 Open Access
Open Access
REVIEW
Machine Intelligence for Mental Health Diagnosis: A Systematic Review of Methods, Algorithms, and Key Challenges
Chitkara University School of Engineering and Technology, Chitkara University, Baddi, 174103, Himachal Pradesh, India
* Corresponding Author: Ashutosh Kumar Dubey. Email:
(This article belongs to the Special Issue: Advanced Medical Imaging Techniques Using Generative Artificial Intelligence)
Computers, Materials & Continua 2026, 86(1), 1-65. https://doi.org/10.32604/cmc.2025.066990
Received 23 April 2025; Accepted 17 September 2025; Issue published 10 November 2025
Abstract
Objective: The increasing global prevalence of mental health disorders highlights the urgent need for the development of innovative diagnostic methods. Conditions such as anxiety, depression, stress, bipolar disorder (BD), and autism spectrum disorder (ASD) frequently arise from the complex interplay of demographic, biological, and socioeconomic factors, resulting in aggravated symptoms. This review investigates machine intelligence approaches for the early detection and prediction of mental health conditions. Methods: The preferred reporting items for systematic reviews and meta-analyses (PRISMA) framework was employed to conduct a systematic review and analysis covering the period 2018 to 2025. The potential impact of machine intelligence methods was assessed by considering various strategies, hybridization of algorithms, tools, techniques, and datasets, and their applicability. Results: Through a systematic review of studies concentrating on the prediction and evaluation of mental disorders using machine intelligence algorithms, advancements, limitations, and gaps in current methodologies were highlighted. The datasets and tools utilized in these investigations were examined, offering a detailed overview of the status of computational models in understanding and diagnosing mental health disorders. Recent research indicated considerable improvements in diagnostic accuracy and treatment effectiveness, particularly for depression and anxiety, which have shown the greatest methodological diversity and notable advancements in machine intelligence. Conclusions: Despite these improvements, challenges persist, including the need for more diverse datasets, ethical issues surrounding data privacy and algorithmic bias, and obstacles to integrating these technologies into clinical settings. This synthesis emphasizes the transformative potential of machine intelligence in enhancing mental healthcare.Keywords
Supplementary Material
Supplementary Material FileIn today’s world, mental health issues are becoming increasingly widespread, showing up in various symptoms such as mood swings, depression, anxiety, stress, paranoia, and shifts in social and dietary patterns [1]. According to the World Health Organization, mental health continues to be one of the most neglected areas in public health, with an estimated one billion people worldwide experiencing a mental disorder [2]. This study investigates the intricate nature of mental disorders. It examines how emerging technologies, particularly artificial intelligence (AI) methods such as machine learning (ML) [3,4], deep learning (DL) [5], and nature-inspired algorithms [4–6], can support clinical decision-making. Recognizing the often ambiguous and uncertain nature of medical diagnosis and early prediction, this study seeks to bridge the existing gaps by uncovering further insights into the factors associated with mental illness and assessing the potential of machine intelligence algorithms for the early detection of mental disorders.
The rise of machine intelligence has instigated a substantial transformation in mental health care, moving from subjective and complex psychological diagnostics to data-driven methodologies [3–7]. These technologies, proficient in processing and analyzing large datasets from genetic, neuroimaging, and electronic health records, reveal patterns and predictors that were previously ignored by human analysts [8–11]. This review examines how computational models and algorithms are revolutionizing mental health care, providing hope for enhanced diagnostics and tailored treatment plans. However, while several reviews have examined the use of AI in mental health, most are focused either on specific disorders or on specific techniques. A clear synthesis across a wider range of disorders and machine intelligence paradigms is lacking. Additionally, many previous reviews fail to address the comparative evaluation of algorithmic performance, dataset diversity, and clinical applicability. This paper fills that gap by presenting a comprehensive, disorder-wise mapping of various machine intelligence models, including ML, DL, and nature-inspired algorithms, applied to six major mental disorders. It also introduces recent developments and evaluates methodological performance, dataset trends, and translational limitations, offering a broader, updated, and translationally impactful perspective.
The motivation for this comprehensive review is driven by the critical need to refine diagnostic precision and deepen our understanding of mental health disorders. Early detection and precise analysis of conditions such as anxiety, stress, depression, bipolar disorder (BD), autism spectrum disorder (ASD), and Alzheimer’s disease (AD) are pivotal for improving treatment outcomes and quality of life. The primary goal of this study is to examine the development and results of various computational models, evaluate the effectiveness and precision of these models, investigate the datasets utilized, and identify trends and gaps.
Numerous machine intelligence techniques, including k-nearest neighbors (kNN), support vector machine (SVM) [12,13], random forests (RF) [14], decision tree (DT) [15,16], convolutional neural network (CNN) [14–17], as well as boosting, bagging methods, and nature-inspired optimization, have been investigated in relation to mental health disorders. These approaches provide a diverse array of methods for comprehending and tackling the intricacies of mental disorders, offering valuable insights into diagnosis, treatment, and management strategies [18–22]. Each algorithm makes a distinct contribution to the field, ranging from pattern recognition and predictive modeling to generating data-driven insights, thereby enhancing the effectiveness of addressing mental health challenges.
The study aim is to provide a systematic exploration of machine intelligence techniques applicability in the detection of mental health disorders. The objectives of this study are as under:
• To analyze various computational models and the development, application, and effectiveness of different machine intelligence algorithms (ML, DL, AI, and data-driven) within the realm of mental health.
• To evaluate the performance of these models considering varying conditions and parameters.
• To explore the datasets used and understand their scope, limitations, insights, and applicability.
• To highlight the advantages, challenges, and limitations and recognize areas that require further research to advance the field.
This study systematically reviewed each study and evaluated the methodologies employed, strategies adopted, outcomes achieved, and datasets used. This process offers a thorough overview of the existing landscape of computational models for understanding and diagnosing mental-health disorders. The goal is to bridge the gap between technological advancements and clinical practice. This review and analysis not only emphasize the significant potential of machine intelligence in transforming mental health care but also considers the challenges and ethical issues linked to its integration. Through this detailed exploration, this study contributes to ongoing efforts to improve accessibility, precision, and effectiveness of mental health care.
The distinct contribution of this review lies in its comprehensive, multi-dimensional analysis of machine intelligence applications across six major mental health disorders namely anxiety, depression, schizophrenia (SZ), BD, ASD, and AD. Unlike existing reviews, this study integrates conventional ML models, DL architectures, and nature-inspired optimization techniques, offering a comparative evaluation of their effectiveness, limitations, and use-case suitability. It also consolidates dataset trends from 2018 to 2025, highlighting their diversity, geographical distribution, and methodological relevance. Furthermore, the inclusion of collaboration networks and dataset method mappings add unique analytical value. By synthesizing technical performance, dataset availability, and translational challenges, this review offers a novel, systematized foundation to inform future research and real-world deployment of AI-driven mental health tools.
The remainder of this paper is organized as follows. Section 2 addresses the research questions for review and mapping. Section 3 defines the scope, outlines the search strategy, specifies the inclusion and exclusion criteria, and details the categorization, data extraction, and analysis. Section 4 discusses and analyzes the methods employed to detect mental disorders, with each category of mental health examined individually. Section 5 provides an analysis based on the datasets accompanied by an in-depth discussion. Section 6 presents an overall discussion and analysis. Finally, Section 7 offers concluding remarks.
2 Review Question Mapping Method
This study focuses on analyzing research related to the detection of mental disorders, understanding various techniques, and the rationale behind their usage. This review and analysis attempt to exemplify the motivations for employing these methods, the experimental protocols used for validation, the parametric variations, and the challenges faced in these studies. The systematic review and meta-analysis are guided by the following mapping questions (MQs) and research questions (RQs), which are formulated to address specific aspects of this investigation.
The MQs and RQs are as follows:
MQ1: What is the publication status and article selection strategy employed for This study?
MQ2: What are the most commonly used machine intelligence algorithms for assessing mental health?
MQ3: How has the collaboration network among researchers in the field of machine intelligence for mental health diagnosis evolved from 2018 to 2025?
RQ1: What are the key methodological studies in the field of mental health?
This question addresses the methods currently used for detecting mental disorders, the primary factors contributing to successful mental health treatment, and how integrating various classifiers, including ML, DL, and other methodological approaches, affects model performance.
RQ2: What are the strengths and weaknesses of these specific methodologies?
This question examines the impacts of previous results and the major challenges encountered. Additionally, it explores the extent to which existing studies address the current challenges and implications in the field.
RQ3: Is the machine intelligence approach effective in identifying mental health issues?
This question investigates the challenges in developing machine intelligence for mental health, the impact of technological interventions on treatment, strategies for monitoring mental health, social media’s effects, and the potential benefits of machine intelligence. These findings emphasize the need for the effective integration of technology in understanding and treating mental health issues. Additionally, it explores how incorporating technology in monitoring progress can assist clinicians in decision-making.
RQ4: What is the current state of dataset availability in this domain?
This question explores the related datasets.
This section includes the scope definition, search strategy, inclusion/exclusion criteria, categorization, data extraction, and analysis.
3.1 MQ1: What is the Publication Status and Article Selection Strategy Employed for This Study?
For this study, a total of 1011 papers were reviewed from various databases, including Google Scholar, PubMed, Springer, ScienceDirect, and IEEE Xplore. The publishers considered included Springer, Elsevier, IEEE, MDPI, Hindawi, Wiley, and Nature. The primary search targeted technological keywords such as “machine learning,” “deep learning,” “data mining,” “classification,” “clustering,” “nature-inspired algorithms,” and “big data.” Additionally, related terms about mental health such as depression, SZ, ASD, BD, AD, and anxiety/stress were also considered in the search strategy. Table 1 outlines the criteria for selecting manuscripts based on their relevance to mental health and technology, specifying required keywords like “artificial intelligence” and “machine learning.” It emphasizes the inclusion of peer-reviewed, full-text articles in English that are available electronically, while excluding non-English papers, those not in electronic format, and those without technological focus.

Fig. 1 presents a data collection chart (from January 2018 to June 2025) summarizing 187 papers concerning mental health categories. The papers were sourced from multiple publishers: 43 from IEEE, 53 from Elsevier, 36 from Springer, 12 from Wiley, 11 from MDPI, 9 from Hindawi, and 12 from Nature. Additionally, 11 papers were obtained from other publishers. The collection includes research papers addressing various mental disorders: 33 focus on anxiety and stress-related disorders, 49 on depression, 28 on SZ, 26 on ASD, 26 on BD, and 25 on AD. The latest trends, including studies from 2024 and 2025, have also been discussed and compared in the Discussion section.

Figure 1: Data collection chart from January 2018 to June 2025, summarizing the paper count (187) across different publishers and categories of mental health issues
A systematic and structured methodology for review has been established to conduct an exploratory study. The online database utilized facilitates the construction of a comprehensive corpus for the study. The preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines were adhered to in the selection of relevant studies [23]. The primary objective was to demonstrate application of this technology in the diagnosis, prediction, and detection of mental health disorders. The impetus for this exploratory study was to address the identified research gap. Key factors were recognized in the prediction and treatment of mental diseases. Fig. 2 presents the PRISMA flow diagram. The flow chart illustrates the process for selecting studies for inclusion in the review. The process began with 1011 records identified. After removing 205 duplicates, 806 records remained for screening. Following the exclusion of non-English papers, poster papers, and EC-2, 720 records were assessed for eligibility. Some reports could not be retrieved, leaving 613 for evaluation. After excluding EC-3, EC-4, and studies with incomplete results, 187 studies met the inclusion criteria and were included in the final review.

Figure 2: Flowchart of the article selection strategy based on the PRISMA style
3.2 MQ2: What are the Most Commonly Used Machine Intelligence Algorithms for Assessing Mental Health?
Machine intelligence algorithms can be broadly categorized into several types on the basis of their learning approach, purpose, and application. Supervised learning algorithms learn from labeled training data, guiding the model to make predictions. Examples include linear regression (LR), logistic regression (LoR), SVM, kNN, DT, RF, gradient boosting machines, and neural networks (NNs) [24–28] (Fig. 3). Unsupervised learning algorithms, which include clustering and association, are used when the data have no labels. Notable methods here include K-means, fuzzy c-means, hierarchical clustering, and density-based spatial clustering of applications with noise [26–28]. Principal component analysis, singular value decomposition, and autoencoders also fall under this category [26–28]. Semi-supervised learning algorithms, such as self-training and co-training models, are trained on a combination of labeled and unlabeled data. These approaches are particularly useful when obtaining a fully labeled dataset is impractical or cost-prohibitive [26–28]. Reinforcement learning algorithms, such as Q-learning, state-action-reward-state-action, deep Q network, proximal policy optimization, and actor-critic methods, learn by interacting with an environment, using feedback from their actions and experiences [26–29]. DL algorithms, a subset of ML that uses NN with many layers (deep networks), include deep convolutional neural networks (DCNNs), and recurrent neural networks (RNNs), which feature long short-term memory (LSTM) networks, generative adversarial networks, and transformers [12–16]. Ensemble learning algorithms combine the predictions of several base estimators, such as bagging (bootstrap aggregating), boosting (e.g., AdaBoost, extreme gradient boosting (XGBoost)), and stacking [12–16], to improve generalizability and robustness. Evolutionary algorithms, including genetic algorithms (GA), particle swarm optimization, ant colony optimization, teaching–learning-based optimization, and artificial bee colony, utilize the principles of natural selection to iteratively select and modify populations of solutions [29,30]. Dimensionality reduction algorithms, which include feature selection techniques (filter, wrapper, and embedded methods) and feature extraction techniques (principal component analysis and t-distributed stochastic neighbor embedding), reduce the number of random variables to consider [16–19]. This simplifies the complexity of the data, aiding in more efficient analysis and interpretation.

Figure 3: Taxonomy of machine intelligence algorithms by learning methodology and function
The predominance of algorithms such as SVM, CNN, and RF in mental health research is largely driven by the nature of the data and the diagnostic tasks involved. SVM is well-suited for high-dimensional datasets with limited samples, a common trait in Electroencephalogram (EEG) and questionnaire-based studies, due to its robustness in handling nonlinear separability and small-sample learning. CNNs excel in processing structured data like brain imaging magnetic resonance imaging (MRI), functional MRI (fMRI) and EEG signals by capturing spatial hierarchies and local patterns, making them ideal for diagnosing conditions like AD, SZ, and ASD. RF offers high interpretability and resistance to overfitting, particularly useful in scenarios involving heterogeneous or tabular data, such as clinical surveys and behavioral markers. These methods were frequently chosen across studies due to their favorable balance between accuracy, computational efficiency, and applicability to varied input modalities. Other models such as LSTM, LoR, kNN, or ensemble methods were included where temporal dynamics, interpretability, or ensemble robustness were required. Thus, the algorithm choices reflect their alignment with the specific nature of mental health data ranging from time-series and spatial data to text and behavioral features.
3.3 MQ3: How Has the Collaboration Network among Researchers in the Field of Machine Intelligence for Mental Health Diagnosis Evolved from 2018 to 2025?
Figs. 4–6 were created using VOSviewer, drawing upon data from the Scopus database for network visualization. Fig. 4 presents a VOSviewer network visualization that demonstrates the linkages between researchers, signified by their respective names during 2018–2025. This is depicted through a color spectrum at the bottom. Each node symbolizes an individual researcher, with the node’s size reflecting their scholarly productivity or citation count. The interconnecting lines represent either collaborative efforts or citation relationships. The nodes’ color variations correspond to different years, illustrating the dynamic nature of collaborative networks over time.
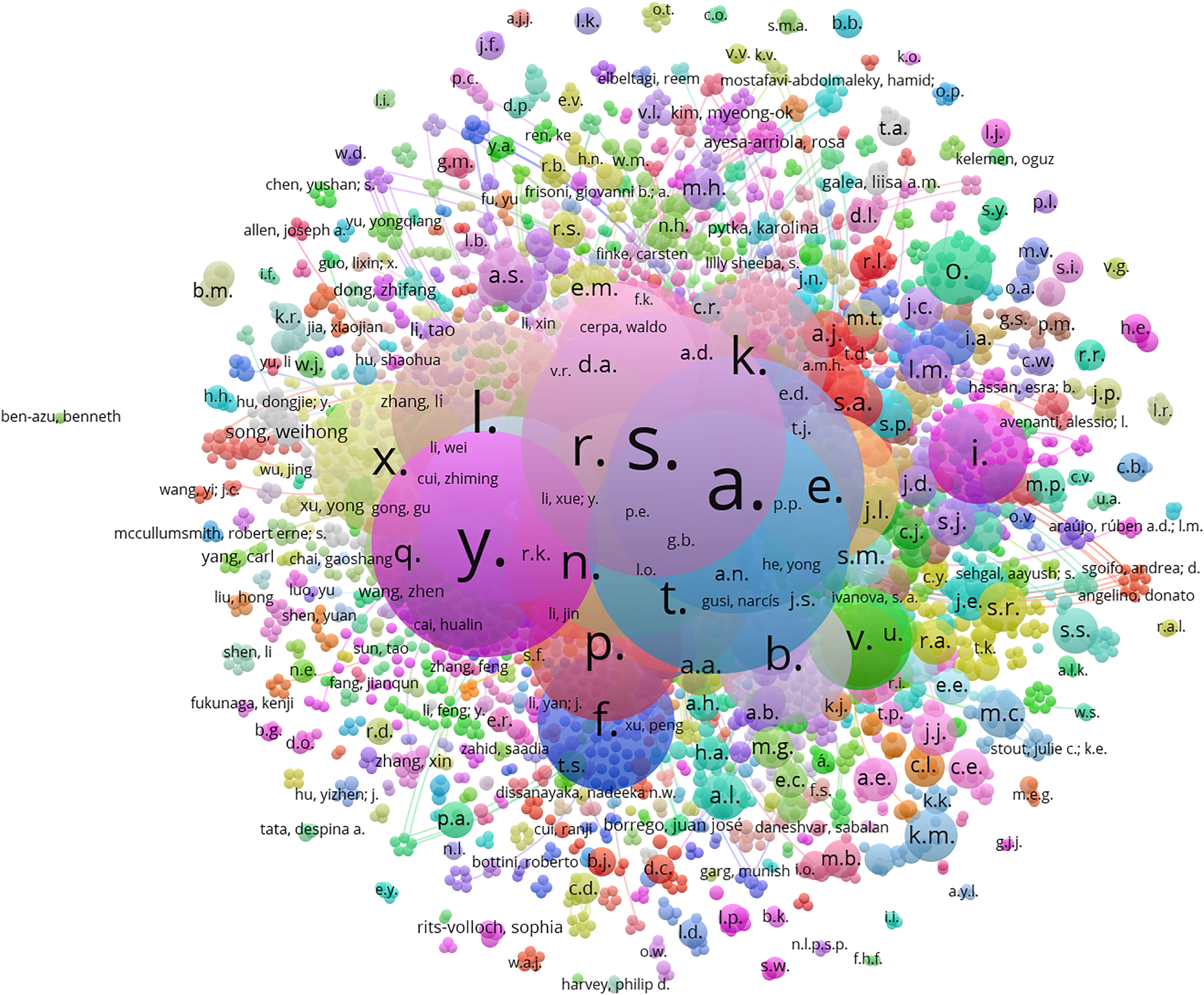
Figure 4: VOSviewer network visualization showing the linkages between researchers, signified by their respective names, spanning from 2018–2025

Figure 5: Network of connections among researchers specializing in the diagnosis of mental health diseases

Figure 6: Interrelationships among various terms associated with AI in mental health
Fig. 5 illustrates the network of connections among researchers specializing in the diagnosis of mental health diseases. The nodes’ diverse colors denote clusters of researchers who frequently collaborate or are jointly cited, whereas the node size may signify the magnitude of their citations or their prominence in the field. The connections between names indicate coauthorship or other collaborative forms. The color gradient at the bottom of the figure, which ranges from 10–60, quantifies the researchers’ impact through their publication and citation counts.
Fig. 6 graphically depicts the interrelationships among various terms associated with AI in mental health. Central terms such as “machine learning,” “artificial intelligence,” “deep learning,” and “neural networks” are indicative of a focus on AI technologies. Adjacent terms reveal AI applications within mental health, covering specific conditions such as depression, anxiety, BD, and SZ. The diagram elucidates the interconnectedness of these topics within contemporary research, with larger nodes suggesting terms that appear more frequently within the scholarly literature, indicating a substantial focus on the application of AI in mental health diagnosis and treatment. The inclusion of terms such as “COVID-19” and “coronavirus disease 2019” signifies the pertinence and investigative interest at the juncture of AI and the pandemic’s effects on mental health. This map infers the application of ML techniques to comprehend and perhaps forecast mental health outcomes during the COVID-19 pandemic. Terms such as “anxiety,” “depression,” and “mental health” situated in proximity to “COVID-19” mirror an upswing in research exploring the pandemic’s impact on mental health disorders, employing AI as an analytical, diagnostic, and management tool. It encapsulates the interdisciplinary essence of contemporary research, bridging AI with healthcare challenges intensified by the pandemic.
The PRISMA framework has been used for the systematic review as it ensures methodological rigor by enforcing a standardized, multi-stage selection process covering identification, screening, eligibility, and inclusion, aligned with predefined inclusion/exclusion criteria for mental health and machine intelligence studies. This structured approach minimizes selection bias, enables reproducible dataset and methodology mapping, and supports transparent reporting of algorithmic performance, dataset diversity, and clinical applicability. By clearly documenting each decision point, PRISMA strengthens the validity of comparative analyses across disorders and facilitates replication.
4.1 RQ1: What Are the Key Methodological Studies in the Field of Mental Health?
This section provides an analysis of the techniques utilized to identify mental disorders, with a focus on each mental health category individually. It further examines the crucial factors that contributed to successful mental health interventions and evaluates the effect of incorporating various classifiers, such as ML, DL, and other methodological approaches, on the performance of models. The mental disorders addressed include anxiety/stress, depression, SZ, BD, ASD, and AD.
To explore the application of machine intelligence in individual mental health conditions, the analysis begins with anxiety and stress. These conditions are among the most commonly reported and have received substantial attention in recent research due to their high prevalence and diverse data availability.
Recent developments in machine intelligence have enabled new strategies for the detection and treatment of mental health disorders. A variety of methods have been employed by researchers globally, including the analysis of electrocardiogram signals and the application of ML algorithms, to better comprehend and tackle the intricacies of mental health issues. This subsection presents prominent studies conducted between 2018 and 2021 that illustrate the advancements and variety within this domain, covering the technological aspects.
In 2018, R-R intervals from electrocardiogram signals were determined by Adheena et al. [31] using the Pan Tompkins method. The identification of anxious states was achieved with accuracies of 69.35% and 71.1% by employing the Kalman filter and SVM, respectively. In 2018, Višnjić et al. [32] found that higher anxiety levels were linked to being younger, texting frequently, and using the internet less, whereas increased stress was associated with making fewer calls and having more phone conversations. In the same year, Wang et al. [33] introduced a model to trigger emotions and pinpointed ideal EEG electrode sites, like Fp2, for evaluating the mental states of miners, leading to the creation of a smart helmet system for real-time monitoring. In 2019, Puli and Kushki [34] introduced a method for the prompt diagnosis and alleviation of negative outcomes from physical activity. By integrating accelerometry and heart rate signals through a novel multiple model Kalman-like filter, an accuracy of 93% was achieved in detecting arousal in children with ASD. Gavrilescu and Vizireanu [35] developed a method that combined active appearance models, an SVM, and a facial action coding system, achieving a 93% accuracy rate in differentiating healthy individuals and an 85% accuracy rate for major depressive disorder (MDD), posttraumatic stress disorder, or generalized anxiety disorder (GAD). Ramakrishnan et al. [36] reached a 96% accuracy in detecting stress conditions using SVMs trained on physiological data, while a DT that incorporated both physiological and self-reported data achieved an 84% accuracy in identifying high trait anxiety. Khouja et al. [37] reported that SVMs accurately identified stress with a 96% success rate. Increased computer use was linked to a slight rise in anxiety and depression risk during weekdays and weekends. Fathi et al. [38] developed a three-stage methodology for diagnosing social anxiety disorder, which included preprocessing steps such as feature selection, normalization, and anomaly detection. An adaptive network-based fuzzy inference system model demonstrated an accuracy of 98.67%, with sensitivity at 97.14% and specificity at 100%. Priya et al. [39] utilized DT, RF trees, naïve Bayes (NB), SVM, and kNN to forecast anxiety, depression, and stress levels using data from the Depression, Anxiety and Stress Scale (DASS21) questionnaire, achieving accuracies as high as 79.8%. Xie et al. [40] introduced an innovative method that combined the functional connectivity of brain networks with CNNs for detecting anxiety and depression through EEG, achieving a classification accuracy of 67.67%. Arsalan et al. [41] gathered EEG data from 28 participants over a three-minute period, extracted features in the time domain, applied a wrapper method for feature selection, and reached an accuracy of 78.5% in classifying anxiety using the RF classifier. In 2020, Chen et al. [42] conducted a survey with 78 couples experiencing recurrent miscarriages and 80 couples without, using a questionnaire. Their multiple regression analysis identified anxiety, stress, social support, and recurrent miscarriage as predictors of depressive symptoms, accounting for 62.9% of the variance. In 2021, Xiong et al. [43] developed YODA, a tool for predicting youth disorders through online questionnaires filled out by parents, using a feature ensemble-based Bayesian neural network (FE-BNN) to achieve area under the curve (AUCs) of 0.8683, 0.8769, and 0.9091 for separation, generalized, and social anxiety disorders, respectively. In 2021, Kruthika et al. [44] applied ML for predicting mental disorders, incorporating Indian classical ragas, with SVM emerging as the most effective classifier, achieving an accuracy of 87.23%. In 2021, Meyerbröker and Morina [45] reviewed the use of virtual reality in assessing and treating anxiety disorders, focusing on patient acceptance and therapist reluctance and their impact on the therapeutic alliance. In 2021, Sharma and Verbeke [46] analyzed biomarkers from 11,081 healthy Dutch citizens’ lifeline biobank data, reducing the dataset to 28 variables. Using ML, relationships among biomarkers and four anxiety disorders were explored, revealing significant but weak correlations (0.17 to 0.3).
Modern approaches to detecting anxiety and stress are increasingly incorporating physiological data, such as electrocardiogram (ECG) readings, along with behavioral patterns from technology use. ML and DL methods, including SVM and NNs, are utilized to examine these datasets, achieving significant accuracy in identifying stress and anxiety indicators. By combining multiple classifiers, the models’ performance is improved, allowing for a more nuanced understanding of mental states by considering both physiological and lifestyle elements. Effective treatment depends on early detection, personalized interventions, and ongoing monitoring, areas where these integrated technological methods can be crucial. These developments encourage a more proactive and individualized strategy for managing anxiety and stress, highlighting the vital role of multimodal data and sophisticated analytical models in enhancing mental health care.
While anxiety and stress have demonstrated strong correlations with behavioral and physiological indicators, depression presents a broader spectrum of symptoms and more heterogeneous data types. The following subsection delves into methodological developments aimed at diagnosing depression using multimodal datasets such as EEG, social media, and facial expression analysis.
In recent years, significant advancements have been made in the application of machine intelligence for diagnosing depression, employing various data sources and analytical techniques. Researchers have developed and validated multiple methods to enhance the detection and understanding of depression, ranging from the analysis of facial features and movements in videos to the utilization of EEG signals and social media data. This subsection summarizes key research findings from 2018 to 2021, highlighting the diverse approaches and technologies employed to assess depression severity, detect depressive symptoms, and predict treatment responses, thereby illustrating the potential of machine intelligence in mental health diagnostics.
In 2018, Wang et al. [47] collected videos from both depressed patients and a control group, utilizing SVMs to analyze facial features and movement variations. The results yielded a recall of 80.77%, precision of 77.75%, accuracy of 78.85%, and specificity of 76.92%. Cai et al. [48] developed a psychophysiological database comprising 213 subjects, including 92 depressed individuals and 121 healthy controls (HCs), recording EEG signals during both resting states and sound stimulation. Various techniques were used to extract features, with kNN achieving the highest accuracy of 79.27%. Jiang et al. [49] analyzed 170 native Chinese individuals, including 85 HCs and 85 depressed patients, and introduced an ensemble LoR model for detecting depression in speech (ELoRDD), which demonstrated favorable sensitivity and specificity ratios. Zhou et al. [50] proposed DepressNet, a deep regression network for assessing depression severity using facial data, which performed well on the Audiovisual Emotional Challenge (AVEC) 2013 and 2014 benchmark datasets, achieving a mean absolute error (MAE) of 6.21 and a root mean square error (RMSE) of 8.39. Wu et al. [51] recorded EEG responses to uplifting images and applied the conformal kernel SVM (CK-SVM) for participant-independent classification of MDD, achieving an accuracy of 83.64%, sensitivity of 87.50%, and specificity of 80.65%. Islam et al. [52] analyzed Facebook data using ML techniques, including DTs, SVMs, ensembles, and kNNs, with DT achieving the highest accuracy of 99%. In 2019, Li et al. [53] employed EEG data from a 128-channel HydroCel Geodesic sensor net for diagnosing depression, attaining an accuracy of 89.02% using an ensemble learning model and power spectral density features. Tadesse et al. [54] used ML and natural language processing (NLP) techniques, achieving optimal depression detection with an accuracy of 91% and an F1-score of 0.93 through a combination of features and multilayer perceptron (MLP) classifier. Ay et al. [55] introduced a hybrid model that combined LSTM and CNN architectures for detecting sadness in EEG signals, achieving notable accuracies of 99.12% for the right hemisphere and 97.66% for the left hemisphere. In 2020, Cai et al. [56] analyzed EEG signals from 86 depressed patients and 92 HCs using linear and nonlinear features along with GA for feature weighting, resulting in kNN achieving 86.98% accuracy. Wang et al. [57] proposed the use of Kinect, a cost-effective depth sensor, for nonintrusive, real-time detection of depression, reaching a classification accuracy of 93.75% across 95 subjects (43 depressed and 52 nondepressed). Wu et al. [58] introduced a DL approach for depression detection with heterogeneous data sources, using RNNs for 1453 active Facebook users, which achieved an F1-score of 76.9%, precision of 83.3%, and recall of 71.4%. Smith et al. [59] conducted semi-structured interviews, incorporating the Patient Health Questionnaire (PHQ-9) with 46 older individuals, where SVM was used to analyze recorded interviews, correctly predicting high and low depression scores between 86% and 92%. In 2021, He et al. [60] introduced an integrated framework combining deep local global attention with CNNs, applied to the AVEC 2013 and 2014 datasets, achieving an RMSE of 8.39 and an MAE of 6.59 on AVEC 2013 and 8.30 and 6.51 on AVEC 2014. Zhou et al. [61] utilized term frequency-inverse document frequency (TF-IDF) and multimodal features from tweets analyzed through linear discriminant analysis (LDA), LR, and Gaussian naive Bayes (GNB), with LDA achieving the highest precision (91.2%) and accuracy (90.4%) in depression detection. Seal et al. [62] introduced DeprNet, a CNN designed for EEG data classification in depression, achieving 99.37% accuracy and 0.999 AUC in record-wise splits, while subject-wise splits yielded an accuracy of 91.4% and an AUC of 0.956. Nemesure et al. [63] utilized 59 features from general health surveys to train a model for MDD and GAD among 4184 students, achieving AUCs of 0.73 for GAD patients and 0.67 for MDD patients. DeSouza et al. [64] recommended using NLP to analyze speech patterns in late-life depression, investigating differences from younger adults with depression and older adults with other conditions. Saeedi et al. [65] proposed a DL framework employing EEG data for automatic discrimination between MDD patients and HCs, achieving an accuracy of 99.24% with one-dimensional CNN-LSTM on effective connectivity images. Kong et al. [66] introduced a spatiotemporal graph convolutional network for the automatic diagnosis of MDD and predicting treatment response, demonstrating superior performance compared to other models.
Depression is diagnosed through clinical interviews and standardized questionnaires, with emerging methods leveraging ML to analyze speech patterns, physical activity, and social media usage for signs of depressive symptoms. DL models, including NLP and sentiment analysis, offer new avenues for identifying depression outside of traditional clinical settings, potentially reaching individuals earlier in their illness. Integrating these models with clinical data can increase diagnostic precision and guide the development of customized treatment plans, emphasizing the importance of early intervention, ongoing monitoring, and a holistic approach to care.
In contrast to depression, SZ is characterized by complex neurocognitive symptoms and often requires advanced neuroimaging and signal processing techniques for diagnosis. The next section explores how ML and DL methods have been applied to identify biomarkers and classify SZ cases.
The exploration of machine intelligence in diagnosing SZ has undergone significant advancements through diverse methodologies and data sources in recent years. Researchers have made strides in identifying biomarkers and symptoms of SZ with notable accuracy from wearable technology that captures autonomic behavior to advanced imaging techniques and EEG signal analysis. This summary encapsulates studies from 2018–2021 and highlights the application of ML algorithms such as SVMs, CNNs, and deep belief networks (DBNs) in distinguishing SZ patients from HCs. These studies not only highlight the potential for more accurate and early diagnosis but also demonstrate the evolving landscape of SZ research, where technology and medicine intersect to offer innovative solutions for mental health diagnostics.
In 2018, wearable technology was utilized by Cella et al. [67] for mobile health applications in patients with SZ. A link was established between parasympathetic dysregulation and symptom severity. The mHealth device effectively measured autonomic behavior and activity. In the same year, Chakraborty et al. [68] conducted interviews with 78 participants, achieving subjective rating predictions with an accuracy ranging from 61% to 85% based on openSMILE acoustic signals. The groups were distinguishable with an accuracy of 79% to 86% using supervised learning techniques. Mikolas et al. [69] employed brain fractional anisotropy data from 77 first-episode SZ patients and 77 age-matched controls, achieving a significant accuracy of 62.34% with a linear SVM in differentiating patients from controls. In 2019, Jahmunah et al. [70] introduced an automated diagnostic tool for evaluating EEG signals from both healthy and SZ subjects. The SVM with radial basis function (SVM-RBF) demonstrated superior performance, attaining an average value of 92.91%. Zhu et al. [71] investigated 960 individuals, finding a correlation between prenatal famine exposure and SZ, with a significant association of the rs2283291 genotype in males. Devia et al. [72] monitored EEG activity in SZ patients viewing nature scenes, achieving an overall accuracy of 71%, with 81% sensitivity for patients and 59% specificity for controls. Oh et al. [73] applied an eleven-layered CNN to analyze EEG signals from 14 healthy volunteers and 14 SZ patients, obtaining accuracies of 98.07% for non-subject-based testing and 81.26% for subject-based testing. Latha and Kavitha [74] analyzed SZ subjects using DBNs based on ventricular regions, achieving a high classification accuracy of 90% with segmented ventricles. The segmented ventricle images exhibited utility, achieving a high AUC (0.899). In 2020, Krishnan et al. [75] applied multivariate empirical mode decomposition to EEG data to evaluate entropy parameters, revealing significant differences between HCs and SZ patients using SVM-RBF, with a 93% F1-score and 0.9831 AUC. Siuly et al. [76] proposed an EEG-based system for SZ identification, achieving a 93.21% correct classification rate using empirical mode decomposition and ensemble bagged trees. Lei et al. [77] achieved 81% accuracy in SZ classification through ML on functional connectivity data from 295 patients and 452 healthy individuals, outperforming other methods. In 2020, Shalbaf et al. [78] attained a 98.6% accuracy in SZ diagnosis using a deep CNN with transfer learning on EEG data, surpassing other models. In 2021, Baygin et al. [79] introduced an innovative automated model for SZ detection through EEG signals, achieving high classification accuracies of 99.47% and 93.58% on two SZ databases. In 2021, Shi et al. [80] implemented multimodal imaging and multilevel characterization with a multiclassifier technique, reaching a classification accuracy of 83.49% for SZ patients and HCs using fMRI and static MRI data without global signal regression. Kadry et al. [81] proposed an automated SZ detection method using T1-weighted brain MRI scans, employing a pretrained visual geometry group (VGG16) and a slime mold algorithm for feature optimization, achieving an accuracy of 94.33% with SVM-C. Khare et al. [82] combined time-frequency analysis and a CNN for SZ detection, reaching an accuracy of 93.36% through the use of spectrograms and CNNs. Sharma et al. [83] introduced a computer-aided diagnosis method using single-channel EEG data for accurate SZ diagnosis, achieving maximum accuracies of 99.21% and 97.2% using wavelet-based features and SVM and kNN, respectively. Karthik et al. [84] identified seven biomarkers from the Gene Expression Omnibus (GEO) database for SZ and BD, with their proposed system achieving accuracies of 97.01% and 95.65% on the respective datasets, surpassing benchmark performance.
SZ detection relies primarily on psychiatric evaluations and the identification of characteristic symptoms, such as delusions and hallucinations. ML models have been applied to EEG and fMRI data to detect abnormalities in brain activity and structure associated with SZ. DL techniques, such as autoencoders and RNNs, have shown promise in identifying patterns indicative of the disorder, potentially leading to earlier diagnosis. Success in treatment hinges on early detection, integrated care approaches, and the personalized adjustment of therapeutic strategies, where AI can play a significant role in predicting treatment outcomes.
Moving from SZ, which typically emerges in early adulthood, the analysis now focuses on ASD, a condition with developmental origins. This section highlights how early screening and behavioral modeling through ML contribute to ASD diagnosis across different age groups.
4.1.4 Autism Spectrum Disorder (ASD)
The application of machine intelligence in the diagnosis and understanding of ASD has advanced significantly in recent years, utilizing a diverse range of data types and analytical methods. EEG signal analysis, MRI imaging, and social media data have been employed by researchers to create models with high accuracy for detecting ASD across different age groups. These studies emphasize the potential of ML algorithms, including SVM, kNN, deep neural network (DNNs), and reinforcement learning, to provide personalized support and early intervention strategies, from early diagnosis in toddlers to screening in adults. This summary presents key contributions from 2018 to 2021, illustrating the varied approaches used to improve ASD diagnosis and understanding, including wearable technology for feature extraction and the development of cognitive computing-based learning models and optimization algorithms for screening. The evolving role of technology in advancing ASD care and research is highlighted.
In 2018, Ibrahim et al. [85] examined EEG feature extraction and classification for epilepsy and ASD. Utilizing various datasets, their methodology attained an accuracy of up to 94.6% through the discrete wavelet transform, Shannon entropy, and kNN algorithms. A cognitive computing-based learning model aimed at enhancing personalized education for ASD patients was proposed by Vijayan et al. [86]. This model employed reinforcement learning and a NN to identify and support autistic students through interactive chatbots and visual aids. Li et al. [87] applied multichannel CNNs along with a patch-level data-expanding technique to detect infants at risk for ASD, achieving an accuracy of 0.7624 on the National Database for Autism Research (NDAR) dataset. Further validation in at-risk infant populations was recommended. In 2019, Kong et al. [88] constructed an individual brain network for feature representation and implemented a deep NN for classification between ASD and typical controls (TC). Their method, using 3000 top features from T1-weighted MRI scans in the autism brain imaging data exchange (ABIDE I), achieved an accuracy of 90.39% and an AUC of 0.9738. In 2019, Akter et al. [89] utilized a Kaggle dataset containing 2009 records to suggest an early diagnosis model for ASD. By employing methods such as AdaBoost, flexible discriminant analysis, C5.0, and SVM, accuracies of 98.77%, 97.20%, 93.89%, and 98.36% were recorded for toddler, child, adolescent, and adult datasets, respectively. An ML model was introduced by Thabtah et al. [90] for screening ASD in adults and adolescents. Through LoR, the model reached accuracies of 99.91% for adults and 97.58% for adolescents. In 2019, Kou et al. [91] investigated 77 children and identified a lower visual preference for dynamic social stimuli as a potential biomarker for ASD in Chinese children. In 2020, Goel et al. [92] presented the modified grasshopper optimization algorithm (MGOA) for ASD screening across all life stages. Testing on datasets from children, adolescents, and adults, the approach utilizing the RF classifier achieved near-perfect accuracy, specificity, and sensitivity for ASD prediction at all developmental stages. In 2020, Yaneva et al. [93] assessed 31 participants with ASD and 40 HC, achieving an accuracy of 74% through LoR classifiers using gaze and non-gaze features. Caution was advised against web-search tasks for very young children due to the limited sample size. In 2020, Tang et al. [94] created a deep multimodal model utilizing two types of connectomic data derived from fMRI scans. Using the ABIDE dataset with 1035 subjects, their method achieved notable results, including a classification accuracy of 74%, a recall of 95%, and an F1-score of 0.805, surpassing unimodal approaches. In 2020, Brueggeman et al. [95] introduced force ASD, an ensemble classifier that integrates brain gene expression and network data. The reliability of prior predictors for estimating gene contributions to ASD etiology was demonstrated. In 2020, Wedyan et al. [96] analyzed thirty children, employing an SVM with features extracted using LDA. Among them, fifteen children were diagnosed with ASD, resulting in a sorting accuracy of 100% and an average accuracy of 93.8%. In 2020, Cantin-Garside et al. [97] implemented SVMs, kNN, and additional algorithms for ASD diagnosis, achieving 99.1% accuracy for individuals and 94.6% accuracy for groups, indicating excellent classification efficiency. In 2021, Negin et al. [98] proposed a nonintrusive vision-assisted technique utilizing YouTube videos for detecting ASD-related behaviors. The best accuracy (79%) was attained through the identification of the histogram of the optical flow descriptor. In 2021, Papakostas et al. [99] adopted an ML-based multimodal approach to evaluate children’s engagement. By employing various ML techniques and ensemble models, AdaBoost and DT reached an accuracy of 93.33% in assessing children’s engagement. In 2021, Akter et al. [100] utilized the Kaggle and University of California Irvine datasets for ASD across age groups. Their multimodel approach incorporated various ML algorithms, with LoR outperforming others. Accuracies of 100%, 99.32%, 94.33%, and 99.74% are achieved for toddlers, kids, teens, and adults, respectively. In 2021, Vakadkar et al. [101] used a toddler dataset from Kaggle, which achieved high accuracies with classifiers: LR (97.15%), NB (94.79%), SVM (93.84%), kNN (90.52%), and RFClassifier (81.52%). Large datasets enhance accuracy.
ASD detection methodologies typically incorporate behavioral assessments and developmental screenings during early childhood. Recent advancements have seen the application of ML algorithms to analyze genetic data, facial expressions, and speech patterns for identifying early signs of ASD. DL models, such as CNNs, have been utilized on neuroimaging data to reveal structural and functional differences in the brains of individuals with autism. These technological methods complement conventional diagnostic approaches, providing the potential for earlier and more accurate diagnoses, which are essential for implementing effective intervention strategies.
While ASD research emphasizes early developmental screening, BD studies often focus on mood fluctuation tracking and episodic pattern recognition. The subsequent section examines how data from neuroimaging, digital phenotyping, and EEG are used to distinguish BD from related mood disorders.
In recent years, substantial advancements have been achieved in the application of ML and data science techniques to enhance the diagnosis and understanding of BD. A variety of methodologies have been investigated, including MRI analysis, EEG data classification, digital phenotyping, and the utilization of NLP in healthcare records. These studies have contributed not only to the identification of biomarkers and the exploration of the neural and genetic foundations of BD but also to innovative methods for monitoring and predicting mood states in individuals with BD. This summary outlines key research efforts from 2018 to 2021, emphasizing how the integration of diverse data sources and advanced analytical techniques has improved the accuracy of BD diagnosis, provided insights into the pathophysiology of BD, and opened new pathways for personalized treatment strategies.
In 2018, Altamura et al. [102] employed 3 Tesla MRI to examine 171 subjects (114 BD and 57 HC). Psychotic BD patients demonstrated gray matter volume deficits in the left frontal and right temporoparietal cortices. The integration of multiple datasets may have affected the results. Alimardani et al. [103] induced steady-state visual evoked potentials in both schizophrenic and BD patients using a 16 Hz visual stimulus. EEG data were classified using various algorithms, with kNN achieving the highest accuracy of 91.30%. Satisfactory accuracies above 70% were maintained with reduced analysis times. Perez et al. [104] utilized a custom smartphone application involving 130 BD individuals and applied signature-based learning, which correctly categorized 75% of participants into the appropriate diagnostic groups and predicted over 70% of future mood evaluations, with an accuracy of 89%–98% among healthy participants. Manzan et al. [105] suggested using MLP networks with orthogonal bipolar vectors for pattern recognition, showing enhanced performance compared to traditional methods across the Iris, Semeion digit, and Australian sign language datasets. Johnson et al. [106] analyzed 29 HCs and 40 bipolar I patients, some of whom were rescanned during different mood states, employing the Montgomery-Asberg depression rating scale, the Young Mania rating scale (YMRS), and 3 Tesla MRI. A key limitation noted was the small sample size. In 2019, Farshad [107] applied the K-means data description method for internal fault detection on a 1000 km bipolar high-voltage direct current line, successfully identifying 4320 internal and 2816 external faults using local measurements. Abaeikoupaei and Al Osman [108] introduced an automatic ternary classification model for BD based on audio features, achieving an unweighted average recall of 59.3% with the stacked ensemble classifier on the test set. Chandran et al. [109] employed NLP to extract information about obsessive and compulsive symptoms from mental healthcare records, attaining precision and recall values of 0.77 and 0.67, respectively. Maes et al. [110] investigated biomarkers related to oxidative and nitrosative stress in individuals with MDD and BDs I and II, seeking to differentiate among these disorders. In 2020, Stanislaus et al. [111] studied 203 newly diagnosed BD patients, 54 unaffected relatives, and 109 HCs, finding significantly higher mood instability ratings in patients compared to HCs. The study noted a lower-than-expected number of unaffected relatives. Lee et al. [112] utilized data science techniques to identify significant single nucleotide polymorphisms associated with the classification of BDs I and II. A classifier using 42 single nucleotide polymorphisms achieved an AUC of 0.9574, resulting in a 3.46% increase in accuracy. Li et al. [113] combined voxel-based morphometry and regional homogeneity analyses with the least absolute shrinkage and selection operator (LASSO) for feature selection, achieving 87.5% accuracy with their SVM model in identifying BD patients. In 2021, Li et al. [114] developed a DL framework utilizing a CNN for the automatic diagnosis of first-episode psychosis (FEP), BD, and HCs based on structural MRI data. The CNN reached high accuracy (99.72%), precision (99.76%), recall (99.74%), and F1-score (99.75%) in distinguishing between FEP patients, BD patients, and HCs. Su et al. [115] utilized LASSO regression and ElasticNet regression to predict Hamilton depression rating scale (HAM-D) and YMRS scores for BD patients based on digital phenotyping data from 84 individuals, achieving prediction errors of 2.73 and 1.06 points for HAM-D and YMRS, respectively. Llamocca et al. [116] employed ML, including RF, DTs, LoR, and SVM, to classify states of BD using various data sources, with RF yielding the highest accuracy.
For BD, detection methods include clinical assessments, self-report questionnaires, and, increasingly, physiological data analysis through wearable technology. ML techniques, including SVMs and NNs, are employed to analyze patterns in mood fluctuations and sleep, offering potential for early detection and differentiation from other mood disorders. The integration of these classifiers with traditional diagnostic tools enhances accuracy and aids in developing personalized treatment strategies, focusing on medication management, psychotherapy, and lifestyle adjustments.
Unlike BD, which involves episodic mood variations, AD is a progressive neurodegenerative condition. The following discussion centers on the use of neuroimaging, biomarker analysis, and smart home data for early AD detection and monitoring.
In recent years, a wide range of ML and DL techniques have been applied to improve the diagnosis and understanding of AD. Studies have utilized various data types, including MRI and fMRI scans, smart home data, and even human plasma, to develop models capable of identifying AD and its stages with remarkable accuracy. From the use of DNN and RF for feature selection to the implementation of novel biomarkers and biosensors, researchers have made significant strides in enhancing AD diagnostics. This summary highlights key research from 2018–2021, highlighting the diversity of approaches and advancements in AD detection. These efforts not only demonstrate the potential for early and accurate diagnosis but also contribute to the development of personalized treatment strategies, indicating the critical role of technology in addressing this complex disease.
In 2018, a DNN and RF feature selection classification model was introduced by Amoroso et al. [117] for 240 subjects across four categories: AD, HC, mild cognitive impairment (MCI), and cMCI. DNN results included recall values of (87.5%, 74.5%, 52.5%, 52.5%) and precision values of (27.5%, 34.3%, 52.5%, 51.2%). The fuzzy and RF models achieved notable recall values of (82.5%, 78.6%, 50%, and 55.9%, respectively) and precision values of (20%, 22.9%, 57.5%, and 47.9%, respectively). In the same year, regression models utilizing SmoteBOOST and a wrapper-based rapidly converging Gibb were developed by Alberdi et al. [118] for symptom prediction, focusing on smart home data of older adults from 2011 to 2016. A clinical assessment was introduced using an activity behavior algorithm to evaluate global cognition and mobility. Among the assessed models, RF exhibited the highest sensitivity (0.92) and F1-score (0.77), while MLP had the lowest values (0.71 sensitivity, 0.69 F1-score). Abd-Elminaam [119] proposed a child safety model in 2018 that employed smartphones equipped with near-field communication, quick response code readers, and optical character recognition features to prevent situations involving missing children, connecting to a cloud-based platform for Internet of Things data storage. An AD diagnosis model was proposed by Islam and Zhang [120] using a deep CNN with brain MRI data, identifying various disease stages, with ensemble networks achieving accuracies of 78% and 77%. In 2019, a DL model for AD and c-MCI diagnosis was developed by Basaia et al. [121] using CNNs on 3D T1-weighted MRI scans from Alzheimer’s disease neuroimaging initiative (ADNI) and institutional datasets, achieving 99% accuracy. A DL framework combining Three Dimensional (3D)-CNN and fully stacked bidirectional LSTM for enhanced AD diagnosis was developed by Feng et al. [122]. The average accuracies of baseline MRI and 18-fluorodeoxyglucose positron emission tomography data from the ADNI dataset were 94.82%, 86.36%, and 65.35%, respectively. In 2019, a nanoplasmonic biosensor utilizing gold nanorods and a chaotropic agent for ultrasensitive AD biomarker detection in human plasma was introduced by Kim et al. [123], which minimized overlapping protein levels to enhance diagnostic accuracy. Acharya et al. [124] proposed a computer-aided brain-diagnosis system for AD detection using MRI and shearlet transform in 2019, achieving accuracy rates of 94.54%, precision of 88.33%, and sensitivity of 96.30%. With a benchmark MRI database, it attained an accuracy of 98.48% and 100% precision. Li et al. [125] introduced a 4-dimensional DL model for AD detection in 2020, achieving accuracy exceeding 92% based on fMRI data. An unsupervised DL method for AD diagnosis utilizing CNN and MRI data was proposed by Bi et al. [126], with the leading model reaching an accuracy of up to 97.01%. A novel biomarker, three-dimensional tortuosity, was introduced by Barbará-Morales et al. [127], with an RF classifier achieving 86.66% accuracy. Al-Shoukry et al. [128] reviewed AD disease through MRI data and ML techniques, emphasizing biomarkers and neuroimaging studies. Ramzan et al. [129] investigated resting-state fMRI for classifying AD stages, achieving high accuracies with a fine-tuned ResNet 18 model. In 2021, Liu et al. [130] utilized open access series of imaging studies (OASIS) structural image data, applying depthwise separable convolution, with classification accuracies ranging from 78.02% to 93.02%. DEMentia NETwork (DEMNET) was presented by Murugan et al. [131] on ADNI data, achieving an overall accuracy of 95.23% and recommending testing on diverse datasets. A CNN and transfer learning approach was employed by Helaly et al. [132] for ADNI dataset classification, achieving accuracy rates of up to 97% using the enhanced VGG19 model.
ASD detection methodologies frequently incorporate behavioral assessments and developmental screenings during early childhood. Recent advancements involve the application of ML algorithms to analyze genetic data, facial expressions, and speech patterns for the identification of early signs of ASD. DL models, particularly CNNs, have been utilized on neuroimaging data to reveal structural and functional differences in the brains of individuals with autism. These technological approaches enhance traditional diagnostic methods, providing the potential for earlier and more accurate diagnoses, which is essential for the initiation of effective intervention strategies.
Although the reviewed techniques have shown substantial promise across multiple disorders, it is essential to examine the methodological limitations and potential biases that may affect their generalizability, interpretability, and clinical integration.
4.1.7 Limitations and Biases of the Chosen Methods
Despite their demonstrated effectiveness, the machine intelligence techniques reviewed—such as SVM, CNN, RF, and LSTM—have certain limitations and biases that may affect generalizability and interpretability in mental health diagnostics. A common concern is the data dependency and overfitting risk inherent in DL models, especially when trained on small or imbalanced datasets. CNNs and LSTMs, while powerful, often require extensive labeled data and may not generalize well across different populations or clinical settings without retraining.
SVM are effective in handling high-dimensional data but are sensitive to kernel choice and parameter tuning. They may also lack interpretability, making them less suitable for clinical decision-making in settings where explainability is critical. Similarly, RF, though robust and interpretable to a degree, can become computationally expensive and are prone to bias when trained on skewed datasets.
Moreover, algorithmic bias can arise when models reflect or amplify patterns in the training data, leading to disparities across demographic groups. This is particularly problematic in mental health, where symptoms manifest heterogeneously across age, gender, and cultural backgrounds. For example, if models are predominantly trained on data from one region or population, they may underperform in other contexts, leading to reduced diagnostic accuracy or missed cases.
Class imbalance, especially between healthy and affected cases, is another pervasive issue that skews model performance metrics. Models may achieve high overall accuracy while failing to detect minority classes such as rare or early-stage disorders. Additionally, lack of transparency in how some DL models derive their outputs makes it difficult to validate or interpret findings in a clinical setting.
Finally, many methods do not account for temporal dynamics of mental health states. Static models may overlook important longitudinal variations or transitions in patient conditions. Incorporating time-series models or hybrid frameworks may offer a path forward but also introduces added complexity and data requirements.
Addressing these limitations is essential for the ethical and effective deployment of machine intelligence in mental health care. Future efforts should prioritize model interpretability, bias mitigation, data diversity, and clinical validation.
With the methodological insights and challenges outlined, the next section provides a comparative evaluation of the strengths and weaknesses of the reviewed ML and DL techniques across anxiety, depression, and stress domains. This sets the stage for synthesizing best practices and identifying gaps for further exploration.
4.2 RQ2: What Are the Strengths and Weaknesses of These Specific Methodologies?
This section presents a comprehensive overview of recent studies that utilize ML and DL techniques for the detection and analysis of mental disorders, specifically focusing on anxiety, depression, and stress. Various methods, including SVM, LoR, DL, and other advanced algorithms, are encapsulated, showing their results, the datasets employed, and the targeted disease categories. Table 2 highlights the advantages and limitations of the respective studies, offering valuable insights into the current state of mental health diagnostics.
Table 2 provides an insightful overview of the progress made in applying ML and DL to diagnose mental health conditions, specifically anxiety, stress, and depression, throughout 2022–2023. This period has seen a diverse array of methodological approaches, ranging from traditional statistical models to advanced NN, applied to various data types and mental health issues. Despite notable achievements and advancements, challenges such as the necessity for larger sample sizes [133–136], the imperative to address bias [137–140], and the integration of these findings into clinical practice remain significant hurdles [141–143]. This not only underscores the immense potential that AI holds for enhancing mental health diagnostics but also emphasizes the ongoing need for further research to adapt these technological advancements for practical, real-world applications.
Several key benefits associated with these technological approaches in mental health diagnostics have been highlighted. High accuracy and precision in identifying conditions such as anxiety, stress, and depression are paramount for early detection and intervention. The application of diverse datasets, ranging from questionnaires and physiological signals to social media interactions, demonstrates the adaptability and versatility of ML models to various data inputs. These methodologies enable a deeper understanding of the intricate patterns and factors related to mental disorders, paving the way for more personalized and effective treatment plans. Furthermore, the integration of sophisticated computational models such as DL facilitates the processing of complex data structures, revealing subtle diagnostic nuances. The exploration of new biomarkers and the development of real-time monitoring systems underscore the innovative potential in preventative mental health care.
Among the algorithms that have demonstrated significant performance, SVM, DT, kNN, RF, and DL models, including CNNs and LSTM networks, have been particularly noteworthy. SVMs excel in classifying mental health conditions due to their effectiveness in managing high-dimensional data with limited training samples. DTs simplify the decision-making process, facilitating the clear identification of mental health indicators. The straightforward implementation and effectiveness of kNN in classification tasks, the improvement in prediction accuracy and mitigation of overfitting offered by RF, and the ability of DL models to extract complex patterns from extensive datasets all contribute to the depth, accuracy, and efficiency of mental health diagnostics.
The combined use of these algorithms presents a promising opportunity for the early identification and customized treatment of mental health disorders. Their application in various data analysis contexts—ranging from physiological monitoring to the analysis of textual and speech information—demonstrates the holistic approach to mental health diagnostics and the significant potential of ML in overcoming its fundamental challenges.
Table 3 illustrates how advanced computational techniques have facilitated a deeper comprehension of complex mental health disorders. For instance, the utilization of LDA to examine thousands of abstracts yields enhanced insights into the pathogenesis of AD, while the implementation of multiple classifiers, including kNN, achieves nearly perfect accuracy in diagnosing AD. This highlights the efficacy of integrating various algorithms to enhance diagnostic precision. The investigation of these computational models across different diseases underscores the versatility and adaptability of ML and DL techniques. For AD, innovative methodologies such as 3D-CNN models and SVMs in conjunction with extreme learning machine (ELM) demonstrate exhibit high effectiveness in identifying disease markers. In the context of ASD, multimodal and fusion techniques, including the integration of a CNN architecture with GA, highlight the effectiveness of combined models in improving diagnostic accuracy. For BD, the application of ensemble models and regression analyses presents new opportunities for identifying treatment targets and understanding disease biomarkers. Research on SZ benefits from the use of DL and ensemble methods, showing the potential for superior classification performance and the investigation of sensory changes and social skill impacts. Despite these promising developments, this study also identifies limitations in current research, such as small sample sizes, potential biases, and challenges in integrating findings into clinical practice. The need for larger, more diverse datasets and the validation of models across varied populations are recurring themes. This underscores the rapid advancements in machine intelligence applications for mental health diagnostics, providing insight into the future of personalized medicine and the ongoing challenge of translating these technological innovations into practical tools for clinicians and patients. This collective effort opens new pathways for early detection, enhanced understanding, and more effective treatment of mental health conditions.
4.3 RQ3: Is the Machine Intelligence Approach Effective in Identifying Mental Health Issues?
The recognition of machine intelligence’s effectiveness in identifying mental health issues has been growing, driven by advancements in algorithms, computational power, and the abundant availability of data. The development and utilization of machine intelligence models in mental health introduce several challenges, including data privacy issues, the requirement for large and diverse datasets to train algorithms effectively, and the integration of these technologies into clinical practice. Nonetheless, technological interventions have demonstrated encouraging results in mental health treatment, presenting new possibilities for diagnosis, monitoring, and therapy.
Machine intelligence has fundamentally changed how mental health conditions are identified, diagnosed, and treated. AI-driven tools and applications can analyze speech patterns, facial expressions, and social media activity to uncover early signs of issues such as depression, anxiety, and stress. These technologies enable the prompt identification of conditions that may not be noticeable to clinicians or patients, thereby facilitating timely intervention [57,61,84,132,133]. Wearable devices and mobile applications powered by AI algorithms are crucial for real-time mental health monitoring. They track physiological indicators [91,97,103], including heart rate variability [115,117], sleep patterns [127,143], and activity levels, providing insights into an individual’s mental state [152]. This continuous tracking allows for the recognition of patterns and triggers associated with mental health conditions, leading to tailored treatment strategies. Additionally, when social media data are analyzed using machine intelligence, it can offer valuable insights into an individual’s mental health. Techniques such as NLP and sentiment analysis can reveal mood changes and signs of depression or anxiety based on users’ posts and interactions [54,64,109]. However, this also brings up important concerns regarding privacy and the ethical use of data.
Fig. 7 illustrates the percentage distribution of methodological usage across various machine intelligence approaches applied in mental health diagnostics. It provides a clear proportional representation of each method’s contribution to the field, highlighting CNN and RF as some of the most frequently employed techniques. The figure also demonstrates the applicability of different methods across key aspects of mental health, including diagnosis, treatment planning, and monitoring. Each method, including SVM, DT, kNN, RF, CNN, LSTM, and LoR, is evaluated for its effectiveness in these areas, as indicated by their utility scores. Notably, CNN and LSTM networks demonstrate high utility across all three domains, underscoring their broad applicability and effectiveness in mental health diagnostics and care. This highlights the diverse capabilities of machine intelligence approaches in improving mental health services, from accurate diagnosis of conditions to the formulation of treatment plans and ongoing patient monitoring. Fig. 8 provides an analysis of methodological performance in cases of anxiety/stress and depression.

Figure 7: Methodological usage percentages and utility of machine intelligence approaches in mental health domains

Figure 8: Analysis of methodological performance in cases of anxiety/stress and depression
Fig. 9 shows the top 10 methods used across various disease categories, including AD, ASD, BD, and SZ. Each method is assessed on the basis of its accuracy, precision, recall and F1-score percentage, highlighting its effectiveness in diagnosing or studying these conditions. This clearly indicates the significant role of these computational techniques in enhancing diagnostic accuracy and efficiency, reflecting their potential to contribute to advanced and personalized mental health care solutions.

Figure 9: Analysis of methodological performance in patients with AD, ASD, BD, and SZ
The most effective machine intelligence algorithms for diagnosing mental health disorders have shown clear trends across conditions. For anxiety and stress, SVM, RF, and kNN excel on physiological and questionnaire-based data, while CNNs and LSTM networks lead in multimodal analysis combining EEG, behavioral, and usage data. For depression, CNNs, spatiotemporal deep models, and attention-based architectures are highly effective on EEG, facial video, and neuroimaging data, whereas transformers, hierarchical attention networks, and RF deliver strong results on text, speech, and social media datasets. SZ diagnosis benefits most from CNNs, DBNs, and hybrid EEG–MRI approaches. For ASD, CNNs, SVMs, and multimodal deep learning models on MRI, EEG, and behavioral data achieve good results with reinforcement learning and optimization algorithms improving early screening. BD detection leverages RF, SVM, and ensemble methods on EEG, MRI, and digital phenotyping data, while CNN-based models differentiate BD from related mood disorders with high precision. AD shows best results from CNNs, hybrid algorithms including LSTM, and ResNet-based transfer learning on MRI/fMRI, often paired with RF or ELM for feature selection. Overall, ensemble and hybrid pipelines that combine DL for complex signals with interpretable ML for structured data consistently outperform single-model approaches, offering superior accuracy and robustness.
4.4 Experimental Setup and Implementation Details
The reviewed studies employed a range of data preprocessing techniques depending on the data modality. Common steps included missing value imputation, outlier detection, data normalization, and feature scaling. Missing values were typically addressed using mean or median imputation for numerical features and mode imputation for categorical variables. In time-series and physiological datasets, outliers were identified using z-score thresholds or interquartile ranges and either excluded or capped. For neural signal data, preprocessing steps often included band-pass filtering, artifact rejection, and baseline correction. Imaging data were preprocessed using standardized pipelines such as skull stripping, intensity normalization, and spatial alignment. For textual data from social media, tokenization, stopword removal, and stemming/lemmatization were commonly applied.
4.4.2 Hyperparameter and Model Tuning
Parameter tuning was a key component in optimizing model performance across studies. For SVM, tuning involved selecting kernel functions and adjusting regularization and gamma values. RFs were optimized by adjusting the number of estimators, tree depth, and minimum samples per split. For CNN and LSTM models, hyperparameters such as the number of layers, filter size, learning rate, dropout rate, batch size, and optimizer were iteratively tuned using grid search, random search, or Bayesian optimization. Many studies reported employing early stopping and learning rate scheduling to prevent overfitting and improve generalization. Table 4 provides a consolidated list of the most common models reviewed, their tuned hyperparameters, and the evaluation metrics used across the reviewed literature. Since this review consolidated results from various published works, computational environments varied.
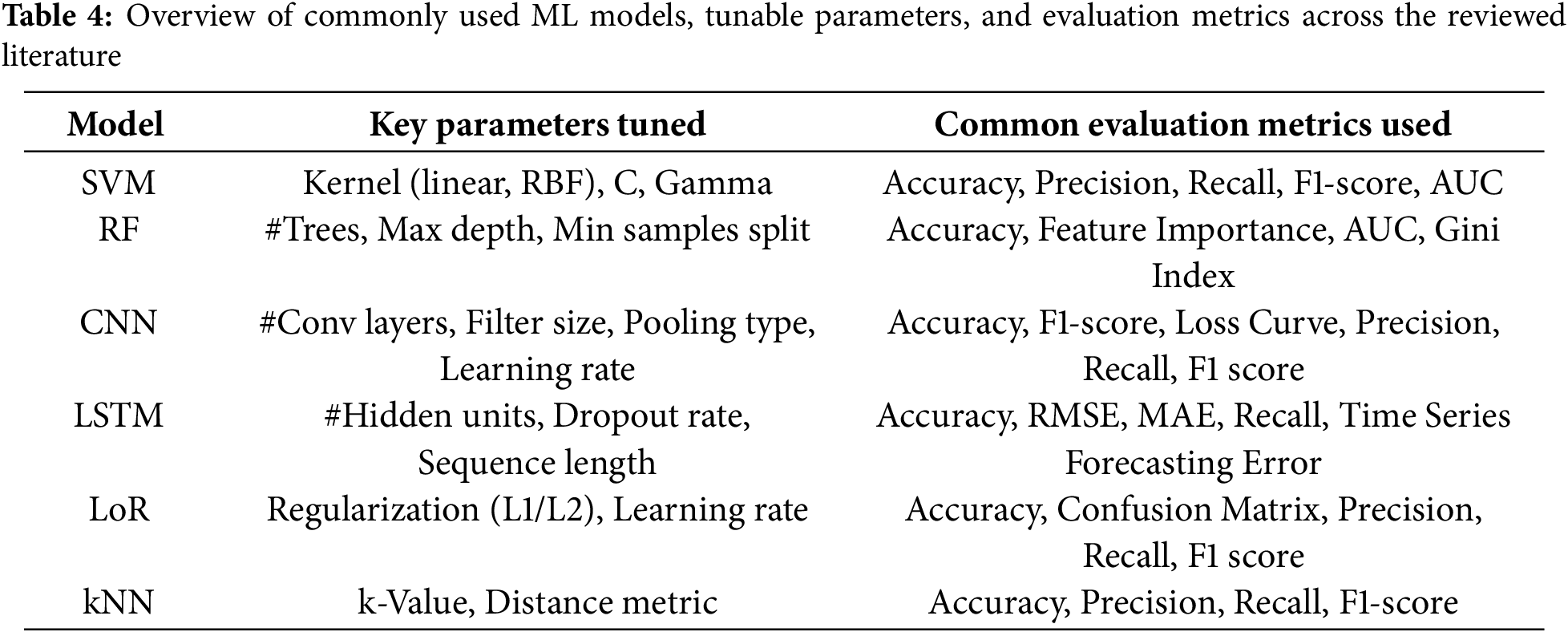
Validation strategies were critical in ensuring model reliability. Most studies employed k-fold cross-validation, with 5-fold and 10-fold setups being the most common. For DL models, hold-out validation and stratified splits were also used to preserve class distribution. Some studies also reported leave-one-subject-out cross-validation, especially in clinical or EEG datasets, to evaluate model robustness on unseen participants.
RQ4: What is the Current State of Dataset Availability in this Domain?
In this section, a variety of datasets pertaining to different mental illnesses is examined. These datasets were organized by type of disorder, including depression, SZ, AD, BD, ASD, and anxiety/stress-related issues (Table 5). They incorporate multiple data collection methods, such as clinical assessments, EEG recordings, video and audio analyses, as well as behavioral patterns observed on social media. The analysis of these datasets aimed to reveal the underlying patterns and indicators of disorders. This strategy sought to enhance the creation of more precise diagnostic tools and tailored treatment plans, utilizing advancements in machine intelligence to tackle the complexities associated with mental health conditions.
Table 6 presents a detailed overview of datasets concerning anxiety, stress, and depression, organized by type of disorder. It encompasses both primary and secondary datasets employed in various research efforts to comprehend and diagnose these mental health conditions. The methodologies applied include LoR, DT, RF, SVM, and CNN, showing the diverse analytical approaches utilized. Application areas range from mobile phone usage in mental health to the functional connectivity of the brain in individuals with anxiety and depression, as well as the detection of depression through gait data and social media behavior analysis. This highlights the interdisciplinary nature of contemporary mental health research, where datasets are derived not only from clinical and EEG recordings but also from digital footprints on social media and mobile sensor data, reflecting the evolving landscape of mental health diagnosis and monitoring. Table 7 enumerates the datasets for ASD, BD, SZ, and AD, demonstrating a variety of methods employed in their analysis and respective application areas. These datasets, gathered through both primary and secondary means, have been crucial in enhancing the understanding and diagnosis of these complex mental health conditions. Techniques such as DNN, RF, CNN, and LoR have been utilized for the analysis of these datasets. The application areas include predicting MCI and its progression to ADs, early diagnosis of ASD in infants, classifying BD based on psychosis, and assessing the severity of SZ symptoms. This illustrates the collaborative effort in mental health research, employing advanced computational methods to extract insights from varied data sources, ultimately aiming to enhance diagnostic accuracy and patient care.
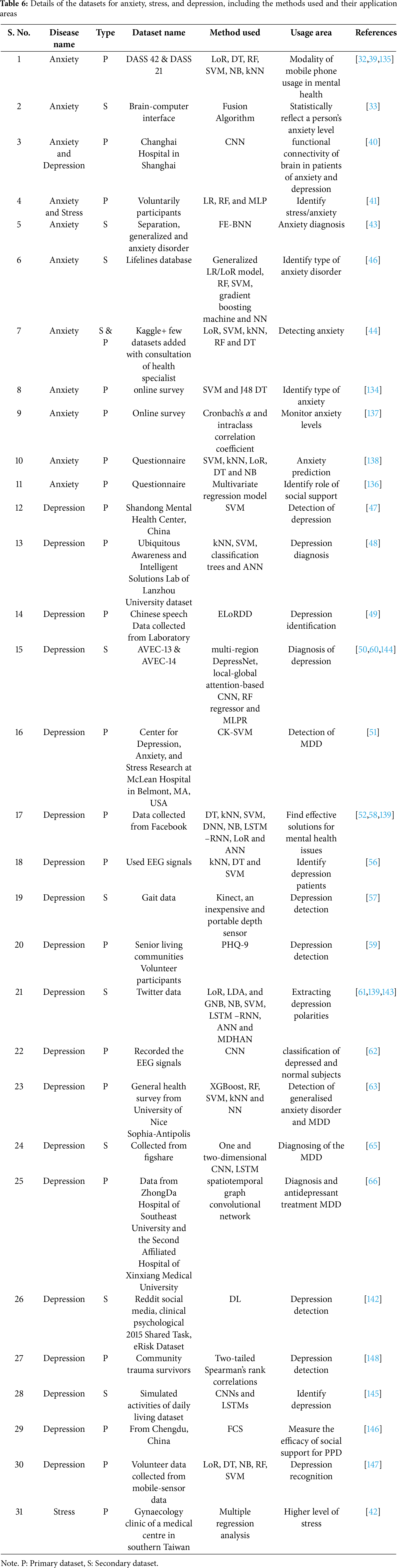
Fig. 10 represents the relationships among mental disorders, the methods applied, and their usage counts, with each method. The methods include LoR, CNN, SVM, RF, kNN, and DT, which show their application across various disorders, such as anxiety, depression, AD, ASD, BD, and SZ.

Figure 10: Relationship between mental disorders and the usage count for the method applied
Fig. 11 displays the combined percentage contributions of the datasets considered in this review paper from various countries for the years 2018–2025. It offers an overview of the geographical distribution of research contributions over the specified years, focusing on different mental disorder datasets. It also illustrates the distribution of datasets considered for the review, which originated from various publishers and spans the years 2018–2025.

Figure 11: Contribution percentages of datasets by country of origin and distribution by publisher for studies considered in this review (2018–2025)
The investigation of machine intelligence in diagnosing and treating mental health disorders signifies a pivotal shift in psychiatry and psychological research. This analysis consolidates essential results from recent studies, emphasizing anxiety, depression, SZ, BD, ASD, and AD. The methodological variety and the incorporation of different classifiers are highlighted, along with their influence on model performance, utilizing supporting evidence to illustrate the advancements and obstacles encountered within each disorder category.
Fig. 12 provides a comparative overview of methodological diversity and its effects on diagnostics and treatment across different mental health disorders. It demonstrates that depression exhibits the highest level of methodological diversity, closely succeeded by anxiety and stress, emphasizing the wide array of machine intelligence techniques utilized in these domains. Additionally, on the impact scale, depression has also ranked highest, signifying substantial progress in diagnostic and treatment effectiveness through machine intelligence.

Figure 12: Comparative view of methodological diversity and its impact on diagnostics and treatment across various mental health disorders
Innovations in the detection and treatment of anxiety utilize physiological and behavioral data, highlighting the integration of ML and DL techniques. The methodologies analyzed suggest that the combination of classifiers can enhance nuanced mental state detection, facilitating personalized interventions that are essential for effective management. Notable advancements in depression research have been achieved through the application of machine intelligence to varied data sources. The employment of SVMs for analyzing facial features and EEG signals has shown high accuracy in detecting depression. DL models such as DepressNet provide new diagnostic opportunities, underscoring the importance of early detection and the formulation of personalized treatment plans. This methodological diversity reflects the evolving landscape of depression diagnostics. Research on SZ benefits from the implementation of SVMs, CNNs, and DBNs, aiding in the differentiation between patients and HCs with significant accuracy. The utilization of wearable technology for symptom identification emphasizes the fusion of technological innovation with psychiatric evaluation, presenting promising pathways for accurate diagnosis and treatment planning. Advancements in BD research involve MRI analysis and EEG data classification, improving diagnostic accuracy and understanding the neural and genetic foundations of the disorder. The investigation of digital phenotyping for mood state monitoring reveals the potential of machine intelligence in providing new insights into BD, enabling personalized treatment strategies. ASD diagnostics have been transformed by ML algorithms, allowing for early and precise detection across various age groups. The efficacy of SVM and kNN in analyzing EEG signals and MRI scans underscores the importance of personalized support and early intervention in ASD management. In AD research, the application of DNN, RF, and novel biomarkers for early and precise disease identification is particularly noteworthy. The comprehensive approach to data integration—from imaging to smart home data—demonstrates the substantial potential of machine intelligence in improving AD diagnostics.
Among various algorithms, noteworthy performance has been demonstrated by SVM, DT, kNN, RF, and DL models, including CNNs and LSTM networks. These technologies excel in the classification of mental health conditions, streamlining decision-making processes, and extracting intricate patterns from large datasets, which enhances the depth, accuracy, and efficiency of diagnostics. The applicability and effectiveness of CNN and LSTM networks in mental health diagnostics and care illustrate the diverse capabilities of machine intelligence in improving mental health services. These advancements, ranging from precise diagnosis of conditions to the formulation of treatment plans and ongoing patient monitoring, suggest a promising future for personalized medicine in mental health, reflecting a comprehensive and progressive approach to diagnostics and treatment planning.
The innovative application of machine intelligence in these disorders constitutes a major strength, providing improved diagnostic accuracy and insights into mental disorders. However, challenges such as the necessity for larger [133,135,147,148], diverse datasets [150,161,164] and ethical considerations related to data privacy and algorithmic bias [166,172,177] persist as significant obstacles. The effectiveness of ML in leveraging nontraditional data sources for mental health diagnostics indicates an expanding scope within psychiatry. This signifies a notable shift toward early detection and intervention of mental health issues, presenting transformative potential for mental health care. The integration of machine intelligence in mental health research represents a crucial transition toward more accurate, personalized, and effective diagnostic and treatment strategies. Despite the existing challenges, the advancements outlined herein provide a promising outlook for the future of mental health care, underscoring the essential role of technology in advancing psychiatric research and practice. This discourse not only emphasizes the considerable progress achieved but also delineates the path forward in utilizing technology to enhance mental health outcomes on a global scale.
A major limitation in existing studies is the insufficient diversity [150,156,161,166] and representativeness of the datasets utilized [172,174,177,179]. Numerous models are trained on relatively uniform populations, which may fail to reflect the broad variability in mental health symptoms across distinct demographic groups, such as age, gender, ethnicity, and socioeconomic background. This shortfall raises concerns about the generalizability of the results, as models may not function as accurately when applied to larger and more diverse populations. Enhancing data diversity is essential for developing models that are both effective and fair for all individuals. Another considerable limitation is the challenge of incorporating ML insights into clinical practice. Although some models demonstrate high accuracy in detecting mental health conditions [32,33,39,43,46], implementing these findings into practical tools for use by clinicians and healthcare systems remains problematic [120,128,130,135,154,155]. Challenges include the necessity for user-friendly systems, clinician education, and addressing skepticism toward AI-driven diagnostics. Furthermore, integrating these tools with existing electronic health records and clinical processes presents additional obstacles, which are crucial for their widespread use and effectiveness in patient care. The application of ML and DL in mental health diagnostics also raises important ethical and privacy concerns [33,37,75] particularly when dealing with sensitive personal data [85,91,114,160]. Issues such as the potential for data misuse, algorithmic bias, and the lack of transparency in model decision-making require urgent attention [32,33,46,92,114]. ML models are prone to algorithmic bias, where they may unintentionally favor certain groups based on the training data [127,135,138,176,183]. This can result in discrepancies in the accuracy of mental health diagnoses across various populations, potentially worsening existing disparities in mental healthcare.
6.1 Real-World Clinical Implications
The findings carry significant implications for clinical deployment. Machine intelligence models capable of non-invasive diagnostics, such as those using EEG or speech data, offer real-time decision support for clinicians, potentially enabling earlier intervention and personalized treatment plans. High-accuracy models for conditions like depression, ASD, and AD can complement traditional psychiatric evaluations and reduce diagnostic subjectivity. Importantly, interpretable models like RF and SVM are more likely to be adopted in clinical settings where explainability is critical for trust and accountability. Integration into electronic health record systems and mobile monitoring platforms can further support long-term patient monitoring and therapy optimization.
6.2 Limitations in Handling Heterogeneous and Multimodal Data
While this review includes studies using diverse datasets, there remains a notable gap in handling heterogeneous and truly multimodal data. Few studies integrate disparate modalities into unified models. Multimodal learning strategies, combining neuroimaging, clinical notes, behavioral patterns, and physiological signals can potentially enhance diagnostic precision, especially for disorders with multifactorial symptoms like SZ and BD. Moreover, real-time applications, such as wearable-integrated monitoring have yet to be tested robustly under clinical constraints like latency, energy use, or patient usability. Future experimental designs should simulate real-world deployment scenarios, including noisy data streams, limited connectivity, and human-in-the-loop decision-making.
Most reviewed studies report end-to-end performance without conducting ablation experiments to understand the contribution of individual components. Ablation studies such as removing feature fusion layers in CNNs, excluding handcrafted features, or disabling dropout layers are crucial for understanding what drives model performance. For instance, in EEG-based models, performance gains may come more from spectral feature preprocessing than from the model architecture itself. Including such evaluations can reveal whether added complexity yields meaningful improvements or leads to overfitting. Future work should emphasize these controlled experiments to improve both interpretability and model design efficiency.
6.4 Interpretation of Model Performance: Current and Emerging Trends
The performance of machine intelligence models in diagnosing mental health disorders reflects a significant advancement in the intersection of AI and clinical psychology. Through the integration of diverse data sources, advanced classification algorithms, and multimodal approaches, these models have demonstrated remarkable accuracy and potential for early diagnosis, real-time monitoring, and personalized intervention strategies across various mental health conditions including anxiety, depression, SZ, BD, ASD, and AD.
In the context of anxiety and stress, studies have leveraged physiological signals, such as ECG and EEG, and behavioral indicators to improve detection accuracy. For instance, Fathi et al. [38] employed an adaptive network-based fuzzy inference system model achieving 98.67% accuracy, with perfect specificity (100%) and a sensitivity of 97.14%. Similarly, Ramakrishnan et al. [36] achieved a 96% accuracy in detecting stress using SVMs trained on physiological data, while Khouja et al. [37] reported similar accuracy using SVMs to classify stress, highlighting the model’s effectiveness in high-dimensional, biomedical data classification. These findings illustrate the robustness of SVMs in managing physiological variability and emphasize the utility of feature engineering in boosting diagnostic performance.
ML models have also demonstrated strong capabilities in diagnosing depression. Seal et al. [62] introduced DeprNet, a CNN model using EEG data, which achieved 99.37% accuracy and an AUC of 0.999 in record-wise splits, while maintaining an accuracy of 91.4% in subject-wise splits. This is complemented by Wu et al. [51], who recorded EEG responses and applied a CK-SVM to achieve an accuracy of 83.64% in classifying MDD, with high sensitivity (87.5%) and specificity (80.65%). These results validate the utility of DL and SVM-based models in handling complex neurophysiological data for mood disorder detection. The integration of NLP and social media analytics, as shown by Tadesse et al. [54] and Islam et al. [52], further expands the diagnostic reach beyond clinical settings, with DT models reaching up to 99% accuracy in identifying depressive traits from Facebook data.
For SZ, models integrating EEG and fMRI data have achieved exceptional results. Shalbaf et al. [78] reported a 98.6% classification accuracy using deep CNNs with transfer learning, and Khare et al. [82] demonstrated a 93.36% accuracy through CNN-based spectrogram analysis. These high accuracies reflect the strength of DL in recognizing complex spatiotemporal brain signal patterns. The use of ensemble learning, as in Siuly et al. [76] (93.21% accuracy), and hybrid approaches, such as that by Karthik et al. [84] achieving 97.01%, highlights the importance of combining multiple algorithms and data features to optimize model robustness and generalizability in SZ diagnostics.
The diagnosis of BD has also benefited from multimodal ML strategies. Li et al. [114] developed a CNN-based model using structural MRI data, achieving 99.72% accuracy, 99.76% precision, and 99.75% F1-score, effectively differentiating between BD and other psychiatric conditions. Similarly, Su et al. [115] utilized digital phenotyping and regression techniques to predict clinical scores like the HAM-D and YMRS, achieving prediction errors as low as 1.06 points. These models illustrate the advantage of incorporating continuous behavioral monitoring with neuroimaging data to predict mood fluctuations and enable targeted intervention strategies.
For ASD, ML models have showcased high predictive capabilities using both neuroimaging and behavioral data. For instance, Akter et al. [100] applied multiple ML models to different age groups, achieving accuracies of up to 100% for toddlers and over 99% for children and adults. Similarly, Nagesh et al. [161] demonstrated the effectiveness of combining CNNs with GA, while Han et al. [159] achieved 95.56% accuracy with a MMSDAE model. These models highlight the benefit of data fusion in revealing subtle behavioral and neurological markers across the autism spectrum.
In the domain of AD, advanced imaging and biomarker-based models have significantly improved diagnostic accuracy. For instance, Menagadevi et al. [154] reported 99.77% accuracy using SVM, while Shamrat et al. [155] achieved 98.67% with CNNs. The application of deep 3D-CNNs, as shown by Etminani et al. [153], and ensemble methods like ResNet18 and DenseNet201 (Odusami et al. [151], accuracy: 98.86%) have proven to be highly effective in identifying subtle anatomical changes in MRI and PET data. These models also highlight the importance of early detection through neuroimaging and smart home data, which can support personalized treatment planning and disease management.
Across all disorders, several algorithms stand out. SVMs consistently deliver high accuracy across diverse conditions due to their strength in managing sparse and high-dimensional data. CNNs and RNNs demonstrate powerful capabilities in processing imaging and sequential data, respectively. Ensemble methods, including RF and gradient boosting, enhance model reliability by aggregating predictions from multiple learners. Furthermore, multimodal approaches, blending physiological, behavioral, and imaging data consistently yield the most robust and generalizable results, as seen in studies across ASD, BD, and AD.
However, limitations persist. Sample size variability, lack of generalization across diverse populations, and ethical considerations in data usage particularly with social media and wearable data pose significant challenges [133,134,152]. Moreover, while several models achieve near-perfect accuracy in controlled settings, their real-world deployment is hindered by concerns over interpretability, clinical integration, and the need for external validation.
Machine intelligence approaches particularly those leveraging SVM, CNN, ensemble learning, and multimodal architectures have demonstrated high efficacy in diagnosing and understanding mental health disorders. These models show immense promise in transforming mental health care through early detection, accurate classification, and personalized treatment. However, future research must address current limitations through larger, diverse datasets and rigorous clinical validation to ensure reliable [133–140], ethical [141–148], and practical application of these technologies in real-world mental health diagnostics [149–168].
Recent advances in ML and signal processing have shown exceptional promise in transforming mental health diagnostics, particularly through the integration of diverse physiological, neuroimaging, behavioral, and social data modalities. These developments are enabling more accurate, scalable, and non-invasive identification of mental disorders.
The study in [184] demonstrated the potential of ECG signals for diagnosing BD, depression, and SZ by applying wavelet scattering networks and a fine kNN classifier. Achieving an average accuracy of 99.8%, this model significantly outperformed traditional CNNs, highlighting its clinical relevance for automated psychiatric screening. Similarly, functional neuroimaging has proven valuable, as shown in [185], where a CNN-based approach utilized wavelet coherence scalograms from rs-fMRI data to classify ASD subtypes. The model achieved 89.8% accuracy in binary classification and 82.1% in multi-class classification, demonstrating the capacity of dynamic functional connectivity features in capturing subtle brain activity patterns.
Graph-based signal processing further enhanced neuroimaging analysis in [186], where graph wavelet packets were applied to diffusion MRI and fMRI data from the BANDA dataset. By analyzing spectral features derived from graph Laplacian eigenvectors, this method outperformed conventional graph signal processing (GSP) techniques, illustrating the power of spectral geometry in modeling complex brain networks.
In wearable sensing, the study in [187] introduced a hybrid approach combining continuous wavelet transform and cardiopulmonary coupling (CPC) to assess mental workload from Photoplethysmography (PPG) signals. With an accuracy of 80.5%, this method not only surpassed traditional heart rate variability-based techniques but also approached ECG-level performance. Different frameworks for the related detection and prevention have also been supported in the related research considering different factors [188–193]. Meanwhile, motion artifact detection and reduction in PPG data, crucial for field deployment, was effectively addressed in [190] using a deep convolutional autoencoder, achieving 93% accuracy and an 88% improvement in signal-to-noise ratio.
EEG-based systems also saw notable advancements. A stress detection framework in [188] integrated a fuzzy attention-based fully convolutional network (FA-FCN), hybrid wavelet–short-time Fourier transform (HW-STFT), recursive least absolute shrinkage and selection operator (RLASSO), and an Adam-optimized sequential generative adversarial network (AOS-GAN), achieving 94% accuracy and demonstrating robustness against imbalanced datasets. Additionally, study [189] introduced a domain adversarial NN with reliable pseudo-label iteration (DANN-RPLI) for cross-subject EEG-based emotion recognition. This model leveraged iterative pseudo-label refinement and domain confusion strategies to achieve state-of-the-art performance across the SEED, SEED-IV, and DEAP datasets.
At the population level, ML was applied in [191] to a dataset of over 61,000 U.S. college students to predict anxiety and depressive disorders, with AUCs of 0.74 and 0.77. Key predictors included financial distress, disability status, and sense of campus belonging. A similar approach was adopted in [194], where XGBoost and nested cross-validation were used on real-world data from adolescents, achieving AUCs of 0.80 (anxiety) and 0.81 (depression), with minimal performance loss even when excluding social determinants of health.
Text and voice-based modalities are also gaining traction. Depression detection from 1.6 million tweets in [195] using NLP and ML achieved up to 97% accuracy. In [196], a voice-based screening model for perinatal mental disorders achieved a sensitivity of 1.00 and recall of 0.82, outperforming the Edinburgh Postnatal Depression Scale. Social media discourse analysis in [197] used SVM, RF, and Bidirectional Encoder Representations from Transformers (BERT) to detect stigma and sentiment in Instagram comments with accuracies up to 96%, showing the utility of AI in tracking public attitudes. Further, study [198] assessed the effectiveness of a single-session stress optimization intervention for adolescents using Bayesian causal forest analysis. The intervention led to significant anxiety reduction, particularly for individuals with high baseline stress, indicating ML’s role in evaluating and personalizing mental health interventions.
These studies illustrate that combining advanced ML techniques with multi-modal data sources is revolutionizing mental health diagnostics. These models not only enhance classification accuracy but also support scalable, real-time, and personalized care strategies in diverse clinical and non-clinical contexts.
6.5 Data Diversity and Algorithmic Bias: Ethical and Practical Considerations for Machine Intelligence Models
The integration of ML and DL into mental health diagnosis and intervention has gained momentum in recent years, offering opportunities for scalable, efficient, and personalized care. However, these advances are accompanied by critical ethical and practical challenges, particularly concerning privacy, informed consent, transparency, data diversity, and bias mitigation.
Quillivic et al. [199] noted that detecting post-traumatic stress disorder (PTSD) from trauma narratives raises ethical issues such as transparency in model decisions, safeguarding sensitive personal data, and ensuring informed consent. Practical barriers include validating performance across different trauma types and integrating AI to support, not replace, clinical judgment. Limited cultural and socioeconomic diversity in the dataset heightened risks of algorithmic bias, highlighting the need for broader, representative data.
Benito et al. [200] found that smartphone-derived behavioral markers offer scalable, low-burden methods for depression detection but face ethical challenges related to obtaining informed consent, ensuring secure handling of sensitive data, and preventing unauthorized use. Practical issues include variability in device usage patterns, performance discrepancies between controlled research and real-world deployment, and difficulties in seamless integration into clinical workflows. They cautioned that reliance on homogeneous participant cohorts can introduce algorithmic bias, recommending inclusive datasets, subgroup performance evaluation, and targeted bias mitigation strategies to enhance reliability across diverse populations.
Beech et al. [201] emphasized that safeguarding sensitive records, securing informed consent, and protecting privacy are key when deploying ML in mental healthcare. Practical hurdles include achieving interoperability, ensuring performance consistency across clinical settings, and fostering clinician trust. Overrepresentation of certain groups risks bias, requiring representative datasets and fairness-aware modeling. Lao et al. [202] explored mobile cognitive behavioral therapy for hepatitis B patients with mental health symptoms, highlighting ethical concerns around privacy, stigma, and sensitive disclosure in infectious disease contexts. Sustaining engagement and integrating with care systems posed practical challenges. Cultural homogeneity risks bias, necessitating representative and culturally adapted datasets. Delgado et al. [203] applied a two-stage AI pipeline for DASS-21 data, achieving high accuracy with interpretable rules. Ethical concerns involved privacy and clinical oversight, while practical challenges included class imbalance, limited demographic diversity, and symptom overlap factors that may bias predictions. Sergio et al. [204] proposed an IoT–Fog–Cloud architecture for real-time stress and anxiety monitoring. While effective in generating timely alerts, limitations included device connectivity issues, notification mismatches, and lack of measurement diversity. Data diversity gaps limit reliability. Sethia and Indu [205] used wearables with genetic algorithm–based feature selection for stress monitoring, identifying Gradient Boosting as most accurate. Challenges included biometric data privacy, clinical oversight, and validating wearable-based assessments beyond lab settings. Limited demographics raised bias concerns. Richter et al. [206] predicted mental health outcomes using cognitive bias tasks and RF regression, with key predictors including interpretation and expectancy bias. Ethical challenges involved consent and data protection, while small, culturally homogeneous samples risked bias. Dell et al. [207] classified depression and PTSD among Hurricane Maria survivors using RF and LR. Ethical risks included sensitive migration-related data handling and automation bias, while demographic concentration limited generalizability. Bastos et al. [208] linked academic stressors to depression scores using epsilon-SVR. Protecting participant confidentiality and avoiding misuse of predictions were key concerns. The small, non-probabilistic sample risked bias. Iturra-Mena et al. [209] predicted anxiety severity in children using EEG and behavioral data. Ethical issues included safeguarding neural data and avoiding overinterpretation, while homogeneous sampling limited applicability. Smith et al. [210] applied multimodal DL for detecting multiple disorders, with joint models outperforming single-disorder models. Ethical concerns involved handling sensitive recordings and informed consent, while demographic imbalances risked inequitable outcomes. Manjunath et al. [211] used CNN and other models to diagnose mental health conditions from clinical questionnaires, with the CNN performing best. Privacy, informed consent, and single-location data bias were key issues. Ramzan et al. [212] employed ML for personalized e-therapy, raising concerns about secure data handling and treatment bias due to demographic imbalances. Likhith et al. [213] predicted smartphone addiction from behavioral and psychological data. Risks included misuse by commercial entities and bias from limited, homogeneous samples. Divya and Devi [214] explored ML/DL for depression detection in women, emphasizing gender-specific privacy concerns, stigma reduction, and the need for culturally inclusive datasets. Lee et al. [215] developed a DL model using activity tracker data for elderly depression and anxiety detection. Risks included misuse of continuous monitoring data and underrepresentation of varied lifestyles. Ding et al. [216] compared ML and DL models for mental health detection from social media posts, noting privacy risks, overreliance dangers, and bias from imbalanced datasets and linguistic diversity.
The integration of machine intelligence into clinical mental healthcare presents intertwined ethical and practical challenges that directly influence model reliability, fairness, and real-world utility. From an ethical perspective, safeguarding privacy and confidentiality is paramount, given the sensitive nature of mental health data sourced from modalities such as audio–video interviews, smartphone logs, wearables, EEG, and social media [199–203]. Risks of re-identification and unauthorized use are amplified by data sharing across contexts, highlighting the need for robust governance, role-based access, and secure computation methods. Informed consent must be ongoing, comprehensible, and revocable, especially for continuous or passive sensing systems. Transparency and explainability are equally critical; clinicians and patients require understandable rationales for AI-driven outputs to avoid automation bias and preserve clinical accountability. Fairness considerations are central, as unequal error rates across demographic or clinical subgroups can exacerbate existing disparities. Safety risks emerge when misclassifications delay care or trigger unnecessary interventions, making clinician oversight and override capabilities non-negotiable. Compliance with regulations such and emerging AI-specific laws further demands meticulous documentation [200–205].
Practical challenges are closely linked to these ethical imperatives. Model robustness often suffers under distribution shifts, when applied to new clinics, devices, languages, or patient profiles not represented in training. Integration into existing workflows is complex, requiring interoperability with EHR systems, adherence to standards, and mitigation of alert fatigue. Trust and usability hinge on calibrated outputs, uncertainty estimates, and intuitive interfaces, backed by clinician training and change management. Data quality remains a persistent hurdle, with reliance on self-reports, imbalanced class distributions, missing data, and noisy sensor readings limiting reliability. Operational constraints, such as real-time processing demands, battery limitations for wearables, and network connectivity issues, further complicate deployment. Lifecycle management, including drift detection [207–209], bias monitoring [210,211], version control [212–214], and periodic revalidation is essential for sustained performance [215,216].
Central to both ethical and practical considerations is the issue of data diversity and algorithmic bias. Many studies rely on socioeconomically, culturally, or demographically homogeneous cohorts, leading to sampling bias that undermines generalizability. Measurement bias arises from device heterogeneity, platform-specific behaviors, or physiological signal variability across populations. Label bias, stemming from self-reports, stigma-driven underreporting, or inconsistent diagnostic criteria further distorts ground truth. Linguistic and cultural variability in mental health expression challenges NLP-based models, while missingness and adherence gaps often correlate with symptom severity or socioeconomic disadvantage, introducing systematic bias. These biases manifest as unequal error rates, poor calibration, and brittle performance when models encounter new contexts or populations. In mental health prediction, such disparities are not merely statistical artifacts but can translate into tangible harm [180,190], including misdiagnosis or inequitable care allocation [197–201].
Mitigating these risks demands deliberate strategies at every stage. Recruitment should prioritize multi-site, multi-ethnic, multilingual representation, with stratified sampling to ensure adequate subgroup sizes. Dataset composition and subgroup-specific metrics like sensitivity, specificity, predictive values, AUC, calibration scores, should be transparently reported alongside confidence intervals. Algorithmic techniques such as reweighting, fairness-constrained loss functions, domain adaptation, and hierarchical modeling can help address imbalance and heterogeneity. Post-processing methods like group-wise thresholding and calibration, coupled with clinician review, add safeguards before deployment. Once operational, active learning pipelines, drift detection, and targeted data acquisition in underrepresented strata help maintain fairness and performance over time. External and prospective validation in diverse clinical settings should precede large-scale adoption, ensuring models are stress-tested against real-world variability.
In synthesis, ethical soundness and practical feasibility in AI-driven mental healthcare are inseparable; privacy, consent, transparency, and fairness must be embedded in system design alongside robust engineering for workflow integration, scalability, and adaptability. Without representative data and bias-aware validation, even high-performing models risk inequitable outcomes and erosion of trust. The safest path forward is to treat these systems as clinician-augmented decision support tools, governed by continuous oversight, staged rollouts, and rigorous monitoring. Inclusive data collection, culturally sensitive design, and standards-based interoperability not only improve reliability but also facilitate regulatory compliance and adoption. Ultimately, sustained value in this domain hinges on harmonizing technical excellence with ethical integrity, ensuring that advances in machine intelligence genuinely expand access, equity, and quality of mental healthcare.
This study primarily focused on methodological interventions using machine intelligence, which may have limited the consideration of integration with real-time environments and clinical applications. Consequently, practical deployment aspects in clinical settings were not comprehensively addressed. Additionally, the emphasis on machine learning and deep learning techniques resulted in limited attention to traditional statistical analyses, which could have provided valuable insights into prevalence rates, associated risk factors, and the long-term effectiveness of various treatment strategies. Furthermore, the review was restricted to studies published in the English language, potentially leading to the exclusion of relevant research available in other languages, thereby limiting the comprehensiveness of the literature coverage.
Future suggestions are as follows:
1. There is a critical need to create and make standardized, large-scale, diverse datasets that represent the global population accessible. Such datasets should include varied demographic, genetic, and environmental factors to enable more comprehensive and generalizable research.
2. As machine intelligence models become more integrated into mental health diagnostics, it is imperative to develop ethical guidelines and privacy-preserving methodologies. These should aim to protect patient data while ensuring algorithmic transparency and fairness.
3. Efforts should be made to bridge the gap between computational models and clinical application. This includes validating machine intelligence-based diagnostic tools in clinical settings, training healthcare professionals to use these technologies, and assessing their impact on patient outcomes.
4. Investigating emerging paradigms, such as federated learning for decentralized data analysis and quantum computing for processing complex datasets, can open new avenues for mental health diagnostics.
5. Conducting long-term studies to assess the efficacy and impact of machine intelligence-based interventions on mental health outcomes will be crucial. These studies can provide insights into the long-term benefits and challenges of integrating machine intelligence into mental health care.
6. Future research can focus on personalized diagnosis by examining attributes specific to the individual. Accordingly, models should be designed to integrate various algorithms, including nature-inspired algorithms, to enable specialized interventions.
This comprehensive review has outlined the notable advancements in the utilization of machine intelligence within mental health diagnostics. A systematic analysis, following the PRISMA guidelines, was performed, covering the years from 2018 to 2025. Key developments, methodologies, and the multifaceted roles of various algorithms in improving the accuracy and effectiveness of mental health disorder predictions have been identified. The investigation into the integration of ML and DL algorithms, along with the examination of diverse datasets, emphasizes the dynamic and evolving nature of computational psychiatry. The results from this review indicate the potential of machine intelligence to transform mental health care by providing detailed insights into early detection, prediction, and personalized treatment strategies. The evaluation of studies utilizing algorithms such as kNN, SVM, and CNN reveals a methodological variety and innovation in addressing mental health challenges. These computational techniques have demonstrated encouraging outcomes, particularly in the diagnosis and treatment of conditions like depression and anxiety, which rank among the most common mental health disorders worldwide. Nonetheless, the review also points out significant obstacles that must be overcome to fully realize the capabilities of machine intelligence in mental health care. Challenges include the lack of diverse and representative datasets, ethical issues related to data privacy and algorithmic bias, and the integration of these technologies into clinical settings. The importance of interdisciplinary collaboration among clinicians, computer scientists, and ethicists is underscored to effectively address these challenges.
Acknowledgement: The authors acknowledge that ChatGPT was utilized in certain sections exclusively for language refinement.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Ravita Chahar: Conceptualization, Investigation, Data collection, Writing—original draft, Writing—review and editing. Ashutosh Kumar Dubey: Conceptualization, Investigation, Data collection, Writing—original draft, Analysis and Interpretation of results, Supervision, Writing—review and editing. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material (PRISMA checklist) is available online at https://www.techscience.com/doi/10.32604/cmc.2025.066990/s1.
References
1. Kirkbride JB, Anglin DM, Colman I, Dykxhoorn J, Jones PB, Patalay P, et al. The social determinants of mental health and disorder: evidence, prevention and recommendations. World Psychiatry. 2024;23(1):58–90. doi:10.1002/wps.21160. [Google Scholar] [PubMed] [CrossRef]
2. Novera CN, Connolly R, Wanke P, Rahman MA, Azad MAK. Past, present and future impact of social media on health workers’ mental health: a text mining approach. J Model Manag. 2024;19(1):1–18. doi:10.1108/jm2-05-2022-0135. [Google Scholar] [CrossRef]
3. Mental Health Report. [cited 2025 Sep 16]. Available from: https://www.who.int/health-topics/mental-health#tab=tab_2. [Google Scholar]
4. Pigoni A, Delvecchio G, Turtulici N, Madonna D, Pietrini P, Cecchetti L, et al. Machine learning and the prediction of suicide in psychiatric populations: a systematic review. Transl Psychiatry. 2024;14(1):140. doi:10.1038/s41398-024-02852-9. [Google Scholar] [PubMed] [CrossRef]
5. Okoro YO, Ayo-Farai O, Maduka CP, Okongwu CC, Sodamade OT. The role of technology in enhancing mental health advocacy: a systematic review. Int J Appl Res Soc Sci. 2024;6(1):37–50. doi:10.51594/ijarss.v6i1.690. [Google Scholar] [CrossRef]
6. Simon GE, Johnson E, Shortreed SM, Ziebell RA, Rossom RC, Ahmedani BK, et al. Predicting suicide death after emergency department visits with mental health or self-harm diagnoses. Gen Hosp Psychiatry. 2024;87(12):13–9. doi:10.1016/j.genhosppsych.2024.01.009. [Google Scholar] [PubMed] [CrossRef]
7. Upadhyay DK, Mohapatra S, Singh NK. An early assessment of Persistent Depression Disorder using machine learning algorithm. Multimed Tools Appl. 2024;83(16):49149–71. doi:10.1007/s11042-023-17369-4. [Google Scholar] [CrossRef]
8. Baba A, Bunji K. Prediction of mental health problem using annual student health survey: machine learning approach. JMIR Ment Health. 2023;10(2):e42420. doi:10.2196/42420. [Google Scholar] [PubMed] [CrossRef]
9. Haghish EF, Obaidi M, Strømme T, Bjørgo T, Grønnerød C. Mental health, well-being, and adolescent extremism: a machine learning study on risk and protective factors. Res Child Adolesc Psychopathol. 2023;51(11):1699–714. doi:10.1007/s10802-023-01105-5. [Google Scholar] [PubMed] [CrossRef]
10. Jonnalagadda A, Rajvir M, Singh S, Chandramouliswaran S, George J, Kamalov F. An ensemble-based machine learning model for emotion and mental health detection. J Info Know Mgmt. 2023;22(2):2250075. doi:10.1142/s0219649222500757. [Google Scholar] [CrossRef]
11. Chahar R, Dubey AK, Narang SK. A review and meta-analysis of machine intelligence approaches for mental health issues and depression detection. Int J Adv Technol Eng Explor. 2021;8(83). doi:10.19101/ijatee.2021.874198. [Google Scholar] [CrossRef]
12. Shobhika, Kumar P, Chandra S. Prediction and comparison of psychological health during COVID-19 among Indian population and Rajyoga meditators using machine learning algorithms. Procedia Comput Sci. 2023;218:697–705. doi:10.1016/j.procs.2023.01.050. [Google Scholar] [PubMed] [CrossRef]
13. Jayanthi S, Priyadharshini V, Kirithiga V, Premalatha S. Mental health status monitoring for people with autism spectrum disorder using machine learning. Int J Inf Technol. 2024;16(1):43–51. doi:10.1007/s41870-023-01524-z. [Google Scholar] [CrossRef]
14. Tyagi A, Singh VP, Gore MM. Towards artificial intelligence in mental health: a comprehensive survey on the detection of schizophrenia. Multimed Tools Appl. 2023;82(13):20343–405. doi:10.1007/s11042-022-13809-9. [Google Scholar] [CrossRef]
15. Alhuwaydi AM. Exploring the role of artificial intelligence in mental healthcare: current trends and future directions-a narrative review for a comprehensive insight. Risk Manag Healthc Policy. 2024;17:1339–48. doi:10.2147/RMHP.S461562. [Google Scholar] [PubMed] [CrossRef]
16. Kim S, Cha J, Kim D, Park E. Understanding mental health issues in different subdomains of social networking services: computational analysis of text-based reddit posts. J Med Internet Res. 2023;25(1):e49074. doi:10.2196/49074. [Google Scholar] [PubMed] [CrossRef]
17. Di Cara NH, Maggio V, Davis OSP, Haworth CMA. Methodologies for monitoring mental health on twitter: systematic review. J Med Internet Res. 2023;25:e42734. doi:10.2196/42734. [Google Scholar] [PubMed] [CrossRef]
18. Chakraborty A, Banerjee JS, Bhadra R, Dutta A, Ganguly S, Das D, et al. A framework of intelligent mental health monitoring in smart cities and societies. IETE J Res. 2024;70(2):1328–41. doi:10.1080/03772063.2023.2171918. [Google Scholar] [CrossRef]
19. Lenton-Brym AP, Collins A, Lane J, Busso C, Ouyang J, Fitzpatrick S, et al. Using machine learning to increase access to and engagement with trauma-focused interventions for posttraumatic stress disorder. Br J Clin Psychol. 2025;64(1):125–36. doi:10.1111/bjc.12468. [Google Scholar] [PubMed] [CrossRef]
20. Garg M. Mental health analysis in social media posts: a survey. Arch Comput Meth Eng. 2023;30(3):1819–42. doi:10.1007/s11831-022-09863-z. [Google Scholar] [PubMed] [CrossRef]
21. Khalil SS, Tawfik NS, Spruit M. Exploring the potential of federated learning in mental health research: a systematic literature review. Appl Intell. 2024;54(2):1619–36. doi:10.1007/s10489-023-05095-1. [Google Scholar] [CrossRef]
22. Patel D, Sumner SA, Bowen D, Zwald M, Yard E, Wang J, et al. Predicting state level suicide fatalities in the United States with realtime data and machine learning. NPJ Ment Health Res. 2024;3(1):3. doi:10.1038/s44184-023-00045-8. [Google Scholar] [PubMed] [CrossRef]
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA, 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi:10.1016/j.ijsu.2021.105906. [Google Scholar] [PubMed] [CrossRef]
24. Wesley R, Pham H. Mental health studies: a review. Applicat Reliab Statist Comput. 2023 Feb;16(2):289–302. doi:10.1007/978-3-031-21232-1_15. [Google Scholar] [CrossRef]
25. Tutun S, Johnson ME, Ahmed A, Albizri A, Irgil S, Yesilkaya I, et al. An AI-based decision support system for predicting mental health disorders. Inf Syst Front. 2023;25(3):1261–76. doi:10.1007/s10796-022-10282-5. [Google Scholar] [PubMed] [CrossRef]
26. Montejo-Ráez A, Molina-González MD, Jiménez-Zafra SM, García-Cumbreras MÁ, García-López LJ. A survey on detecting mental disorders with natural language processing: literature review, trends and challenges. Comput Sci Rev. 2024;53(2):100654. doi:10.1016/j.cosrev.2024.100654. [Google Scholar] [CrossRef]
27. Aabdalla ID, Vasumathi D. A novel hybrid deep learning and reinforcement learning framework for multimodal cardiovascular disease prediction. Int J Adv Comput Res. 2025;15(73):73. doi:10.19101/ijacr.2024.1466030. [Google Scholar] [CrossRef]
28. Bhatnagar S, Agarwal J, Sharma OR. Detection and classification of anxiety in university students through the application of machine learning. Procedia Comput Sci. 2023;218(7):1542–50. doi:10.1016/j.procs.2023.01.132. [Google Scholar] [CrossRef]
29. Khoo LS, Lim MK, Chong CY, McNaney R. Machine learning for multimodal mental health detection: a systematic review of passive sensing approaches. Sensors. 2024;24(2):348. doi:10.3390/s24020348. [Google Scholar] [PubMed] [CrossRef]
30. Steyve N, Ndjakomo S, Steve P, Pierre E. Hybrid chaotic whale-shark optimization algorithm to improve artificial neural network: application to the skin neglected tropical diseases diagnosis. Int J Adv Comput Res. 2023;13(63):8. doi:10.19101/ijacr.2021.1152068. [Google Scholar] [CrossRef]
31. Adheena MA, Sindhu N, Jerritta S. Physiological detection of anxiety. In: 2018 International Conference on Circuits and Systems in Digital Enterprise Technology (ICCSDET); 2018 Dec 21; Kottayam, India: IEEE. p. 1–5. doi:10.1109/ICCSDET.2018.8821162. [Google Scholar] [CrossRef]
32. Višnjić A, Veličković V, Sokolović D, Stanković M, Mijatović K, Stojanović M, et al. Relationship between the manner of mobile phone use and depression, anxiety, and stress in university students. Int J Environ Res Public Health. 2018;15(4):697. doi:10.3390/ijerph15040697. [Google Scholar] [PubMed] [CrossRef]
33. Wang M, Zhang S, Lv Y, Lu H. Anxiety level detection using BCI of miner’s smart helmet. Mob Netw Appl. 2018;23(2):336–43. doi:10.1007/s11036-017-0935-5. [Google Scholar] [CrossRef]
34. Puli A, Kushki A. Toward automatic anxiety detection in autism: a real-time algorithm for detecting physiological arousal in the presence of motion. IEEE Trans Biomed Eng. 2020;67(3):646–57. doi:10.1109/TBME.2019.2919273. [Google Scholar] [PubMed] [CrossRef]
35. Gavrilescu M, Vizireanu N. Predicting depression, anxiety, and stress levels from videos using the facial action coding system. Sensors. 2019;19(17):3693. doi:10.3390/s19173693. [Google Scholar] [PubMed] [CrossRef]
36. Ramakrishnan A, Pardes A, Lynch W, Molaro C, Platt ML. A machine learning approach to identifying objective biomarkers of anxiety and stress. BioRxiv. 2019 Aug 24;14(7):745315. doi:10.1101/745315. [Google Scholar] [CrossRef]
37. Khouja JN, Munafò MR, Tilling K, Wiles NJ, Joinson C, Etchells PJ, et al. Is screen time associated with anxiety or depression in young people? Results from a UK birth cohort. BMC Public Health. 2019;19(1):82. doi:10.1186/s12889-018-6321-9. [Google Scholar] [PubMed] [CrossRef]
38. Fathi S, Ahmadi M, Birashk B, Dehnad A. Development and use of a clinical decision support system for the diagnosis of social anxiety disorder. Comput Methods Programs Biomed. 2020;190(5):105354. doi:10.1016/j.cmpb.2020.105354. [Google Scholar] [PubMed] [CrossRef]
39. Priya A, Garg S, Tigga NP. Predicting anxiety, depression and stress in modern life using machine learning algorithms. Procedia Comput Sci. 2020;167(6):1258–67. doi:10.1016/j.procs.2020.03.442. [Google Scholar] [CrossRef]
40. Xie Y, Yang B, Lu X, Zheng M, Fan C, Bi X, et al. Anxiety and depression diagnosis method based on brain networks and convolutional neural networks. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC); 2020 Jul 20–24; Montreal, QC, Canada: IEEE. p. 1503–6. doi:10.1109/embc44109.2020.9176471. [Google Scholar] [PubMed] [CrossRef]
41. Arsalan A, Majid M, Anwar SM. Electroencephalography based machine learning framework for anxiety classification. In: Intelligent technologies and applications. Singapore: Springer Singapore; 2020. p. 187–97. doi:10.1007/978-981-15-5232-8_17. [Google Scholar] [CrossRef]
42. Chen SL, Chang SM, Kuo PL, Chen CH. Stress, anxiety and depression perceived by couples with recurrent miscarriage. Int J Nurs Pract. 2020;26(2):e12796. doi:10.1111/ijn.12796. [Google Scholar] [PubMed] [CrossRef]
43. Xiong H, Berkovsky S, Romano M, Sharan RV, Liu S, Coiera E, et al. Prediction of anxiety disorders using a feature ensemble based Bayesian neural network. J Biomed Inform. 2021;123(1):103921. doi:10.1016/j.jbi.2021.103921. [Google Scholar] [PubMed] [CrossRef]
44. Kruthika G, Kuruba P, Dushyantha ND. A system for anxiety prediction and treatment using Indian classical music therapy with the application of machine learning. In: Intelligent data communication technologies and internet of things. Singapore: Springer Singapore; 2021. p. 345–59. doi:10.1007/978-981-15-9509-7_30. [Google Scholar] [CrossRef]
45. Meyerbröker K, Morina N. The use of virtual reality in assessment and treatment of anxiety and related disorders. Clin Psychol Psychother. 2021;28(3):466–76. doi:10.1002/cpp.2623. [Google Scholar] [PubMed] [CrossRef]
46. Sharma A, Verbeke WJMI. Understanding importance of clinical biomarkers for diagnosis of anxiety disorders using machine learning models. PLoS One. 2021;16(5):e0251365. doi:10.1371/journal.pone.0251365. [Google Scholar] [PubMed] [CrossRef]
47. Wang Q, Yang H, Yu Y. Facial expression video analysis for depression detection in Chinese patients. J Vis Commun Image Represent. 2018;57(10):228–33. doi:10.1016/j.jvcir.2018.11.003. [Google Scholar] [CrossRef]
48. Cai H, Han J, Chen Y, Sha X, Wang Z, Hu B, et al. A pervasive approach to EEG-based depression detection. Complexity. 2018;2018(1):5238028. doi:10.1155/2018/5238028. [Google Scholar] [CrossRef]
49. Jiang H, Hu B, Liu Z, Wang G, Zhang L, Li X, et al. Detecting depression using an ensemble logistic regression model based on multiple speech features. Comput Math Methods Med. 2018;2018:6508319. doi:10.1155/2018/6508319. [Google Scholar] [PubMed] [CrossRef]
50. Zhou X, Jin K, Shang Y, Guo G. Visually interpretable representation learning for depression recognition from facial images. IEEE Trans Affect Comput. 2020;11(3):542–52. doi:10.1109/TAFFC.2018.2828819. [Google Scholar] [CrossRef]
51. Wu CT, Dillon DG, Hsu HC, Huang S, Barrick E, Liu YH. Depression detection using relative EEG power induced by emotionally positive images and a conformal kernel support vector machine. Appl Sci. 2018;8(8):1244. doi:10.3390/app8081244. [Google Scholar] [CrossRef]
52. Islam MR, Kabir MA, Ahmed A, Kamal ARM, Wang H, Ulhaq A. Depression detection from social network data using machine learning techniques. Health Inf Sci Syst. 2018;6(1):8. doi:10.1007/s13755-018-0046-0. [Google Scholar] [PubMed] [CrossRef]
53. Li X, Zhang X, Zhu J, Mao W, Sun S, Wang Z, et al. Depression recognition using machine learning methods with different feature generation strategies. Artif Intell Med. 2019;99:101696. doi:10.1016/j.artmed.2019.07.004. [Google Scholar] [PubMed] [CrossRef]
54. Tadesse MM, Lin H, Xu B, Yang L. Detection of depression-related posts in reddit social media forum. IEEE Access. 2019;7:44883–93. doi:10.1109/ACCESS.2019.2909180. [Google Scholar] [CrossRef]
55. Ay B, Yildirim O, Talo M, Baloglu UB, Aydin G, Puthankattil SD, et al. Automated depression detection using deep representation and sequence learning with EEG signals. J Med Syst. 2019;43(7):205. doi:10.1007/s10916-019-1345-y. [Google Scholar] [PubMed] [CrossRef]
56. Cai H, Qu Z, Li Z, Zhang Y, Hu X, Hu B. Feature-level fusion approaches based on multimodal EEG data for depression recognition. Inf Fusion. 2020;59(3):127–38. doi:10.1016/j.inffus.2020.01.008. [Google Scholar] [CrossRef]
57. Wang T, Li C, Wu C, Zhao C, Sun J, Peng H, et al. A gait assessment framework for depression detection using kinect sensors. IEEE Sens J. 2021;21(3):3260–70. doi:10.1109/JSEN.2020.3022374. [Google Scholar] [CrossRef]
58. Wu MY, Shen CY, Wang ET, Chen ALP. A deep architecture for depression detection using posting, behavior, and living environment data. J Intell Inf Syst. 2020;54(2):225–44. doi:10.1007/s10844-018-0533-4. [Google Scholar] [CrossRef]
59. Smith M, Dietrich BJ, Bai EW, Bockholt HJ. Vocal pattern detection of depression among older adults. Int J Ment Health Nurs. 2020;29(3):440–9. doi:10.1111/inm.12678. [Google Scholar] [PubMed] [CrossRef]
60. He L, Chan JC, Wang Z. Automatic depression recognition using CNN with attention mechanism from videos. Neurocomputing. 2021;422(7):165–75. doi:10.1016/j.neucom.2020.10.015. [Google Scholar] [CrossRef]
61. Zhou J, Zogan H, Yang S, Jameel S, Xu G, Chen F. Detecting community depression dynamics due to COVID-19 pandemic in Australia. IEEE Trans Comput Soc Syst. 2021;8(4):982–91. doi:10.1109/TCSS.2020.3047604. [Google Scholar] [PubMed] [CrossRef]
62. Seal A, Bajpai R, Agnihotri J, Yazidi A, Herrera-Viedma E, Krejcar O. DeprNet: a deep convolution neural network framework for detecting depression using EEG. IEEE Trans Instrum Meas. 2021;70:2505413. doi:10.1109/TIM.2021.3053999. [Google Scholar] [CrossRef]
63. Nemesure MD, Heinz MV, Huang R, Jacobson NC. Predictive modeling of depression and anxiety using electronic health records and a novel machine learning approach with artificial intelligence. Sci Rep. 2021;11(1):1980. doi:10.1038/s41598-021-81368-4. [Google Scholar] [PubMed] [CrossRef]
64. DeSouza DD, Robin J, Gumus M, Yeung A. Natural language processing as an emerging tool to detect late-life depression. Front Psychiatry. 2021;12:719125. doi:10.3389/fpsyt.2021.719125. [Google Scholar] [PubMed] [CrossRef]
65. Saeedi A, Saeedi M, Maghsoudi A, Shalbaf A. Major depressive disorder diagnosis based on effective connectivity in EEG signals: a convolutional neural network and long short-term memory approach. Cogn Neurodyn. 2021;15(2):239–52. doi:10.1007/s11571-020-09619-0. [Google Scholar] [PubMed] [CrossRef]
66. Kong Y, Gao S, Yue Y, Hou Z, Shu H, Xie C, et al. Spatio-temporal graph convolutional network for diagnosis and treatment response prediction of major depressive disorder from functional connectivity. Hum Brain Mapp. 2021;42(12):3922–33. doi:10.1002/hbm.25529. [Google Scholar] [PubMed] [CrossRef]
67. Cella M, Okruszek Ł, Lawrence M, Zarlenga V, He Z, Wykes T. Using wearable technology to detect the autonomic signature of illness severity in schizophrenia. Schizophr Res. 2018;195(4):537–42. doi:10.1016/j.schres.2017.09.028. [Google Scholar] [PubMed] [CrossRef]
68. Chakraborty D, Yang Z, Tahir Y, Maszczyk T, Dauwels J, Thalmann N, et al. Prediction of negative symptoms of schizophrenia from emotion related low-level speech signals. In: 2018 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP); 2018 Apr 15–20; Calgary, AB, Canada: IEEE. p. 6024–8. doi:10.1109/ICASSP.2018.8462102. [Google Scholar] [CrossRef]
69. Mikolas P, Hlinka J, Skoch A, Pitra Z, Frodl T, Spaniel F, et al. Machine learning classification of first-episode schizophrenia spectrum disorders and controls using whole brain white matter fractional anisotropy. BMC Psychiatry. 2018;18(1):97. doi:10.1186/s12888-018-1678-y. [Google Scholar] [PubMed] [CrossRef]
70. Jahmunah V, Oh SL, Rajinikanth V, Ciaccio EJ, Cheong KH, Arunkumar N, et al. Automated detection of schizophrenia using nonlinear signal processing methods. Artif Intell Med. 2019;100(5–6):101698. doi:10.1016/j.artmed.2019.07.006. [Google Scholar] [PubMed] [CrossRef]
71. Zhu X, Li R, Kang G, Kang Q, Rao W, Yang M, et al. CACNA1C polymorphism (rs2283291) is associated with schizophrenia in Chinese males: a case-control study. Dis Markers. 2019;2019:8062397. doi:10.1155/2019/8062397. [Google Scholar] [PubMed] [CrossRef]
72. Devia C, Mayol-Troncoso R, Parrini J, Orellana G, Ruiz A, Maldonado PE, et al. EEG classification during scene free-viewing for schizophrenia detection. IEEE Trans Neural Syst Rehabil Eng. 2019;27(6):1193–9. doi:10.1109/TNSRE.2019.2913799. [Google Scholar] [PubMed] [CrossRef]
73. Oh SL, Vicnesh J, Ciaccio EJ, Yuvaraj R, Acharya UR. Deep convolutional neural network model for automated diagnosis of schizophrenia using EEG signals. Appl Sci. 2019;9(14):2870. doi:10.3390/app9142870. [Google Scholar] [CrossRef]
74. Latha M, Kavitha G. Detection of Schizophrenia in brain MR images based on segmented ventricle region and deep belief networks. Neural Comput Appl. 2019;31(9):5195–206. doi:10.1007/s00521-018-3360-1. [Google Scholar] [CrossRef]
75. Krishnan PT, Joseph Raj AN, Balasubramanian P, Chen Y. Schizophrenia detection using MultivariateEmpirical Mode Decomposition and entropy measures from multichannel EEG signal. Biocybern Biomed Eng. 2020;40(3):1124–39. doi:10.1016/j.bbe.2020.05.008. [Google Scholar] [CrossRef]
76. Siuly S, Khare SK, Bajaj V, Wang H, Zhang Y. A computerized method for automatic detection of schizophrenia using EEG signals. IEEE Trans Neural Syst Rehabil Eng. 2020;28(11):2390–400. doi:10.1109/TNSRE.2020.3022715. [Google Scholar] [PubMed] [CrossRef]
77. Lei D, Pinaya WHL, van Amelsvoort T, Marcelis M, Donohoe G, Mothersill DO, et al. Detecting schizophrenia at the level of the individual: relative diagnostic value of whole-brain images, connectome-wide functional connectivity and graph-based metrics. Psychol Med. 2020;50(11):1852–61. doi:10.1017/S0033291719001934. [Google Scholar] [PubMed] [CrossRef]
78. Shalbaf A, Bagherzadeh S, Maghsoudi A. Transfer learning with deep convolutional neural network for automated detection of schizophrenia from EEG signals. Phys Eng Sci Med. 2020;43(4):1229–39. doi:10.1007/s13246-020-00925-9. [Google Scholar] [PubMed] [CrossRef]
79. Baygin M, Yaman O, Tuncer T, Dogan S, Barua PD, Acharya UR. Automated accurate schizophrenia detection system using Collatz pattern technique with EEG signals. Biomed Signal Process Control. 2021;70(8):102936. doi:10.1016/j.bspc.2021.102936. [Google Scholar] [CrossRef]
80. Shi D, Li Y, Zhang H, Yao X, Wang S, Wang G, et al. Machine learning of schizophrenia detection with structural and functional neuroimaging. Dis Markers. 2021;2021:9963824. doi:10.1155/2021/9963824. [Google Scholar] [PubMed] [CrossRef]
81. Kadry S, Taniar D, Damasevicius R, Rajinikanth V. Automated detection of schizophrenia from brain MRI slices using optimized deep-features. In: 2021 Seventh International conference on Bio Signals, Images, and Instrumentation (ICBSII); 2021 Mar 25–27; Chennai, India: IEEE. p. 1–5. doi:10.1109/icbsii51839.2021.9445133. [Google Scholar] [CrossRef]
82. Khare SK, Bajaj V, Acharya UR. SPWVD-CNN for automated detection of schizophrenia patients using EEG signals. IEEE Trans Instrum Meas. 2021;70:2507409. doi:10.1109/TIM.2021.3070608. [Google Scholar] [CrossRef]
83. Sharma M, Acharya UR. Automated detection of schizophrenia using optimal wavelet-based l1 norm features extracted from single-channel EEG. Cogn Neurodyn. 2021;15(4):661–74. doi:10.1007/s11571-020-09655-w. [Google Scholar] [PubMed] [CrossRef]
84. Karthik S, Sudha M. Predicting bipolar disorder and schizophrenia based on non-overlapping genetic phenotypes using deep neural network. Evol Intell. 2021;14(2):619–34. doi:10.1007/s12065-019-00346-y. [Google Scholar] [CrossRef]
85. Ibrahim S, Djemal R, Alsuwailem A. Electroencephalography (EEG) signal processing for epilepsy and autism spectrum disorder diagnosis. Biocybern Biomed Eng. 2018;38(1):16–26. doi:10.1016/j.bbe.2017.08.006. [Google Scholar] [CrossRef]
86. Vijayan A, Janmasree S, Keerthana C, Baby Syla L. A framework for intelligent learning assistant platform based on cognitive computing for children with autism spectrum disorder. In: 2018 International CET Conference on Control, Communication, and Computing (IC4); 2018 Jul 5–7; Thiruvananthapuram, India: IEEE. p. 361–5. doi:10.1109/CETIC4.2018.8530940. [Google Scholar] [CrossRef]
87. Li G, Liu M, Sun Q, Shen D, Wang L. Early diagnosis of autism disease by multi-channel CNNs. In: Machine learning in medical imaging. Cham, Switzerland: Springer International Publishing; 2018. p. 303–9. doi:10.1007/978-3-030-00919-9_35. [Google Scholar] [CrossRef]
88. Kong Y, Gao J, Xu Y, Pan Y, Wang J, Liu J. Classification of autism spectrum disorder by combining brain connectivity and deep neural network classifier. Neurocomputing. 2019;324(3):63–8. doi:10.1016/j.neucom.2018.04.080. [Google Scholar] [CrossRef]
89. Akter T, Shahriare Satu M, Khan MI, Ali MH, Uddin S, Lió P, et al. Machine learning-based models for early stage detection of autism spectrum disorders. IEEE Access. 2019;7:166509–27. doi:10.1109/ACCESS.2019.2952609. [Google Scholar] [CrossRef]
90. Thabtah F, Abdelhamid N, Peebles D. A machine learning autism classification based on logistic regression analysis. Health Inf Sci Syst. 2019;7(1):12. doi:10.1007/s13755-019-0073-5. [Google Scholar] [PubMed] [CrossRef]
91. Kou J, Le J, Fu M, Lan C, Chen Z, Li Q, et al. Comparison of three different eye-tracking tasks for distinguishing autistic from typically developing children and autistic symptom severity. Autism Res. 2019;12(10):1529–40. doi:10.1002/aur.2174. [Google Scholar] [PubMed] [CrossRef]
92. Goel N, Grover B, Anuj, Gupta D, Khanna A, Sharma M. Modified grasshopper optimization algorithm for detection of autism spectrum disorder. Phys Commun. 2020;41:101115. doi:10.1016/j.phycom.2020.101115. [Google Scholar] [CrossRef]
93. Yaneva V, Ha LA, Eraslan S, Yesilada Y, Mitkov R. Detecting high-functioning autism in adults using eye tracking and machine learning. IEEE Trans Neural Syst Rehabil Eng. 2020;28(6):1254–61. doi:10.1109/TNSRE.2020.2991675. [Google Scholar] [PubMed] [CrossRef]
94. Tang M, Kumar P, Chen H, Shrivastava A. Deep multimodal learning for the diagnosis of autism spectrum disorder. J Imaging. 2020;6(6):47. doi:10.3390/jimaging6060047. [Google Scholar] [PubMed] [CrossRef]
95. Brueggeman L, Koomar T, Michaelson JJ. Forecasting risk gene discovery in autism with machine learning and genome-scale data. Sci Rep. 2020;10(1):4569. doi:10.1038/s41598-020-61288-5. [Google Scholar] [PubMed] [CrossRef]
96. Wedyan M, Al-Jumaily A, Crippa A. Early diagnose of autism spectrum disorder using machine learning based on simple upper limb movements. In: Hybrid Intelligent Systems: 18th International Conference on Hybrid Intelligent Systems (HIS 2018); 2018 Dec 13–15; Porto, Portugal. p. 491–500. [Google Scholar]
97. Cantin-Garside KD, Kong Z, White SW, Antezana L, Kim S, Nussbaum MA. Detecting and classifying self-injurious behavior in autism spectrum disorder using machine learning techniques. J Autism Dev Disord. 2020;50(11):4039–52. doi:10.1007/s10803-020-04463-x. [Google Scholar] [PubMed] [CrossRef]
98. Negin F, Ozyer B, Agahian S, Kacdioglu S, Ozyer GT. Vision-assisted recognition of stereotype behaviors for early diagnosis of Autism Spectrum Disorders. Neurocomputing. 2021;446(1):145–55. doi:10.1016/j.neucom.2021.03.004. [Google Scholar] [CrossRef]
99. Papakostas GA, Sidiropoulos GK, Lytridis C, Bazinas C, Kaburlasos VG, Kourampa E, et al. Estimating children engagement interacting with robots in special education using machine learning. Math Probl Eng. 2021;2021:9955212. doi:10.1155/2021/9955212. [Google Scholar] [CrossRef]
100. Akter T, Khan MI, Ali MH, Satu MS, Uddin MJ, Ali Moni M. Improved machine learning based classification model for early autism detection. In: 2021 2nd International Conference on Robotics, Electrical and Signal Processing Techniques (ICREST); 2021 Jan 5–7; Dhaka, Bangladesh: IEEE. p. 742–7. doi:10.1109/icrest51555.2021.9331013. [Google Scholar] [CrossRef]
101. Vakadkar K, Purkayastha D, Krishnan D. Detection of autism spectrum disorder in children using machine learning techniques. SN Comput Sci. 2021;2(5):386. doi:10.1007/s42979-021-00776-5. [Google Scholar] [PubMed] [CrossRef]
102. Altamura AC, Maggioni E, Dhanoa T, Ciappolino V, Paoli RA, Cremaschi L, et al. The impact of psychosis on brain anatomy in bipolar disorder: a structural MRI study. J Affect Disord. 2018;233:100–9. doi:10.1016/j.jad.2017.11.092. [Google Scholar] [PubMed] [CrossRef]
103. Alimardani F, Cho JH, Boostani R, Hwang HJ. Classification of bipolar disorder and schizophrenia using steady-state visual evoked potential based features. IEEE Access. 2018;6:40379–88. doi:10.1109/ACCESS.2018.2854555. [Google Scholar] [CrossRef]
104. Perez Arribas I, Goodwin GM, Geddes JR, Lyons T, Saunders KEA. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274. doi:10.1038/s41398-018-0334-0. [Google Scholar] [PubMed] [CrossRef]
105. Manzan JRG, Yamanaka K, Santos Peretta I, Pinto ER, Oliveira TEC, Nomura S. A mathematical discussion concerning the performance of multilayer perceptron-type artificial neural networks through use of orthogonal bipolar vectors. Comput Appl Math. 2018;37(2):932–53. doi:10.1007/s40314-016-0377-x. [Google Scholar] [CrossRef]
106. Johnson CP, Christensen GE, Fiedorowicz JG, Mani M, Shaffer JJJr, Magnotta VA, et al. Alterations of the cerebellum and basal ganglia in bipolar disorder mood states detected by quantitative T1ρ mapping. Bipolar Disord. 2018;20(4):381–90. doi:10.1111/bdi.12581. [Google Scholar] [PubMed] [CrossRef]
107. Farshad M. Detection and classification of internal faults in bipolar HVDC transmission lines based on K-means data description method. Int J Electr Power Energy Syst. 2019;104(14):615–25. doi:10.1016/j.ijepes.2018.07.044. [Google Scholar] [CrossRef]
108. AbaeiKoupaei N, Al Osman H. A multi-modal stacked ensemble model for bipolar disorder classification. IEEE Trans Affect Comput. 2023;14(1):236–44. doi:10.1109/TAFFC.2020.3047582. [Google Scholar] [CrossRef]
109. Chandran D, Robbins DA, Chang CK, Shetty H, Sanyal J, Downs J, et al. Use of Natural Language Processing to identify Obsessive Compulsive Symptoms in patients with schizophrenia, schizoaffective disorder or bipolar disorder. Sci Rep. 2019;9(1):14146. doi:10.1038/s41598-019-49165-2. [Google Scholar] [PubMed] [CrossRef]
110. Maes M, Landucci Bonifacio K, Morelli NR, Vargas HO, Barbosa DS, Carvalho AF, et al. Major differences in neurooxidative and neuronitrosative stress pathways between major depressive disorder and types I and II bipolar disorder. Mol Neurobiol. 2019;56(1):141–56. doi:10.1007/s12035-018-1051-7. [Google Scholar] [PubMed] [CrossRef]
111. Stanislaus S, Faurholt-Jepsen M, Vinberg M, Coello K, Kjærstad HL, Melbye S, et al. Mood instability in patients with newly diagnosed bipolar disorder, unaffected relatives, and healthy control individuals measured daily using smartphones. J Affect Disord. 2020;271(2):336–44. doi:10.1016/j.jad.2020.03.049. [Google Scholar] [PubMed] [CrossRef]
112. Lee CY, Zeng JH, Lee SY, Lu RB, Kuo PH. SNP data science for classification of bipolar disorder I and bipolar disorder II. IEEE/ACM Trans Comput Biol Bioinform. 2021;18(6):2862–9. doi:10.1109/TCBB.2020.2988024. [Google Scholar] [PubMed] [CrossRef]
113. Li H, Cui L, Cao L, Zhang Y, Liu Y, Deng W, et al. Identification of bipolar disorder using a combination of multimodality magnetic resonance imaging and machine learning techniques. BMC Psychiatry. 2020;20(1):488. doi:10.1186/s12888-020-02886-5. [Google Scholar] [PubMed] [CrossRef]
114. Li Z, Li W, Wei Y, Gui G, Zhang R, Liu H, et al. Deep learning based automatic diagnosis of first-episode psychosis, bipolar disorder and healthy controls. Comput Med Imaging Graph. 2021;89(12):101882. doi:10.1016/j.compmedimag.2021.101882. [Google Scholar] [PubMed] [CrossRef]
115. Su HY, Wu CH, Liou CR, Lin EC, See Chen P. Assessment of bipolar disorder using heterogeneous data of smartphone-based digital phenotyping. In: ICASSP, 2021-2021 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP); 2021 Jun 6–11; Toronto, ON, Canada: IEEE. p. 4260–4. doi:10.1109/icassp39728.2021.9415008. [Google Scholar] [CrossRef]
116. Llamocca P, López V, Santos M, Čukić M. Personalized characterization of emotional states in patients with bipolar disorder. Mathematics. 2021;9(11):1174. doi:10.3390/math9111174. [Google Scholar] [CrossRef]
117. Amoroso N, Diacono D, Fanizzi A, La Rocca M, Monaco A, Lombardi A, et al. Deep learning reveals Alzheimer’s disease onset in MCI subjects: results from an international challenge. J Neurosci Meth. 2018;302:3–9. doi:10.1016/j.jneumeth.2017.12.011. [Google Scholar] [PubMed] [CrossRef]
118. Alberdi A, Weakley A, Schmitter-Edgecombe M, Cook DJ, Aztiria A, Basarab A, et al. Smart home-based prediction of multidomain symptoms related to Alzheimer’s disease. IEEE J Biomed Health Inform. 2018;22(6):1720–31. doi:10.1109/JBHI.2018.2798062. [Google Scholar] [PubMed] [CrossRef]
119. Abd-Elminaam DS. Smart life saver system for alzheimer patients, down syndromes, and child missing using IoT. Asian J Appl Sci. 2018 Feb 16;6(1):21–37. [Google Scholar]
120. Islam J, Zhang Y. Brain MRI analysis for Alzheimer’s disease diagnosis using an ensemble system of deep convolutional neural networks. Brain Inform. 2018;5(2):2. doi:10.1186/s40708-018-0080-3. [Google Scholar] [PubMed] [CrossRef]
121. Basaia S, Agosta F, Wagner L, Canu E, Magnani G, Santangelo R, et al. Automated classification of Alzheimer’s disease and mild cognitive impairment using a single MRI and deep neural networks. Neuroimage Clin. 2019;21:101645. doi:10.1016/j.nicl.2018.101645. [Google Scholar] [PubMed] [CrossRef]
122. Feng C, Elazab A, Yang P, Wang T, Zhou F, Hu H, et al. Deep learning framework for Alzheimer’s disease diagnosis via 3D-CNN and FSBi-LSTM. IEEE Access. 2019;7:63605–18. doi:10.1109/ACCESS.2019.2913847. [Google Scholar] [CrossRef]
123. Kim H, Lee JU, Kim S, Song S, Sim SJ. A nanoplasmonic biosensor for ultrasensitive detection of Alzheimer’s disease biomarker using a chaotropic agent. ACS Sensors. 2019;4(3):595–602. doi:10.1021/acssensors.8b01242. [Google Scholar] [PubMed] [CrossRef]
124. Acharya UR, Fernandes SL, WeiKoh JE, Ciaccio EJ, Fabell MKM, Tanik UJ, et al. Automated detection of Alzheimer’s disease using brain MRI images—a study with various feature extraction techniques. J Med Syst. 2019;43(9):302. doi:10.1007/s10916-019-1428-9. [Google Scholar] [PubMed] [CrossRef]
125. Li W, Lin X, Chen X. Detecting Alzheimer’s disease Based on 4D fMRI: an exploration under deep learning framework. Neurocomputing. 2020;388(6):280–7. doi:10.1016/j.neucom.2020.01.053. [Google Scholar] [CrossRef]
126. Bi X, Li S, Xiao B, Li Y, Wang G, Ma X. Computer aided Alzheimer’s disease diagnosis by an unsupervised deep learning technology. Neurocomputing. 2020;392(1):296–304. doi:10.1016/j.neucom.2018.11.111. [Google Scholar] [CrossRef]
127. Barbará-Morales E, Pérez-González J, Rojas-Saavedra KC, Medina-Bañuelos V. Evaluation of brain tortuosity measurement for the automatic multimodal classification of subjects with Alzheimer’s disease. Comput Intell Neurosci. 2020;2020:4041832. doi:10.1155/2020/4041832. [Google Scholar] [PubMed] [CrossRef]
128. Al-Shoukry S, Rassem TH, Makbol NM. Alzheimer’s diseases detection by using deep learning algorithms: a mini-review. IEEE Access. 2020;8:77131–41. doi:10.1109/access.2020.2989396. [Google Scholar] [CrossRef]
129. Ramzan F, Khan MUG, Rehmat A, Iqbal S, Saba T, Rehman A, et al. A deep learning approach for automated diagnosis and multi-class classification of Alzheimer’s disease stages using resting-state fMRI and residual neural networks. J Med Syst. 2019;44(2):37. doi:10.1007/s10916-019-1475-2. [Google Scholar] [PubMed] [CrossRef]
130. Liu J, Li M, Luo Y, Yang S, Li W, Bi Y. Alzheimer’s disease detection using depthwise separable convolutional neural networks. Comput Methods Programs Biomed. 2021;203(1):106032. doi:10.1016/j.cmpb.2021.106032. [Google Scholar] [PubMed] [CrossRef]
131. Murugan S, Venkatesan C, Sumithra MG, Gao XZ, Elakkiya B, Akila M, et al. DEMNET: a deep learning model for early diagnosis of Alzheimer diseases and dementia from MR images. IEEE Access. 2021;9:90319–29. doi:10.1109/ACCESS.2021.3090474. [Google Scholar] [CrossRef]
132. Helaly HA, Badawy M, Haikal AY. Deep learning approach for early detection of Alzheimer’s disease. Cogn Comput. 2022;14(5):1711–27. doi:10.1007/s12559-021-09946-2. [Google Scholar] [PubMed] [CrossRef]
133. Srinath KS, Kiran K, Pranavi S, Amrutha M, Shenoy PD, Venugopal KR. Prediction of depression, anxiety and stress levels using dass-42. In: 2022 IEEE 7th International conference for Convergence in Technology (I2CT); 2022 Apr 7–9; Mumbai, India: IEEE; 2022. p. 1–6. doi:10.1109/I2CT54291.2022.9824087. [Google Scholar] [CrossRef]
134. Albagmi FM, Alansari A, Al Shawan DS, AlNujaidi HY, Olatunji SO. Prediction of generalized anxiety levels during the Covid-19 pandemic: a machine learning-based modeling approach. Inform Med Unlocked. 2022;28(4):100854. doi:10.1016/j.imu.2022.100854. [Google Scholar] [PubMed] [CrossRef]
135. Shdaifat EA. Quality of life, depression, and anxiety in patients undergoing renal replacement therapies in Saudi Arabia. Sci World J. 2022;2022(1):7756586. doi:10.1155/2022/7756586. [Google Scholar] [PubMed] [CrossRef]
136. Razgulin J, Argustaitė-Zailskienė G, Šmigelskas K. The role of social support and sociocultural adjustment for international students’ mental health. Sci Rep. 2023;13(1):893. doi:10.1038/s41598-022-27123-9. [Google Scholar] [PubMed] [CrossRef]
137. Jang SJ, Chung SJ, Lee H. Validation of the climate change anxiety scale for Korean adults. Perspect Psychiatr Care. 2023;2023(1):9718834. doi:10.1155/2023/9718834. [Google Scholar] [CrossRef]
138. Malik SS, Khan A. Anxiety, depression and stress prediction among college students using machine learning algorithms. In: 2023 Second International Conference on Electrical, Electronics, Information and Communication Technologies (ICEEICT); 2023 Apr 5–7; Trichirappalli, India: IEEE. p. 1–5. doi:10.1109/iceeict56924.2023.10157693. [Google Scholar] [CrossRef]
139. Joshi ML, Kanoongo N. Depression detection using emotional artificial intelligence and machine learning: a closer review. Mater Today Proc. 2022;58(7):217–26. doi:10.1016/j.matpr.2022.01.467. [Google Scholar] [CrossRef]
140. Khare SK, Bajaj V. A hybrid decision support system for automatic detection of Schizophrenia using EEG signals. Comput Biol Med. 2022;141(14):105028. doi:10.1016/j.compbiomed.2021.105028. [Google Scholar] [PubMed] [CrossRef]
141. Wanderley Espinola C, Gomes JC, Mônica Silva Pereira J, dos Santos WP. Detection of major depressive disorder, bipolar disorder, schizophrenia and generalized anxiety disorder using vocal acoustic analysis and machine learning: an exploratory study. Res Biomed Eng. 2022;38(3):813–29. doi:10.1007/s42600-022-00222-2. [Google Scholar] [CrossRef]
142. Ansari L, Ji S, Chen Q, Cambria E. Ensemble hybrid learning methods for automated depression detection. IEEE Trans Comput Soc Syst. 2023;10(1):211–9. doi:10.1109/TCSS.2022.3154442. [Google Scholar] [CrossRef]
143. Zogan H, Razzak I, Wang X, Jameel S, Xu G. Explainable depression detection with multi-aspect features using a hybrid deep learning model on social media. World Wide Web. 2022;25(1):281–304. doi:10.1007/s11280-021-00992-2. [Google Scholar] [PubMed] [CrossRef]
144. Casado CÁ, Cañellas ML, López MB. Depression recognition using remote photoplethysmography from facial videos. IEEE Trans Affective Comput. 2023;14(4):3305–16. doi:10.1109/taffc.2023.3238641. [Google Scholar] [CrossRef]
145. Zamzami IF. The key criteria for predicting unusual behavior in the elderly with deep learning models under 5G technology. IEEE Access. 2023;11:18921–37. doi:10.1109/ACCESS.2023.3248287. [Google Scholar] [CrossRef]
146. Cheng B, Roberts N, Zhou Y, Wang X, Li Y, Chen Y, et al. Social support mediates the influence of cerebellum functional connectivity strength on postpartum depression and postpartum depression with anxiety. Transl Psychiatry. 2022;12(1):54. doi:10.1038/s41398-022-01781-9. [Google Scholar] [PubMed] [CrossRef]
147. Thati RP, Dhadwal AS, Kumar P, Sainaba P. A novel multi-modal depression detection approach based on mobile crowd sensing and task-based mechanisms. Multimed Tools Appl. 2023;82(4):4787–820. doi:10.1007/s11042-022-12315-2. [Google Scholar] [PubMed] [CrossRef]
148. Kimble M, Cappello O, Fleming K. Hypervigilance and depression as predictors of eye tracking to ambiguous pictures in trauma survivors. Int J Psychophysiol. 2023;187(8):27–33. doi:10.1016/j.ijpsycho.2023.01.007. [Google Scholar] [PubMed] [CrossRef]
149. Martinelli DD. Evolution of Alzheimer’s disease research from a health-tech perspective: insights from text mining. Int J Inf Manag Data Insights. 2022;2(2):100089. doi:10.1016/j.jjimei.2022.100089. [Google Scholar] [CrossRef]
150. AlSharabi K, Bin Salamah Y, Abdurraqeeb AM, Aljalal M, Alturki FA. EEG signal processing for Alzheimer’s disorders using discrete wavelet transform and machine learning approaches. IEEE Access. 2022;10:89781–97. doi:10.1109/ACCESS.2022.3198988. [Google Scholar] [CrossRef]
151. Odusami M, Maskeliūnas R, Damaševičius R. An intelligent system for early recognition of Alzheimer’s disease using neuroimaging. Sensors. 2022;22(3):740. doi:10.3390/s22030740. [Google Scholar] [PubMed] [CrossRef]
152. Chedid N, Tabbal J, Kabbara A, Allouch S, Hassan M. The development of an automated machine learning pipeline for the detection of Alzheimer’s Disease. Sci Rep. 2022;12(1):18137. doi:10.1038/s41598-022-22979-3. [Google Scholar] [PubMed] [CrossRef]
153. Etminani K, Soliman A, Davidsson A, Chang JR, Martínez-Sanchis B, Byttner S, et al. A 3D deep learning model to predict the diagnosis of dementia with Lewy bodies, Alzheimer’s disease, and mild cognitive impairment using brain 18F-FDG PET. Eur J Nucl Med Mol Imag. 2022;49(2):563–84. doi:10.1007/s00259-021-05483-0. [Google Scholar] [PubMed] [CrossRef]
154. Menagadevi M, Mangai S, Madian N, Thiyagarajan D. Automated prediction system for Alzheimer detection based on deep residual autoencoder and support vector machine. Optik. 2023;272:170212. doi:10.1016/j.ijleo.2022.170212. [Google Scholar] [CrossRef]
155. Shamrat FMJM, Akter S, Azam S, Karim A, Ghosh P, Tasnim Z, et al. AlzheimerNet: an effective deep learning based proposition for Alzheimer’s disease stages classification from functional brain changes in magnetic resonance images. IEEE Access. 2023;11(1):16376–95. doi:10.1109/ACCESS.2023.3244952. [Google Scholar] [CrossRef]
156. Balaji P, Chaurasia MA, Bilfaqih SM, Muniasamy A, Alsid LEG. Hybridized deep learning approach for detecting Alzheimer’s disease. Biomedicines. 2023;11(1):149. doi:10.3390/biomedicines11010149. [Google Scholar] [PubMed] [CrossRef]
157. Liu S, Cao Y, Liu J, Ding X, Coyle D. For the Alzheimer’s Disease Neuroimaging Initiative. A novelty detection approach to effectively predict conversion from mild cognitive impairment to Alzheimer’s disease. Int J Mach Learn Cybern. 2023;14(1):213–28. doi:10.1007/s13042-022-01570-2. [Google Scholar] [CrossRef]
158. Zhang F, Wei Y, Liu J, Wang Y, Xi W, Pan Y. Identification of Autism spectrum disorder based on a novel feature selection method and Variational Autoencoder. Comput Biol Med. 2022;148(19):105854. doi:10.1016/j.compbiomed.2022.105854. [Google Scholar] [PubMed] [CrossRef]
159. Han J, Jiang G, Ouyang G, Li X. A multimodal approach for identifying autism spectrum disorders in children. IEEE Trans Neural Syst Rehabil Eng. 2022;30:2003–11. doi:10.1109/TNSRE.2022.3192431. [Google Scholar] [PubMed] [CrossRef]
160. Bala M, Ali MH, Satu MS, Hasan KF, Ali Moni M. Efficient machine learning models for early stage detection of autism spectrum disorder. Algorithms. 2022;15(5):166. doi:10.3390/a15050166. [Google Scholar] [CrossRef]
161. Nagesh N, Patil P, Patil S, Kokatanur M. An architectural framework for automatic detection of autism using deep convolution networks and genetic algorithm. Int J Electr Comput Eng IJECE. 2022;12(2):1768. doi:10.11591/ijece.v12i2.pp1768-1775. [Google Scholar] [CrossRef]
162. Barik K, Watanabe K, Bhattacharya J, Saha G. A fusion-based machine learning approach for autism detection in young children using magnetoencephalography signals. J Autism Dev Disord. 2023;53(12):4830–48. doi:10.1007/s10803-022-05767-w. [Google Scholar] [PubMed] [CrossRef]
163. Wawer A, Chojnicka I, Okruszek L, Sarzynska-Wawer J. Single and cross-disorder detection for autism and schizophrenia. Cogn Comput. 2022;14(1):461–73. doi:10.1007/s12559-021-09834-9. [Google Scholar] [CrossRef]
164. Liu X, Wu J, Li W, Liu Q, Tian L, Huang H. Domain adaptation via low rank and class discriminative representation for autism spectrum disorder identification: a multi-site fMRI study. IEEE Trans Neural Syst Rehabil Eng. 2023;31:806–17. doi:10.1109/TNSRE.2022.3233656. [Google Scholar] [PubMed] [CrossRef]
165. Grazioli S, Crippa A, Rosi E, Candelieri A, Ceccarelli SB, Mauri M, et al. Exploring telediagnostic procedures in child neuropsychiatry: addressing ADHD diagnosis and autism symptoms through supervised machine learning. Eur Child Adolesc Psychiatry. 2024;33(1):139–49. doi:10.1007/s00787-023-02145-4. [Google Scholar] [PubMed] [CrossRef]
166. Schulte-Rüther M, Kulvicius T, Stroth S, Wolff N, Roessner V, Marschik PB, et al. Using machine learning to improve diagnostic assessment of ASD in the light of specific differential and co-occurring diagnoses. J Child Psychol Psychiatry. 2023;64(1):16–26. doi:10.1111/jcpp.13650. [Google Scholar] [PubMed] [CrossRef]
167. Selcuk Nogay H, Adeli H. Diagnostic of autism spectrum disorder based on structural brain MRI images using, grid search optimization, and convolutional neural networks. Biomed Signal Process Control. 2023;79(3):104234. doi:10.1016/j.bspc.2022.104234. [Google Scholar] [CrossRef]
168. Bennett CC, Ross MK, Baek E, Kim D, Leow AD. Predicting clinically relevant changes in bipolar disorder outside the clinic walls based on pervasive technology interactions via smartphone typing dynamics. Pervasive Mob Comput. 2022;83(2):101598. doi:10.1016/j.pmcj.2022.101598. [Google Scholar] [CrossRef]
169. Humyra Islam AM, Rahman MH, Bristy SA, Salim Andalib KM, Khan U, Awal MA, et al. Identification of molecular signatures and pathways common to blood cells and brain tissue based RNA-Seq datasets of bipolar disorder: insights from comprehensive bioinformatics approach. Inform Med Unlocked. 2022;29(1):100881. doi:10.1016/j.imu.2022.100881. [Google Scholar] [CrossRef]
170. Zain SM, Mumtaz W. Tri-model ensemble with grid search optimization for bipolar disorder diagnosis. In: 2022 International Conference on Frontiers of Information Technology (FIT); 2022 Dec 12–13; Islamabad, Pakistan: IEEE. p. 24–9. doi:10.1109/FIT57066.2022.00015. [Google Scholar] [CrossRef]
171. Ahmed AY, Kahya MA, Altamir SA. Classification improvement of gene expression for bipolar disorder using weighted sparse logistic regression. Bulletin EEI. 2022;11(2):1062–8. doi:10.11591/eei.v11i2.3594. [Google Scholar] [CrossRef]
172. Mateo-Sotos J, Torres AM, Santos JL, Quevedo O, Basar C. A machine learning-based method to identify bipolar disorder patients. Circuits Syst Signal Process. 2022;41(4):2244–65. doi:10.1007/s00034-021-01889-1. [Google Scholar] [CrossRef]
173. Ceccarelli F, Mahmoud M. Multimodal temporal machine learning for Bipolar Disorder and Depression Recognition. Pattern Anal Appl. 2022;25(3):493–504. doi:10.1007/s10044-021-01001-y. [Google Scholar] [CrossRef]
174. Xi C, Li A, Lai J, Huang X, Zhang P, Yan S, et al. Brain-gut microbiota multimodal predictive model in patients with bipolar depression. J Affect Disord. 2023;323(1):140–52. doi:10.1016/j.jad.2022.11.026. [Google Scholar] [PubMed] [CrossRef]
175. Løchen AR, Kolskår KK, de Lange AG, Sneve MH, Haatveit B, Lagerberg TV, et al. Visual processing deficits in patients with schizophrenia spectrum and bipolar disorders and associations with psychotic symptoms, and intellectual abilities. Heliyon. 2023;9(2):e13354. doi:10.1016/j.heliyon.2023.e13354. [Google Scholar] [PubMed] [CrossRef]
176. Ouali U, Zgueb Y, Jouini L, Aissa A, Jomli R, Ouertani A, et al. Accuracy of the Arabic HCL - 32 and MDQ in detecting patients with bipolar disorder. BMC Psychiatry. 2023;23(1):70. doi:10.1186/s12888-023-04529-x. [Google Scholar] [PubMed] [CrossRef]
177. Huang YJ, Lin YT, Liu CC, Lee LE, Hung SH, Lo JK, et al. Assessing schizophrenia patients through linguistic and acoustic features using deep learning techniques. IEEE Trans Neural Syst Rehabil Eng. 2022;30:947–56. doi:10.1109/TNSRE.2022.3163777. [Google Scholar] [PubMed] [CrossRef]
178. Amadeo MB, Esposito D, Escelsior A, Campus C, Inuggi A, Pereira Da Silva B, et al. Time in schizophrenia: a link between psychopathology, psychophysics and technology. Transl Psychiatry. 2022;12(1):331. doi:10.1038/s41398-022-02101-x. [Google Scholar] [PubMed] [CrossRef]
179. Huberts LCE, Does RJMM, Ravesteijn B, Lokkerbol J. Predictive monitoring using machine learning algorithms and a real-life example on schizophrenia. Qual Reliab Eng Int. 2022;38(3):1302–17. doi:10.1002/qre.2957. [Google Scholar] [CrossRef]
180. Cella M, Sedgwick O, Lawrence M, Grant N, Tsapekos D, Harrison L, et al. Evaluating the mechanisms of social cognition intervention in schizophrenia: a proof-of-concept trial. Psychiatry Res. 2023;319(1):114963. doi:10.1016/j.psychres.2022.114963. [Google Scholar] [PubMed] [CrossRef]
181. Grover N, Chharia A, Upadhyay R, Longo L. Schizo-net: a novel schizophrenia diagnosis framework using late fusion multimodal deep learning on electroencephalogram-based brain connectivity indices. IEEE Trans Neural Syst Rehabil Eng. 2023;31:464–73. doi:10.1109/TNSRE.2023.3237375. [Google Scholar] [PubMed] [CrossRef]
182. Göker H. 1D-convolutional neural network approach and feature extraction methods for automatic detection of schizophrenia. Signal Image Video Process. 2023;17(5):2627–36. doi:10.1007/s11760-022-02479-7. [Google Scholar] [CrossRef]
183. Nsugbe E. Enhanced recognition of adolescents with schizophrenia and a computational contrast of their neuroanatomy with healthy patients using brainwave signals. Appl AI Lett. 2023;4(1):e79. doi:10.1002/ail2.79. [Google Scholar] [CrossRef]
184. Telangore H, Sharma N, Sharma M, Acharya UR. A novel ECG-based approach for classifying psychiatric disorders: leveraging wavelet scattering networks. Med Eng Phys. 2025;135:104275. doi:10.1016/j.medengphy.2024.104275. [Google Scholar] [PubMed] [CrossRef]
185. Al-Hiyali MI, Yahya N, Faye I, Hussein AF. Identification of autism subtypes based on wavelet coherence of BOLD FMRI signals using convolutional neural network. Sensors. 2021;21(16):5256. doi:10.3390/s21165256. [Google Scholar] [PubMed] [CrossRef]
186. Dam S, Maurel P, Coloigner J. Graph wavelet packets for the classification of brain data in anxiety and depression. In: 2024 32nd European Signal Processing Conference (EUSIPCO); 2024 Aug 26–30; Lyon, France: IEEE. p. 1436–40. [Google Scholar]
187. Zhang H, Wang Z, Zhuang Y, Yin S, Chen Z, Liang Y. Assessment of mental workload level based on PPG signal fusion continuous wavelet transform and cardiopulmonary coupling technology. Electronics. 2024;13(7):1238. doi:10.3390/electronics13071238. [Google Scholar] [CrossRef]
188. Rai RK, Singh DK. Stress detection through wearable EEG technology: a signal-based approach. Comput Electr Eng. 2025;126(5):110478. doi:10.1016/j.compeleceng.2025.110478. [Google Scholar] [CrossRef]
189. Ju X, Su J, Dai S, Wu X, Li M, Hu D. Domain Adversarial Neural Network with Reliable Pseudo-labels Iteration for cross-subject EEG emotion recognition. Knowl Based Syst. 2025;316(2):113368. doi:10.1016/j.knosys.2025.113368. [Google Scholar] [CrossRef]
190. Gautam Y, Jebelli H. Autoencoder-based Photoplethysmography (PPG) signal reliability enhancement in construction health monitoring. Autom Constr. 2024;165(1):105537. doi:10.1016/j.autcon.2024.105537. [Google Scholar] [CrossRef]
191. Zhai Y, Zhang Y, Chu Z, Geng B, Almaawali M, Fulmer R, et al. Machine learning predictive models to guide prevention and intervention allocation for anxiety and depressive disorders among college students. J Couns Dev. 2025;103(1):110–25. doi:10.1002/jcad.12543. [Google Scholar] [CrossRef]
192. Lucasius C, Mai A, Patel T, Kundur D, Szatmari P, Strauss J, et al. A procedural overview of why, when and how to use machine learning for psychiatry. Nat Ment Health. 2025;3(1):8–18. doi:10.1038/s44220-024-00367-2. [Google Scholar] [CrossRef]
193. Vannucci A, Fields A, Heleniak C, Bloom PA, Harmon C, Nikolaidis A, et al. Machine learning for identifying caregiving adversities associated with greatest risk for mental health problems in children. Nat Ment Health. 2025;3(1):71–82. doi:10.1038/s44220-024-00355-6. [Google Scholar] [CrossRef]
194. Mardini MT, Khalil GE, Bai C, DivaKaran AM, Ray JM. Identifying adolescent depression and anxiety through real-world data and social determinants of health: machine learning model development and validation. JMIR Ment Health. 2025;12(7):e66665. doi:10.2196/66665. [Google Scholar] [PubMed] [CrossRef]
195. Ullah W, Oliveira-Silva P, Nawaz M, Zulqarnain RM, Siddique I, Sallah M. Identification of depressing tweets using natural language processing and machine learning: application of grey relational grades. J Radiat Res Appl Sci. 2025;18(1):101299. doi:10.1016/j.jrras.2025.101299. [Google Scholar] [CrossRef]
196. Ooba H, Maki J, Masuyama H. Voice analysis and deep learning for detecting mental disorders in pregnant women: a cross-sectional study. Discov Ment Health. 2025;5(1):12. doi:10.1007/s44192-025-00138-0. [Google Scholar] [PubMed] [CrossRef]
197. Merayo N, Ayuso-Lanchares A, González-Sanguino C. Machine learning algorithms to address the polarity and stigma of mental health disclosures on instagram. Expert Syst. 2025;42(2):e13832. doi:10.1111/exsy.13832. [Google Scholar] [CrossRef]
198. Liu J, Hu J, Qi Y, Wu X, Gan Y. Personalized stress optimization intervention to reduce adolescents’ anxiety: a randomized controlled trial leveraging machine learning. J Anxiety Disord. 2025;110(1):102964. doi:10.1016/j.janxdis.2024.102964. [Google Scholar] [PubMed] [CrossRef]
199. Quillivic R, Gayraud F, Auxéméry Y, Vanni L, Peschanski D, Eustache F, et al. Interdisciplinary approach to identify language markers for post-traumatic stress disorder using machine learning and deep learning. Sci Rep. 2024;14(1):12468. doi:10.1038/s41598-024-61557-7. [Google Scholar] [PubMed] [CrossRef]
200. Benito GV, Goldberg X, Brachowicz N, Castaño-Vinyals G, Blay N, Espinosa A, et al. Machine learning for anxiety and depression profiling and risk assessment in the aftermath of an emergency. Artif Intell Med. 2024;157(2):102991. doi:10.1016/j.artmed.2024.102991. [Google Scholar] [PubMed] [CrossRef]
201. Beech A, Fan H, Shu J, Oyarzun J, Nadel P, Karaman OT, et al. Using natural language processing to identify patterns associated with depression, anxiety, and stress symptoms during the COVID-19 pandemic. J Affect Disord. 2025;376(4):113–21. doi:10.1016/j.jad.2025.01.139. [Google Scholar] [PubMed] [CrossRef]
202. Lao CK, Wang X, Li X, Wang Z, Zhou G. A culturally adapted mobile cognitive behavioral therapy for individuals with Hepatitis B on depression, anxiety and stress: a pilot randomized controlled trial. Internet Interv. 2025;41(1):100862. doi:10.1016/j.invent.2025.100862. [Google Scholar] [PubMed] [CrossRef]
203. Delgado S, Vignola RCB, Sassi RJ, Belan PA, de Araújo SA. Symptom mapping and personalized care for depression, anxiety and stress: a data-driven AI approach. Comput Biol Med. 2024;182(7):109146. doi:10.1016/j.compbiomed.2024.109146. [Google Scholar] [PubMed] [CrossRef]
204. Sergio WL, Ströele V, Dantas M, Braga R, Macedo DD. Enhancing well-being in modern education: a comprehensive eHealth proposal for managing stress and anxiety based on machine learning. Internet Things. 2024;25(10):101055. doi:10.1016/j.iot.2023.101055. [Google Scholar] [CrossRef]
205. Shikha S, Sethia D, Indu S. Optimization of wearable biosensor data for stress classification using machine learning and explainable AI. IEEE Access. 2024;12(10):169310–27. doi:10.1109/ACCESS.2024.3463742. [Google Scholar] [CrossRef]
206. Richter T, Stahi S, Mirovsky G, Hel-Or H, Okon-Singer H. Disorder-specific versus transdiagnostic cognitive mechanisms in anxiety and depression: machine-learning-based prediction of symptom severity. J Affect Disord. 2024;354(1):473–82. doi:10.1016/j.jad.2024.03.035. [Google Scholar] [PubMed] [CrossRef]
207. Dell NA, Salas-Wright CP, Vaughn MG, Maldonado-Molina MM, Oh S, Bates M, et al. A machine learning approach using migration-related cultural stress to classify depression and post-traumatic stress disorder among hurricane survivors. J Affect Disord. 2024;347(5):77–84. doi:10.1016/j.jad.2023.11.055. [Google Scholar] [PubMed] [CrossRef]
208. Bastos AF, Fernandes O Jr, Liberal SP, Pires AJL, Lage LA, Grichtchouk O, et al. Academic-related stressors predict depressive symptoms in graduate students: a machine learning study. Behav Brain Res. 2025;478:115328. doi:10.1016/j.bbr.2024.115328. [Google Scholar] [PubMed] [CrossRef]
209. Iturra-Mena AM, Wall M, Chen SYH, Moser JS, Muzik M, Rosenblum K, et al. Data-driven identification of brain-behavioral and sociodemographic predictors of anxiety severity in children using machine learning. JAACAP Open. 2025;27(1):281. doi:10.1016/j.jaacop.2025.05.005. [Google Scholar] [CrossRef]
210. Smith A, Miller JJ, Anthony DC, Radford-Smith DE. Machine learning-based prediction of anxiety disorders using blood metabolite and social trait data from the UK Biobank. Brain Behav Immun Health. 2025;46:101010. doi:10.1016/j.bbih.2025.101010. [Google Scholar] [PubMed] [CrossRef]
211. Manjunath HR, Kolekar VK, Bhuvaneshwari KS, Jain R, Aravind A, Dev S. Comprehensive detection and analysis of depression in women through advanced machine learning techniques. In: 2024 IEEE 4th International Conference on ICT in Business Industry & Government (ICTBIG); 2024 Dec 13–14; Indore, India: IEEE. p. 1–5. [Google Scholar]
212. Ramzan HA, Abdulah F, Ahmad M, Ramzan S, Ashraf M. AI-driven personalization of E-therapy interventions for anxiety, stress, and depression. In: 2024 18th International Conference on Open Source Systems and Technologies (ICOSST); 2024 Dec 26–27; Lahore, Pakistan: IEEE; 2024. p. 1–6. doi:10.1109/ICOSST64562.2024.10871158. [Google Scholar] [CrossRef]
213. Likhith S, Chitteti C, Dharani M, Nivedhitha V, Geethika NG, Godwin V. Machine learning model for prediction of smartphone addiction. In: 2024 International Conference on Expert Clouds and Applications (ICOECA); 2024 Apr 18–19; Bengaluru, India: IEEE. p. 924–9. doi:10.1109/ICOECA62351.2024.00163. [Google Scholar] [CrossRef]
214. Divya P, Sri Devi R. Empowering women’s mental health: a critical examination of depression detection. In: 2024 7th International Conference on Circuit Power and Computing Technologies (ICCPCT); 2024 Aug 8–9; Kollam, India: IEEE. p. 158–64. doi:10.1109/ICCPCT61902.2024.10673296. [Google Scholar] [CrossRef]
215. Lee TR, Kim GH, Choi MT. Geriatric depression and anxiety screening via deep learning using activity tracking and sleep data. Int J Geriatr Psychiatry. 2024;39(2):e6071. doi:10.1002/gps.6071. [Google Scholar] [PubMed] [CrossRef]
216. Ding Z, Wang Z, Zhang Y, Cao Y, Liu Y, Shen X, et al. Trade-offs between machine learning and deep learning for mental illness detection on social media. Sci Rep. 2025;15(1):14497. doi:10.1038/s41598-025-99167-6. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2026 The Author(s). Published by Tech Science Press.
Copyright © 2026 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF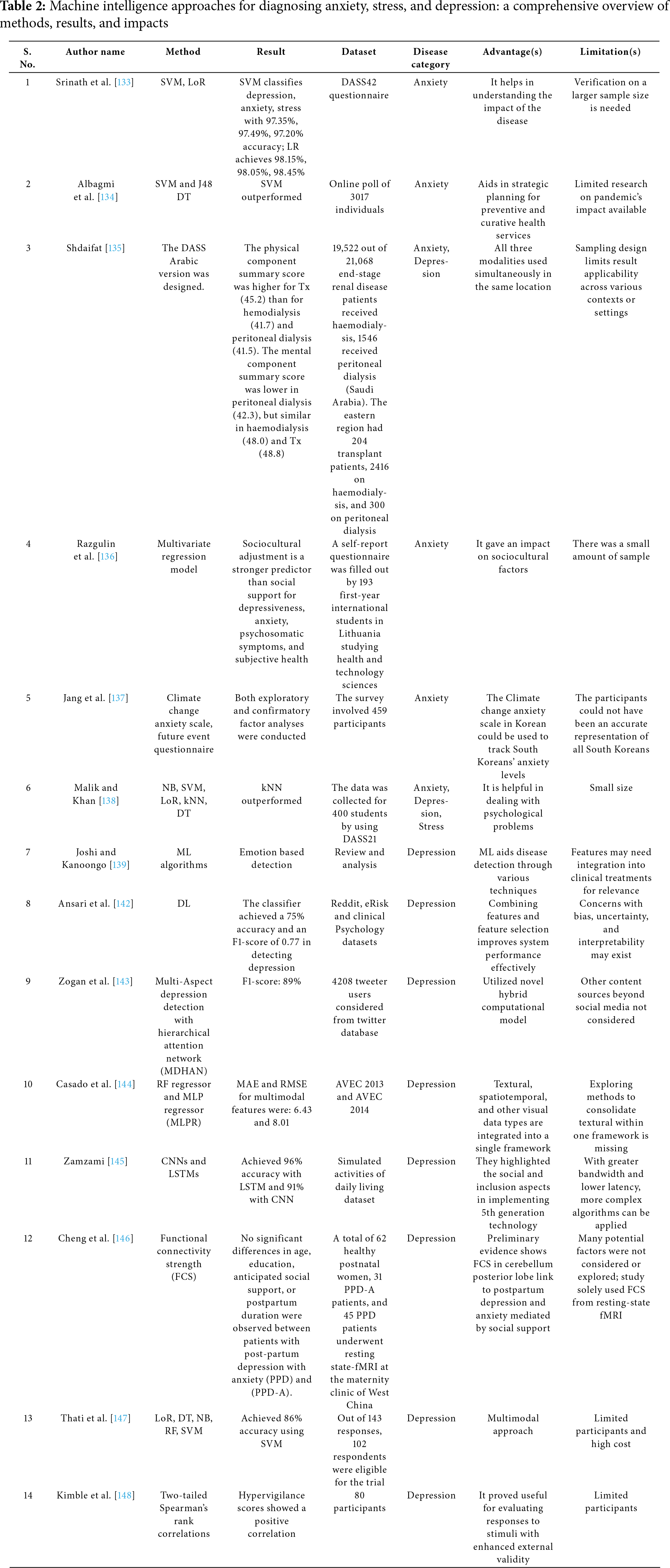
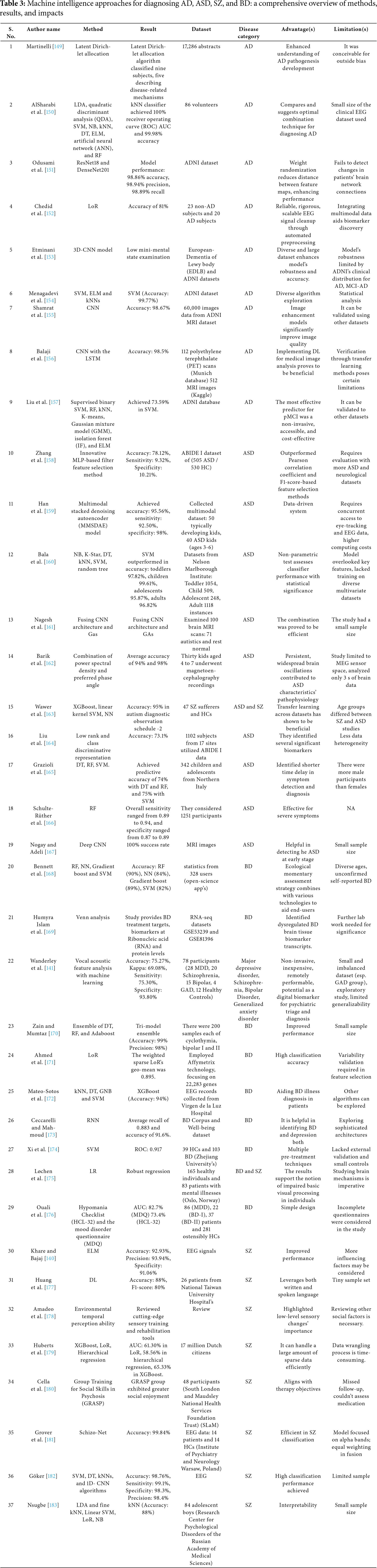
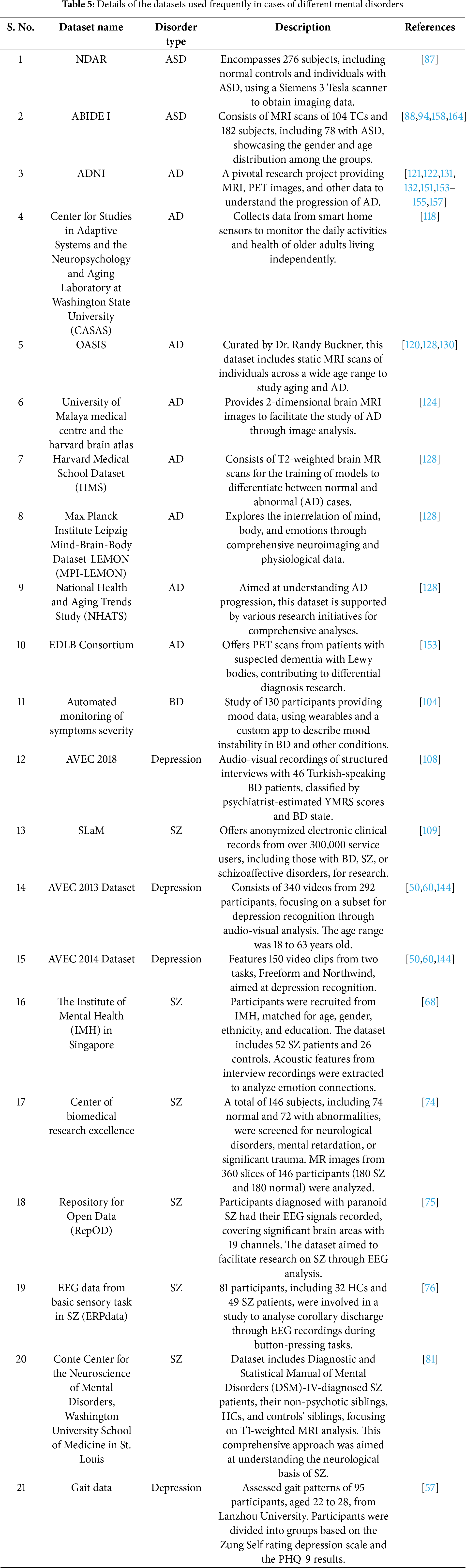
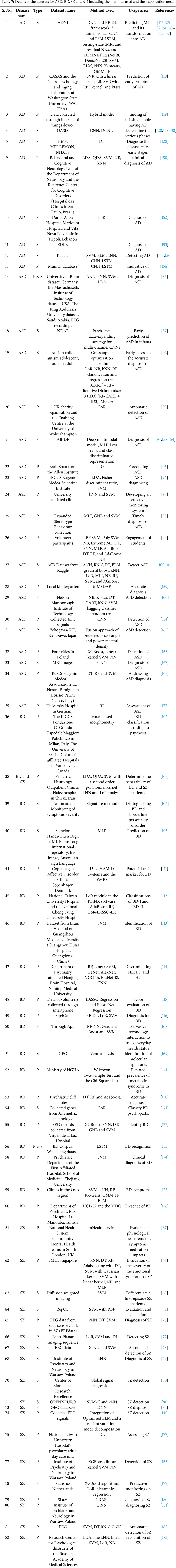
 Downloads
Downloads
 Citation Tools
Citation Tools