 Open Access
Open Access
ARTICLE
Comparative Clinical Outcomes of Right Lateral Thoracotomy and Totally Thoracoscopic Surgery for Adult Patients with Atrial Septal Defect: A Single Center, Retrospective Study
1 Department of Cardiovascular Surgery, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, 510080, China
2 Guangdong Provincial Key Laboratory of South China Structural Heart Disease, Guangzhou, 510080, China
* Corresponding Authors: Haiyun Yuan. Email: ; Xiaobing Liu. Email:
(This article belongs to the Special Issue: Novel Methods and Techniques for the Management of Congenital Heart Disease)
Congenital Heart Disease 2025, 20(3), 357-368. https://doi.org/10.32604/chd.2025.066817
Received 18 April 2025; Accepted 25 June 2025; Issue published 11 July 2025
Abstract
Background: Totally thoracoscopic surgery (TTS) and right lateral thoracotomy (RLT) are both extensively utilized in the surgical repair for atrial septal defect (ASD). However, RLT is generally considered in low-weight pediatric patients as a result of restricted surgical exposure. This study aims to introduce an RLT approach for ASD repair in adults and compare its clinical outcomes with TTS. Methods: We conducted a retrospective analysis of the clinical data of 23 adult patients who underwent ASD repair at Guangdong Provincial People’s Hospital between June and October 2024. Patients were divided into two groups based on the surgical approach they adopted: group totally thoracoscopic surgery (TTS, n = 12) and group right lateral thoracotomy (RLT, n = 11). All individuals finished a follow-up three months after surgery. Operative parameters, postoperative courses, echocardiographic measurements and laboratory investigations were compared between the two groups. Results: The total surgical duration was significantly longer in group RLT compared with group TTS [(234.00 ± 47.93) min vs. (175.17 ± 52.36) min, p = 0.011]. Group RLT exhibited a significantly higher respiratory index (RI) at <6 h postoperatively (1.00 ± 0.58 vs. 0.30 ± 0.37, p = 0.01) and significantly lower levels of soluble suppression of tumorigenicity 2 (sST2) [(136.61 ± 43.12) ng/mL vs. (199.08 ± 33.56) ng/mL, p = 0.037] and cardiac troponin (cTnT) [(277.04 ± 89.85) pg/mL vs. (343.30 ± 482.40) pg/mL, p = 0.047] at 12–24 h postoperatively. Echocardiographic measurements showed no significant differences between two groups, except for a more pronounced reduction in left atrial (LA) size at discharge in group TTS [(5.00 ± 3.64) mm vs. (0.09 ± 4.44) mm, p = 0.008]. Conversely, group RLT demonstrated a less significant decrease in glutamyl transpeptidase (GGT) [(1.00 ± 6.00) U/L vs. (5.25 ± 3.86) U/L, p = 0.026] but a more significant decrease in blood urea nitrogen (BUN) [(1.81 ± 1.10) mg/dL vs. (0.81 ± 1.07) mg/dL, p = 0.038]. Conclusions: RLT for ASD repair in adults demonstrated comparable clinical outcomes to TTS in terms of postoperative recovery and cardiac function and also produced fewer scars than TTS. Our study proved the feasibility, safety and cosmetic effects of uniport RLT for ASD repair in adults when compared with TTS.Keywords
Atrial septal defect (ASD), a common congenital cardiac anomaly, is characterized by significant hemodynamic disturbances and potential long-term complications when left untreated [1,2]. Surgical intervention remains the primary therapeutic approach, with evolving techniques offering diverse treatment options [3]. While conventional open-heart surgery has historically been the standard of care, the emergence of minimally invasive approaches, particularly totally thoracoscopic surgery (TTS) and right lateral thoracotomy (RLT), has transformed the surgical landscape by demonstrating superior outcomes in terms of reduced operative trauma and accelerated postoperative recovery [4,5]. Currently, tri-port TTS is the standard minimally invasive approach for surgical closures of ASD in adults while uniport RLT is mostly applied to children [6,7,8,9]. This study introduces an optimized RLT technique for ASD repair in adult patients and systematically evaluates its clinical efficacy in comparison with the established TTS approach, which aims at broadening the application of uniport RLT and seeking novel experience in minimally invasive cardiac surgery for adults.
This study was approved by the Ethics Review Committee of Guangdong Provincial People’s Hospital (Grant number: XJS2024-089-01), was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the patients. A retrospective analysis was conducted on clinical data from 23 consecutive patients who underwent ASD repair through either TTS or RLT approach at Guangdong Provincial People’s Hospital between June 2024 and October 2024. Comprehensive perioperative data were systematically collected, including operative parameters, postoperative courses, echocardiographic measurements, and laboratory findings. These data were subjected to comparative analysis between the TTS and RLT groups to evaluate the efficacy, identify potential advantages, and assess limitations associated with the RLT approach. Patients in the 2 groups had to meet the following selection criteria: (i) ASD unsuitable for percutaneous transcatheter closure; (ii) systolic pulmonary arterial pressure (sPAP) < 60 mmHg (measured by echocardiograms); (iii) no history of a surgical procedure in the right chest cavity or of any other lung disease or thoracic deformities; (iv) no concurrence of serious aortic or coronary artery disease; (v) weight > 40 kg, BMI: 17.1–23.9 kg/m2. The same surgical team performed all procedures for both groups.
All procedures were performed under standardized general anesthesia with single-lumen endotracheal intubation general anesthesia to deliver single-lung ventilation in all patients. Arrested-heart ASD repair was carried out under mild hypothermic cardiopulmonary bypass (CBP) (28–32°C) in both groups, which was realized by administration of Del Nido cardioplegia. The defects were closed by either autologous pericardium or a bioprosthetic patch (0.6 mm, Balance Medical, Beijing, China), with an annuloplasty ring (26–30 mm, Balance Medical) installed when moderate or critical tricuspid regurgitation exists.
For the TTS approach, patients were settled in a 45° left lateral decubitus position (Fig. 1A), with the primary surgical access established along the right anterior 4th to 5th intercostal space (5–7 cm) to allow for suture needles handled by the right hand of the operator, grasping forceps in the left hand, strings for pericardium retraction, surgical aspirators and scissors, complemented by two additional ports (1–1.5 cm) for thoracoscope (Karl Storz, Tuttlingen, BW, Germany), perfusion cannula and other instrumental manipulations such as Chitwood clamp and retraction strings (Fig. 1B). CBP was achieved through a peripheral strategy: the superior vena cava (SVC) drainage catheter (15–18 Fr, Weigao, Weihai, China) was accessed placed via right internal jugular vein cannulation, while the inferior vena cava (IVC) (straight, 20–24 Fr, Weigao) and aorta drainage catheters (15–18 Fr, Medtronic, Minneapolis, MN, USA) were established through the right femoral vein and artery, respectively (Fig. 1C). Three surgeons were involved in the operation, including a head surgeon on the right side of patient, an assistant surgeon opposite to the head surgeon to cope with the strings and another assistant next to the head surgeon to manipulate the thoracoscope. Operated region projected onto the Storz screen was available to all the participants (Fig. 1D). The drainage tube was inserted through the inferior port site and secured with non-absorbable sutures using an instant knotting technique (Fig. 1F).
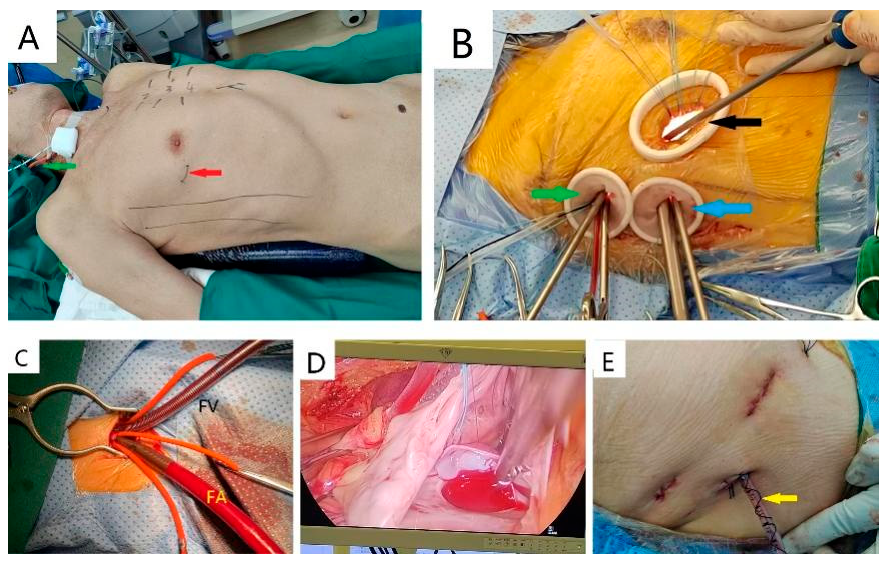
Figure 1: Surgical technique in the TTS group: (A). Primary incision of TTS locates at 4th or 5th intercostal space below the pectoralis major (red arrow) and SVC cannulation is accessed via right jugular vein (green arrow); (B). TTS allows the head surgeons to operate through primary incision (black arrow) and the upper port (green arrow), while thoracoscopes are place through the lower port (blue arrow); (C). Aortic and IVC cannulation are administered via femoral artery (FA) and femoral vein (FV), respectively; (D). Images projected onto the screen show the ASD and patch clearly; (E). A coiled non-absorbable suture to secure the drainage tube (yellow arrow).
In the RLT group, the patients were positioned at 65° left lateral decubitus (Fig. 2A). There was a single longitudinal incision at right lateral 4th intercostal space on the thorax surface (4–5 cm) and a deeper incision along the intercostal muscles (5–7 cm) with two thoracic retractors vertically placed for adequate exposure (Fig. 2B). The only surgical incision allowed direct cannulation for both IVC (right-angled, 20–24 Fr, Weigao) and aorta (15–18 Fr, Medtronic) as well as a chain clamp, a tube for cardioplegia delivery and all the surgical manipulations. Two surgeons were required: a head surgeon on the right side of patient and an assistant surgeon opposite to the head surgeon. The surgical region was also excellent in exposing the defect and allowing the surgeon to manipulate it (Fig. 2C). This approach employed a unique drainage strategy where the tube was placed through a single incision and stabilized using two absorbable fishbone-like sutures, allowing for subsequent removal through instant traction (Fig. 2D).
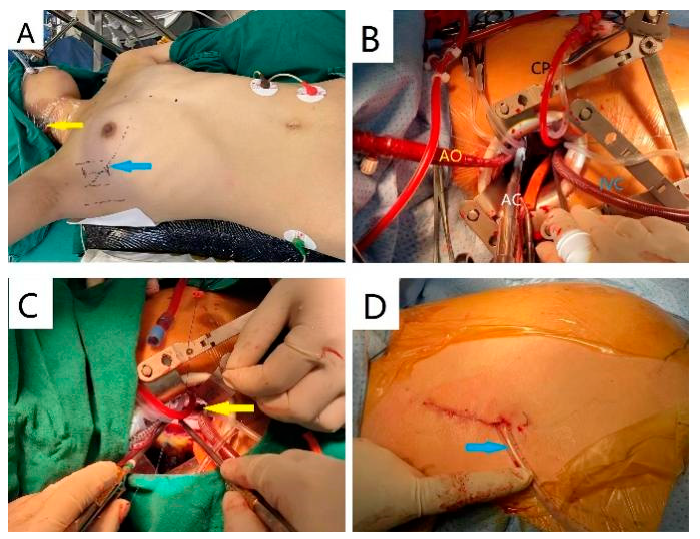
Figure 2: Surgical technique in the RLT group: (A) The only surgical access of RLT locates at right lateral 4th intercostal space (blue arrow) and SVC cannulation is delivered in the same manner to TTS (yellow arrow); (B) Cannulation for aorta (AO), cardioplegic solution (CP), IVC and aortic clamp (AC) are arranged clockwise and two distractors are vertically placed; (C) RLT allows the head surgeon to maneuver through the only incision and ASD is also well exposed (yellow arrow); (D) Two absorbable fishbone-like sutures to secure the drainage tube (blue arrow) in RLT.
The study evaluated comprehensive outcome measures across multiple domains:
- (i) Operative parameters: total surgical duration, CPB time, aortic cross-clamp (ACC) time.
- (ii) Postoperative courses: intensive care unit (ICU) length of stay, mechanical ventilation time (MVT), vasoactive-inotropic score (VIS), total hospital stay duration, thoracic drainage time (TDT), post-extubation complications (pneumothorax, pleural effusion, subcutaneous emphysema).
- (iii) Echocardiographic measurements: cardiac chamber dimensions, left ventricular ejection fraction (LVEF) and valvular flow velocities.
- (iv) Laboratory findings: hematological profile, brain natriuretic peptide (BNP), soluble suppression of tumorigenicity (sST2), cardiac enzymes: creatine kinase (CK), CK-MB isotype and lactate dehydrogenase (LDH), cardiac troponin T (cTnT), blood gas analysis (BGA) [including respiratory index (RI)], renal and hepatic function tests.
VIS was calculated using the validated Belletti formula [10]. Drainage tube was removed when the drainage volume was below 100 mL per day. Echocardiographic measurements were assessed at discharge and 3-month follow-up. RI and sST2 were introduced to evaluate the early pulmonary function and risk of adverse events post-surgery, respectively. RI refers to the ratio of P(A-a) O2 to PaO2 (see Eq. (1)), serves as a simple and reliable indicator for the evaluation of post-CPB pulmonary function and isn’t influenced by the concentration of inhaled oxygen [11,12]. Higher level of RI relates to worse capacity of oxygenation owing to significantly increased pulmonary water content, decreased pulmonary compliance, surface active substances and functional residual capacity and diffuse alveolar atelectasis after CPB [11]. sST2 is a member of the interleukin-1 (IL-1) receptor family that serves as a decoy for IL-33, interfering with IL-33’s ability to protect against hypertrophy and fibrosis. Recent researches have confirmed its association with high incidence of early cardiovascular events or mortality after cardiac surgery [13,14].
P(A-a) O2: alveolar-arterial oxygen tension gradient, PaO2: arterial oxygen tension.
The continuous data were presented as mean ± standard deviation (SD) if normally distributed, or as median with interquartile range (IQR) if non-normally distributed. Categorical data were presented as frequency with percentage. Statistical significance of results between the two groups were made through following steps: using the Shapiro-Wilk test for clarifying normality of continuous variables, using the Student’s t-test to calculate p value for normally distributed data, or Mann-Whitney U test for non-normally distributed data; chi-square test, or using Fisher’s exact test to calculate p value for categorical variables, as appropriate. All statistical analyses were performed using IBM SPSS Statistics version 25.0. A p value of less than 0.05 was considered to indicate statistical significance.
In the present study, a total of 23 patients were included, with 12 undergoing TTS for repairing ASD with or without tricuspid annuloplasty, and 11 undergoing RLT. The baseline characteristics of the patients are summarized in Table 1. The two groups were well-matched, with no significant differences observed in age, female gender, height, weight, body mass index (BMI), levels of hematological profiles, BNP, cTnT, myocardial enzymes, indicators of hepatic and renal function except for blood urea nitrogen, and New York Heart Association (NYHA) classification for cardiac function (all p > 0.05).
Table 1: Baseline characteristics.
| Group | TTS (n = 12) | RLT (n = 11) | p Value |
|---|---|---|---|
| Age (year) | 25.61 ± 6.51 | 30.45 ± 16.39 | 0.377 |
| Female gender [n (%)] | 8 (66.67) | 8 (72.73) | >0.99 |
| BMI (kg/m2) | 19.40 ± 2.34 | 20.66 ± 2.87 | 0.259 |
| Weight (kg) | 51.83 ± 7.65 | 52.82 ± 10.73 | 0.801 |
| RBC (×1012/L) | 4.46 ± 0.34 | 4.65 ± 0.31 | 0.178 |
| Hb (g/L) | 133.00 ± 10.95 | 129.45 ± 15.27 | 0.526 |
| HCT | 0.407 ± 0.033 | 0.400 ± 0.037 | 0.667 |
| PLT (×1012/L) | 256.33 ± 49.69 | 259.00 ± 63.00 | 0.806 |
| BNP (pg/mL) | 63.25 ± 86.05 | 104.44 ± 86.15 | 0.926 |
| cTnT (pg/mL) | 3.75 ± 1.67 | 4.20 ± 1.15 | 0.283 |
| CK (U/L) | 70.50 ± 39.00 | 71.00 ± 59.00 | 0.926 |
| CKMB (U/L) | 10.00 ± 0.60 | 10.00 ± 1.70 | 0.521 |
| LDH (U/L) | 156.27 ± 21.73 | 152.00 ± 26.00 | 0.972 |
| ALT (g/L) | 16.83 ± 1.78 | 14.00 ± 6.02 | 0.319 |
| AST (g/L) | 20.33 ± 3.55 | 19.91 ± 5.99 | 0.837 |
| GGT (g/L) | 22.75 ± 10.19 | 16.50 ± 6.96 | 0.116 |
| TBil (umol/L) | 11.05 ± 2.23 | 10.87 ± 4.35 | 0.242 |
| DBil (umol/L) | 2.30 ± 0.48 | 2.00 ± 1.30 | 0.107 |
| UA (umol/L) | 339.52 ± 89.91 | 394.74 ± 94.80 | 0.171 |
| Scr (mmol/L) | 63.39 ± 13.90 | 64.93 ± 12.41 | >0.99 |
| BUN (mmol/L) | 4.57 ± 1.32 | 6.03 ± 1.53 | 0.023 |
| K+ (mmol/L) | 3.74 ± 0.27 | 3.82 ± 0.20 | 0.418 |
| Ca2+ (mmol/L) | 2.39 ± 0.10 | 2.37 ± 0.11 | 0.636 |
| Na+ (mmol/L) | 140.59 ± 0.71 | 141.02 ± 0.78 | 0.472 |
| NYHA classification | 1.92 ± 0.29 | 2.09 ± 0.30 | 0.166 |
| SpO2 (%) | 98.75 ± 1.22 | 99.36 ± 0.81 | 0.202 |
As shown in Table 2, the preoperative echocardiographic measurements, including cardiac chamber size, ASD diameter, valvular blood velocity, LVEF, and systolic pulmonary artery pressure, were comparable between the two groups (all p > 0.05). There were no significant differences in the incidence of tricuspid valvuloplasty (TVP), CPB time, and ACC time between the two groups, either. However, group RLT exhibited a significantly longer total surgical duration compared with group TTS (p = 0.011).
Table 2: Perioperative data.
| Group | TTS (n = 12) | RLT (n = 11) | p Value |
|---|---|---|---|
| Preoperative echo measurements | |||
| RA (mm) | 50.08 ± 6.05 | 51.27 ± 6.69 | 0.659 |
| RV (mm) | 59.17 ± 10.51 | 61.00 ± 8.89 | 0.658 |
| RVOT (mm) | 35.00 ± 5.39 | 34.73 ± 7.10 | 0.918 |
| LA (mm) | 32.00 ± 2.83 | 31.45 ± 5.16 | 0.761 |
| LVd (mm) | 39.58 ± 3.75 | 38.27 ± 6.00 | 0.533 |
| LVs (mm) | 24.75 ± 2.56 | 23.18 ± 3.37 | 0.221 |
| AAO (mm) | 25.42 ± 3.50 | 25.55 ± 3.64 | 0.932 |
| MPA (mm) | 25.83 ± 3.04 | 27.00 ± 2.49 | 0.328 |
| MVv (m/s) | 0.93 ± 0.15 | 0.93 ± 0.27 | >0.99 |
| AVv (m/s) | 1.11 ± 0.17 | 1.03 ± 0.13 | 0.177 |
| PVv (m/s) | 1.34 ± 0.26 | 1.30 ± 0.26 | 0.758 |
| TVv (m/s) | 0.83 ± 0.29 | 0.86 ± 0.31 | 0.790 |
| LVEF (%) | 66.17 ± 3.74 | 66.00 ± 5.25 | 0.930 |
| Diameter of ASD (four-chamber) (mm) | 18.00 ± 6.67 | 22.70 ± 9.32 | 0.177 |
| Diameter of ASD (short-axis) (mm) | 17.99 ± 7.42 | 23.05 ± 9.39 | 0.164 |
| sPAP (mmHg) | 31.91 ± 8.36 | 35.90 ± 8.49 | 0.292 |
| ASD with edge [n (%)] | 7 (58.33) | 8 (72.73) | 0.667 |
| Operative parameters | |||
| TVP [n (%)] | 1 (8.33) | 3 (27.27) | 0.317 |
| Overall surgery time (min) | 175.17 ± 52.36 | 234.00 ± 47.93 | 0.011 |
| CPB time (min) | 150.79 ± 44.18 | 154.00 ± 50.44 | 0.746 |
| ACC time (min) | 88.57 ± 34.19 | 95.84 ± 33.73 | 0.320 |
As presented in Table 3, a significantly higher proportion of patients undergoing RLT exhibited more elevated RI at less than 6 h postoperatively (p = 0.01), lower levels of sST2 (p = 0.037) and ΔcTnT (p = 0.047) at 12 to 24 h post-surgery compared with those undergoing TTS. No significant differences were observed in postoperative courses, hematological profiles, indicators of hepatic and renal function, lactate acid, or myocardial enzymes between the two groups (all p > 0.05). Notably, GGT showed less while BUN showed more reduction in group RLT than group TTS (p = 0.026 and p = 0.038, respectively).
Table 3: Early postoperative outcomes.
| Group | TTS (n = 12) | RLT (n = 11) | p Value |
|---|---|---|---|
| Postoperative courses | |||
| ICU stay (d) | 0.92 ± 1.03 | 0.82 ± 0.30 | 0.175 |
| MVT (h) | 2.80 ± 3.03 | 4.00 ± 3.50 | 0.218 |
| VIS | 2.38 ± 2.98 | 1.45 ± 2.16 | 0.444 |
| TDT (d) | 2.92 ± 1.15 | 2.75 ± 0.73 | 0.679 |
| In-hospital stay (d) | 3.80 ± 2.45 | 3.90 ± 2.00 | 0.558 |
| Pneumothorax [n (%)] | 1 (8.33) | 3 (27.27) | 0.317 |
| Pleural effusion [n (%)] | 5 (41.67) | 6 (54.55) | 0.684 |
| Subcutaneous emphysema [n (%)] | 5 (41.67) | 2 (18.18) | 0.640 |
| Laboratory findings | |||
| <6 h post-surgery | |||
| ΔRBC (×1012/L) | 0.49 ± 0.34 | 0.56 ± 0.31 | 0.628 |
| ΔHb (g/L) | 14.58 ± 10.50 | 15.27 ± 9.68 | 0.872 |
| ΔHCT | 0.054 ± 0.038 | 0.053 ± 0.032 | 0.949 |
| ΔPLT (×1012/L) | 91.42 ± 34.96 | 78.45 ± 30.92 | 0.359 |
| Lac (mmol/L) | 2.08 ± 1.26 | 1.60 ± 3.00 | 0.622 |
| RI | 0.30 ± 0.37 | 1.00 ± 0.58 | 0.010 |
| sST2 (ng/mL) | 60.22 ± 24.56 | 50.06 ± 28.45 | 0.392 |
| 12~24 h post-surgery | |||
| ΔRBC (×1012/L) | 0.55 ± 0.35 | 0.57 ± 0.39 | 0.919 |
| ΔHb (g/L) | 15.92 ± 10.87 | 14.27 ± 9.23 | > 0.99 |
| ΔHCT 12~24 h | 0.056 ± 0.032 | 0.048 ± 0.025 | 0.500 |
| ΔPLT (×1012/L) | 74.33 ± 30.73 | 55.91 ± 31.67 | 0.172 |
| Lac (mmol/L) | 1.87 ± 1.88 | 2.22 ± 1.68 | 0.440 |
| RI | 0.31 ± 0.14 | 0.48 ± 0.49 | 0.565 |
| sST2 (ng/mL) | 199.08 ± 33.56 | 136.61 ± 43.12 | 0.037 |
| ΔBNP (pg/mL) | 573.60 ± 647.33 | 536.38 ± 444.31 | 0.817 |
| ΔcTnT (pg/mL) | 343.30 ± 482.40 | 227.04 ± 89.85 | 0.047 |
| ΔCK (U/L) | 867.83 ± 319.51 | 1173.64 ± 501.84 | 0.093 |
| ΔCKMB (U/L) | 31.46 ± 19.21 | 30.53 ± 25.52 | 0.806 |
| ΔLDH (U/L) | 108.33 ± 73.91 | 160.18 ± 104.50 | 0.116 |
| ΔALB (g/L) | 36.18 ± 2.77 | 34.30 ± 2.80 | 0.122 |
| ΔALT (U/L) | 0.42 ± 5.65 | 3.09 ± 5.82 | 0.276 |
| ΔAST (U/L) | 42.50 ± 21.15 | 38.91 ± 22.24 | 0.695 |
| ΔGGT (U/L) | −5.25 ± 3.86 | −1.00 ± 6.00 | 0.026 |
| ΔTBil (umol/L) | 6.21 ± 8.50 | 6.78 ± 4.77 | 0.844 |
| ΔDBil (umol/L) | 1.36 ± 1.61 | 1.55 ± 0.81 | 0.371 |
| ΔUA (umol/L) | −27.09 ± 66.41 | −40.92 ± 121.99 | 0.736 |
| ΔScr (mmol/L) | −4.55 ± 6.37 | −3.77 ± 9.02 | 0.812 |
| ΔBUN (mmol/L) | −0.81 ± 1.07 | −1.81 ± 1.10 | 0.038 |
| ΔK+ (mmol/L) | 0.51 ± 0.40 | 0.20 ± 0.35 | 0.059 |
| ΔCa2+ (mmol/L) | −0.25 ± 0.11 | −0.27 ± 0.11 | 0.595 |
| ΔNa+ (mmol/L) | 1.67 ± 3.31 | 1.69 ± 3.08 | 0.989 |
Table 4 illustrates the variations in echocardiographic parameters at discharge and 3-month post-surgery. The results indicate that, with the exception of the LA size at discharge (p = 0.008), all the measurements were comparable between the two groups.
Table 4: Postoperative echocardiographic measurements.
| Group | TTS (n = 12) | RLT (n = 11) | p Value |
|---|---|---|---|
| At discharge | |||
| ΔRA (mm) | 9.25 ± 4.67 | 4.27 ± 7.47 | 0.067 |
| ΔRV (mm) | 8.75 ± 9.49 | 6.45 ± 6.17 | 0.504 |
| ΔRVOT (mm) | 11.67 ± 4.85 | 7.91 ± 5.13 | 0.085 |
| ΔLA (mm) | 5.00 ± 3.64 | 0.09 ± 4.44 | 0.008 |
| ΔLVd (mm) | −0.17 ± 4.24 | −3.82 ± 4.02 | 0.064 |
| ΔLVs (mm) | −0.92 ± 2.71 | −3.82 ± 4.02 | 0.054 |
| ΔAAO (mm) | 0.50 ± 1.88 | −0.36 ± 7.24 | 0.303 |
| ΔMPA (mm) | 4.25 ± 2.56 | 2.64 ± 4.54 | 0.301 |
| ΔMVv (m/s) | 0.25 ± 0.14 | 0.22 ± 0.26 | 0.677 |
| ΔAVv (m/s) | −0.02 ± 0.22 | −0.1 ± 0.17 | 0.335 |
| ΔPVv (m/s) | 0.39 ± 0.28 | 0.39 ± 0.52 | 0.458 |
| ΔTVv (m/s) | 0.30 ± 0.29 | 0.32 ± 0.35 | 0.918 |
| ΔLVEF (%) | 0.58 ± 5.88 | 0.18 ± 7.88 | 0.891 |
| At 3-month follow-up | |||
| ΔRA (mm) | 11.33 ± 5.02 | 7.50 ± 6.88 | 0.147 |
| ΔRV (mm) | 11.00 ± 9.12 | 10.30 ± 9.01 | 0.945 |
| ΔRVOT (mm) | 11.25 ± 5.79 | 9.50 ± 5.40 | 0.475 |
| ΔLA (mm) | 0.75 ± 3.77 | 1.10 ± 4.09 | 0.837 |
| ΔLVd (mm) | −3.25 ± 4.71 | −2.80 ± 4.87 | 0.828 |
| ΔLVs (mm) | −1.83 ± 4.00 | −3.00 ± 3.06 | 0.690 |
| ΔAAO (mm) | −0.17 ± 2.17 | 0.60 ± 5.82 | 0.676 |
| ΔMPA (mm) | 3.25 ± 2.49 | 3.90 ± 2.51 | 0.551 |
| ΔMVv (m/s) | 0.06 ± 0.21 | 0.20 ± 0.28 | 0.226 |
| ΔAVv (m/s) | −0.10 ± 0.25 | 0.04 ± 0.21 | 0.185 |
| ΔPVv (m/s) | 0.49 ± 0.25 | 0.57 ± 0.30 | 0.538 |
| ΔTVv (m/s) | 0.30 ± 0.27 | 0.23 ± 0.42 | 0.629 |
| ΔLVEF (%) | 0.67 ± 5.77 | −2.50 ± 9.86 | 0.359 |
In our study, patients who adopted right lateral thoracotomy underwent a successful repair of ASD with or without TVP and obtained equivalent effect to totally thoracoscopic surgery in terms of operative parameters, postoperative courses, echocardiographic measurements, and laboratory findings. However, prolonged total surgical duration, lower sST2 and cTnT levels within 6 h after surgery in group RLT indicated inexperience but milder myocardial injury of the trial regarding this novel minimally invasive approach.
TTS and RLT are both minimally invasive approaches that have been adopted in heart surgery for decades [4,5]. It’s generally acknowledged that TTS can be applied for congenital heart diseases in patients with wide range of weight and age while RLT is mostly used in children or low-weight population [8,9]. Our research proved the feasibility and safety to administer RLT to repair ASD for adults or children weighing over 40 kg.
In this retrospective study, RLT was related to significantly longer total surgical duration but showed similar results of ACC and CBP time with TTS. It might be a consequence of limited exposure to cannulate IVC via the 3rd–5th intercostal incision while TTS delivered IVC cannulation via femoral vein, which is an ordinary and time-saving technique. Once extracorporeal circulation and right atrial incision were established, RLT provided direct insight of ASD and tricuspid valves that resembles TTS, thus leading to little difference to install the patch and annuloplasty ring.
Our study revealed that RI within 6 h after surgery was significantly higher in group RLT, which is supposed to result from single lateral ventilation to achieve ideal exposure of operated region. Oppression of right lung also contributed to the pulmonary injury as 65° left lateral position might result in sag of lung and covered right atrium. Considering that the incision of skin was tiny, two distractors vertically placed retractors were applied to create a square space to ensure the insight (Fig. 2B) but caused longer laceration of intercostal muscle and atelectasis after closure of chest cavity. However, RI of group RLT was consistently below the normal level of 1.5 and there was no significance between two groups 12 h after the surgery, which revealed that increase of RI in RLT was transient and spare no clinical significance to indicate pulmonary injury.
In terms of the CPB pattern, we adopted central cannulation (via aorta) in group RLT and peripheral cannulation (via femoral artery) in group TTS. Previous researches had confirmed the advantages of central cannulation over peripheral cannulation regarding in-hospital mortality, neurologic outcomes and myocardial injury [15,16,17]. These findings might explain why the level of sST2 and cTnT 12 h post-surgery was lower in RLT. Underlying mechanism might resemble differential oxygenation (also known as Harlequin syndrome) in patients receiving peripheral venoarterial extracorporeal membrane oxygenation, which features hypoxia of upper part of the body, especially the brain and heart [18]. During arrested-heart surgery in the TTS group, peripheral CBP take charge of the perfusion of the whole body, but the length of the femoral vein cannula usually creates increased resistance to effective drainage, worsening the ischemia of heart [19]. Furthermore, exemption from peripheral cannulation provides antegrade perfusion and avoids the occurrence of iatrogenic damages such as retrograde dissection of arteries, ipsilateral limb ischemia venous perforation and subsequent entrapment of air with airlock [19,20,21]. RLT can also serve as an alternative for patients with abdominal-iliac-femoral aneurysm or atherosclerosis that are not appropriate for peripheral cannulation, and doesn’t require preoperative examinations to rule out these conditions [19,22].
As for predischarge and follow-up echocardiographic measurements, no remarkable difference was observed between two groups except for variation of LA at discharge. We don’t think different approaches could bring diverse short-term outcomes.
Considerable cosmetic effect is another superiority of RLT. For TTS, a major incision along the lower margin of breast and two operative ports were inevitable to let thoracoscope, strings and various instruments through the thoracic wall (Fig. 3A) while RLT only produced one incision at right lateral thoracic wall (Fig. 3B), which is even tinier than that previously reported in children [9].
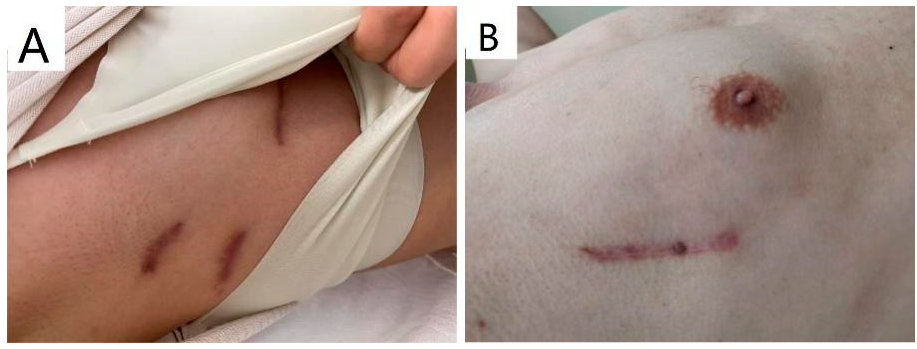
Figure 3: Scars of the thoracic wall three months post-surgery: (A). TTS creates three separate scars on right anterior and lateral wall. (B). RLT creates one scar on right lateral wall.
Thoracoscope can provide an ideal image of operation for all the participants (Fig. 1D) while surgical vision of RLT is available to the head surgeon exclusively (Fig. 2C). However, it does not compromise the clarity of the surgical view or the feasibility of the procedure. Moreover, it eliminates the need to invest significant time into mastering the learning curve and migrating the risks associated with thoracoscopic surgical techniques.
Patient selection is a paramount factor for a smooth procedure and satisfactory outcomes. Apart from ordinary criteria for TTS such as acceptable pulmonary arterial pressure and free of previous intrapleural surgeries or chest deformities, figure of patients also has an influence on the operation. Average level of BMI in both groups was approximately 20 kg/m2 as vertical and narrow intercostal spaces in skinny individuals and thick thoracic wall of obese patients will magnify the difficulties in exposure, cannulation and suture.
Major limitation to this study is inadequate sample amount and short follow-up period. Attempt to perform ASD closure for adults with uniport RLT was recently started in our center, and resulted in a small sample size, which might increase the risk of false negatives or spurious correlations and limit the generalizability of findings. A brief observation window may miss long-term outcomes and reflect temporary fluctuations rather than sustained trends, leading to biased conclusions. In the following research, we plan to carry on the right lateral thoracotomy for ASD repair in large scale and prolong the follow-up period to draw the problems above. Inconsistency of several vital indicators weakened the rigor and reliability of the study. Preoperative sST2, RI and postoperative echo-estimated sPAP should be collected upon patient enrollment. Patient satisfactory score is required to evaluate the cosmetic effects quantitatively at due follow-up in the future.
RLT offered equivalent surgical outcomes and more outstanding cosmetic effects to TTS for the repair of atrial septal defects in adult patients. In this setting, RLT seemed to be associated with longer total surgical duration but less elevation of cTnT and sST2 after surgery. Our study has broadened the applicable scope of right lateral thoracotomy for ASD repair and provides an alternative for patients who seek for minimally invasive surgical effects.
Acknowledgement:
Funding Statement: This research was funded by the Natural Science Foundation of Guangdong Province (2023A1515012501), Guangzhou Municipal Science and Technology Planning Project (2023B03J1254), Congenital Heart Disease Medical Talent Cultivation and Education Fund (2023QT0009).
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization, Xiaobing Liu; methodology, Haiyun Yuan and Yushen Fang; software, Yushen Fang; validation, Xiaobing Liu and Haiyun Yuan; formal analysis, Yushen Fang; investigation, Zechen Li and Jiahong Li; resources, Xiaobing Liu, Haiyun Yuan and Gang Xu; data curation, Kaiyu Wang; writing—original draft preparation, Yushen Fang; writing—review and editing, Shusheng Wen, Jimei Chen and Jian Zhuang; visualization, Yushen Fang and Haiyun Yuan; supervision, Xiaobing Liu and Haiyun Yuan; project administration, Xiaobing Liu; funding acquisition, Haiyun Yuan and Shusheng Wen. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data of this study were available from the corresponding authors upon reasonable request.
Ethics Approval: This study has been approved by the Ethics Committee of the Guangdong Provincial People’s Hospital (Grant number: XJS2024-089-01), was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the patients.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Jain S, Dalvi B. Atrial septal defect with pulmonary hypertension: when/how can we consider closure? J Thorac Dis. 2018;10(Suppl 24):S2890–8. doi:10.21037/jtd.2018.07.112. [Google Scholar] [CrossRef]
2. Engelfriet PM, Duffels MG, Möller T, Boersma E, Tijssen JG, Thaulow E, et al. Pulmonary arterial hypertension in adults born with a heart septal defect: the Euro heart survey on adult congenital heart disease. Heart. 2007;93(6):682–7. doi:10.1136/hrt.2006.098848. [Google Scholar] [CrossRef]
3. Grieshaber P, Jaschinski C, Farag M, Fonseca-Escalante E, Gorenflo M, Karck M, et al. Surgical treatment of atrial septal defects. Rev Cardiovasc Med. 2024;25(10):350. doi:10.31083/j.rcm2510350. [Google Scholar] [CrossRef]
4. Chiu KM, Chen RJ. Videoscope-assisted cardiac surgery. J Thorac Dis. 2014;6(1):22–30. doi:10.3978/j.issn.2072-1439.2014.01.04. [Google Scholar] [CrossRef]
5. Lamelas J. Minimally invasive concomitant aortic and mitral valve surgery: the “Miami Method”. Ann Cardiothorac Surg. 2015;4(1):33–7. doi:10.3978/j.issn.2225-319X.2014.08.17. [Google Scholar] [CrossRef]
6. Jung JC, Kim KH. Minimally invasive cardiac surgery versus conventional median sternotomy for atrial septal defect closure. Korean J Thorac Cardiovasc Surg. 2016;49(6):421–6. doi:10.5090/kjtcs.2016.49.6.421. [Google Scholar] [CrossRef]
7. Mishaly D, Ghosh P, Preisman S. Minimally invasive congenital cardiac surgery through right anterior minithoracotomy approach. Ann Thorac Surg. 2008;85(3):831–5. doi:10.1016/j.athoracsur.2007.11.068. [Google Scholar] [CrossRef]
8. Liu G, Qiao Y, Ma L, Ni L, Zeng S, Li Q. Totally thoracoscopic surgery for the treatment of atrial septal defect without of the robotic Da Vinci surgical system. J Cardiothorac Surg. 2013;8:119. doi:10.1186/1749-8090-8-119. [Google Scholar] [CrossRef]
9. Shinkawa T, Yamagishi M, Shuntoh K, Miyazaki T, Hisaoka T, Inoue T, et al. Atrial septal defect repair through limited lateral thoracotomy in children. Jpn J Thorac Cardiovasc Surg. 2006;54(11):469–71. doi:10.1007/s11748-006-0037-y. [Google Scholar] [CrossRef]
10. Belletti A, Lerose CC, Zangrillo A, Landoni G. Vasoactive-inotropic score: evolution, clinical utility, and pitfalls. J Cardiothorac Vasc Anesth. 2021;35(10):3067–77. doi:10.1053/j.jvca.2020.09.117. [Google Scholar] [CrossRef]
11. Yuan SM. Postperfusion lung syndrome: respiratory mechanics, respiratory indices and biomarkers. Ann Thorac Med. 2015;10(3):151–7. doi:10.4103/1817-1737.150736. [Google Scholar] [CrossRef]
12. Jiang T, Wang LX, Teng HK, Xu LT, Lou XK, Wang Y, et al. Ultra-fast-track cardiac anesthesia in minimally invasive cardiac surgery: a retrospective observational study. Cardiovasc Diagn Ther. 2024;14(5):740–52. doi:10.21037/cdt-24-175. [Google Scholar] [CrossRef]
13. Patel DM, Thiessen-Philbrook H, Brown JR, McArthur E, Moledina DG, Mansour SG, et al. Association of plasma-soluble ST2 and galectin-3 with cardiovascular events and mortality following cardiac surgery. Am Heart J. 2020;220:253–63. doi:10.1016/j.ahj.2019.11.014. [Google Scholar] [CrossRef]
14. Wozolek A, Jaquet O, Donneau AF, Lancellotti P, Legoff C, Cavalier E, et al. Cardiac biomarkers and prediction of early outcome after heart valve surgery: a prospective observational study. J Cardiothorac Vasc Anesth. 2022;36(3):862–9. doi:10.1053/j.jvca.2021.06.028. [Google Scholar] [CrossRef]
15. Tiwari KK, Murzi M, Bevilacqua S, Glauber M. Which cannulation (ascending aortic cannulation or peripheral arterial cannulation) is better for acute type A aortic dissection surgery? Interact Cardiovasc Thorac Surg. 2010;10(5):797–802. doi:10.1510/icvts.2009.230409. [Google Scholar] [CrossRef]
16. Benedetto U, Raja SG, Amrani M, Pepper JR, Zeinah M, Tonelli E, et al. The impact of arterial cannulation strategy on operative outcomes in aortic surgery: evidence from a comprehensive meta-analysis of comparative studies on 4476 patients. J Thorac Cardiovasc Surg. 2014;148(6):2936–43.e1–4. doi:10.1016/j.jtcvs.2014.05.082. [Google Scholar] [CrossRef]
17. Raja SG. Cannulation strategies for aortic surgery: which is the best one? J Thorac Dis. 2017;9(Suppl 6):S428–9. doi:10.21037/jtd.2017.03.99. [Google Scholar] [CrossRef]
18. Contento C, Battisti A, Agrò B, De Marco M, Iaiza A, Pietraforte L, et al. A novel veno-arteriovenous extracorporeal membrane oxygenation with double pump for the treatment of Harlequin syndrome. Perfusion. 2020;35(1 suppl):65–72. doi:10.1177/0267659120908409. [Google Scholar] [CrossRef]
19. Kandakure PR, Batra M, Garre S, Banovath SN, Shaikh F, Pani K. Direct cannulation in minimally invasive cardiac surgery with limited resources. Ann Thorac Surg. 2020;109(2):512–6. doi:10.1016/j.athoracsur.2019.05.075. [Google Scholar] [CrossRef]
20. Muhs BE, Galloway AC, Lombino M, Silberstein M, Grossi EA, Colvin SB, et al. Arterial injuries from femoral artery cannulation with port access cardiac surgery. Vasc Endovascular Surg. 2005;39(2):153–8. doi:10.1177/153857440503900204. [Google Scholar] [CrossRef]
21. Liu Z, Maimaitiaili A, Ma X, Dong S, Wei W, Wang Q, et al. Initial experience and favorable outcomes on cannulation strategies and surgical platform construction in fully video-assisted thoracoscopic cardiac surgery. Front Cardiovasc Med. 2024;11:1414333. doi:10.3389/fcvm.2024.1414333. [Google Scholar] [CrossRef]
22. İstar H, Sevuk U. Minimally invasive cardiac surgery in low-resource settings: right vertical infra-axillary mini-thoracotomy without peripheral cannulation—the first 100 cases. Eur Rev Med Pharmacol Sci. 2023;27(13):6247–55. doi:10.26355/eurrev_202307_32984. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools