 Open Access
Open Access
ARTICLE
Efficacy of Descending Aortic Retrograde Flow Area to Forward Flow Area Ratio on Echocardiography in Perioperative Management after Blalock-Thomas-Taussig Shunt
1 Division of Pediatric Cardiology, Saitama Children’s Medical Center, Saitama, 330-8777, Japan
2 Department of Pediatrics, The Jikei University School of Medicine, Tokyo, 105-8471, Japan
* Corresponding Author: Kentaro Kogawa. Email:
(This article belongs to the Special Issue: Novel Methods and Techniques for the Management of Congenital Heart Disease)
Congenital Heart Disease 2025, 20(4), 451-461. https://doi.org/10.32604/chd.2025.068006
Received 19 May 2025; Accepted 29 August 2025; Issue published 18 September 2025
Abstract
Objective: To investigate the usefulness of the descending aortic retrograde flow area to forward flow area (dAO RF) ratio using echocardiography in managing pulmonary blood flow during the perioperative period following Blalock-Thomas-Taussig shunt (BTTS) surgery. Methods: This retrospective study analyzed patient characteristics, surgical procedures, and perioperative courses. The dAO RF ratio was measured upon admission to the pediatric intensive care unit (PICU) and 12, 24, 48, and 72 h post-surgery. Blood pressure, percutaneous oxygen saturation, arterial blood gas values, and lactate levels were measured at the same time as the dAO RF ratio. Results: Seventy-one cases were analyzed. Excessive and low pulmonary blood flow occurred in 17 and two patients, respectively. Four patients required resuscitation within 72 h post-surgery, due to excessive pulmonary blood flow. No deaths occurred within 72 h. The dAO RF ratio was significantly higher in patients with excessive pulmonary blood flow at PICU admission and at its maximum (p = 0.049 and p < 0.01, respectively). The maximum dAO RF ratio was significantly correlated with lactate levels when measured concurrently; however, there was clinical variability in the resuscitation cases. The dAO RF ratio cutoff of 0.61 was highly accurate (area under the receiver operating characteristic curve: 0.91 [95% confidence interval: 0.82–1.00]), sensitive (82.4%), and specific (94.4%) for detecting excessive pulmonary blood flow. Conclusions: Measuring the dAO RF ratio postoperatively can prevent deterioration in patients with BTTS by accurately detecting excessive pulmonary blood flow, offering a minimally invasive and accurate assessment of perioperative pathophysiology.Keywords
In congenital heart disease (CHD) with ductus arteriosus-dependent pulmonary blood flow, a surgical systemic-to-pulmonary artery shunt is the approach to establish stable pulmonary circulation. This procedure serves as a palliative treatment for CHD with reduced or absent pulmonary blood flow. Considerable advancements have been made in diagnosis, intensive care, and intraoperative management in recent decades; however, overall outcomes with modified Blalock-Thomas-Taussig shunts (BTTS) remain unsatisfactory. Recent studies report mortality rates of 5.9%–13.5% (14.6% in single ventricle circulation) [1,2,3,4,5,6,7] and approximately 10.0%–31.3% in developing countries [8,9,10]. The 2021 report from the Japanese Association for Thoracic Surgery indicated a hospital mortality rate of 2.2% for 405 systemic-to-pulmonary shunt surgeries as primary procedures [11]. Factors contributing to mortality include low body weight, shunt size, shunt location, single ventricle physiology, and increased pulmonary vascular resistance, which is often heightened in the perioperative phase [12,13]. Therefore, proper evaluation of whether pulmonary blood flow is excessive or insufficient in the acute postoperative period is essential. This is typically assessed using indicators such as percutaneous oxygen saturation (SpO2), lactate levels, and urine output. Reports indicate that in neonates with patent ductus arteriosus (PDA), retrograde flow in the descending aorta, detected by echocardiography, is useful for evaluating systemic perfusion [14].
The present study aimed to determine whether the ratio of the descending aortic retrograde flow area to forward flow area (dAO RF ratio) on echocardiography could assist in perioperative management strategies for patients undergoing BTTS surgery. Moreover, we aimed to identify a cutoff value for the dAO RF ratio that indicates excessive pulmonary blood flow.
The present study comprised cases in which modified BTTS was performed for CHD at our hospital between 01 January 2018, and 31 March 2024. Patients with aortic stenosis, greater than mild aortic regurgitation, preoperative extracorporeal membrane oxygenation management, and insufficient data were excluded from the study.
2.2 Postoperative Hemodynamic Assessment in Patients
Medical records were reviewed retrospectively to analyze patient characteristics, surgical procedures, and perioperative courses. The dAO RF ratio was calculated by echocardiography upon admission to the pediatric intensive care unit (PICU) (immediately after surgery) and 12, 24, 48, and 72 h postoperatively. Other parameters, including blood pressure (systolic/diastolic), SpO2, partial pressure of oxygen (pO2), partial pressure of carbon dioxide (pCO2), and lactate levels, were measured at the same time as the dAO RF ratio. The dAO RF ratio measured at PICU admission served as the baseline for assessing dynamic changes in the postoperative period.
Postoperatively, patients were provided with optimal sedation and analgesia using dexmedetomidine, midazolam, and fentanyl (in some cases, rocuronium) until hemodynamic stability was attained. For perioperative anticoagulation, heparin therapy was initiated the day after surgery, following an assessment of drainage and other bleeding conditions. Heparin was initiated at 10 U/kg/h after an initial bolus dose of 30–40 U/kg, with a maximum flow rate of 30 U/kg/h and a target-activated partial thromboplastin time of 50–70 s. The BTTS material was expanded polytetrafluoroethylene.
2.4 Echocardiographic Techniques Used
The dAO RF ratio was measured using pulse-wave Doppler in the subcostal window in the sagittal plane of the descending aorta at the level of the diaphragm, parallel to the descending aorta (within an angle of 20°), and averaged over three heartbeats in the transthoracic echocardiographic images (Fig. 1). Pediatric cardiologists performed all examinations. Echocardiographic data were reviewed by a pediatric cardiologist. Cardiac ultrasound was performed using a Philips Affiniti 70 (Tokyo, Japan) ultrasound machine equipped with a 6–12-MHz probe while the patient was sedated.
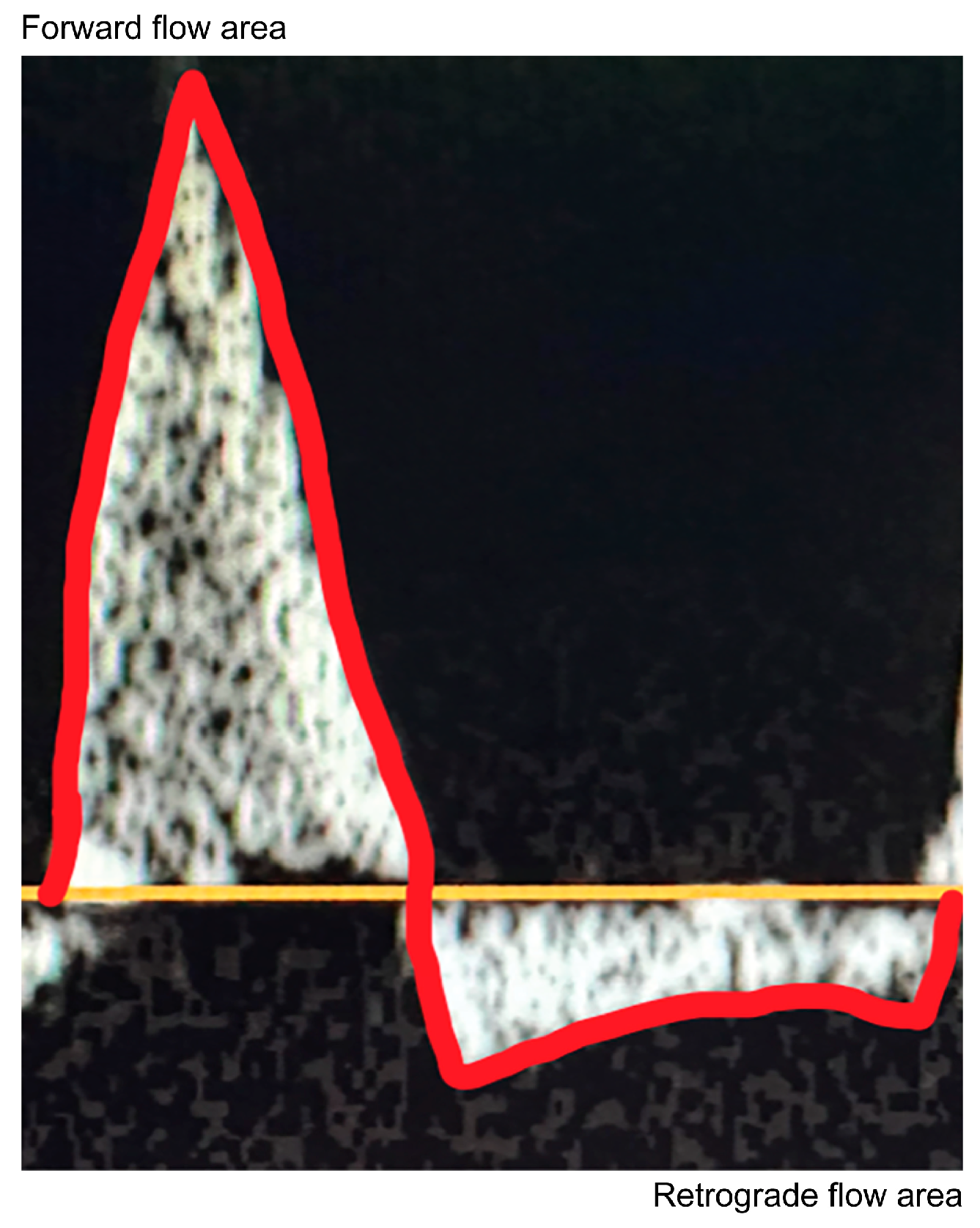
Figure 1: Assessment of diastolic retrograde flow in the descending aorta by Doppler echocardiography. Velocity-time integrals of systolic forward flow and diastolic retrograde flow were measured by tracing the curve above and below the baseline, averaging over three heartbeats.
2.5 Definition of Excessive and Low Pulmonary Blood flow and Resuscitation
Excessive pulmonary blood flow was defined as requiring surgical intervention, such as BTTS clip adjustment or extracorporeal membrane ventilation to control pulmonary blood flow, or complications such as cardiac arrest. Low pulmonary blood flow was defined as requiring surgical intervention, such as emergency chest opening, BTTS revision, extracorporeal membrane ventilation due to thrombosis in the BTTS, or BTTS or pulmonary artery stenosis. Resuscitation was defined as requiring open chest procedures, cardiopulmonary resuscitation, and vasopressor administration. Preoperative ductus arteriosus dependence was defined as requiring a PDA to maintain systemic or pulmonary circulation and the use of prostaglandin E1.
The primary outcome was assessed by calculating the dAO RF ratio and evaluating whether the patient had excessive pulmonary blood flow.
2.7 Ethical Compliance Statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (Ethical Guidelines for Epidemiological Research of the Ministry of Health, Labor, and Welfare of Japan) [15] and with the Helsinki Declaration of 1975, as revised in 2008. The study has been approved by the institutional committee of Saitama Children’s Medical Center (approval number: 2023-05-001; approval date: 11 January 2024). The requirement for written informed consent was waived owing to the retrospective nature of the study.
All statistical analyses were conducted using Easy R (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). Easy R is a modified version of R Commander with additional statistical functions commonly used in biostatistics [16]. The Kolmogorov-Smirnov test was used to assess the normality of distributions. Statistical significance was set at a two-tailed p < 0.05. The Mann-Whitney U test was used to compare categorical and continuous variables. Continuous and categorical variables are presented as medians (ranges) and numbers (percentages), respectively. Correlation analyses were performed using Spearman’s rank correlation coefficient.
We identified 90 relevant cases, of which 19 were excluded (two for extracorporeal membrane oxygenation management; 17 for incomplete data), and 71 were enrolled. Table 1 and Table 2 list the patient characteristics and shunt procedure details, respectively. Among the patients, 45 (63.3%) were male, with a median age of 2.2 months (range: 0.1–19.6 months) and a median weight of 4.2 kg (range: 2.3–9.1 kg). Furthermore, 23 (32.4%) patients had antegrade pulmonary blood flow. Preoperative ductus arteriosus dependence was present in >60% of patients, and single ventricle circulation in approximately 60% (Table 1). A cardiopulmonary bypass was used in 34 cases (47.8%), and the most common shunt size was 4.0 mm, used in 50 cases (70.4%). Shunt inflow was via the brachiocephalic artery in 48 cases (67.6%), and clipping during surgery was performed in nine cases (12.6%) (Table 2). During the perioperative period, 24 patients (33.8%) were admitted to the PICU with an open chest, 17 (23.9%) had excessive pulmonary blood flow, and two (2.8%) had low pulmonary blood flow. Four patients required resuscitation within 72 h post-surgery, all due to excessive pulmonary blood flow. There were no deaths within 72 h. Of the two deaths during hospitalization, one was due to excessive pulmonary blood flow (Table 3). The dAO RF ratio (interquartile range) was significantly higher in patients with excessive pulmonary blood flow (0.44 [0.38–0.58] vs. 0.40 [0.33–0.45], p = 0.049) at PICU admission, and at its maximum (0.68 [0.63–0.72] vs. 0.49 [0.42–0.56], p < 0.01) (Fig. 2).
Table 1: Patient characteristics.
| Sex, male/female, n | 45/26 |
| Age at surgery, months, median (range) | 2.2 (0.1–19.6) |
| Weight at surgery, kg, median (range) | 4.2 (2.3–9.1) |
| Diagnosis, n (%) | |
| Tetralogy of Fallot | 18 (25.4) |
| Single ventricle | 13 (18.3) |
| Double-outlet right ventricle | 10 (14.1) |
| Pulmonary atresia with intact ventricular septum | 7 (9.9) |
| Hypoplastic left heart syndrome | 6 (8.5) |
| Common atrioventricular septal defect | 6 (8.5) |
| Critical pulmonary stenosis | 5 (7.0) |
| Tricuspid atresia | 4 (5.6) |
| Transposition of the great arteries (type III) | 2 (2.8) |
| Genetic abnormality/asplenia/polysplenia, n (%) | 7 (9.9)/8 (11.3)/5 (7.0) |
| Presence of antegrade pulmonary blood flow, n (%) | 23 (32.4) |
| Duct-dependent pulmonary circulation before surgery, n (%) | 44 (62.0) |
| Single ventricle circulation, n (%) | 42 (59.2) |
| Previous systemic-to-pulmonary artery shunt, n (%) | 9 (12.7) |
Table 2: Details of the shunt procedure.
| Surgical approach, n (%) | |
| Median sternotomy/thoracotomy | 54 (76.1)/17 (23.9) |
| Cardiopulmonary bypass, n (%) | 34 (47.9) |
| Shunt size, n (%) | |
| 3.5 mm/4.0 mm/5.0 mm | 12 (16.9)/50 (70.4)/9 (12.7) |
| Shunt inflow, n (%) | |
| Brachiocephalic/subclavian/others | 48 (67.6)/21 (29.6)/2 (2.8) |
| Shunt outflow, n (%) | |
| Central pulmonary/right pulmonary/left pulmonary | 8 (11.3)/48 (67.6)/15 (21.1) |
| Control of blood flow with the clip during systemic-to-pulmonary artery SP shunt, n (%) | 9 (12.7) |
| Concomitant procedure, n (%) | |
| Branch pulmonary artery plasty | 19 (26.8) |
| Norwood | 5 (7.0) |
| Main pulmonary artery banding | 4 (5.6) |
| Damus–Kaye–Stansel anastomosis | 3 (4.2) |
| Total anomalous pulmonary venous connection repair | 1 (1.4) |
| Coarctation of the aorta repair | 1 (1.4) |
| Common atrioventricular valve plasty | 1 (1.4) |
| Glenn take-down | 1 (1.4) |
Table 3: Perioperative details.
| Open-chest management at the point of PICU admission, n (%) | 24 (33.8) |
| Excessive pulmonary blood flow, n (%) | 17 (23.9) |
| Low pulmonary blood flow, n (%) | 2 (2.8) |
| Resuscitation within 72 h postoperatively, n (%) | 4 (5.6) |
| Death within 72 h postoperatively, n (%) | 0 (0.0) |
| Death during hospitalization, n (%) | 2 (2.8) |
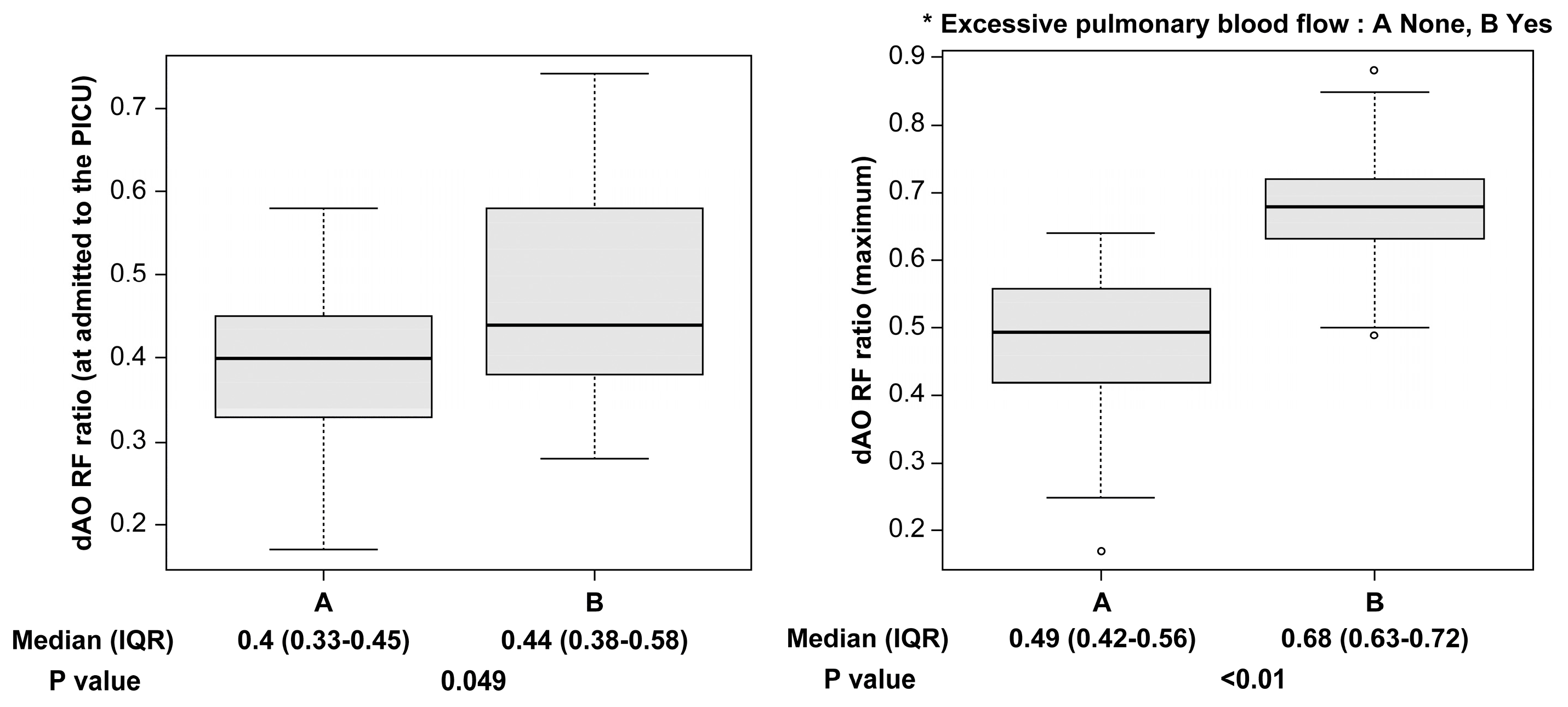
Figure 2: The dAO RF ratio is significantly higher in patients with excessive pulmonary blood flow when admitted to the PICU, and at its maximum. dAO RF = descending aortic retrograde flow area to forward flow area; IQR = interquartile range; PICU = pediatric ICU.
The maximum dAO RF ratio correlated with lactate levels (r = 0.34, p = 0.004), but not with SpO2 (r = 0.18, p = 0.14), the diastolic/systolic blood pressure ratio (r = −0.21, p = 0.08), pO2 (r = 0.14, p = 0.25), or pCO2 (r = 0.15, p = 0.21). All parameters were measured at the same time.
In cases of excessive pulmonary blood flow, a cutoff value of 0.61 for the dAO RF ratio was highly accurate, with a sensitivity of 82.4% and a specificity of 94.4% (area under the receiver operating characteristic curve: 0.91 [95% confidence interval: 0.82–1.0]) (Fig. 3). All four patients who required resuscitation within 72 h had excessive pulmonary blood flow, with dAO RF ratios of 0.78, 0.72, 0.85, and 0.88. In two of the four patients, concomitant lactate levels were not elevated (1.23 and 1.79 mmol/L). Table 4 presents their respective courses; none had undergone clip adjustment before deterioration.
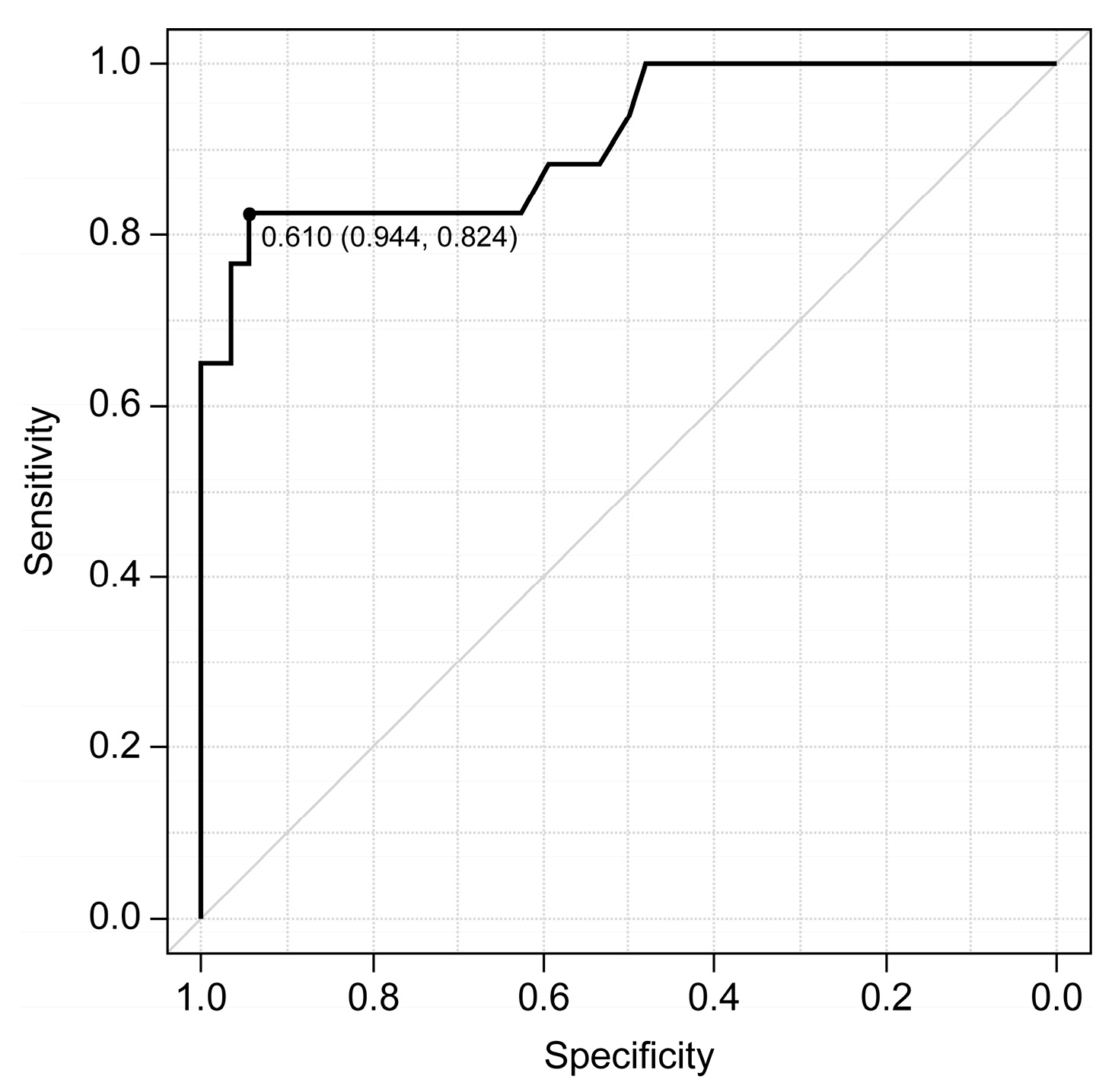
Figure 3: In excessive pulmonary blood flow, a cutoff value of 0.61 for the descending aortic retrograde flow area to forward flow area ratio is highly accurate (area under the receiver operating characteristic curve: 0.91 [95% confidence interval: 0.82–1.0]), sensitive (82.4%), and specific (94.4%).
Table 4: Patients’ demographic data.
| No. | Diagnosis | Age (Days) | Body Weight (g) | Shunt Route | Size (mm) | Clip | Antegrade Pulmonary Blood Flow | Cardiopulmonary Bypass | Concomitant Procedure | dAO RF Ratio | SpO2 (%) | Lactate (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tetralogy of Fallot | 244 | 8290 | BCA-LPA | 4 | None | Yes | None | None | 0.78 | 93 | 1.23 |
| 2 | Single right ventricle, mitral atresia, double-outlet right ventricle, pulmonary stenosis | 65 | 6120 | LSCA-LPA | 4 | None | None | Yes | Pulmonary artery plasty. Pulmonary artery division. Ductus arteriosus ligation | 0.72 | 84 | 1.79 |
| 3 | Single right ventricle, pulmonary atresia | 37 | 4482 | LSCA-LPA | 4 | None | None | Yes | Pulmonary artery plasty. Ductus arteriosus ligation | 0.85 | 88 | 4.84 |
| 4 | Double-outlet right ventricle, pulmonary stenosis, atrial septal defect | 15 | 2540 | BCA-RPA | 3.5 | None | Yes | None | Main pulmonary artery banding. Ductus arteriosus ligation | 0.88 | 92 | 10.04 |
Pulmonary blood flow can change suddenly to become excessive in the acute postoperative phase of BTTS, which can be fatal. Therefore, monitoring pulmonary blood flow over time and maintaining a balance between pulmonary and systemic blood flow is crucial. Postoperatively, pulmonary blood flow may gradually increase or fluctuate rapidly due to changes in pulmonary vascular resistance after reducing the volume load or cardiopulmonary bypass [17]. In this study, we highlighted the usefulness of the dAO RF ratio in assessing pulmonary blood flow during the acute postoperative phase of BTTS. Previous studies have shown the value of using echocardiography to assess retrograde blood flow in neonatal ductus arteriosus. Groves et al. [14] reported a strong correlation between retrograde blood flow and elevated left ventricular output. Furthermore, Carlo et al. [18] reported that persistent diastolic retrograde blood flow in the abdominal aorta was linked to a higher risk of necrotizing enterocolitis in infants with CHD. An elevated dAO RF ratio detected via echocardiography was significantly associated with postoperative complications linked to high-flow systemic-to-pulmonary shunts, suggesting it could be a valuable marker for assessing pulmonary blood flow through these shunts. In this study, a dAO RF ratio >0.6 was associated with excessive pulmonary blood flow.
The dAO RF ratio, calculated over time, can help identify gradual increases in pulmonary blood flow and hemodynamic changes that may lead to shock, prompting early surgical interventions, shunt clipping, adjustments in respiratory management, and the administration of various medications (e.g., cardioactive drugs and vasodilators) before the shock occurs. The maximum dAO RF ratio was not correlated with SpO2 and pO2. This may suggest that the dAO RF ratio can be useful in cases in which pulmonary blood flow cannot be assessed solely by oxygenation, such as when the lung itself is in poor condition or when there is mixing of arteriovenous blood in the atrium or ventricle.
We found a correlation between the dAO RF ratio and lactate levels. However, lactate levels can increase during the postoperative acute phase due to various factors, such as the effects of surgical invasion; therefore, especially in the hyperacute phase, it may be difficult to assess excessive pulmonary blood flow based solely on lactate levels. Additionally, not all four patients requiring resuscitation had elevated lactate levels (Table 4), suggesting that lactate levels increase as a result of circulatory failure and that in the early stages of excessive pulmonary blood flow, the dAO RF ratio might be a more sensitive marker than lactate levels. It may also be useful to determine the presence of excessive pulmonary blood flow by echocardiography if lactate levels and SpO2 tend to increase over time. Using this indicator may be particularly useful in single ventricle circulation, which is considered to have a high mortality rate [7].
Recent reports have shown an increasing trend in the use of PDA stenting and a declining trend in the use of BTTS [19]. A meta-analysis indicated that PDA stenting is associated with lower complication rates, shorter hospital stays, and improved survival compared to BTTS in neonates and infants with duct-dependent pulmonary circulation [20]. However, in urgent situations such as ductal closure or anoxic spells, or when anatomical factors make stenting unfeasible, BTTS remains the preferred option. In the present study, the dAO RF ratio was evaluated in patients undergoing BTTS. Given that the dAO RF ratio reflects the balance between retrograde and antegrade flow in the descending aorta, it may serve as a surrogate marker of pulmonary blood flow. While this parameter may be useful for postoperative monitoring following BTTS, it may also have broader applications. For instance, in preoperative ductus-dependent circulation, the dAO RF ratio may help to optimize prostaglandin E1 dosing to ensure adequate pulmonary perfusion. In addition, its measurement after PDA stenting may also aid in evaluating pulmonary blood flow. Future studies should assess its clinical utility across different palliative surgical strategies and stages.
Limitations of the present study include its single-center, retrospective design and several methodological constraints. Statistical limitations included the small sample size, especially for patients with excessive pulmonary blood flow (n = 17), which prevented the dAO RF ratio from being combined with other hemodynamic parameters without risking overfitting in the multivariable analysis, and precluded calculation of separate cutoff values for different patient subgroups. Methodological limitations included the lack of echocardiographic measurement reliability assessment (inter- and intra-observer variability) and potential selection bias from excluding patients with incomplete dAO RF ratio data, although none of the excluded patients experienced adverse events. We did not investigate the relationship between the dAO RF ratio and the pulmonary to systemic blood flow ratio as this is not practical in the acute postoperative phase, nor did we separate cases with and without antegrade pulmonary blood flow. The limited number of cases with low pulmonary blood flow (n = 2) prevented the establishment of management values for this condition. Finally, while the dAO RF ratio can be influenced by aortic regurgitation, none of the patients in the present study had significant aortic regurgitation affecting postoperative outcomes.
Based on our findings, we suggest measuring the dAO RF ratio at PICU admission, every 12 h for the first 24 h, and every 24 h thereafter until 72 h postoperatively. If an increasing trend is detected, serial monitoring may be beneficial to track progression. A cutoff of >0.61 may prompt surgical intervention. Future studies are needed to establish intermediate thresholds and validate these recommendations in larger cohorts.
In conclusion, if the degree and trend of pulmonary blood flow can be evaluated by measuring the dAO RF ratio in the acute postoperative phase of BTTS, deterioration related to pulmonary blood flow can be identified early and potentially avoided. It may also be useful for time-series comparisons in individual cases. It may also be an important indicator for minimally invasive and continuous monitoring of perioperative conditions.
Acknowledgement:
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization, Kentaro Kogawa; methodology, Kentaro Kogawa; validation, Kentaro Kogawa; formal analysis, Kentaro Kogawa, Kenji Hoshino and Daishi Hirano; investigation, Kentaro Kogawa; data curation, Kentaro Kogawa; writing—original draft preparation, Kentaro Kogawa; writing—review and editing, Kentaro Kogawa, Kenji Hoshino and Reiji Ito; visualization, Kentaro Kogawa; supervision, Reiji Ito and Daishi Hirano; project administration, Kentaro Kogawa. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the Corresponding Author, Kentaro Kogawa, upon reasonable request.
Ethics Approval: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (Ethical Guidelines for Epidemiological Research of the Ministry of Health, Labor, and Welfare of Japan) [15] and with the Helsinki Declaration of 1975, as revised in 2008. The study has been approved by the institutional committee of Saitama Children’s Medical Center (approval number: 2023-05-001; approval date: 11 January 2024). The requirement for written informed consent was waived owing to the retrospective nature of the study.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Glossary
| Blalock-Thomas-Taussig shunt | |
| congenital heart disease | |
| descending aortic retrograde flow area to forward flow area | |
| partial pressure of carbon dioxide | |
| patent ductus arteriosus | |
| pediatric intensive care unit | |
| partial pressure of oxygen | |
| percutaneous oxygen saturation |
References
1. Valencia E, Staffa SJ, Kuntz MT, Zaleski KL, Kaza AK, Maschietto N, et al. Transcatheter ductal stents versus surgical systemic-pulmonary artery shunts in neonates with congenital heart disease with ductal-dependent pulmonary blood flow: trends and associated outcomes from the pediatric health information system database. J Am Heart Assoc. 2023;12(17):e030528. doi:10.1161/JAHA.123.030528. [Google Scholar] [CrossRef]
2. Headrick AT, Qureshi AM, Ghanayem NS, Heinle J, Anders M. In-hospital morbidity and mortality after modified Blalock–Taussig–Thomas shunts. Ann Thorac Surg. 2022;114(1):168–75. doi:10.1016/j.athoracsur.2021.11.003. [Google Scholar] [CrossRef]
3. Bove T, Vandekerckhove K, Panzer J, De Groote K, De Wolf D, François K. Disease-specific outcome analysis of palliation with the modified Blalock-Taussig shunt. World J Pediatr Congenit Heart Surg. 2015;6(1):67–74. doi:10.1177/2150135114558690. [Google Scholar] [CrossRef]
4. Tarca A, Peacock G, McKinnon E, Andrews D, Saundankar J. A single-centre retrospective review of modified Blalock–Taussig shunts: a 22-year experience. Heart Lung Circ. 2023;32(3):405–13. doi:10.1016/j.hlc.2022.12.005. [Google Scholar] [CrossRef]
5. McMullan DM, Permut LC, Jones TK, Johnston TA, Rubio AE. Modified Blalock-Taussig shunt versus ductal stenting for palliation of cardiac lesions with inadequate pulmonary blood flow. J Thorac Cardiovasc Surg. 2014;147(1):397–401. doi:10.1016/j.jtcvs.2013.07.052. [Google Scholar] [CrossRef]
6. Glatz AC, Petit CJ, Goldstein BH, Kelleman MS, McCracken CE, McDonnell A, et al. Comparison between patent ductus arteriosus stent and modified Blalock-Taussig shunt as palliation for infants with ductal-dependent pulmonary blood flow: insights from the congenital catheterization research collaborative. Circulation. 2018;137(6):589–601. doi:10.1161/circulationaha.117.029987. [Google Scholar] [CrossRef]
7. Santro T, d’Udekem Y, Zannino D, Hobbes B, Konstantinov IE, Brizard C, et al. Determinants of acute events leading to mortality after shunt procedure in univentricular palliation. J Thorac Cardiovasc Surg. 2019;158(4):1144–53.e6. doi:10.1016/j.jtcvs.2019.03.126. [Google Scholar] [CrossRef]
8. Shaikh S, Al-Mukhaini KS, Al-Rawahi AH, Al-Dafie O. Outcomes of infants undergoing modified Blalock–Taussig shunt procedures in Oman: a retrospective study. Sultan Qaboos Univ Med J. 2021;21(3):457–64. doi:10.18295/squmj.8.2021.125. [Google Scholar] [CrossRef]
9. Amelia P, Advani N, Pulungan AB, Djer MM, Hegar B, Prawira Y, et al. Predicting factors for mortality in patients after the modified Blalock-Taussig shunt procedure in developing countries: a retrospective study. Int J Gen Med. 2023;16:5291–300. doi:10.2147/IJGM.S432855. [Google Scholar] [CrossRef]
10. Oofuvong M, Tanasansuttiporn J, Wasinwong W, Chittithavorn V, Duangpakdee P, Jarutach J, et al. Predictors of death after receiving a modified Blalock-Taussig shunt in cyanotic heart children: a competing risk analysis. PLoS One. 2021;16(1):e0245754. doi:10.1371/journal.pone.0245754. [Google Scholar] [CrossRef]
11. Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery, Yoshimura N, Sato Y, Takeuchi H, Abe T, et al. Thoracic and cardiovascular surgeries in Japan during 2021: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2024;72(4):254–91. doi:10.1007/s11748-023-01997-6. [Google Scholar] [CrossRef]
12. Hasegawa T, Oshima Y, Tanaka T, Maruo A, Matsuhisa H. Clinical assessment of diastolic retrograde flow in the descending aorta for high-flow systemic-to-pulmonary artery shunting. J Thorac Cardiovasc Surg. 2016;151(6):1540–6. doi:10.1016/j.jtcvs.2016.02.028. [Google Scholar] [CrossRef]
13. Leopold C, von Ohain J, Wolf C, Beran E, Lange R, Cleuziou J, et al. Reasons for failure of systemic-to-pulmonary artery shunts in neonates. Thorac Cardiovasc Surg. 2019;67(1):2–7. doi:10.1055/s-0037-1621706. [Google Scholar] [CrossRef]
14. Groves AM, Kuschel CA, Knight DB, Skinner JR. Does retrograde diastolic flow in the descending aorta signify impaired systemic perfusion in preterm infants? Pediatr Res. 2008;63(1):89–94. doi:10.1203/PDR.0b013e31815b4830. [Google Scholar] [CrossRef]
15. Eba J, Nakamura K. Overview of the ethical guidelines for medical and biological research involving human subjects in Japan. Jpn J Clin Oncol. 2022;52(6):539–44. doi:10.1093/jjco/hyac034. [Google Scholar] [CrossRef]
16. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8. doi:10.1038/bmt.2012.244. [Google Scholar] [CrossRef]
17. Saini A, Delius RE, Seshadri S, Walters H 3rd, Mastropietro CW. Passive peritoneal drainage improves fluid balance after surgery for congenital heart disease. Eur J Cardiothorac Surg. 2012;41(2):256–60. doi:10.1016/j.ejcts.2011.05.037. [Google Scholar] [CrossRef]
18. Carlo WF, Kimball TR, Michelfelder EC, Border WL. Persistent diastolic flow reversal in abdominal aortic Doppler-flow profiles is associated with an increased risk of necrotizing enterocolitis in term infants with congenital heart disease. Pediatrics. 2007;119(2):330–5. doi:10.1542/peds.2006-2640. [Google Scholar] [CrossRef]
19. Lemley BA, Wu L, Roberts AL, Shinohara RT, Quarshie WO, Qureshi AM, et al. Trends in ductus arteriosus stent versus Blalock-Taussig-Thomas shunt use and comparison of cost, length of stay, and short-term outcomes in neonates with ductal-dependent pulmonary blood flow: an observational study using the pediatric health information systems database. J Am Heart Assoc. 2023;12(23):e030575. doi:10.1161/JAHA.123.030575. [Google Scholar] [CrossRef]
20. Alsagheir A, Koziarz A, Makhdoum A, Contreras J, Alraddadi H, Abdalla T, et al. Duct stenting versus modified Blalock-Taussig shunt in neonates and infants with duct-dependent pulmonary blood flow: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2021;161(2):379–90.e8. doi:10.1016/j.jtcvs.2020.06.008. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools