 Open Access
Open Access
CASE REPORT
Giant-Size Main Pulmonary Artery Aneurysm in an Adult Patient with Ebstein Anomaly and Dextrocardia
1 Department of Cardiac Surgery, University Hospital Centre Zagreb, Zagreb, Croatia
2 Department of Cardiovascular Diseases, University Hospital Centre Zagreb, Zagreb, Croatia
* Corresponding Author: Kristina Krzelj. Email:
# Authors contributed equally to this work (co-first authorship)
(This article belongs to the Special Issue: Nightmare Case Reports in Congenital Heart Disease)
Congenital Heart Disease 2023, 18(2), 207-212. https://doi.org/10.32604/chd.2023.027453
Received 03 November 2022; Accepted 12 January 2023; Issue published 15 March 2023
Abstract
Main pulmonary artery aneurysms are rare, mostly asymptomatic and discovered accidentally. The main pulmonary artery aneurysms may be idiopathic or secondary to underlying diseases such as pulmonary hypertension, Behcet’s disease, connective tissue disorders, congenital heart disease, vasculitis, syphilis, tuberculosis and endocarditis. There are some indices that dextrocardia is associated with anomalies of the pulmonary arteries and pulmonary valve. A rare occurrence of main pulmonary artery aneurysms results in a lack of recommendations, so the remaining challenges are whether main pulmonary artery aneurysms should be treated, how, and when. The decision on surgical intervention or conservative treatment of the main pulmonary artery aneurysms depends on its size, etiology and accompanying diseases and includes a multidisciplinary heart team. Our case of the main pulmonary artery aneurysm and pulmonary valve abnormality associated with Ebstein anomaly and dextrocardia in a 67-year-old male patient brings causality considerations and treatment options in such a unique condition.Graphic Abstract
Keywords
The main pulmonary artery aneurysm is a rare entity with unknown prevalence in the general population [1]. The pulmonary artery aneurysm represents focal dilatation of the pulmonary artery involving all 3 layers of the vessel wall with an upper limit of the main pulmonary artery diameter of 29 mm and an upper limit of the interlobar pulmonary artery of 17 mm [2]. The majority of main pulmonary artery aneurysms are asymptomatic and discovered accidentally on chest radiographs and computed tomography (CT) scans undertaken due to other causes or in autopsies. Among published cases, there is equal sex incidence [3]. Described main pulmonary artery aneurysms were either idiopathic or secondary to a specific underlying disease such as pulmonary hypertension, Behcet’s disease, connective tissue disorders, congenital heart disease (CHD), syphilis, tuberculosis, endocarditis, neoplasms [4]. More than 50% of cases of pulmonary aneurysms are associated with CHDs such as persistent ductus arteriosus, ventricular septal defect, atrial septal defects, hypoplastic aortic valve, bicuspid aortic valve, and anomalies of the pulmonary valve [4]. Furthermore, there are some indices that dextrocardia is associated with anomalies of the pulmonary arteries and pulmonary valve [5]. The Ebstein anomaly is a congenital malformation of the tricuspid valve with failure of the leaflet delamination from the underlying myocardium, apical displacement of the septal leaflet hinge point by 20 mm or 8 mm/m2 of body surface area, anterior rotation of the tricuspid valve and right ventricular myopathy [6]. The Ebstein anomaly represents 1% of all CHDs [7]. Patients with less severe forms of Ebstein anomaly mostly reach adult age before symptoms develop requiring surgical intervention. The association of a large main pulmonary artery aneurysm, pulmonary valve abnormality, dextrocardia, and Ebstein anomaly in an adult patient has not been described yet. It is difficult to find the exact correlation between those entities, but it is worth reporting about such a unique case considering possible causality and treatment options. Hereby, we bring a case of successful surgical treatment of a giant-size main pulmonary artery aneurysm associated with Ebstein anomaly and dextrocardia in a 67-year-old male patient.
A 67-year-old male patient with a history of Ebstein anomaly and main pulmonary artery aneurysm was referred to our institution from a local hospital due to scheduled cardiac surgery. He had no known connective tissue disorder or history of syphilis or tuberculosis.
He was followed by a cardiologist since early childhood owing to Ebstein anomaly, but he had no relevant medical documentation available.
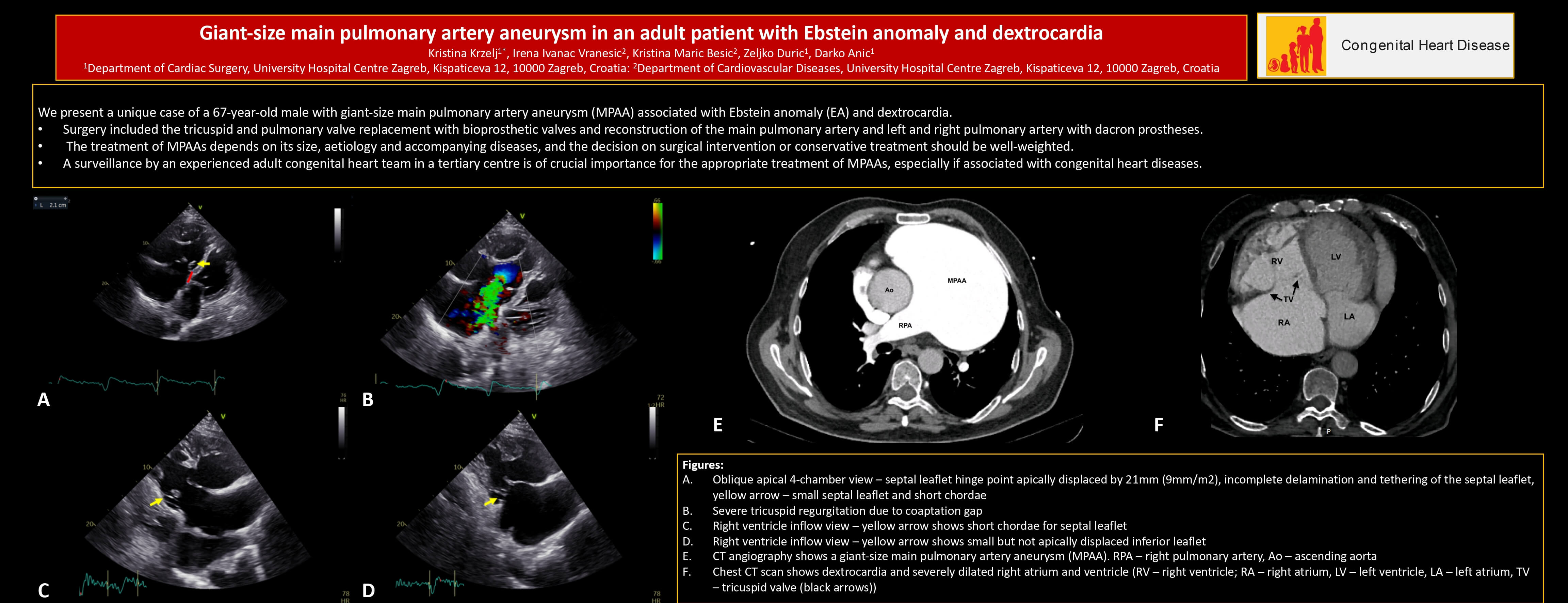
Four months before admission, he was admitted to the emergency department of our institution due to acute right heart failure exacerbation. During the in-hospital stay, echocardiography was performed and revealed situs solitus, dextrocardia, and severe tricuspid regurgitation due to a mild form of Ebstein anomaly (type A by Carpentier classification) with incomplete delamination of the septal leaflet and malcoaptation due to severe annular and right ventricle and right atrium dilatation in long-standing disease (Fig. 1). There was no displacement of the inferior leaflet hinge point and no significant rotation of the tricuspid valve, but the septal leaflet offset was apically displaced by 21 mm (indexed by body surface area (BSA) 9 mm/m2) in comparison with the mitral valve, which is slightly above the cut-off value for establishing Ebstein anomaly diagnosis (Fig. 1). Additionally, echocardiography showed an abnormal pulmonary valve with moderate pulmonary regurgitation, mild pulmonary stenosis, and severely dilated main pulmonary artery and pulmonary artery branches (Fig. 2). The patent foramen ovale with left-to-right shunting was established as well. On the ECG atrial flutter and left bundle branch block were observed. Right heart catheterization showed an absence of pulmonary hypertension (mean pulmonary artery pressure 17 mmHg, wedge pressure 22 mmHg, Rp/Rs 0.07, pulmonary vascular resistance 3 Wood units) and confirmed patent foramen ovale with left-to-right shunting. The peripheral oxygen saturation was 94% without oxygen supplementation. Chest CT scan showed a large main pulmonary artery aneurysm, 10.4 cm in diameter, with dilated left and right pulmonary arteries (2.8 and 3.2 cm in diameter, respectively) (Figs. 3 and 4).
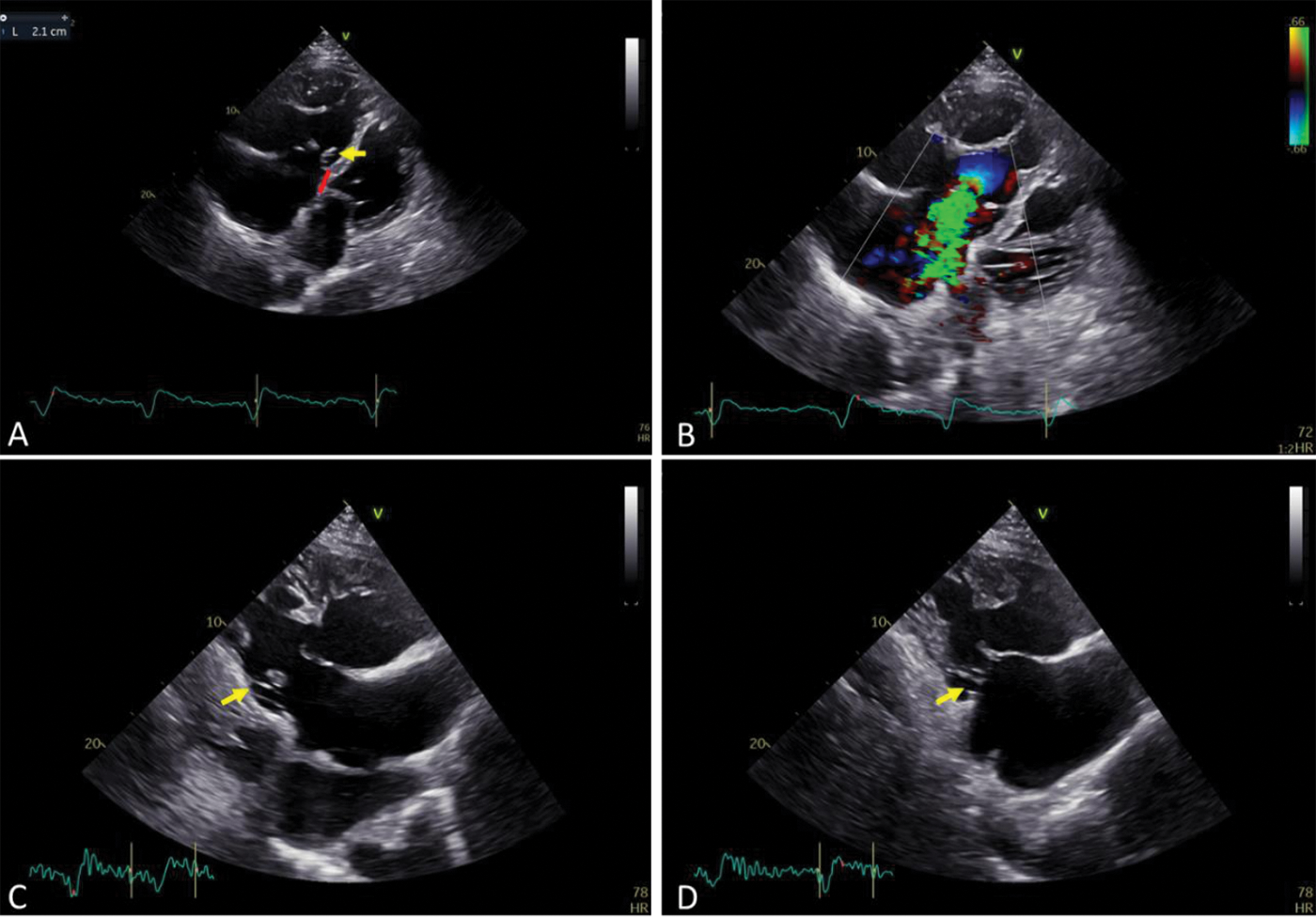
Figure 1: (A) Oblique apical four-chamber view (right lateral decubitus position)–septal leaflet hinge point apically displaced by 21 mm (9 mm/m2), incomplete delamination and tethering of the septal leaflet, yellow arrow–small septal leaflet and short chordae; (B) Severe tricuspid regurgitation due to coaptation gap; (C) Right ventricle inflow view–yellow arrow shows short chordae for septal leaflet; (D) Right ventricle inflow view–yellow arrow shows small but not apically displaced inferior leaflet
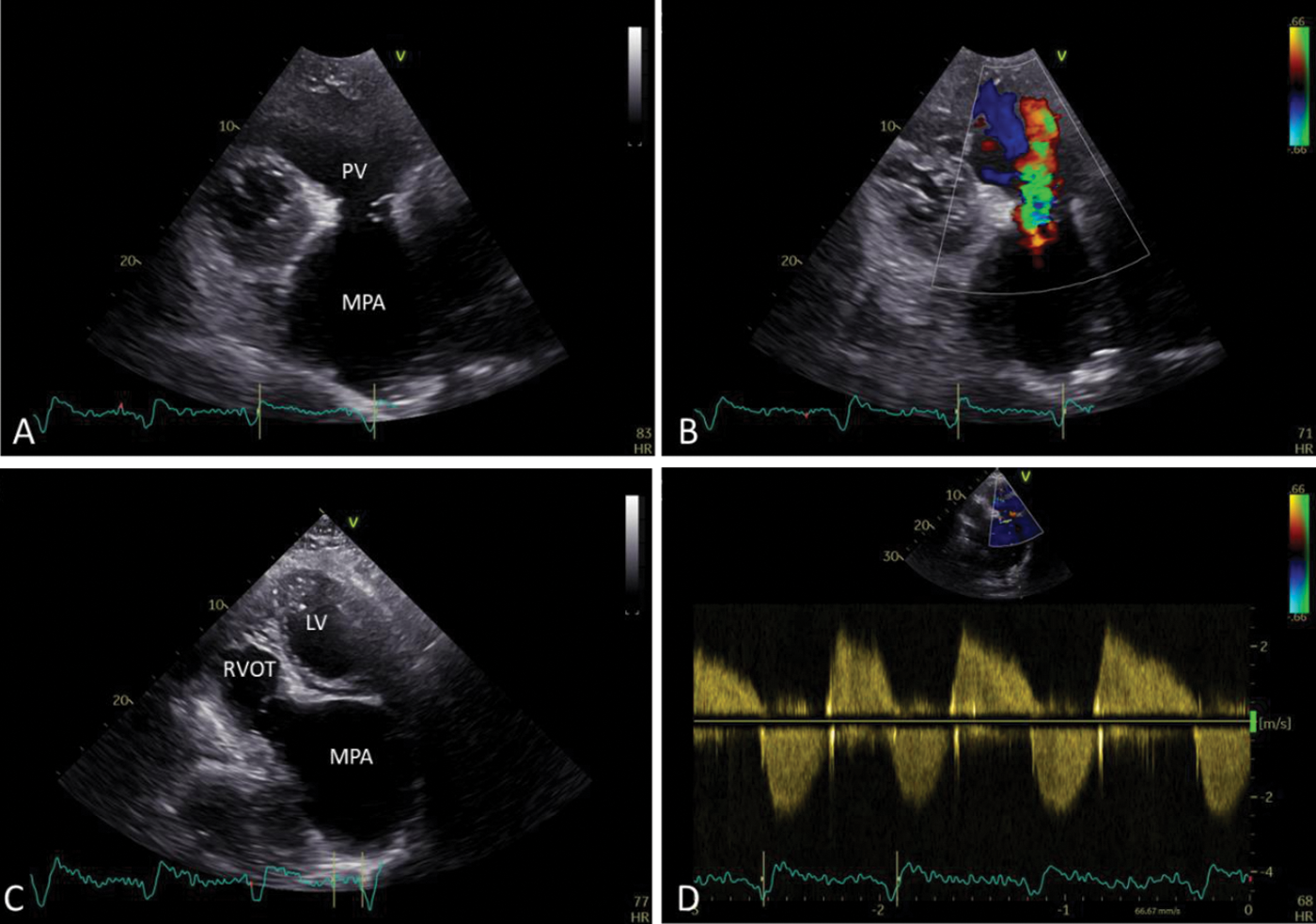
Figure 2: (A) Short axis view–abnormal pulmonary valve (PV), severely dilated main pulmonary artery (MPA); (B) Moderate pulmonary regurgitation; (C) Apical five-chamber view with the further anterior tilt of the probe–giant pulmonary artery (LV-left ventricle; RVOT-right ventricle outflow tract; MPA-main pulmonary artery); (D) Spectral doppler shows moderate pulmonary regurgitation and mild pulmonary stenosis
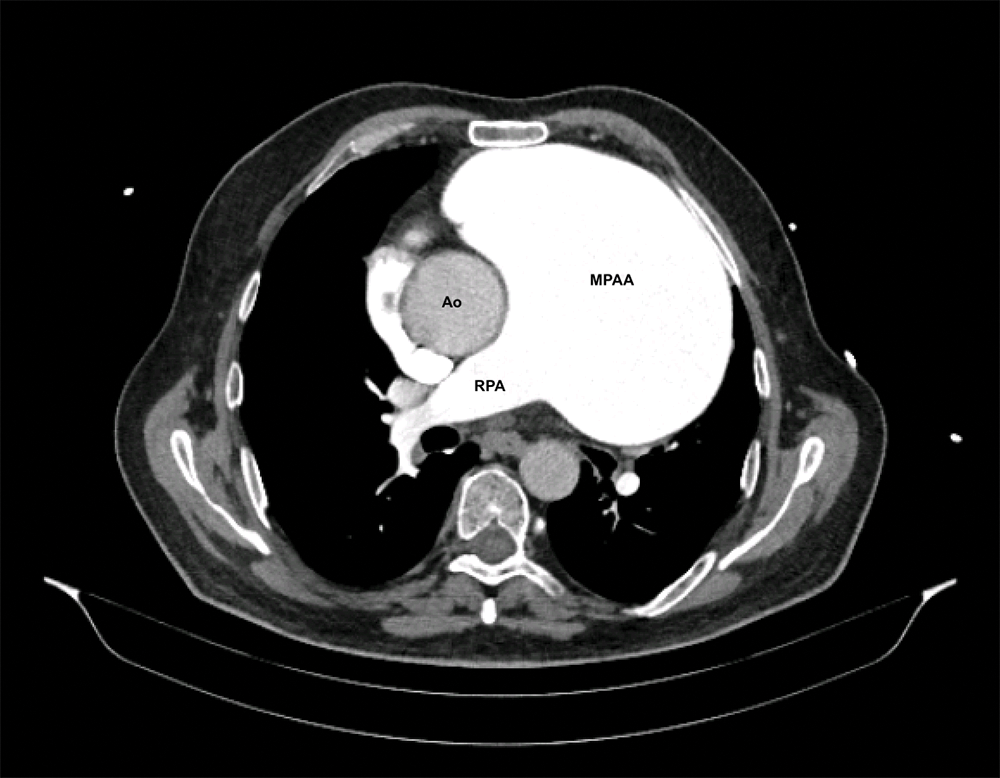
Figure 3: CT angiography shows a giant-size main pulmonary artery aneurysm (MPAA–main pulmonary artery aneurysm, RPA–right pulmonary artery, Ao–ascending aorta)
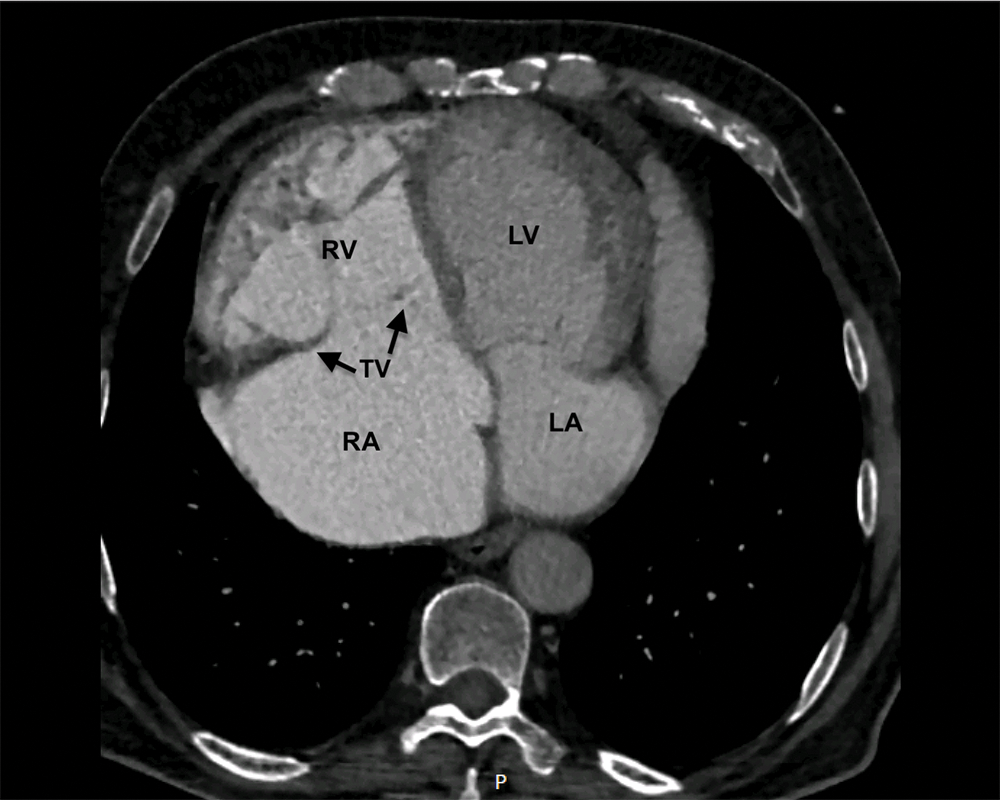
Figure 4: Chest CT scan shows dextrocardia and severely dilated right atrium and ventricle (RV–right ventricle; RA–right atrium, LV–left ventricle, LA–left atrium, TV–tricuspid valve (black arrows))
The heart team concluded the patient is a candidate for surgical treatment, but the patient refused to undergo surgery. Despite optimal medical therapy, the patient reached NYHA III-IV status, so he changed his decision and accepted surgical intervention.
Surgery included the tricuspid and pulmonary valve replacement with bioprosthetic valves (St. Jude Medical Biocor 33 mm (St. Jude Medical, One Lillehei Plaza, St. Paul, MN, US 55117-1799) bioprosthesis at the tricuspid position and Carpentier-Edwards Perimount Magna Ease 25 mm (Edwards Lifesciences, One Edwards Way Irvine, CA, US 92614) at the pulmonary position) and reconstruction of the main pulmonary artery and left and right pulmonary artery with Dacron prostheses following cardiopulmonary bypass establishment and cardiac arrest achievement. There were no signs of tricuspid or pulmonary valve endocarditis. The wrapping of the main pulmonary artery dacron prosthesis was performed with the remaining arterial wall, and the sample of the arterial wall was not taken for the histopathological exam. A histopathological exam of the pulmonary and tricuspid leaflets showed myxomatous changes in both valves. The postoperative course went uneventfully. The atrial flutter was rate controlled with beta-blocker bisoprolol and metildigoxin, whereas adequate anticoagulation was achieved with apixaban. The patient was discharged on the 13th postoperative day.
The main pulmonary artery aneurysms can be idiopathic or secondary to many previously mentioned causes [4]. Our patient had no known connective tissue disorder, history of syphilis, tuberculosis, or signs of Behcet’s disease. The patient had patent foramen ovale accompanied by small left-to-right shunting and absent pulmonary hypertension, so the pathophysiology of the main pulmonary artery aneurysm in the circumstances of absent pulmonary hypertension and present severe tricuspid regurgitation is quite unclear.
Although congenital heart diseases are closely related to main pulmonary artery aneurysms, we did not find any case in the literature reporting on the Ebstein anomaly accompanied by main pulmonary artery aneurysm, thus the causality between the natural course of the Ebstein anomaly and pulmonary artery aneurysm appearance is unknown. The potential explanation for Ebstein anomaly and pulmonary artery aneurysm occurrence may be eventual endocarditis of the tricuspid valve in medical history because the tricuspid valve in Ebstein anomaly is prone to endocarditis. However, we did not find any signs of tricuspid valve endocarditis intraoperatively.
Additional intriguing information, in this case, is an association with situs solitus dextrocardia. The reported incidence of dextrocardia is 0.37–0.53 per 10,000 live births [5]. Among three types of dextrocardia (situs solitus, situs inversus, situs ambiguous), situs solitus was reported as most commonly associated with abnormalities of the pulmonary valve and pulmonary arteries [5]. Although the appearance of pulmonary arteries abnormalities in patients with dextrocardia was observed in a certain number of cases [5], molecular mechanisms and the pathophysiology of such association are unexplored. Therefore, the question of why pulmonary arteries abnormalities are present in some cases of dextrocardia and in some do not, remains unanswered. We hypothesize that volume overload due to pulmonary valve abnormalities along with unusual blood flow streaming patterns in dextrocardia might be a potential explanation for a giant-size main pulmonary artery aneurysm development in our patient. However, owing to the rareness of both anomalies and the accompanying lack of evidence regarding molecular mechanisms and pathophysiology, the exact causality cannot be established.
A rare occurrence of main pulmonary artery aneurysms results in a scarcity of evidence-based recommendations for conservative vs. surgical treatment. Owing to the mostly asymptomatic nature of main pulmonary artery aneurysms the question is whether main pulmonary artery aneurysms should be treated, how, and when. Despite low pulmonary pressure, giant-size main pulmonary artery aneurysm and unknown diameter growth rate in our case, suggest a potentially increased risk of severe complications such as dissection or rupture. However, the patient was a candidate for tricuspid valve replacement, thus we performed main pulmonary artery aneurysm reconstruction as well. In cases of giant-size main pulmonary artery aneurysms (>6.5 cm in diameter), associated with pulmonary hypertension, anatomical anomalies, rapid growth, and compression of neighbouring structures, surgery should always be considered, otherwise conservative treatment with strict follow-up is recommended [1].
In planning surgical intervention in this unique case, another question was whether to perform tricuspid valve replacement or reconstruct the tricuspid valve according to the Da Silva cone procedure [8]. We decided to replace the tricuspid and pulmonary valve because in our institution Da Silva cone procedure is preferred in younger patients who are suitable candidates for this complex operation, and in the setting of dextrocardia and situs solitus, the Da Silva procedure was considered too complex for our institutional experience and therefore, as not safe enough. Furthermore, our patient was assessed as poorly compliant regarding the need for warfarin therapy and INR control if the mechanical valves would be implanted, thus we decided to implant two bioprosthetic valves.
The treatment of main pulmonary artery aneurysms depends on its size, aetiology and accompanying diseases, and the decision on either surgical intervention or conservative treatment should be well-weighted. Our case is the only reported occurrence of the association of giant-size main pulmonary artery aneurysm, Ebstein anomaly, and dextrocardia with unclear causality. Heart failure was a striving indication for surgery in our case, although the precise guidelines and recommendations for such an association do not exist. However, strict surveillance by an experienced adult congenital heart team in a tertiary centre is of crucial importance for the appropriate treatment of main pulmonary artery aneurysms, especially if associated with congenital heart diseases.
Funding Statement: The authors received no specific funding for this work.
Author Contributions: All listed authors should have substantially contributed to the manuscript and have approved the final submitted version. The authors confirm their contribution to the paper as follows: manuscript conception and design: Kristina Krzelj, Zeljko Duric, Darko Anic; data collection: Kristina Krzelj, Irena Ivanac Vranesic, and Kristina Maric Besic; analysis and interpretation of results: Kristina Krzelj, Zeljko Duric, Irena Ivanac Vranesic, Kristina Maric Besic, Darko Anic; draft manuscript preparation: Kristina Krzelj, Irena Ivanac Vranesic, Zeljko Duric; review and editing: Irena Ivanac Vranesic, Zeljko Duric. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: This research does not involve human and/or animal experimentation. The permission to publish this case report was granted by the subject patient.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Hou, R., Ma, G. T., Liu, X. R., Zhang, C. J., Liu, J. Z. et al. (2016). Surgical treatment of pulmonary artery aneurysm: An institutional experience and literature review. Interactive CardioVascular and Thoracic Surgery, 23(3), 438–442. https://doi.org/10.1093/icvts/ivw157 [Google Scholar] [PubMed] [CrossRef]
2. Fraser, R. S., Müller, N. L., Colman, N., Paré, P. D. (1999). Pulmonary hypertension and edema. Diagnosis of Diseases of the Chest, 3, 1935–1937. [Google Scholar]
3. Blades, B., Ford, W., Clark, P. (1950). Pulmonary artery aneurysms: Report of a case treated by surgical intervention. Circulation, 2(4), 565–571. https://doi.org/10.1161/01.CIR.2.4.565 [Google Scholar] [PubMed] [CrossRef]
4. Kreibich, M., Siepe, M., Kroll, J., Höhn, R., Grohmann, J. et al. (2015). Aneurysms of the pulmonary artery. Circulation, 131(3), 310–316. https://doi.org/10.1161/CIRCULATIONAHA.114.012907 [Google Scholar] [PubMed] [CrossRef]
5. Bohun, C. M., Potts, J. E., Casey, B. M., Sandor, G. G. S. (2007). A population-based study of cardiac malformations and outcomes associated with dextrocardia. American Journal of Cardiology, 100(2), 305–309. https://doi.org/10.1016/j.amjcard.2007.02.095 [Google Scholar] [PubMed] [CrossRef]
6. Fuchs, M. M., Connolly, H. M. (2020). Ebstein anomaly in the adult patient. Cardiology Clinics, 38(3), 353–363. https://doi.org/10.1016/j.ccl.2020.04.004 [Google Scholar] [PubMed] [CrossRef]
7. Cherry, C., DeBord, S., Moustapha-Nadler, N. (2009). Ebstein’s anomaly: A complex congenital heart defect. AORN Journal, 89(6), 1098–1114. https://doi.org/10.1016/j.aorn.2009.03.003 [Google Scholar] [PubMed] [CrossRef]
8. da Silva, J. P., Viegas, M., Castro-Medina, M., da Fonseca da Silva, L. (2020). The Da silva cone operation after the starnes procedure for ebstein’s anomaly: New surgical strategy and initial results. JTCVS Techniques, 3, 281–283. https://doi.org/10.1016/j.xjtc.2020.05.011 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools