 Open Access
Open Access
REVIEW
Contemporary Management of Failing Modified Fontan after the Total Cavopulmonary Connection
Department of Cardiovascular Surgery, Chinese Academy of Medical Sciences and Peking Union Medical College, National Center for Cardiovascular Diseases, Fuwai Hospital, Beijing, 100037, China
* Corresponding Author: Shoujun Li. Email:
# These authors contributed equally to this work
(This article belongs to the Special Issue: Novel Methods and Techniques for the Management of Congenital Heart Disease)
Congenital Heart Disease 2025, 20(3), 287-303. https://doi.org/10.32604/chd.2025.067619
Received 08 May 2025; Accepted 27 June 2025; Issue published 11 July 2025
Abstract
Congenital heart disease (CHD) stands as the most common cardiovascular disorder among children, exerting a profound impact on the growth, development, and quality of life of the affected pediatric population. The modified Fontan procedure, the total cavopulmonary connection (TCPC), has become a pivotal palliative or definitive surgical method for treating complex CHD cases, including single ventricle and tricuspid valve atresia. Through staged surgical processes, this technique directly diverts vena cava blood into the pulmonary artery, thus improving the patient’s oxygenation status. Despite the initial success of the Fontan circulation in providing a means for survival in patients with complex CHD, a significant proportion of patients will eventually experience Fontan failure. Fontan failure is a complex syndrome characterized by a constellation of symptoms and signs, including heart failure, arrhythmia, protein-losing enteropathy, and plastic bronchitis. Understanding the contemporary management of failing modified Fontan after TCPC is crucial for optimizing patient outcomes, as the number of adult patients with Fontan circulation continues to grow due to improved surgical techniques and postoperative care.Graphic Abstract
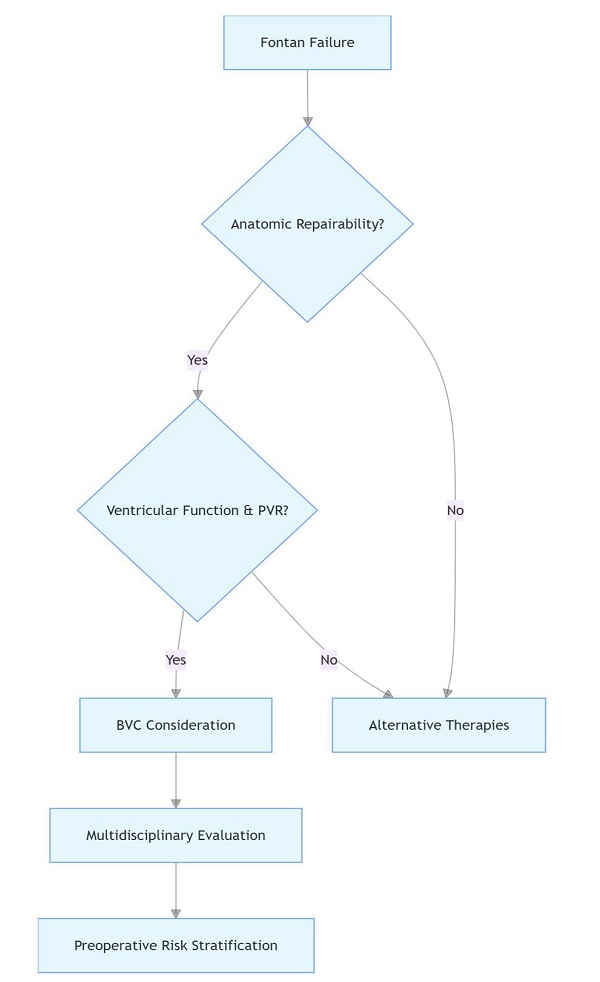
Keywords
The Fontan procedure holds great significance in the field of congenital heart disease (CHD) treatment, with its origin closely tied to the early understanding of the mammalian circulatory system. The concept of cavopulmonary circulation was introduced by Carlo Adolfo Carlon in the early 1950s [1]. This discovery, along with Rodbard and Wagner’s hypothesis during the same era that the driving force from the left or systemic ventricle could propel systemic venous blood through the pulmonary circulation, laid the crucial theoretical groundwork for subsequent surgical innovations [2]. In 1968, Francis Fontan aimed to divert both superior and inferior vena cava blood directly to the pulmonary artery by passing the non-functional right ventricle and creating a circulation similar to that of fish (vena cava-pulmonary artery-pulmonary vein-left atrium-left ventricle-aorta) [3]. This innovative procedure, named the “Fontan procedure”, inaugurated a new era in the surgical treatment of functional single-ventricle hearts [4]. Despite significant progress in surgical outcomes, Fontan patients remain at lifelong risk of complications such as heart failure, protein-losing enteropathy (PLE), plastic bronchitis (PB), arrhythmia, cyanosis, hepatic dysfunction. These complications can lead to circulatory decompensation, known as the “Failing Fontan” [5,6] (Table 1).
Table 1: Definition and diagnostic criteria for Fontan failure-related complications.
| Complications | Definition and Diagnostic Criteria |
|---|---|
| Heart Failure | Inability of the single ventricle to maintain adequate perfusion, with signs of circulatory congestion/forward failure. |
| NYHA class III–IV. | |
| CVP > 12 mmHg. | |
| Cardiac index < 2.0 L/min/m2. | |
| PLE | Intestinal protein loss leading to hypoalbuminemia. Table 2 shows the details. |
| PB | Airway obstruction by fibrin-mucin casts, diagnosed via bronchoscopy. |
| Chest CT shows bronchial filling defects-Acute dyspnea, wheezing, hypoxemia. | |
| Arrhythmias | Hemodynamically significant atrial/ventricular rhythm disorders. Atrial tachycardia or fibrillation, Ventricular tachycardia. ECG/Holter documented arrhythmia with hemodynamic compromise. |
| Cyanosis | Arterial oxygen saturation (SpO2) < 85%; Arterial PaO2 < 60 mmHg, echocardiography/angiography showing right-to-left shunt. |
| FALD | Chronic liver injury with fibrosis/cirrhosis. Mild to moderate elevations in ALT and AST (usually <300 U/L), hypoalbuminemia (<35 g/L), and abnormal coagulation (INR > 1.5). FibroScan > 12.5 kPa (liver cirrhosis) Portal hypertension (varices, splenomegaly). |
| PVR elevation | PVR > 4 Wood (measured via cardiac catheterization). Pulmonary-to-systemic resistance ratio > 0.3. Preoperative mean pulmonary artery pressure > 18 mmHg or postoperative mean pulmonary artery pressure > 15 mmHg. |
| Thromboembolic Events | Intravascular thrombosis causing organ dysfunction. Stroke, pulmonary embolism, or conduit thrombosis, Imaging confirmed thrombus, D-dimer > 500 ng/mL. |
| AVVR | Moderate-severe regurgitation affecting ventricular function. Echocardiographic regurgitation grade ≥ 3+, Effective regurgitant orifice area > 0.4 cm2, Ventricular dilation. |
| EDS | Hormonal abnormalities (growth, thyroid, bone, gonadal). |
Table 2: Diagnostic criteria for PLE.
| Diagnostic Aspects | Clinical Symptom Manifestations | Laboratory Indicators |
|---|---|---|
| Specific Content | 1. Abdominal distension or discomfort. 2. Chronic diarrhea 3. Ascites 4. Edema 5. Pleural effusion or pericardial effusion 6. Severe malnutrition | 1. Hypoproteinemia: serum albumin <3.5 g/dL; Total proteins level <6.0 g/dL 2. Elevated Ratio of α-1-Antitrypsin Clearance to Creatinine Clearance: Ratio > 0.08 3. Spot Fecal alpha-1-antitrypsin concentration >54 mg/dL |
| Diagnostic Precautions | 1. Both clinical symptom manifestations and laboratory Indicators are required for diagnosis. 2. Other causes that may cause similar symptoms and laboratory changes need to be ruled out to ensure an accurate diagnosis | |
This article is intended to comprehensively review the historical development, practical experiences, and inherent limitations of the modified Fontan procedure specifically in pediatric patients. Moreover, it will systematically summarize the most recent surgical techniques and advancements in managing the failing modified Fontan.
2 Influence of Patient-Specific Factors on the Efficacy of Total Cavopulmonary Connection (TCPC)
The efficacy of TCPC is significantly shaped by various patient-specific factors, which are pivotal in determining surgical success and long-term prognosis.
- (1) Age is a key determinant. The optimal age for TCPC surgery is 2–4 years, when children have stable cardiovascular development, good surgical tolerance, and strong recovery ability. Infants under 2 years usually undergo a bidirectional cavopulmonary shunt at 4–6 months first, followed by TCPC later. For patients over 15 years, outcomes are generally satisfactory, but those over 30 face risks due to long-term left ventricular volume overload-induced myocardial changes and postoperative cardiac dysfunction [7].
- (2) Cardiac rhythm matters, with sinus rhythm being ideal. A pacemaker can be implanted post-operation for heart block patients. Atrial flutter and fibrillation are more controllable after surgery, and CHD-related ones originating from the right atrium can be corrected by the right maze procedure [8].
- (3) Abnormal venous connections, though correctable during surgery, often lead to suboptimal outcomes. Preoperative mean pulmonary artery pressure is a critical indicator; the classic threshold for TCPC surgery was <15 mmHg, now relaxed to 18 mmHg due to surgical advancements.
- (4) Preoperative mean pulmonary artery pressure serves as a critical indicator of surgical feasibility. An elevation in preoperative mean pulmonary artery pressure is strongly associated with both early and late mortality. Initially, the classic indications for TCPC surgery emphasized that the preoperative mean pulmonary artery pressure should be less than 15 mmHg. With the evolution of surgical techniques, this threshold has been relaxed to 18 mmHg [9].
- (5) High pulmonary vascular resistance (PVR) increases surgical risk by impeding oxygenation and elevating the cardiac burden. Pulmonary artery development is crucial; patients with a McGoon ratio < 1.5 and Nakata < 150 mm2/m2 have poor prognoses, but local pulmonary artery stenosis may render these indices inaccurate [10].
- (6) Atrioventricular valve (AVV) function affects TCPC prognosis. Severe AVV regurgitation can reduce pulmonary blood flow and cause Fontan failure; patients with degenerated valve function after TCPC have a Fontan failure rate twice as high as others [10].
3 Pathophysiology of Failing Fontan
Patients with CHD may experience seemingly good short-term outcomes after the Fontan procedure. However, the Fontan circulation is inherently abnormal compared to normal physiological circulation. At its core, the Fontan procedure creates a portal-like circulatory pattern. To achieve sufficient left ventricular pre-load, central venous pressure (CVP) must be increased, which is the root cause of many Fontan-related complications [11]. For adequate pulmonary blood flow, CVP needs to be at least as high as pulmonary artery pressure, usually 12–14 mmHg. But a CVP that is too high causes lymphatic stasis and edema, while a low CVP impairs pulmonary blood flow. This contradiction is known as the “Fontan paradox” [12]. The newly formed portal system reduces left ventricular pre-load, leading to decreased cardiac output. Without a sub-pulmonary pumping mechanism, even small changes in the resistance of this system can cause significant fluctuations in cardiac output. Chronic CVP elevation and persistently low cardiac output gradually lead to physiological abnormalities, ultimately resulting in Fontan failure [13,14,15] (Fig. 1).
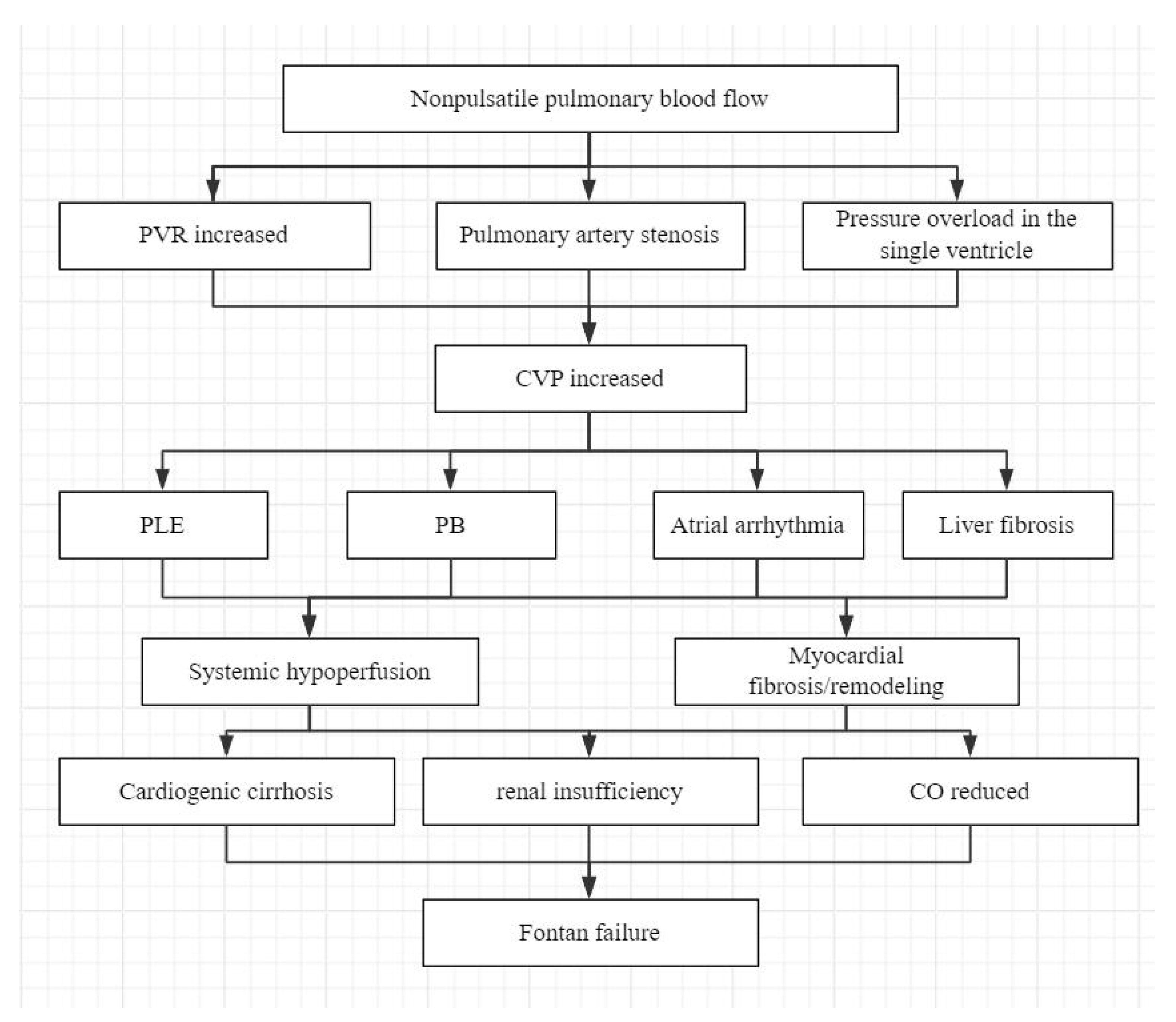
Figure 1: Flowchart of the pathophysiological cascade of Fontan failure. PVR, pulmonary vascular resistance; CVP, central venous pressure; PLE, protein-losing enteropathy; PB, plastic bronchitis; CO, cardiac output.
4 Clinical manifestations and Treatment
Indications: New York Heart Association (NYHA) class I-II, no obvious structural abnormalities, PVR ≤18 mmHg and hemodynamic stability.
- (1) Treatment of Heart Failure
As previously discussed, the core challenge in Fontan circulation resides primarily in obstructive pathophysiology within the newly established cavopulmonary pathway rather than intrinsic cardiac dysfunction. Consequently, traditional heart failure therapies may be less effective than interventions specifically targeting resistance in the Fontan circulatory circuit. Most current medical treatments are extrapolated from biventricular heart failure protocols. In acute decompensation, phosphodiesterase inhibitors such as milrinone offer lusitropic, inotropic, and vasodilatory effects beneficial for Fontan failure [16]. Clinical experience suggests milrinone provides maximal benefit in systolic dysfunction and can augment diuresis in Fontan patients with preserved left ventricular ejection fractions (LVEF) and circulatory failure. However, caution is warranted: vasodilators should be avoided in cirrhosis or low systemic vascular resistance [17].
Fontan failure is often characterized by upregulation of the renin-angiotensin-aldosterone system (RAAS), increasing pulmonary and systemic vascular resistance, elevating end-diastolic pressures, and reducing cardiac output. Angiotensin-converting enzyme (ACE) inhibitors (ACEIs) are frequently prescribed to counteract RAAS activation, with aldosterone antagonists offering adjunctive benefit [18]. Chronic heart failure also drives sympathetic overactivation, necessitating β-blockers to inhibit neurohumoral dysfunction, often used in combination with ACEIs. Notably, ACEIs has limitations in Fontan failure. Common side effects include dry cough (10–20% incidence), hypotension, and potential renal dysfunction. Dry cough is hypothesized to result from bradykinin accumulation due to ACE inhibition, stimulating respiratory receptors. Hypotension may occur early in therapy, while baseline renal insufficiency requires close monitoring. Mineralocorticoid receptor antagonists represent a viable alternative, particularly given the high prevalence of liver dysfunction in Fontan patients. Nesiritide, a recombinant brain natriuretic peptide, binds vascular guanylate cyclase receptors to increase cGMP, inducing arteriovenous dilation, inhibiting RAAS, promoting natriuresis, and reducing cardiac preload/afterload. These effects make nesiritide a valuable option for Fontan-associated heart failure [19]. Milrinone and nesiritide have high levels of evidence in acute decompensation and can be used as first-line therapy, provided that hemodynamics and organ function are monitored. The evidence for RAAS inhibitors (ACEIs/aldosterone antagonists) and β-blockers is of low level, mainly based on extrapolation of biventricular heart failure, and should be used with caution in Fontan patients, especially those with liver and kidney dysfunction.
- (2) Treatment of Increased PVR
Pulmonary vasodilators are essential in treating Fontan failure, as they enhance oxygenation by dilating pulmonary vessels, reducing pulmonary vascular resistance, and increasing pulmonary blood flow. Key classes include: (1) Phosphodiesterase type 5 inhibitors (PDE5is): Sildenafil and tadalafil inhibit PDE5, increasing intracellular cGMP to relax pulmonary vascular smooth muscle and reduce resistance, PDE5 inhibitors may be used as a first-line option, especially in patients with lymphatic circulation disorders such as PLE, due to the convenience of oral administration and relatively safe side effect profile. [20]. Pulmonary vasodilation may benefit patients with lymphatic stasis and potentially alleviate PLE. (2) Endothelin receptor antagonists: Bosentan and ambrisentan block endothelin-receptor binding, inhibiting vasoconstriction and promoting pulmonary vessel dilation. Endothelin receptor antagonists should be alert to hepatotoxicity, and it is recommended for patients with poor response to PDE5 inhibitors or pulmonary vascular remodeling. Liver function should be closely monitored during treatment. (3) Prostacyclin analogues: Epoprostenol and treprostinil directly relax pulmonary vascular smooth muscle and inhibit platelet aggregation. Prostacyclin analogues are indicated in patients with acute decompensation or severe elevated pulmonary vascular resistance, but long-term use is limited by the route of administration and side effects [21,22].
- (3) Treatment of Fontan Associated Liver Disease (FALD)
Long-term follow-up investigations after the Fontan procedure have unearthed that both elevated CVP and systemic hypoperfusion are causative factors for congestive liver disease. This hepatic condition often undergoes a progressive course, with the potential to transform into liver fibrosis, cirrhosis, and, in extreme scenarios, hepatocellular carcinoma.
Diuretics such as furosemide and spironolactone are critical in managing patients by alleviating systemic congestion. Through diuresis, they reduce circulatory fluid volume, lowering hepatic venous pressure to improve FALD. For inadequate cardiac output, positive inotropes like digoxin enhance myocardial contractility, increasing cardiac output and improving liver perfusion to maintain hepatic function and prevent deterioration in patients with underlying cardiac conditions.
Monitoring potential hepatotoxic medications is essential, with prompt discontinuation of any identified agents. Concurrent liver function surveillance—including serum transaminase, bilirubin, and albumin levels—detects early hepatic impairment. Hepatoprotective therapies, such as polyene phosphatidylcholine (stabilizing hepatocyte membranes for repair) and reduced glutathione (neutralizing free radicals via antioxidant pathways), support hepatocyte regeneration and mitigate liver injury.
- (4) Treatment of PLE
Unfortunately, therapeutic options for PLE remain limited, with treatment goals focused on improving ventricular function, stabilizing intestinal mucosa, and reversing protein loss (Table 2). As previously discussed, ACE inhibitors, diuretics, and β-blockers are used to optimize cardiac function. Aldosterone receptor antagonists have shown efficacy in reducing intestinal protein loss. Glucocorticoids have been reported to alleviate PLE symptoms in multiple cases, supporting the role of inflammation in mucosal damage and protein leakage. Heparin therapy may benefit PLE by replenishing sulfated glycosaminoglycans in mucosal cells, which regulate intestinal albumin loss. The commonly used dose for children is 100–200 U/kg/d, divided into 2–3 subcutaneous injections. Adults can use 5000–10,000 U/d, and the dose is adjusted according to activated partial thromboplastin time (aPTT), aiming to maintain aPTT at 1.5–2 times the upper limit of normal [23]. Somatostatin analogues such as octreotide reduce gastrointestinal secretions, decrease intestinal mucosal permeability, promote lymphatic constriction, and minimize protein loss [24]. Intravenous immunoglobulin is used to address immunodeficiency secondary to severe protein depletion [25].
Nutritional strategies include high-protein diets and medium-chain triglyceride diets to counteract protein wasting. Enteral or parenteral nutrition support is administered as needed to ensure adequate nutrient intake.
- (5) Treatment of PB
Medical management of PB includes bronchodilators, mucolytic agents, steroids, antibiotics, and inhaled tissue plasminogen activator has been shown to be effective. Pulmonary vasodilators and macrolides may also be effective [25]. Bronchoscopy is used for diagnosis and treatment.
- (6) Treatment of Cyanosis
The main cause of cyanosis after Fontan procedure is the change of circulatory structure leading to hypoxemia, which is mainly treated by interventional or surgical treatment, without targeted drug treatment.
- (7) Treatment of Arrhythmia
Arrhythmias significantly impair Fontan hemodynamics and require prompt management. Anti-arrhythmic drug selection depends on arrhythmia type: verapamil or propafenone for supraventricular tachycardia, and lidocaine or mexiletine for ventricular arrhythmias. However, close monitoring of electrocardiogram and cardiac function is critical due to potential drug adverse effects. Compared to catheter ablation— the preferred modality due to lower recurrence rates—medical therapy alone has higher relapse risks. Short-term studies support exercise therapy as an adjunct to improve arrhythmia management. Antiarrhythmic drugs: Low level of evidence (level C), used only as initial therapy or as an alternative when catheter ablation is not feasible, with close monitoring of side effects and electrocardiographic changes. Catheter ablation is currently the preferred method for rhythm control (level B), especially for patients with drug failure or high recurrence rate. It is recommended to carry out in experienced centers. Exercise therapy: it can be used as an auxiliary method (level B) [26,27].
- (8) Treatment of Atrioventricular Valve Regurgitation (AVVR)
The medical management of AVVR predominantly involves the utilization of diuretics. Agents like furosemide and spironolactone are administered to decrease the heart’s volume load. By promoting diuresis, these diuretics effectively alleviate symptoms associated with pulmonary congestion and systemic congestion. Vasodilators, such as ACEIs, play a crucial role in this treatment paradigm. ACEIs act by reducing the heart’s afterload. This reduction in afterload leads to a decrease in the regurgitant fraction, thereby enhancing cardiac function. Concurrently, β-blockers can be incorporated into the treatment regimen. β-blockers function by slowing the heart rate. Additionally, they contribute to improving myocardial remodeling and reducing the cardiac load. As a result, β-blockers can, to a certain degree, relieve the symptoms associated with AVVR. Drug treatment of AVVR is generally effective, the evidence level is low (level C), and it mainly relies on surgical treatment.
- (9) Treatment of Thromboembolic Complications
In the management of Fontan patients, clinical practice demonstrates that aspirin and warfarin are the most prescribed long-term antithrombotic agents. A meta-analysis revealed the overall thromboembolism (TE) incidence among Fontan patients on aspirin or warfarin ranges from 8.6–9%, significantly lower than the 18.6% rate in those without thromboprophylaxis [28]. The CHEST Guidelines recommend initiating aspirin (1–5 mg/kg/day) or unfractionated heparin in pediatric Fontan patients, transitioning to vitamin K antagonists to maintain an international normalized ratio (INR) target of 2.5 (range: 2.0–3.0), although the optimal treatment duration remains undefined (level B).
For warfarin use in Fontan patients, a loading dose of 0.1 mg/kg/day is recommended, with individualized adjustment of maintenance dosing to sustain INR within 2.0–3.0 [29]. INR monitoring frequency may be reduced to at least monthly. Notably, all thromboprophylactic agents carry an elevated bleeding risk, with warfarin-associated bleeding rates higher than aspirin (level B) [30]. Beyond bleeding, warfarin use may correlate with low bone mineral density and increased fracture risk, mitigated by interventions such as weight-bearing exercise, vitamin D/calcium supplementation, and bisphosphonate therapy [31]. While case reports describe successful novel oral anticoagulant (NOAC) use in adult Fontan patients, insufficient safety/efficacy data preclude their recommendation in pediatric populations (level C) [13].
- (10) Treatment of Endocrine Dysregulation Syndromes (EDS)
The etiologies of EDS after Fontan procedure are complex and multifactorial. Medical management primarily involves symptom-targeted therapy tailored to individual patients, with a core focus on correcting nutrient deficiencies. For example, vitamin D and calcium supplementation are critical for preventing and managing abnormal bone metabolism, which is often disrupted in EDS. Additionally, replenishing trace elements such as zinc and iron can optimize nutritional status, thereby supporting normal hormonal synthesis and metabolism.
EDS significantly impacts psychological well-being, frequently precipitating anxiety and depression psychological factors that can further exacerbate endocrine dysfunction. Thus, comprehensive psychological support and counseling are essential. These interventions not only alleviate EDS related psychological distress but also mitigate the bidirectional exacerbation between mental health and endocrine imbalance, ultimately improving quality of life and long-term prognosis.
Indications: patients with NYHA III–IV, presence of Fontan pathway stenosis/thrombosis, moderate to severe AVVR, refractory hypoxemia or multiple organ dysfunction, or no response to medical treatment.
Early-onset failure is often attributed to intraoperative myocardial injury, suboptimal surgical technique, or undiagnosed preoperative PVR elevation, with higher mortality compared to late-onset cases. Reoperation to revise or remove the Fontan pathway is frequently necessary [32,33]. Late-onset failure arises from chronic hemodynamic stress-induced structural remodeling, requiring vigilant surveillance for clinical deterioration. If medical management fails, surgical or interventional strategies become critical (Table 3).
- (1) Surgical treatment of the Fontan pathway
Post-Fontan circulatory abnormalities often involve stenosis in extracardiac conduits, vena cava anastomoses, or pulmonary arteries, compromising blood flow. Percutaneous balloon dilation, stenting, or surgical conduit replacement may restore patency. Conduit thrombosis necessitates prompt thrombolytic therapy, though bleeding risk is significant; surgical thrombectomy remains a definitive option for refractory cases [33,34].
- (2) Surgical treatment of heart failure
Following the Fontan procedure, the single ventricle is subjected to prolonged dual systemic and pulmonary circulatory burdens, rendering it susceptible to progressive myocardial hypertrophy, strain, and deteriorative ventricular function. Patients with Fontan failure exhibit a gravely compromised prognosis, with only approximately 40% surviving without requiring death or transplantation within a 10-year follow-up period [35,36]. The decision of heart transplantation for patients with Fontan failure should be based on the International Society for Heart and Lung Transplantation (ISHLT) guidelines, combined with multi-dimensional indicators such as cardiac function, complications, age stratification, and so on, and individualized treatment should be achieved through multidisciplinary team evaluation (Table 4). Patients with Fontan failure often require additional repair surgery when a heart transplant is required. When the original Fontan conduit is disconnected, the conduit may have serious adhesion and calcification with the surrounding tissue, which increases the difficulty of separation and the risk of bleeding. The perianastomotic tissue may become very fragile and prone to additional damage. The control of the Angle and tension of the anastomosis is crucial, otherwise it is easy to lead to anastomotic stenosis or blood leakage [37].
Table 3: Classification and comparison of surgical interventions for Fontan failure.
| Complication Type | Pathophysiological Mechanism | Surgical/Interventional Modalities | Contemporary Advances |
|---|---|---|---|
| Fontan Pathway Obstruction | Flow-limiting stenosis/thrombosis in extracardiac conduit, cavopulmonary anastomoses, or pulmonary arteries | Balloon angioplasty, stent implantation, conduit replacement, thrombectomy | Bioresorbable stents under trial for pediatric patients |
| End-Stage Ventricular Dysfunction | Chronic volume/pressure overload → myocardial fibrosis, LVEF < 35%, NYHA III-IV | Heart transplantation, combined heart-liver/kidney transplantation, MCS | Miniaturized MCS devices for adolescents |
| PLE/PB | CVP-mediated mesenteric venous congestion → intestinal lymphangiectasia; bronchial lymphatic obstruction → fibrin cast formation | Lymphovenous anastomosis, thoracic duct decompression, Fontan fenestration | Fluorescent lymphography for real-time lymphatic mapping |
| Cyanosis from Intracardiac/Extracardiac Shunts | Right-to-left shunting: Patent fenestration, pulmonary arteriovenous malformations, systemic venous collaterals; Reduced pulmonary flow: Branch PA stenosis | Anastomosis repair, collateral embolization, pulmonary angioplasty | 3D-printed PA stents for complex branch stenosis |
| Refractory Atrial Arrhythmias | Macroreentry due to atrial dilation and fibrosis | Radiofrequency ablation, cryoablation, surgical Maze procedure | AI-driven arrhythmia prediction models for early intervention |
| Severe Atrioventricular Valve Regurgitation | Annular dilation, leaflet prolapse, or chordal rupture | Valve repair (annuloplasty, chordal shortening), valve replacement | TVI in experimental stages |
Table 4: Indications for heart transplantation after Fontan failure.
| Indication Category | Criterion | Evidence Grade |
|---|---|---|
| Cardiac function and hemodynamic failure | 1. Dependence on intravenous inotropic agents (dopamine > 5 μg/kg/min or milrinone) or requirement for mechanical circulatory support (ECMO, VAD) 2. CI < 2.0 L/min/m2 for >2 weeks with ineffective medical therapy | Class I, B |
| Functional Status | 1. NYHA Class IV heart failure 2. Peak oxygen consumption (VO2peak) ≤ 12 mL/kg/min (with β-blockers) or ≤14 mL/kg/min (without β-blockers) 3. 6-min walk distance < 300 m, or VO2peak ≤ 50% of predicted value with anaerobic threshold ≤ 11 mL/kg/min | Class I, B |
| Irreversible Hepatorenal Dysfunction | Liver: Child-Pugh score ≥ B (bilirubin > 34 μmol/L, albumin < 30 g/L, prothrombin time prolongation > 4 s) or liver fibrosis elastography > 20 kPa with ascites Kidney: eGFR < 30 mL/min/1.73 m2 with diuretic resistance (24-h urine output < 1 mL/kg/h) | Class I, C |
| Refractory PLE | Ineffective treatment with octreotide + heparin for 3 months | Class IIa, B |
| Refractory Malignant Arrhythmias | Recurrent ventricular tachycardia/ventricular fibrillation requiring ≥ 2 cardioversions per month and failed ICD therapy | Class IIa, B |
| Chronic Thromboembolic Pulmonary Hypertension | PVR > 6 Wood units/m2 with no response to NO challenge, or concurrent repeated cardiac thrombus embolization | Class IIa, B |
| Special Indications for Pediatric Patients | Growth retardation (height/weight < 5th percentile) or neurodevelopmental delay (Wechsler Intelligence Scale IQ < 70) | Class IIa, C |
| Special Indications for Adult Patients | Cardiac MRI delayed enhancement > 30% or recurrent atrial tachycardia (frequency > 1 episode/week) | Class IIa, C |
Advanced Fontan failure often involves multiorgan dysfunction (MOT), including heart-liver or heart-liver-kidney transplantation, is considered for: end-stage cardiac failure (NYHA class IV); evere hepatic decompensation (Child-Pugh C, intractable ascites, hepatic encephalopathy); end-stage renal disease (uremia requiring dialysis) Patient selection requires adequate functional status to tolerate complex surgery and the absence of irreversible comorbidities. Mechanical Circulatory Support (MCS) has emerged as a bridge to transplantation, reducing pediatric waitlist mortality by up to 50% [38,39,40]. The latest research suggests that intracorporeal left ventricular assist device (LVAD) offer an alternative to “bridging transplantation” or “long-term support” for such patients. The latest clinical data from Fuwai Hospital of Chinese Academy of Medical Sciences showed that the 1-year survival rate of CH-VAD device using full magnetic levation technology in patients with single ventricle can reach 95%, and the 3-year survival rate is maintained at 91%, which is significantly better than that of traditional LVAD device and close to the long-term effect of heart transplantation [41,42]. At the same time, MCS report released by the ISHLT in 2024 pointed out that the 5-year overall survival rate of fully magnetically levitated LVAD in patients with single ventricle has reached 64%, among which the subgroup analysis of patients with Fontan circulation failure showed that the prognosis of patients with fine hemodynamic management was not statistically different from that of patients with biventricular failure.
- (3) Surgical treatment of PLE and PB
Elevated CVP and lymphatic hypertension are strongly correlated with the development of PLE and PB. Reducing CVP is critical and may be achieved through strategies such as: relief of Fontan pathway obstructions; repair of AVVR to optimize ventricular function; creation or revision of a Fontan fenestration to decompress systemic venous pressure; innominate vein-to-atrial appendage shunt placement to redirect venous flow [43,44].For lymphatic decompression, surgical interventions include: lymphaticovenous anastomosis to establish alternative lymphatic drainage pathways; thoracic duct (TD) decompression via surgical or percutaneous techniques; selective lymphatic embolization, TD stent placement, iodized oil lymphatic embolization, or hepatic lymphatic embolization to disrupt abnormal lymphatic pathways [45,46,47].
When medical and interventional therapies fail, heart transplantation emerges as a definitive treatment. Nearly all transplant survivors exhibit significant resolution of PLE/PB symptoms and other Fontan-related complications, underscoring its role as a life-saving intervention for end-stage disease.
- (4) Surgical treatment of cyanosis
Cyanosis following the Fontan procedure arises from multifactorial mechanisms. Inadequate pulmonary blood flow is a primary cause, often due to anastomotic stenosis or vascular compression by surrounding tissues, which reduces pulmonary perfusion. Surgical correction of these anatomical abnormalities is typically required to restore adequate pulmonary blood flow. Abnormal shunts represent another key etiology, including systemic-pulmonary venous collaterals, conduit/baffle-to-atrial communications, and pulmonary arteriovenous malformations (PAVMs). Catheter-based interventions effectively occlude venovenous collaterals and abnormal systemic-pulmonary venous connections. However, residual intracardiac shunts or PAVMs refractory to endovascular treatment necessitate surgical intervention to resolve hypoxemia and improve oxygenation [48,49].
- (5) Surgical treatment of arrhythmia
For patients with atrial tachycardia or atrial fibrillation who fail to respond to drug therapy or are intolerant to antiarrhythmic drugs, radiofrequency ablation and cryoablation can be used to ablation the arrhythmogenic focus and eliminate abnormal electrical circuits [50]. The maze procedure is a clear alternative when ablation fails. Many studies have shown that Maze procedure can effectively control the arrhythmia in most patients and obtain a high recurrence rate of sinus rhythm. Another important benefit of the Maze procedure is the reduction in the incidence of embolic complications by reducing atrial stasis and thrombosis [33,51]. The dual efficacy of the Maze procedure for arrhythmia control and thromboembolism prevention makes it a cornerstone intervention in refractory cases when risks and benefits are carefully weighed. The hybrid operation mode of radiofrequency ablation combined with Maze procedure has achieved good prognosis in the treatment of patients with complex refractory arrhythmia.
- (6) Surgical treatment of AVVR
Moderate-to-severe AVVR with clinical symptoms (exertional dyspnea, fatigue, palpitations, declining cardiac function) and echocardiographic evidence of ventricular dilation with reduced contractility warrants surgical evaluation. Valve repair is preferred for structurally preserved AVVs (annuloplasty for annular dilation, leaflet plication for prolapse, chordal repair for laxity), preserving native valve function and avoiding long-term anticoagulation risks. This approach is associated with lower rates of TE and bleeding compared to replacement [52]. Valve replacement is indicated for irreparably damaged valves (severe leaflet calcification, chordal/papillary muscle dysfunction). While surgical replacement remains standard, the percutaneous edge-to-edge repair (PETER) procedure has shown safety and efficacy in adult degenerative mitral/tricuspid regurgitation. Although proposed for congenital AVVR, its use in Fontan patients lacks supportive data. Such investigations could align with advancements in transcatheter valve therapies for CHD, potentially offering minimally invasive alternatives to open surgery.
5 Biventricular Conversion (BiVC)
While survival after Fontan surgery has improved substantially, long-term complications remain inevitable. For patients with Fontan failure, heart transplantation is often the ultimate treatment, though the field faces challenges including donor shortages, prolonged waitlists, and elevated post-transplant mortality. A single-center retrospective study by Doulamis et al. demonstrated that BiVC is a safe and effective salvage therapy for Fontan failure, all (n = 23) who underwent elective BiVC survived, whereas 2-year survival rate for patients with a failing Fontan circulation was 72.7% (95% confidence interval, 37%–90%) [53]. Primary BiVC is feasible in patients with mild hypoplastic left heart syndrome, whereas a staged approach with ventricular recruitment is preferred for those with moderate-severe left ventricular dysplasia. Fig. 2 is the computed tomography (CT) data of a patient who underwent TCPC followed by BiVC in our hospital.
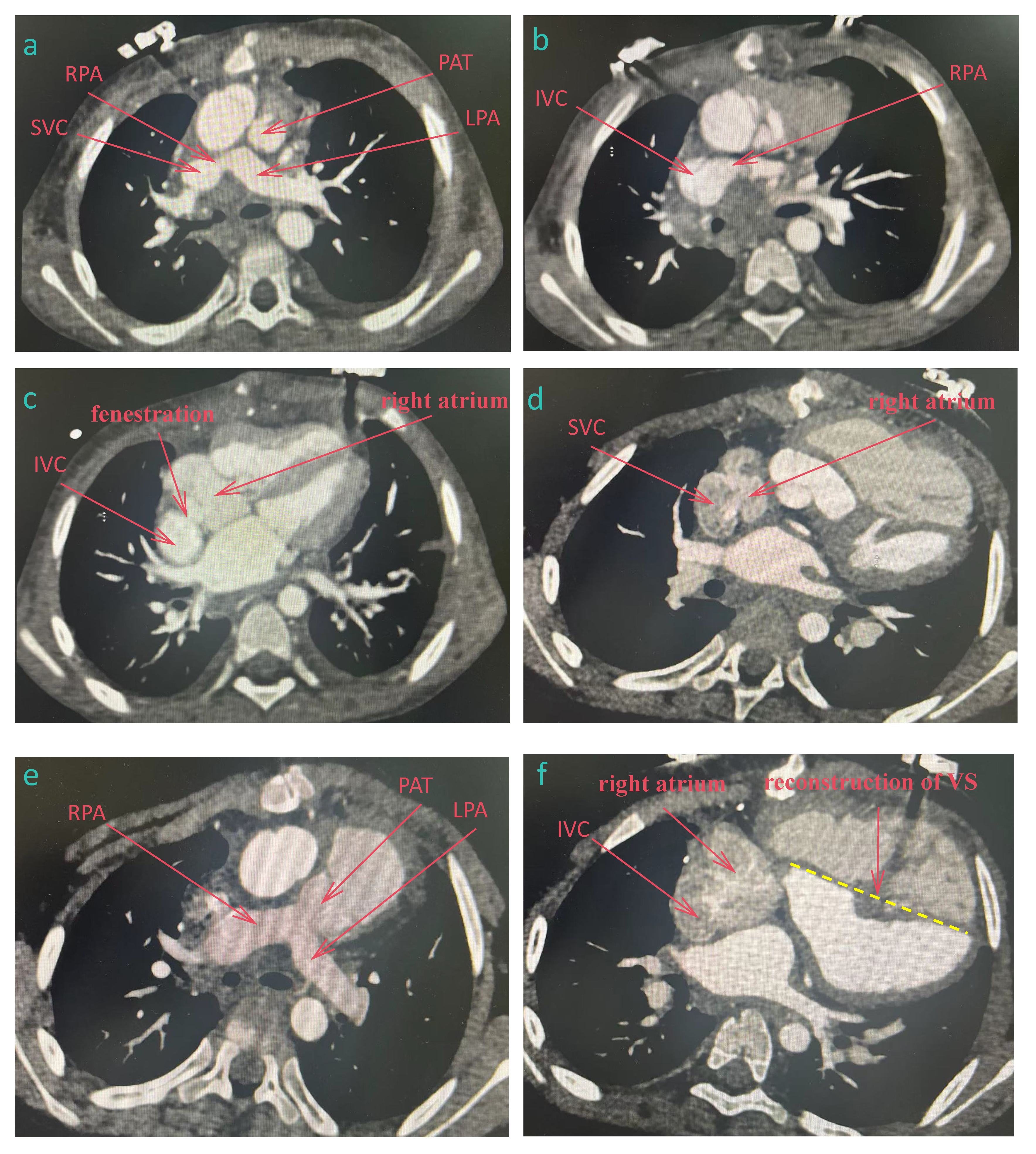
Figure 2: CT data of a patient who underwent total cavopulmonary connection followed by Biventricular conversion. (a–c) after TCPC, (d–f) after biventricular conversion. (a): The superior vena cava was connected to the right pulmonary artery, and the pulmonary artery trunk was cut off. (b): The inferior vena cava was connected to the right pulmonary artery. (c): The right atrium was fenestrated and communicated with the inferior vena cava. (d): The superior vena cava was reconnected to the right atrium. (e): Pulmonary artery trunk and pulmonary valve were reconstructed. (f): The inferior vena cava was reconnected to the right atrium and the ventricular septum was reconstructed. SVC, superior vena cava; IVC, inferior vena cava; RPA, right pulmonary artery; LPA, right pulmonary artery; PAT, pulmonary artery trunk; VS, ventricular septum.
Given the suboptimal long-term outcomes even in stable Fontan patients, emerging research explores BiVC not only as rescue therapy but also as an elective intervention in well-compensated circulations. Scholars argue that BiVC should be considered proactively, before the onset of end-stage complications, to mitigate the deleterious effects of single-ventricle physiology. However, the optimal timing for BiVC, particularly in asymptomatic patients, requires further clarification.
Psychological Problems and Treatment
The psychological problems of patients with Fontan failure are multi-dimensional and complex, and individualized intervention should be carried out according to the stage of disease progression, age characteristics and social support system. Existing studies have clarified the standardized process of psychological assessment, such as Hospital Anxiety and Depression Scale (HADS) initial screening-psychologist consultation-multidisciplinary collaboration and hierarchical intervention strategies, such as basic support-cognitive behavioral therap-drug combined with trauma therapists [54].
6 Prognosis and Future Directions
Future research directions in the management of Fontan failure include the development of novel medical therapies, such as gene-based therapies and cell-based therapies, to improve ventricular function and reduce the burden of pulmonary vascular disease. The use of artificial intelligence and machine learning algorithms may also help in predicting Fontan failure earlier and optimizing treatment strategies. In addition, efforts to improve the availability of donor organs for heart transplantation, such as the development of bioengineered hearts or the use of mechanical circulatory support devices as a bridge to transplantation, may offer hope for patients with end-stage Fontan failure. With the interdisciplinary integration of biomedical engineering, materials science, imaging and other disciplines, significant progress has been made in the in vitro research of transcatheter devices to assist Fontan patients, which also points out the direction for future technical development.
The contemporary management of failing modified Fontan after TCPC requires a comprehensive, multidisciplinary approach. Early diagnosis, appropriate medical and interventional management, and careful consideration of surgical options when necessary are crucial for improving the outcomes and quality of life of these complex patients.
Acknowledgement:
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Honghao Fu: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Writing—original draft. Zhangwei Wang, Shoujun Li: Writing—review & editing. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that supports the findings of this study are available from the first author upon reasonable request.
Ethics Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Stellin G. A tribute to the pioneers of right heart bypass: an historical review. World J Pediatr Congenit Heart Surg. 2020;11(2):198–203. doi:10.1177/2150135119894478. [Google Scholar] [CrossRef]
2. Rodbard S, Wagner D. By-passing the right ventricle. Proc Soc Exp Biol Med. 1949;71(1):69. doi:10.3181/00379727-71-17082. [Google Scholar] [CrossRef]
3. Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26(3):240–8. doi:10.1136/thx.26.3.240. [Google Scholar] [CrossRef]
4. Kreutzer G, Galíndez E, Bono H, De Palma C, Laura JP. An operation for the correction of tricuspid atresia. J Thorac Cardiovasc Surg. 1973;66(4):613–21. [Google Scholar]
5. Gewillig M, Brown SC, van de Bruaene A, Rychik J. Providing a framework of principles for conceptualising the Fontan circulation. Acta Paediatr. 2020;109(4):651–8. doi:10.1111/apa.15098. [Google Scholar] [CrossRef]
6. Mazza GA, Gribaudo E, Agnoletti G. The pathophysiology and complications of fontan circulation. Acta Biomed. 2021;92(5):e2021260. doi:10.23750/abm.v92i5.10893. [Google Scholar] [CrossRef]
7. Kato A, Sato J, Yoshii K, Yoshida S, Nishikawa H, Ohashi N, et al. The mid-term outcome of Fontan conversion compared with primary total cavopulmonary connection. J Cardiol. 2021;78(3):213–8. doi:10.1016/j.jjcc.2021.02.005. [Google Scholar] [CrossRef]
8. Baroutidou A, Otountzidis N, Papazoglou AS, Moysidis DV, Kartas A, Mantziari L, et al. Atrial fibrillation ablation in congenital heart disease: therapeutic challenges and future perspectives. J Am Heart Assoc. 2024;13(2):e032102. doi:10.1161/JAHA.123.032102. [Google Scholar] [CrossRef]
9. Lehner A, Schuh A, Herrmann FEM, Riester M, Pallivathukal S, Dalla-Pozza R, et al. Influence of pulmonary artery size on early outcome after the Fontan operation. Ann Thorac Surg. 2014;97(4):1387–93. doi:10.1016/j.athoracsur.2013.11.068. [Google Scholar] [CrossRef]
10. Bove T, Grootjans E, Naessens R, Martens T, De Wolf D, Vandekerckhove K, et al. Long-term follow-up of atrioventricular valve function in Fontan patients: effect of atrioventricular valve surgery. Eur J Cardiothorac Surg. 2023;64(4):ezad305. doi:10.1093/ejcts/ezad305. [Google Scholar] [CrossRef]
11. Deal BJ, Jacobs ML. Management of the failing Fontan circulation. Heart. 2012;98(14):1098–104. doi:10.1136/heartjnl-2011-301133. [Google Scholar] [CrossRef]
12. Alsaied T, Rathod RH, Aboulhosn JA, Budts W, Anderson JB, Baumgartner H, et al. Reaching consensus for unified medical language in Fontan care. ESC Heart Fail. 2021;8(5):3894–905. doi:10.1002/ehf2.13294. [Google Scholar] [CrossRef]
13. Veldtman GR, Opotowsky AR, Wittekind SG, Rychik J, Penny DJ, Fogel M, et al. Cardiovascular adaptation to the Fontan circulation. Congenit Heart Dis. 2017;12(6):699–710. doi:10.1111/chd.12526. [Google Scholar] [CrossRef]
14. Rösner A, Khalapyan T, Dalen H, McElhinney DB, Friedberg MK, Lui GK. Classic-pattern dyssynchrony in adolescents and adults with a Fontan circulation. J Am Soc Echocardiogr. 2018;31(2):211–9. doi:10.1016/j.echo.2017.10.018. [Google Scholar] [CrossRef]
15. Rychik J. The relentless effects of the Fontan paradox. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2016;19(1):37–43. doi:10.1053/j.pcsu.2015.11.006. [Google Scholar] [CrossRef]
16. Miwa K, Iwai S, Nagashima T. Anticoagulation therapy after the Fontan procedure. Pediatr Cardiol. 2022;43(6):1271–6. doi:10.1007/s00246-022-02848-6. [Google Scholar] [CrossRef]
17. Téllez L, Rodríguez de Santiago E, Albillos A. Fontan-associated liver disease: pathophysiology, staging, and management. Semin Liver Dis. 2021;41(4):538–50. doi:10.1055/s-0041-1732355. [Google Scholar] [CrossRef]
18. Menon SC, Al-Dulaimi R, McCrindle BW, Goldberg DJ, Sachdeva R, Goldstein BH, et al. Delayed puberty and abnormal anthropometry and its associations with quality of life in young Fontan survivors: a multicenter cross-sectional study. Congenit Heart Dis. 2018;13(3):463–9. doi:10.1111/chd.12597. [Google Scholar] [CrossRef]
19. Driesen BW, Voskuil M, Grotenhuis HB. Current treatment options for the failing Fontan circulation. Curr Cardiol Rev. 2022;18(4):e060122200067. doi:10.2174/1573403X18666220106114518. [Google Scholar] [CrossRef]
20. Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of Sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J. 2008;29(13):1681–7. doi:10.1093/eurheartj/ehn215. [Google Scholar] [CrossRef]
21. Goldberg DJ, Zak V, Goldstein BH, Schumacher KR, Rhodes J, Penny DJ, et al. Results of the FUEL trial. Circulation. 2020;141(8):641–51. doi:10.1161/circulationaha.119.044352. [Google Scholar] [CrossRef]
22. Kim YH, Chae MH, Choi DY. Inhaled iloprost for the treatment of patient with Fontan circulation. Korean J Pediatr. 2014;57(10):461–3. doi:10.3345/kjp.2014.57.10.461. [Google Scholar] [CrossRef]
23. Peled Y, Ducharme A, Kittleson M, Bansal N, Stehlik J, Amdani S, et al. International society for heart and lung transplantation guidelines for the evaluation and care of cardiac transplant candidates—2024. J Heart Lung Transplant. 2024;43(10):1529–628.e54. doi:10.1016/j.healun.2024.05.010. [Google Scholar] [CrossRef]
24. Sharma VJ, Iyengar AJ, Zannino D, Gentles T, Justo R, Celermajer DS, et al. Protein-losing enteropathy and plastic bronchitis after the Fontan procedure. J Thorac Cardiovasc Surg. 2021;161(6):2158–65.e4. doi:10.1016/j.jtcvs.2020.07.107. [Google Scholar] [CrossRef]
25. Elder RW, Wu FM. Clinical approaches to the patient with a failing Fontan procedure. Curr Cardiol Rep. 2016;18(5):44. doi:10.1007/s11886-016-0716-y. [Google Scholar] [CrossRef]
26. Rychik J, Atz AM, Celermajer DS, Deal BJ, Gatzoulis MA, Gewillig MH, et al. Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation. 2019;140(6):e234–84. doi:10.1161/CIR.0000000000000696. [Google Scholar] [CrossRef]
27. Scheffers LE, Berg LEMV, Ismailova G, Dulfer K, Takkenberg JJM, Helbing WA. Physical exercise training in patients with a Fontan circulation: a systematic review. Eur J Prev Cardiol. 2021;28(11):1269–78. doi:10.1177/2047487320942869. [Google Scholar] [CrossRef]
28. Sethasathien S, Phinyo P, Sittiwangkul R, Silvilairat S. Comparative effectiveness among thromboprophylaxis strategies after the Fontan operation: a systematic review and network meta-analysis. Thromb Res. 2024;241:109093. doi:10.1016/j.thromres.2024.109093. [Google Scholar] [CrossRef]
29. Giglia TM, Massicotte MP, Tweddell JS, Barst RJ, Bauman M, Erickson CC, et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease. Circulation. 2013;128(24):2622–703. doi:10.1161/01.cir.0000436140.77832.7a. [Google Scholar] [CrossRef]
30. Iyengar AJ, Winlaw DS, Galati JC, Wheaton GR, Gentles TL, Grigg LE, et al. No difference between aspirin and warfarin after extracardiac Fontan in a propensity score analysis of 475 patients. Eur J Cardiothorac Surg. 2016;50(5):980–7. doi:10.1093/ejcts/ezw159. [Google Scholar] [CrossRef]
31. Barnes C, Newall F, Ignjatovic V, Wong P, Cameron F, Jones G, et al. Reduced bone density in children on long-term warfarin. Pediatr Res. 2005;57(4):578–81. doi:10.1203/01.PDR.0000155943.07244.04. [Google Scholar] [CrossRef]
32. Mayer JE Jr, Bridges ND, Lock JE, Hanley FL, Jonas RA, Castaneda AR. Factors associated with marked reduction in mortality for Fontan operations in patients with single ventricle. J Thorac Cardiovasc Surg. 1992;103(3):444–52. [Google Scholar]
33. Huddleston CB. The failing Fontan: options for surgical therapy. Pediatr Cardiol. 2007;28(6):472–6. doi:10.1007/s00246-007-9008-z. [Google Scholar] [CrossRef]
34. Agasthi P, Jain CC, Egbe AC, Hagler DJ, Cabalka AK, Taggart NW, et al. Clinical outcomes of percutaneous Fontan stenting in adults. Can J Cardiol. 2023;39(10):1358–65. doi:10.1016/j.cjca.2023.04.023. [Google Scholar] [CrossRef]
35. Kamsheh AM, O’Connor MJ, Rossano JW. Management of circulatory failure after Fontan surgery. Front Pediatr. 2022;10:1020984. doi:10.3389/fped.2022.1020984. [Google Scholar] [CrossRef]
36. Weisert M, Menteer J, Durazo-Arvizu R, Wood J, Su J. Early prediction of failure to progress in single ventricle palliation: a step toward personalizing care for severe congenital heart disease. J Heart Lung Transplant. 2022;41(9):1268–76. doi:10.1016/j.healun.2022.06.002. [Google Scholar] [CrossRef]
37. Pradegan N, Cattapan C, Tessari C, Toscano G, D’Onofrio A, Tarzia V, et al. Anatomical aspects and long-term outcomes of additional surgical repair during heart transplantation in adult congenital heart disease. ASAIO J. 2025;71(7):e107–9. doi:10.1097/MAT.0000000000002353. [Google Scholar] [CrossRef]
38. Taner T, Hilscher MB, Broda CR, Drenth JPH. Issues in multi-organ transplantation of the liver with kidney or heart in polycystic liver-kidney disease or congenital heart disease: current practices and immunological aspects. J Hepatol. 2023;78(6):1157–68. doi:10.1016/j.jhep.2023.02.012. [Google Scholar] [CrossRef]
39. Hogue SJ, Greenberg JW, Mehdizadeh-Shrifi A, Villa CR, Chin C, Lorts A, et al. Heart transplantation outcomes for Fontan patients when using contemporary strategies: heart-liver transplantation and ventricular assist device therapy. World J Pediatr Congenit Heart Surg. 2025;16(3):368–75. doi:10.1177/21501351241305127. [Google Scholar] [CrossRef]
40. Butto A, Wright LK, Dyal J, Mao CY, Garcia R, Mahle WT. Impact of ventricular assist device use on pediatric heart transplant waitlist mortality: analysis of the scientific registry of transplant recipients database. Pediatr Transplant. 2024;28(4):e14787. doi:10.1111/petr.14787. [Google Scholar] [CrossRef]
41. Wang X, Zhou X, Chen H, Du J, Qing P, Zou L, et al. Long-term outcomes of a novel fully magnetically levitated ventricular assist device for the treatment of advanced heart failure in China. J Heart Lung Transplant. 2024;43(11):1806–15. doi:10.1016/j.healun.2024.05.004. [Google Scholar] [CrossRef]
42. Lu Y, Zhao S, Han J, Lv Q, Du X, Hua Z, et al. Multicenter study for CH-VAD as a fully magnetically levitated left ventricular assist device. iScience. 2025;28(2):111764. doi:10.1016/j.isci.2025.111764. [Google Scholar] [CrossRef]
43. Hraška V. Decompression of thoracic duct: new approach for the treatment of failing Fontan. Ann Thorac Surg. 2013;96(2):709–11. doi:10.1016/j.athoracsur.2013.02.046. [Google Scholar] [CrossRef]
44. Polomska AK, Proulx ST. Imaging technology of the lymphatic system. Adv Drug Deliv Rev. 2021;170:294–311. doi:10.1016/j.addr.2020.08.013. [Google Scholar] [CrossRef]
45. Itkin M, Piccoli DA, Nadolski G, Rychik J, DeWitt A, Pinto E, et al. Protein-losing enteropathy in patients with congenital heart disease. J Am Coll Cardiol. 2017;69(24):2929–37. doi:10.1016/j.jacc.2017.04.023. [Google Scholar] [CrossRef]
46. Gray M, Kovatis KZ, Stuart T, Enlow E, Itkin M, Keller MS, et al. Treatment of congenital pulmonary lymphangiectasia using ethiodized oil lymphangiography. J Perinatol. 2014;34(9):720–2. doi:10.1038/jp.2014.71. [Google Scholar] [CrossRef]
47. Sagray E, Johnson JN, Schumacher KR, West S, Lowery RE, Simpson K. Protein-losing enteropathy recurrence after pediatric heart transplantation: multicenter case series. Pediatr Transplant. 2022;26(5):e14295. doi:10.1111/petr.14295. [Google Scholar] [CrossRef]
48. McElhinney DB, Marx GR, Marshall AC, Mayer JE, Del Nido PJ. Cavopulmonary pathway modification in patients with heterotaxy and newly diagnosed or persistent pulmonary arteriovenous malformations after a modified Fontan operation. J Thorac Cardiovasc Surg. 2011;141(6):1362–70.e1. doi:10.1016/j.jtcvs.2010.08.088. [Google Scholar] [CrossRef]
49. Nguyen Cong MBH, Schaeffer T, Osawa T, Palm J, Georgiev S, Di Padua C, et al. Impact of veno-venous collaterals on outcome after the total cavopulmonary connection. Int J Cardiol. 2024;410:132229. doi:10.1016/j.ijcard.2024.132229. [Google Scholar] [CrossRef]
50. Moore BM, Anderson R, Nisbet AM, Kalla M, du Plessis K, d’Udekem Y, et al. Ablation of atrial arrhythmias after the atriopulmonary Fontan procedure: mechanisms of arrhythmia and outcomes. JACC Clin Electrophysiol. 2018;4(10):1338–46. doi:10.1016/j.jacep.2018.08.012. [Google Scholar] [CrossRef]
51. Houck CA, de Groot NMS, Kardys I, Niehot CD, Bogers AJJC, Mouws EMJP. Outcomes of atrial arrhythmia surgery in patients with congenital heart disease: a systematic review. J Am Heart Assoc. 2020;9(19):e016921. doi:10.1161/JAHA.120.016921. [Google Scholar] [CrossRef]
52. Jalal Z, Gewillig M, Boudjemline Y, Guérin P, Pilati M, Butera G, et al. Transcatheter interventions in patients with a Fontan circulation: current practice and future developments. Front Pediatr. 2022;10:965989. doi:10.3389/fped.2022.965989. [Google Scholar] [CrossRef]
53. Doulamis IP, Marathe SP, Piekarski B, Beroukhim RS, Marx GR, Del Nido PJ, et al. Biventricular conversion after fontan completion: a preliminary experience. J Thorac Cardiovasc Surg. 2022;163(3):1211–23. doi:10.1016/j.jtcvs.2021.04.076. [Google Scholar] [CrossRef]
54. Marshall KH, D’Udekem Y, Winlaw DS, Zannino D, Celermajer DS, Justo R, et al. Wellbeing in children and adolescents with Fontan physiology. J Pediatr. 2024;273:114156. doi:10.1016/j.jpeds.2024.114156. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools