 Open Access
Open Access
ARTICLE
Using Pharmacokinetic Modeling and Electronic Health Record Data to Predict Clinical and Safety Outcomes after Methylprednisolone Exposure during Cardiopulmonary Bypass in Neonates
1 Department of Pediatrics, Duke University, Durham, USA
2 Duke Clinical Research Institute, Durham, USA
3 Department of Pediatrics, Medical University of South Carolina, Charleston, USA
* Corresponding Author: Karan R. Kumar. Email:
Congenital Heart Disease 2023, 18(3), 295-313. https://doi.org/10.32604/chd.2023.026262
Received 01 October 2022; Accepted 08 February 2023; Issue published 09 June 2023
Abstract
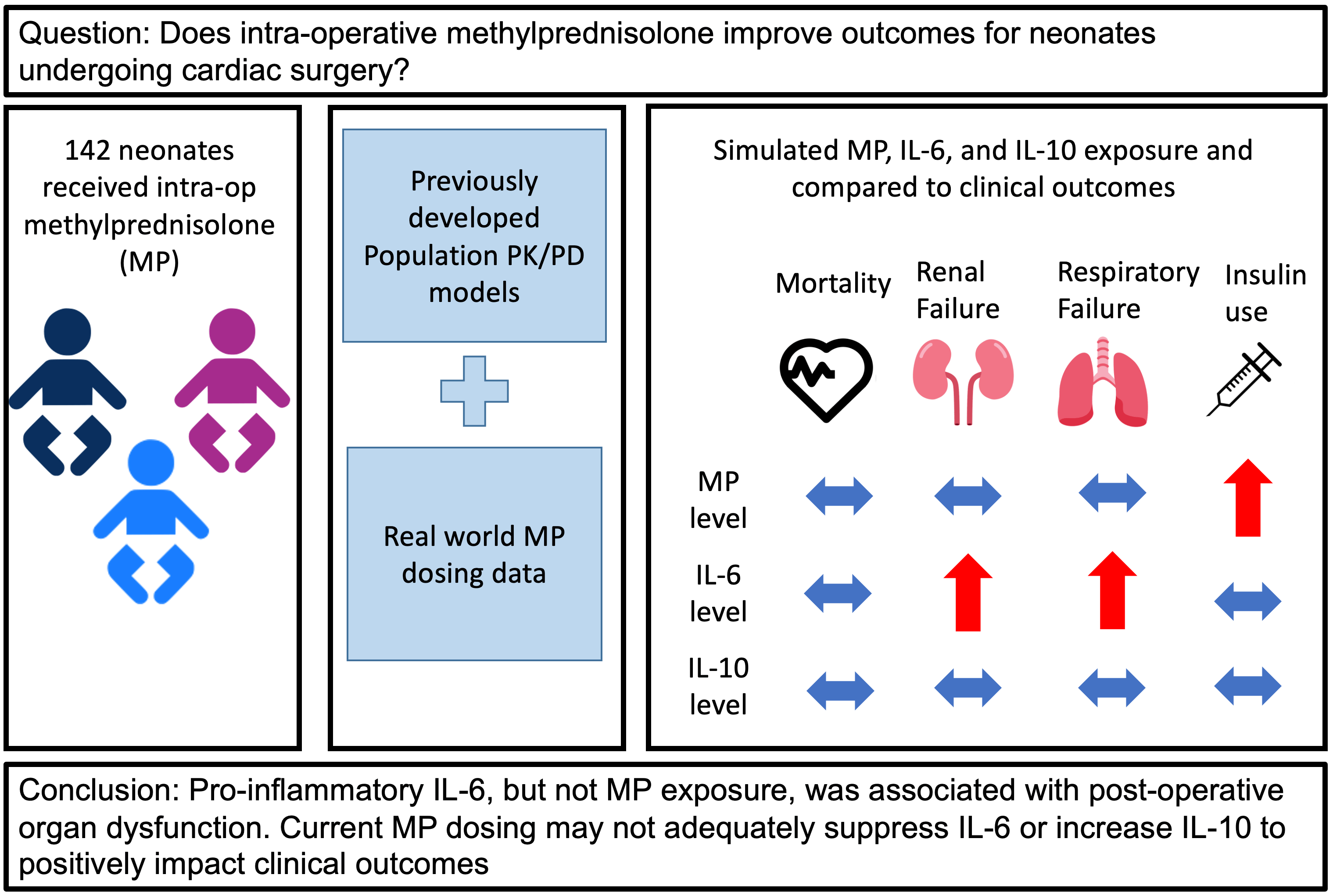
Background: Infants undergoing cardiac surgery with cardiopulmonary bypass (CPB) frequently receive intra-operative methylprednisolone (MP) to suppress CPB-related inflammation; however, the optimal dosing strategy and efficacy of MP remain unclear. Methods: We retrospectively analyzed all infants under 90 days-old who received intra-operative MP for cardiac surgery with CPB from 2014–2017 at our institution. We combined real-world dosing data from the electronic health record (EHR) and two previously developed population pharmacokinetic/pharmacodynamic models to simulate peak concentration (Cmax) and area under the concentration-time curve for 24 h (AUC24) for MP and the inflammatory cytokines interleukin-6 (IL-6) and interleukin-10 (IL-10). We evaluated the relationships between post-operative, safety, and other clinical outcomes obtained from the EHR with each predicted exposure using non-parametric tests. Results: A total of 142 infants with median post-natal age 8 (interquartile range [IQR]: 5, 37) days received a total dose of 30 (19, 49) mg/kg of MP. Twelve (8%) died, 37 (26%) met the composite post-operative outcome, 114 (80%) met the composite safety outcome, and 23 (16%) had a major complication. Predicted median Cmax and AUC24 IL-6 exposure was significantly higher for infants meeting the composite post-operative outcome and those with major complications. Predicted median Cmax and AUC24 MP exposure was significantly higher for infants requiring insulin. No exposure was associated with death or other safety outcomes. Conclusions: Pro-inflammatory IL-6, but not MP exposure, was associated with post-operative organ dysfunction, suggesting current MP dosing may not adequately suppress IL-6 or increase IL-10 to impact clinical outcomes. Prospective study will be required to define the optimal exposure-efficacy and exposure-safety profiles in these infants.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools