
 | Oncologie |  |
DOI: 10.32604/oncologie.2022.022521
ARTICLE
Development and Validation of a Nomogram Model to Predict the Prognosis of Intrahepatic Cholangiocarcinoma
1Department of Pathology, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, 350025, China
2Department of Pathology, The School of Basic Medical Sciences, Fujian Medical University, Fuzhou, 350122, China
*Corresponding Authors: Lihong Chen. Email: drlhchen@sina.com; Bin Wang. Email: doctorwang2001@163.com
#Yi Chen and Liyun Huang contributed equally to this work
Received: 16 March 2022; Accepted: 19 May 2022
Abstract: Background: The effective method for predicting prognosis of ICC is still lack. This study aims to establish and verify an effective prognostic nomogram model for intrahepatic cholangiocarcinoma (ICC) after partial hepatectomy. Materials and Methods: A nomogram model was developed in a cohort of 127 patients from January 2015 to December 2019. General clinical characteristics including preoperative physical examination data and postoperative pathological features were obtained. The independent risk factors identified by univariate and multivariate COX proportional hazards regression models were used to construct nomogram model. Predictive accuracy and discriminative ability were determined using a concordance index and a calibration curve. In addition, the clinical significance of postoperative pathological subtypes was analyzed by Kaplan-Meier survival analysis. Results: Univariate analysis and multivariate COX regression analysis revealed that CEA, maximum diameter, tumor number, and large duct type ICC was the independent risk factors. These variables were incorporated into the nomogram and the C-index for one year and three year overall survival prediction was 0.765 (95% CI: 0.672–0.814) and 0.695 (95% CI: 0.672–0.814), respectively. Postoperative pathological analysis showed that the large duct ICC had a distinct clinicopathological features and poor outcome. Conclusion: The proposed nomogram enables a prognostic prediction for patients with ICC and postoperative subclassification of ICC is of great significant to the prognosis of ICC.
Keywords: Intrahepatic cholangiocarcinoma; prognostic factors; large duct type intrahepatic cholangiocarcinoma
Nomenclature
| ICC | Intrahepatic cholangiocarcinoma |
| HBV | Hepatitis B virus |
| NCAM | Neural cell adhesion molecule |
| CA19-9 | Carbohydrate antigen 19-9 |
| CA125 | Carbohydrate antigen 125 |
| CEA | Carcinoembryonic antigen |
| IDH1/2 | Isocitrate dehydrogenase 1/2 |
| KRAS | Kirsten ratsarcoma viral oncogene homolog 2 |
Intrahepatic cholangiocarcinoma (ICC), which originates from the secondary bile duct and its branch bile duct epithelium, accounts for 10%–15% of primary liver cancer and is now becoming a highly aggressive malignant tumor [1,2]. The incidence of ICC has continued to increase in recent years, and most patients lose their best treatment opportunity due to the lack of early ICC diagnosis methods [3]. Although ICC patients receive surgical resection and postoperative chemotherapy, postoperative recurrence and extrahepatic metastasis still occur, which seriously affects the prognosis of patients and imposes a heavy medical burden on families and the public medical system [4]. Therefore, early prediction of surgical prognosis of ICC patients is an urgent need in clinical decision-making and early postoperative intervention for ICC recurrence.
At present, numerous factors identified by clinicians can influence the prognosis of ICC patients after resection [5–7]. However, there is no international consensus on the prognostic factors that can significantly and independently affect the survival and recurrence rates in ICC patients. Currently, the American Joint Committee on Cancer (AJCC) TNM staging system introduced a new staging system for ICC, which subdivided ICC based on a size cutoff of 5 cm, lymph node metastasis, vascular invasion, and extrahepatic metastasis. However, this staging system ignores other significant clinical information, such as pathological subtype and preoperative serological indicators, which makes it difficult for clinicians to accurately evaluate the prognosis of ICC [8]. Two pathological subtypes of ICC, including large- and small-duct types, with unique clinicopathological and genetic characteristics were also proposed in the 2019 World Health Organization guidelines [9]. Large-duct ICC possesses features of tall columnar tumor cells with a low nucleocytoplasmic ratio and abundant, clear, eosinophilic, or mucinous cytoplasm. Their nuclei are usually high-grade and arranged in a large-sized glandular or papillary structure with abundant extracellular mucus [10]. Small-duct ICC is composed of low columnar to cuboidal tumor cells with mild or moderate heteromorphic nuclei. The tumor cell structure is similar, with epithelial cells of small bile ducts and forms trabecular, cribriform, micro-papillary, or solid structures [10]. Compared to large-duct ICC, mutations of isocitrate dehydrogenase 1/2 (IDH1/2) and fibroblast growth factor receptor 2 (FGFR2) rearrangement are more common in small-duct ICC, while the KRAS mutation rate is relatively lower [10,11]. Therefore, the ICC classification standard plays a key role in evaluating the prognosis of patients. However, the effects of pathological subtypes on the postoperative outcome in Chinese ICC patients need to be confirmed by nomogram analysis.
In this study, preoperative serological indicators and postoperative pathological indicators were included to construct a nomogram to predict the prognosis of postoperative ICC patients. To the best of our knowledge, this is the first nomogram statistical method used to evaluate the prognosis of ICC patients by incorporating the pathological classification of ICC.
A retrospective study was performed on a total of 127 patients diagnosed with ICC from January 2015 to December 2019 at Mengchao Hepatobiliary Hospital of Fujian Medical University (Fuzhou, China). The inclusion criteria were as follows: (1) patients with radical resection of intrahepatic bile duct tumors at our hospital (R0) and complete resection of liver tumors, (2) patients with full records of clinicopathological data, (3) postoperatively histopathologically proven ICC, (4) no history of other anti-tumor therapies before surgery, and (5) no history of other malignancies. Exclusion criteria included the following: (1) death within 30 days after surgical operation and (2) combined hepatocellular carcinoma–cholangiocarcinoma. This study was approved by the ethics committee at Mengchao Hepatobiliary Hospital of Fujian Medical University (Approval No. 2021–044–01). The clinical information and characteristics were recorded and analyzed after written consent was obtained from the patients and their families.
After baseline history-taking and a detailed physical examination, blood was obtained from patients in order to detect serological indicators, including hepatitis B surface antigen (HBsAg), hepatitis B virus DNA level, anti-hepatitis C virus (HCV) antibody, serum albumin, total bilirubin, alanine transaminase, carbohydrate antigen 125 (CA125), carbohydrate antigen 19-9 (CA 19-9), and carcinoembryonic antigen (CEA). All patients were assessed with contrast-enhanced computed tomography or magnetic resonance imaging, and positron emission tomography was chosen to determine whether intrahepatic or extrahepatic metastases existed. According to tumor features and anatomy of the liver, partial liver resection combined with regional lymph node dissection in the hepatoduodenal ligament and retropancreatic and/or para-aortic lymph nodes was performed.
2.3 Postoperative Pathological Diagnosis and Classification of ICC Pathological Subtypes
Serial slides were cut from representative formalin-fixed paraffin-embedded (FFPE) samples, stained with hematoxylin and eosin (H&E), and then observed under the microscope by 2 independent pathologists. Tumor pathological indicators were recorded, including tumor size, number, tumor envelope, gross classification (mass type, peritubular-infiltration type, and intratubular-growth type), tumor differentiation (poorly differentiated, moderately differentiated, and well-differentiated), extrahepatic metastasis, bile duct invasion, lymph node metastasis, and liver cirrhosis. According to histologic appearance and immunohistochemical index, ICC was sub-classified into large and small duct types [12,13].
2.4 KRAS and IDH1/2 Gene Mutation Detection
Since IDH1/2 and KRAS mutations were reported to be the most frequent genetic alterations according to several large-scale genomic analyses, tumor tissues were macro-dissected from FFPE tissue blocks. Then, the total DNA of ICC tissues was extracted using a commercial kit (DNB400-50RXN; Merck, Darmstadt, Germany) according to the manufacturer’s instructions. This study focused on the mutation hotspots of exon 4 at codon 132 of IDH1, exon 4 at codon 172 of IDH2, and exon 2 of KRAS by polymerase chain reaction (PCR). The primer pairs used are as follows: IDH1-R132: F: 5’-GATGAGAAGAGGGTTGAGGAGTT-3’ and R: 5’-TACCTTGCTTAATGGGTGTAGATAC-3’; IDH2-R172: F: 5’-AGCTGAAGAAGATGTGGAAAAGTC-3’ and R: 5’-TTTGGGGTGAAGACCATTTTG-3’; and KRAS: F: 5’-ACGTCTGCAGTCAACTGGAATT-3’ and R: 5’-TCTGTATCAAAGAATGGTCCTGC-3’. The PCR productions were analyzed with the 3730xl DNA Analyzer (Thermo Fisher Scientific, Waltham, MA, USA).
ICC patients underwent follow-up examinations every 3 months after surgery. At each post-surgery visit, physical examinations were carried out. Blood was collected to detect serum levels of CA19-9, CA125, and CEA, and contrast-enhanced CT or magnetic resonance imaging was also performed. When tumor recurrence or metastasis was suspected, contrast-enhanced CT or magnetic resonance imaging was performed earlier. In this study, the endpoint of follow-up was defined as tumor recurrence confirmed by radiologic imaging or death caused by ICC. Overall survival (OS) was defined as the interval between the date of surgery and death or the last date of follow-up.
Categorical variables are displayed as mean ± standard deviation values, and categorical variables are expressed as frequencies. All recorded variables associated with prognosis were first analyzed by univariate COX regression analysis. Subsequently, the potential variables associated with prognosis at a significant level by univariate COX regression analysis were further enrolled in multivariate COX regression analysis to verify the independent risk factors. Nomogram was plotted based on these independent differential factors using the rms package in R version 4.0.3 (http://www.r-project.org/; R Foundation for Statistical Computing, Vienna, Austria). The performance of the nomogram was measured using the concordance index (C-index) and assessed by 1000 bootstrap samples to compare nomogram-predicted vs. observed Kaplan–Meier estimates of survival probability. Then, receiver operating characteristic (ROC) curve analysis was used for comparisons between our nomogram and AJCC Cancer Staging Manual (eighth edition). Survival curves were calculated using the Kaplan–Meier method and compared using the log-rank test. P < 0.05 was considered to be statistically significant.
3.1 Characteristics of Patients and Postoperative Recurrence and Survival
The basic characteristics of the entire cohort are shown in Table 1. Approximately half of the cases were male (n = 68 [53.54%]), tumor size ≥ 5 cm (n = 71 [55.91%]), and poorly or moderately differentiated (n = 105 [82.68%]). Some patients were negative for HBsAg (n = 42 [33.07%]), and no patients were found to be seropositive for HCV infection. Vascular invasion and lymph node metastasis were found in 67 (52.79%) and 46 (36.22%) patients, respectively. The mean follow-up time and range were 462 days and 61–1915 days, respectively. Moreover, the numbers of dead or recurrent cases during follow-up were 44 and 34, respectively.
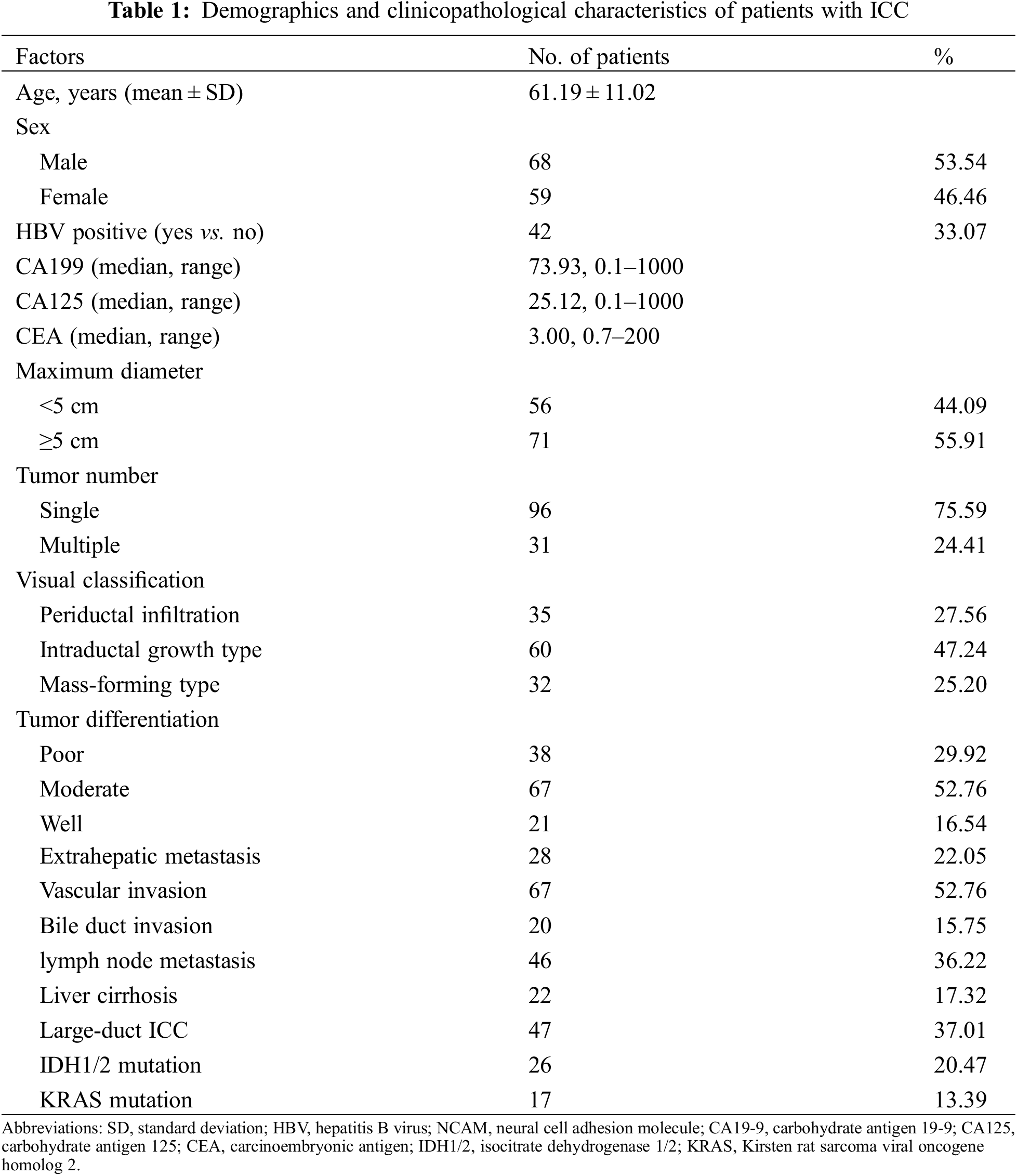
3.2 Univariate and Multivariate COX Regression Results
As showed in Table 2, univariate and multivariate COX analyses identified several independent risk factors for OS as follows: CEA (hazard ratio [HR], 1.010; 95% confidence interval [CI], 1.003–1.017; P = 0.004), maximum diameter (HR, 1.303; 95% CI, 1.176–1.443; P < 0.001), tumor number (HR, 2.094; 95% CI, 1.153–3.801; P = 0.015), and large-duct ICC (HR, 2.831; 95% CI, 1.298–6.175; P = 0.009).
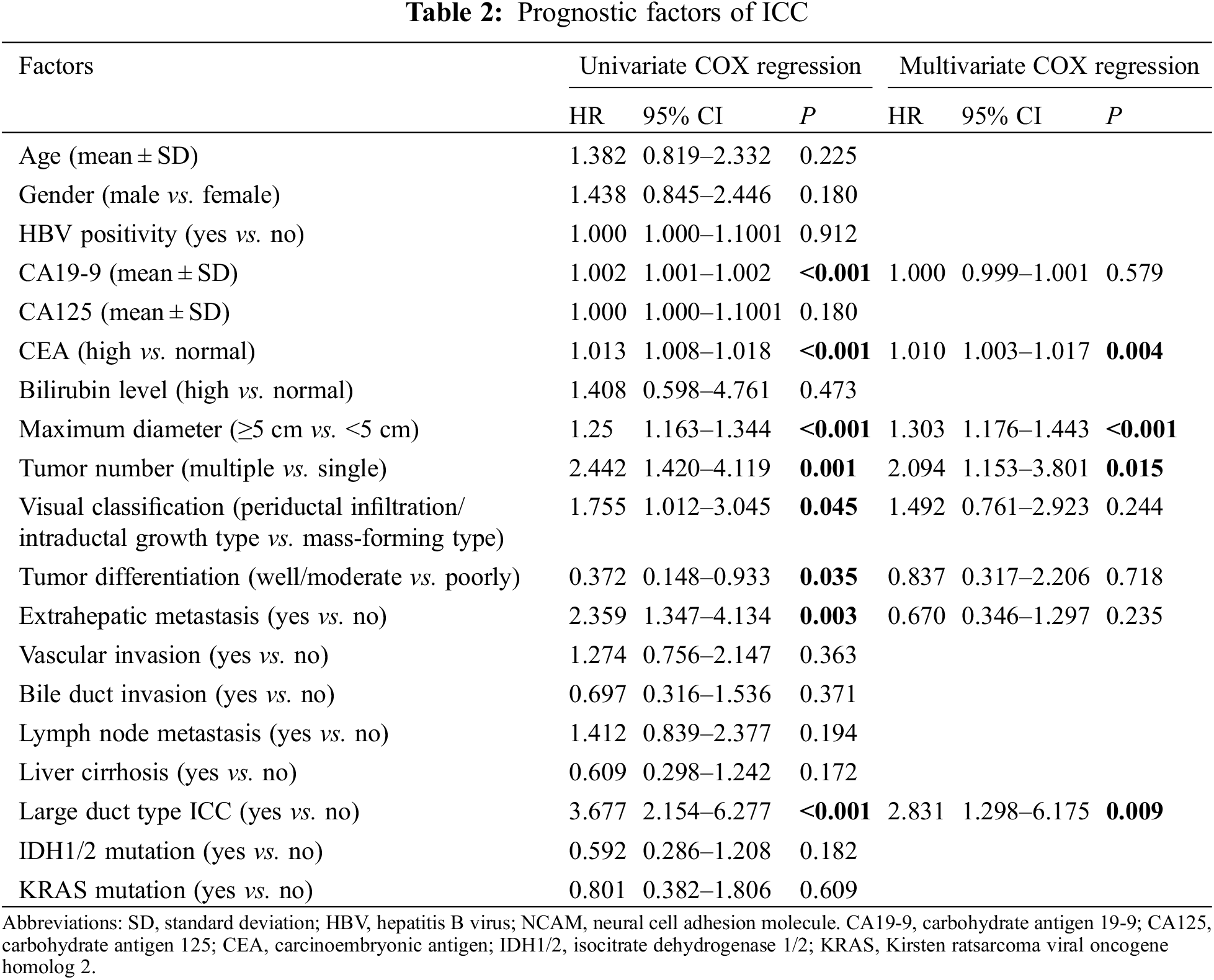
3.3 Development and Validation of Nomogram
The prognostic nomogram that integrated all significant independent factors for OS during univariate and multivariate COX regression is shown in Fig. 1. In this model, every patient’s score was calculated, and the ones with a higher total score had a worse prognosis for OS. The C-indices for 1- and 3-year OS prediction were 0.765 (95% CI, 0.672–0.814) and 0.695 (95% CI, 0.672–0.814), respectively. In comparison, the area under the receiver operating characteristic curve (AUROC) to predict the prognosis of ICC patients based on our model was significantly higher than that of the AJCC Cancer Staging Manual (eighth edition) in the time nodes (1- and 3-year survival rates) (Fig. 2).
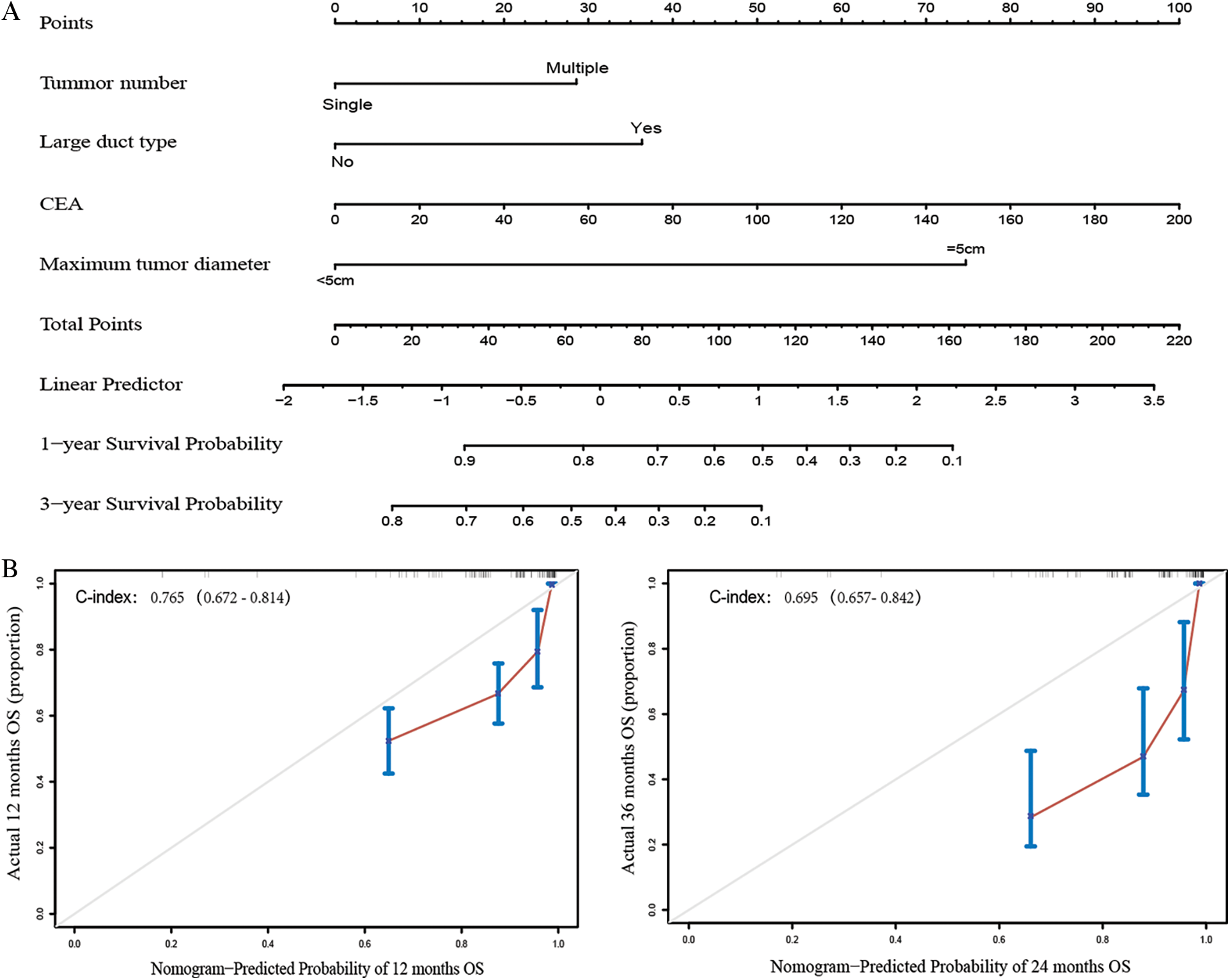
Figure 1: Construction and evaluation of nomogram structure. A. ICC survival nomogram based on tumor number, large-duct type, CEA, and maximum tumor diameter. B. The calibration curve for predicting patient survival within 1 or 3 years
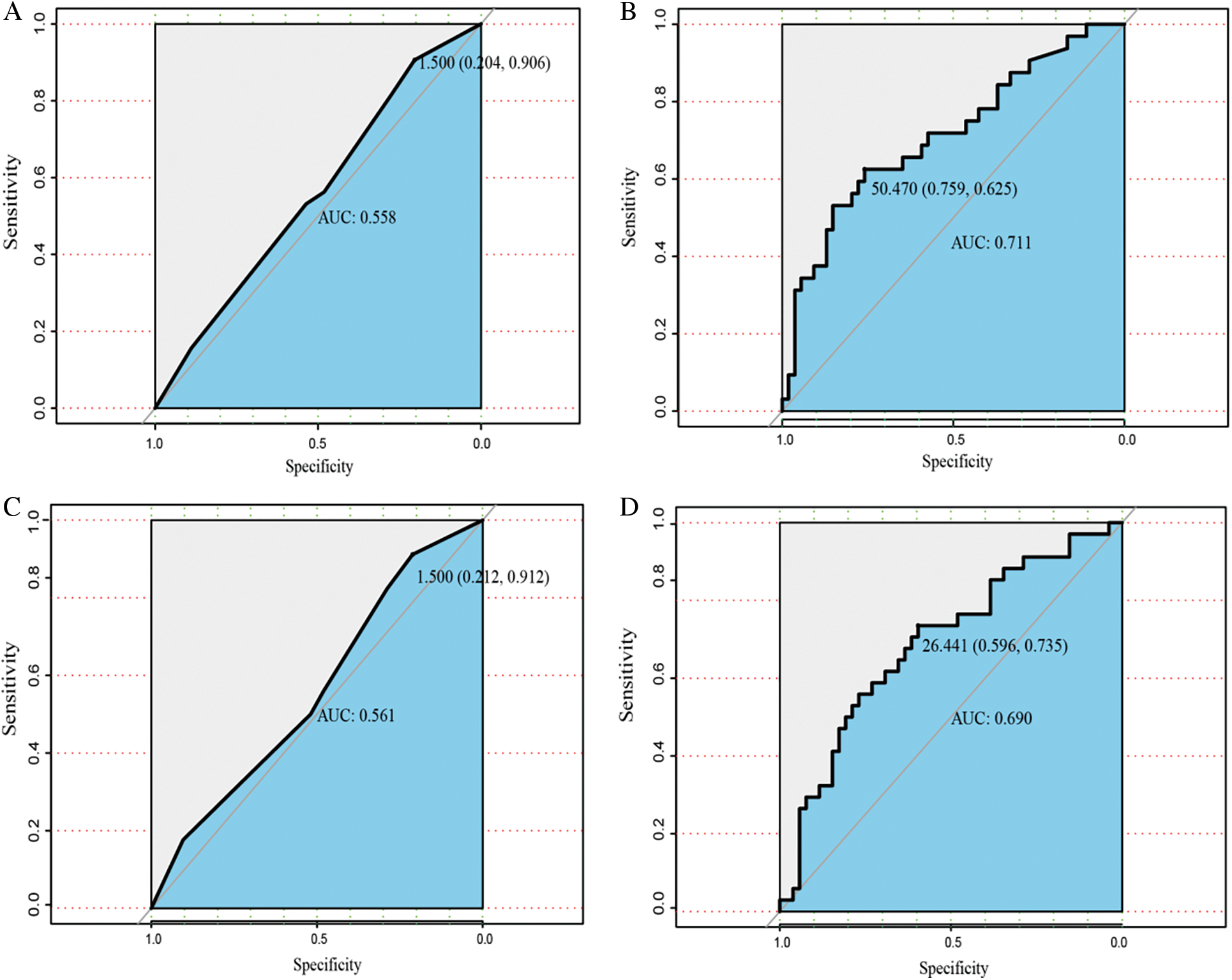
Figure 2: The AUROC of nomogram compared with AJCC TNM classification. A, B. The AUROC to predict the prognosis of ICC patients based on our model and the AJCC Cancer Staging Manual in 1 year after surgery. C, D. The AUROC to predict the prognosis of ICC patients based on our model and the AJCC Cancer Staging Manual (eighth edition) in 3 years after surgery
3.4 Clinicopathological and Prognostic Characteristics in Subtypes of ICC
The typical morphologic spectrum of large-and small-duct subtypes of ICC is shown in Fig. 3A. The tumor cells in large-duct ICC were arranged in a large-sized glandular or papillary structure with abundant extracellular mucus. Large-duct ICC stains positive for S100P and negative for N-cadherin and CD56. The tumor cell structure in small-duct ICC is similar to epithelial cells of small bile ducts and forms trabecular, cribriform, micro-papillary, or solid structures. The tumor lacks columnar tumor cells that can produce mucin. Small-duct ICC always stained positive for N-cadherin and CD56 but negative for S100P.
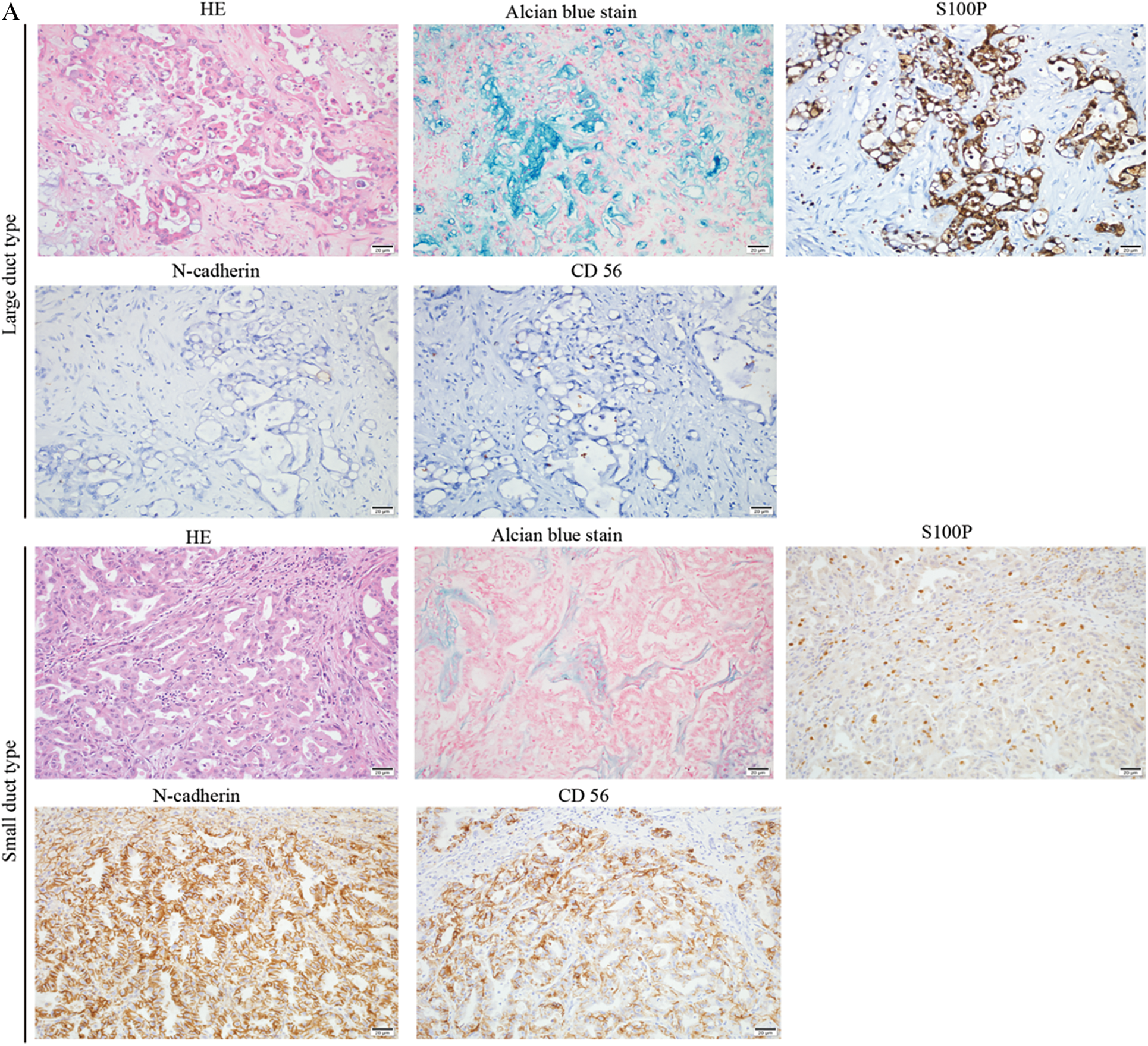
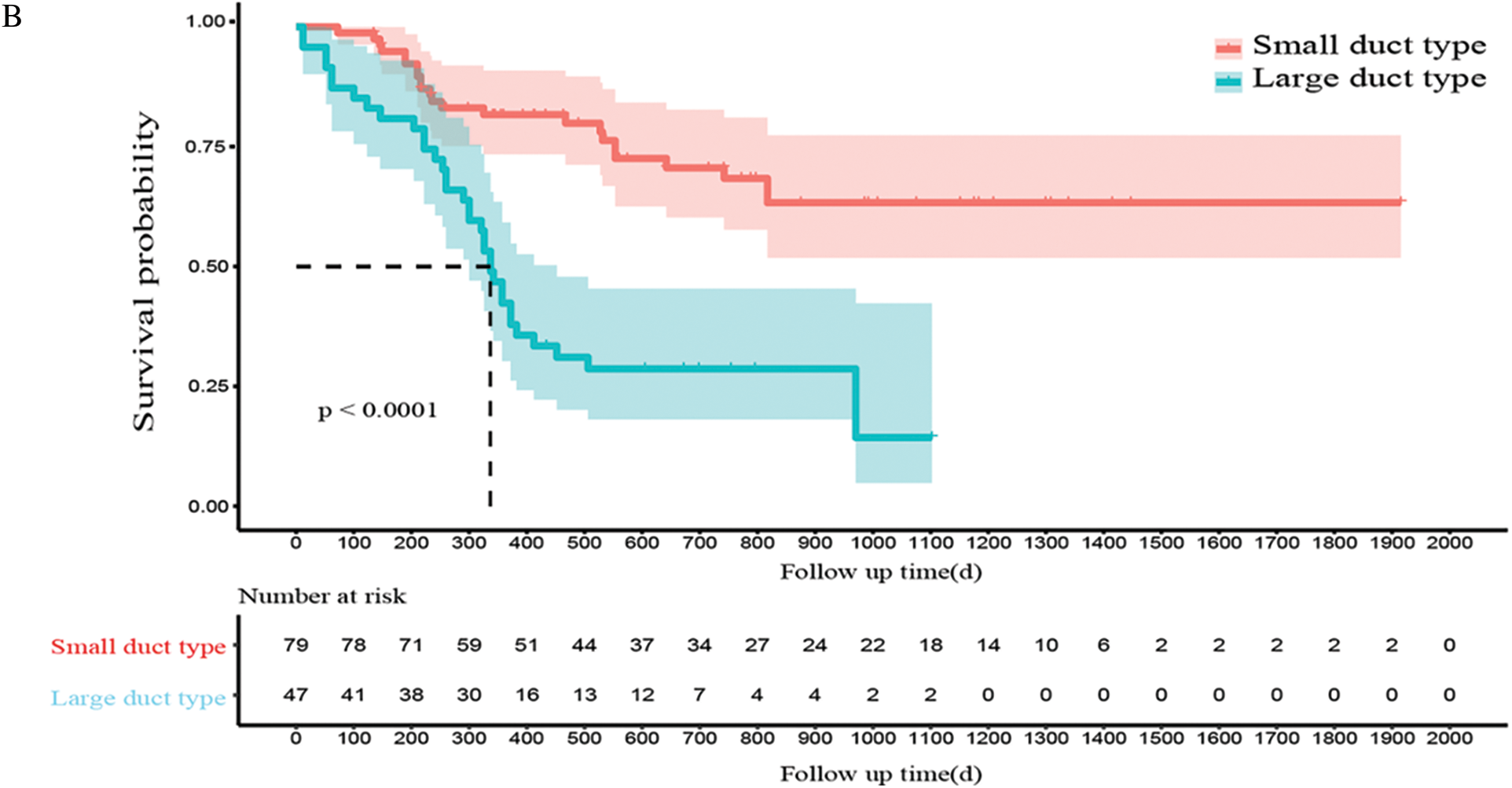
Figure 3: Clinicopathological and prognostic characteristics in subtypes of ICC. A. The expression patterns of mucin production, S100P, N-cadherin, and NCAM between large- and small-duct ICC. B. Survival analysis of OS for large and small-duct ICC
In addition, patients with large-duct ICC had a significantly worse recurrence-free and overall prognostic outcome than those with small-duct ICC (P < 0.0001, Fig. 3B).
Despite the lower incidence in liver cancers, intrahepatic cholangiocarcinoma (ICC) has a poorer prognosis. Therefore, early prediction of postoperative prognosis is significant in predicting postoperative recurrence and early intervention in key populations. Currently, several studies have been carried out to predict the prognosis of ICC patients by constructing a nomogram statistical model. It was found that indicators, including lymph node metastasis, tumor size, tumor stage, and serum CA19-9, had a large impact on the clinical outcome of ICC patients. Despite the significant role of pathological subtypes in ICC, none of these studies included the pathological subtypes of ICC into the nomogram model. Hence, we included preoperative serological indicators, postoperative pathological characteristics, and pathological staging factors into univariate and multivariate regression analyses in the current study. Additionally, CEA, large-duct ICC, multiple tumors, and tumors with diameters >5 cm were all incorporated into the nomogram model to predict the 1-year and 3-year survival of ICC patients after radical resection. Our results suggested that the nomogram model has good predictive performance in predicating ICC prognosis.
Due to its insidious onset and poor prognosis, ICC is prone to recurrence after surgery. The 5-year survival rate and 5-year recurrence rate of patients undergoing ICC surgery in our hospital were 40.30% and 57.01%, respectively, which were slightly higher than rates at other centers [7]. The reason for this divergence may be attributed to the small sample size and patients lost to follow-up. Nomograms can incorporate a variety of potential influencing factors to construct a prognostic prediction model, which can assist doctors in evaluating the prognosis of patients. Several clinical studies based on a statistical model have shown that nomograms display greater application value than traditional AJCC staging. For example, compared to a traditional AJCC staging and scoring system developed by the Liver Cancer Study Group of Japan, a single-center clinical study of 367 patients found that the nomogram built by incorporating CA 19-9, diameter and number of tumors, vascular invasion, lymph node metastasis, and local extrahepatic metastasis was more powerful (C-index, 0.74 vs. 0.65 vs. 0.64) [7]. In addition, another study focused on revealing factors affecting postoperative recurrence in ICC patients showed that the nomogram model built by tumor diameter, hepatitis B virus infection, and lymph node metastasis could predict the prognosis of patients [14]. Nevertheless, apart from lymph node metastasis, tumor diameter, and other well-known indicators, pathological subtypes of ICC were not included in these studies. In this study, ICC subtypes and other common prognostic factors were included in the multivariate analysis, and we found that CEA, large-duct ICC, multiple tumors, and tumors with diameters >5 cm were independent risk factors affecting the prognosis of patients. Furthermore, the nomogram model incorporating the pathological subtypes had higher C-indices (C-index, 0.765; 95% CI, 0.672–0.814) in prognosis prediction, indicating that the classification of postoperative ICC subtypes could improve the predictive efficiency of a nomogram model for evaluating ICC prognosis.
The diameter of tumors has always been a prognostic factor of concern among clinical workers, but the definite impact of various tumor sizes on prognosis remains inconclusive [15,16]. Specifically, the tumor size was subdivided further in AJCC TNM staging of intrahepatic cholangiocarcinoma (eighth edition), where the T1 stage was divided into a T1a stage and T1b stage with a single tumor diameter of 5 cm as the boundary [9]. However, according to the LCSGJ staging scheme raised by the Liver Cancer Study Group of Japan, ICC patients with tumor sizes of >2 cm had a poorer prognosis [17]. This study showed that tumor diameter ≥5 cm was an independent risk factor affecting the prognosis of patients. This may be attributed to the fact that larger tumors not only indicate higher tumor staging but also result in longer surgery times, and both these factors are disadvantageous for prognosis. Similarly, multiple tumors are extensive and invade both the left and right liver lobes, which can be difficult for radical resection. Since residual cancer tissues are more likely to remain at the resection margins, the cancer cell would be radiographically negative micrometastases in the remnant liver. All of these could increase the probability of postoperative recurrence and metastasis and thus result in poor prognosis.
As a result of significant tissue heterogeneity, ICC presents different growth patterns [13]. In order to further carry out precise molecular typing of ICC and adopt targeted treatment measures, the World Health Organization Classification of Tumors of the Digestive System (fifth edition) divides ICC into two special histological subtypes (large duct and small duct), which differ in histological characteristics and gene mutation characteristics [9]. Liau and Aishima confirmed that patients with large-duct ICC had the poorest prognosis in different ICC patient cohorts, and this type ICC was closely related to the pathological features of malignant tumors, including lymph node metastasis and vascular infiltration [12,18]. This study also showed that large-duct ICC was an independent risk factor affecting the prognosis of ICC patients, and large-duct ICC patients had significantly poorer prognoses than those with the small-duct variation. Compared to large-duct ICC, small-duct ICC has a greater mutation frequency of IDH1/2 and FGFR2 [10]. Thus, targeted drugs for IDH1/2 mutation and FGFR2 would improve the therapeutic outcome in ICC patients [19,20]. These findings collectively suggested that, for patients with advanced ICC who have lost the opportunity for treatment, targeted genetic testing can be performed to provide patients with potential targeted therapy strategies after the diagnosis of small-duct ICC by needle biopsy. However, this study also has limitations. First, the population included was small and the study was a single-center one; thus, statistical results may be biased. Second, loss to follow-up would also result in differences between our statistical results and those of previous studies.
In conclusion, we provide a new nomogram model that can improve the predictive efficiency of prognosis for ICC patients, and postoperative subclassification of ICC is of great significance to the prognosis of ICC.
Author Contributions: LC and WH conceived and designed the experiments. YC and LH performed the experiments. YC and LH analyzed the data. YC, LC, and WH wrote the paper. All authors contributed to the article and approved the submitted version.
Ethics Approval and Informed Consent Statement: This study was approved by the Ethics Committee at Mengchao Hepatobiliary Hospital of Fujian Medical University (Approval No. 2021–044–01). The clinical information and characteristics were recorded and analyzed after written consent was obtained from the patients and their families.
Availability of Data and Materials: All data are presented in the article and can be accessed by communicating with the corresponding author.
Acknowledgement: We thank LetPub (https://www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding Statement: This study was sponsored by Startup Found for scientific research, Fujian Medical University (Grant No. 2019QH1295); Fuzhou Health Technology Project (Grant No. 2021-S-wq27); Natural Science Foundation of Fujian Province (Fujian Provincial Natural Science Foundation). (Grant No. 2020J01605); High-Level Hospital Foster Grants from Fujian Provincial Hospital, Fujian Province, China (2019HSJJ08).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Mazzaferro, V., Gorgen, A., Roayaie, S., Droz, D. B. M., Sapisochin, G. (2020). Liver resection and transplantation for intrahepatic cholangiocarcinoma. Journal of Hepatology, 72(2), 364–377. DOI 10.1016/j.jhep.2019.11.020. [Google Scholar] [CrossRef]
2. Sapisochin, G., Ivanics, T., Subramanian, V., Doyle, M., Heimbach, J. K. et al. (2020). Multidisciplinary treatment for hilar and intrahepatic cholangiocarcinoma: A review of the general principles. International Journal of Surgery, 82, 77–81. DOI 10.1016/j.ijsu.2020.04.067. [Google Scholar] [CrossRef]
3. Hamaoka, M., Kozaka, K. (2019). Early detection of intrahepatic cholangiocarcinoma. Japanese Journal of Radiology, 37(10), 669–684. DOI 10.1007/s11604-019-00860-0. [Google Scholar] [CrossRef]
4. Kodali, S., Shetty, A., Shekhar, S. (2021). Management of intrahepatic cholangiocarcinoma. Journal of Clinical Medicine, 10(11), 2368. DOI 10.3390/jcm10112368. [Google Scholar] [CrossRef]
5. Liang, J., Zhou, H., Huang, X. Q., Liu, Y. F., Zhang, L. et al. (2021). A myeloid signature-based nomogram predicts the postoperative recurrence of intrahepatic cholangiocarcinoma. Frontiers in Molecular Biosciences, 8, 742953. DOI 10.3389/fmolb.2021.742953. [Google Scholar] [CrossRef]
6. Wang, T., Wang, W., Zhang, J., Yang, X., Shen, S. et al. (2020). Development and validation of a nomogram for differentiating combined hepatocellular cholangiocarcinoma from intrahepatic cholangiocarcinoma. Frontiers in Oncology, 10, 598433. DOI 10.3389/fonc.2020.598433. [Google Scholar] [CrossRef]
7. Wang, Y., Li, J., Xia, Y., Gong, R., Wang, K. et al. (2013). Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. Journal of Clinical Oncology, 31(9), 1188–1195. DOI 10.1200/JCO.2012.41.5984. [Google Scholar] [CrossRef]
8. Lee, A. J., Chun, Y. S. (2018). Intrahepatic cholangiocarcinoma: The AJCC/UICC 8th edition updates. Chinese Clinical Oncology, 7(5), 52. DOI 10.21037/cco. [Google Scholar] [CrossRef]
9. Nagtegaal, I. D., Odze, R. D., Klimstra, D., Paradis, V., Rugge, M. et al. (2020). The 2019 who classification of tumours of the digestive system. Histopathology, 76(2), 182–188. DOI 10.1111/his.13975. [Google Scholar] [CrossRef]
10. Ma, B., Meng, H., Tian, Y., Wang, Y., Song, T. et al. (2020). Distinct clinical and prognostic implication of IDH1/2 mutation and other most frequent mutations in large duct and small duct subtypes of intrahepatic cholangiocarcinoma. BMC Cancer, 20(1), 318. DOI 10.1186/s12885-020-06804-6. [Google Scholar] [CrossRef]
11. Hayashi, A., Misumi, K., Shibahara, J., Arita, J., Sakamoto, Y. et al. (2016). Distinct clinicopathologic and genetic features of 2 histologic subtypes of intrahepatic cholangiocarcinoma. The American Journal of Surgical Pathology, 40(8), 1021–1030. DOI 10.1097/PAS.0000000000000670. [Google Scholar] [CrossRef]
12. Liau, J. Y., Tsai, J. H., Yuan, R. H., Chang, C. N., Lee, H. J. et al. (2014). Morphological subclassification of intrahepatic cholangiocarcinoma: Etiological, clinicopathological, and molecular features. Modern Pathology, 27(8), 1163–1173. DOI 10.1038/modpathol.2013.241. [Google Scholar] [CrossRef]
13. Kendall, T., Verheij, J., Gaudio, E., Evert, M., Guido, M. et al. (2019). Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver International, 39 (Suppl 1), 7–18. DOI 10.1111/liv.14093. [Google Scholar] [CrossRef]
14. Jeong, S., Cheng, Q., Huang, L., Wang, J., Sha, M. et al. (2017). Risk stratification system to predict recurrence of intrahepatic cholangiocarcinoma after hepatic resection. BMC Cancer, 17(1), 464. DOI 10.1186/s12885-017-3464-5. [Google Scholar] [CrossRef]
15. Yamasaki, S. (2003). Intrahepatic cholangiocarcinoma: Macroscopic type and stage classification. Journal of Hepato-Biliary-Pancreatic Surgery, 10(4), 288–291. DOI 10.1007/s00534-002-0732-8. [Google Scholar] [CrossRef]
16. Spolverato, G., Ejaz, A., Kim, Y., Sotiropoulo, G. C., Pau, A. et al. (2014). Tumor size predicts vascular invasion and histologic grade among patients undergoing resection of intrahepatic cholangiocarcinoma. Journal of Gastrointestinal Surgery, 18(7), 1284–1291. DOI 10.1007/s11605-014-2533-1. [Google Scholar] [CrossRef]
17. Sirica, A. E., Gores, G. J., Groopman, J. D., Selaru, F. M., Strazzabosco, M. et al. (2019). Intrahepatic cholangiocarcinoma: Continuing challenges and translational advances. Hepatology, 69(4), 1803–1815. DOI 10.1002/hep.30289. [Google Scholar] [CrossRef]
18. Aishima, S., Kuroda, Y., Nishihara, Y., Iguchi, T., Taguchi, K. et al. (2007). Proposal of progression model for intrahepatic cholangiocarcinoma: Clinicopathologic differences between hilar type and peripheral type. The American Journal of Surgical Pathology, 31(7), 1059–1067. DOI 10.1097/PAS.0b013e31802b34b6. [Google Scholar] [CrossRef]
19. Molenaar, R. J., Maciejewski, J. P., Wilmink, J. W., van Noorden, C. J. F. (2018). Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene, 37(15), 1949–1960. DOI 10.1038/s41388-017-0077-z. [Google Scholar] [CrossRef]
20. Pu, X., Zhu, L., Li, F., Zheng, J., Wu, H. et al. (2020). Target molecular treatment markers in intrahepatic cholangiocarcinoma based on Chinese population. Pathology, Research and Practice, 216(9), 153116. DOI 10.1016/j.prp.2020.153116. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |