 Open Access
Open Access
ARTICLE
Leveraging Blockchain with Optimal Deep Learning-Based Drug Supply Chain Management for Pharmaceutical Industries
Department of Networking and Communications, School of Computing, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu, 603 203, India
* Corresponding Author: Venkatesh Kaliyamurthy. Email:
Computers, Materials & Continua 2023, 77(2), 2341-2357. https://doi.org/10.32604/cmc.2023.040269
Received 11 March 2023; Accepted 15 June 2023; Issue published 29 November 2023
Abstract
Due to its complexity and involvement of numerous stakeholders, the pharmaceutical supply chain presents many challenges that companies must overcome to deliver necessary medications to patients efficiently. The pharmaceutical supply chain poses different challenging issues, encompasses supply chain visibility, cold-chain shipping, drug counterfeiting, and rising prescription drug prices, which can considerably surge out-of-pocket patient costs. Blockchain (BC) offers the technical base for such a scheme, as it could track legitimate drugs and avoid fake circulation. The designers presented the procedure of BC with fabric for creating a secured drug supply-chain management (DSCM) method. With this motivation, the study presents a new blockchain with optimal deep learning-enabled DSCM and recommendation scheme (BCODL-DSCMRS) for Pharmaceutical Industries. Firstly, Hyperledger fabric is used for DSC management, enabling effective tracking processes in the smart pharmaceutical industry. In addition, a hybrid deep belief network (HDBN) model is used to suggest the best or top-rated medicines to healthcare providers and consumers. The spotted hyena optimizer (SHO) algorithm is used to optimize the performance of the HDBN model. The design of the HSO algorithm for tuning the HDBN model demonstrates the novelty of the work. The presented model is tested on the UCI repository’s open-access drug reviews database.Keywords
Over several years, pharmaceutical industries have faced the problem of tracking products through the supply chain. These drawbacks have become easier for fraudsters to launch fake drugs into the market [1,2]. A new technique for tracking and tracing drugs is obligatory to overcome these problems. Research workers believe Blockchain (BC) could give the technical basis for those systems because it tracks legal drugs and prevents the circulation of fake ones [3]. Counterfeit drugs are determined by the World Health Organization (WHO) as those that “are manufactured fraudulently, mislabelled of low quality, hiding the source details or identity, and the defined standard”. Despite being consumed, they might cause severe health problems. Sometimes the maker of fake drugs uses legitimate companies’ logos to get their products into the market [4,5]. Even though a global challenge, this practice extremely affects well-developed countries. Fake medications are distributed over an extremely complex network, making them challenging to remove and detect [6]. A system is needed to track and trace drug delivery at all stages to avoid their distribution. BC is the modern innovation that promises to accomplish these objectives [7,8]. Fig. 1 represents the pipeline of supply chain management (SCM) in the medical industry.
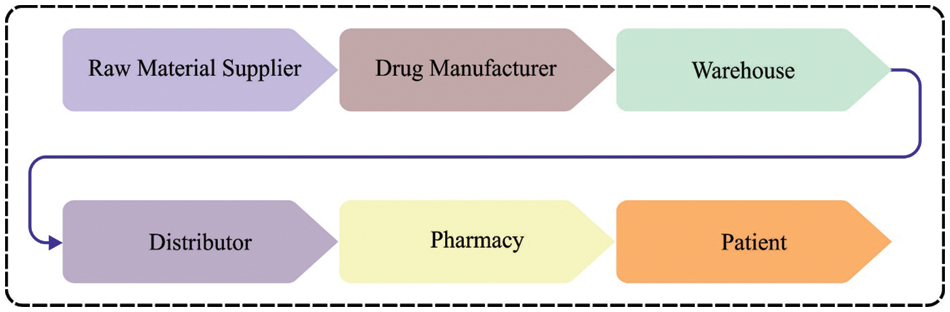
Figure 1: SCM in pharmaceutical industry
The medical industry is a critical sector that should be controlled for optimization to be a part of the global trade mechanism [9]. In that regard, it is essential to mobilize the resource to make sure that the predominant economic sector can be controlled for optimization [10,11]. Even though the drug distribution method has considerably improved over the last few years, it is still necessary to increase the availability of tablets, authentication, and market dynamics of real-time price management. The system allows loopholes to become structural inefficiencies and market failures. This inefficiency creates credibility issues, drug shortages, etc. [12]. This problem is the major cause of market failures and macro instability. As technology progresses, the present social structure of society does not support the technological pace. Consequently, a new measure needs to be taken to readapt the dynamics. The presented architecture is driven by the motivation to resolve those automation problems [13]. The healthcare field regularizes itself and receives benefits from blockchain technology (BCT). BC was created initially to serve as the transaction log for Bitcoin [14]. It gives a distributed ledger for storing data records arranged in various “blocks.” The collected data involves the participants, time, date, and price included in all the transactions [15]. Once employed in the medical supply chain, BC provides an electronic ledger where everyone in the network can see and validate data.
Existing deep learning (DL) enabled drug supply chain (DSC) management and recommendation systems have made significant progress in improving the efficiency and safety of DSCs. However, several limitations still need to be addressed to fully realize the potential of these systems. Some of the limitations are discussed here. The pharmaceutical industry is highly regulated, and each country or region may have different regulations for DSCs. The lack of standardization can make it challenging to develop DL models that can be applied globally. In addition, implementing DL-enabled DSC management and recommendation systems can be expensive and require significant computing resources. Scaling the system to handle large volumes of data can also be challenging. Most existing systems focus on optimizing specific aspects of the DSC, such as inventory management or logistics. However, end-to-end SCM requires a holistic approach that considers all aspects of the SCM, from manufacturing to delivery to the end customer.
This study develops a new BC with optimal DL-enabled drug supply chain management and recommendation scheme (BCODL-DSCMRS) for Pharmaceutical Industries. The proposed BCODL-DSCMRS technique mainly focuses on the incessant observation and tracing of the drug supply for addressing forging problems. The BCODL-DSCMRS technique comprises two significant modules: BC-enabled DSC management and a DL-based consumer recommendation system. Firstly, Hyperledger fabric is used for DSC management, enabling effective tracking processes in the smart medical industry. In addition, a hybrid deep belief network (HDBN) model is used to suggest the best or top-rated drugs to the medical industry customer. The spotted hyena optimizer (SHO) algorithm is exploited to optimize the performance of the HDBN model. The presented method is tested on the UCI repository’s open-access drug reviews database. The key contribution of the paper is summarized below:
• An intelligent BCODL-DSCMRS method encompassing BC-enabled DSC management, HDBN-based drug recommendation, and SHO-based parameter tuning is provided for pharmaceutical industries. To our knowledge, the BC-enabled DSC method has never been presented in the literature.
• A Hyperledger fabric approach is designed for addressing forging problems and DSC management in the pharmaceutical industry.
• Hyperparameter optimization of the HDBN approach using the SHO technique with cross-validation assists to increase the prediction outcome of the BCODL-DSCMRS approach for hidden data.
The rest of the paper is organized as follows. Section 2 gives the related works, Section 3 provides the presented method, Section 4 provides the result analysis, and Section 5 concludes the paper.
In [16], the authors presented and executed a new BC and ML-based DSC management and recommendation (DSCMR) method. The presented method comprises the BC-based DSCM and ML-based medicine recommendation model for users. During the primary element, the DSCM method used Hyperledger fabrics to constantly monitor and track the medicine delivery procedure from the smart medicine companies. However, the N-gram and Light Gradient Boosting Machine (LightGBM) techniques are utilized in the ML element for recommending the popular or optimum prescription to users of medicine companies. Musamih et al. [17] examined an Ethereum BC-based system leveraging smart contracts and decentralizing off-chain storing to effectively trace the product from the medical field. The smart contract assurance data source removes the necessity for mediators and offers every stakeholder a secured, immutable transaction history.
Ahmadi et al. [18] inspected the novel medical governance dependent upon the Internet of Things (IoTs) and BCT. IoT-based BC is a form of distributed ledger (DLT) which keeps immutable records of the transaction, which is inefficient of existence fabricated and is noticeable to every participant. Executing an IoT-based BC technique offers the tools for the medical sector to optimize medicine governance along the SCM, which makes healthcare further reliable and effectual. Huang et al. [19] introduced a scenario-related BC technique for medicine traceability and regulation named Drugledger that recreates the entire service infrastructure with separate service providers as three independent service elements and ensures the authenticity and confidentiality of information. Additionally, Drugledger is effectually pruning their storing, attaining a stable and suitable BC storing. Jamil et al. [20] examined a new DSCM utilizing Hyperledger Fabric dependent upon BCT for handling secure DSC records. The presented technique resolves these challenges by carrying out drug record transactions on BC to construct a smart healthcare ecosystem with DSC.
Agrawal et al. [21] proposed a BC-enabled network that permits manufacturers to monitor medicines efficiently but the SCM with enhanced safety and transparency over the procedure. This analysis also attempts to minimise the cost and time reliant on the manufacturing company to transfer the medicine to consumers by offering forward and backward SCM mathematical methods. In detail, the forward chain method maintains medicine delivery in the company to consumers in the shortest time with consistent transport mode. Uddin [22] presented a novel and new tracking and tracing BC-enabled Medledger method that leverages the Hyperledger Fabric BC environment utilizing chain codes (smart contracts). The presented Medledger method supports securely and efficiently applying DSC transactions from fabric, allowing a private permissioned, distributed network of distinct medicine stakeholders. The chain code can be planned, coded, and executed utilizing sequence diagrams for governing and controlling the interaction betwixt the contributing stakeholder from the DSC system.
In [23], the authors developed a secure blockchain-based Proposed Application (PA) to generate, maintain, and validate healthcare certificates. Peng et al. [24] devised VFChain, an auditable and verifiable federated learning structure that depends on the BC system. Firstly, to offer verifiability, a committee was chosen using the BC to aggregate methods and record verifiable proof in the BC. After, a new authenticated data structure was introduced for BC’s auditability to augment the search efficacy of verifiable proof and supported a secure rotation of committees. Peng et al. [25] devised a method based on BC, a privacy-preserving verifiable data-sharing system. With a novel authenticated data structure method, the author designed a new BC-related structure to verify proficiently any part of the data record shared in a decentralized way. Wu et al. [26] devised a Verifiable Query Layer (VQL) deployed on the cloud to render verifiable and efficient data query services for BC systems. The middleware layer extracted data from basic BC technology and proficiently reorganize them in databases. A cryptographic fingerprint can be computed depending on all constructed databases to avoid fake datasets from being saved in the middleware.
Although numerous ML and DL approaches for DSC management are available in this study, but still, it is essential to improve the overall performance. The amount of parameters of DL approaches also quickly increases, leading to overfitting the model because of continuous deepening. The metaheuristic algorithm can be employed since the trial and error model for hyperparameter tuning is a difficult process. Thus, the SHO algorithm is exploited for the parameter selection of DBN.
This article introduced a new BCODL-DSCMRS technique for automated DSC management and recommendation processes in the smart pharmaceutical industry. The presented BCODL-DSCMRS technique intends to the incessant observing and tracing of the drug supply for addressing forging problems. The BCODL-DSCMRS technique comprises two significant modules: BC-enabled DSC management and a DL-based consumer recommendation system. Fig. 2 represents the overall workflow of the BCODL-DSCMRS method.
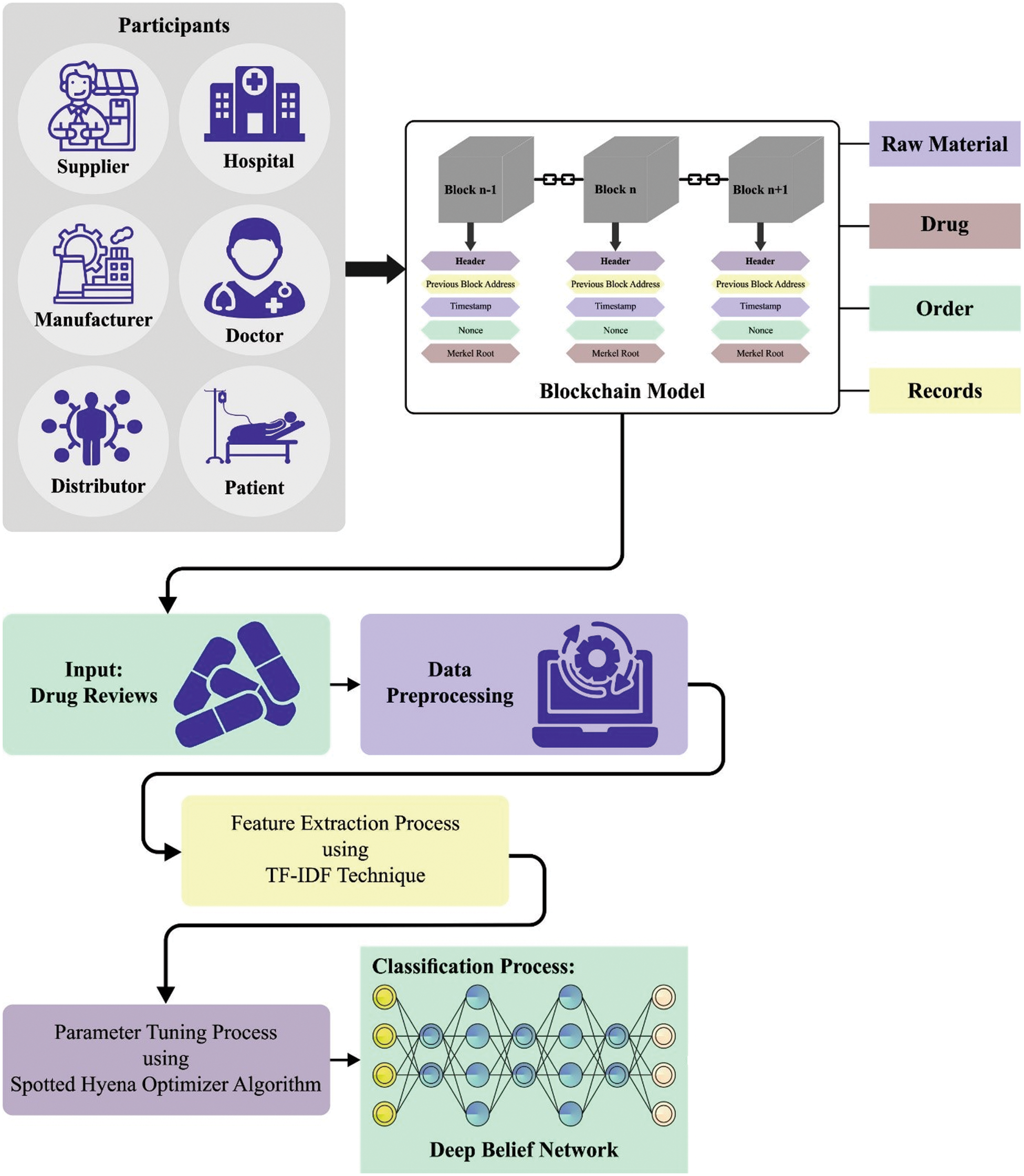
Figure 2: Overall workflow of BCODL-DSCMRS algorithm
3.1 BC-Based Distributed Ledger
This work uses the Hyperledger fabric for DSC management, enabling an effective tracking process in the smart medical industry. The Hyperledger Fabric is established on the source of DLT technologies containing two parts: BC and world state parts [27]. Hyperledger Fabric configures several world state datasets for maintaining the group of present values or states of procedures to provide the utility for accessing the present ledger states at some phase. The world state dataset part was capable of storing the states of the ledger effectively and recovering the applications or requirements of the users. Therefore, it stores the present state automatically, and the method developer verifies it, never seeing the complete transaction log. The data from the key-value pair can be kept inside the world state dataset. The database (DB) upgrades the state value automatically once the state has been altered or executed any transactions have.
Two suitable choices of Level DB and Couch DB are accessible. A primary choice means level DB has a default state DB that maintains data of smart contracts from the key-value pair and occurs in all the pair nodes of networks. The outcomes of queries from Couch DB provide real data content. It also assists each type of query for accessing data content with the REST Application Programming Interface (API). Because of these features, couch DB can be utilized in the presented method for storing the data connected to our network. But the second part is BC which is the capability of storing the group of functions and alters that take place in the world state DB from the procedure of the transaction log. Next, this transaction has been saved in the procedure of blocks and is related to chain-like infrastructure. The transaction is saved in order from the BC network. The BC offers a data immutability feature; nobody alters or deletes the data once saved.
3.2 Drug Recommendation Module
Before proceeding to the recommendation process, the drug review dataset comprises noisy data initially pre-processed to improve its quality. Next, the Term Frequency-Inverse Document Frequency (TF-IDF) model is utilized for the feature extraction. TF-IDF is a statistical measure used to determine the mathematical impact of words in a document [28]. The vectorization method is related to One Hot Encoding. On the other hand, the value equivalent to the word has been allocated a TF-IDF value rather than 1. The TF-IDF value has been attained by multiplying the TF and IDF values. In this work, the DBN model is applied to recommending medicines. Restricted Boltzmann Machine (RBM) comprises the hidden layer (HL) and visible layer (VL). The VL is accountable for input, and the HL also learn higher-level semantic feature from the input dataset.
In Eq. (1),
where
The probability function of
where
The RBM mechanism can be trained by iteration, and the variable
In Eq. (5),
In Eq. (6),
Next, the variables of RBM are attuned to the suitable value to prevent the local optima solution. RBM has a stronger feature learning capability and is utilized for extracting data. But the RBM efficiency for FE is constrained once used for complex non-linear data. Therefore, the DBN explores a deep hierarchical depiction of the training sample. Both nearby layers of DBN are regarded as a single RBM.
3.3 Hyperparameter Tuning Module
The SHO technique is a hyperparameter optimizer to improve the DBN model’s performance. A spotted hyena (SH), or a laughing hyena, is considered the biggest among every hyena species [29]. This hyena is a larger carnivore currently innate to Africa and originated in Asia. This species demonstrates higher societal attachment, complicated performance, competitive social patterns, and the most enormous clan size. While they lived major in number from the clan, it can be assumed that they were a great prosperous species because of their adaptability and cunning nature. It chases its chosen prey with a pack for long distances. The entire performance design of SHs is mathematically mapped to overcome various optimized complications. In the SHO implementation, prey location can be considered an instantaneous best for nearer to optimum as the searching area was unexplored earlier. After setting the best searching agent over the optimum solution, the rest of the searching agents change the location. An arithmetical illustration of performance was formulated by Eqs. (10) and (11).
whereas
whereas
Mathematically, the track and hunt patterns are outlined as follows. Victim location was detected to a superlative agent (hyena) that is preserved as optimal. Eqs. (12)–(14) are liable for transforming the hyena’s position.
whereas
whereas
In which

The presented method is simulated by Python 3.6.5 tool on PC i5-8600k, 16 GB RAM, GeForce 1050Ti 4 GB, 1 TB HDD, and 250 GB SSD. The parameter settings are as follows: dropout: 0.5, learning rate: 0.01, epoch count 50, activation: ReLU, and batch size: 5.
In this section, the experimental analysis of the BCODL-DSCMRS method on DSC management is investigated in detail. Table 1 and Fig. 3 report the BCODL-DSCMRS method’s overall results with other existing DSC management approaches. The experimental results specified that the DSC-Gcoin model had reported lower performance than other techniques. Along with that, a slightly improvised outcome is obtained by the DSCIM-SH technique. Moreover, the RDRSCM-Hyperledger technique has accomplished reasonable outcomes over other models. However, the BCODL-DSCMRS model surpassed other models and attained maximum performance under all users.
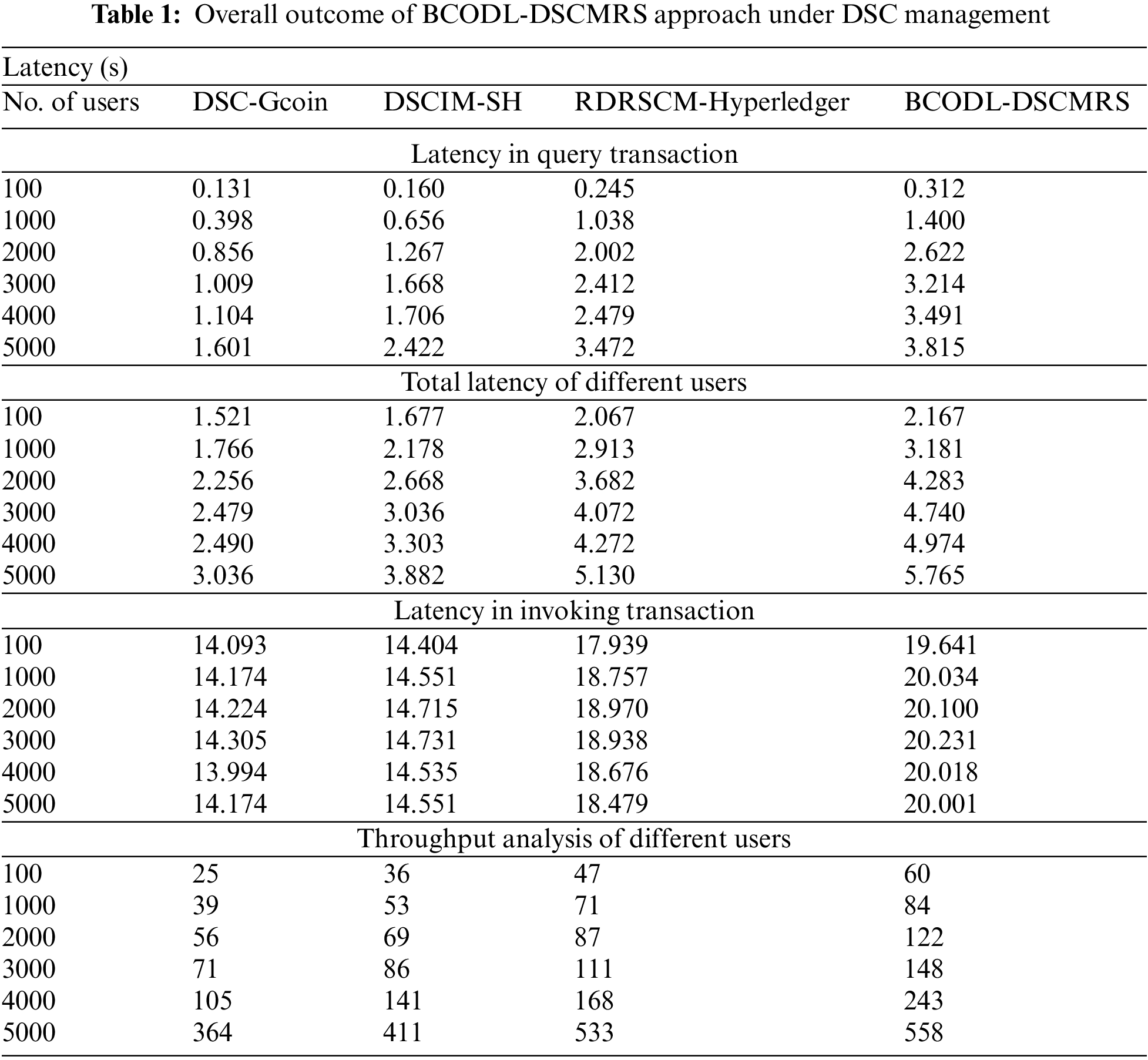
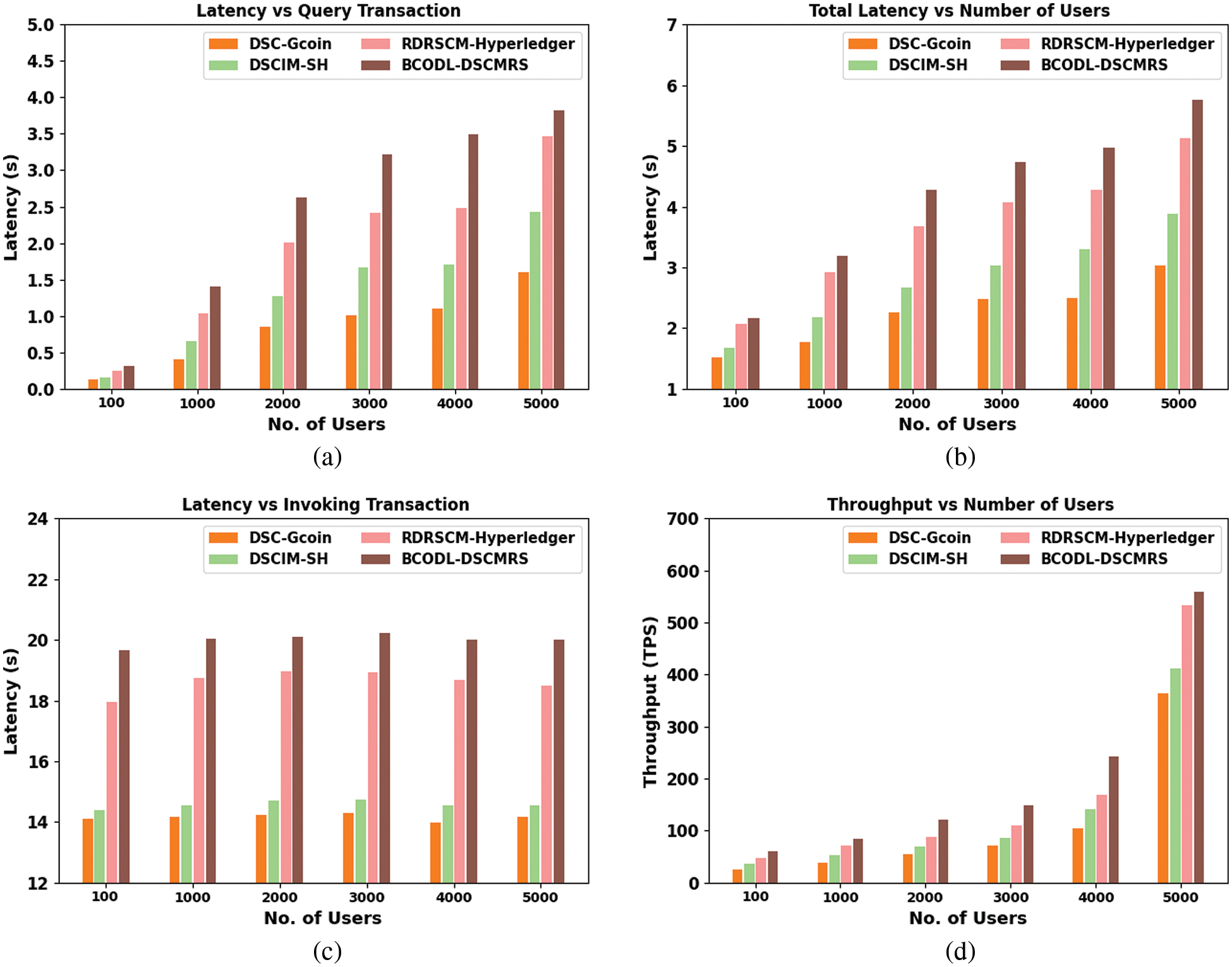
Figure 3: Latency analysis (a) latency in query transaction (b) total latency of different users (c) latency in invoking transaction (d) throughput analysis of different users
The drug recommendation results of the BCODL-DSCMRS model are examined on the drug review dataset from the UCI repository (https://www.kaggle.com/datasets/jessicali9530/kuc-hackathon-winter-2018). The dataset includes 20000 samples with two classes, as given in Table 2.
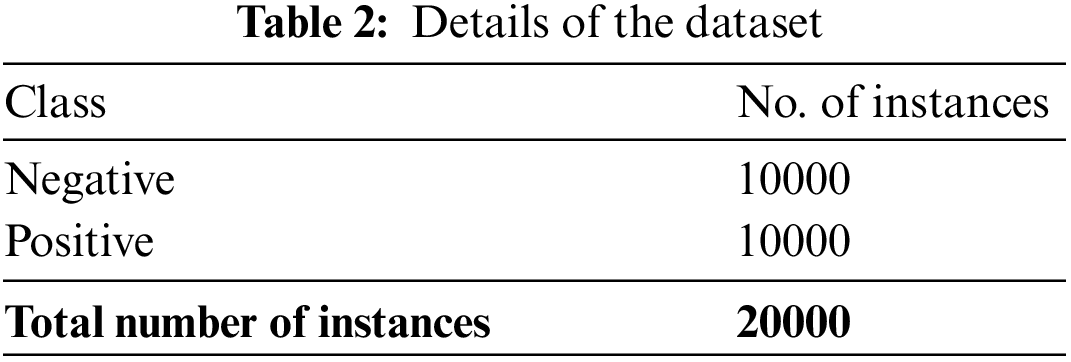
The confusion matrix of the BCODL-DSCMRS model under the drug recommendation process is depicted in Fig. 4. With 80% of TRD; the BCODL-DSCMRS model has recognized 7808 samples as negative and 7886 samples as positive. Eventually, with 20% of TSD, the BCODL-DSCMRS technique recognized 1950 samples as negative and 1993 as positive. Meanwhile, with 30% of TSD, the BCODL-DSCMRS technique has recognized 3030 samples as negative and 2885 as positive.
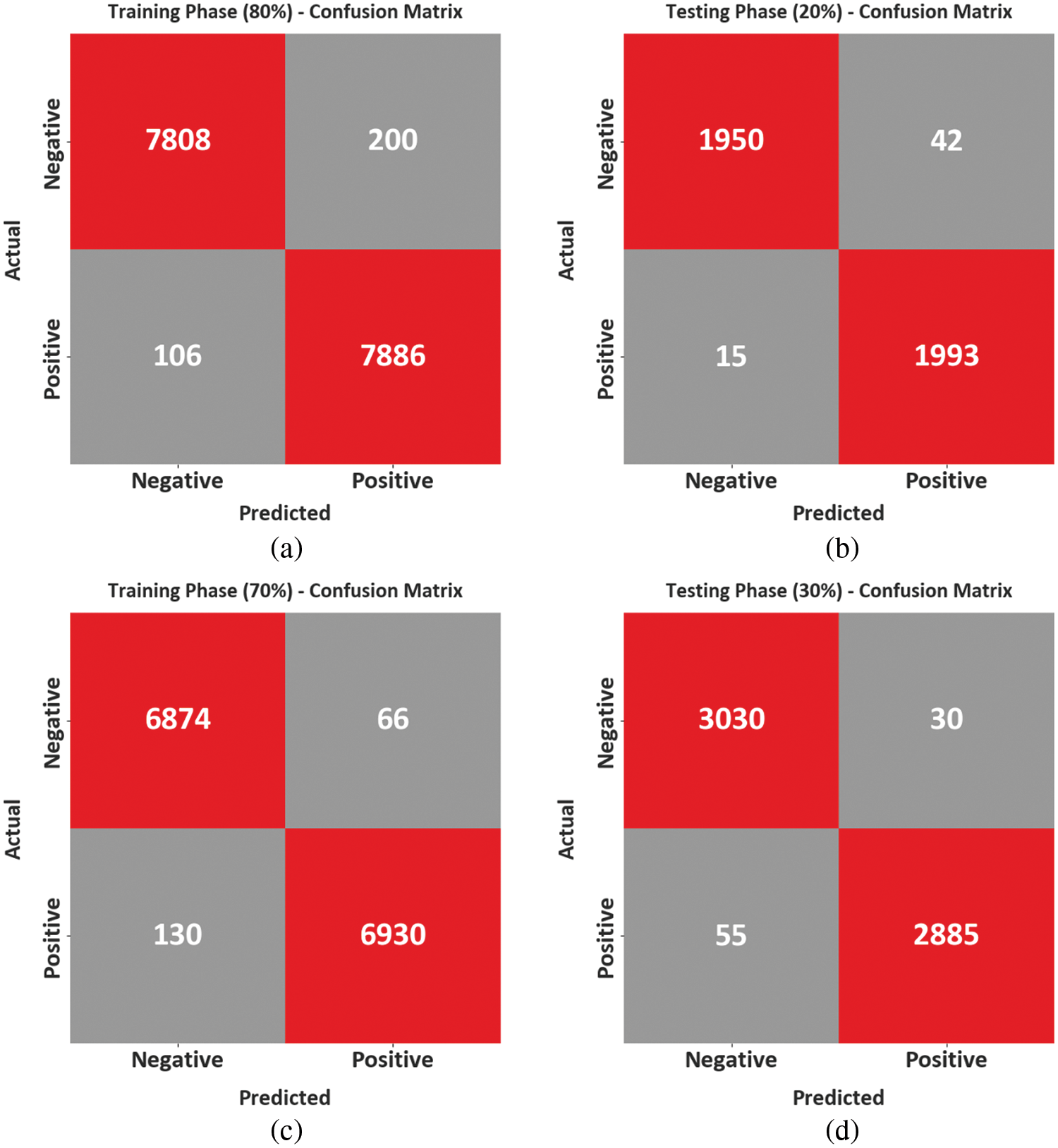
Figure 4: Confusion matrices of BCODL-DSCMRS system (a and b) TRD/TSD of 80:20 and (c and d) TRD/TSD of 70:30
In Table 3, the overall outcomes of the BCODL-DSCMRS method are investigated under different sizes of the training dataset (TRD) and testing dataset (TSD). The experimental values stated that the BCODL-DSCMRS model has properly recommended the drugs. With 80% of TRD, the BCODL-DSCMRS model reaches an average

Simultaneously, with 70% of TRD, the BCODL-DSCMRS method obtains an average
The training accuracy (TAY) and validation accuracy (VAY) of the BCODL-DSCMRS system are performed in Fig. 5. The figure implies that the BCODL-DSCMRS method had shown superior outcomes with the highest values of TAY and VAY. It is visible that the BCODL-DSCMRS technique has attained the maximum TAY outcomes.
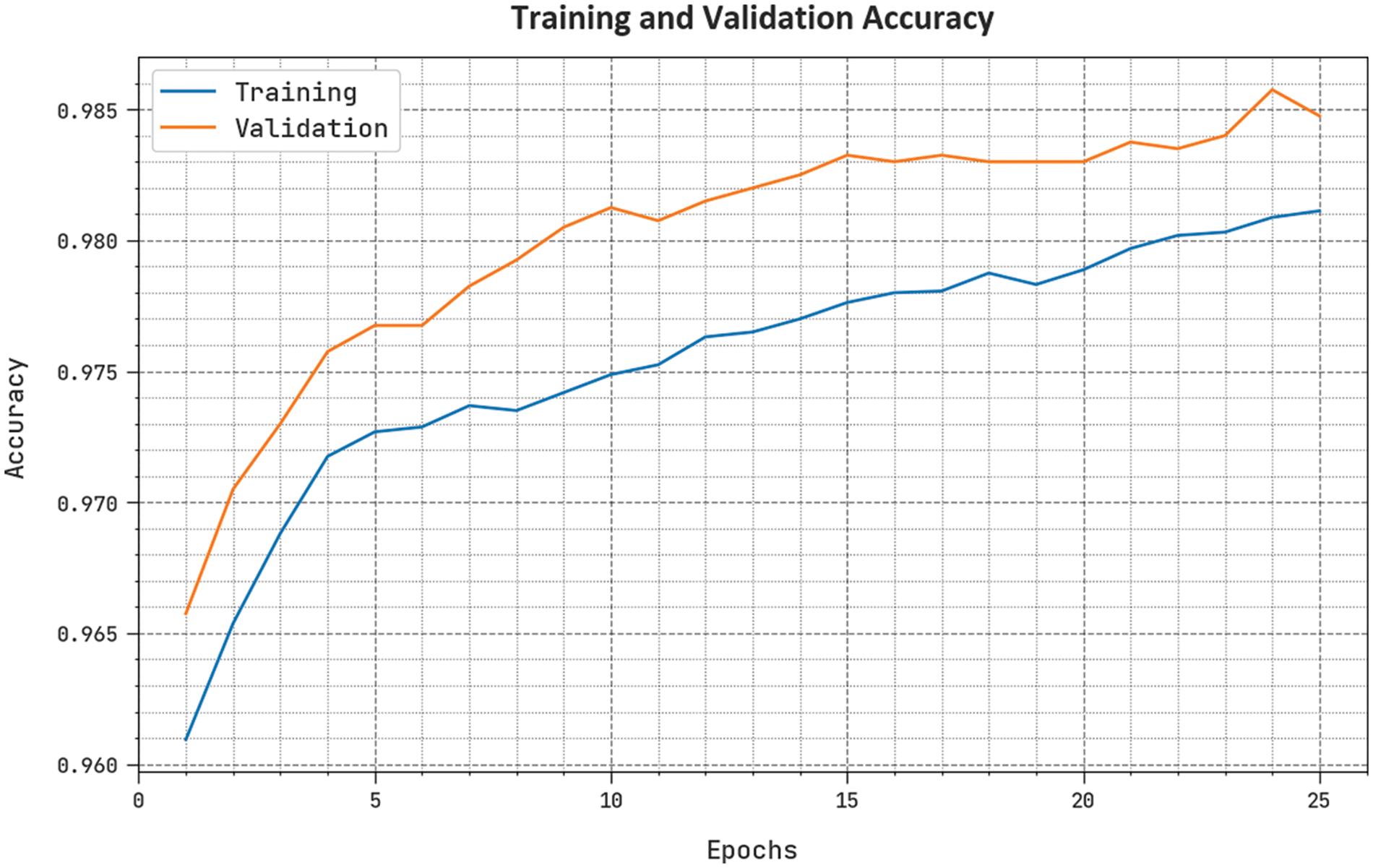
Figure 5: TAY and VAY outcome of BCODL-DSCMRS method
The training loss (TLSS) and validation loss (VLSS) of the BCODL-DSCMRS method are tested in Fig. 6. The figure shows that the BCODL-DSCMRS system demonstrated superior performance with the lowest values of TLSS and VLSS. The BCODL-DSCMRS technique has resulted in the lowest VLSS outcomes.
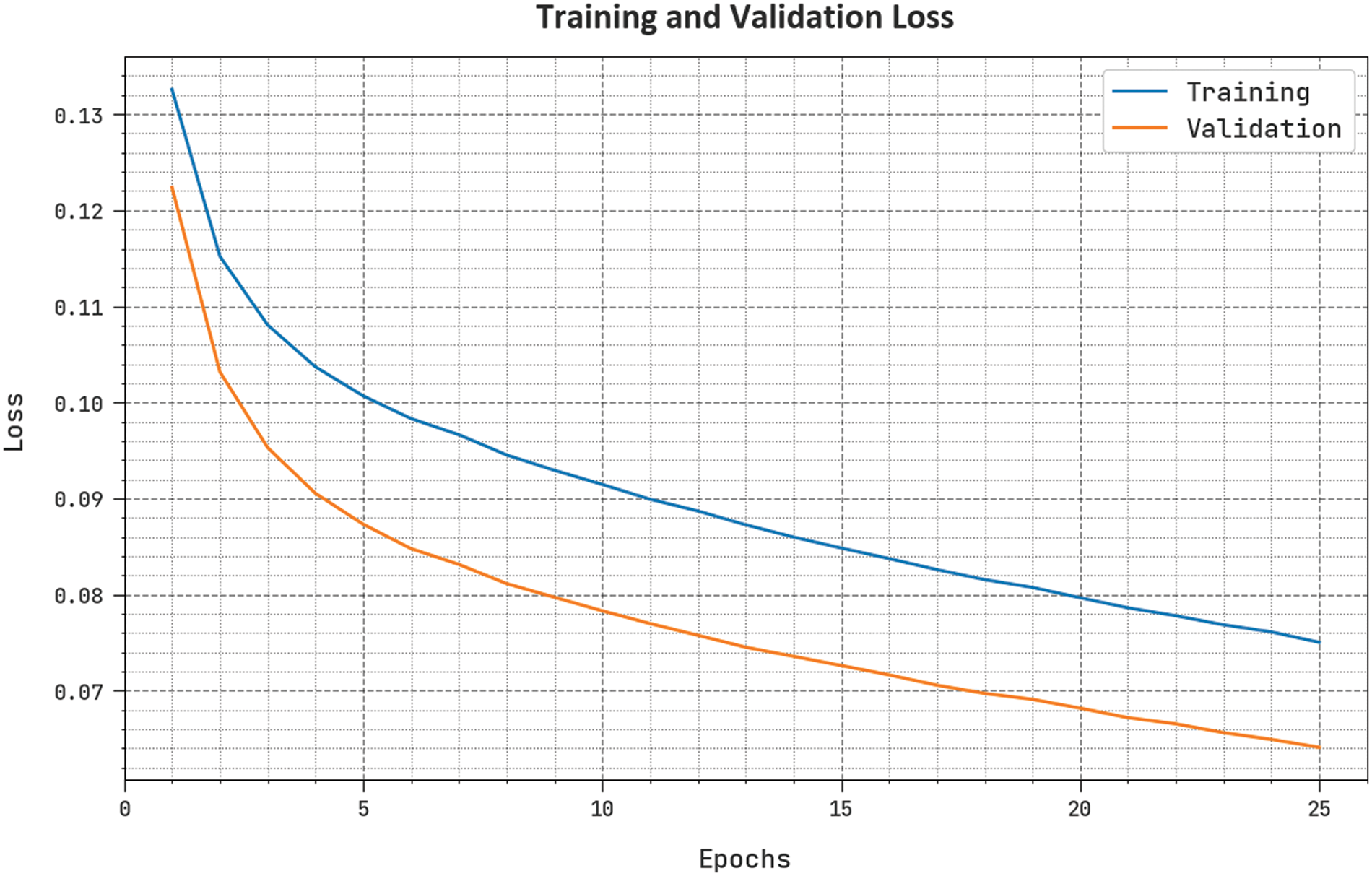
Figure 6: TLSS and VLSS outcome of BCODL-DSCMRS method
Table 4 represents the overall drug recommendation results of the BCODL-DSCMRS model [30]. In Fig. 7, a comparative
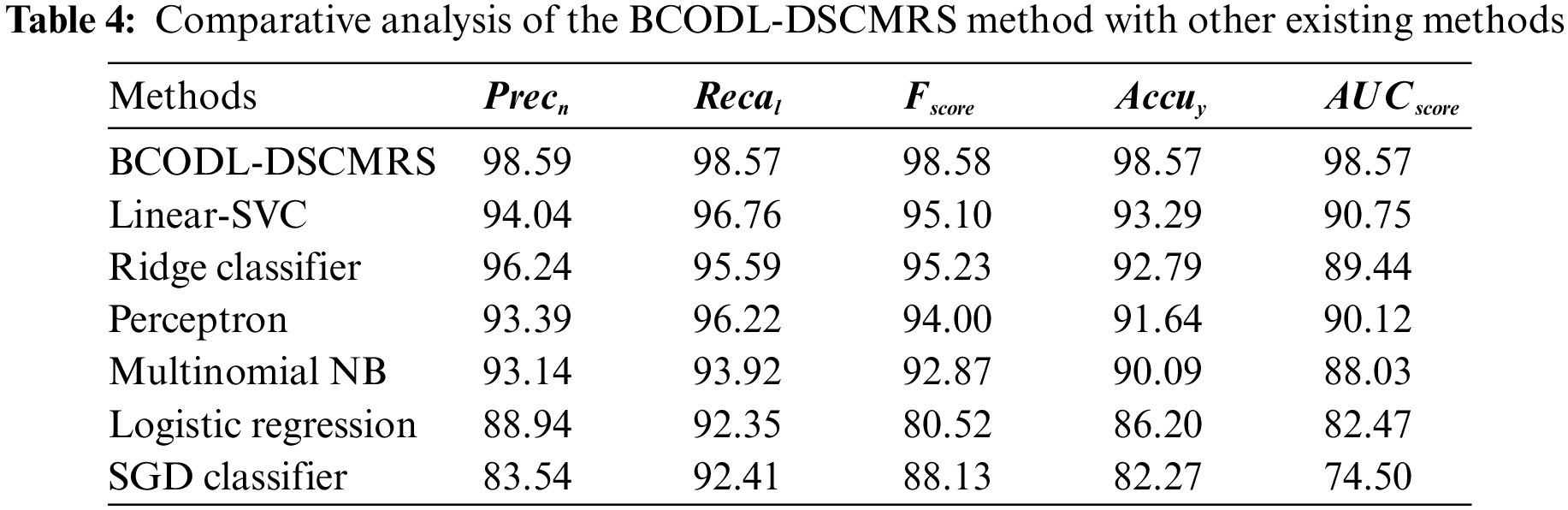
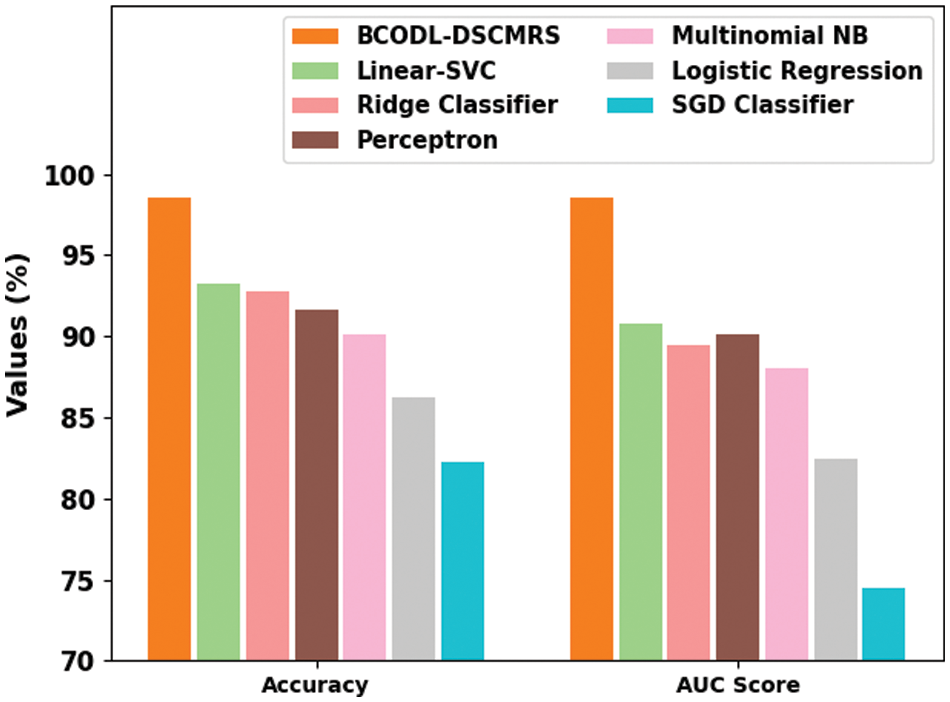
Figure 7:
In Fig. 8, a comparative
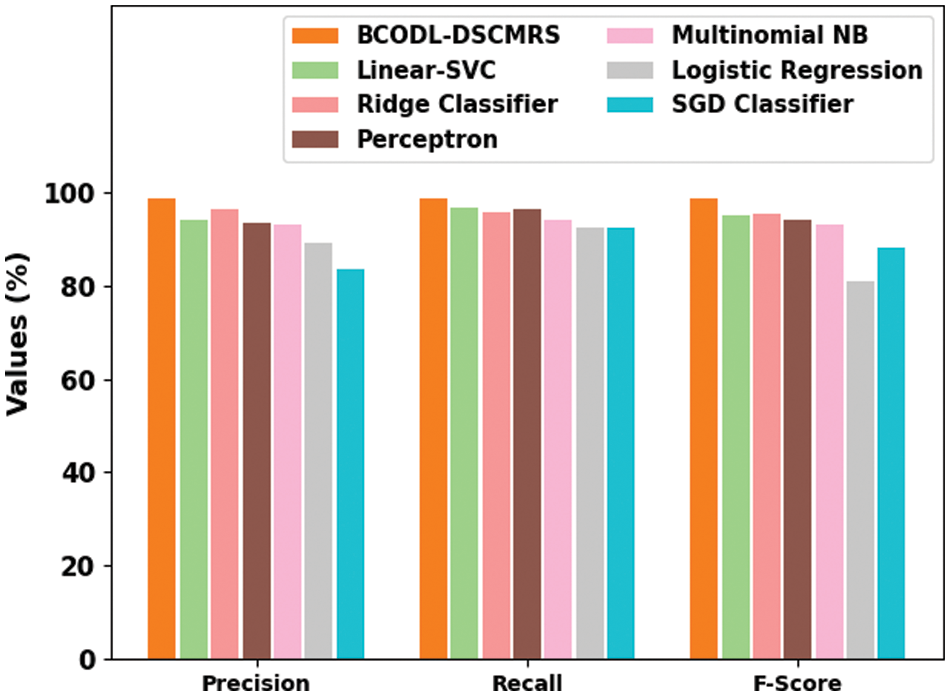
Figure 8: Comparative analysis of BCODL-DSCMRS approach with other existing systems
This article introduced a new BCODL-DSCMRS method for automated DSC management and recommendation processes in the smart pharmaceutical industry. The presented BCODL-DSCMRS technique intends to the incessant observing and tracing of the drug supply for addressing forging problems. The BCODL-DSCMRS technique comprises two significant modules: BC-enabled DSC management and a DL-based consumer recommendation system. Firstly, Hyperledger fabric is used for DSC management, enabling effective tracking processes in the smart medical industry. In addition, a hybrid DBN model is used to suggest the best or top-rated medicines to the pharmaceutical industry customer. The SHO algorithm can be used to improve the performance of the HDBN method. The proposed method is tested on the UCI repository’s open-access drug reviews database. The simulation results of the BCODL-DSCMRS technique show promising performance. In future, the performance of the proposed model can be improved by a hybrid metaheuristic algorithm. Besides, the results of the proposed model can be investigated on a large scale real time database.
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Study conception and design: Shanthi Perumalsamy; data collection: Shanthi Perumalsamy; analysis and interpretation of results: Shanthi Perumalsamy, Venkatesh Kaliyamurthy; draft manuscript preparation: Shanthi Perumalsamy, Venkatesh Kaliyamurthy. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. B. Subashini and D. Hemavathi, “Detecting the traceability issues in supply chain industries using blockchain technology,” in 2022 Int. Conf. on Advances in Computing, Communication and Applied Informatics (ACCAI), Chennai, India: IEEE, pp. 1–8, 2022. [Google Scholar]
2. X. Liu, A. V. Barenji, Z. Li, B. Montreuil and G. Q. Huang, “Blockchain-based smart tracking and tracing platform for drug supply chain,” Computers & Industrial Engineering, vol. 161, pp. 107669, 2021. [Google Scholar]
3. S. Sarkar, “Digital Traceability of pharmaceutical drugs in supply chain,” International Journal of Advance Research in Computer Science and Management, vol. 10, no. 2, pp. 39–44, 2022. [Google Scholar]
4. Z. Zhu, Z. Yao, G. Qi, N. Mazur and B. Cong, “Associative learning mechanism for drug-target interaction prediction,” CAAI Transactions on Intelligence Technology, vol. 7, no. 3, pp. 121, 2023. https://doi.org/10.1049/cit2.12194 [Google Scholar] [CrossRef]
5. B. Azzouz, M. Abou Taam, A. Morel and T. Trenque, “Psoriasis during angiotensin receptor blocker exposure: An underestimated adverse drug reaction,” Expert Opinion on Drug Safety, vol. 17, no. 9, pp. 853–857, 2018. [Google Scholar] [PubMed]
6. T. Yi, M. Shi and H. Zhu, “Medical data publishing based on average distribution and clustering,” CAAI Transactions on Intelligence Technology, vol. 7, no. 3, pp. 381–394, 2022. [Google Scholar]
7. M. J. Labaran and M. Hamma-Adama, “The Nigerian pharmaceutical supply chain: Blockchain adoption, counterfeit drugs and successful deployment of COVID-19 vaccine in Nigeria,” Journal of Scientific Research and Reports, vol. 27, no. 2, pp. 20–36, 2021. [Google Scholar]
8. F. J. Abdullayeva, “Internet of things-based healthcare system on patient demographic data in Health 4.0,” CAAI Transactions on Intelligence Technology, vol. 7, no. 4, pp. 644–657, 2022. [Google Scholar]
9. Y. Kalakoti, S. Yadav and D. Sundar, “TransDTI: Transformer-based language models for estimating DTIs and building a drug recommendation workflow,” ACS Omega, vol. 7, no. 3, pp. 2706–2717, 2022. [Google Scholar] [PubMed]
10. N. Duborija-Kovacevic, J. Djapic, M. Nedovic-Vukovic and Z. Besovic, “Point-prevalence survey on drug shortage characteristics in a developing country with a very small pharmaceutical market,” Acta Poloniae Pharmaceutica-Drug Research, vol. 78, no. 5, pp. 731–739, 2022. [Google Scholar]
11. C. G. Guzman Trujllo, “The role of blockchain in the pharmaceutical industry supply chain as a tool for reducing the flow of counterfeit drugs,” Ph.D. dissertation, Dublin Business School, Dublin, Ireland, 2018. [Google Scholar]
12. S. K. Panda and S. C. Satapathy, “Drug traceability and transparency in medical supply chain using blockchain for easing the process and creating trust between stakeholders and consumers,” Personal and Ubiquitous Computing, vol. 9, pp. 1–17, 2021. https://doi.org/10.1007/s00779-021-01588-3 [Google Scholar] [CrossRef]
13. A. Vishwakarma, G. S. Dangayach, M. L. Meena, S. Gupta and S. Luthra, “Adoption of blockchain technology enabled healthcare sustainable supply chain to improve healthcare supply chain performance,” MEQ, vol. 34, no. 4, pp. 1111–1128, 2023. [Google Scholar]
14. S. Bali, V. Bali, R. P. Mohanty and D. Gaur, “Analysis of critical success factors for blockchain technology implementation in healthcare sector,” BIJ, vol. 30, no. 4, pp. 1367–1399, 2023. [Google Scholar]
15. M. Hölbl, M. Kompara, A. Kamišalić and L. Nemec Zlatolas, “A systematic review of the use of blockchain in healthcare,” Symmetry, vol. 10, no. 10, pp. 470, 2018. https://doi.org/10.3390/sym10100470 [Google Scholar] [CrossRef]
16. K. Abbas, M. Afaq, T. Ahmed Khan and W. C. Song, “A blockchain and machine learning-based drug supply chain management and recommendation system for smart pharmaceutical industry,” Electronics, vol. 9, no. 5, pp. 852, 2020. [Google Scholar]
17. A. Musamih, K. Salah, R. Jayaraman, J. Arshad, M. Debe et al., “A blockchain-based approach for drug traceability in healthcare supply chain,” IEEE Access, vol. 9, pp. 9728–9743, 2021. [Google Scholar]
18. V. Ahmadi, S. Benjelloun, M. El Kik, T. Sharma, H. Chi et al., “Drug governance: IoT-based blockchain implementation in the pharmaceutical supply chain,” in Sixth Int. Conf. on Mobile And Secure Services (MobiSecServ), Miami Beach, FL, USA, pp. 1–8, 2020. https://doi.org/10.1109/MobiSecServ48690.2020.9042950 [Google Scholar] [CrossRef]
19. Y. Huang, J. Wu and C. Long, “Drugledger: A practical blockchain system for drug traceability and regulation,” in IEEE Int. Conf. Internet of Things (iThings) and IEEE Green Computing and Communications (GreenCom) and IEEE Cyber, Physical and Social Computing (CPSCom) and IEEE Smart Data (SmartData), Halifax, NS, Canada, pp. 1137–1144, 2018. [Google Scholar]
20. F. Jamil, L. Hang, K. Kim and D. Kim, “A novel medical blockchain model for drug supply chain integrity management in a smart hospital,” Electronics, vol. 8, no. 5, pp. 505, 2019. [Google Scholar]
21. D. Agrawal, S. Minocha, S. Namasudra and A. H. Gandomi, “A robust drug recall supply chain management system using hyperledger blockchain ecosystem,” Computers in Biology and Medicine, vol. 140, pp. 105100, 2022. [Google Scholar]
22. M. Uddin, “Blockchain Medledger: Hyperledger fabric enabled drug traceability system for counterfeit drugs in pharmaceutical industry,” International Journal of Pharmaceutics, vol. 597, pp. 120235, 2021. [Google Scholar] [PubMed]
23. P. Sharma, S. Namasudra, R. Gonzalez Crespo, J. Parra-Fuente and M. Chandra Trivedi, “EHDHE: Enhancing security of healthcare documents in IoT-enabled digital healthcare ecosystems using blockchain,” Information Sciences, vol. 629, pp. 703–718, 2023. [Google Scholar]
24. Z. Peng, J. Xu, X. Chu, S. Gao and Y. Yao, “VFChain: Enabling verifiable and auditable federated learning via blockchain systems,” IEEE Transactions on Network Science and Engineering, vol. 9, no. 1, pp. 173–186, 2022. [Google Scholar]
25. Z. Peng, J. Xu, H. Hu, L. Chen and H. Kong, “BlockShare: A blockchain empowered system for privacy-preserving verifiable data sharing,” Bulletin of the IEEE Computer Society Technical Committee on Data Engineering, vol. 1, pp. 14–24, 2022. [Google Scholar]
26. H. Wu, Z. Peng, S. Guo, Y. Yang and B. Xiao, “VQL: Efficient and verifiable cloud query services for blockchain systems,” IEEE Transactions on Parallel and Distributed Systems, vol. 33, no. 6, pp. 1393–1406, 2022. [Google Scholar]
27. J. H. Tseng, Y. C. Liao, B. Chong and S. W. Liao, “Governance on the drug supply chain via Gcoin Blockchain,” International Journal of Environmental Research and Public Health, vol. 15, no. 6, pp. 1055, 2018. https://doi.org/10.3390/ijerph15061055 [Google Scholar] [PubMed] [CrossRef]
28. Z. Li, H. Huang, Z. Zhang and G. Shi, “Manifold-based multi-deep belief network for feature extraction of hyperspectral image,” Remote Sensing, vol. 14, no. 6, pp. 1484, 2022. [Google Scholar]
29. N. Panda, S. K. Majhi and R. Pradhan, “A hybrid approach of spotted hyena optimization integrated with quadratic approximation for training wavelet neural network,” Arabian Journal for Science and Engineering, vol. 47, pp. 1–17, 2022. https://doi.org/10.1007/s13369-022-06564-4 [Google Scholar] [PubMed] [CrossRef]
30. S. Garg, “Drug recommendation system based on sentiment analysis of drug reviews using machine learning,” in 11th Int. Conf. on Cloud Computing, Data Science & Engineering (Confluence), Noida, India, pp. 175–181, 2021. https://doi.org/10.1109/Confluence51648.2021.9377188 [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools