 Open Access
Open Access
CASE REPORT
Case Report: A Third Patch as a Saviour in an Unsuccessful Complete Atrioventricular Septal Defect Repair
1 Section of Paediatric Cardiovascular Surgery, Department of Cardiovascular Surgery, Eskişehir Osmangazi University Faculty of Medicine, Eskişehir, 26040, Türkiye
2 Section of Paediatric Cardiology, Department of Paediatrics, Eskişehir Osmangazi University Faculty of Medicine, Eskişehir, 26040, Türkiye
* Corresponding Author: Emrah Şişli. Email:
(This article belongs to the Special Issue: Novel Methods and Techniques for the Management of Congenital Heart Disease)
Congenital Heart Disease 2025, 20(4), 531-537. https://doi.org/10.32604/chd.2025.067271
Received 29 April 2025; Accepted 25 June 2025; Issue published 18 September 2025
Abstract
Residual atrioventricular valve regurgitation after correction of complete atrioventricular septal defect (cAVSD) is still not ideal. As a modification of the double-patch method, our technique comprises a suture-bite-wide strip of a third patch that is incorporated to the upper margin of the left side of the ventricular septal defect (VSD) patch. This third patch counteracts not only the valvular tissue loss caused by the suture bites but also the rightward displacement of the VSD patch in a bulged fashion that occurs with increased left ventricular pressure after weaning from cardiopulmonary bypass. This unfavorable outcome was addressed with the current technique through augmentation of the left-sided bridging leaflets serving to prevent the separation of them from their corresponding mural leaflets. The concept was applied in two cases with Down syndrome aged 5 months and 6 months, respectively, as a rescue procedure in the same session just after a failed cAVSD repair. Since the immediate- and short-term outcomes of the atrioventricular valves in regard to regurgitation are satisfying, we believe that the technique proposed herein holds promise for the future in terms of tackling residual atrioventricular valve regurgitation.Keywords
Supplementary Material
Supplementary Material FileDespite the existence of a variety of techniques applied for complete atrioventricular septal defect (cAVSD) repair, left atrioventricular valve regurgitation (LAVVR) is one of the most challenging sequels that causes many hospital readmissions and re-operations [1,2,3,4,5]. Thus, we aimed to share our experience with post-repair LAVVR in two cases, based on our hypothesis regarding the lateral displacement of left-sided bridging leaflets.
Before surgery, written informed consent was obtained from the parents about the details, potential additional surgical interventions and outcomes of the surgical procedures. Immediately after cAVSD repair, the status of the atrioventricular valves is evaluated intra-operatively via saline test under asystole and epicardial echocardiography right after weaning from cardiopulmonary bypass (CPB). Valvar regurgitation severity was graded using an integrative approach, incorporating color flow Doppler jet characteristics, vena contracta width (mm), and chamber quantification including left atrial and left ventricular diameters [6]. Following detection of a moderate-to-severe LAVVR, CPB is re-instituted. Under mild hypothermia, after the application of an aortic cross clamping (ACC) and delivery of antegrade cold-blood cardioplegia for cardiac electrical quiescence, previous right atriotomy is reopened. The atrial septal defect (ASD) patch is excised, whereas the ventricular septal defect (VSD) patch remains intact, except for the superior margin. The left-sided cleft is reidentified and suspended. As for the Rastelli type A disease, while the superior bridging leaflet (SBL) is naturally organized into two sections based on the attachments of corda tending, the inferior bridging leaflet (IBL) requires division according to the chordal attachments over the septal crest.
The concept of the technique is revealed in Fig. 1. Right-sided bridging leaflets are reattached to the top edge of the VSD patch using double-armed 6/0 polypropylene sutures. A 3–5 mm broad oval strip of patch (the third patch) is prepared at the same length as the upper edge of the VSD patch. The right-sided sutures are then threaded through the right side of the third patch, and then through the ASD patch. Afterward, the left-sided bridging leaflets are sutured using a 6/0 polypropylene in a continuous fashion to the third patch’s left side followed by the completion of the left-sided cleft repair. In order to avoid valvar stenosis, it is important to consider the z-scores of the opening of the left valvar orifice while closing the cleft.
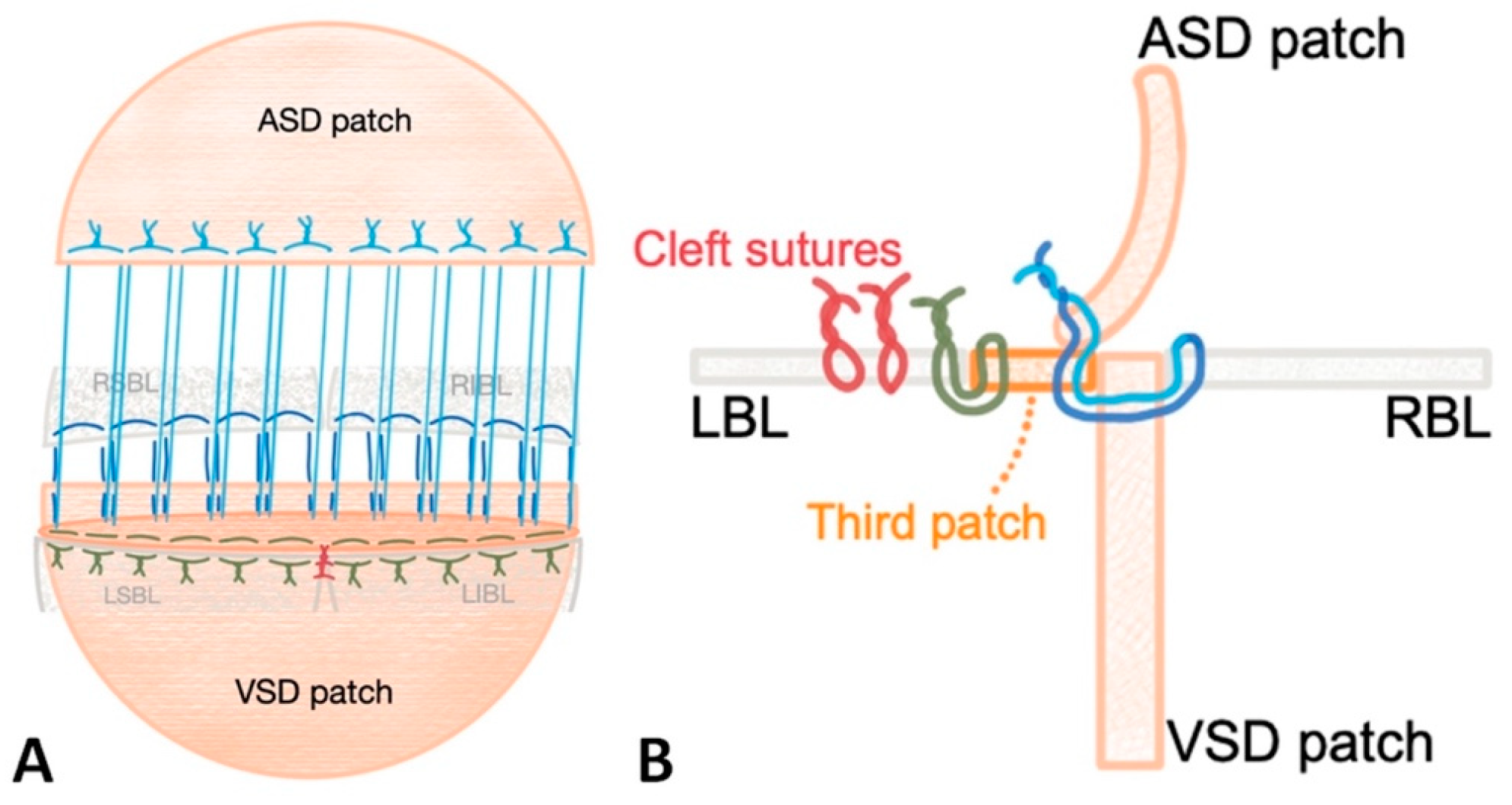
Figure 1: Schematic drawing of the triple-patch repair technique through front (A) and lateral (B) views. Yellow stitch corresponds to the connection of the left bridging leaflets with the left side of the third patch. Red sutures represent cleft sutures. Note that dark blue and light blue stitches are the same sutures. Abbreviations: LBL: left bridging leaflet, RBL: right bridging leaflet, LSBL and LIBL: left superior and inferior bridging leaflet. RSBL and RIBL: right superior and inferior bridging leaflet (drawn by EŞ).
A 6-month-old female with Down syndrome weighing 6.8 kg was the initial case (Fig. 2). Using a bovine pericardium, the patient had a triple patch repair of cAVSD (Rastelli type A) as a rescue procedure after a failed double-patch repair which was detected via saline test and epicardial echocardiography. After the re-institution of CPB and application of ACC and cardioplegic electrical quiescence, the right atriotomy was reopened and the saline test was performed following the take-down of the ASD patch. The saline test revealed a divergence of the left bridging leaflets from the mural leaflet. The ACC and CPB times were 132 min and 180 min, respectively. Following an uneventful intensive care unit stay of five days, the patient was discharged on postoperative day 14. The results were favorable in the immediate- and short-term, with trivial eccentric LAVVR and no right-sided atrioventricular valve regurgitation (AVVR) observed at the 16-month follow-up (Supplementary Video S1).
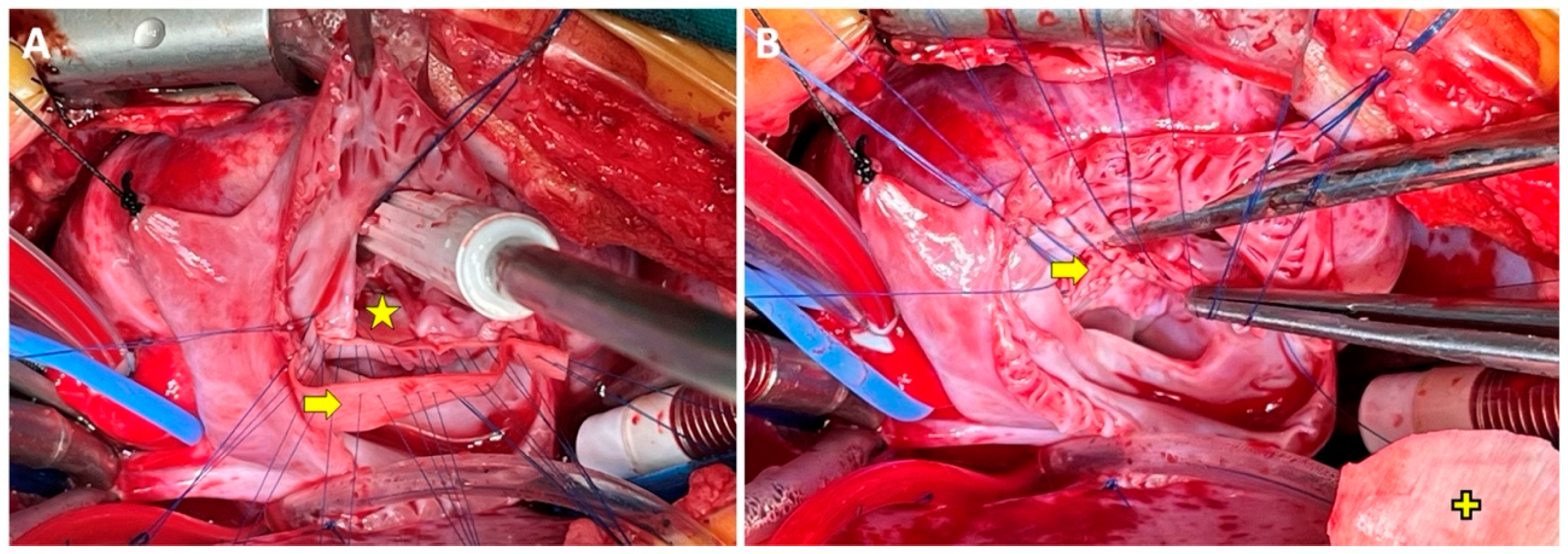
Figure 2: The first case’s intraoperative surgeon’s angle of view. The yellow star and arrow signs signify the VSD and the third patches, respectively. Note that the right side of the third patch is sutured from the right-sided bridging leaflet to the VSD patch (A) and then the third patch’s left side is sutured to left-sided bridging leaflets (B). Yellow plus sign indicates ASD patch (archive of EŞ).
A 5-month-old boy with Down syndrome, who weighed 5.1 kg, was the second case in whom, the decision was made to repair the cAVSD (Rastelli type A) using the triple patch technique. The primary reason for triple patch repair was the severe LAVVR identified by intra-operative saline test and epicardial echocardiography (Fig. 3) after a failed double-patch repair. Resembling to the first case, the left bridging leaflets were diverging from the mural leaflet. The duration of ACC and CPB were 83 min and 122 min, respectively. The postoperative course was uneventful with four days of intensive care unit stay and 12 days of hospital stay. The outcome was encouraging in that there was no LAVVR, and the right AVVR was mild on the 13-month follow-up (Supplementary Video S2).
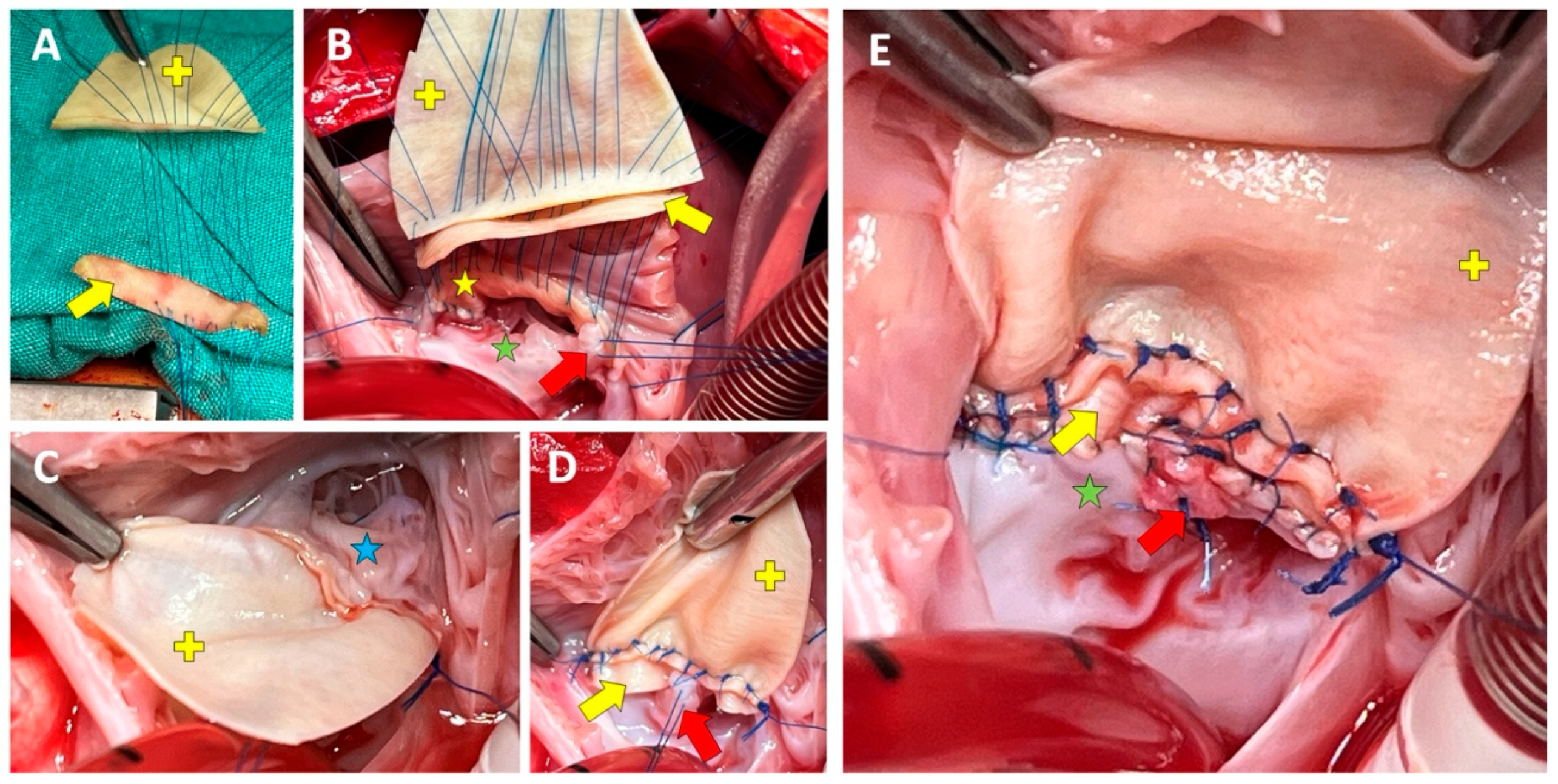
Figure 3: Second case’s intraoperative surgeon’s view. Sequence of right-sided sutures are presented in (A,B). View of the right (C) and left (B) sides after the third and ASD patches are advanced into place. Note that in (D), the left side of the third patch has not yet been sutured to the left-sided bridging leaflets. The final left-sided view of the repair (E). Signs: The yellow star, arrow, and plus signs denotes the VSD, the third, and the ASD patches, respectively. The blue and green stars indicate right- and left-sided bridging leaflets, respectively. The red arrow indicates the first cleft suture (archive of EŞ).
In the postoperative period, after homeostasis is achieved, both patients received anticoagulation with low molecular weight heparin by prophylactic dose at 1 mg/kg, which was continued throughout the intensive care unit stay. Along with anticoagulation, anti-platelet treatment with acetylsalicylic acid was initiated dosing between 3–5 mg/kg, which was continued for three months after surgery.
In cAVSD repair, the double-patch repair technique is effective in satisfying immediate- and long-term reoperation rates. However, the LAVVR remains the issue yet to be solved today based on the fact that while the rate of post-repair LAVVR was more than and equal to moderate at 45% before discharge, freedom from reoperation at five and 10 years was reported to be 91.8% and 86.9%, respectively [1].
Irrespective of the initial technique applied for cAVSD repair, we hypothesize that there is a loss of distance roughly around 3 mm or more in the bridging leaflets of the left atrioventricular valve (LAVV) as regards the midline corresponding to the VSD rim, which results in restriction of the proximity of the bridging leaflets’ lateral section from their corresponding left-sided lateral (mural) leaflet. Accordingly, we intraoperatively observed via saline test that the mechanisms behind this distancing are two. In the first place, it is the distance lost by the suture bites and secondly, the right-sided bulging of the VSD patch accompanied by the increase in left ventricular pressure after weaning from CPB (Fig. 4A). In our opinion, the coaptation zone between bridging and lateral leaflets appears to be the crux of a successful cAVSD repair in order to obtain a more favorable outcome. Therefore, the present approach was based on addressing the divergence of the bridging leaflets from their corresponding lateral leaflets, which leads to a lack of proper alignment (Fig. 4B) and was the main reason for LAVVR in our cases.
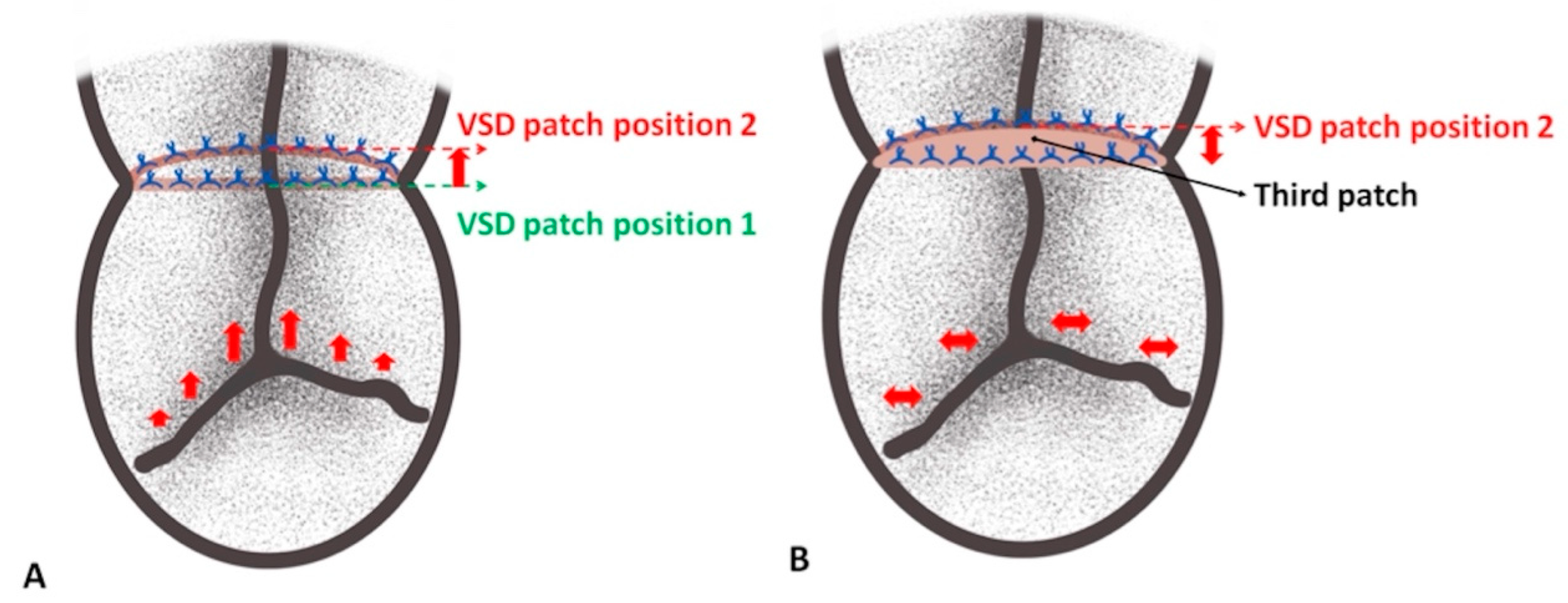
Figure 4: Schematic depiction of the distance loss of the left-sided bridging leaflets (A) and addressing of this issue by advancement of the third patch (B). Note that bulging of the VSD patch leads to pulling of and deficient approximation of the left-sided bridging leaflets with their corresponding lateral leaflet (A). The position of the VSD patch during surgery (position 1) and after weaning from cardiopulmonary bypass (position 2) is underscored (drawn by EŞ).
In our technique, two of the three principles of valve reconstructions were addressed. Firstly, the full motion of the bridging leaflets was preserved and restored thanks to the augmented left-sided leaflet. Secondly, the length of the coaptation zone was maintained by approximating the bridging leaflets to the lateral leaflets, which, in our opinion, is essential for addressing postoperative AVVR.
It is highly likely that the above-mentioned pathophysiological mechanisms are the underlying reason for what we have observed in the literature regarding the novel techniques applied to date in order to tackle with post-repair LAVVR [2,3,4,5]. For instance, Sughimoto et al. [4] suggested patch augmentation of the cleft as a reliable method in situations where ‘the cause of the regurgitation is the lack of coaptation between the reconstructed bridging leaflet and the tip of the left lateral leaflet’. Sun et al. [5] applied a V-shaped double-layer patch technique. In their concept, while one of the opposing arms of the patch is sutured to the LAVV, the other one is firstly connected to the right-sided atrioventricular valve and then, used as an ASD patch. In the Poirier et al.’s technique, the LAVV is also patch-augmented but in a crescent-shaped style. Resembling our technique, it can be easily seen that the common goal of all four novel techniques is to gain distance laterally to the LBL.
Moreover, in their technique, Najm et al. [2] augmented LAVV with the VSD patch of which, an extra 3–4 mm length of patch was fashioned beyond the annular plane and sutured to the divided SBL and IBL of the LAVV. The Najm’s technique would be quite simpler and time -saving but the most apparent difference to Najm’s technique is that the technique applied herein requires take-down of the cleft sutures and ASD patch. Replenishment of the VSD patch would have been harmful particularly for the suture line of the VSD rim. Therefore, a strip of patch was used for augmentation of the left bridging leaflets while the VSD was left in place.
Although there is no data about the immediate postoperative LAVV status in the article of Sughimoto et al., the freedom from a second reoperation at 10 years was 59.4% in the patients with normal papillary anatomy [4]. Najm et al. reported 5.7% moderate-to-severe LAVVR after their novel technique, and 3.8% of their patients required a second redo surgery for persistent LAVVR [2]. In the cohort of Sun et al. [5], while 6.5% of the patients had mil-to-moderate LAVVR at discharge, the reoperation rate for a 5.2-year period of follow-up was 3.4%. Reoperation for LAVVR in the Poirier’s cohort was 25% within 2.3 years [3]. In our series, the first case was symptom-free and revealed an accelerated weight gain during a follow-up period of 16 months. Additionally, the 16th-month echocardiographic evaluation revealed good ventricular function with trivial eccentric LAVVR and no right-sided AVVR. The second case’s follow-up has also been encouraging in terms of both symptomatology and weight gain. Likewise, the 13th-month echocardiography showed trivial LAVVR and mild right-sided AVVR. While it is impossible to compare our short-term outcomes with the other novel techniques’, our short-term results seem to be promising in terms of LAVVR.
Based on the superior bridging leaflet’s morphology, degree of bridging, and chordal attachments, Rastelli classified cAVSD as type A, B, and C [7]. In type A, the SBL is effectively split into two at the interventricular septal plane, and the chordal attachments are conveniently attached to the sides of the septal crest. Type B involves unequal splitting of the SBL with anomalous papillary muscle attachment. Finally, type C comprises marked bridging of the SBL, which is free-floating without chordal attachments. Both of our patients had Rastelli type A cAVSD in which, the SBL did not require division but the IBL. Accordingly, it can be argued that the current technique can readily be performed in the cases with type C disease necessitating division of the SBL. However, it is quite arguable to implement this technique on the cases with type B disease, which requires translocation of the anomalous papillary muscle of the left SBL attaching to the right side of the septal crest.
We think that the closure of the cleft on the left side is another crucial step in the procedure, although it entails the risk of stenosis. Hence, it is imperative to take into account and verify the z-scores throughout the surgical procedure. Another dispute would be the potential immobility of leaflets and subsequent valve dysfunction caused by unavoidable calcification of heterologous pericardial patches in the long-term. Said that, disorganization of collagen fibers, significant calculus formation and ossification in a heterologous pericardial patch was shown to be faster than that of an autologous pericardial patch. Therefore, an autologous pericardium would have been a superior alternative due to its enhanced resistance to calcification [8], as well as its ease of use and technical reliability in terms of tissue conformity, particularly considering the delicate nature of the tissue in the atrioventricular valve. On the other hand, the main reason for using a bovine pericardial patch as the third patch in the current cases was to reduce the time loss caused by treating the autologous pericardium with glutaraldehyde because, at that point in time, the CPB and ACC times had already been prolonged.
In conclusion, a new research question we derived from these cases is the validation of our hypothesis regarding the distance loss observed in the LAVV due to the rightward bulging of the VSD patch using advanced imaging techniques. Additionally, the current technique would be complicated and require longer CPB and aortic cross-clamp times, which are undesirable. Although the evidence is scarce to support the current technique’s superiority over the previously established methods, the short-term results for LAVVR were promising, requiring further comparative study with a larger number of patients and longer follow-up periods.
Acknowledgement:
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Emrah Şişli, Pelin Köşger; data collection: Emrah Şişli, Pelin Köşger; analysis and interpretation of results: Emrah Şişli, Pelin Köşger; draft manuscript preparation: Emrah Şişli, Pelin Köşger; preparation of visual materials: Emrah Şişli, Pelin Köşger. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data supporting the findings of the current study is available from the corresponding author, Emrah Şişli.
Ethics Approval: This study was reviewed by the Ethics Committee of Eskişehir Osmangazi University, and was waived from full ethical review (2025-261). A written consent was obtained from the parents for publication of the visual materials (intra-operative photographs and echocardiographic video recordings). Additionally, a parental written informed consent was obtained prior to the initiation of the study.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://www.techscience.com/doi/10.32604/chd.2025.067271/s1.
Abbreviations
| Aortic cross clamp | |
| Atrial septal defect | |
| Complete atrioventricular septal defect | |
| Cardiopulmonary bypass | |
| Inferior bridging leaflet | |
| Superior bridging leaflet | |
| Left atrioventricular valve regurgitation | |
| Ventricular septal defect |
References
1. Schumacher K, Marin Cuartas M, Meier S, Aydin MI, Borger MA, Dahnert I, et al. Long-term results following atrioventricular septal defect repair. J Cardiothorac Surg. 2023;18(1):250. doi:10.1186/s13019-023-02355-6. [Google Scholar] [CrossRef]
2. Najm HK. Routine leaflet augmentation of left atrioventricular valve in the repair of atrioventricular septal defect. J Saudi Heart Assoc. 2009;21(4):209–13. doi:10.1016/j.jsha.2009.10.002. [Google Scholar] [CrossRef]
3. Poirier NC, Williams WG, Van Arsdell GS, Coles JG, Smallhorn JF, Omran A, et al. A novel repair for patients with atrioventricular septal defect requiring reoperation for left atrioventricular valve regurgitation. Eur J Cardiothorac Surg. 2000;18(1):54–61. doi:10.1016/S1010-7940(00)00402-4. [Google Scholar] [CrossRef]
4. Sughimoto K, d’Udekem Y, Konstantinov IE, Brizard CP. Mid-term outcome with pericardial patch augmentation for redo left atrioventricular valve repair in atrioventricular septal defectdagger. Eur J Cardiothorac Surg. 2016;49(1):157–66. doi:10.1093/ejcts/ezv013. [Google Scholar] [CrossRef]
5. Sun S, Sun Y, Huang J, Zou P, Rao J, Xu W, et al. The V-shaped double-layer patch technique for complete atrioventricular septal defect: a novel surgical technique. J Thorac Cardiovasc Surg. 2023;165(3):1237–43. doi:10.1016/j.jtcvs.2022.04.028. [Google Scholar] [CrossRef]
6. Cantinotti M, Giordano R, Koestenberger M, Voges I, Santoro G, Franchi E, et al. Echocardiographic examination of mitral valve abnormalities in the paediatric population: current practices. Cardiol Young. 2020;30(1):1–11. doi:10.1017/S1047951119003196. [Google Scholar] [CrossRef]
7. Rastelli G, Kirklin JW, Titus JL. Anatomic observations on complete form of persistent common atrioventricular canal with special reference to atrioventricular valves. Mayo Clin Proc. 1966;41(5):296–308. [Google Scholar]
8. Jiang WJ, Cui YC, Li JH, Zhang XH, Ding HH, Lai YQ, et al. Is autologous or heterologous pericardium better for valvuloplasty? A comparative study of calcification propensity. Tex Heart Inst J. 2015;42(3):202–8. doi:10.14503/THIJ-14-4296. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools