DOI:10.32604/biocell.2022.018026

| BIOCELL DOI:10.32604/biocell.2022.018026 |  |
| Viewpoint |
Implant surface features as key role on cell behavior
1Federal University of Rio de Janeiro, Rio de Janeiro, 21941-617, Brazil
2São Paulo State University, Araraquara, 14801-930, Brazil
*Address correspondence to: Erica Dorigatti de Avila, erica.avila@unesp.br
Received: 24 June 2021; Accepted: 26 July 2021
Abstract: It has been recognized that physical and chemical properties of biomaterial surfaces mediate the quality of extracellular matrix (ECM) that may affect cell behaviors. In nature, ECM is a heterogeneous three-dimensional superstructure formed by three major components, glycosaminoglycan, glycoconjugate, and protein, that anchors cellular compartments in tissues and regulates the function and the behavior of cells. Changes in the biointerface alter the quality of ECM and morphology through cell surface receptors, which, in turn, enable it to trigger specific cell signaling and different cellular responses. In fact, a number of strategies have been used to improve the functionality of surfaces and direct cell behavior through precisely designed environments. Herein, we aimed to discuss, through a science-based viewpoint, the biomaterial surface features on cell behavior and analyze the impact of cell physical modification on dental implant development.
Keywords: Biomaterial; Cell adhesion; Surface chemistry; Surface topography
Overall surface features are responsible for determining cellular phenotype, behavior and extracellular matrix (ECM) secretion, and therefore can be considered as the ruler of the environment (Diener et al., 2005). In turn, ECM is a heterogeneous three-dimensional superstructure formed by three major components, glycosaminoglycan, glycoconjugate, and protein, that anchors cellular compartments in tissues and regulates the function and the behavior of cells (Kusindarta and Wihadmadyatami, 2018).
Within the dental implant field, biomaterials are developed to restore, maintain or improve damaged tissues. In this sense, it is expected that the dental implanted biomaterials stimulate the ECM production and its replacement by the host tissue (Diener et al., 2005; Dvir et al., 2011). However, for tissue regeneration successfully takes place and contributes to the host cellular and tissue responses, it is necessary to understand the required biomaterial surface properties of implanted medical devices according to the native cell profile (Amani et al., 2019).
Surface features might induce the total number of cells, their morphology, size, cytoskeletal organization and nuclearity, through the quality of proteins immediately adsorbed onto the implanted material (Amani et al., 2019). After a few seconds of biomaterial implantation, the surface becomes rapidly covered by a variety of proteins from blood and interstitial fluids, forming the blood clot (Kikuchi and Okano, 2005; Anselme et al., 2010). Hence, the adsorbed protein layer is responsible for modifying the surface chemistry and energy of the biomaterial implanted substrate and, therefore, to influence the cell layer organization. In other words, properties, such as roughness, wettability and surface free energy (SFE), drive the quality and quantity of proteins adsorbed, as well as protein composition; and the adsorbed protein represents a key mediator of cell’s surface receptors.
In fact, the absorbed protein is a key mediator of cell’s surface receptors (Anselme et al., 2010). Just after the protein layer formation onto implant material, transmembrane linkers, known as integrins, act facilitating the interactions between the cytoskeleton from cells and the protein layer from the ECM (Siebers et al., 2005; Keselowsky et al., 2007). Remarkably, differences on cell phenotypes might affect and alter gene expression, and consequently, control how cells will respond to the respective environment.
In view of the aforementioned remarks, a crucial point is to control the structure of the adsorbed layer formed on biomaterial through the physical and chemical surface modification. Therefore, understanding how cells interact with each surface profile enables the creation of straightforward strategies and improves the biological performance of biomaterial devices (Feldmann et al., 2013). Herein, we aimed to discuss through a science-based viewpoint the biomaterial surface features on cell behavior and analyze the impact of cell physical modification on dental implant development.
Nowadays, it is already known that biomaterial profile might directly affect cells spreading, migration, proliferation and differentiation, and favor or damage surface-cell interaction. All physicochemical properties inherent to each type of material are initially responsible for the surface-protein interactions (Anselme et al., 2010; Ayala et al., 2011; Rahmati et al., 2020). Therefore, the quality of cell attachment is driven by the structure of the surface exposed to several molecular species during the protein adsorption process, which means that any material construction must consider such physical and chemical material properties (Ayala et al., 2011). Within the dental implant field, this knowledge has affected the development of surfaces to favor and increase the implants’ survival.
Surface modification can be conveniently achieved by modification of original material through physical (roughness) (Xu et al., 2004; Zhou et al., 2015) and chemical (wetting and SFE) properties or by coating construction onto original substrate. (Lim et al., 2008; Arima and Iwata, 2007).
Regarding, the first possibility, we can share an important finding regarding the role of specific surface roughness on osteoclast phenotypes. In the past, osteoclast’s functions were widely accepted for their ability to resorb bone only. However, studies have revealed that osteoclasts also contribute to bone formation by communicating with osteoblastic cells and osteogenic differentiation through the secretion of coupling factors (Zhang et al., 2018; Sims and Martin, 2014). In this process, surface roughness is responsible for different functional states of osteoclasts and modulates osteogenic differentiation through the induction of different cell morphologies (Fig. 1). Interesting findings have revealed a small number of osteoclast cells with higher number of nuclei per osteoclast on smooth surfaces. By contrast, a high number of osteoclast cells with a low number of nuclei were found on rough surfaces (Zhang et al., 2018). These differences on cell morphology reflect in the catabolic enzyme activity and gene expression of osteoclastogenic markers, which means that the increase in surface roughness, around 1−2 μm, decrease osteoclast-associated features, such as resorption capacity.
In this sense, porosity and pore size in polymeric biomaterials also play critical roles in determining cellular phenotype onto the surface (Oliviero et al., 2012; Perez and Mestres, 2016).
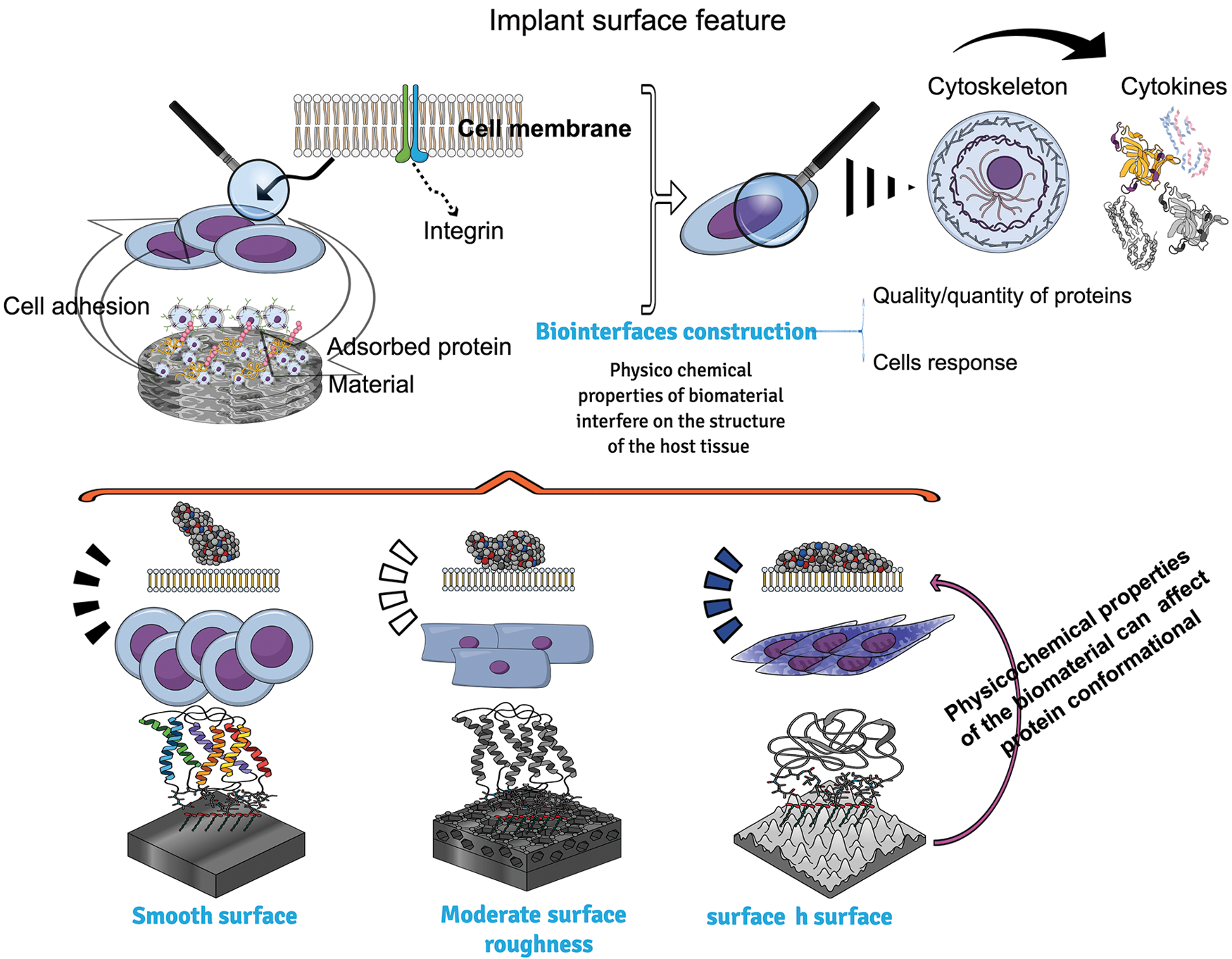
Figure 1: An overview of biomaterial surface features and how roughness can affect cell behavior. Different surface topographies may interfere on the quality of protein adhered onto them. The adsorbed protein layer is responsible to modify the surface chemistry and energy of substrate of biomaterial implanted and therefore to influence the cell layer organization. Cells attached on the surface express cytoskeleton proteins and integrin, which interact together to regulate gene expression.
The difference in pore size will determine the quality of cell growth throughout extensive and rapid angiogenesis and the quantity of protein adsorbed. Indeed, the ideal pore size favorable for application depends on the tissue that the biomaterial is intended to replace. For bone regeneration, for example, it was found that pore diameters above 300 µm are advantageous for bone migration (Murphy et al., 2010). By contrast, for soft tissue, it was found that porosity of 60 µm increases the number of cells and tissue infiltration and provides excellent deposition of collagen and elastin, and superior fibrotic tissue distribution (Osorio et al., 2010). From the soft tissue perspective, it was found that smooth surfaces stimulate different kinds of cell responses. Fibroblast and epithelial cells, for example, attach better to flat surfaces in the absence of roughness, lumps, or holes (Xu et al., 2004; Cochran et al., 1994).
Apart from roughness, surface wettability and surface free energy are important chemical factors, which also determine the surface protein adsorption (Guo et al., 2016), the quality of cell–material interaction and subsequent cell attachment (Cai et al., 2020; Alves et al., 2010). Indeed, protein adsorption is the first event after the implantation of a biomaterial into the body and its subsequent contact with biological fluids (Olivares-Navarrete et al., 2008). Each protein is composed of at least 20 amino acids. Each amino acid has a general core network of {–NH–CaHR–CO–}, where R describes a specific functional property. Depending on the R structure, the amino acids can disclose nonpolar, polar, and charged amino acids, and therefore, it determines the biological response through the affinity by specific types of cells (Hirsh et al., 2013). It has been recognized that super hydrophilic surfaces (contact angle between surface and water less than 5°) accelerate and enhance fibronectin and albumin adsorption and, consequently, osteoblast cells attachment. It occurs because both proteins and osteoblastic cells are negatively charged and a super hydrophilic surface exhibits cationic sites, facilitating the interaction (Aita et al., 2009).
Although surface wettability exerts different effects on the adhesion of different types of cells, it is known that overall cells are more likely to adhere to hydrophilic surfaces rather than hydrophobic ones (Wei et al., 2007; Gittens et al., 2014). It can be also explained by the composition of the cell membranes, which are composed by phospholipids from lipid bilayer, embedded proteins, and water. Even though phospholipids are composed by amphipathic molecules (molecules that have both polar and nonpolar parts), polar groups are always prevalent. It means that with the increase of wettability, hydroxyl and carboxyl groups from material surface might attract the cell surface lipids and ions through intermolecular force (hydrogen bonds), and thus improve the adhesion, growth, and cell proliferation.
Hydrophilic surfaces interact closely with blood and biological fluids, allowing normal protein adsorption in a conformation that exposes adhesion motifs and enhances bone cell adhesion (Gittens et al., 2014; Huang et al., 2012). This knowledge has made great contributions to the advancement of new commercial dental implant surfaces. From these discoveries, overall dental implant companies have been racing to fabricate the most hydrophilic surface with superior long-term performance. On the flip side, super-hydrophobic surfaces, with a contact angle of more than 150° are unfavorable to cell growth and adhesion (Cai et al., 2020).
Contact angle between surface and wetting agent reflects the chemical nature of the tested material and consequently how wettable is that surface according to the characteristics of the liquid used for such evaluation. The numerical values corresponding to the contact angle are used to measure the surface free energy (SFE). Surface free energy is defined as the available energy from atoms displaced from the bulk of a material to the surface after intermolecular bonds disruption. The type of dangling bonds exposed at a material’s surface determines the SFE categories, i.e., high or low. Materials, which are covalently, ionically, or metallically bonded, disclose high SFE and materials that are bonded by van der Waals bonds disclose low SFE (Gentleman and Gentleman, 2014). Similarly to wetting properties, SFE also affects protein adsorption and controls the early stages of cell adhesion and tissue formation at the material interface; however, the quality of surface-cell interaction will always depend on the chemical nature of local cells analyzed (Gentleman and Gentleman, 2014).
Focusing on chemical substrate, different types of materials have been investigated as implant surface possibilities. Titanium (Ti) and its alloys are still widely used for medical and dental implant field (Rossi et al., 2021; Tendero et al., 2021). The reason for that assumption is due to inherent Ti properties such as excellent corrosion resistance and good biocompatibility (Bosshardt et al., 2017). Zirconia and polyetheretherketone (PEEK) have been also tested in order to improve the esthetic, the performance of implant materials and to create a biomimetic cellular microenvironment (Dong et al., 2020; Rigolin et al., 2017; Guillot et al., 2016). PEEK-based coating has been applied to improve mechanical strength and reduce elastic modulus, in case of zirconia for example, as well as to confer good wear resistance and chemical stability (Qin et al., 2021).
Controlling cell behaviors can be also achieved by polymeric coatings construction (Alves et al., 2010; Tilkin et al., 2020; Chen et al., 2018). Within the tissue regeneration field, polymeric materials have emerged to fabricate artificial biomaterials that can effectively mimic cell–ECM interactions. Among them, poly(hexamethyldisiloxane) (PHMDSO) may exhibit different surface wettability (from hydrophobic to superhydrophilic) by altering the duration of oxygen-plasma treatment, according to the convenience. The greater surface hydrophilicity, the better adhesion and spreading fibroblast cells (Wei et al., 2007). Others important polymers used to film construction are poly-l-lactic acid and polystyrene (PLLA, PS). Both biomaterials were found to stimulate osteoblastic cell adhesion and spreading (Lim et al., 2005). With regards to responsive polymers, thermoresponsive polymers, such as poly[oligo (ethylene glycol) methacrylates] (POEGMA) are frequently used to manipulate cell adhesion (Nagase et al., 2009). In this sense, several methods and strategies to develop polymeric surfaces can be highlight: layer-by-layer (LbL) assembly (de Avila et al., 2019; Verza et al., 2021), lithographic surface modification techniques, electrospun fibers, spin coating, 3D bioprinting, self-assembled monolayers (SAMs) and polymer brush (Cai et al., 2020).
After all, independent of the technology used to construct polymeric thin films, the central idea is to create a non-cytotoxic environment by decorating the polymeric material with suitable chemical, mechanical, and topographical cues for controlling cell adhesion, stem cell differentiation, and cell-cell interactions.
Vision of the future
The development of biomaterials needs to focus on the biointerface construction to match the structure of the host tissue and to meet the biophysical and biochemical requirements of specific cell types. In order to do that, it is critical to manipulate the surface by physical and chemical parameters to achieve the clinical purpose of the biomaterial. From the clinical standpoint, dental implant survival has advanced from a fairly unpredictable procedure, to a very predictable practice. Two factors, which have been claimed to influence the biological response, are physical and chemical modifications of implant surface properties, which provide an effective and straightforward strategy to improve cell attachment, spreading, and differentiation.(Schneider et al., 2003) Focusing on polymeric coating construction, up to date, the knowledge regarding this subject has been limited to in vitro and in vivo investigations.
However, surface-cell interaction possibilities have encouraged the development of desired surfaces through polymeric surfaces with rational designs in chemical and topographical cues for controlling cell behaviors. Above all, the understanding regarding all factors that generate a response to signal cell events for subsequent control of cell behaviors and functions is essential for materials and life sciences, such as advanced biomedical engineering and tissue engineering.
Acknowledgement: Figure created in the Mind the Graph platform (www.mindthegraph.com).
Availability of Data and Materials: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Authors’ Contribution: The authors confirm contribution to the paper as follows: study conception and design: RSM and EDA; draft manuscript preparation: RSM, MMAP and EDA. All authors reviewed the article text and approved the final version of the manuscript.
Funding Statement: Pereira MMA is currently supported by grant provided by Coordination for the Improvement of Higher Education Personnel (CAPES #88887.573209/2020-00) finance code 001. de Molon RS was supported by grant provided by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) Grant #88887.583248/2020-00. de Avila ED was supported by grant provided by FAPESP (The São Paulo Research Foundation) grant #2018/20719-3.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Aita H, Hori N, Takeuchi M, Suzuki T, Yamada M, Anpo M, Ogawa T (2009). The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 30: 1015–1025. DOI 10.1016/j.biomaterials.2008.11.004. [Google Scholar] [CrossRef]
Alves NM, Pashkuleva I, Reis RL, Mano JF (2010). Controlling cell behavior through the design of polymer surfaces. Small 6: 2208–2220. DOI 10.1002/smll.201000233. [Google Scholar] [CrossRef]
Amani H, Arzaghi H, Bayandori M, Dezfuli AS, Pazoki-Toroudi H, Shafiee A, Moradi L (2019). Controlling cell behavior through the design of biomaterial surfaces: A focus on surface modification techniques. Advanced Materials Interfaces 6: 1900572. DOI 10.1002/admi.201900572. [Google Scholar] [CrossRef]
Anselme K, Ponche A, Bigerelle M (2010). Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 2: Biological aspects. The Proceedings of the Institution of Mechanical Engineers, Part H 224: 1487–1507. [Google Scholar]
Arima Y, Iwata H (2007). Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 28: 3074–3082. DOI 10.1016/j.biomaterials.2007.03.013. [Google Scholar] [CrossRef]
Ayala R, Zhang C, Yang D, Hwang Y, Aung A, Shroff SS, Arce FT, Lal R, Arya G, Varghese S (2011). Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials 32: 3700–3711. DOI 10.1016/j.biomaterials.2011.02.004. [Google Scholar] [CrossRef]
Bosshardt DD, Chappuis V, Buser D (2017). Osseointegration of titanium, titanium alloy and zirconia dental implants: current knowledge and open questions. Periodontology 2000 73: 22–40. DOI 10.1111/prd.12179. [Google Scholar] [CrossRef]
Cai SX, Wu CX, Yang WG, Liang WF, Yu HB, Liu LQ (2020). Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnology Reviews 9: 971–989. DOI 10.1515/ntrev-2020-0076. [Google Scholar] [CrossRef]
Chen LN, Yan C, Zheng ZJ (2018). Functional polymer surfaces for controlling cell behaviors. Materials Today 21: 38–59. DOI 10.1016/j.mattod.2017.07.002. [Google Scholar] [CrossRef]
Cochran D, Simpson J, Weber HP, Buser D (1994). Attachment and growth of periodontal cells on smooth and rough titanium. International Journal of Oral and Maxillofacial Implants 9: 289–297. [Google Scholar]
de Avila ED, Castro AGB, Tagit O, Krom BP, Löwik D, van Wellf V, Bannenberg LJ, Vergani CE, van den Beucken JJJP (2019). Anti-bacterial efficacy via drug-delivery system from layer-by-layer coating for percutaneous dental implant components. Applied Surface Science 488: 194–204. DOI 10.1016/j.apsusc.2019.05.154. [Google Scholar] [CrossRef]
Diener A, Nebe B, Luthen F, Becker P, Beck U, Neumann HG, Rychly J (2005). Control of focal adhesion dynamics by material surface characteristics. Biomaterials 26: 383–392. DOI 10.1016/j.biomaterials.2004.02.038. [Google Scholar] [CrossRef]
Dong H, Liu H, Zhou N, Li Q, Yang G, Chen L, Mou Y (2020). Surface modified techniques and emerging functional coating of dental implants. Coatings 10: 1012. DOI 10.3390/coatings10111012. [Google Scholar] [CrossRef]
Dvir T, Timko BP, Kohane DS, Langer R (2011). Nanotechnological strategies for engineering complex tissues. Nature Nanotechnology 6: 13–22. DOI 10.1038/nnano.2010.246. [Google Scholar] [CrossRef]
Feldmann EM, Sundberg JF, Bobbili B, Schwarz S, Gatenholm P, Rotter N (2013). Description of a novel approach to engineer cartilage with porous bacterial nanocellulose for reconstruction of a human auricle. The Journal of Biomaterials Applications 28: 626–640. DOI 10.1177/0885328212472547. [Google Scholar] [CrossRef]
Gentleman MM, Gentleman E (2014). The role of surface free energy in osteoblast-biomaterial interactions. International Materials Reviews 59: 417–429. DOI 10.1179/1743280414Y.0000000038. [Google Scholar] [CrossRef]
Gittens RA, Scheideler L, Rupp F, Hyzy SL, Geis-Gerstorfer J, Schwartz Z, Boyan BD (2014). A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomaterialia 10: 2907–2918. DOI 10.1016/j.actbio.2014.03.032. [Google Scholar] [CrossRef]
Guillot R, Pignot-Paintrand I, Lavaud J, Decambron A, Bourgeois E, Josserand V, Logeart-Avramoglou D, Viguier E, Picart C (2016). Assessment of a polyelectrolyte multilayer film coating loaded with BMP-2 on titanium and PEEK implants in the rabbit femoral condyle. Acta Biomaterialia 36: 310–322. DOI 10.1016/j.actbio.2016.03.010. [Google Scholar] [CrossRef]
Guo S, Zhu X, Li M, Shi L, Ong JL, Janczewski D, Neoh KG (2016). Parallel control over surface charge and wettability using polyelectrolyte architecture: Effect on protein adsorption and cell adhesion. ACS Applied Materials & Interfaces 8: 30552–30563. DOI 10.1021/acsami.6b09481. [Google Scholar] [CrossRef]
Hirsh SL, McKenzie DR, Nosworthy NJ, Denman JA, Sezerman OU, Bilek MM (2013). The Vroman effect: Competitive protein exchange with dynamic multilayer protein aggregates. Colloids and Surfaces B: Biointerfaces 103: 395–404. DOI 10.1016/j.colsurfb.2012.10.039. [Google Scholar] [CrossRef]
Huang Q, Lin L, Yang Y, Hu R, Vogler EA, Lin C (2012). Role of trapped air in the formation of cell-and-protein micropatterns on superhydrophobic/superhydrophilic microtemplated surfaces. Biomaterials 33: 8213–8220. DOI 10.1016/j.biomaterials.2012.08.017. [Google Scholar] [CrossRef]
Keselowsky BG, Wang L, Schwartz Z, Garcia AJ, Boyan BD (2007). Integrin alpha(5) controls osteoblastic proliferation and differentiation responses to titanium substrates presenting different roughness characteristics in a roughness independent manner. Journal of Biomedical Materials Research Part A 80: 700–710. [Google Scholar]
Kikuchi A, Okano T (2005). Nanostructured designs of biomedical materials: Applications of cell sheet engineering to functional regenerative tissues and organs. Journal of Controlled Release 101: 69–84. DOI 10.1016/j.jconrel.2004.08.026. [Google Scholar] [CrossRef]
Kusindarta DL, Wihadmadyatami H (2018). The role of extracellular matrix in tissue regeneration. In: Abdelhay H, Kaoud E (eds.Tissue Regeneration, pp. 65–73. Norderstedt: Books on Demand. [Google Scholar]
Lim JY, Hansen JC, Siedlecki CA, Hengstebeck RW, Cheng J, Winograd N, Donahue HJ (2005). Osteoblast adhesion on poly(L-lactic acid)/polystyrene demixed thin film blends: Effect of nanotopography, surface chemistry, and wettability. Biomacromolecules 6: 3319–3327. DOI 10.1021/bm0503423. [Google Scholar] [CrossRef]
Lim JY, Shaughnessy MC, Zhou Z, Noh H, Vogler EA, Donahue HJ (2008). Surface energy effects on osteoblast spatial growth and mineralization. Biomaterials 29: 1776–1784. DOI 10.1016/j.biomaterials.2007.12.026. [Google Scholar] [CrossRef]
Murphy CM, Haugh MG, O’Brien FJ (2010). The effect of mean pore size on cell attachment, prolifetion and migration in collagenglycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 31: 461–466. DOI 10.1016/j.biomaterials.2009.09.063. [Google Scholar] [CrossRef]
Nagase K, Kobayashi J, Okano T (2009). Temperature-responsive intelligent interfaces for biomolecular separation and cell sheet engineering. Journal of the Royal Society Interface 6: S293–309. DOI 10.1098/rsif.2008.0499.focus. [Google Scholar] [CrossRef]
Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, Chaudhri RA, Ornoy A, Boyan BD, Schwartz Z (2008). Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proceedings of the National Academy of Sciences USA 105: 15767–15772. DOI 10.1073/pnas.0805420105. [Google Scholar] [CrossRef]
Oliviero O, Ventre M, Netti PA (2012). Functional porous hydrogels to study angiogenesis under the effect of controlled release of vascular endothelial growth factor. Acta Biomaterialia 8: 3294–3301. DOI 10.1016/j.actbio.2012.05.019. [Google Scholar] [CrossRef]
Osorio M, Cañas A, Puerta J, Díaz L, Naranjo T, Ortiz I, Castro C (2010). Ex vivo and in vivo biocompatibility assessment (blood and tissue) of three-dimensional bacterial nanocellulose biomaterials for soft tissue implants. Scientific Reports 9: 1–14. [Google Scholar]
Perez RA, Mestres G (2016). Role of pore size and morphology in musculo-skeletal tissue regeneration. Materials Science & Engineering: C 61: 922–939. DOI 10.1016/j.msec.2015.12.087. [Google Scholar] [CrossRef]
Qin W, Ma J, Liang Q, Li J, Tang B (2021). Tribological, cytotoxicity and antibacterial properties of graphene oxide/carbon fibers/polyetheretherketone composite coatings on Ti-6Al-4V alloy as orthopedic/dental implants. Journal of the Mechanical Behavior of Biomedical Materials 122: 104659. [Google Scholar]
Rahmati M, Silva EA, Reseland JE, Heyward CA, Haugen HJ (2020). Biological responses to physicochemical properties of biomaterial surface. Chemical Society Reviews 49: 5178–5224. DOI 10.1039/D0CS00103A. [Google Scholar] [CrossRef]
Rigolin MSM, de Avila ED, Basso FG, Hebling J, de S Costa CA, Mollo Junior FA (2017). Effect of different implant abutment surfaces on OBA-09 epithelial cell adhesion. Microscopy Research and Technique 80: 1304–1309. DOI 10.1002/jemt.22941. [Google Scholar] [CrossRef]
Rossi MC, Bayerlein DL, Brandão JS, Pfeifer J, Rosa G, Silva WM, Martinez LG, Saeki MJ, Alves A (2021). Physical and biological characterizations of TiNbSn/(Mg) system produced by powder metallurgy for use as prostheses material. Journal of the Mechanical Behavior of Biomedical Materials 115: 104260. DOI 10.1016/j.jmbbm.2020.104260. [Google Scholar] [CrossRef]
Schneider GB, Perinpanayagam H, Clegg M, Zaharias R, Seabold D, Keller J, Stanford C (2003). Implant surface roughness affects osteoblast gene expression. Journal of Dental Research 82: 372–376. DOI 10.1177/154405910308200509. [Google Scholar] [CrossRef]
Siebers MC, ter Brugge PJ, Walboomers XF, Jansen JA (2005). Integrins as linker proteins between osteoblasts and bone replacing materials. A critical review. Biomaterials 26: 137–146. DOI 10.1016/j.biomaterials.2004.02.021. [Google Scholar] [CrossRef]
Sims NA, Martin TJ (2014). Coupling the activities of bone formation and resorption: A multitude of signals within the basic multicellular unit. BoneKEy Report 3: 481. DOI 10.1038/bonekey.2013.215. [Google Scholar] [CrossRef]
Tendero I, Rossi MC, Viera M, Amado Jé M, Tobar MD, Vicente Á, Yañez A, Amigó V (2021). Laser surface modification in Ti-xNb-yMo alloys prepared by powder metallurgy. Metals 11: 367. DOI 10.3390/met11020367. [Google Scholar] [CrossRef]
Tilkin RG, Regibeau N, Lambert SD, Grandfils C (2020). Correlation between surface properties of polystyrene and polylactide materials and fibroblast and osteoblast cell line behavior: A critical overview of the literature. Biomacromolecules 21: 1995–2013. DOI 10.1021/acs.biomac.0c00214. [Google Scholar] [CrossRef]
Verza BS, van den Beucken JJJP, Brandt JV, Jafelicci Junior M, Barão VAR, Piazza RD, Tagit O, Spolidorio DMP, Vergani CE, de Avila ED (2021). A long-term controlled drug-delivery with anionic beta cyclodextrin complex in layer-by-layer coating for percutaneous implants devices. Carbohydrate Polymers 257: 117604. DOI 10.1016/j.carbpol.2020.117604. [Google Scholar] [CrossRef]
Wei J, Yoshinari M, Takemoto S, Hattori M, Kawada E, Liu B, Oda Y (2007). Adhesion of mouse fibroblasts on hexamethyldisiloxane surfaces with wide range of wettability. Journal of Biomedical Materials Research Part B: Applied Biomaterials 81: 66–75. DOI 10.1002/(ISSN)1552-4981. [Google Scholar] [CrossRef]
Xu C, Yang F, Wang S, Ramakrishna S (2004). In vitro study of human vascular endothelial cell function on materials with various surface roughness. Journal of Biomedical Materials Research Part A 71: 154–161. [Google Scholar]
Zhang Y, Chen SE, Shao J, van den Beucken J (2018). Combinatorial surface roughness effects on osteoclastogenesis and osteogenesis. ACS Applied Materials & Interfaces 10: 36652–36663. DOI 10.1021/acsami.8b10992. [Google Scholar] [CrossRef]
Zhou R, Wei D, Cao J, Feng W, Cheng S, Du Q, Li B, Wang Y, Jia D, Zhou Y (2015). Synergistic effects of surface chemistry and topologic structure from modified microarc oxidation coatings on Ti implants for improving osseointegration. ACS Applied Materials & Interfaces 7: 8932–8941. DOI 10.1021/acsami.5b02226. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |