 Open Access
Open Access
ARTICLE
Cardiothoracic Ratio for Assessment of Ventricular Volumes and Function in Patients with Repaired Tetralogy of Fallot
1 Department of Paediatrics and Adolescent Medicine, Hong Kong Children’s Hospital, Hong Kong SAR, China
2 Department of Radiology, Hong Kong Children’s Hospital, Hong Kong SAR, China
3 Department of Paediatrics and Adolescent Medicine, School of Clinical Medicine, The University of Hong Kong, Hong Kong SAR, China
* Corresponding Author: Yiu-fai Cheung. Email:
Congenital Heart Disease 2025, 20(2), 201-211. https://doi.org/10.32604/chd.2025.063217
Received 08 January 2025; Accepted 13 March 2025; Issue published 30 April 2025
Abstract
Background: The role of cardiothoracic ratio (CTR) from the chest radiograph for assessment of ventricular enlargement and function in repaired tetralogy of Fallot (TOF) is conflicting. This study aimed to determine the associations between CTR and cardiac magnetic resonance (CMR)-derived ventricular volumes and indices of ventricular function in adolescents and young adults with repaired TOF. Methods: The CTR and CMR findings, performed within 12 months of each other, were reviewed in 76 patients aged 22.1 ± 6.4 years. Associations between CTR and CMR parameters including right (RV) and left ventricular (LV) volumes and ejection fraction were determined. Diagnostic accuracies of CTR in identifying moderate to severe RV or LV dilation were assessed by calculation of area under the receiver operator characteristic curves (AUC). Results: Patients with normal CTR and those with increased CTR > 0.5 had similar right and left ventricular volumes, ejection fraction, and pulmonary regurgitant fraction (all p > 0.05). There were no significant correlations between CTR and RV end-diastolic (r = 0.06, p = 0.65) and end-systolic (r = 0.06, p = 0.65) volumes, LV end-diastolic (r = 0.23, p = 0.08) and end-systolic (r = 0.18, p = 0.16) volumes, and LV (r = −0.07, p = 0.60) and RV (r < −0.01, p = 0.97) ejection fraction. The CTR failed to distinguish between patients with moderate to severe RV (AUC 0.50) or LV (AUC 0.46) dilation from patients without ventricular dilation. Conclusions: The CTR based on the chest radiograph failed to reflect dilation or reduced ejection fraction of either the right or the left ventricle in adolescents and young adults with repaired TOF.Keywords
Surgical repair of tetralogy of Fallot (TOF) involves the closure of the ventricular septal defect and relief of right ventricular (RV) outflow obstruction by muscle resection and placement of a transannular patch in the majority of patients. Chronic pulmonary regurgitation (PR), RV dilation and dysfunction resulting from the surgery necessitate long-term follow-up and monitoring [1,2]. Importantly, patients with RV volume overload are at risk of sudden death, ventricular arrhythmias, worse New York Heart Association class, and decreased exercise tolerance [3]. Additionally, studies have shown that left ventricular (LV) dysfunction is an independent predictor of adverse cardiovascular events in repaired TOF patients [3,4]. Replacement of the pulmonary valve is conducive to RV and LV remodeling with the restoration of function, the timing of which depends to a large extent on the severity of RV volume overload and systolic dysfunction [5]. Regular assessment of the size and function of the right ventricle in patients with repaired TOF is hence of paramount importance [1,2].
While cardiac magnetic resonance imaging (CMR) is considered the gold standard for the assessment of RV and LV volumes and function and quantification of PR in patients with repaired TOF [6], its high cost and limited availability restrict its frequent use solely for the purpose monitoring of ventricular volume load in patients with repaired TOF. Echocardiography has also been used to evaluate RV size and function, albeit with documented poor interobserver agreement and difficulties in imaging the entire RV cavity due to its crescentic shape and retrosternal position [7,8]. Hence, chest radiograph is still commonly performed in the clinical setting to assess heart size as a surrogate of ventricular volume load in this setting.
Cardiothoracic ratio (CTR), defined as the ratio of the greatest dimension of the heart to the greatest dimension of the chest cavity in the transverse dimension of the posteroanterior chest radiograph, can be measured reproducibly and is commonly used as a simple marker of cardiac enlargement. A CTR of greater than 0.50 suggests cardiomegaly [9]. Limited studies to evaluate the associations between CTR and CMR-derived ventricular size in patients with repaired TOF have, however, yielded inconsistent results [10,11]. Given the paucity of data, this study aims to determine the associations between CTR and CMR-derived ventricular volumes and indices of ventricular function in adolescents and young adults after repair of TOF.
This was a retrospective study of patients with repaired TOF aged ≥10 years followed up in the paediatric cardiac clinic. Only patients with CMR performed within 12 months of the available chest radiograph were recruited. Exclusion criteria included 1) patients with anteroposterior chest radiographs, pleural effusion, or pulmonary consolidation, 2) those with incomplete CMR data, and 3) patients who had surgical or catheter intervention between the chest radiographic and CMR examination. The study was approved by Institutional Review Board and the need for consent was waived because of the retrospective nature of the study.
Chest radiographs were evaluated as technically adequate if there were no gross rotational or thoracic abnormalities. All posteroanterior films, regardless of the depth of inspiration, were included. Patients with coexisting lung pathology or pleural effusion where the cardiac silhouette was indistinct were excluded. The CTR was measured as the ratio of the maximal dimension of the cardiac silhouette to the transverse diameter of the rib cage at the level of the right hemidiaphragm using CMS radiology viewer (Fig. 1) and a CTR of >0.5 was defined as abnormal [9]. All measurements of CTR were performed by a single investigator (JPH) who was blinded to the CMR findings.
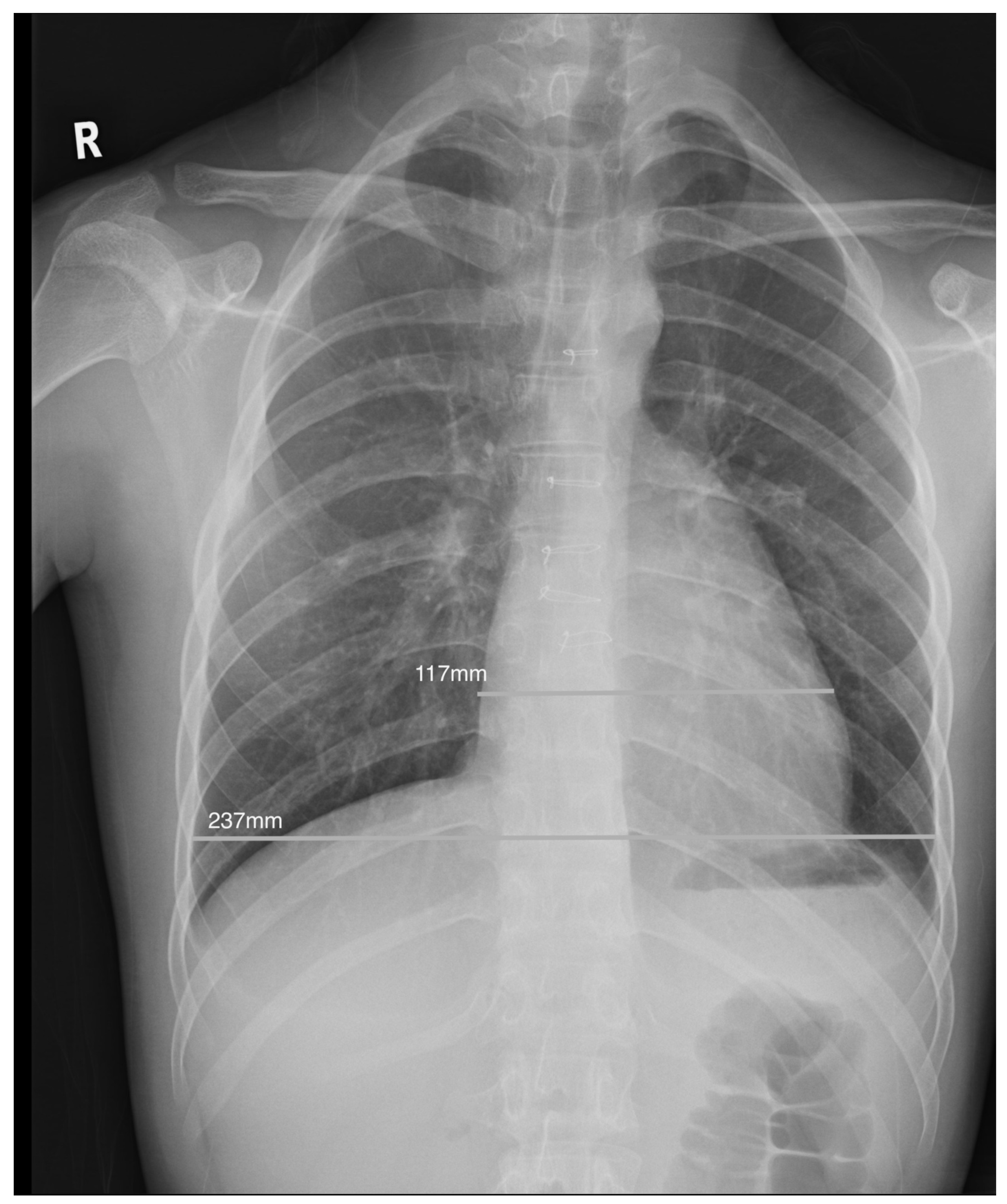
Figure 1: Illustration of a normal cardiothoracic ratio of 0.49 in an adolescent with increased indexed RV end-diastolic volume of 163 mL/m2 after repair of tetralogy of Fallot.
2.3 Assessment of Ventricular Volume and Function
Cardiac magnetic resonance was performed using a 1.5 T unit (MAGNETOM Aera, Siemens, Erlangen, Germany). A series of vector electrocardiographically (ECG) gated breath-hold or free-breathing balanced steady-state free precession stack of short-axis images encompassing both ventricles were acquired. Imaging parameters are as follows: retrospective gating, repetition time 2.9 ms, echo time 1.24 ms, temporal resolution 25 ms–45 ms, 63-degree flip angle, slice thickness 6 mm–7 mm, gap 20%–25% (1.2 mm–1.8 mm), and voxel size 1.3 mm × 1.3 mm × 7 mm. Manual delineation of epicardial and endocardial contours at end-diastole and end-systole of the left ventricle and endocardial contours of the right ventricle at end-diastole and end-systole was performed. The LV and RV end-systolic volume (ESV) and end-diastolic volume (EDV), and stroke volume (SV) were determined by the dedicated software (CVi42, Circle CVI, Calgary, AB, Canada). The ventricular volumes were indexed by the body surface area. Pulmonary regurgitant fraction was calculated as retrograde flow volume divided by antegrade flow volume in the proximal main pulmonary artery using ECG-gated, free-breathing cine phase contrast sequence obtained in the short-axis of the proximal main pulmonary artery [12].
For the interpretation of RV and LV volume overload, the following criteria were used [10]: (i) a normal size refers to indexed RV EDV of ≤110 mL/m2 and LV EDV of ≤90 mL/m2, (ii) mild dilation refers to indexed RV EDV of >110 mL/m2 to ≤140 mL/m2 and LV EDV of >90 mL/m2 to ≤120 mL/m2, (iii) moderate dilation refers to indexed RV EDV of >140 mL/m2 to ≤170 mL/m2 and LV EDV of >120 to ≤150 mL/m2, and iv) severe dilation refers to indexed RV EDV of >170 mL/m2 and LV EDV of >150 mL/m2 [10]. Impairment of systolic function of the right ventricle was defined by an ejection fraction <45% and that of the left ventricle by an ejection fraction <55%. Moderate to severe PR was defined as pulmonary regurgitant fraction ≥25%.
Data are presented as mean ± SD or median (interquartile range) where appropriate. Normality was assessed using Kolmogorov-Smirnov test. Demographic and CMR parameters of patients with normal and those with increased CTR were compared by unpaired t-test and Mann-Whitney U test for normally distributed and non-normal data, respectively. Associations between CTR and CMR-derived ventricular volume and functional parameters were assessed by Pearson correlation. The diagnostic accuracies of CTR in identifying moderate to severe RV or LV dilation and any degree of RV or LV dilation were assessed by receiver operator characteristic (ROC) analysis with calculation of area under the curve (AUC). A p value <0.05 was regarded as statistically significant. All analyses were performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY, USA).
A total of 76 (54 males) patients aged 22.1 ± 6.4 years were included in this study (Table 1). Of these 76 patients, 9 (11.9%) had DiGeorge syndrome, and one each with Noonan syndrome, Holt-Oram syndrome, and Miller-Dieker syndrome. Twenty-five patients required palliative shunt operation followed by total surgical repair performed at a median of 2.9 years (range, 0.6 years to 4.2 years).
Table 1: Demographic and cardiac magnetic resonance parameters*.
| All | CTR ≤ 0.5 | CTR > 0.5 | p Value | |
|---|---|---|---|---|
| (n = 76) | (n = 22) | (n = 54) | ||
| Age (year) | 22.1 ± 6.4 | 23.6 ± 5.0 | 21.54 ± 6.8 | 0.19 |
| Gender (M/F) | 54/22 | 17/5 | 37/17 | 0.58 |
| Age at TOF repair (year) | 2.9 (0.6–4.2) | 3.2 (2.2–4.2) | 2.7 (0.2–4.2) | 0.17 |
| Interval between CXR and CMR (month) | 6.0 (2.5–8.0) | 6.0 (2.0–8.0) | 5.5 (3.0–8.0) | 0.36 |
| Follow-up duration (year) | 20.7 ± 6.4 | 21.7 ± 4.5 | 20.3 ± 7.1 | 0.33 |
| Indexed RV EDV (mL/m²) | 129.7 ± 34.7 | 125.3 ± 31.4 | 131.4 ± 36.1 | 0.52 |
| Indexed RV ESV (mL/m²) | 66.4 ± 24.8 | 64.2 ± 23.7 | 67.2 ± 25.4 | 0.65 |
| RV EF (%) | 48.4 (42.4–53.1) | 46.7 (42.0–52.0) | 48.6 (42.8–53.1) | 0.61 |
| Indexed LVEDV (mL/m²) | 74.0 (66.3–85.8) | 70.6 (59.2–81.4) | 74.3 (69.4–89.5) | 0.57 |
| Indexed LVESV (mL/m²) | 30.8 (26.4–39.6) | 31.7 (27.2–33.9) | 30.8 (26.4–41.0) | 0.88 |
| LV EF (%) | 58.4 ± 7.7 | 59.5 ± 7.5 | 58.0 ± 7.7 | 0.48 |
| Sum of LV and RV EDV (mL/m²) | 208.1 ± 47.8 | 191.8 ± 42.4 | 214.1 ± 48.7 | 0.11 |
| PR fraction (%) | 33.7 ± 16.4 | 35.1 ± 15.4 | 33.2 ± 16.9 | 0.68 |
The CTR of the entire patient cohort was 0.5 ± 0.1. Of the 76 patients, 22 (28.9%) patients had CTR ≤ 0.5 while 54 (71%) had CTR >0.5. The average absolute time interval between chest radiograph and CMR was 6.0 months (range, 2.5 months to 8.0 months).
With regard to the CMR findings, the RV EDV for the entire cohort was 129.7 ± 34.7 mL/m² and the pulmonary regurgitant fraction was 33.7% ± 16.4%. The right ventricle was dilated in 50 (65.8%) patients, being mild in 24 (31.6%), moderate in 17 (22.4%), and severe in 9 (11.8%) patients. On the other hand, the left ventricle was only mildly dilated in 10 (13.2%) patients and moderately to severely dilated in 2 (2.6%) patients. For the functional CMR parameters, 27 (35.5%) patients had impaired RV ejection fraction and 23 (30%) patients had impaired LV ejection fraction. Eleven (14.5%) patients had impairment of both RV and LV ejection fraction. Forty-six (76%) patients had significant PR.
The CMR-derived ventricular volume and functional parameters were compared between patients with increased and those with normal CTR (Table 1). The two groups did not show significant differences in CMR volumetric and functional parameters and severity of PR (all p > 0.05). A significant proportion of patients with RV dilation based on CMR assessment had normal CTR. Among the 22 patients with CTR ≤ 0.5, 13 (59.1%) had varying degrees of RV dilation, 7 patients (31.8%) had moderate or severe RV dilation, and 1 (4.5%) had LV dilation. Among the 54 patients with CTR >0.5, 12 patients (22%) had normal indexed RV EDV, 33 patients (61.1%) had normal indexed LV EDV, and 11 patients (20.3%) had both normal indexed LV and RV EDV.
Fig. 2 shows the scatterplots of CTR versus CMR-derived volumetric and functional parameters. The CTR did not correlate with indexed RV EDV (r = 0.06, p = 0.65), RV ESV (r = 0.06, p = 0.65), LV EDV (r = 0.23, p = 0.08), and LV ESV (r = 0.18, p = 0.16). Furthermore, there were no associations between CTR and LV (r = −0.07, p = 0.6) or RV (r < −0.01, p = 0.97) ejection fraction. Further subgroup analyses found that even in patients with moderate to severe RV dilation, the CTR did not show correlation with RV or LV volumes (all p > 0.05). When stratified by age, patients aged <18 years were found to have CTR being significantly correlated with indexed LV EDV (r = 0.47, p = 0.03) but not LV ESV and RV volumes (all p > 0.05).
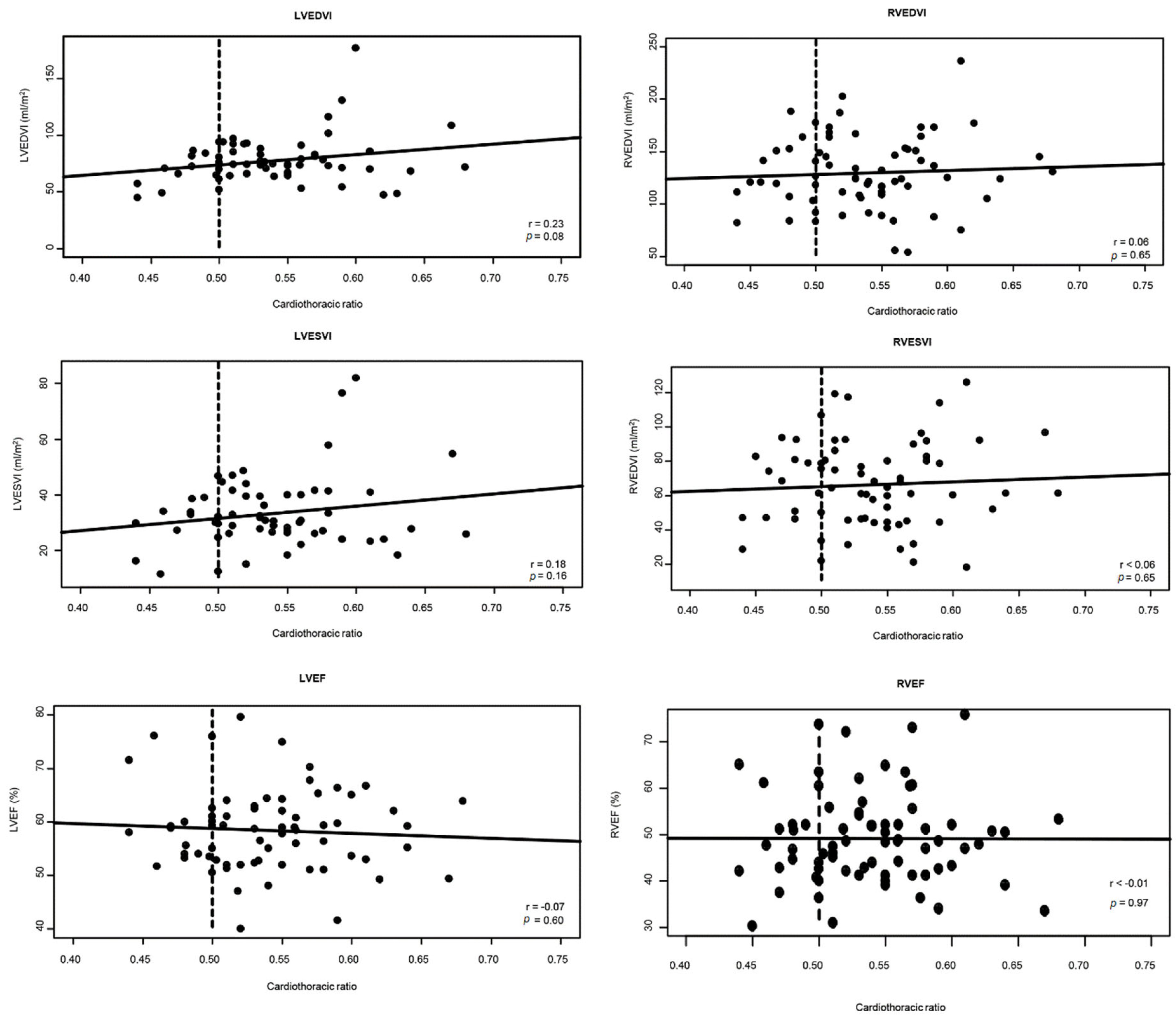
Figure 2: Scatterplots showing the absence of correlations between cardiothoracic ratio and right and LV volumes and ejection fraction. Abbreviations: EDVI = indexed end-diastolic volume; EF = ejection fraction; ESVI = indexed end-systolic volume; LV = left ventricular; RV = right ventricular. *Dashed lines represent the cut-off value for increased cardiothoracic ratio.
The ROC curves and AUC to determine cutoffs of CTR for prediction of RV or LV dilation are shown in Fig. 3. Overall, CTR could not discriminate patients with any degree of RV (AUC 0.49) or LV (AUC 0.50) dilation or those with moderate to severe degree of RV (AUC 0.50) or LV (AUC 0.46) dilation from patients without ventricular dilation.
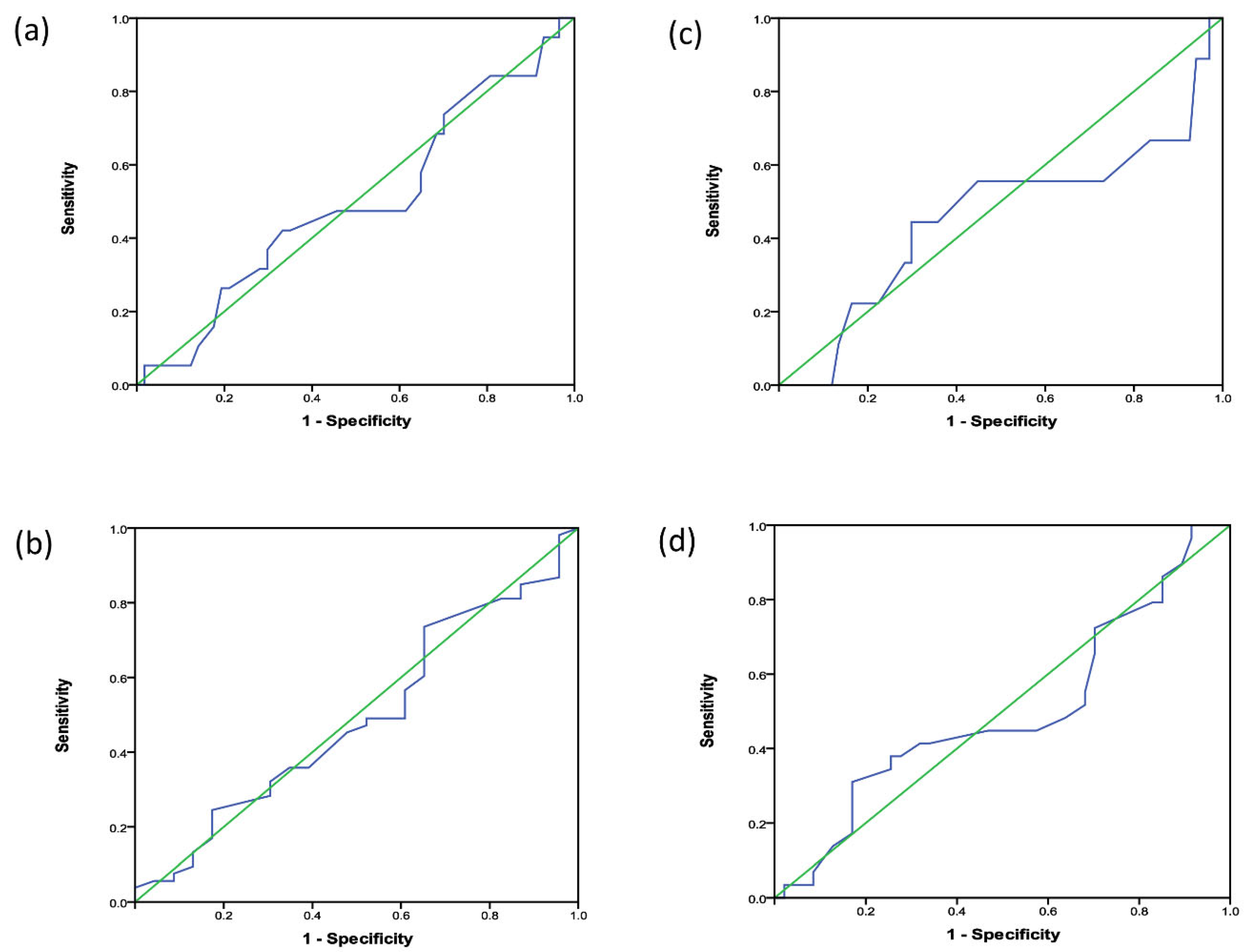
Figure 3: ROC curves for determination of CTR cut-off values to predict (a) increased LV end-diastolic volume of any degree, (b) moderate to severely increased LV end-diastolic volume, (c) increased RV end-diastolic volume of any degree, and (d) moderately to severely increased right ventricular end-diastolic volume.
The present study demonstrates the failure of CTR based on the chest radiograph to reflect dilation or reduced ejection fraction of either the right or the left ventricle in adolescents and young adults with repaired TOF. The ROC analysis shows that CTR has a poor discriminatory ability to differentiate patients with from those without RV or LV dilation or dysfunction. Our data caution against the use of CTR solely for the monitoring of ventricular dilation, in particular RV volume overload, in patients after repair of TOF.
Previous studies on the relationship between CTR and ventricular volume and function in patients with repaired tetralogy of Fallot are limited and have yielded inconsistent results. Our findings agree with those reported by Spiewak et al. [10], who performed a retrospective study of 82 repaired TOF patients with a median age of 24.7 years. Similar to our results, they showed that CTR did not correlate with indexed RV and LV EDV and ejection fraction. Indeed, their finding of 64.9% of patients having normal CTR but with severe RV dilation is consistent with our finding of about 60% of our cohort with normal CTR yet having varying degrees of RV dilation. Although CTR is generally used as an indicator to evaluate LV volume [13], the RV volume overload with distortion of the septal geometry may have limited its usefulness in this regard in patients with repaired TOF. While the cause is unclear, interestingly, we found that in patients <18 years of age, the CTR is found to correlated with indexed LV EDV but not RV volumes. The present study has additionally shown that CTR is not associated with ejection fraction of both the right and left ventricles in these patients. In another study, Grotenhuis et al. examined the usefulness of assessing CTR in 127 children with different cardiac pathologies, 76 of whom had repaired TOF [11]. They found, on the other hand, that CTR correlated significantly with indexed RV EDV and ESV. Nonetheless, on closer inspection of their results, patients with CTR less than 0.5 could have an indexed end-diastolic RV volumes ranging from less than 100 mL/m2 to more than 200 mL/m2. Taken together, the findings of the present and previous studies do not support the usefulness of CTR in the monitoring of ventricular volume overloading in patients with repaired TOF.
There are probable reasons to explain why CTR may not accurately estimate RV volume overloading in repaired TOF patients. In the posteroanterior chest radiograph, the right heart border comprises the relatively straight wall of superior caval vein and the mildly convex right atrial wall [14]. The left heart contour, on the other hand, comprises four segments, namely from cranial to caudal, the aortic knob, main pulmonary artery, left atrial appendage, and lateral wall of the left ventricle [14]. When the right ventricle dilates, it expands anteriorly, superiorly, and leftwards to push the left ventricle and rotate the heart [10]. The change in the anteroposterior and anterosuperior dimensions with RV dilation cannot be reflected by the CTR. On the other hand, it is perhaps not unexpected of a weak correlation between right atrial volume and CTR shown previously in repaired TOF patients [10]. In the present study, we did not assess the right atrial volume.
It is unclear whether there exists better alternative X-ray measurement to enhance the diagnostic utility in patients with repaired TOF. Lateral cardiothoracic ratio has been found to have no additional value for quantification of cardiac size in patients with repaired TOF, aortic regurgitation, isolated left-to-right shunt and hypertrophic cardiomyopathy [11]. Browne et al. reported that the two-dimensional cardiothoracic ratio, being extracted by defining a region of interest around the cardiac and thoracic areas and calculating a ratio of the two pixel counts, has better correlation with LV ejection fraction than the conventional one-dimensional cardiothoracic ratio [15]. However, it remains unclear whether this applies also to the assessment RV ejection fraction and volumes.
Notwithstanding the findings of our study, the chest radiograph remains to have a role in the follow-up of patients with repaired TOF. For this patient population, the chest radiographic information on pulmonary vasculature, lung parenchyma, conduit calcification, and stent integrity are of clinical relevance. In patients with congenital heart disease other than TOF, CTR has commonly been used to quantify LV dilation in left-to-right shunts and left-sided valvar regurgitation. Interestingly though, Grotenhuis et al. found that CTR, while correlating with LV volume in aortic regurgitation, did not correlate with cardiac chamber volumes in patients with left-to-right shunts [11].
Nevertheless, in the present era, the multimodality imaging approach with utilization of echocardiography, computed tomography, and CMR in addition to a plain chest radiograph should be adopted for comprehensive evaluation of this at-risk TOF patient population [16,17,18,19]. Echocardiography is readily available and provides information on RV volume overload, biventricular function, residual RV outflow obstruction and ventricular septal defect, severity of PR, aortic root size, and parameters of myocardial deformation and ventricular dyssynchrony [18]. However, it has limitations in the accurate quantification of RV size and severity of PR. Furthermore, the quality of echocardiographic images in adolescents and adults with poor acoustic windows can be suboptimal. Cardiac magnetic resonance overcomes these limitations and provides high-quality, reproducible measurements of biventricular size and function, myocardial fibrosis, and pulmonary blood flow without exposure to radiation. Furthermore, CMR is independent of acoustic windows and is ideal for long-term follow-up [19]. However, it is costly, less accessible, lacks portability, and is contraindicated in patients with non-CMR compatible pacemakers or defibrillators and claustrophobia. Metallic implants in patients may further cause artifacts. In these latter groups of patient, computed tomography may have a role.
There are several limitations to this study. First, inadequate inspiratory effort in the paediatric population would affect the accuracy of assessing the CTR, given that CTR is larger during expiration and smaller at inspiration [14]. Second, CTR was interpreted by a single investigator who was not blinded to the patient status. Nevertheless, the assessment of CTR is standard and performed with the investigator blinded to the results of CMR. Third, there may be interval changes in CTR and CMR parameters, albeit the changes are likely to be small, as the longest time interval between two imaging modality was 12 months for our patient cohort. Indeed, Hoelscher et al. reported that most of the patients with repaired TOF and severe PR late after surgery, RV dilation did not progress over a three-year follow-up [20]. Finally, the small sample size in the present study remains an important limitation that needs to be addressed in future studies with larger number of patients.
Cardiothoracic ratio as measured from the chest radiograph does not reflect RV or LV dilation or systolic dysfunction in patients with repaired tetralogy of Fallot. Further research to validate the findings of the present study should include a large sample size. For the purpose of monitoring the long-term complications of repaired TOF, multimodality assessment with echocardiography and CMR is indicated.
Acknowledgement:
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Jacob PL Ho and Yiu-fai Cheung; data collection: Jacob PL. Ho and Carol WK Ng; analysis and interpretation of results: Jacob PL Ho, Carol WK Ng and Wilfred HS Wong; drafting manuscript preparation: Jacob PL Ho and Yiu-fai Cheung. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: The review of clinical records was approved by the authors’ Central Institutional Review Board of Hospital Authority (Ref No.: PAED-2022-011) and was conducted in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The requirement for informed patient consent was waived by the Board due to the retrospective nature of the research.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, et al. 2020 ESC guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42(6):563–645. doi:10.1093/eurheartj/ehaa554. [Google Scholar] [CrossRef]
2. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(14):e698–800. [Google Scholar]
3. Khairy P, Aboulhosn J, Gurvitz MZ, Opotowsky AR, Mongeon FP, Kay J, et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010;122(9):868–75. doi:10.1161/CIRCULATIONAHA.109.928481. [Google Scholar] [CrossRef]
4. Ghai A, Silversides C, Harris L, Webb GD, Siu SC, Therrien J. Left ventricular dysfunction is a risk factor for sudden cardiac death in adults late after repair of tetralogy of Fallot. J Am Coll Cardiol. 2002;40(9):1675–80. doi:10.1016/S0735-1097(02)02344-6. [Google Scholar] [CrossRef]
5. Van den Eynde J, Sá M, Vervoort D, Roever L, Meyns B, Budts W, et al. Pulmonary valve replacement in tetralogy of Fallot: an updated meta-analysis. Ann Thorac Surg. 2022;113(3):1036–46. doi:10.1016/j.athoracsur.2020.11.040. [Google Scholar] [CrossRef]
6. Fogel MA, Anwar S, Broberg C, Browne L, Chung T, Johnson T, et al. Society for Cardiovascular Magnetic Resonance/European Society of Cardiovascular Imaging/American Society of Echocardiography/Society for Pediatric Radiology/North American Society for Cardiovascular Imaging Guidelines for the Use of Cardiac Magnetic Resonance in Pediatric Congenital and Acquired Heart Disease: Endorsed by The American Heart Association. Circ Cardiovasc Imaging. 2022;15(6):e014415. [Google Scholar]
7. Srinivasan C, Sachdeva R, Morrow WR, Greenberg SB, Vyas HV. Limitations of standard echocardiographic methods for quantification of right ventricular size and function in children and young adults. J Ultrasound Med. 2011;30(4):487–93. doi:10.7863/jum.2011.30.4.487. [Google Scholar] [CrossRef]
8. Dutta T, Aronow WS. Echocardiographic evaluation of the right ventricle: clinical implications. Clin Cardiol. 2017;40(8):542–8. doi:10.1002/clc.22694. [Google Scholar] [CrossRef]
9. Truszkiewicz K, Poręba R, Gać P. Radiological cardiothoracic ratio in evidence-based medicine. J Clin Med. 2021;10(9):2016. doi:10.3390/jcm10092016. [Google Scholar] [CrossRef]
10. Spiewak M, Małek LA, Biernacka EK, Kowalski M, Michałowska I, Hoffman P, et al. Cardiothoracic ratio may be misleading in the assessment of right- and left-ventricular size in patients with repaired tetralogy of Fallot. Clin Radiol. 2014;69(7):e1–8. doi:10.1016/j.crad.2014.03.009. [Google Scholar] [CrossRef]
11. Grotenhuis HB, Zhou C, Tomlinson G, Isaac KV, Seed M, Grosse-Wortmann L, et al. Cardiothoracic ratio on chest radiograph in pediatric heart disease: how does it correlate with heart volumes at magnetic resonance imaging? Pediatr Radiol. 2015;45(11):1616–23. doi:10.1007/s00247-015-3386-9. [Google Scholar] [CrossRef]
12. Samyn MM, Powell AJ, Garg R, Sena L, Geva T. Range of ventricular dimensions and function by steady-state free precession cine MRI in repaired tetralogy of Fallot: right ventricular outflow tract patch vs. conduit repair. J Magn Reson Imaging. 2007;26(4):934–40. doi:10.1002/jmri.21094. [Google Scholar] [CrossRef]
13. Loomba RS, Shah PH, Nijhawan K, Aggarwal S, Arora R. Cardiothoracic ratio for prediction of left ventricular dilation: a systematic review and pooled analysis. Future Cardiol. 2015;11(2):171–5. doi:10.2217/fca.15.5. [Google Scholar] [CrossRef]
14. Baron MG. The cardiac silhouette. J Thorac Imaging. 2000;15(4):230–42. doi:10.1097/00005382-200010000-00003. [Google Scholar] [CrossRef]
15. Browne RF, O’Reilly G, McInerney D. Extraction of the two-dimensional cardiothoracic ratio from digital PA chest radiographs: correlation with cardiac function and the traditional cardiothoracic ratio. J Digit Imaging. 2004;17(2):120–3. doi:10.1007/s10278-003-1900-3. [Google Scholar] [CrossRef]
16. Moscatelli S, Pergola V, Motta R, Fortuni F, Borrelli N, Sabatino J, et al. Multimodality imaging assessment of tetralogy of Fallot: from diagnosis to long-term follow-up. Children. 2023;10(11):1747. doi:10.3390/children10111747. [Google Scholar] [CrossRef]
17. Valente AM, Cook S, Festa P, Ko HH, Krishnamurthy R, Taylor AM, et al. Multimodality imaging guidelines for patients with repaired tetralogy of fallot: a report from the AmericanSsociety of Echocardiography: developed in collaboration with the Society for Cardiovascular Magnetic Resonance and the Society for Pediatric Radiology. J Am Soc Echocardiogr. 2014;27(2):111–41. doi:10.1016/j.echo.2013.11.009. [Google Scholar] [CrossRef]
18. Huntgeburth M, Germund I, Geerdink LM, Sreeram N, Udink Ten Cate FEA. Emerging clinical applications of strain imaging and three-dimensional echocardiography for the assessment of ventricular function in adult congenital heart disease. Cardiovasc Diagn Ther. 2019;9(Suppl 2):S326–45. doi:10.21037/cdt.2018.11.08. [Google Scholar] [CrossRef]
19. Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson. 2011;13(1):9. doi:10.1186/1532-429X-13-9. [Google Scholar] [CrossRef]
20. Hoelscher M, Bonassin F, Oxenius A, Seifert B, Leonardi B, Kellenberger CJ, et al. Right ventricular dilatation in patients with pulmonary regurgitation after repair of tetralogy of Fallot: how fast does it progress? Ann Pediatr Cardiol. 2020;13(4):294–300. doi:10.4103/apc.APC_140_19. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools