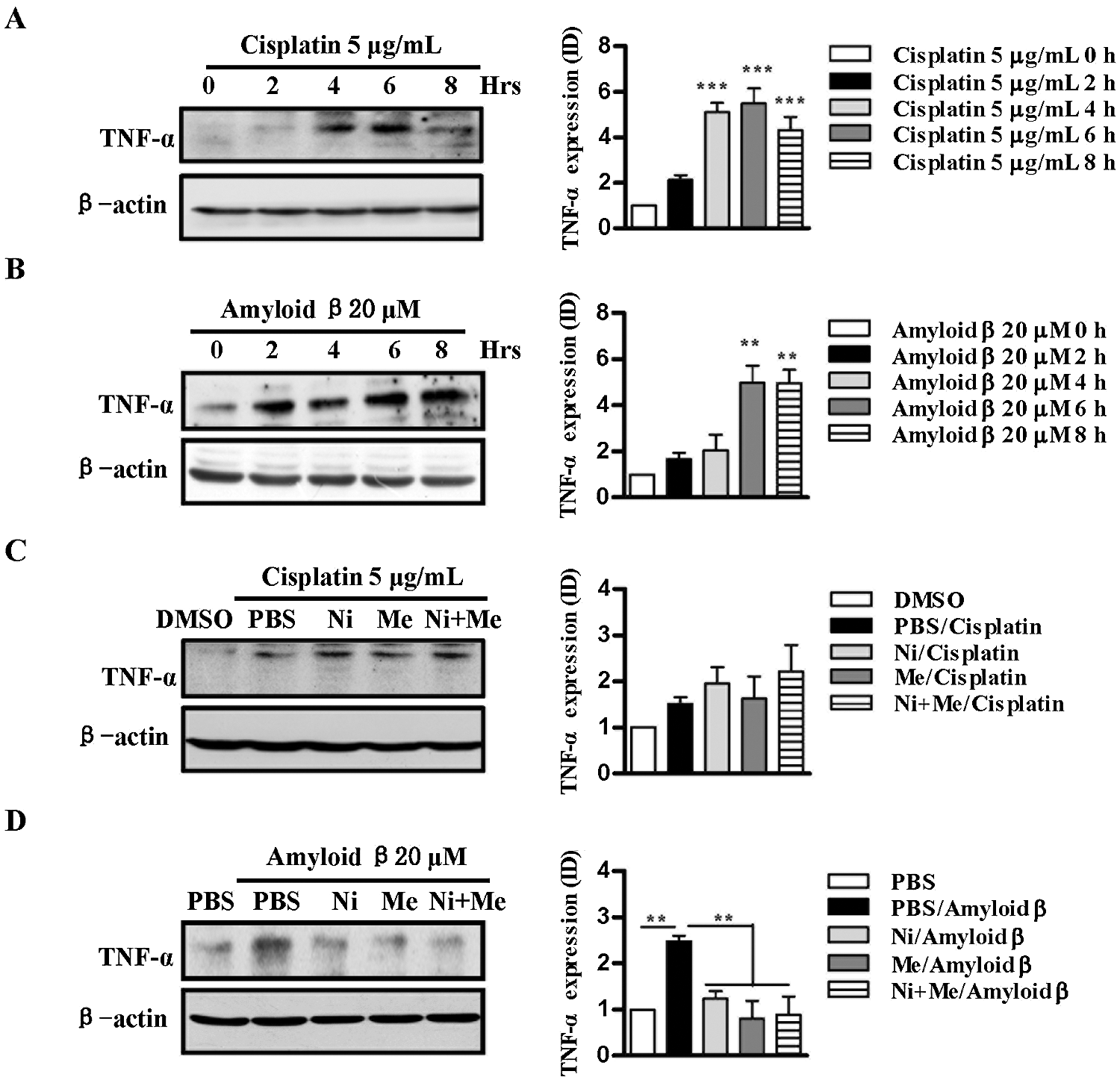
Figure 3: Nicotine and menthol abolish the effect of cisplatin and amyloid β1-42 on TNF-α up-regulation in SH-SY5Y cells.
Nicotine and menthol alleviate cisplatin and amyloid β25-35 induced caspase-3 activations in SH-SY5Y cells
Caspase-3 serves a vital role in the intrinsic and extrinsic pathways of apoptosis (Herr, 2018). SH-SY5Y cells were incubated with nicotine, menthol, or nicotine and menthol used together prior to cisplatin and amyloid β25-35 stimulation. The activity of caspase 3 was assessed by Western blot analyses. Whereas the treatments with cisplatin and amyloid β25-35 obviously increased the level of cleaved caspase 3 in a time-dependent manner (Figs. 4A and 4B), the pretreatments with nicotine, menthol, or nicotine and menthol used together efficiently abrogated the effects of cisplatin (Fig. 4C) and amyloid β25-35 (Fig. 4D) on caspase 3 activation. These results indicate that as agonists of nAChR and TRPM8, nicotine exhibit no synergistic inhibitory effect with menthol on cisplatin and amyloid β induced caspase 3 activation.
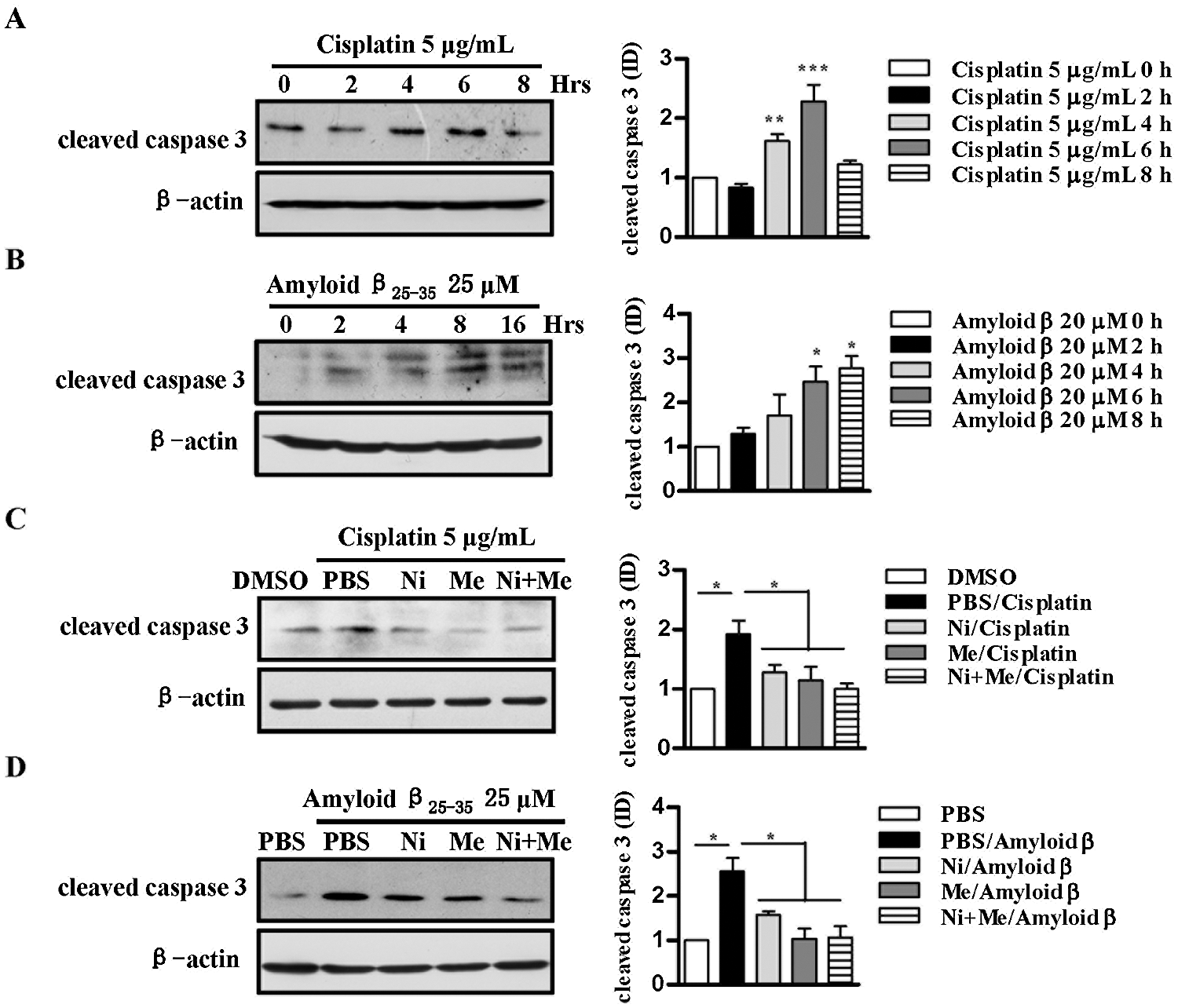
Figure 4: Nicotine and menthol abrogate cisplatin, and amyloid β25-35 induces caspase 3 activation in SH-SY5Y cells.
The treatments with nicotine and menthol alleviate cisplatin and amyloid β1-42 induced the collapse of mitochondrial membrane potential in SH-SY5Y cells
In the earlier stage of apoptosis, the outer membrane of the mitochondria disrupts, and intermembrane space proteins are released into the cytosol (Vringer and Tait, 2019). Hence, the collapse of mitochondrial membrane potential, which reflects mitochondrial function, serves as an earlier indicator of cell health (Sakamuru et al., 2016). Whereas the treatment with cisplatin decreased 25.1% mitochondrial membrane potential (Fig. 5A), amyloid β1-42 treatment attenuated 29.5% mitochondrial membrane potential (Fig. 5B), indicating that cisplatin and amyloid β1-42 efficiently induced the collapse of the mitochondrial membrane potential. While the pretreatment with nicotine, menthol attenuated about 29.9%, 32.1% cisplatin-decreased mitochondrial membrane potential, respectively, nicotine and menthol used together alleviated 20.1% of the collapse of the mitochondrial membrane potential (Fig. 5C). Similar results can be derived in amyloid β1-42 treated condition (Fig. 5D). All these findings indicate that as agonists of nAChR and TRPM8, nicotine and menthol exhibited an anti-apoptotic effect by stabilizing the mitochondrial membrane potential.
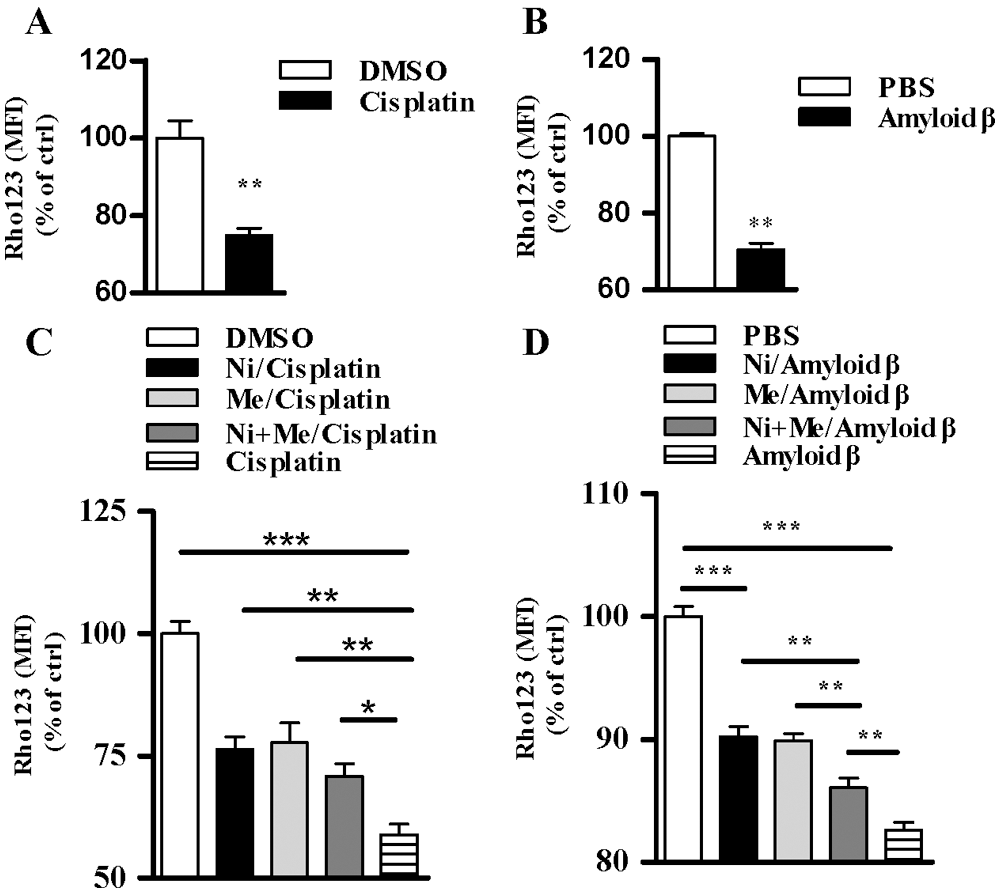
Figure 5: Nicotine and menthol alleviate cisplatin and amyloid β1-42 induced the collapse of mitochondrial membrane potential in SH-SY5Y cells.
Nicotine and menthol increase Bcl-xl expression via JNK activation in SH-SY5Y cells
JNK activation has a positive correlation with the nicotine-mediated anti-apoptotic ability (Xue et al., 2014). Left-handed nicotine, which has a powerful ability for cell proliferation (Wang et al., 2014), was used as a positive control. We incubated SH-SY5Y cells with nicotine, menthol, or nicotine and menthol used together and found that both the JNK phosphorylation (Fig. 6A) and the expressions of Mcl-1 and Bcl-xl (Fig. 6B) were efficiently increased by these treatments. The inhibition of JNK activation with SP600125 obviously abolished the effects of nicotine and menthol on Bcl-xl up-regulation (Fig. 6C). Despite the fact that SP600125 administration actually inhibited JNK activation, the inhibition of JNK phosphorylation had no effect on nicotine and menthol up-regulating Mcl-1 expression (Fig. 6C). All these results indicate that JNK activation is involved in an increase of Bcl-xl expression by nicotine and menthol.

Figure 6: Nicotine and menthol augment Bcl-xl expression via JNK activation in SH-SY5Y cells. (A, B). SH-SY5Y cells were treated with PBS, nicotine (1 µM), menthol (1 µM), and nicotine and menthol used together (1 µM, respectively) for 30 min. (C) SH-SY5Y cells were pre-treated with DMSO or SP600125 (10 µM) prior to nicotine (1 µM), menthol (1 µM) or nicotine and menthol used together (1 µM, respectively) stimulation. The whole cellular protein was extracted, and the levels of phosphorylated JNK (A, C), Mcl-1 and Bcl-xl (B, C) were assessed using Western blot analysis. β-actin was used as an internal control. Data are representative of three independent experiments. Ni, nicotine; Me, menthol; Ni (−), left-handed nicotine.
Neurodegenerative diseases, which are involved in the processes of inflammation and cell viability, exhibit increased prevalence in the developed community (Patel et al., 2017). As agonists of nAChR and TRPM8 (Dong et al., 2020; Lombardo and Maskos, 2015), nicotine and menthol have therapeutic benefits on neurodegenerative diseases (Pogocki et al. 2007), the synergistic effect of nicotine and menthol is definitely important for dealing with these neurodegenerative diseases. In the present study, we demonstrated that as agonists of nAChR and TRPM8, nicotine and menthol exerted neuroprotective effects by up-regulating Bcl-xl expression via JNK activation in SH-SY5Y cells. Interestingly, mitochondrial membrane potential, an indicator of the functional metabolic status of mitochondria, was found to underlie the mechanism of nicotine and menthol-enhanced cell viability. These findings indicate that Bcl-xl and JNK might be potential therapeutic target molecules in nAChR and TRPM8 associated diseases. Mitochondrial membrane potential might be useful for monitoring the progress of neurodegenerative diseases. Despite the fact that SH-SY5Y cell is a better cell model for Parkinson’s disease (Krishna et al., 2014; Xicoy et al., 2017), a number of documents have conferred this cell line as a cell model of Alzheimer’s disease (Prasad and Rao, 2015; Yang et al., 2014). Whether nicotine exerts an anti-apoptotic effect and the potential synergistic effect with menthol in Parkinson’s disease still requires further investigations that therefore formed the basis of the present study.
The B cell lymphoma-2 (Bcl-2) family consists of a number of proteins containing Bcl-2 homology domains that regulate apoptosis by controlling mitochondrial membrane permeability and releasing cytochrome c (Kvansakul et al., 2017). The Bcl-2 family can be separated into pro-survival proteins, pro-apoptotic proteins, and “BH3 only” proteins (Kvansakul et al., 2017). Interactions between death-promoting and death-suppressing members lead to a rheostat model in which the ratio of pro-apoptotic and anti-apoptotic proteins controls the fate of acute myeloid leukemia (Ashkenazi et al., 2017). Bcl-xl localizes in the outer mitochondrial membrane and protects cells against apoptotic stimuli (Kale et al., 2012). Although nicotine increases Bcl-xl expression and facilitates multiple drug resistance of lung cancer cells (Ke et al., 2013), the mechanisms by which nicotine up-regulates other anti-apoptotic proteins or increasing the interactions between death-promoting and death-suppressing members require further exploration. In the present study, the collapse of mitochondrial membrane potential was found to contribute to cisplatin- and amyloid β-induced apoptosis (Fig. 4). The treatments with nicotine and menthol increased Bcl-xl up-regulation (Fig. 5B), whereas the effects of cisplatin and amyloid β on the mitochondrial membrane potential were partly abolished by nicotine and menthol pretreatments (Fig. 4). As Bcl-xl and other Bcl-2 family proteins help preserve the mitochondrial potential by reducing mitochondrial membranes permeability (Suh et al., 2013), the findings that nicotine up-regulating Bcl-xl expression indicate that mitochondrial membrane permeability contributes to nicotine-enhanced cell viability and the neuroprotective effect.
SP600125 abolishes the effect of nicotine on the anti-apoptotic ability by inhibiting JNK phosphorylation in Raw264.7 and El4 cells (Wang et al., 2014). The treatment with amyloid β increases TNF-α expression via NF-κB pathway activation in the brain of TNF transgenic mice (Paouri et al., 2017). In this study, we treated SH-SY5Y cells with nicotine and menthol and found that as agonists of nAChR and TRPM8, nicotine and menthol increased Bcl-xl expression via JNK signaling (Fig. 6). These findings indicate that JNK kinase might be an upstream molecule of the nuclear factor kappa-B (NF-κB) pathway activation. The precise roles of JNK phosphorylation in nicotine and menthol-inhibited TNF-α expression and in the protective effect on cell viability need to be clarified.
The treatment with menthol contributes to nicotine intake and addiction via its TRPM8 in C57BL/6 mice (Fait et al., 2017; Fan et al., 2016). Menthol administration enhances nicotine’s effect by inducing neuron excitability and the expression of nicotinic acetylcholine receptors (Henderson et al., 2017). Compared with nicotine exposure alone, chronic (±)-menthol plus nicotine exposure increases β2 nAChR levels in the hypothalamus (Mulcahy et al., 2019). In the present study, despite the fact that the administrations of menthol and nicotine increased anti-apoptotic abilities by up-regulating Bcl-xl via JNK activation, the exact interactions between nAChR and TRPM8, as well as the up-regulation of β2 nAChR by menthol, require further studies.
Bhadania et al. (2012) documented that menthol administration (400 mg/kg, s.c.) successively for 10 days significantly improved spatial learning and memory ability of β-amyloid peptide-induced cognitive deficits by revealing that the higher dose of menthol showed improvement in both short-term and long-term memory. Also, when administered with nicotine, menthol showed psychoactive properties by affecting brain activity and behavior and facilitates dopamine-releasing effect in the mouse model (Thompson et al., 2018; Zhang et al., 2018). All these documents indicate that the effects of menthol and nicotine administration are attributable to the comprehensive function of the central nervous system. Aicher et al. (2003) found that 10−7 M nicotine has a better effect on surface molecules’ expression than 10−5–10−6 M nicotine does in dendritic cells. Our previous studies demonstrated that 10−6–10−7 M nicotine has a similar effect on the cross-presentation in dendritic cells and the neuroprotective ability in SH-SY5Y cells (Gao and Gu, 2007; Xue et al., 2014). In the present study, despite the short-term incubation of the SH-SY5Y cells with nicotine and menthol revealing an independent effect on cell viability, menthol’s actions on nAchR increasing nicotine bioavailability in the body were also documented (Wickham, 2015). Hence. the exact interactions among different doses of nicotine and menthol in either the short-term exposure or the long-term exposure in the body need to be elucidated.
In the present study, despite the fact that nicotine and menthol independently decreased cisplatin and amyloid β1-42 induced TNF-α expression and the collapse of mitochondrial membrane potential, the exact relationship between cytokine release and mitochondrial function still uncertain. A rapid increase of reactive oxygen species (ROS) occurs in acute ischemic stroke and overwhelms antioxidant defense, causing further tissue damage (Rodrigo et al., 2013). The reduction of antioxidants was documented to produce an oxidation-reduction imbalance and lead to mitochondrial dysfunction (Poprac et al., 2017). Whereas a lower concentration of ROS is essential for normal cellular signaling, a high concentration of ROS causes damage to cellular macromolecules and results in necrosis and apoptosis (Singh et al., 2019). Hence, despite the fact we found that nicotine and menthol independently alleviate cisplatin and amyloid β1-42-induced TNF-α expression and the collapse of mitochondrial membrane potential, further study should focus on the exact effects of nicotine and menthol on ROS production.
In conclusion, in the present study, as agonists of nAChR and TRPM8, nicotine and menthol exerted their neuroprotective effects by up-regulating anti-apoptotic protein Bcl-xl via JNK activation. These findings indicate that Bcl-xl and JNK might be potential therapeutic target molecules for the prevention and treatment of neurodegenerative diseases.
Acknowledgement: We thank Ruiling Chen and Yinan Wang for excellent Laboratory Equipment maintenance.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contribution: The authors confirm contribution to the paper as follows: Study conception and design: RB Ruan and J Liu; Western blot: ZM Xie; microscope observation: Q Liu; flow cytometry analyses: LX Zhang; CCK-8 assay: XK Han; analysis and interpretation of results: XY Liao; draft manuscript preparation: FG Gao. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: Not Applicable.
Funding Statement: This work was supported by the Young Elite Scientists Sponsorship Program of China Association for Science and Technology (No. 2016QNRC001) and the National Natural Science Foundation of China (Nos. 81771669, 81273203). The fund had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Aicher A, Heeschen C, Mohaupt M, Cooke JP, Zeiher AM, Dimmeler S (2003). Nicotine strongly activates dendritic cell-mediated adaptive immunity: Potential role for progression of atherosclerotic lesions. Circulation 107: 604–611. DOI 10.1161/01.CIR.0000047279.42427.6D. [Google Scholar] [CrossRef]
Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ (2017). From basic apoptosis discoveries to advanced selective Bcl-2 family inhibitors. Nature Reviews Drug Discovery 16: 273–284. DOI 10.1038/nrd.2016.253. [Google Scholar] [CrossRef]
Bhadania M, Joshi H, Patel P, Kulkarni VH (2012). Protective effect of menthol on β-amyloid peptide induced cognitive deficits in mice. European Journal of Pharmacology 681: 50–54. DOI 10.1016/j.ejphar.2012.01.035. [Google Scholar] [CrossRef]
Bono F, Mutti V, Fiorentini C, Missale C (2020). Dopamine D3 receptor heteromerization: Implications for neuroplasticity and neuroprotection. Biomolecules 10: 1016. DOI 10.3390/biom10071016. [Google Scholar] [CrossRef]
Dong Y, Bi W, Zheng K, Zhu E, Wang S et al. (2020). Nicotine prevents oxidative stress-induced hippocampal neuronal injury through α7-nAChR/Erk1/2 signaling pathway. Frontiers in Molecular Neuroscience 13: 961. DOI 10.3389/fnmol.2020.557647. [Google Scholar] [CrossRef]
Fait BW, Thompson DC, Mose TN, Jatlow P, Jordt SE, et al. (2017). Menthol disrupts nicotine’s psychostimulant properties in an age and sex-dependent manner in C57BL/6J mice. Behavioural Brain Research 334: 72–77. DOI 10.1016/j.bbr.2017.07.027. [Google Scholar] [CrossRef]
Fan L, Balakrishna S, Jabba SV, Bonner PE, Taylor SR et al. (2016). Menthol decreases oral nicotine aversion in C57BL/6 mice through a TRPM8-dependent mechanism. Tobacco Control 25: ii50–ii54. DOI 10.1136/tobaccocontrol-2016-053209. [Google Scholar] [CrossRef]
Gao FG, Gu JR (2007). Ex vivo nicotine stimulation augments the efficacy of therapeutic bone marrow-derived dendritic cell vaccination. Clinical Cancer Research 13: 3706–3712. DOI 10.1158/1078-0432.CCR-07-0028. [Google Scholar] [CrossRef]
Go YY, Mun JY, Chae SW, Chang J, Song JJ (2020). Comparison between in vitro toxicities of tobacco-and menthol-flavored electronic cigarette liquids on human middle ear epithelial cells. Scientific Reports 10: 83. DOI 10.1038/s41598-020-59290-y. [Google Scholar] [CrossRef]
Henderson BJ, Wall TR, Henley BM, Kim CH, Mckinney S, Lester HA (2017). Menthol enhances nicotine reward-related behavior by potentiating nicotine-induced changes in nAChR function, nAChR upregulation, and DA neuron excitability. Neuropsychopharmacology 42: 2285–2291. DOI 10.1038/npp.2017.72. [Google Scholar] [CrossRef]
Herr AB (2018). Evolution of an allosteric “off switch” in apoptotic caspases. Journal of Biological Chemistry 293: 5462–5463. DOI 10.1074/jbc.H118.002379. [Google Scholar] [CrossRef]
Islam MS, Ahmed MU, Sayeed MSB, Al Maruf A, Mostofa A et al. (2013). Lung cancer risk in relation to nicotinic acetylcholine receptor, CYP2A6 and CYP1A1 genotypes in the Bangladeshi population. Clinica Chimica Acta 416: 11–19. DOI 10.1016/j.cca.2012.11.011. [Google Scholar] [CrossRef]
Jiang YN, Ni XY, Yan HQ, Shi L, Lu NN et al. (2019). Interleukin 6-triggered ataxia-telangiectasia mutated kinase activation facilitates epithelial-to-mesenchymal transition in lung cancer by upregulating vimentin expression. Experimental Cell Research 381: 165–171. DOI 10.1016/j.yexcr.2019.05.011. [Google Scholar] [CrossRef]
Kale J, Liu Q, Leber B, Andrews DW (2012). Shedding light on apoptosis at subcellular membranes. Cell 151: 1179–1184. DOI 10.1016/j.cell.2012.11.013. [Google Scholar] [CrossRef]
Ke SZ, Ni XY, Zhang YH, Wang YN, Wu B, Gao FG (2013). Camptothecin and cisplatin upregulate ABCG2 and MRP2 expression by activating the ATM/NF-κB pathway in lung cancer cells. International Journal of Oncology 42: 1289–1296. DOI 10.3892/ijo.2013.1805. [Google Scholar] [CrossRef]
Krishna A, Biryukov M, Trefois C, Antony PM, Hussong R et al. (2014). Systems genomics evaluation of the SH-SY5Y neuroblastoma cell line as a model for Parkinson’s disease. BMC Genomics 15: 1154. DOI 10.1186/1471-2164-15-1154. [Google Scholar] [CrossRef]
Kvansakul M, Caria S, Hinds MG (2017). The Bcl-2 family in host-virus interactions. Viruses 9: 290. DOI 10.3390/v9100290. [Google Scholar] [CrossRef]
Lombardo S, Maskos U (2015). Role of the nicotinic acetylcholine receptor in Alzheimer’s disease pathology and treatment. Neuropharmacology 96: 255–262. DOI 10.1016/j.neuropharm.2014.11.018. [Google Scholar] [CrossRef]
Mulcahy MJ, Huard SM, Paulo JA, Wang JH, Mckinney S et al. (2019). Brain region-specific nAChR and associated protein abundance alterations following chronic nicotine and/or menthol exposure. Journal of Proteome Research 19: 36–48. DOI 10.1021/acs.jproteome.9b00286. [Google Scholar] [CrossRef]
Muruganathan U, Srinivasan S, Vinothkumar V (2017). Antidiabetogenic efficiency of menthol, improves glucose homeostasis and attenuates pancreatic β-cell apoptosis in streptozotocin-nicotinamide induced experimental rats through ameliorating glucose metabolic enzymes. Biomedicine & Pharmacotherapy 92: 229–239. DOI 10.1016/j.biopha.2017.05.068. [Google Scholar] [CrossRef]
Nalivaeva NN, Turner AJ (2013). The amyloid precursor protein: A biochemical enigma in brain development, function and disease. FEBS Letters 587: 2046–2054. DOI 10.1016/j.febslet.2013.05.010. [Google Scholar] [CrossRef]
Nonnemaker J, Feirman SP, Macmonegle A, Ambrose BK, Jackson KJ et al. (2019). Examining the role of menthol cigarettes in progression to established smoking among youth. Addictive Behaviors 98: 106045. DOI 10.1016/j.addbeh.2019.106045. [Google Scholar] [CrossRef]
Paouri E, Tzara O, Kartalou GI, Zenelak S, Georgopoulos S (2017). Peripheral tumor necrosis factor-alpha (TNF-α) modulates amyloid pathology by regulating blood-derived immune cells and glial response in the brain of AD/TNF transgenic mice. Journal of Neuroscience 37: 5155–5171. DOI 10.1523/JNEUROSCI.2484-16.2017. [Google Scholar] [CrossRef]
Patel H, Mcintire J, Ryan S, Dunah A, Loring R (2017). Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-κB pathway and activation of the Nrf2 pathway. Journal of Neuroinflammation 14: 399. DOI 10.1186/s12974-017-0967-6. [Google Scholar] [CrossRef]
Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G (2016). Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY) 8: 603–619. DOI 10.18632/aging.100934. [Google Scholar] [CrossRef]
Pogocki D, Ruman T, Danilczuk M, Danilczuk M, Celuch M, Wałajtys-Rode E (2007). Application of nicotine enantiomers, derivatives and analogues in therapy of neurodegenerative disorders. European Journal of Pharmacology 563: 18–39. DOI 10.1016/j.ejphar.2007.02.038. [Google Scholar] [CrossRef]
Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M (2017). Targeting free radicals in oxidative stress-related human diseases. Trends in Pharmacological Sciences 38: 592–607. DOI 10.1016/j.tips.2017.04.005. [Google Scholar] [CrossRef]
Prasad H, Rao R (2015). The Na+/H+ exchanger NHE6 modulates endosomal pH to control processing of amyloid precursor protein in a cell culture model of Alzheimer disease. Journal of Biological Chemistry 290: 5311–5327. DOI 10.1074/jbc.M114.602219. [Google Scholar] [CrossRef]
Rodrigo R, Fernández-Gajardo R, Gutiérrez R, Manuel Matamala J Carrasco R et al. (2013). Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS & Neurological Disorders Drug Targets 12: 698–714. [Google Scholar]
Rosenberg RN, Lambracht-Washington D (2013). DNA Aβ42 vaccination as possible alternative immunotherapy for Alzheimer disease. JAMA Neurology 70: 772–773. DOI 10.1001/jamaneurol.2013.1502. [Google Scholar] [CrossRef]
Rozza AL, de Faria FM, Brito ARS, Pellizzon CH (2014). The gastroprotective effect of menthol: Involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PLoS One 9: e86686. DOI 10.1371/journal.pone.0086686. [Google Scholar] [CrossRef]
Sakamuru S, Attene-Ramos MS, Xia M (2016). Mitochondrial membrane potential assay. High-Throughput Screening Assays in Toxicology. New York, NY: Humana Press. [Google Scholar]
Singh A, Kukreti R, Saso L, Kukreti S (2019). Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 24: 1583. DOI 10.3390/molecules24081583. [Google Scholar] [CrossRef]
Suh DH, Kim MK, Kim HS, Chung HH, Song YS (2013). Mitochondrial permeability transition pore as a selective target for anti-cancer therapy. Frontiers in Oncology 3: 00041. DOI 10.3389/fonc.2013.00041. [Google Scholar] [CrossRef]
Thompson MF, Poirier GL, Dávila-García MI, Huang W, Tam K et al. (2018). Menthol enhances nicotine-induced locomotor sensitization and in vivo functional connectivity in adolescence. Journal of Psychopharmacology 32: 332–343. DOI 10.1177/0269881117719265. [Google Scholar] [CrossRef]
van Opdenbosch N, Lamkanfi M (2019). Caspases in cell death, inflammation, and disease. Immunity 50: 1352–1364. DOI 10.1016/j.immuni.2019.05.020. [Google Scholar] [CrossRef]
Vringer E, Tait SW (2019). Mitochondria and inflammation: Cell death heats up. Frontiers in Cell and Developmental Biology 7: 6627. DOI 10.3389/fcell.2019.00100. [Google Scholar] [CrossRef]
Wang YY, Liu Y, Ni XY, Bai ZH, Chen QY et al. (2014). Nicotine promotes cell proliferation and induces resistance to cisplatin by α7 nicotinic acetylcholine receptor-mediated activation in Raw264.7 and El4 cells. Oncology Reports 31: 1480–1488. DOI 10.3892/or.2013.2962. [Google Scholar] [CrossRef]
Wickham R (2015). Focus: Addiction: How menthol alters tobacco-smoking behavior: A biological perspective. Yale Journal of Biology and Medicine 88: 279. [Google Scholar]
Xicoy H, Wieringa B, Martens GJ (2017). The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Molecular Neurodegeneration 12: 384. DOI 10.1186/s13024-017-0149-0. [Google Scholar] [CrossRef]
Xue MQ, Liu XX, Zhang YL, Gao FG (2014). Nicotine exerts neuroprotective effects against β-amyloid-induced neurotoxicity in SH-SY5Y cells through the Erk1/2-p38-JNK-dependent signaling pathway. International Journal of Molecular Medicine 33: 925–933. DOI 10.3892/ijmm.2014.1632. [Google Scholar] [CrossRef]
Yan HQ, Huang XB, Ke SZ, Jiang YN, Zhang YH et al. (2014). Interleukin 6 augments lung cancer chemotherapeutic resistance via ataxia-telangiectasia mutated/NF-kappaB pathway activation. Cancer Science 105: 1220–1227. DOI 10.1111/cas.12478. [Google Scholar] [CrossRef]
Yang W, Ma K, Chen X, Shi L, Bu G et al. (2014). Mitogen-activated protein kinase signaling pathways are involved in regulating α7 nicotinic acetylcholine receptor-mediated amyloid-β uptake in SH-SY5Y cells. Neuroscience 278: 276–290. DOI 10.1016/j.neuroscience.2014.08.013. [Google Scholar] [CrossRef]
Zhang M, Harrison E, Biswas L, Tran T, Liu X (2018). Menthol facilitates dopamine-releasing effect of nicotine in rat nucleus accumbens. Pharmacology Biochemistry and Behavior 175: 47–52. DOI 10.1016/j.pbb.2018.09.004. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |