 Open Access
Open Access
ARTICLE
Surgical Treatment of Anomalous Left Coronary Artery Originating from the Pulmonary Artery: A Single-Center Experience
1 Department of Cardiothoracic Surgery, Children’s Hospital of Chongqing Medical University, National Clinical Medical Research Center for Children’s Health and Diseases, Key Laboratory of the Ministry of Education for the Study of Childhood Developmental Diseases, Chongqing, 401122, China
2 Chongqing Key Laboratory of Structural Birth Defects and Organ Repair and Reconstruction, Chongqing, 401122, China
* Corresponding Author: Yong An. Email:
Congenital Heart Disease 2025, 20(3), 347-355. https://doi.org/10.32604/chd.2025.065354
Received 10 March 2025; Accepted 25 June 2025; Issue published 11 July 2025
Abstract
Background: Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital anomalous coronary artery origin disorder. Objective: We sought to summarize the clinical experience and prognostic characteristics of surgical treatment of ALCAPA. Methods: We retrospectively analyzed clinical information on patients who had ALCAPA and underwent surgery at our center from February 2016 to October 2023. Results: This comparative study of 23 ALCAPA patients (9 infants <1 year; 14 children >1 year) demonstrated significant age-dependent outcomes. Infant patients exhibited markedly prolonged mechanical ventilation (183 ± 105.6 vs. 48.5 ± 62.2 min, p = 0.001) and hospitalization (30.8 ± 8.2 vs. 19.5 ± 6.2 days, p = 0.001), despite comparable operative times (p > 0.05). The perioperative mortality rate was 8.7% (2/23). Early postoperative mortality showed a non-significant trend in infants (22.2% vs. 0%, p = 0.11). Serial follow-up revealed substantial functional improvement, with abnormal left ventricular ejection fractions decreasing from 56.5% preoperatively to 14.3% at 1-month (p < 0.01), and severe mitral regurgitation declining from 34.7% to 14.3%. However, persistent left ventricular enlargement (81% at follow-up) and moderate mitral regurgitation (52.4%) were frequently observed. Conclusion: Surgical correction of ALCPA effectively restores coronary perfusion and reduces severe mitral regurgitation, though residual ventricular dilation and moderate valvular dysfunction persist in the short-term postoperative period. Nevertheless, the overall prognosis remains favorable when timely intervention is performed.Keywords
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital anomalous coronary artery origin disorder. With advances in ultrasound, computed tomography (CT) imaging, and other technologies, an increasing number of asymptomatic patients with ALCAPA are diagnosed and treated, and the population incidence of the disease has risen to 0.021% [1]. The disease usually exists as an isolated anomaly, though it may occasionally be associated with other cardiac malformations. The mortality rate reaches 90% in newborns up to one year of age, and the rate of sudden death in adults up to 35 years of age reaches 80% to 90%. Early diagnosis and surgical treatment in children can completely correct these anatomical abnormalities at an early stage, thereby improving cardiac function and long-term prognosis [2]. This study aimed to retrospectively analyze clinical information on patients who had ALCAPA and underwent surgery at our single center, and compared the demographic and surgical variables of ALCAPA.
We retrospectively analyzed 23 patients with ALCAPA who underwent surgical treatment at the Children’s Hospital of Chongqing Medical University between June 2016 and November 2023. Intraoperative findings confirmed the preoperative diagnoses in all cases.
All patients underwent electrocardiogram (ECG), Echo, chest radiography. CT angiography (CTA) was performed in the majority of cases to assess coronary artery anatomy, including abnormal vessel lengths and diameters. Although invasive coronary angiography remains the diagnostic gold standard, its risks are particularly elevated in infants and young children, including radiation exposure, anesthetic drugs, and contrast agent-related complications. Therefore, when non-invasive imaging modalities provide sufficient diagnostic information for surgical planning, we typically avoid coronary angiography to minimize risks. Multidisciplinary discussions were conducted for complex cases. We usually have some discussions about the complex situation. Representative imaging findings are illustrated in Fig. 1. In addition, myocardial nuclear imaging and magnetic resonance coronary angiography (MRCA) can be used as complementary tests, but not as routine tests [3].
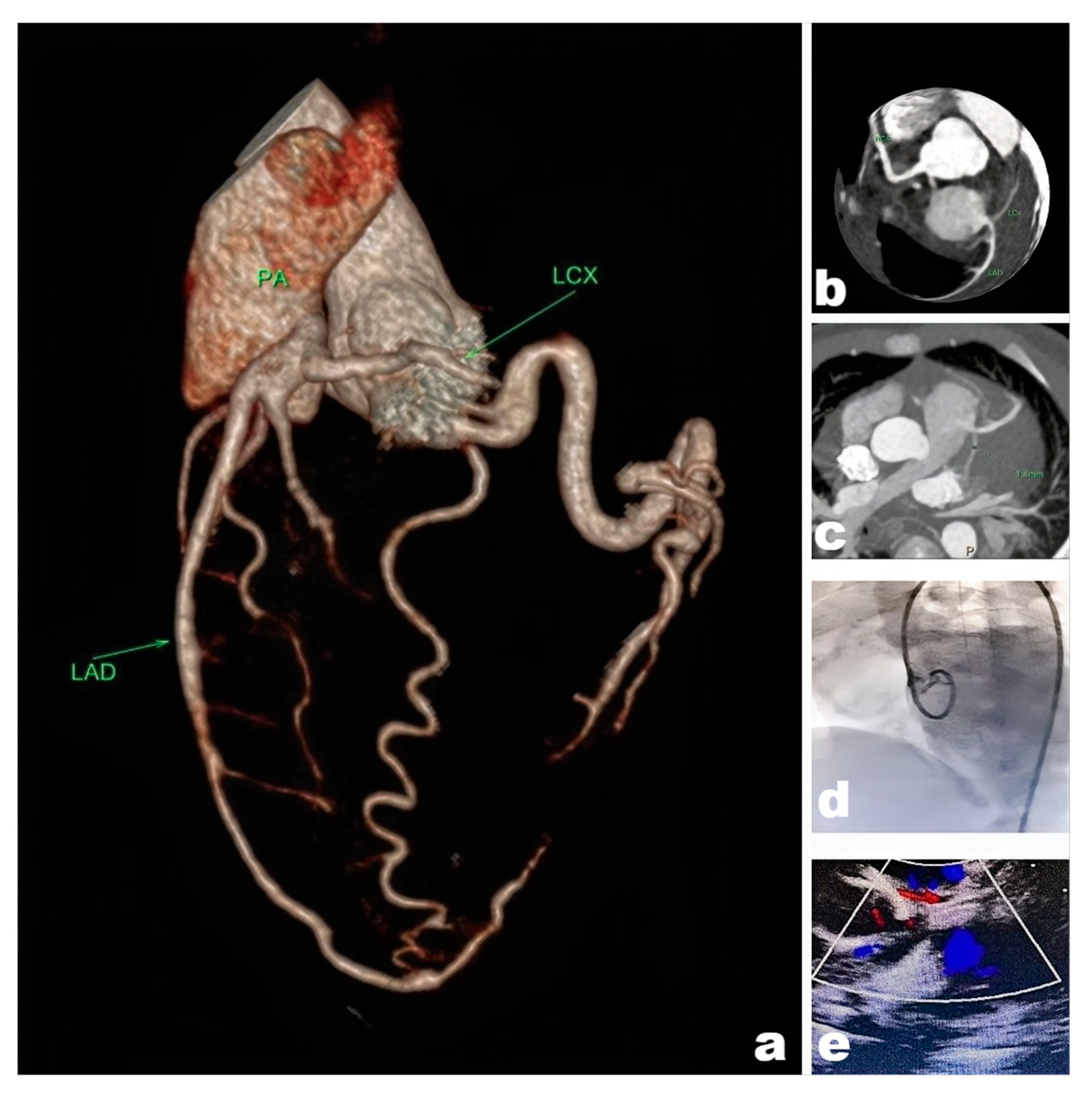
Figure 1: Picture (a). CTA reconstruction revealed an Anomalous origin of the left coronary artery from the pulmonary artery. (b,c). Images of coronary suggesting that the left coronary artery anomaly originated from the pulmonary artery. (d). Invasive coronary angiography of the left coronary artery was disappeared, indicating that the hemodynamic abnormality was present in the left coronary artery. (e). Ultrasound suggesting that abnormal blood flow signals were present in the pulmonary artery, the “blood-stealing syndrome” was happened.
The key steps of the surgery are as follows:
- Median sternotomy: The standard surgical approach, providing optimal operative exposure. Incision extent: Typically from 1 cm below the suprasternal notch to the xiphoid process, with length adjusted according to patient anatomy.
- Cardiopulmonary bypass (CPB) establishment: Cannulation of the aorta and both superior and inferior venae cavae to maintain systemic perfusion.
- Myocardial protection: Administration of cold blood cardioplegia to induce cardiac arrest, minimizing ischemic myocardial injury.
- Direct coronary reimplantation (preferred method): a. Identification and mobilization of the anomalous left coronary artery (LCA) based on preoperative imaging, preserving adequate length. b. Creation of a button-shaped aortotomy at the left coronary sinus. c. Anastomosis of the LCA to the aortic root using continuous polypropylene sutures. d. Pulmonary artery reconstruction: If the LCA harvest site involves the pulmonary artery wall, repair is performed using autologous pericardium or prosthetic patch material.
- Intrapulmonary artery tunnel (Takeuchi procedure): (Indicated when the LCA ostium is distant from the aorta). a. Creation of an aortopulmonary window (4–5 mm diameter) in the interatrial septum. b. Construction of an intrapulmonary tunnel using a flap from the anterior pulmonary artery wall to connect the LCA to the aorta. c. Pulmonary arterioplasty with patch augmentation to prevent stenosis.
- Mitral valve repair is indicated only when Cardiac ultrasound and surgical inspection demonstrate structural pathology; otherwise, it should be avoided
The key technical notes: All anastomoses should be performed under tension-free conditions to ensure patency. Intraoperative transesophageal echocardiography (TEE) is mandatory to verify coronary flow and ventricular function. The key steps in the surgery are illustrated in Fig. 2.

Figure 2: Intraoperative exploration of the left coronary opening, freeing of the left coronary artery, and reconstruction of the left coronary system.
Data were statistically analyzed using statistical software. Continuous variables with normal distribution were expressed as mean ± standard deviation (SD) and analyzed using Student’s t-test (SPSS version 26; IBM Corp.). Non-normally distributed variables were presented as median (interquartile range). Categorical variables were summarized as n (%). The p-value < 0.05 was considered statistically significant.
The study was approved by the Ethics Committee of the Affiliated Children’s Hospital of Chongqing Medical University [approval No. 2023(515)], was conducted in accordance with the Declaration of Helsinki, and waived the need for informed consent from the patients who were retrospectively analyzed in the study.
All 23 patients underwent surgical repair, direct coronary reimplantation (include pulmonary artery reconstruction) in 22 cases and intrapulmonary tunnel creation (Takeuchi procedure) in 1 case. Concomitant mitral valve was repaired in 5 patients. Postoperative management included: intravenous inotropic support (dopamine, milrinone, and epinephrine), and guideline-directed medical therapy for heart failure (β-blockers, ACE inhibitors, and diuretics). Mechanical circulatory support (venoarterial extracorporeal membrane oxygenation [VA-ECMO] in n = 3) was instituted for refractory cardiogenic shock. The preoperative clinical manifestations of the patients and relevant data are shown in Table 1, Table 2 and Table 3.
Table 1: 23 patients’ age and presenting with different clinical manifestations preoperatively (mean ± standard deviation, months).
| Number of People (n%) | Age (months) | ||
|---|---|---|---|
| Infants under 1 year of age | Feeding difficulties | 6 | 6.1 ± 2.3 |
| cyanosis | 8 | 4.2 ± 1.1 | |
| Children over 1 year of age | chest distress | 6 | 29.3 ± 20.6 |
| fainting | 4 | 56.8 ± 24.1 | |
| Other symptoms | Heart murmur | 16 (16/23) | 40.5 ± 26.3 |
| Expansion of the Heart Sphere | 14 (14/23) | 38.2 ± 30.7 | |
| Reduced mobility | 16 (16/23) | 34.2 ± 29.4 | |
Table 2: Imaging data of ALCAPA patients (n%).
| Preoperative (n%) 23 People | Review at Discharge (n%) 21 People | 1-Month Discharge Follow-up (n%) 21 People | |
|---|---|---|---|
| LVEF anomaly | 13 (56.5) | 4 (19.0) | 3 (14.3) |
| left ventricle enlargement | 20 (86.9) | 17 (80.9) | 17 (81.0) |
| Decreased systolic function | 15 (65.2) | 13 (68.4) | 10 (47.6) |
| Mild mitral valve regurgitation | 5 (21.7) | 7 (33.3) | 7 (33.3) |
| Moderate mitral valve regurgitation | 10 (43.3) | 10 (47.6) | 11 (52.4) |
| Severe mitral regurgitation | 8 (34.7) | 4 (19.0) | 3 (14.3) |
| Organic mitral valve lesions | 5 (21.7) | 3 (14.2) | 3 (14.3) |
| Enrichment of collateral circulation | 13 (56.5) | 12 (57.1) | 12 (57.1) |
| Anomalous site on the CTA: Originating at the beginning of the main pulmonary artery | 4 (17.4) | ||
| Originating from the main sinus | 7 (30.4) | ||
| Originating from the left wall of the MPA | 9 (39.1) | ||
| Originating from the right wall of the MPA | 3 (13.1) |
Table 3: Demographic and Surgical Data.
| Infants (<1 year, n = 9) | Children (>1 year, n = 14) | p | |
|---|---|---|---|
| Operative time (h) | 4.6 ± 1.1 | 3.9 ± 1.3 | p > 0.05 |
| CPB time (min) | 170 ± 56.7 | 164.4 ± 69.2 | p > 0.05 |
| ACC time (min) | 133.2 ± 31.1 | 119.3 ± 32.4 | p > 0.05 |
| Mechanical ventilation duration (min) | 183 ± 105.6 | 48.5 ± 62.2 | p = 0.01 |
| Mean hospital stay (D) | 30.8 ± 8.2 | 19.5 ± 6.2 | p = 0.01 |
| Early postoperative | 2 | 0 |
Postoperatively, 3 patients required VA-ECMO support, of whom 1 died and 2 were successfully weaned. Early postoperative mortality showed a non-significant trend in infants (22.2% vs. 0%, p = 0.11). The overall perioperative mortality was 2 cases, which the mortality rate was 8.7% (2/23), both occurring in infants aged <1 year: One patient expired from refractory ventricular arrhythmias despite maximal resuscitation efforts under ECMO support, while the other succumbed to irreversible low cardiac output syndrome (LCOS) unresponsive to optimized pharmacologic therapy.
3.3 The data of Surgical Treatment
This comparative study of 23 ALCAPA patients (9 infants <1 year; 14 children >1 year) demonstrated significant age-dependent outcomes. Infant patients exhibited markedly prolonged mechanical ventilation (183 ± 105.6 vs. 48.5 ± 62.2 min, p = 0.001) and hospitalization (30.8 ± 8.2 vs. 19.5 ± 6.2 days, p = 0.001), despite comparable operative times (p > 0.05). Early postoperative mortality showed a non-significant trend in infants (22.2% vs. 0%, p = 0.11). Serial follow-up revealed substantial functional improvement, with abnormal left ventricular ejection fractions (LVEF) decreasing from 56.5% preoperatively to 14.3% at 1-month (p < 0.01), and severe mitral regurgitation declining from 34.7% to 14.3%. Nevertheless, persistent left ventricular enlargement (81% at follow-up) and moderate mitral regurgitation (52.4%) were frequently observed.
All 21 surviving patients (follow-up during 1 month to 5 years) maintained clinical improvement without coronary anastomotic stenosis or major complications. A 4 year old child required redo mitral valve repair due to persistent regurgitation from intrinsic valve pathology, ultimately undergoing mechanical valve replacement with satisfactory mid-term function (though long-term outcomes remain uncertain).
Notably: Significant improvements in ejection fraction, systolic function, and cardiac chamber dimensions were sustained (all p < 0.05). No late deaths or reinterventions occurred. Longer follow-up is needed to evaluate delayed complications and durability.
For ALCAPA, the embryology may be due to abnormal development and migration of angioblast buds [4]. During the neonatal period (first 8 weeks of life), the predominant clinical manifestations include heart failure and secondary mitral regurgitation, resulting from inadequate collateral circulation between the right and left coronary systems following the physiologic decline in pulmonary vascular resistance. Affected infants typically present with nonspecific symptoms including failure to thrive and tachypnea, whereas Echo demonstrates marked left ventricular dilation and severely impaired systolic function. This condition follows a rapidly progressive course, with an untreated mortality rate approaching 90% within the first year of life [5,6]. Adult survivors typically demonstrate either right coronary artery dominance or well-developed intercoronary collaterals established in early infancy. When abundant collaterals are present, the right coronary artery provides chronic retrograde perfusion to the left coronary system, while blood shunts from the left coronary artery into the pulmonary artery due to the pressure gradient, creating the characteristic “blood-stealing syndrome”, resulting in chronic myocardial ischemia, progressive mitral valve dysfunction, and elevated risk of sudden cardiac death and life-threatening arrhythmias [6].Initial clinical screening typically involves ECG and Echo, which can reliably identify ALCAPA in experienced centers. Although invasive coronary angiography remains the diagnostic gold standard, CTA and Echo provide accurate noninvasive alternatives for initial evaluation in most cases.
Surgical intervention represents the only definitive treatment for ALCAPA and should be performed promptly upon diagnosis, regardless of patient age. The surgical objective is to establish a dual-coronary arterial system. Primary techniques include coronary reimplantation and the intrapulmonary tunnel creation (Takeuchi procedure) [7,8]. Palliative coronary ligation, while historically described, carries substantial risks of chronic myocardial ischemia and accelerated coronary atherosclerosis. Coronary reimplantation is the preferred approach, achieving anatomical correction in most cases with favorable postoperative outcomes [9]. The Takeuchi procedure remains an alternative option, though it fails to restore physiological anatomy and is associated with significant long-term complications. Late complications including tunnel leakage and supravalvular pulmonary stenosis frequently occur [10]. The Del Nido cardioplegia solution serves as a cornerstone of myocardial protection, offering superior efficacy and prolonged duration of action compared to conventional formulations. Its ability to minimize perfusion frequency renders it particularly advantageous for complex pediatric cardiac surgical procedures. Moreover, the combined aortic cross-clamping and pulmonary artery occlusion strategy provides another two critical physiological advantages: (1) hemodynamic stabilization by eliminating retrograde flow into the low-pressure pulmonary circulation (2) optimization of cardioplegia delivery by preventing washout through the anomalous coronary ostium. Systematic venting protocols before pulmonary artery reperfusion are mandatory to avoid air embolism-induced myocardial injury. In high-risk patients with severe left ventricular dysfunction and mitral valve regurgitation, perioperative extracorporeal membrane pulmonary oxygenation contributes to improved survival, and the rate of ECMO use at our institution was 13.0% (3/23), which is slightly higher than the reported average rate of 8.7% [11]. Significant debate persists in the literature about the indications for concomitant mitral valvuloplasty during primary ALCAPA repair, with particular moderate regurgitation. Isomatsu et al. [12] reported that concomitant mid-upper annuloplasty in all patients with mitral regurgitation, regardless of the degree of severity (mild-severe), was effective with a re-intervention rate of 4.2%. As described by Kudumula et al. and in the European multicenter study, only patients with moderate to severe mitral regurgitation undergo concomitant mitral valvuloplasty intraoperatively, but only when combined with organic mitral valve disease, because mitral regurgitation diminishes as cardiac function recovers [13,14]. Our institutional protocol aligns with European guidelines, advocating concomitant mitral valve repair exclusively for moderate-to-severe regurgitation with demonstrable structural abnormalities (e.g., leaflet prolapse). Among 8 such cases, 5 exhibited significant improvement, including one requiring subsequent valve replacement with favorable mid-term outcomes. For mild-to-moderate regurgitation, conservative management is preferred, as most pediatric patients demonstrate remarkable ventricular recovery (improved left ventricle dimensions and ejection fractions) within one month postoperatively. While international consensus on optimal surgical timing remains undefined, our experience strongly supports early intervention upon diagnosis to mitigate progressive myocardial injury. Although our follow-up duration precludes definitive long-term assessment, extant literature reports 20-year survival rates of 86 ± 4% [15], with outcomes continuing to improve through advancing surgical techniques. Future studies should address late coronary complications including stenosis and atherosclerotic disease. The current evidence demonstrates that postoperative EF improvement in ALCAPA patients is significantly age-dependent, with infants (<1 year) showing superior recovery compared to older children and adults. This is attributed to greater myocardial plasticity and reduced fibrosis in immature hearts, allowing near-normalization of EF when surgery is performed early [16]. In contrast, delayed repair (>1 year) often results in only marginal EF gains due to irreversible ischemic damage from coronary blood stealing. A critical window appears to exist before 6 months of age, beyond which residual ventricular dysfunction becomes more prevalent. This aligns with studies showing faster left ventricular end-diastolic diameter (LVEDD) normalization in infants [16], supporting early intervention to prevent maladaptive remodeling. Long-term survival remains favorable (86 ± 4% at 20 years), but delayed correction risks persistent ventricular dysfunction and late-onset heart failure. These findings underscore ALCAPA as a time-sensitive surgical condition, where prompt anatomical restoration optimizes myocardial salvage and functional recovery [17,18]. From a pathophysiological perspective, neonates exhibit reduced surgical tolerance due to immature organ systems, prolonged operative duration, and heightened postoperative care requirements. These factors necessitate meticulous risk-benefit assessment when determining optimal surgical timing for ALCAPA repair. This study has some limitations. As this was a single-center, retrospective study, while a high risk of selection bias maybe existed in patient selection, and the number of patients included was too small to evaluate the overall ALCAPA population. Meanwhile, the interpretation of the surgical intervention and postoperative management for patients may differ between hospitals. Therefore, further multi-center study is warranted. Moreover, we evaluated ALCAPA conditions together in infants and children with possible other coexisting symptoms, which may have influenced the outcomes. Thus, further studies with larger and more specific populations is needed.
Surgical correction of ALCPA effectively restores coronary perfusion and reduces severe mitral regurgitation, though residual ventricular dilation and moderate valvular dysfunction persist in the short-term postoperative period. Compared to older children, infant ALCAPA patients demonstrate more complex recovery trajectories, characterized by significantly prolonged ventilator dependence and hospitalization duration. Nevertheless, the overall prognosis remains favorable when timely intervention is performed.
Acknowledgement:
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: Methodology, software validation, formal analysis, data curation, investigation, supervision: Guozhen Wang. Conceptualization, visualization, project administration, funding acquisition: Yong An. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data in this article were obtained from all patients in clinical practice in our center. The data that support the findings of this study are available from the corresponding author, Yong An, upon reasonable request.
Ethics Approval: This study was agreed by the Medical Research Ethics Committee of the Children’s Hospital of Chongqing Medical University [approval number: 2023 (515)], was conducted in accordance with the Declaration of Helsinki, and waived the need for informed consent from the patients who were retrospectively analyzed in the study.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Catheter Cardiovasc Diagn. 1990;21:28–40. doi:10.1002/ccd.1810210110. [Google Scholar] [CrossRef]
2. Cashen K, Kwiatkowski DM, Riley CM, Buckley J, Sassalos P, Gowda KN, et al. Anomalous origin of the left coronary artery from the pulmonary artery: a retrospective multicenter study. Pediatr Crit Care Med. 2021;22:e626–35. doi:10.1097/PCC.0000000000002820. [Google Scholar] [CrossRef]
3. Silverman NH. Echocardiographic presentation of anomalous origin of the left coronary artery from the pulmonary artery. Cardiol Young. 2015;25:1512–23. doi:10.1017/S1047951115002565. [Google Scholar] [CrossRef]
4. Xiao Y, Jin M, Han L, Ding W, Zheng J, Sun C, et al. Two congenital coronary abnormalities affecting heart function: anomalous origin of the left coronary artery from the pulmonary artery and congenital left main coronary artery atresia. Chin Med J. 2014;127:3724–31. [Google Scholar]
5. Hu R, Zhang W, Yu X, Zhu H, Zhang H, Liu J. Midterm surgical outcomes for ALCAPA repair in infants and children. Thorac Cardiovasc Surg. 2022;70:2–9. doi:10.1055/s-0041-1725978. [Google Scholar] [CrossRef]
6. Feng T, Zhangke G, Song B, Fan F, Jia Z, Xiaofeng L. Outcomes of surgical repair of anomalous origin of the left coronary artery from the pulmonary artery in infants and children. Cardiol Young. 2022;32:36–41. doi:10.1017/S104795112100161X. [Google Scholar] [CrossRef]
7. Yuan X, Li B, Sun H, Yang Y, Meng H, Xu L, et al. Surgical outcome in adolescents and adults with anomalous left coronary artery from pulmonary artery. Ann Thorac Surg. 2018;106:1860–7. doi:10.1016/j.athoracsur.2018.05.051. [Google Scholar] [CrossRef]
8. Takeuchi S, Imamura H, Katsumoto K, Hayashi I, Katohgi T, Yozu R, et al. New surgical method for repair of anomalous left coronary artery from pulmonary artery. J Thorac Cardiovasc Surg. 1979;78:7–11. [Google Scholar]
9. Triglia LT, Guariento A, Zanotto L, Zanotto L, Cattapan C, Hu R, et al. Anomalous left coronary artery from pulmonary artery repair: outcomes from the European Congenital Heart Surgeons Association Database. J Card SURGERY. 2021;36:1910–6. doi:10.1111/jocs.15448. [Google Scholar] [CrossRef]
10. Yokohama F, Toh N, Kotani Y, Watanabe N, Takaya Y, Akagi T, et al. Multiple late complications after Takeuchi repair of anomalous left coronary artery from the pulmonary artery. JACC Case Rep. 2021;3:731–5. doi:10.1016/j.jaccas.2021.02.035. [Google Scholar] [CrossRef]
11. Karimi M, Kirshbom PM. Anomalous origins of coronary arteries from the pulmonary artery: a comprehensive review of literature and surgical options. World J Pediatr Congenit Heart Surg. 2015;6:526–40. doi:10.1177/2150135115596584. [Google Scholar] [CrossRef]
12. Isomatsu Y, Imai Y, Shin’Oka T, Aoki M, Iwata Y. Surgical intervention for anomalous origin of the left coronary artery from the pulmonary artery: the Tokyo experience. J Thorac Cardiovasc Surg. 2001;121:792–7. doi:10.1067/mtc.2001.112834. [Google Scholar] [CrossRef]
13. Weixler VHM, Zurakowski D, Baird CW, Guariento A, Piekarski B, Del Nido PJ, et al. Do patients with anomalous origin of the left coronary artery benefit from an early repair of the mitral valve? Eur J Cardio-Thorac Surg. 2020;57:72–7. doi:10.1093/ejcts/ezz158. [Google Scholar] [CrossRef]
14. Neumann A, Sarikouch S, Bobylev D, Meschenmoser L, Breymann T, Westhoff-Bleck M, et al. Long-term results after repair of anomalous origin of left coronary artery from the pulmonary artery: Takeuchi repair versus coronary transfer. Eur J Cardio-Thorac Surg. 2017;51:308–15. doi:10.1093/ejcts/ezw268. [Google Scholar] [CrossRef]
15. Lange R, Cleuziou J, Krane M, Ewert P, Pabst Von Ohain J, Beran E, et al. Long-term outcome after anomalous left coronary artery from the pulmonary artery repair: a 40-year single-centre experience. Eur J Cardio-Thorac Surg. 2018;53:732–9. doi:10.1093/ejcts/ezx407. [Google Scholar] [CrossRef]
16. Ye L, Qiu L, Zhang H, Chen H, Jiang C, Hong H, et al. Cardiomyocytes in young infants with congenital heart disease: a three-month window of proliferation. Sci Rep. 2016;6:23188. doi:10.1038/srep23188. [Google Scholar] [CrossRef]
17. Bhushan R, Mallik M, Potey K, Grover V, Aiyer P, Jhajhria NS. Anomalous origin of the left coronary artery from the pulmonary artery: a midterm experience of a rare entity at a tertiary care center. J Cardiovasc Thorac Res. 2023;15:181–5. doi:10.34172/jcvtr.2023.31651. [Google Scholar] [CrossRef]
18. Dehaki MG, Al-Dairy A, Rezaei Y, Ghavidel AA, Omrani G, Givtaj N, et al. Mid-term outcomes of surgical repair for anomalous origin of the left coronary artery from the pulmonary artery: in infants, children and adults. Ann Pediatr Cardiol. 2017;10:137–43. doi:10.4103/0974-2069.205140. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools