 Open Access
Open Access
REVIEW
3D Printing of Plant Fiber-Based Materials and Quality Evaluation of Their Products: A Review
1 Department of Printing & Packaging, University of Shanghai for Science and Technology, Shanghai, 200093, China
2 School of Intelligent Emergency Management, University of Shanghai for Science and Technology, Shanghai, 200093, China
* Corresponding Author: Jiangping Yuan. Email:
(This article belongs to the Special Issue: Next-Generation 3D Printing: Material Innovation and Computational Methodologies)
Computers, Materials & Continua 2025, 84(2), 1951-1979. https://doi.org/10.32604/cmc.2025.065836
Received 22 March 2025; Accepted 15 May 2025; Issue published 03 July 2025
Abstract
Additive manufacturing (AM) and Three-dimensional (3D) printing build complex structures layer by layer, greatly expanding design possibilities. Traditional thermoplastics like Polylactic Acid (PLA), Acrylonitrile Butadiene Styrene (ABS), and Polyethylene Terephthalate Glycol (PETG) are widely used in 3D printing, but their non-renewable nature and limited biodegradability have driven research into plant fiber-based materials. These materials, mainly cellulose and lignin, come from sources like wood and agricultural waste, offering renewability, biodegradability, and biocompatibility. This paper reviews recent advances in plant fiber-based materials for 3D printing, covering their development from raw materials to applications. It highlights the sources, modification methods, and unique properties of cellulose and lignin in 3D printing, and examines processes like fused deposition modeling (FDM), direct ink writing (DIW), stereolithography (SLA), and digital light processing (DLP). The paper discusses key evaluation metrics, including mechanical properties, thermal stability, interlayer bonding strength, and biodegradability, and explores innovative applications in biomedicine (tissue engineering, wound healing), food (personalized nutrition), packaging (smart monitoring), and electronics and energy (flexible devices). Finally, it addresses challenges and future directions in material innovation, process optimization, and large-scale production, emphasizing the potential of interdisciplinary approaches and technology integration for sustainable manufacturing.Keywords
Additive manufacturing (AM) and Three-dimensional (3D) printing refer to various processes that build 3D structures and prototypes layer by layer based on digital models. Solid modeling in computer-aided design (CAD) provides the foundational data for AM. These models are sliced into thin cross-sectional layers, which are stacked to form the final shape. This method can create complex structures and fine surfaces that traditional manufacturing struggles to achieve, greatly expanding design freedom and possibilities [1]. Materials used in 3D printing are critical. They affect the performance of printed objects, as well as the efficiency, cost, and scalability of the printing process [2]. Common 3D printing thermoplastics include Polylactic Acid (PLA), Acrylonitrile Butadiene Styrene (ABS), and Polyethylene Terephthalate Glycol (PETG). PLA is easy to use, biodegradable, and prints at low temperatures. ABS offers durability, impact resistance, and heat resistance for functional parts [3–5]. PETG combines ABS’s strength with PLA’s ease of printing, providing good adhesion and chemical resistance. Other materials like PC, nylon, and TPU offer flexibility, strength, or wear resistance, serving industries such as automotive and medical manufacturing [6–9]. However, thermoplastics have drawbacks, including non-renewability, environmental pollution, and limited biodegradability. This has led to a shift toward renewable, biodegradable plant fiber-based materials to meet green manufacturing needs.
Plant fibers for 3D printing come from various sources, such as wood, bamboo, flax, ramie, hemp, cotton, and coconut shells. Agricultural waste, like rice husks, wheat straw, and sugarcane bagasse, can also be used to extract fibers. Plant fiber-based materials mainly consist of natural polymers like cellulose and lignin. Cellulose, the primary load-bearing polymer in plants, is widely studied for its excellent mechanical properties and biocompatibility [10]. Lignin, with its unique aromatic structure and amphiphilic nature, offers new possibilities for material functionalization [11]. Combining plant fiber-based materials with 3D printing not only utilizes abundant biomass resources to reduce costs but also leverages the unique properties of cellulose and lignin for efficient manufacturing of complex structures. This provides innovative solutions for fields like biomedicine, food, packaging, electronics, and energy. However, using plant fiber-based materials in 3D printing faces challenges, including optimizing material properties, adapting printing processes, and evaluating product quality systematically.
This paper reviews the latest research progress on plant fiber-based materials in 3D printing, covering the entire process from raw materials to applications. It first describes the characteristics of plant fiber-based materials, focusing on the sources, properties, and advantages of cellulose and lignin in 3D printing. It then explains the use of extrusion-based 3D printing (e.g., fused deposition modeling (FDM) and direct ink writing (DIW)) and vat photopolymerization 3D printing (e.g., stereolithography (SLA) and digital light processing (DLP)) for plant fiber-based materials, analyzing the features and suitability of each process. For product performance evaluation, it highlights testing methods and application value for key metrics like mechanical properties, thermal stability, interlayer bonding strength, and biodegradability. It also discusses specific application cases of plant fiber-based materials in biomedicine (e.g., tissue engineering and wound repair), food (e.g., personalized nutrition), packaging (e.g., smart monitoring), and electronics and energy (e.g., flexible devices). Finally, it addresses the challenges and future directions for material innovation and process optimization in this field. The overall pathway of 3D printing with plant fiber-based materials, from raw material sources and printing processes to application scenarios, is shown in Fig. 1.


Figure 1: 3D printing of plant fiber-based materials from raw materials to applications. (A) Sources of cellulose and lignin [12]; (B) 3D printing technologies for plant fiber-based materials [13]; (C) Applications of 3D-printed plant fiber-based material products [14–18]. Reprinted with permission from reference [14]. Copyright 2021, copyright Elsevier. Reprinted with permission from reference [16]. Copyright 2024, copyright Elsevier
2 Characteristics of Plant Fiber-Based Materials
Cellulose is a polysaccharide composed of β-1,4-linked glucose units [19], which can be obtained from various biomass sources, such as hardwood (e.g., poplar, acacia, or eucalyptus), softwood (e.g., pine or spruce), forestry residues, agricultural waste, or grass [20]. Cellulose-based materials have key features like biocompatibility, renewability, and sustainability. However, they also have drawbacks, such as poor solubility in common solvents, lack of thermoplasticity, and limited antibacterial properties. To address cellulose’s limitations, chemical modification can produce cellulose derivatives, divided into macromolecular derivatives and nanoscale particles [21]. Etherification is the main modification method. Organic groups like methyl or ethyl react with cellulose hydroxyl groups to form water-soluble derivatives, such as carboxymethyl cellulose (CMC, Width: 10–15 nm, Aspect ratio < 2), hydroxypropyl methyl cellulose (HPMC), methyl cellulose (MC), hydroxyethyl cellulose (HEC), ethyl cellulose (EC), and cellulose acetate (CA). Nanoscale cellulose particles include cellulose nanofibrils (CNF, Width: 5–30 nm, Aspect ratio > 50), cellulose nanocrystals (CNC, Width: 3–10 nm, Aspect ratio > 5) [22], and bacterial cellulose (BC). Bacterial cellulose nanocrystals (BCNC) can be prepared by acid hydrolysis of BC [21,23].
The performance of cellulose-based materials depends heavily on fiber source, processing method, and fiber morphology. Variations in fiber properties can lead to inconsistent performance in 3D-printed products. To ensure quality and performance stability of 3D-printed cellulose-based products, standardized processes for fiber treatment and cellulose-based filament production are needed. These include uniform protocols for cellulose extraction, modification, and filament preparation. Standardization ensures consistency across different batches and applications, improving the reliability and market competitiveness of 3D-printed products. This expands their use in biomedicine (e.g., drug carriers, wound dressings), environmental engineering (e.g., oil-water separation membranes), and electronic devices (e.g., flexible thermal conductive materials) [24].
Cellulose is widely used to reinforce polymer materials due to its excellent mechanical properties, good shape and size, and ease of surface functionalization [25]. Spray-dried cellulose nanofibrils (SDCNF) have small particle sizes and excellent dispersion and distribution in polymer matrices. As a reinforcing filler in thermoplastics, SDCNF significantly improves the mechanical properties, thermal stability, and 3D printing suitability of polypropylene [26]. Detailed studies of cellulose’s physical, chemical, and mechanical properties and its crystalline structure enable its broad use in new bio-derived composite materials. These include inorganic composites [27,28], polymer composites [29,30], nanocomposites [31], hydrogels [32], and electronic products [33].
Lignin is the second most abundant renewable biomass resource in plants and a key component in sustainable alternatives to traditional materials [34]. It can be used to synthesize chemicals, fuels, and low-molecular-weight compounds, aligning with sustainability goals and helping reduce carbon footprints [35]. Lignin can be extracted using methods like organic solvent extraction, alkaline treatment, acid treatment, sulfate processing, and ionic liquid methods [36]. Different extraction methods produce lignin with varying structures, molecular weights, chemical reactivities, and compositions. Common lignin types include kraft lignin, sodium lignin, lignosulfonates, and organosolv lignin [37]. To ensure consistent performance of lignin-based 3D printing materials, lignin extraction and modification processes need standardization to control molecular weight distribution and functional group uniformity.
Lignin decomposes into rigid carbon at high temperatures, but its use is limited by high thermal transition temperatures and flow resistance during processing [38]. To improve its melt and flow properties, lignin must be blended with other polymers. Lignin contains active functional groups like phenolic hydroxyl, carboxyl, carbonyl, and methyl groups. Its aromatic ring structure provides antioxidant properties and thermoplasticity, making it suitable for developing 3D lignin-based bio-inks with high strength, low shrinkage, and functional properties [39]. Additionally, the numerous aromatic rings and conjugated functional groups in lignin’s molecular structure give it unique optical properties, such as aggregation-induced emission, UV absorption, and sustainable photothermal conversion [40]. The combination of hydrophilic functional groups (e.g., carboxyl and hydroxyl) with the hydrophobic aromatic backbone and carbon chains gives lignin unique amphiphilic properties [41]. This allows lignin to blend or crosslink with various materials, especially 3D printing materials like PLA and polyvinyl alcohol (PVA), improving material performance, reducing production costs, and decreasing plastic use.
Adding too much lignin can cause lignin particle aggregation and reduce mechanical properties [42]. Modifying lignin by introducing more active sites (e.g., phenolic hydroxyl or ester groups) and adding plasticizers can improve its compatibility and dispersion in polymer matrices. This enables high-lignin-content composites, enhancing 3D printing material performance. Zhang et al. [43,44] increased phenolic hydroxyl content in lignin to promote hydrogen bonding with polyamide 12, improving dispersion. Mohan et al. [45] esterified lignin and used it as a filler in PLA, acting as a plasticizer to enhance PLA’s performance, reduce costs, and improve biodegradability, making it more environmentally friendly. Ren [46] used citrate ester plasticizers to toughen PLA, improving lignin dispersion and PLA chain mobility, achieving a PLA/lignin 3D printing biocomposite with 50 wt% lignin content [46].
3 3D Printing Techniques of Plant Fiber-Based Materials
As 3D printing technology advances, plant fiber-based materials are increasingly used in various printing methods, creating sustainable and functional manufacturing pathways. Table 1 compares the basic features of two common 3D printing technologies. Table 2 summarizes the roles of plant fiber materials in these technologies, key technical parameters, and typical application examples.

3.1 Extrusion-Based 3D Printing
Extrusion-based 3D printing works by extruding material from a nozzle under constant pressure and depositing it along a digital path for shaping. It is widely used due to its simplicity and low cost [47]. Processes such as FDM, DIW, and micro-extrusion 3D bioprinting all fall under the material extrusion category.
3.1.1 Fused Deposition Modeling (FDM)
FDM is the most widely used 3D printing technology. The ink used in FDM-based extrusion processes is typically made from thermoplastic materials, which offer good flowability and processability. PLA is a commonly used polymer material in 3D printing due to its environmental friendliness, renewability, biocompatibility, and biodegradability [48]. Compositing cellulose with PLA can improve the brittleness and poor thermal stability of PLA [49]. Zhang et al. [50] developed a green and efficient dispersion process based on the mixing of CNF aqueous suspension and PLA micropowder followed by air-drying. This process, combined with silane coupling agents and surface modification using polyethylene glycol (PEG), was used to prepare 3D printing filaments through twin-screw extrusion, as shown in Fig. 2. The experiments demonstrated that the modified CNF significantly improved the melt flowability and mechanical properties of PLA (attributed to the uniform dispersion of CNF, increased PLA crystallinity, and reduced defects). Touchard et al. [51] increased the tensile strength of polyether block amide (Pebax®) by four times and the elastic modulus by 20 times by continuous regenerated cellulose fibres (RCF), and processed this composite material into 3D printing filaments.

Figure 2: Manufacturing of CNF/PLA filaments and 3D printing [50]. Reprinted with permission from reference [50]. Copyright 2023, copyright Elsevier
3.1.2 Direct Ink Writing (DIW)
DIW uses viscoelastic ink or slurry to deposit materials layer by layer through a nozzle or fine orifice to build 3D objects. Compared to traditional manufacturing, DIW allows precise deposition of viscoelastic ink to create custom geometries layer by layer, improving the efficiency of prototyping and manufacturing functional parts [52]. DIW inks are typically hydrogels, pastes, dispersions, or solutions made from synthetic polymers, natural polymers, and additives [53,54].
Increasing the ink viscosity or using crosslinkable inks, such as those induced to undergo sol-gel transitions by light crosslinking, temperature changes, pH variations, or ion crosslinking, can improve the shape fidelity of DIW-printed products. However, increasing the ink viscosity often reduces the printability [55]. CNF, due to their shear-thinning and thixotropic properties, biocompatibility, and renewability, have been widely explored as components in DIW inks [56]. Yu et al. [57] synthesized an aqueous polyurethane ink for DIW 3D printing by incorporating CNF, which enabled the direct printing of complex, monolithic elastomeric structures at room temperature, while maintaining the designed shape.
3.2 Vat Photopolymerization Additive Manufacturing (VPAM)
VPAM 3D printing has the advantages of high resolution and fast manufacturing [58]. It uses photosensitive resins as printing materials, which are cured by UV or visible light exposure to build precise products layer by layer [59], as shown in Fig. 3A.

Figure 3: (A) Schematic of VPAM 3D printing technology [65]; (B) SLA 3D printed lignin-based samples [66]; (C) DLP 3D printed photopolymerizable allyl cellulose (AC) derivative hydrogels [62]. Reprinted with permission from reference [65]. Copyright 2024, copyright Elsevier
SLA uses a photopolymerization process, selectively curing liquid photopolymer resin with UV lasers or other light sources [60]. SLA is favored for its ability to create smooth, detailed, and complex geometric shapes, making it particularly suitable for product development, rapid prototyping, and the creation of fine models in various industries [61]. Fig. 3B shows a lignin-based sample printed using SLA 3D printing.
3.2.2 Digital Light Processing (DLP)
DLP technology is widely used for creating high-precision, small-sized objects, with accuracy reaching even the micron level. Compared to other 3D printing methods, the advantages of DLP printing lie in its high precision and relatively short printing time. The resin used for DLP printing is a liquid resin that forms a crosslinked polymer material when triggered by UV light. However, most commercially available UV-curable resins are petroleum-based materials, and the products printed with these resins cannot be reprocessed or degraded, posing environmental pollution at the end of their lifecycle. Silva et al. [62] developed a fully cellulose resin modified through alkali/urea dissolution and photopolymerization, using Avicel® and industrial cellulose pulp. This innovation overcomes the limitations of fossil-based materials in sustainable DLP 3D printing. The resulting low-concentration (2.5–5 wt%) single-component resin produces dimensionally stable hydrogels with rapid curing kinetics (compressive stress ≤ 135 kPa), excellent water retention (427%), pH stability, hydrolytic stability (>4 weeks), and fibroblast cell compatibility, as shown in Fig. 3C. Wan et al. [63] used lignin-derived vanillin and guaiacol to prepare pure bio-based resins for DLP printing. Johnson et al. [64] combined lignin methacrylate (LMA), vanillin, and soybean oil to prepare a DLP resin with dynamic functionality, enabling a self-healing process.
4 Quality Evaluation of 3D Printed Products
Quality evaluation of 3D printing is a multidimensional process that involves performance assessment across various aspects. Accurate quality evaluation not only helps optimize the printing process but also ensures the reliability and durability of 3D printed parts in real-world applications.
Mechanical properties are key factors that determine the practical value of 3D printed products, directly affecting core metrics such as strength, toughness, fatigue resistance, and environmental adaptability. They directly affect strength, toughness, fatigue resistance, and environmental adaptability. In complex conditions, such as aerospace [77], biomedical [78], or industrial manufacturing [79], poor mechanical properties can lead to structural failure or loss of function. For example, aerospace requires a high strength-to-weight ratio [80] and fatigue resistance, while biomedical applications need suitable elastic modulus and biocompatibility [81]. Evaluating the mechanical properties of 3D-printed products is necessary to optimize printing parameters (e.g., layer thickness, temperature, infill rate) [82,83] and to verify the compatibility of materials and processes. Common evaluation methods include standardized mechanical tests such as ASTM D638 tensile tests [84,85], ASTM D790 bending tests [86], and Dynamic Mechanical Analysis (DMA) [87].
In recent years, plant fiber-based composites have become a research focus for enhancing the mechanical properties of 3D printed products due to their sustainability and lightweight characteristics [88]. The mechanical properties of 3D-printed plant fiber-based products are influenced by multiple factors, including process parameters [89] (e.g., layer thickness, printing speed [90], build orientation [91]), material properties, and post-processing techniques [92,93]. Plant fiber-based composites are a research focus for enhancing 3D-printed product performance due to their sustainable and lightweight characteristics [88]. Trivedi [94] found that varying cellulose nanocrystal (CNC) content (0–5 wt%) affects the crystallization and mechanical properties of PLA. Adding 1 wt% CNC increased tensile strength by 22.3% and modulus by 64.17%, while 5 wt% CNC improved impact strength by 53.95% and dynamic storage modulus by 34.50%, with a significant increase in dynamic mechanical properties (loss modulus increased by 53.95%) without affecting the glass transition temperature. Ambone [95] added 1 wt% CNF to PLA, increasing the tensile strength of 3D-printed PLA by 84% and elastic modulus by 63%. Vidakis [96] used SLA 3D printing to create CNF-reinforced nanocomposites, studying the effects of CNF content (0–1.0 wt%) on mechanical, thermal, and microstructural properties. Results showed that 1.0 wt% CNF increased tensile strength by over 100%, as shown in Fig. 4B, with significant improvements in impact toughness and Vickers hardness (though brittleness and rigidity also increased). Qiao et al. [97] developed continuous bamboo fiber-reinforced polyethylene (T-CBF/PE) composites using alkali treatment (to improve bamboo fiber surface wettability) combined with 3D printing and localized hot-press impregnation. Alkali treatment enhanced fiber-matrix interface bonding, increasing composite fiber tensile strength to 15.6 MPa (102.6% higher than pure PE). The 3D-printed bulk tensile strength and elastic modulus reached 1.77 and 2.76 times that of pure PE, respectively, as shown in Fig. 4A. These studies show that adjusting fiber type, content, or fiber-matrix interface compatibility can significantly improve the mechanical properties and thermal stability of plant fiber-reinforced materials, enhancing the overall performance of 3D-printed products.


Figure 4: (A) Tensile stress-strain curve, tensile strength, and toughness of bamboo fiber [97]; (B) Relationship between Average Tensile Strength (MPa) and Average Tensile Elastic Modulus (MPa) with CNF Concentration for Biomed Clear and its CNF nanocomposites [96]
The thermal properties of 3D printing materials are critical for their performance, especially in applications requiring durability under high temperatures or significant thermal stress [98]. Common evaluation methods include thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) [99]. Thermal analysis (TA) is a key method for assessing the thermal properties of polymer materials. Important parameters include the initial decomposition temperature (Ti) and the temperature at 5% mass loss (T5%). In multi-stage thermal degradation systems, Ti is heavily influenced by the slope of the thermogravimetric curve, so T5% is considered more stable and reliable. The glass transition temperature (Tg), measured by DSC, indicates the point where a material shifts from a rigid state to a rubbery, more flexible state. It reflects the material’s ability to maintain structural integrity and dimensional stability [100].
In recent years, researchers have improved the thermal stability of 3D printing materials by blending modified lignin or its derivatives with various polymer matrices. TGA has shown good results in evaluating the thermal properties of these composites. For example, Kassaun et al. [101] found that using lignin-caprolactone composites in FDM 3D printing improves printing performance. TGA results showed that lignin-caprolactone grafting copolymerization increased the thermal decomposition temperature (To), 50% mass loss temperature (T50%), and maximum weight loss rate temperature (DTGmax), indicating improved thermal stability. Wan et al. [102] prepared lignin nanoparticles (LNPs) using ultrasonic treatment and added them to an acrylate matrix to create LNPs/acrylate UV-curable resin. After curing, the mechanical, thermal, and morphological properties were studied. TGA curves showed that increasing LNP content improved the heat resistance of LNPs/acrylate composites, with residual content rising from 6.9 to 8.4 wt%. This suggests that LNPs can form an effective carbonized layer at high temperatures to protect the LNPs/acrylate composite.
4.3 Inter Layer Bonding Strength
Interlayer bonding strength is a key parameter that determines the structural integrity and mechanical properties of 3D printed products. It directly affects delamination resistance, load-bearing efficiency, and anisotropic behavior. Conventional evaluation methods include peel tests, interlayer shear strength tests (three-point bending tests), and microscopic morphology analysis (e.g., scanning electron microscopy to observe interlayer voids, fiber distribution, and interface bonding conditions) [103,104]. For most composite materials, the interface region is one of the most significant challenges. Weak interfaces cause stress concentration, leading to defects and damage in the material. Internal defects caused by weak bonding between fibers and the polymer matrix or voids created by gas diffusion are the primary reasons for weak interfaces [105].
Surface modification is one of the most common methods to improve interface bonding performance. Choosing appropriate polymer matrices and controlling the appropriate fiber addition ratio can help improve interface performance and mechanical strength [106]. Material modifications that enhance interfacial flexural strength, appropriate compatibilizers, and natural fibers can also improve interface compatibility, thereby influencing the mechanical performance of printed parts [107]. Ye et al. [108] designed a lignin-g-maleic anhydride-g-PLA (LMP) using a two-step method to act as a bridge, improving intermolecular interactions in P/L composites. Fig. 5A shows the reinforcement mechanism of LMP in P/L composites, enhancing compatibility. For a composite with 20% lignin, adding 5 wt% LMP increased tensile strength by 22%, elongation at break by 68%, and toughness by 114%. Microscopic morphology analysis confirmed the interface strengthening mechanism. As shown in Fig. 5B, as LMP content increased from 3% to 5%, the fracture interface of P/LMP/L became smoother. Demir et al. [109] used alkali treatment, hydrogen peroxide, stearic acid, and ionic liquids to modify the surface of cotton, flax, and jute fabrics. Combined with FDM printing and mechanical testing of interfacial adhesion (DIN 53530 standard), the effects of fiber type and treatment on PLA-fabric interfacial bonding strength were studied. Alkali treatment significantly improved the bonding strength of jute and cotton fabrics (plain weave structures showed the best adhesion), while hydrogen peroxide reduced flax adhesion. Stearic acid and ionic liquid treatments generally weakened interfacial bonding. Battisto et al. [110] treated TEMPO-oxidized CNFs (TOCNFs) with the hydrochloride salt of lauroyl arginate ethyl ester (LAE⋅HCl), to improve compatibility with the resin interface. By comparing the rheological curves of resin with 1% (w/w) coated and uncoated TOCNFs, as shown in Fig. 5C, the coated resin exhibited more uniform dispersion. Even with 8% (w/w) coated TOCNFs, the resin viscosity (at 30 Hz shear rate) remained lower than that with 1% (w/w) uncoated TOCNFs. This indicates that the LAE coating effectively reduced CNF aggregation, improving TOCNF dispersion in the composite resin and preventing sharp viscosity increases at high concentrations.

Figure 5: (A) The enhancement mechanism of LMP in P/L composites [108]; (B) Fracture surfaces of 3D-printed tensile models of composites containing 3%, 5%, and 10% LMP [108]; (C) Rheological curves of base commercial resin (gray square), composite resins containing 1%, 2%, 3%, 4%, 6%, and 8% (w/w) coated TOCNF (green circles with increasing saturation), and composite resin containing 1% (w/w) uncoated TOCNF (red circles with no fill) [110]. Reprinted with permission from reference [108]. Copyright 2023, copyright Elsevier
4.4 Biodegradability and Sustainability
Biodegradability is a core indicator for evaluating the environmental friendliness and sustainability of 3D printed products. It directly affects their ability to be converted into harmless products through microbial action at the end of their lifecycle. Conventional evaluation methods include standard composting tests (ASTM D5338 [111]), enzymatic degradation experiments (lipase, protease catalyzed degradation) [112], and soil burial weight loss analysis [113], among others.
Plant fiber-based composites, due to their natural degradable properties, have become a research focus for improving the biodegradability of 3D printed products. Murillo-Morales et al. [114] used Enzymatically modified lignin (EL) as a nucleating agent to prepare PLA/TPU blends. The experiments showed that the addition of EL increased the elastic modulus by up to 2.5 times compared to the control group. The biodegradation rate after 6 months of soil burial reached 15%, as shown in Fig. 6A. The printed part also exhibited a smooth surface, high geometric accuracy, and an adjustable wood-like color tone. Zhang et al. [115] found that adding 0.5 wt% CNF could accelerate the degradation of PLA (with the number-average and weight-average molecular weight reductions being 7.96% and 4.91% higher than pure PLA, respectively). This addition increased the tensile modulus by 18.4%, but decreased the strength by 7.4% (attributed to weak interfacial bonding). An equivalent degradation mapping relationship was established between 60 days at 37°C and 14 days at 50°C. Fig. 6B shows the macroscopic and microscopic morphology of printed products before and after degradation at 50°C.

Figure 6: (A) Representative samples and weight loss percentage of 3D-printed dog-bone specimens degraded in soil [114]. (B) Macroscopic and microscopic morphology of printed products before and after degradation at 50°C. (a,c) Macroscopic and microscopic morphology of pure PLA and (b,d) CNF/PLA composites before and after degradation at 50°C [115]. Reprinted with permission from reference [114]. Copyright 2023, copyright Elsevier
Life cycle assessment (LCA) is an effective tool for evaluating environmental impacts related to raw material extraction, manufacturing, use, and disposal of final products. LCA of 3D printing materials PLA, ABS, and PETG shows different environmental impacts. PETG has the least impact on global warming, water use, and ecotoxicity. PLA is biodegradable but harms water resources and freshwater ecosystems. ABS has the greatest environmental impact, especially in fossil fuel use and human health risks [116]. According to Foroughi et al. [23], plant fiber-based composites, such as nanocellulose and cellulose fibers, are more sustainable. LCA shows that enzymatic and TEMPO oxidation processes use less energy and produce fewer emissions than methods relying heavily on chemicals, though water use remains a concern. Full LCA of cellulose composites (e.g., epoxy/BC and CNF) indicates lower environmental impact than PLA and glass fiber-reinforced polypropylene, especially with recycling and reduced material demand. Plant fiber composites are better suited for 3D printing than PLA, ABS, and PETG because they are renewable, biodegradable, and cause less environmental harm during production, making them an ideal choice for sustainable manufacturing.
5 Plant Fiber-Based Materials in 3D Printing Applications
3D printing technology is increasingly being applied across various industries, especially in sectors such as biomedicine, food, packaging, and electronics [117].
5.1 Biomedicine: Tissue Engineering and Wound Repair
3D bioprinting is a product of the fusion of bio-manufacturing, additive manufacturing, tissue engineering, and regenerative medicine [118]. 3D bioprinting methods use computer-aided programming technology to create precise biological structures, which can accurately simulate the natural anatomical structure of organs/tissues [119].
Tissue engineering is a branch of biomedical engineering focused on the development of tissue regeneration scaffolds, organoid culture, and both in vitro and in vivo therapies [120]. Compared to traditional tissue engineering techniques, 3D printing technology offers more precise control. Traditional methods struggle to accurately control pore size, anatomical design, and the distribution of cells and growth factors, all of which are crucial for the formation of new tissue. Through 3D printing, the structure and function of hydrogel inks can be precisely controlled to create scaffolds with specific pore sizes and anatomical designs, providing strong support for new tissue formation, as shown in Fig. 7.

Figure 7: 3D bioprinted scaffold used for bone tissue engineering [14]. Reprinted with permission from reference [14]. Copyright 2021, copyright Elsevier
The application of 3D printing in tissue engineering must not only meet printability requirements to ensure shape fidelity and resolution of printed structures but also possess biological activity to support cell adhesion, proliferation, differentiation, and other functions [81]. CNCs can effectively balance the mechanical and biological properties of the scaffold [121]. Goyal et al. [122] constructed a polyacrylamide/alginate/cellulose nanofiber (PAM/ALG/CNF) double-network (UV/Fe3+ crosslinked) composite hydrogel. By combining extrusion printing with finite element simulation, the study systematically explored the regulatory mechanism of CNF content (optimal 3 wt%) and fiber orientation on anisotropic mechanical properties. The results showed that the Fe3+ crosslinked PAM/ALG1.5/3CNF composite material exhibited significantly enhanced tensile strength (~285 kPa) and toughness (~200 kJ/m3), along with excellent swelling, thermal stability, and cell compatibility. Monfared et al. [123] improved the mechanical properties of CNF hydrogels by controlling printing viscosity (increased by more than 1.5 times) and using a visible light-triggered PEG photopolymerization dual strategy. This created 3D printed scaffolds with an adjustable Young’s modulus of 10–30 kPa. Kumar et al. [124] regulated the shear-thinning behavior of alginate (SA) hydrogel ink by the carboxyl interaction between CNCs and xanthan gum (XG), enhancing the rheological performance and printing accuracy, and the resulting ink exhibited both mechanical properties and cell activity.
The structural differences in cellulose may affect the printability, cartilage formation ability, or biocompatibility of bioinks. Therefore, Jovic et al. [125] selected three types of nanocellulose (fibrous NFC, crystalline NCC, and mixed NCB) derived from pulp with varying pore geometry and mechanical properties to prepare bioinks (75:25 v/v) with alginate. The study systematically evaluated the printing resolution, shape fidelity, cartilage generation ability, and biocompatibility of the inks. The results showed that NFC exhibited the best printing resolution, while NCB had the best shape fidelity after printing. NCC significantly upregulated the expression of cartilage-related genes and promoted extracellular matrix synthesis. Both NCC and NCB showed enhanced cell proliferation and metabolic activity within 21 days, indicating their functional adaptability and biological activity advantages in cartilage bioprinting.
Wound healing is a complex physiological process that occurs after trauma or injury, which involves the removal of damaged tissue and the regeneration of healthy tissue to restore the skin’s integrity [126]. Wound dressings form a protective barrier to prevent contamination of the wound, maintain appropriate humidity, regulate the release of exudates, and promote wound healing. Fig. 8A shows the process of printing wound dressings using 3D printing technology. Wound dressings embedded with bioactive molecules or nanoparticles can possess antibacterial effects and accelerate wound repair. Fig. 8B shows the preparation of PCL/lignin-based wound dressings containing curcumin and D-panthenol using 3D printing technology. These dressings exhibit excellent antioxidant and antibacterial properties, promoting wound healing. However, when multiple bioactive components are embedded, it becomes difficult to independently control their release. Alizadehgiashi et al. [127] designed hydrogel dressings with different structures using 3D printing technology, and by regulating drug concentration, adjusting regional distribution, and controlling the swelling and degradation of the hydrogel matrix, the independent and controlled release of multiple active ingredients was achieved.

Figure 8: (A) Schematic of 3D printed wound dressing manufacturing [127]; (B) Comparison of 3D printed antimicrobial wound dressing and the status of rat wounds on day 13, with and without the dressing [15]
5.2 Food: Personalized Nutrition and Rheological Control
3D printing technology has gradually become a hotspot in food research due to its advantages, such as the ability to achieve diverse colors, innovative shape designs, and comprehensive customization of nutritional components [128]. DIW technology, when printing food pastes, has the drawback of insufficient structural self-supporting ability due to the low viscosity of the raw materials. Armstrong et al. [129] used CNCs as rheological modifiers for food-based inks such as spinach puree, tomato puree, and applesauce, successfully achieving the manufacture of high-precision self-supporting edible structures, as shown in Fig. 9. Zhang et al. [16], addressing the dietary needs of patients with swallowing disorders, developed a protein/polysaccharide aqueous food ink based on CNC. The addition of 1.5 wt% CNC significantly increased the ink’s viscosity, yield stress, and self-supporting capability, which not only improved the shape fidelity of the printed structures but also ensured good shape stability even after 96 h of storage at 4°C.

Figure 9: Schematic of the 3D printing food process [129]. Reprinted with permission from reference [129]. Copyright 2022, copyright Elsevier
5.3 Packaging: Smart Monitoring and High Barrier Properties
Packaging films, as physical barriers, create a microenvironment for food, helping to slow down biological and chemical reactions [130]. Dermol et al. [131] developed a 3D printable hydrogel system by optimizing the ratio of bacterial nanocellulose (BNC) to cationic starch, creating a film that meets packaging requirements with high strength, low density, and excellent barrier properties.
With the rise of healthy diet management, smart packaging systems have attracted wide attention due to their ability to enable rapid and intuitive quality detection during food storage and transportation [132]. The customization capability of coaxial 3D printing technology allows for precise design and regulation of the material’s microstructure according to different application scenarios, thus obtaining the customized structures and properties required for packaging materials. Zhou et al. [133] was the first to apply coaxial 3D printing in smart food packaging, developing fruit preservation and visual monitoring labels based on nanocellulose. Through coaxial 3D printing, controlled release of 1-methylcyclopropene was achieved, extending the shelf life of lychees by 6 days, as shown in Fig. 10A. Later, Zhou et al. [134] used coaxial 3D printing to create food packaging aerogels with dual functions of antimicrobial and buffering properties, as shown in Fig. 10B, for buffering packaging used in the storage and transportation of fruits and vegetables. Popoola et al. [17] used 3D printing to produce food freshness indicator labels (Fig. 10C) made from a composite of 8% sodium alginate, 10% gelatin, and CNC. The addition of CNC enhanced the material’s mechanical properties and stabilized pH-responsive dyes. The film sensitively indicated the freshness of meat and fish through color changes within 3 days at room temperature, while also exhibiting flexibility, shape-memory, and biodegradability.

Figure 10: (A) Schematic of the preparation process of 3D printed CNF-based labels and their application in food preservation and monitoring [133]; (B) Process diagram of 3D printed dual-function food packaging [134]; (C) 3D printed food freshness detection labels [17]. Reprinted with permission from reference [133]. Copyright 2021, copyright Elsevier
5.4 Electronics and Energy: Flexible Devices
With the development of flexible electronics and energy storage devices, the sustainability, customizability, and environmental friendliness of materials have become key issues. CNF, due to its excellent mechanical strength, flexibility, and renewability, has been widely applied in 3D printing flexible devices. Flexible sensors play an important role in wearable health monitoring and smart medical devices. However, traditional hydrogels, due to their high viscosity, pose challenges in printing and are prone to water loss and structural instability after molding [135]. To address this issue, Guo et al. [136] proposed a hybrid printing process combining DIW and Vat Photopolymerization (VPP). By introducing cellulose to regulate the mechanical and rheological properties of hydrogels and combining dual-core coaxial printing technology, hydrogel-elastomer composite packaging was achieved. This breakthrough overcame the printing limitations of low-viscosity hydrogels, expanding the adjustable viscosity range of the system while extending the lifespan of hydrogel sensors. The 3D printed hydrogel can be applied to flexible sensing in parts such as fingers and wrists, showing excellent mechanical adaptability and environmental stability, as shown in Fig. 11A.
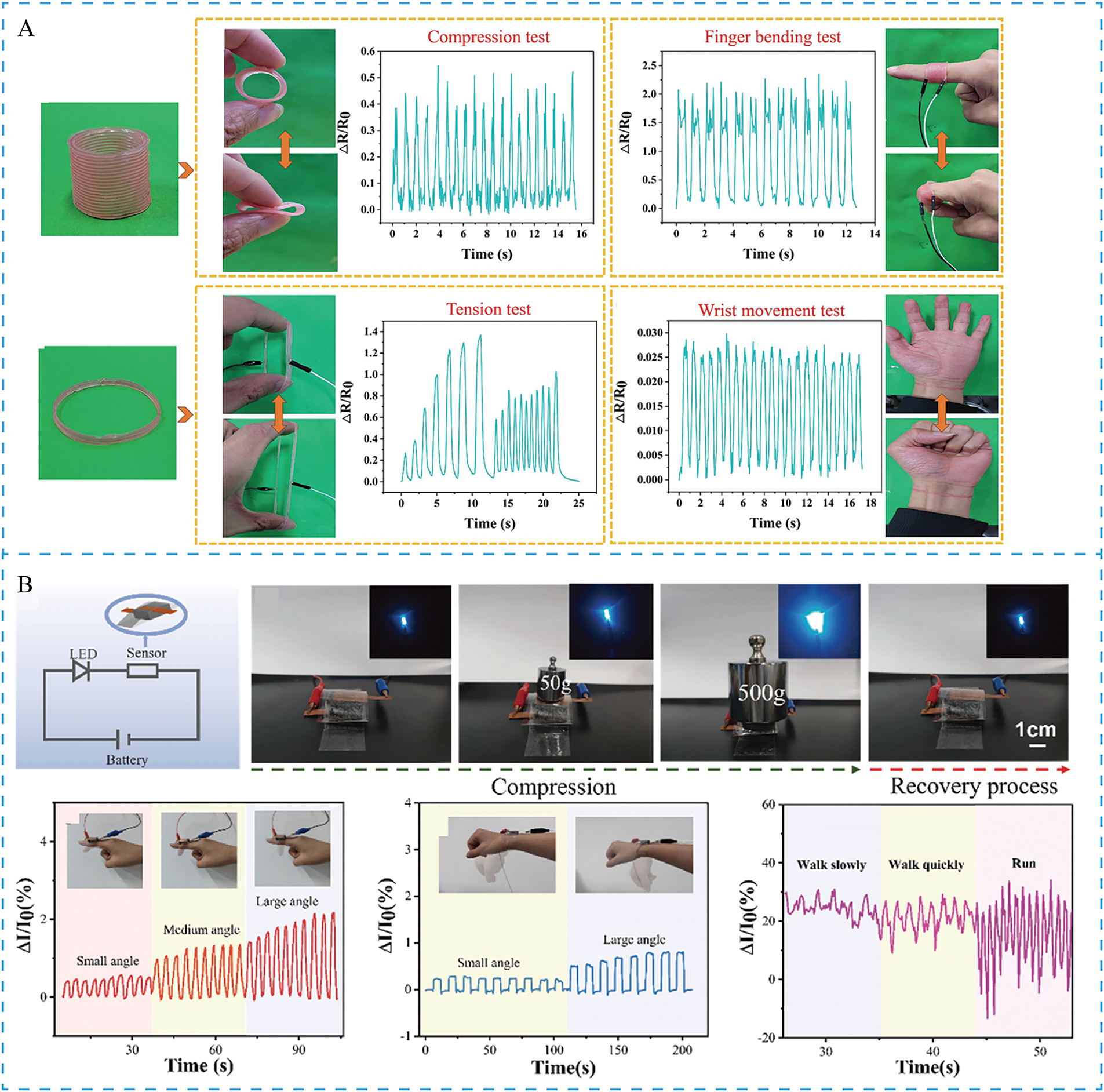
Figure 11: (A) Sensor applications based on different printing structures [136]; (B) Application of LCNT/CNF-15 composite aerogel as a piezoresistive pressure sensor [138]. Reprinted with permission from reference [136]. Copyright 2025, copyright Elsevier
Additionally, Zhan et al. [137] and others developed a hydrogel sensor based on a PVA/polyvinylpyrrolidone (PVP) composite wood pulp nanocellulose (CNF-P-Na+) using printing technology. By integrating stress-sensitive nanostructured silica, the sensor achieved high sensitivity (able to respond to micro-movement signals), high stretchability (>100% strain), and long-term stability (>1000 cycles). This sensor shows great potential for monitoring the rehabilitation of patients with nerve damage, capable of real-time detection of electromyography (EMG) signals, providing a new material option for portable, low-cost EMG-BF (electromyography biofeedback) therapeutic devices.
Although nanocellulose has good biocompatibility and flexibility, its low electrical conductivity limits its application in flexible electronics. To improve its conductivity, Amini et al. [138] used lignin-derived carbon nanotubes (LCNT) as conductive components and combined them with CNF, using 3D printing technology to create flexible aerogel sensors. The resulting LCNT/CNF composite aerogel (mass ratio 85:15) exhibited a uniform porous structure and stable conductive network, providing stable signal output within a pressure range of 0.2–9.8 kPa, maintaining 92.5% stress stability after 1000 cycles of compression. This sensor can be used for monitoring biological signals, such as finger and wrist bending detection and gait analysis, as shown in Fig. 11B.
Soft robotics, known for their high flexibility and excellent biocompatibility, have been widely used in medical and environmental monitoring applications. However, traditional silicone-based soft actuators are non-degradable and can cause environmental pollution. Therefore, Müller et al. [18] developed a 3D printable biodegradable composite material based on CNF reinforced with gelatin. By incorporating CNF to regulate the material’s rheological properties and mechanical performance (with tensile modulus improved to the level of silicone), a pneumatic actuator was successfully constructed, achieving an 80° bending angle and 0.1 N blocking force under 15 kPa pressure. Compared to traditional silicone soft actuators, this biodegradable actuator not only has self-healing capabilities but also degrades naturally in soil, demonstrating excellent environmental adaptability.
6 Future Challenges and Prospects
Plant-fiber-based 3D printing technology shows great potential in sustainable manufacturing and versatile applications. However, its future development faces challenges in material performance, process accuracy, and large-scale production. To address these issues and advance the technology, clear research priorities and interdisciplinary strategies are needed. The following sections outline a structured roadmap and interdisciplinary approaches to highlight key development directions and breakthroughs for achieving high-performance, sustainable 3D printing applications.
6.1 Structured Roadmap for Research Priorities
(1) Development of Hybrid Composite Materials: Future material innovation should focus on creating complex and responsive hybrid composites by combining plant fibers (e.g., cellulose, lignin) with synthetic polymers or inorganic nanoparticles to balance sustainability and performance. For example, integrating cellulose nanofibers (CNF) and lignin with conductive nanoparticles can improve mechanical strength, thermal stability, and conductivity, enabling applications in flexible sensors, tissue engineering, and high-performance biocomposites. Efforts should prioritize improving compatibility and synergy between components to ensure consistent performance across applications like wearable devices and biomedical scaffolds. Additionally, using 4D printing technology [139] to develop plant-fiber-based materials that respond to external stimuli (e.g., temperature, light, humidity) will create new opportunities for wearable devices, soft robotics, and smart packaging. These responsive materials will enhance the adaptability of 3D-printed products to dynamic environments and expand the potential of high-performance biocomposites in diverse applications.
(2) Optimization of Printing Parameters: Improving the accuracy and efficiency of 3D printing processes requires systematic optimization of printing parameters. For extrusion-based technologies like FDM, future research should focus on adjusting nozzle diameter, layer thickness, printing speed, and temperature to enhance printing precision, interlayer adhesion, and material compatibility. For photopolymerization technologies (SLA/DLP), the focus should be on increasing printing speed, expanding material compatibility (e.g., incorporating plant-fiber-based resins), and achieving higher resolution for complex structures. These improvements will support applications in industrial manufacturing and biomedical fields where precision and functionality are critical.
(3) Exploration of New Fiber Processing Methods: Developing innovative fiber processing methods can enhance the functionality and printability of plant-fiber-based materials. Techniques such as plasma treatment, enzymatic modification, or advanced chemical grafting (e.g., silanization or acetylation) should be explored to improve fiber-matrix adhesion, thermal stability, and hydrophobicity. For instance, enzymatic treatment can reduce energy-intensive processing steps while improving fiber compatibility with polymer matrices, leading to better dispersion and mechanical performance in 3D-printed products. These new processing methods will broaden the use of plant fibers in smart packaging, soft robotics, and environmental engineering.
6.2 Interdisciplinary Approaches and Technological Integration
Combining computational materials science, machine learning, and synthetic biology will accelerate progress in plant-fiber-based 3D printing technology. Machine learning can predict material behavior under different printing conditions, enabling precise parameter adjustments to improve printing accuracy and material efficiency. Synthetic biology can engineer microbial strains to produce high-quality cellulose and lignin, reducing reliance on traditional plant resources and improving material consistency. Multi-scale simulations can optimize printing processes by modeling fiber-matrix interactions at various scales. These interdisciplinary approaches will drive intelligent design and process optimization, promoting the use of plant-fiber-based 3D printing technology in sustainable manufacturing and other fields.
Plant fiber-based materials in 3D printing show great potential for sustainable manufacturing, offering new solutions for biomedicine, food, packaging, and electronics and energy fields. Cellulose and lignin, as renewable and biodegradable natural polymers, improve mechanical properties, thermal stability, and printability through chemical modification and composite material design. 3D printing processes like FDM, DIW, SLA, and DLP enable efficient production of complex structures with plant fiber-based materials. Optimizing process parameters and fiber treatment methods enhances product performance and precision. These 3D-printed products exhibit excellent mechanical properties, thermal stability, interlayer bonding strength, and biodegradability, with notable advantages in tissue engineering, wound healing, personalized food, smart packaging, and flexible electronic devices. However, challenges remain in material consistency, process compatibility, and large-scale production.
In the future, plant fiber-based 3D printing should focus on developing hybrid composites, optimizing printing parameters, and exploring new fiber treatment methods. Combining plant fibers with synthetic polymers or inorganic nanoparticles can balance sustainability and high performance. Introducing 4D printing will advance responsive material applications. Interdisciplinary approaches, such as computational materials science, machine learning, and synthetic biology, will speed up material design and process optimization. Multi-scale simulations will support fiber-matrix interaction improvements. These advances will promote the widespread use of plant fiber-based 3D printing in sustainable manufacturing, laying the foundation for high-performance, eco-friendly additive manufacturing. Despite challenges, this technology’s environmental friendliness and versatility make it a key driver of green manufacturing and innovative applications, with a promising future.
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization, Jiangping Yuan; methodology, Weili Liu and Fayi Hao; resources, Jiangping Yuan; writing—original draft preparation, Weili Liu; writing—review and editing, Fayi Hao and Jiangping Yuan; supervision, Jiangping Yuan; project administration, Fayi Hao. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Jandyal A, Chaturvedi I, Wazir I, Raina A, Ul Haq MI. 3D printing—a review of processes, materials and applications in Industry 4.0. Sustain Operat Comput. 2022;3(43):33–42. doi:10.1016/j.susoc.2021.09.004. [Google Scholar] [CrossRef]
2. Pei E, Bernard A, Gu D, Klahn C, Monzón M, Petersen M, et al. Springer handbook of additive manufacturing. Cham, Switzerland: Springer Nature; 2023. [Google Scholar]
3. Żur A, Żur P, Michalski P, Baier A. Preliminary study on mechanical aspects of 3D-printed PLA-TPU composites. Materials. 2022;15(7):2364. doi:10.3390/ma15072364. [Google Scholar] [PubMed] [CrossRef]
4. Sztorch B, Konieczna R, Pakuła D, Frydrych M, Marciniec B, Przekop RE. Preparation and characterization of composites based on ABS modified with polysiloxane derivatives. Materials. 2024;17(3):561. doi:10.3390/ma17030561. [Google Scholar] [PubMed] [CrossRef]
5. Fales A, Černohlávek V, Štěrba J, Dian M, Suszyński M. Innovative approaches to material selection and testing in additive manufacturing. Materials. 2025;18(1):144. doi:10.3390/ma18010144. [Google Scholar] [PubMed] [CrossRef]
6. Talecka J, Kluczyński J, Jasik K, Szachogłuchowicz I, Torzewski J. Strength and electrostatic discharge resistance analysis of additively manufactured polyethylene terephthalate glycol (PET-G) parts for potential electronic application. Materials. 2024;17(16):4095. doi:10.3390/ma17164095. [Google Scholar] [PubMed] [CrossRef]
7. Valvez S, Silva AP, Reis PNB. Optimization of printing parameters to maximize the mechanical properties of 3D-printed PETG-based parts. Polymers. 2022;14(13):2564. doi:10.3390/polym14132564. [Google Scholar] [PubMed] [CrossRef]
8. Acierno D, Patti A. Fused deposition modelling (FDM) of thermoplastic-based filaments: process and rheological properties—an overview. Materials. 2023;16(24):7664. doi:10.3390/ma16247664. [Google Scholar] [PubMed] [CrossRef]
9. León-Calero M, Reyburn Valés SC, Marcos-Fernández Á, Rodríguez-Hernandez J. 3D printing of thermoplastic elastomers: role of the chemical composition and printing parameters in the production of parts with controlled energy absorption and damping capacity. Polymers. 2021;13(20):3551. doi:10.3390/polym13203551. [Google Scholar] [PubMed] [CrossRef]
10. Marakana PG, Dey A, Saini B. Isolation of nanocellulose from lignocellulosic biomass: synthesis, characterization, modification, and potential applications. J Environ Chem Eng. 2021;9(6):106606. doi:10.1016/j.jece.2021.106606. [Google Scholar] [CrossRef]
11. Zheng Y, Moreno A, Zhang Y, Sipponen MH, Dai L. Harnessing chemical functionality of lignin towards stimuli-responsive materials. Tren Chem. 2024;6(2):62–78. doi:10.1016/j.trechm.2023.12.001. [Google Scholar] [CrossRef]
12. Garemark J, Perea-Buceta JE, Rico del Cerro D, Hall S, Berke B, Kilpeläinen I, et al. Nanostructurally controllable strong wood aerogel toward efficient thermal insulation. ACS Appl Mat Interf. 2022;14(21):24697–707. doi:10.1021/acsami.2c04584. [Google Scholar] [PubMed] [CrossRef]
13. Albelo I, Raineri R, Salmon S. Materials and methods for all-cellulose 3D printing in sustainable additive manufacturing. Sustain Chem. 2024;5(2):98–115. doi:10.3390/suschem5020008. [Google Scholar] [CrossRef]
14. Dutta SD, Hexiu J, Patel DK, Ganguly K, Lim K-T. 3D-printed bioactive and biodegradable hydrogel scaffolds of alginate/gelatin/cellulose nanocrystals for tissue engineering. Int J Biol Macromol. 2021;167(4):644–58. doi:10.1016/j.ijbiomac.2020.12.011. [Google Scholar] [PubMed] [CrossRef]
15. Domínguez-Robles J, Cuartas-Gómez E, Dynes S, Utomo E, Anjani QK, Detamornrat U, et al. Poly(caprolactone)/lignin-based 3D-printed dressings loaded with a novel combination of bioactive agents for wound-healing applications. Sustain Mater Technol. 2023;35:e00581. doi:10.1016/j.susmat.2023.e00581. [Google Scholar] [CrossRef]
16. Zhang C, Wang C-S, Girard M, Therriault D, Heuzey M-C. 3D printed protein/polysaccharide food simulant for dysphagia diet: impact of cellulose nanocrystals. Food Hydrocoll. 2024;148:109455. doi:10.1016/j.foodhyd.2023.109455. [Google Scholar] [CrossRef]
17. Popoola O, Finny A, Dong I, Andreescu S. Smart and sustainable 3D-printed nanocellulose-based sensors for food freshness monitoring. ACS Appl Mat Interf. 2024;16(44):60920–32. doi:10.1021/acsami.4c10304. [Google Scholar] [PubMed] [CrossRef]
18. Müller O, Poulin A, Aeby X, Emma R, Kanno R, Nagai T, et al. 3D printed biodegradable soft actuators from nanocellulose reinforced gelatin composites (Adv. Sustainable Syst. 2/2025). Adv Sustain Syst. 2025;9(2):2570021. doi:10.1002/adsu.202570021. [Google Scholar] [CrossRef]
19. Alves LC. Cellulose solutions: dissolution, regeneration, solution structure and molecular interactions [Ph.D. thesis]. Coimbra, Portugal: University of Coimbra; 2015. [Google Scholar]
20. Magalhães S, Fernandes C, Pedrosa JFS, Alves L, Medronho B, Ferreira PJT, et al. Eco-friendly methods for extraction and modification of cellulose: an overview. Polymers. 2023;15(14):3138. doi:10.3390/polym15143138. [Google Scholar] [PubMed] [CrossRef]
21. Koshani R, Tavakolian M, van de Ven TGM. Cellulose-based dispersants and flocculants. J Mater Chem B. 2020;8(46):10502–26. doi:10.1039/D0TB02021D. [Google Scholar] [PubMed] [CrossRef]
22. Dufresne A. Nanocellulose: a new ageless bionanomaterial. Mater Today. 2013;16(6):220–7. doi:10.1016/j.mattod.2013.06.004. [Google Scholar] [CrossRef]
23. Foroughi F, Rezvani Ghomi E, Morshedi Dehaghi F, Borayek R, Ramakrishna S. A review on the life cycle assessment of cellulose: from Properties to the potential of making it a low carbon material. Materials. 2021;14(4):714. doi:10.3390/ma14040714. [Google Scholar] [PubMed] [CrossRef]
24. Aziz T, Farid A, Haq F, Kiran M, Ullah A, Zhang K, et al. A review on the modification of cellulose and its applications. Polymers. 2022;14(15):3206. doi:10.3390/polym14153206. [Google Scholar] [PubMed] [CrossRef]
25. Xu B, Hu X, Lu S, Wang T, Chen Z, Bai C, et al. 3D printing of cellulose nanocrystal-based Pickering foams for removing microplastics. Sep Purif Technol. 2024;339:126642. doi:10.1016/j.seppur.2024.126642. [Google Scholar] [CrossRef]
26. Hwang S, Han Y, Gardner DJ. Melt compounding of spray-dried cellulose nanofibrils/polypropylene and their application in 3D printing. Cellulose. 2024;31(12):7531–52. doi:10.1007/s10570-024-06038-w. [Google Scholar] [CrossRef]
27. Singh D. Research in polymeric materials—current status and future potential. In: National Conference on Advances in Applied Sciences and Mathematics, NCASM-20; 2020 Sep 24–25; Rajpura, India. [Google Scholar]
28. Yu J, Wang AC, Zhang M, Lin Z. Water treatment via non-membrane inorganic nanoparticles/cellulose composites. Mater Today. 2021;57:329–57. doi:10.1016/j.mattod.2021.03.024. [Google Scholar] [CrossRef]
29. Mo Y, Huang X, Yue M, Hu L, Hu C. Preparation of nanocellulose and application of nanocellulose polyurethane composites. RSC Adv. 2024;14(26):18247–57. doi:10.1039/d4ra01412j. [Google Scholar] [PubMed] [CrossRef]
30. Reale Batista MD, Drzal LT, Kiziltas A, Mielewski D. Hybrid cellulose-inorganic reinforcement polypropylene composites: lightweight materials for automotive applications. Polym Compos. 2020;41(3):1074–89. doi:10.1002/pc.25439. [Google Scholar] [CrossRef]
31. Hubbe MA, Rojas OJ, Lucia LA, Sain MM. Cellulosic nanocomposites: a review. BioResources. 2008;3:929–80. [Google Scholar]
32. Du Y, Feng G. When nanocellulose meets hydrogels: the exciting story of nanocellulose hydrogels taking flight. Green Chem. 2023;25(21):8349–84. doi:10.1039/d3gc01829f. [Google Scholar] [CrossRef]
33. Zhao D, Zhu Y, Cheng W, Chen W, Wu Y, Yu H. Cellulose-based flexible functional materials for emerging intelligent electronics. Adv Mater. 2021;33(28):2000619. doi:10.1002/adma.202000619. [Google Scholar] [PubMed] [CrossRef]
34. Zhang S, Xiao J, Wang G, Chen G. Enzymatic hydrolysis of lignin by ligninolytic enzymes and analysis of the hydrolyzed lignin products. Bioresour Technol. 2020;304:122975. doi:10.1016/j.biortech.2020.122975. [Google Scholar] [PubMed] [CrossRef]
35. Coral Medina JD, Woiciechowski A, Zandona Filho A, Noseda MD, Kaur BS, Soccol CR. Lignin preparation from oil palm empty fruit bunches by sequential acid/alkaline treatment—a biorefinery approach. Bioresour Technol. 2015;194:172–8. doi:10.1016/j.biortech.2015.07.018. [Google Scholar] [PubMed] [CrossRef]
36. Acosta J, Torres-Chavez PI, Carvajal-Millan E, Ramirez-Wong B, Bello-Pérez L, Montano Leyva B. Ionic liquids and organic solvents for recovering lignin from lignocellulosic biomass. BioResources. 2014;9:3660–87. doi:10.15376/biores.9.2.3660-3687. [Google Scholar] [CrossRef]
37. Köhnke J, Gierlinger N, Prats-Mateu B, Unterweger C, Solt P, Mahler AK, et al. Comparison of four technical lignins as a resource for electrically conductive carbon particles. BioResources. 2019;14(1):1091–109. doi:10.15376/biores.14.1.1091-1109. [Google Scholar] [CrossRef]
38. Nguyen NA, Barnes SH, Bowland CC, Meek KM, Littrell KC, Keum JK, et al. A path for lignin valorization via additive manufacturing of high-performance sustainable composites with enhanced 3D printability. Sci Adv. 2018;4(12):eaat4967. doi:10.1126/sciadv.aat4967. [Google Scholar] [PubMed] [CrossRef]
39. Böcherer D, Montazeri R, Li Y, Tisato S, Hambitzer L, Helmer D. Decolorization of lignin for high-resolution 3D printing of high lignin-content composites. Adv Sci. 2024;11(39):2406311. doi:10.1002/advs.202406311. [Google Scholar] [PubMed] [CrossRef]
40. Zhao X, Huang C, Xiao D, Wang P, Luo X, Liu W, et al. Melanin-inspired design: preparing sustainable photothermal materials from lignin for energy generation. ACS Appl Mat Interf. 2021;13(6):7600–7. doi:10.1021/acsami.0c21256. [Google Scholar] [PubMed] [CrossRef]
41. Yang J, Liu L, An X, Seta FT, Li C, Zhang H, et al. Facile preparation of lignosulfonate induced silver nanoparticles for high efficient removal of organic contaminants in wastewater. Ind Crops Prod. 2021;169(13):113644. doi:10.1016/j.indcrop.2021.113644. [Google Scholar] [CrossRef]
42. Xiong S-J, Bo P, Zhou S-J, Li M-K, Yang S, Wang Y-Y, et al. Economically competitive biodegradable PBAT/lignin composites: effect of lignin methylation and compatibilizer. ACS Sustain Chem Eng. 2020;8(13):5338–46. doi:10.1021/acssuschemeng.0c00789. [Google Scholar] [CrossRef]
43. Zhang S, Ji A, Meng X, Bhagia S, Yoo CG, Harper DP, et al. Structure-property relationship between lignin structures and properties of 3D-printed lignin composites. Compos Sci Technol. 2024;249(7633):110487. doi:10.1016/j.compscitech.2024.110487. [Google Scholar] [CrossRef]
44. Zhang S, Meng X, Bhagia S, Ji A, Dean Smith M, Wang YY, et al. 3D printed lignin/polymer composite with enhanced mechanical and anti-thermal-aging performance. Chem Eng J. 2024;481:148449. doi:10.1016/j.cej.2023.148449. [Google Scholar] [CrossRef]
45. Mohan MK, Krasnou I, Lukk T, Karpichev Y. Novel softwood lignin esters as advanced filler to PLA for 3D printing. ACS Omega. 2024;9(44):44559–67. doi:10.1021/acsomega.4c06680. [Google Scholar] [PubMed] [CrossRef]
46. Ren Z, Zhou X, Ding K, Ji T, Sun H, Chi X, et al. Design of sustainable 3D printable polylactic acid composites with high lignin content. Int J Biol Macromol. 2023;253:127264. doi:10.1016/j.ijbiomac.2023.127264. [Google Scholar] [PubMed] [CrossRef]
47. Jiang Z, Diggle B, Tan ML, Viktorova J, Bennett CW, Connal LA. Extrusion 3D printing of polymeric materials with advanced properties. Adv Sci. 2020;7(17):2001379. doi:10.1002/advs.202001379. [Google Scholar] [PubMed] [CrossRef]
48. Liu Z, Wang Y, Wu B, Cui C, Guo Y, Yan C. A critical review of fused deposition modeling 3D printing technology in manufacturing polylactic acid parts. Int J Adv Manuf Technol. 2019;102(9):2877–89. doi:10.1007/s00170-019-03332-x. [Google Scholar] [CrossRef]
49. Wang Q, Ji C, Sun J, Zhu Q, Liu J. Structure and properties of polylactic acid biocomposite films reinforced with cellulose nanofibrils. Molecules. 2020;25(14):3306. doi:10.3390/molecules25143306. [Google Scholar] [PubMed] [CrossRef]
50. Zhang Z, Wang W, Li Y, Fu K, Tong X, Cao B, et al. 3D printing of cellulose nanofiber/polylactic acid composites via an efficient dispersion method. Compos Commun. 2023;43:101731. doi:10.1016/j.coco.2023.101731. [Google Scholar] [CrossRef]
51. Touchard F, Marchand D, Chocinski-Arnault L, Fournier T, Magro C. 3D printing of continuous cellulose fibre composites: microstructural and mechanical characterisation. Rapid Prototyp J. 2023;29(9):1879–87. doi:10.1108/rpj-04-2023-0121. [Google Scholar] [CrossRef]
52. Chen Y, Xu J, Chen Y, Wang C, Wang H, Wu J. Poly(butylene succinate) filaments for fused deposition modelling (FDM) 3D-printing. Polym Chem. 2025;16(9):1072–84. doi:10.1039/D4PY01351D. [Google Scholar] [CrossRef]
53. Li J, Pumera M. 3D printing of functional microrobots. Chem Soc Rev. 2021;50(4):2794–838. doi:10.1039/d0cs01062f. [Google Scholar] [PubMed] [CrossRef]
54. Bahcegul EG, Bahcegul E, Özkan N. 3D printing of hemicellulosic biopolymers extracted from lignocellulosic agricultural wastes. ACS Appl Polym Mater. 2020;2(7):2622–32. doi:10.1021/acsapm.0c00256. [Google Scholar] [CrossRef]
55. Kam D, Braner A, Abouzglo A, Larush L, Chiappone A, Shoseyov O, et al. 3D printing of cellulose nanocrystal-loaded hydrogels through rapid fixation by photopolymerization. Langmuir. 2021;37(21):6451–8. doi:10.1021/acs.langmuir.1c00553. [Google Scholar] [PubMed] [CrossRef]
56. Ee LY, Yau Li SF. Recent advances in 3D printing of nanocellulose: structure, preparation, and application prospects. Nanoscale Adv. 2021;3(5):1167–208. doi:10.1039/d0na00408a. [Google Scholar] [PubMed] [CrossRef]
57. Yu Z, Sun X, Zhu Y, Zhou E, Cheng C, Zhu J, et al. Direct ink writing 3D printing elastomeric polyurethane aided by cellulose nanofibrils. ACS Nano. 2024;18(41):28142–53. doi:10.1021/acsnano.4c07681. [Google Scholar] [PubMed] [CrossRef]
58. Pazhamannil RV, Govindan P. Current state and future scope of additive manufacturing technologies via vat photopolymerization. Mat Today Proc. 2021;43(1):130–6. doi:10.1016/j.matpr.2020.11.225. [Google Scholar] [CrossRef]
59. Ligon SC, Liska R, Stampfl J, Gurr M, Mülhaupt R. Polymers for 3D printing and customized additive manufacturing. Chem Rev. 2017;117(15):10212–90. doi:10.1021/acs.chemrev.7b00074. [Google Scholar] [PubMed] [CrossRef]
60. Maines EM, Porwal MK, Ellison CJ, Reineke TM. Sustainable advances in SLA/DLP 3D printing materials and processes. Green Chem. 2021;23(18):6863–97. doi:10.1039/d1gc90138a. [Google Scholar] [CrossRef]
61. Lakkala P, Munnangi SR, Bandari S, Repka M. Additive manufacturing technologies with emphasis on stereolithography 3D printing in pharmaceutical and medical applications: a review. Int J Pharmac X. 2023;5:100159. doi:10.1016/j.ijpx.2023.100159. [Google Scholar] [PubMed] [CrossRef]
62. Silva R, Rebelo RC, Paula CTB, Pereira P, Fonseca AC, Serra AC, et al. All-cellulose resin for 3D printing hydrogels via digital light processing (DLP). Int J Biol Macromol. 2025;306:141389. doi:10.1016/j.ijbiomac.2025.141389. [Google Scholar] [PubMed] [CrossRef]
63. Wan Z, Zhang H, Niu M, Zhang W, Guo Y, Li H. Development of lignin-derived UV-curable resin for DLP 3D printing. Ind Crops Prod. 2024;221:119243. doi:10.1016/j.indcrop.2024.119243. [Google Scholar] [CrossRef]
64. Johnson RM, Cortés-Guzmán KP, Perera SD, Parikh AR, Ganesh V, Voit WE, et al. Lignin-based covalent adaptable network resins for digital light projection 3D printing. J Polym Sci. 2024;62(12):2585–96. doi:10.1002/pol.20230026. [Google Scholar] [CrossRef]
65. Gao W, Guo Y, Cui J, Liang C, Lu Z, Feng S, et al. Dual-curing polymer systems for photo-curing 3D printing. Addit Manuf. 2024;85:104142. doi:10.1016/j.addma.2024.104142. [Google Scholar] [CrossRef]
66. Ruiz Deance AL, Siersema B, Yoe LEA-C, Wurm FR, Gojzewski H. Copolymerizing lignin for tuned properties of 3D-printed PEG-based photopolymers. ACS Appl Polym Mat. 2023;5(12):10021–31. doi:10.1021/acsapm.3c01875. [Google Scholar] [CrossRef]
67. N’Gatta KM, Belaid H, El Hayek J, Assanvo EF, Kajdan M, Masquelez N, et al. 3D printing of cellulose nanocrystals based composites to build robust biomimetic scaffolds for bone tissue engineering. Sci Rep. 2022;12(1):21244. doi:10.21203/rs.3.rs-1754000/v1. [Google Scholar] [CrossRef]
68. Aumnate C, Soatthiyanon N, Makmoon T, Potiyaraj P. Polylactic acid/kenaf cellulose biocomposite filaments for melt extrusion based-3D printing. Cellulose. 2021;28(13):8509–25. doi:10.1007/s10570-021-04069-1. [Google Scholar] [CrossRef]
69. Lecoublet M, Ragoubi M, Leblanc N, Koubaa A. Sustainable 3D-printed cellulose-based biocomposites and bio-nano-composites: analysis of dielectric performances. Ind Crops Prod. 2024;221:119332. doi:10.1016/j.indcrop.2024.119332. [Google Scholar] [CrossRef]
70. Nagel Y, Sivaraman D, Neels A, Zimmermann T, Zhao S, Siqueira G, et al. Anisotropic, strong, and thermally insulating 3D-printed nanocellulose-PNIPAAM aerogels. Small Struct. 2023;4(12):2300073. doi:10.1002/sstr.202300073. [Google Scholar] [CrossRef]
71. Zheng Y, Zhu Y, Yu Z, Zhu J, Zhang Y, Ye Y, et al. Passive thermal regulation with 3D printed phase change material/cellulose nanofibrils composites. Compos Part B Eng. 2022;247(6):110332. doi:10.1016/j.compositesb.2022.110332. [Google Scholar] [CrossRef]
72. Nocheseda CJC, Liza FP, Collera AKM, Caldona EB, Advincula RC. 3D printing of metals using biodegradable cellulose hydrogel inks. Addit Manuf. 2021;48:102380. doi:10.1016/j.addma.2021.102380. [Google Scholar] [CrossRef]
73. Baniasadi H, Polez RT, Kimiaei E, Madani Z, Rojas OJ, Österberg M, et al. 3D printing and properties of cellulose nanofibrils-reinforced quince seed mucilage bio-inks. Int J Biol Macromol. 2021;192:1098–107. doi:10.1016/j.ijbiomac.2021.10.078. [Google Scholar] [PubMed] [CrossRef]
74. Wang Q, Liu X, Qiang Z, Hu Z, Cui X, Wei H, et al. Cellulose nanocrystal enhanced, high dielectric 3D printing composite resin for energy applications. Compos Sci Technol. 2022;227:109601. doi:10.1016/j.compscitech.2022.109601. [Google Scholar] [CrossRef]
75. Palucci Rosa R, Rosace G, Arrigo R, Malucelli G. Preparation and characterization of 3D-printed biobased composites containing micro- or nanocrystalline cellulose. Polymers. 2022;14(9):1886. doi:10.3390/polym14091886. [Google Scholar] [PubMed] [CrossRef]
76. Cafiso D, Septevani AA, Noè C, Schiller TL, Pirri CF, Roppolo I, et al. 3D printing of fully cellulose-based hydrogels by digital light processing. Sustain Mater Technol. 2022;32:e00444. doi:10.1016/j.susmat.2022.e00444. [Google Scholar] [CrossRef]
77. Nian Y, Wan S, Avcar M, Wang X, Hong R, Yue R, et al. Nature-inspired 3D printing-based double-graded aerospace negative Poisson’s ratio metastructure: design, fabrication, investigation, optimization. Compos Struct. 2024;348:118482. doi:10.1016/j.compstruct.2024.118482. [Google Scholar] [CrossRef]
78. Moreno R, Carou D, Carazo-Álvarez D, Gupta MK. Statistical models for the mechanical properties of 3D printed external medical aids. Rapid Prototyp J. 2021;27(1):176–86. doi:10.1108/rpj-02-2020-0033. [Google Scholar] [CrossRef]
79. Chand R, Sharma V, Trehan R, Gupta M. A physical investigation of dimensional and mechanical characteristics of 3D printed nut and bolt for industrial applications. Rapid Prototyp J. 2022;28(5):953–66. doi:10.1108/RPJ-09-2021-0250. [Google Scholar] [CrossRef]
80. Srivastava M, Jayakumar V, Udayan Y, Sathishkumar M, Muthu SM, Gautam P, et al. Additive manufacturing of Titanium alloy for aerospace applications: insights into the process, microstructure, and mechanical properties. Appl Mater Today. 2024;41:102481. doi:10.1016/j.apmt.2024.102481. [Google Scholar] [CrossRef]
81. Tarassoli SP, Jessop ZM, Jovic T, Hawkins K, Whitaker IS. Candidate bioinks for extrusion 3D bioprinting—a systematic review of the literature. Front Bioeng Biotechnol. 2021;9:616753. doi:10.3389/fbioe.2021.616753. [Google Scholar] [PubMed] [CrossRef]
82. Benamira M, Benhassine N, Ayad A, Dekhane A. Investigation of printing parameters effects on mechanical and failure properties of 3D printed PLA. Eng Fail Anal. 2023;148:107218. doi:10.1016/j.engfailanal.2023.107218. [Google Scholar] [CrossRef]
83. Liu Y, Bai W, Cheng X, Tian J, Wei D, Sun Y, et al. Effects of printing layer thickness on mechanical properties of 3D-printed custom trays. J Prosthet Dent. 2021;126(5):671.e1–7. doi:10.1016/j.prosdent.2020.08.025. [Google Scholar] [PubMed] [CrossRef]
84. Anuar H, Rahman NAA, Manshor MR, Alli YA, Alimi OA, Alif F, et al. Novel soda lignin/PLA/EPO biocomposite: a promising and sustainable material for 3D printing filament. Mater Today Commun. 2023;35:106093. doi:10.1016/j.mtcomm.2023.106093. [Google Scholar] [CrossRef]
85. Ding R, Duan Z, Sun Y, Yuan Q, Tien TT, Zúniga MG, et al. Enhancement of 3D printability and mechanical properties of polylactic acid/lignin biocomposites via interface engineering. Ind Crops Prod. 2023;194(7):116286. doi:10.1016/j.indcrop.2023.116286. [Google Scholar] [CrossRef]
86. Jiang Y, Latif M, Kim J. Three-dimensional printing of lignocellulose structures: improving mechanical properties and shape fidelity. ACS Omega. 2024;9(22):23442–50. doi:10.1021/acsomega.3c10101. [Google Scholar] [PubMed] [CrossRef]
87. Frasca S, Katsiotis CS, Henrik-Klemens Å, Larsson A, Strømme M, Lindh J, et al. Compatibility of kraft lignin and phenol-organosolv lignin with PLA in 3D printing and assessment of mechanical recycling. ACS Applied Polymer Materials. 2024;6(22):13574–84. doi:10.1021/acsapm.4c02208. [Google Scholar] [CrossRef]
88. Kabir SMF, Mathur K, Seyam A-FM. The road to improved fiber-reinforced 3D printing technology. Technologies. 2020;8(4):51. doi:10.3390/technologies8040051. [Google Scholar] [CrossRef]
89. Doshi M, Mahale A, Kumar Singh S, Deshmukh S. Printing parameters and materials affecting mechanical properties of FDM-3D printed parts: perspective and prospects. Mat Today: Proc. 2022;50(9):2269–75. doi:10.1016/j.matpr.2021.10.003. [Google Scholar] [CrossRef]
90. Lyu Y, Zhao H, Wen X, Lin L, Schlarb AK, Shi X. Optimization of 3D printing parameters for high-performance biodegradable materials. J Appl Polym Sci. 2021;138(32):50782. doi:10.1002/app.50782. [Google Scholar] [CrossRef]
91. Utz J, Zubizarreta J, Geis N, Immonen K, Kangas H, Ruckdäschel H. 3D printed cellulose-based filaments—processing and mechanical properties. Materials. 2022;15(19):6582. doi:10.3390/ma15196582. [Google Scholar] [PubMed] [CrossRef]
92. Guessasma S, Stephant N, Durand S, Belhabib S. Digital light processing route for 3D printing of acrylate-modified PLA/lignin blends: microstructure and mechanical performance. Polymers. 2024;16(10):1342. doi:10.3390/polym16101342. [Google Scholar] [PubMed] [CrossRef]
93. Gauss C, Pickering KL. A new method for producing polylactic acid biocomposites for 3D printing with improved tensile and thermo-mechanical performance using grafted nanofibrillated cellulose. Addit Manuf. 2023;61:103346. doi:10.1016/j.addma.2022.103346. [Google Scholar] [CrossRef]
94. Trivedi AK, Gupta MK. 3D printed PLA based bionanocomposites with improved mechanical and dynamic mechanical properties: effect of varying CNC reinforcements. Cellulose. 2025;32:2303–19. doi:10.1007/s10570-025-06432-y. [Google Scholar] [CrossRef]
95. Mandala R, Prasad BA, Akella S. Enhancing the mechanical properties of 3D-Printed polylactic acid through pellet additive manufacturing: a grey relational analysis based on entropy weights. Int J Lightweig Mat Manuf. 2025;8(3):331–40. doi:10.1016/j.ijlmm.2025.02.003. [Google Scholar] [CrossRef]
96. Vidakis N, Petousis M, Michailidis N, Kechagias JD, Mountakis N, Argyros A, et al. High-performance medical-grade resin radically reinforced with cellulose nanofibers for 3D printing. J Mech Behav Biomed Mater. 2022;134:105408. doi:10.1016/j.jmbbm.2022.105408. [Google Scholar] [PubMed] [CrossRef]
97. Qiao H, Li Q, Chen Y, Liu Y, Jiang N, Wang C. Mechanical and thermal properties of 3D-printed continuous bamboo fiber-reinforced PE composites. Materials. 2025;18(3):593. doi:10.3390/ma18030593. [Google Scholar] [PubMed] [CrossRef]
98. Kalaš D, Šíma K, Kadlec P, Polanský R, Soukup R, Řeboun J, et al. FFF 3D printing in electronic applications: dielectric and thermal properties of selected polymers. Polymers. 2021;13(21):3702. doi:10.3390/polym13213702. [Google Scholar] [PubMed] [CrossRef]
99. Ma H, Zhang Q, Meng T, Yin J, Fang X, Yin S, et al. Design and mechanical/thermal properties of in-situ synthesized mullite in SLA 3D printing Al2O3-SiO2 ceramic. Ceram Int. 2025;51(8):10726–37. doi:10.1016/j.ceramint.2024.12.503. [Google Scholar] [CrossRef]
100. Blanco I. A brief review of the applications of selected thermal analysis methods to 3D printing. Thermo. 2022;2(1):74–83. doi:10.3390/thermo2010006. [Google Scholar] [CrossRef]
101. Kassaun BB, Wang L, Backman O, Xu C, Fatehi P. 3D printable lignin-caprolactone material. Green Chem. 2025;27(13):3451–64. doi:10.1039/d4gc06179a. [Google Scholar] [CrossRef]
102. Wan Z, Zhang H, Niu M, Zhang W, Guo Y, Li H. Preparation of lignin nanoparticles by ultrasonication and its incorporation in DLP 3D printing UV-curable resin as bio-filler. Ind Crops Prod. 2025;224:120394. doi:10.1016/j.indcrop.2024.120394. [Google Scholar] [CrossRef]
103. Liaw C-Y, Tolbert JW, Chow LW, Guvendiren M. Interlayer bonding strength of 3D printed PEEK specimens. Soft Matter. 2021;17(18):4775–89. doi:10.1039/d1sm00417d. [Google Scholar] [PubMed] [CrossRef]
104. Li G, Feng L, Zhai Z, Wang F. Study on enhancing effect of carbon nanotubes on the interlayer strength of 3D printing of plastic powder. AIP Adv. 2020;10:065106. doi:10.1063/5.0007088. [Google Scholar] [CrossRef]
105. Yang J, An X, Lu B, Cao H, Cheng Z, Tong X, et al. Lignin: a multi-faceted role/function in 3D printing inks. Int J Biol Macromol. 2024;267(5):131364. doi:10.1016/j.ijbiomac.2024.131364. [Google Scholar] [PubMed] [CrossRef]
106. Bi X, Huang R. 3D printing of natural fiber and composites: a state-of-the-art review. Mat Design. 2022;222:111065. doi:10.1016/j.matdes.2022.111065. [Google Scholar] [CrossRef]
107. Caminero MA, Chacón JM, García-Moreno I, Reverte JM. Interlaminar bonding performance of 3D printed continuous fibre reinforced thermoplastic composites using fused deposition modelling. Polym Test. 2018;68:415–23. doi:10.1016/j.polymertesting.2018.04.038. [Google Scholar] [CrossRef]
108. Ye H, He Y, Li H, You T, Xu F. Customized compatibilizer to improve the mechanical properties of polylactic acid/lignin composites via enhanced intermolecular interactions for 3D printing. Ind Crops Prod. 2023;205:117454. doi:10.1016/j.indcrop.2023.117454. [Google Scholar] [CrossRef]
109. Demir M, Seki Y. Interfacial adhesion strength between FDM-printed PLA parts and surface-treated cellulosic-woven fabrics. Rapid Prototyp J. 2023;29(6):1166–74. doi:10.1108/rpj-10-2022-0369. [Google Scholar] [CrossRef]
110. Battisto EW, Sarsfield SR, Lele SR, Williams T, Catchmark JM, Chmely SC. Enhancing the matrix-fiber interface with a surfactant leads to improved performance properties of 3D printed composite materials containing cellulose nanofibrils. ACS Appl Mat Interf. 2022;14(39):44841–8. doi:10.1021/acsami.2c12363. [Google Scholar] [PubMed] [CrossRef]
111. Choe S, Kim Y, Park G, Lee DH, Park J, Mossisa AT, et al. Biodegradation of 3D-printed biodegradable/non-biodegradable plastic blends. ACS Appl Poly Mat. 2022;4(7):5077–90. doi:10.1021/acsapm.2c00600. [Google Scholar] [CrossRef]
112. Wang G, Wang S, Hu T, Shi F. Multifunctional hydrogel with 3D printability, fluorescence, biodegradability, and biocompatibility for biomedical microrobots. Molecules. 2024;29(14):3351. doi:10.3390/molecules29143351. [Google Scholar] [PubMed] [CrossRef]
113. Mazur J, Sobczak P, Panasiewicz M, Łusiak P, Krajewska M, Findura P, et al. Mechanical properties and biodegradability of samples obtained by 3D printing using FDM technology from PLA filament with by-products. Sci Rep. 2025;15(1):5847. doi:10.1038/s41598-025-89984-0. [Google Scholar] [PubMed] [CrossRef]
114. Murillo-Morales G, Sethupathy S, Zhang M, Xu L, Ghaznavi A, Xu J, et al. Characterization and 3D printing of a biodegradable polylactic acid/thermoplastic polyurethane blend with laccase-modified lignin as a nucleating agent. Int J Biol Macromol. 2023;236:123881. doi:10.1016/j.ijbiomac.2023.123881. [Google Scholar] [PubMed] [CrossRef]
115. Zhang Z, Cao B, Jiang N. The mechanical properties and degradation behavior of 3D-printed cellulose nanofiber/polylactic acid composites. Materials. 2023;16(18):6197. doi:10.3390/ma16186197. [Google Scholar] [PubMed] [CrossRef]
116. Kumar R, Sharma H, Saran C, Tripathy TS, Sangwan KS, Herrmann C. A comparative study on the life cycle assessment of a 3D printed product with PLA, ABS & PETG materials. Procedia CIRP. 2022;107:15–20. doi:10.1016/j.procir.2022.04.003. [Google Scholar] [CrossRef]
117. Firmanda A, Syamsu K, Sari YW, Cabral J, Pletzer D, Mahadik B, et al. 3D printed cellulose based product applications. Mat Chem Front. 2022;6(3):254–79. doi:10.1039/d1qm00390a. [Google Scholar] [CrossRef]
118. Decante G, Costa JB, Silva-Correia J, Collins MN, Reis RL, Oliveira JM. Engineering bioinks for 3D bioprinting. Biofabrication. 2021;13(3):032001. doi:10.1088/1758-5090/abec2c. [Google Scholar] [PubMed] [CrossRef]
119. Sundaramurthi D, Rauf S, Hauser CAE. 3D bioprinting technology for regenerative medicine applications. IJB. 2016;2(2):9–26. doi:10.18063/ijb.2016.02.010. [Google Scholar] [CrossRef]
120. Zennifer A, Senthilvelan P, Sethuraman S, Sundaramurthi D. Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications. Carbohydr Polym. 2021;256:117561. doi:10.1016/j.carbpol.2020.117561. [Google Scholar] [PubMed] [CrossRef]
121. Janmohammadi M, Nourbakhsh MS, Bahraminasab M. 3D printed polycaprolactone scaffold incorporated with tragacanth gum/bioactive glass and cellulose nanocrystals for bone tissue engineering. Int J Biol Macromol. 2025;305:141114. doi:10.1016/j.ijbiomac.2025.141114. [Google Scholar] [PubMed] [CrossRef]
122. Goyal R, Mahapatra SN, Yadav R, Mitra S, Samanta A, Kumar A, et al. 3D printed cellulose nanofiber-reinforced and iron-crosslinked double network hydrogel composites for tissue engineering applications: mechanical properties and cellular viability. Bioprinting. 2025;46(24):e00392. doi:10.1016/j.bprint.2025.e00392. [Google Scholar] [CrossRef]
123. Monfared M, Mawad D, Rnjak-Kovacina J, Stenzel MH. 3D bioprinting of dual-crosslinked nanocellulose hydrogels for tissue engineering applications. J Mater Chem B. 2021;9(31):6163–75. doi:10.1039/d1tb00624j. [Google Scholar] [PubMed] [CrossRef]
124. Kumar A, I Matari IA, Han SS. 3D printable carboxylated cellulose nanocrystal-reinforced hydrogel inks for tissue engineering. Biofabrication. 2020;12(2):025029. doi:10.1088/1758-5090/ab736e. [Google Scholar] [PubMed] [CrossRef]
125. Jovic TH, Nicholson T, Arora H, Nelson K, Doak SH, Whitaker IS. A comparative analysis of pulp-derived nanocelluloses for 3D bioprinting facial cartilages. Carbohydr Polym. 2023;321:121261. doi:10.1016/j.carbpol.2023.121261. [Google Scholar] [PubMed] [CrossRef]
126. Gouripriya DA, Adhikari J, Debnath P, Ghosh S, Ghosh P, Thomas S, et al. 3D printing of bacterial cellulose for potential wound healing applications: current trends and prospects. Int J Biol Macromol. 2024;279(11):135213. doi:10.1016/j.ijbiomac.2024.135213. [Google Scholar] [PubMed] [CrossRef]
127. Alizadehgiashi M, Nemr CR, Chekini M, Pinto Ramos D, Mittal N, Ahmed SU, et al. Multifunctional 3D-printed wound dressings. ACS Nano. 2021;15(7):12375–87. doi:10.1021/acsnano.1c04499. [Google Scholar] [PubMed] [CrossRef]
128. Cheng Y, Fu Y, Ma L, Yap PL, Losic D, Wang H, et al. Rheology of edible food inks from 2D/3D/4D printing, and its role in future 5D/6D printing. Food Hydrocolloids. 2022;132:107855. doi:10.1016/j.foodhyd.2022.107855. [Google Scholar] [CrossRef]
129. Armstrong CD, Yue L, Deng Y, Qi HJ. Enabling direct ink write edible 3D printing of food purees with cellulose nanocrystals. J Food Eng. 2022;330(9):111086. doi:10.1016/j.jfoodeng.2022.111086. [Google Scholar] [CrossRef]
130. Chen K, Zhang M, Bhandari B, Deng D. 3D printed cinnamon essential oil/banana peel carbon dots loaded corn starch/gelatin bilayer film with enhanced functionality for food packaging application. Food Chem. 2024;448(1):139176. doi:10.1016/j.foodchem.2024.139176. [Google Scholar] [PubMed] [CrossRef]
131. Dermol Š, Borin B, Gregor-Svetec D, Slemenik Perše L, Lavrič G. The development of a bacterial nanocellulose/cationic starch hydrogel for the production of sustainable 3D-printed packaging foils. Polymers. 2024;16(11):1527. doi:10.3390/polym16111527. [Google Scholar] [PubMed] [CrossRef]
132. Zhao Y, Du J, Zhou H, Zhou S, Lv Y, Cheng Y, et al. Biodegradable intelligent film for food preservation and real-time visual detection of food freshness. Food Hydrocolloids. 2022;129:107665. doi:10.1016/j.foodhyd.2022.107665. [Google Scholar] [CrossRef]
133. Zhou W, Wu Z, Xie F, Tang S, Fang J, Wang X. 3D printed nanocellulose-based label for fruit freshness keeping and visual monitoring. Carbohydr Polym. 2021;273(2):118545. doi:10.1016/j.carbpol.2021.118545. [Google Scholar] [PubMed] [CrossRef]
134. Zhou W, Fang J, Tang S, Wu Z, Wang X. 3D-printed nanocellulose-based cushioning-antibacterial dual-function food packaging aerogel. Molecules. 2021;26(12):3543. doi:10.3390/molecules26123543. [Google Scholar] [PubMed] [CrossRef]
135. Roy A, Afshari R, Jain S, Zheng Y, Lin M-H, Zenkar S, et al. Advances in conducting nanocomposite hydrogels for wearable biomonitoring. Chem Soc Rev. 2025;54(5):2595–652. doi:10.1039/d4cs00220b. [Google Scholar] [PubMed] [CrossRef]
136. Guo Z, Xie W, Liu W. Hybrid three-dimensional printing and encapsulation process for cellulose hydrogel sensors. Int J Biol Macromol. 2025;302:140571. doi:10.1016/j.ijbiomac.2025.140571. [Google Scholar] [PubMed] [CrossRef]
137. Zhan J, Kong Y, Zhou X, Gong H, Chen Q, Zhang X, et al. 3D printing of wearable sensors with strong stretchability for myoelectric rehabilitation. Biomater Sci. 2025;13(4):1021–32. doi:10.1039/D4BM01434K. [Google Scholar] [PubMed] [CrossRef]
138. Amini M, Hashemi SA, Yu Z, Ghaffarkhah A, Kamkar M, Jiang F, et al. Flexible 3D-printed cellulosic constructs for EMI shielding and piezoresistive sensing. Adv Mater Technol. 2025;454:146. doi:10.1002/admt.202401898. [Google Scholar] [CrossRef]
139. Wu T, Sugiarto S, Yang R, Sathasivam T, Weerasinghe UA, Chee PL, et al. From 3D to 4D printing of lignin towards green materials and sustainable manufacturing. Mater Horiz. 2025;12(9):2789–819. doi:10.1039/d4mh01680g. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools