 Open Access
Open Access
ARTICLE
AMSFuse: Adaptive Multi-Scale Feature Fusion Network for Diabetic Retinopathy Classification
1 School of Computer Science and Engineering, Central South University, Changsha, 410083, China
2 School of Humanities, Central South University, Changsha, 410083, China
3 Department of Human Anatomy and Histology & Embryology, Basic Medical Sciences, Changsha Health Vocational College, Changsha, 410100, China
4 Glaucoma Institute, Changsha Aier Eye Hospital, Changsha, 410000, China
5 Department of Computer Science and Artificial Intelligence, College of Computing and Information Technology, University of Bisha, Bisha, 67714, Saudi Arabia
* Corresponding Author: Yalong Xiao. Email:
Computers, Materials & Continua 2025, 82(3), 5153-5167. https://doi.org/10.32604/cmc.2024.058647
Received 17 September 2024; Accepted 28 November 2024; Issue published 06 March 2025
Abstract
Globally, diabetic retinopathy (DR) is the primary cause of blindness, affecting millions of people worldwide. This widespread impact underscores the critical need for reliable and precise diagnostic techniques to ensure prompt diagnosis and effective treatment. Deep learning-based automated diagnosis for diabetic retinopathy can facilitate early detection and treatment. However, traditional deep learning models that focus on local views often learn feature representations that are less discriminative at the semantic level. On the other hand, models that focus on global semantic-level information might overlook critical, subtle local pathological features. To address this issue, we propose an adaptive multi-scale feature fusion network called (AMSFuse), which can adaptively combine multi-scale global and local features without compromising their individual representation. Specifically, our model incorporates global features for extracting high-level contextual information from retinal images. Concurrently, local features capture fine-grained details, such as microaneurysms, hemorrhages, and exudates, which are critical for DR diagnosis. These global and local features are adaptively fused using a fusion block, followed by an Integrated Attention Mechanism (IAM) that refines the fused features by emphasizing relevant regions, thereby enhancing classification accuracy for DR classification. Our model achieves 86.3% accuracy on the APTOS dataset and 96.6% RFMiD, both of which are comparable to state-of-the-art methods.Keywords
Diabetic retinopathy (DR) is a condition that affects the retina as a result of diabetes complications, resulting in permanent eye damage and, in some cases, vision loss. If left untreated, this form of issue among patients has a high priority of people going blind. DR is a leading cause of vision impairment and blindness among working-age adults globally. Early detection and management are crucial in preventing irreversible damage.
According to recent statistics, around 537 million adults globally have diabetes as of 2023, with the number expected to increase to 783 million by 2045 and potentially 1.3 billion by 2050. This increase emphasizes the rising worldwide impact of diabetes [1]. As shown in Fig. 1, DR is classified into five stages, reflecting the progression of the disease:
1. Normal: where there are no retinopathy symptoms.
2. Mild Non-Proliferative Retinopathy: characterized by the presence of microaneurysms. At this stage, patients may not experience symptoms, but microaneurysms indicate the onset of retinal damage. Regular monitoring is essential to prevent progression.
3. Moderate Non-Proliferative Retinopathy: where some blood vessels become distorted and swollen. Blockages in retinal vessels begin to occur, potentially leading to noticeable visual changes. Early intervention can help manage the condition.
4. Severe Non-Proliferative Retinopathy: involving significant blockage of blood vessels. A larger number of vessels are blocked, signaling the retina to grow new vessels, which can lead to more severe complications if not addressed promptly.
5. Proliferative Retinopathy: an advanced stage marked by the growth of new blood vessels. These fragile new vessels can bleed into the vitreous, causing vision loss or blindness. Immediate treatment is critical at this stage to prevent severe outcomes.

Figure 1: Retinal fundus images with various stages of DR
Each stage of DR has distinct characteristics and features that doctors may dismiss, potentially resulting in misdiagnosis. Accurate classification of these stages is vital for determining appropriate treatment strategies, such as laser therapy or surgical interventions, to prevent vision deterioration. This difficulty emphasizes the necessity for an automated approach to assist in the correct detection and classification of DR. It is estimated that timely and appropriate treatment and regular screening of the eyes can reduce the incidence of new cases of this disease by at least 56% [2]. With the goal of early detection, regular screening is advised by the International Council of Ophthalmology, and the American Academy of Ophthalmology [3]. These organizations recommend screening intervals of 12 to 24 months for patients without or with mild DR. While regular screening is vital for preventing blindness, the anticipated rise in diabetes patients poses a significant challenge for the screening and follow-up processes. To address this growing demand, automated DR detection systems leveraging artificial intelligence offer a scalable and efficient solution. Unlike traditional manual screening methods, AI approaches can analyze large volumes of retinal images rapidly, ensuring timely diagnosis and reducing the burden on healthcare professionals.
The diagnosis of DR has advanced significantly in the last ten years in the field of deep learning [4]. Convolutional Neural Networks (CNNs) have been proposed in a number of different forms to automate the grading of DR [5]. Recently, Vision Transformers (ViTs) have become a powerful tool that enhances deep learning models’ capabilities [6]. ViTs have demonstrated their effectiveness with DR classification, such as [7] and computed tomography [8].
However, in these models, images are represented as one-dimensional token sequences, which leads to ignoring either their actual local or global structures. Combining multi-scale global and local features which are necessary for tasks like segmentation and image classification is the first step toward solving this problem [9]. Although significant progress has been made by recent works, including ViTAE [10], StoHisNet [11], Transfuse [12], CMT [13], and Comformer [14], which integrated features derived from convolutional and self-attention processes [15], challenges remain in adaptively fusing multi-scale features without compromising their individual strengths.
To address this issue, we introduced AMSFuse, which integrates global and local blocks to concurrently extract and adapt global and local features. These are followed by fusion blocks that merge these features at various semantic scales and then comes an Integrated Attention Mechanism (IAM) that refines and weights fused features to enhance representation. This approach enhances our model’s performance compared to earlier classification techniques that primarily depend on either transformer or convolutional models. Results show that our approach achieves better results on common DR datasets. Our contributions can be listed as follows:
1. We proposed an AMSFuse network that integrates global and local features at multiple scales. This network adaptively captures both semantic information and local spatial features through global and local feature blocks.
2. We introduced an integrated attention mechanism to refine the fused global and local features, highlighting critical pathological regions for enhanced diagnostic precision.
3. The proposed model achieves state-of-the-art performance with 86.3% accuracy on the APTOS dataset and 96.6% on the RFMiD dataset, demonstrating competitive or superior performance compared to existing methods.
2.1 DR Classification Based on CNN
Due to the complexity of eye fundus images, traditional approaches for classifying diabetic retinopathy have faced difficulties, which have limited early-stage identification. Convolutional neural networks (CNNs), one deep learning technique, has demonstrated potential in tackling these issues by leveraging artificial intelligence for classification [3]. Deep learning methods have shown great potential for diagnosing and categorizing DR, which is a leading cause of blindness in patients with diabetes. These techniques are particularly beneficial for the early detection and classification of DR, several studies have suggested using deep learning models, including MobileNetV2 [4], VGG16 [5], DenseNet121 [16], and ResNet50 [16]. These models have shown to be useful in automating the diagnosis process, showing good results when identifying DR based on the retinal fundus.
2.2 DR Classification Based on ViT
ViT has shown promising results in medical image classification tasks [6], including DR classification. With new findings, researchers have investigated the application of transformer-based models such as BEiT [7], DeiT [8], CaiT [17], TransMIL [18], and MIL-ViT [19] for automated comprehension of DR severity from fundus images. Transformer models have achieved significant success in the field of computer vision applications, despite their superior performance in natural language processing [20]. Recent models such as Mvitv2 [21], ViT-CoMer [22], and EfficientViT [22] have enhanced the performance of the ViT architecture, facilitating the extraction of global features. Furthermore, compared to conventional convolution-based techniques, the use of pre-trained transformers that have been optimized for use on DR datasets.
2.3 Multi-Scale Feature Fusion
Multi-scale feature fusion is vital for accurately classifying medical images. To tackle the limitation of insufficient local features, DeiT [8] introduced a distillation token to combine CNN features with ViT. Additionally, T2TViT [23] proposed a designed method to improve the Vision Transformer’s ability to capture local features. Methods such as CMT [13], Conformer [14], VitAE [10], and StoHis [11] demonstrate that integrating local features with global representations significantly improves the Transformer’s ability to notice small local features. Furthermore, models such as MFFM [24] and SegR-Net [25] Although these models perform exceptionally well on common datasets like ImageNet and various downstream tasks, they do not achieve the same success in the medical imaging domain. This shortfall is due to the insufficient datasets of medical images, where pathological features are more scattered and challenging to detect compared to ordinary images.
In order to improve DR lesion classification, [26] offers multi-scale feature fusion. However, it primarily focuses on local features, limiting its ability to capture the global feature context, which is necessary for accurate DR classification.
Therefore, we decided to leverage adaptive features along with multi-scale global-local characteristics. Our novel approach, AMSFuse, presents an adaptive parallel fusion network that is developed to maintain a parallel structure and prevent interference between local and global features. Convolutional layers specialize in detecting and extracting local patterns and features within images, such as edges and textures. On the other hand, ViT utilizes a transformer architecture, enabling it to analyze the entire image at once, capturing global context at different parts of the image simultaneously without the need for sequential processing. These features are efficiently integrated within this network by an adaptive feature fusion block, which maintains the integrity of both global and local blocks. Furthermore, we incorporate IAM to refine the fused features, emphasizing relevant regions and enhancing the overall classification accuracy for DR.
As shown in Fig. 2, the AMSFuse network, introduced as a new method for DR classification, aims to adaptively capture both local spatial details and global semantic representations of fundus images at varying scales. This is achieved by employing a parallel structure that extracts global and local information through distinct feature blocks. The features are then adaptively fused using the AMSFuse block, and a downsampling step is performed. This process is repeated across four stages. Finally, the output from the last AMSFuse Block is processed through an integrated attention mechanism, followed by a Global Average Pooling layer, a Layer Norm layer, and a Linear layer to produce the classification result. This network facilitates a comprehensive integration of multi-scale global and local features, significantly enhancing the accuracy of DR classification.

Figure 2: Proposed AMSFuse network
Local spatial features in fundus images are crucial. The local feature block, depicted in (1), utilizes a
• Depthwise Convolution: A
• Linear Transformation and Addition: The result of the depthwise convolution is passed through a linear transformation (f_
The global feature block denoted by
The AMSFuse block, indicated by
after matching the spatial dimensions, the global and local features are combined through element-wise addition. This operation effectively merges the high-level semantic information from the global features with the fine-grained details from the local features, providing a richer and more comprehensive feature representation the process is depicted in (3):
3.5 Integrated Attention Mechanism (IAM)
To further refine the fused features, we propose an Integrated Attention Mechanism. This mechanism integrates features from various stages of the model, including the global features block, local features block, and adaptively fused features from the AMSFuse block, depicted in (6), the reduced feature map, indicated as R, is created by concatenating the global features (
After reducing the feature map, it is passed through the IAM. The query, key, and value tensors are obtained from the input feature map R using
The attention mechanism calculates the output by taking the dot product of queries Q and keys K, scaling it by the square root of the key dimension
the final output from the IAM, after applying the attention mechanism is computed as (9).
where
The Integrated Attention Mechanism (IAM) effectively leverages the combined feature map, by dynamically focusing on different parts of the input feature map, (IAM) enhances the model’s ability to capture complex dependencies and relationships within the data, leading to improved performance. The final output fed to the classifier to obtain the classification.
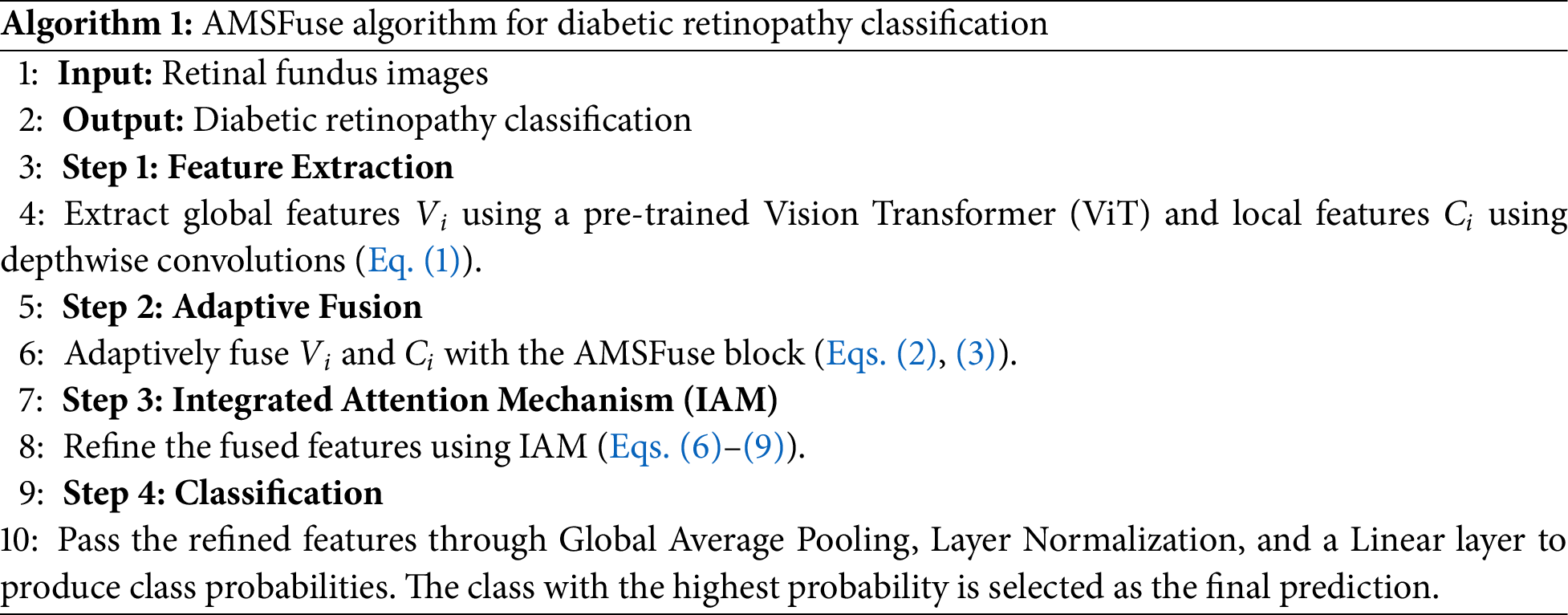
Algorithm 1 provides a comprehensive overview of the AMSFuse network. The methodology begin with the extraction of local and global features through dedicated feature extraction blocks. These features are subsequently fused within the AMSFuse block using an adaptive fusion strategy, ensuring a seamless integration of multi-scale information. The Integrated IAM further refines the fused features by prioritizing critical regions and suppressing irrelevant information, enhancing the model's focus on diagnostically significant areas. This systematic approach leverages both global contextual and localized spatial information, resulting in a robust and highly accurate classification of DR severity.
4 Experimental Results and Visualization
The AMSFuse model is trained using the cross-entropy loss function, which is commonly used for classification tasks. This loss function measures the difference between the predicted class probabilities and the actual class labels, and its formula is as follows:
In Eq. (10),
We ran a series of experiments with two datasets to evaluate the AMSFuse model’s effectiveness and reliability. The outcomes of these trials show that, in terms of classification performance, our method performs better than earlier state-of-the-art network models. In the subsequent sections, we will offer an in-depth analysis of the datasets used, outline the evaluation metrics utilized, and describe the experimental setup. Following this, we will present the results of our experiments across all datasets, including a comprehensive examination of our model’s performance through a series of ablation studies conducted on the APTOS dataset.
We utilized two publicly available datasets for evaluating the performance of the AMSFuse model: APTOS2019 and RFMiD.
The APTOS2019 Blindness Detection Dataset [27] includes 5590 fundus images with five DR classifications labeled into various stages of DR: No DR (label 0), Mild (label 1), Moderate (label 2), Severe (label 3), and Proliferative (label 4). Only the annotations of the training set (3662 photos) are publicly accessible. These annotations were randomly divided into 70% for training, 15% for validation, and 15% for testing.
The RFMiD Retinal Fundus Multi-disease Dataset [28] contains a total of 1900 fundus images, and we exclusively utilize it for diagnosing diabetic retinopathy. In both datasets, we followed standardized protocols commonly used in the research community to ensure consistency and fairness in our study. All utilized images are of high quality, with clear visibility of retinal features necessary for accurate DR classification.
A ViT model trained on a dataset of 345,271 fundus samples is used as the pre-trained model for retinal disease diagnosis tasks. This dataset includes samples annotated as normal (208,733), diabetic retinopathy (38,284), age-related macular degeneration (21,962), glaucoma (24,082), and cataract (67,230) [19].
4.3 Evaluation Metrics and Implementation Information
We chose ACC, F1, Precision, and Kappa as the main classification metrics, all derived from the confusion matrix. This matrix consists of True Positive (TP), True Negative (TN), False Positive (FP), and False Negative (FN) counts. Thus, Accuracy (ACC) is calculated using the following formula Eq. (11), which represents the proportion of correctly identified samples:
Precision, reflecting the model’s prediction accuracy, is computed using Eq. (12), representing the ratio of true positive samples among those predicted to be positive:
Recall is calculated using the Eq. (13):
this equation calculates the recall rate, which measures the percentage of actual positive cases that the model accurately identifies.
The F1 score, defined by Eq. (14), balances Precision and the proportion of actual positives:
The Kappa coefficient, used to measure classifier consistency, ranges from 0 (random agreement) to 1 (perfect agreement), and is calculated by Eq. (15):
where
4.4 Parameter Tuning and Analysis
To optimize AMSFuse, we conducted a grid search for key hyperparameters. The learning rate was set to
4.5 Comparison with Other Advanced Models
DR Classification with APTOS Dataset. The comparison on the APTOS2019 dataset, shown in Table 2, clearly shows that the AMSFuse network outperforms several state-of-the-art methods for diabetic retinopathy (DR) grading. AMSFuse stands out with an impressive accuracy of 86.3%, an F1 score of 86.2%, and a Kappa coefficient of 92.8%, which are the highest among the models compared. This indicates that AMSFuse not only accurately identifies DR cases but also provides reliable and balanced performance. The improvements over other models, including DLI [29], BiFormer-B [30], EfficientViT [31], and even advanced architectures like ViT-CoMer [22] and MIL-ViT [19]. Fig. 3 shows the experimental results visualization.

Figure 3: PR curve, ROC curve, confusion matrix, and t-SNE visualization on APTOS
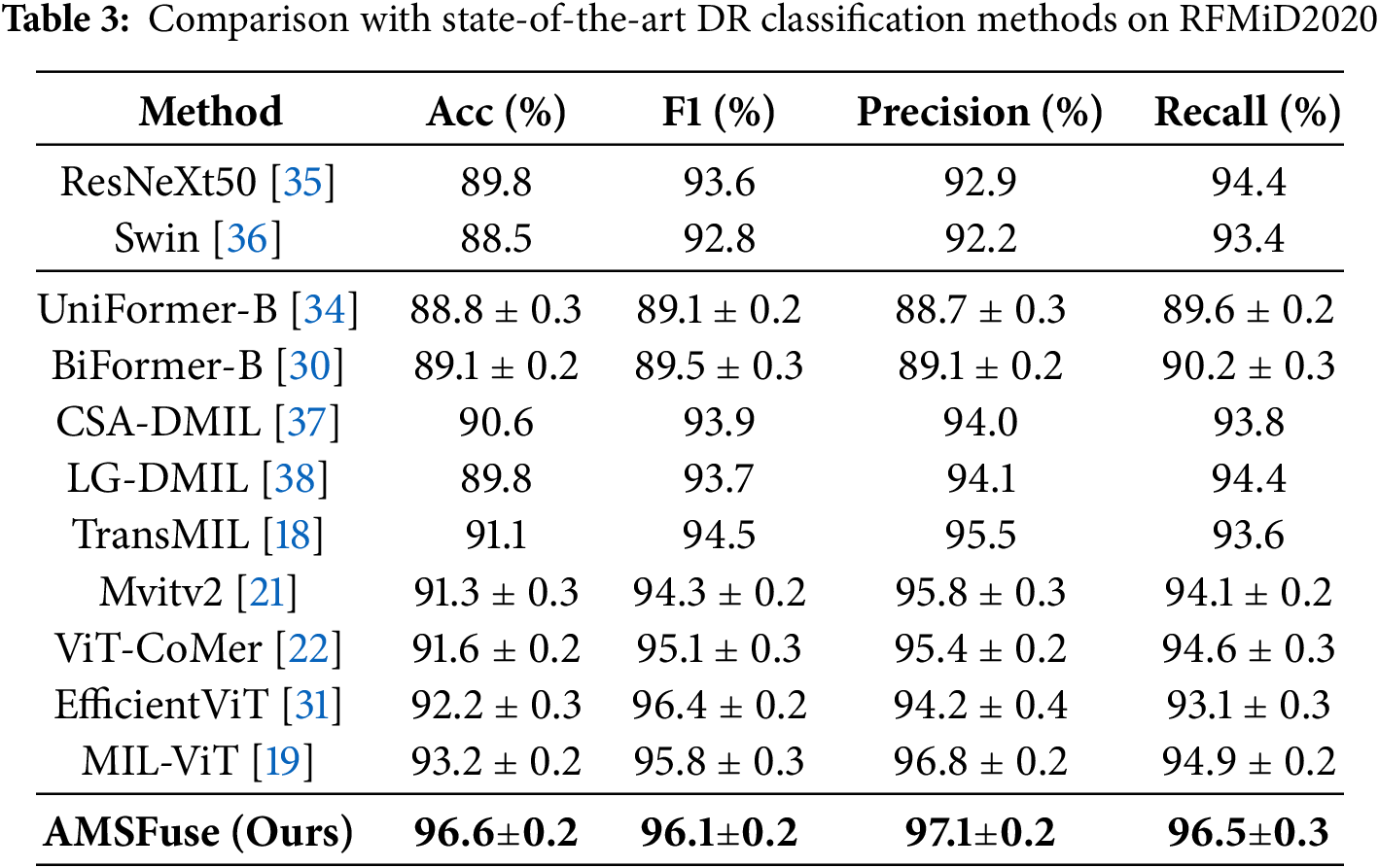
DR Classification with RFMiD Dataset. In the comparison of the RFMiD2020 dataset, shown in Table 3, the AMSFuse network outperforms various leading diabetic retinopathy (DR) classification methods. AMSFuse has the highest metrics, with an accuracy of 96.6%, F1 score of 96.1%, precision of 97.1%, and recall of 96.5%. These results demonstrate the model’s performance to accurately and reliably categorize DR instances. Other models, such as ResNeXt50 [35] show lower performance metrics, with ResNet34 obtaining 87.8% accuracy and ResNeXt50 achieving 89.8%. Mvitv2 [21] and BiFormer-B [34] perform well, but fall short of AMSFuse, with accuracies of 88.0% and 88.5%, respectively. Advanced models such as TransMIL [18], EfficientViT [31], and ViT-CoMer [22] demonstrate improvements, with CSA-DMIL reaching 90.6% accuracy. However, the AMSFuse model shows better results compared to these models. Notably, the MIL-ViT [19] model achieves competitive results with 93.2% accuracy, but it does not surpass the performance of AMSFuse. Fig. 4 shows the experimental results visualization.
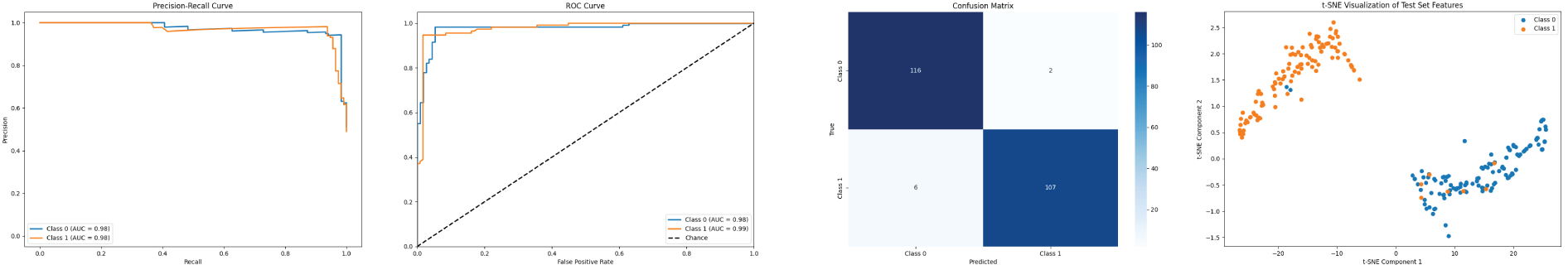
Figure 4: PR curve, ROC curve, confusion matrix, and t-SNE visualization on RFMiD
We conducted an ablation study to evaluate the impact of the different branches of our network on the APTOS dataset. The study is divided into three levels that examine the contributions of the local block, global block, AMSFuse, and IAM.
Effectiveness of Local Block, Global Block, and AMSFuse. We started by comparing the performance of running the local and global blocks individually using AMSFuse. The results demonstrate that the global block outperforms the local block, and the application of AMSFuse considerably increases performance. The local block obtained 75.0% accuracy, 60.0% F1 score, and 65.0% Kappa, whereas the global block increased these metrics to 78.0%, 68.0%, and 70.0%. The integration of AMSFuse led to significant gains, obtaining the greatest metrics with an accuracy of 86.3%, F1 score of 86.2%, and Kappa of 92.8%, shown in Table 4.

Impact of AMSFuse Block and IAM. An additional experiment was conducted to further assess the influence of the AMSFuse Block and IAM in the performance of the model. For this ablation experiment, three configurations were tested:
• Model with the IAM disabled: with only global and local branches without AMSFuse block.
• Model with AMSFuse block disabled: with only global and local branches with IAM.
• Model with AMSFuse and IAM disabled.
• Model with AMSFuse and IAM enabled.
Table 5 the results indicate that the AMSFuse block, along with IAM, significantly contributes to improved performance. The model with concat and the IAM disabled achieved 79.4% accuracy, 79.6% F1 score, and 83.3% Kappa. On the other hand, the model without the AMSFuse block obtained 80.1% accuracy, 80.5% F1 score, and 92.0% Kappa. In contrast, the model that integrates both blocks exhibited remarkable improvements with an accuracy of 86.3%, F1 score of 86.2%, and Kappa of 92.8%, which demonstrates the benefits of adaptively fusing multi-scale features without compromising their individual representation.

The ablation study results indicate that both the AMSFuse Block and IAM play crucial roles in enhancing the performance of the proposed network. The global block consistently outperforms the local block when used individually, and the integration of these blocks via AMSFuse further boosts accuracy. The IAM further refines the feature fusion, leading to significant gains in classification metrics.
While AMSFuse has demonstrated performance in grading DR, there are several constraints to consider. Variability in datasets, such as differences in image quality and patient demographics, may influence how well the model performs in different clinical contexts.
To better understand AMSFuse’s effectiveness, it’s helpful to compare its performance with current clinical standards. For instance, research has shown that deep-learning algorithms can sometimes match the accuracy of manual grading by retinal specialists, particularly in terms of sensitivity and specificity across various stages of DR classification [39]. In this regard, AMSFuse’s results suggest that it has the potential to improve conventional diagnostic techniques in healthcare settings.
Interpretability is crucial for clinical acceptance. Making the model’s decisions more transparent will help to build trust among healthcare professionals. Future work will focus on integrating more interpretable components. With the potential to adapt AMSFuse for related tasks, such as medical image segmentation, we expect that our approach could improve segmentation accuracy compared to traditional methods, and we plan to investigate this further. By capturing global context and local details through adaptive multi-scale feature fusion, and highlighting important regions with the IAM, we hope to enhance the model’s ability to focus on critical anatomical features. This could potentially lead to better segmentation performance, but further research is needed to confirm this. Furthermore, AMSFuse’s computational demands and the need for high-performance hardware may limit its use in resource-constrained environments. By addressing these areas, we aim to make AMSFuse a reliable and versatile tool for clinical use.
We introduced AMSFuse, an Advanced Multi-Scale Fusion framework designed to improve the classification of diabetic retinopathy (DR) from retinal fundus images. The core innovation lies in the AMSFuse block, which combines multi-scale feature fusion with an Integrated Attention Mechanism (IAM) to capture both global and local features. This approach enables the model to focus on critical regions in the images, enhancing classification accuracy across different DR stages. Our experiments on the APTOS2019 and RFMiD2020 datasets demonstrated that AMSFuse outperforms existing state-of-the-art models, achieving higher accuracy, precision, recall, and F1 scores. These results highlight the effectiveness of our multi-scale fusion strategy and attention mechanisms in capturing the complex patterns associated with DR. By improving the accuracy of DR classification, AMSFuse has significant implications for medical image analysis and automated DR screening. It can assist ophthalmologists in early detection and treatment planning, potentially reducing the risk of vision loss among diabetic patients. Its scalability and efficiency make it suitable for integration into clinical workflows, meeting the growing demand for automated screening solutions. For future work, we plan to extend our framework to other retinal diseases, explore additional modalities like optical coherence tomography (OCT) images, and assess the generalizability of AMSFuse in diverse clinical settings.
Acknowledgement: The authors are thankful to the Deanship of Graduate Studies and Scientific Research at University of Bisha for supporting this work through the Fast-Track Research Support Program.
Funding Statement: This work is supported by the National Natural Science Foundation of China (No. 62376287), the International Science and Technology Innovation Joint Base of Machine Vision and Medical Image Processing in Hunan Province (2021CB1013), the Natural Science Foundation of Hunan Province (Nos. 2022JJ30762, 2023JJ70016).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Chengzhang Zhu, Ahmed Alasri; data collection: Tao Xu, Abdulrahman Noman, Raeed Alsabri; analysis and interpretation of results: Ahmed Alasri, Xuanchu Duan, Monir Abdullah, Raeed Alsabri; draft manuscript preparation: Ahmed Alasri, Yalong Xiao, Chengzhang Zhu, Raeed Alsabri; supervision and project administration: Chengzhang Zhu. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: In this study, we used public datasets, which can be downloaded from the website if needed.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. International Diabetes Federation. Diabetes facts and figures. IDF Diabetes Atlas. 2023. p. 1–12. Available from: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html. [Accessed 2024 Oct 27]. [Google Scholar]
2. American Diabetes Association Professional Practice Committee. 12. Retinopathy, neuropathy, and foot care: standards of medical care in diabetes—2022. Diabetes Care. 2021;45:S185–94. [Google Scholar]
3. Bhulakshmi D, Rajput S. A systematic review on diabetic retinopathy detection and classification based on deep learning techniques using fundus images. PeerJ Comput Sci. 2024;10(11):e1947. doi:10.7717/peerj-cs.1947. [Google Scholar] [PubMed] [CrossRef]
4. Dong K, Zhou C, Ruan Y, Li Y. MobileNetV2 model for image classification. In: 2nd International Conference on Information Technology and Computer Application (ITCA); 2020. p. 476–80. [Google Scholar]
5. Rocha Da DA, Ferreira FMF, Peixoto ZMA. Diabetic retinopathy classification using VGG16 neural network. Res Biomed Eng. 2022;38(2):761–72. doi:10.1007/s42600-022-00200-8. [Google Scholar] [CrossRef]
6. Dosovitskiy A. An image is worth 16x16 words: transformers for image recognition at scale. arXiv:2010.11929. 2020. [Google Scholar]
7. Bao H, Dong L, Piao S, Wei F. BEiT: BERT pre-training of image transformers. arXiv:2106.08254. 2021. [Google Scholar]
8. Touvron H, Cord M, Jégou H. DeiT III: revenge of the ViT. In: European Conference on Computer Vision; 2022. p. 516–33. [Google Scholar]
9. Huo X, Sun G, Tian S, Wang Y, Yu L, Long J, et al. HiFuse: hierarchical multi-scale feature fusion network for medical image classification. Biomed Signal Process Control. 2024;87(7660):105534. doi:10.1016/j.bspc.2023.105534. [Google Scholar] [CrossRef]
10. Xu Y, Zhang Q, Zhang J, Tao D. ViTAE: vision transformer advanced by exploring intrinsic inductive bias. Adv Neural Inf Process Syst. 2021;34:28522–35. [Google Scholar]
11. Fu B, Zhang M, He J, Cao X. StoHisNet: a hybrid multi-classification model with CNN and transformer for gastric pathology images. Comput Methods Programs Biomed. 2022;221(6):106924. doi:10.1016/j.cmpb.2022.106924. [Google Scholar] [PubMed] [CrossRef]
12. Zhang Y, Liu H, Hu Q. TransFuse: fusing transformers and CNNs for medical image segmenta- tion. In: Medical Image Computing and Computer Assisted Intervention–MICCAI 2021; 2021. p. 14–24. [Google Scholar]
13. Guo J, Han K, Wu H, Tang Y, Chen X. CMT: convolutional neural networks meet vision transformers. In: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition; 2022. p. 12175–85. [Google Scholar]
14. Peng Z, Huang W, Gu S. Conformer: local features coupling global representations for visual recognition. In: Proceedings of the IEEE/CVF International Conference on Computer Vision; 2021. p. 367–76. [Google Scholar]
15. Vaswani A, Shazeer N, Parmar N, Uszkoreit J, Jones L, Gomez AN, et al. Attention is all you need. In: Advances in neural information processing systems. USA: Curran Associates, Inc.; 2017. Vol. 30, p. 1–12. [Google Scholar]
16. Zhang J, Xie B, Wu X, Ram R, Liang D. Classification of diabetic retinopathy severity in fundus images with DenseNet121 and ResNet50. arXiv:2108.08473. 2021. [Google Scholar]
17. Touvron H, Cord M, Sablayrolles A, Synnaeve G, Jégou H. Going deeper with image transformers. In: Proceedings of the IEEE/CVF International Conference on Computer Vision; 2021. p. 32–42. [Google Scholar]
18. Yang F, Yang H, Fu J. Learning texture transformer network for image super-resolution. In: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition; 2020. p. 5791–800. [Google Scholar]
19. Bi Q, Sun X, Yu S, Ma K, Bian C, Ning M, et al. MIL-ViT: a multiple instance vision transformer for fundus image classification. J Vis Commun Image Represent. 2023;97(12):103956. doi:10.1016/j.jvcir.2023.103956. [Google Scholar] [CrossRef]
20. Zhou SK, Greenspan H, Davatzikos C, Duncan J. Imaging traits, technology trends, case studies with progress highlights, and future promises. Proc IEEE. 2021;109(5):820–38. doi:10.1109/JPROC.2021.3054390. [Google Scholar] [PubMed] [CrossRef]
21. Li Y, Wu C-Y, Fan H. MViTv2: improved multiscale vision transformers for classification and detection. In: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition; 2022. p. 4804–14. [Google Scholar]
22. Xia C, Wang X. ViT-CoMer: vision transformer with convolutional multi-scale feature interaction for dense predictions. In: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition; 2024. p. 5493–502. [Google Scholar]
23. Yuan L, Chen Y, Wang T. Tokens-to-token ViT: training vision transformers from scratch on ImageNet. In: Proceedings of the IEEE/CVF International Conference on Computer Vision; 2021. p. 558–67. [Google Scholar]
24. Chen F, Ma S, Hao J, Liu W. Dual-path and multi-scale enhanced attention network for retinal diseases classification using ultra-wide-field images. IEEE Access. 2023;11(1106):45405–15. doi:10.1109/ACCESS.2023.3273613. [Google Scholar] [CrossRef]
25. Ryu J, Rehman MU, Nizami IF, Chong KT. SegR-Net: a deep learning framework with multi-scale feature fusion for robust retinal vessel segmentation. Comput Biol Med. 2023;163(2):107132. doi:10.1016/j.compbiomed.2023.107132. [Google Scholar] [PubMed] [CrossRef]
26. Fan R, Liu Y, Zhang R. Multi-scale feature fusion with adaptive weighting for diabetic retinopathy severity classification. Electronics. 2021;10(12):1369. doi:10.3390/electronics10121369. [Google Scholar] [CrossRef]
27. Karthik M, Sohier D. Aptos 2019 blindness detection. 2019. p. 1–12. Available from: https://kaggle.com/competitions/aptos2019-blindness-detection. [Accessed 2024 Oct 27]. [Google Scholar]
28. Pachade S, Porwal P, Thulkar M, Deshmukh G. Retinal fundus multi-disease image dataset (RFMiDa dataset for multi-disease detection research. Data. 2021;6(2):14. doi:10.3390/data6020014. [Google Scholar] [CrossRef]
29. Rakhlin A. Diabetic retinopathy detection through integration of deep learning classification framework. bioRxiv. 2018;225508. doi:10.1101/225508. [Google Scholar] [CrossRef]
30. Zhu L, Wang X, Ke Z, Zhang W, Lau RW. BiFormer: vision transformer with bi-level routing attention. In: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition; 2023. p. 10323–33. [Google Scholar]
31. Liu X, Peng H, Zheng N, Yang Q. EfficientViT: memory efficient vision transformer with cascaded group attention. In: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition; 2023. p. 14420–30. [Google Scholar]
32. Gong L, Ma K, Zheng Y. Distractor-aware neuron intrinsic learning for generic 2D medical image classifications. In: International Conference on Medical Image Computing and Computer-Assisted Intervention; 2020. p. 591–601. [Google Scholar]
33. Liu S, Gong L, Ma K, Zheng Y. GREEN: a graph residual re-ranking network for grading diabetic retinopathy. In: Medical Image Computing and Computer Assisted Intervention; 2020. p. 585–94. [Google Scholar]
34. Li K, Wang Y, Zhang J, Liu Y, Li H, Qiao Y. UniFormer: unifying convolution and self-attention for visual recognition. IEEE Trans Pattern Anal Mach Intell. 2023;45(10):12581–600. doi:10.1109/TPAMI.2023.3282631. [Google Scholar] [PubMed] [CrossRef]
35. Xie S, Girshick P, Ross R, Tu Z, He K. Aggregated residual transformations for deep neural networks. In: Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition; 2017. p. 1492–500. [Google Scholar]
36. Liu Z, Lin Y, Cao Y, Hu H, Wei Y, Zhang Z. Swin transformer: hierarchical vision transformer using shifted windows. In: Proceedings of the IEEE/CVF International Conference on Computer Vision; 2021. p. 10012–22. [Google Scholar]
37. Bi Q, Qin K, Li Z, Zhang H, Xu K, Xia G-S. A multiple-instance densely-connected con- vnet for aerial scene classification. IEEE Trans Image Process. 2020;29:4911–26. doi:10.1109/TIP.2020.2975718. [Google Scholar] [PubMed] [CrossRef]
38. Bi Q, Yu S, Ji W, Bian C, Gong, Liu. Local-global dual perception based deep multiple instance learning for retinal disease classification. In: Medical image computing and computer assisted intervention–MICCAI 2021. MICCAI 2021. Lecture Notes in Computer Science; 2021; Cham: Springer. p. 55–64. doi:10.1007/978-3-030-87237-3_6. [Google Scholar] [CrossRef]
39. Gulshan V, Rajan RP, Widner K, Wu P, Rhodes J. Performance of a deep-learning algorithm vs. manual grading for detecting diabetic retinopathy. JAMA Ophthalmol. 2019;137(9):987–93. doi:10.1001/jamaophthalmol.2019.2004. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF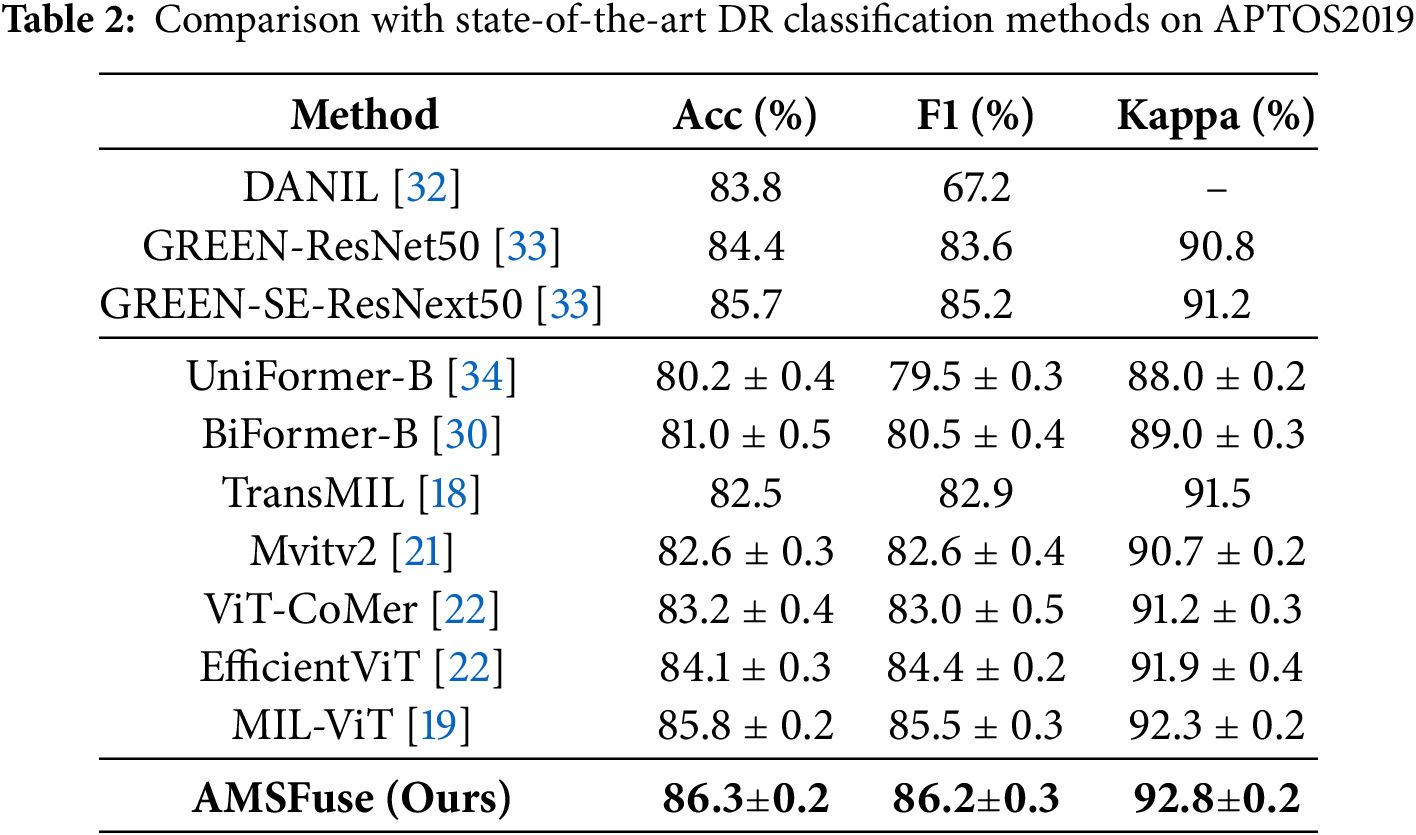
 Downloads
Downloads
 Citation Tools
Citation Tools