 Open Access
Open Access
REVIEW
Immunotherapy in gastric cancer—A systematic review
1 Polytechnic University of Coimbra, ESTESC, UCPCBL, Rua 5 de Outubro, SM Bispo, Apartado, Coimbra, 3046-854, Portugal
2 H&TRC–Health & Technology Research Center, Coimbra Health School, Polytechnic University of Coimbra, Coimbra, 3046-854, Portugal
3 Coimbra Institute for Clinical and Biomedical Research (iCBR) Area of Environment Genetics and Oncobiology (CIMAGO), Biophysics Institute of Faculty of Medicine, University of Coimbra, Coimbra, 3000-548, Portugal
4 Center for Innovative Biomedicine and Biotechnology (CIBB), University of Coimbra, Coimbra, 3000-548, Portugal
5 European Association of Biomedical Scientists, Brussels, 1000, Belgium
* Corresponding Author: FERNANDO MENDES. Email:
Oncology Research 2025, 33(2), 263-281. https://doi.org/10.32604/or.2024.052207
Received 26 March 2024; Accepted 22 November 2024; Issue published 16 January 2025
Abstract
Background: Gastric Cancer (GC) is the 5th most prevalent and 4th most deadly neoplasm globally. Immunotherapy has emerged as a promising treatment approach in GC, potentially improving positive clinical outcomes while addressing the limitations of conventional therapies. GC immunotherapy modalities consist of adoptive cell therapy (ACT), cancer vaccines, and immune checkpoint inhibitors (ICI). Objectives: This systematic review aims to provide an overview of the advances in immune-based therapeutic approaches in GC, highlighting the potential of this therapy as a strategy for GC treatment. Methods: Key studies investigating several immunotherapeutic agents and combination therapies were searched in PUBMED and included in this study. Specific cancer outcomes related to disease progression or survival were analyzed. Results: After screening 236 studies, the results revealed that immunotherapy, particularly the ICI pembrolizumab, demonstrated promising efficacy in the treatment of GC, as several studies reported improved OS, PFS, and objective response rate with the use of pembrolizumab alone or in combination with other treatment modalities. Conclusion: Safety analysis showed that immunotherapy was mostly well-tolerated, with manageable adverse events and relatively good safety profiles. Nonetheless, further research is required to understand the mechanisms of tumor resistance better and identify predictive biomarkers that can direct treatment optimization.Graphic Abstract
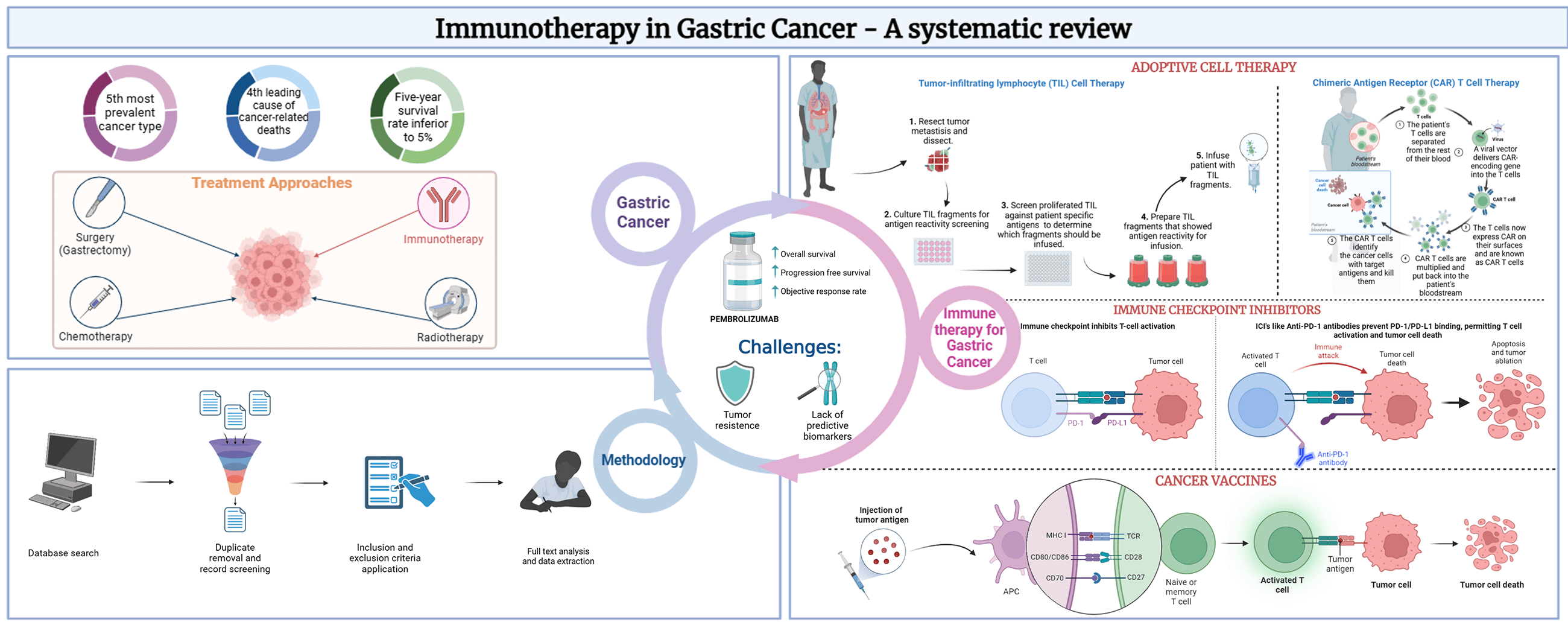
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools