 Open Access
Open Access
ARTICLE
Hypoxia-induced exosomal lncRNA-PVT1 as a biomarker and mediator of EMT in hepatocellular carcinoma
1 General Practice Medical Center, West China Hospital, Sichuan University, Chengdu, 610041, China
2 Department of Laboratory Medicine, West China Hospital, Sichuan University, Chengdu, 610041, China
3 Sichuan Clinical Research Center for Laboratory Medicine, Chengdu, 610041, China
4 Clinical Laboratory Medicine Research Center of West China Hospital, Chengdu, 610041, China
5 Medical Big Data Centre, Sichuan University, Chengdu, 610041, China
6 State Key Laboratory of Oral Diseases & National Center for Stomatology & National Clinical Research Center for Oral Diseases & Department of Information Management & Department of Stomatology Informatics, West China Hospital of Stomatology, Sichuan University, Chengdu, 610041, China
* Corresponding Author: GA LIAO. Email:
Oncology Research 2025, 33(6), 1405-1421. https://doi.org/10.32604/or.2024.056708
Received 29 July 2024; Accepted 14 November 2024; Issue published 29 May 2025
Abstract
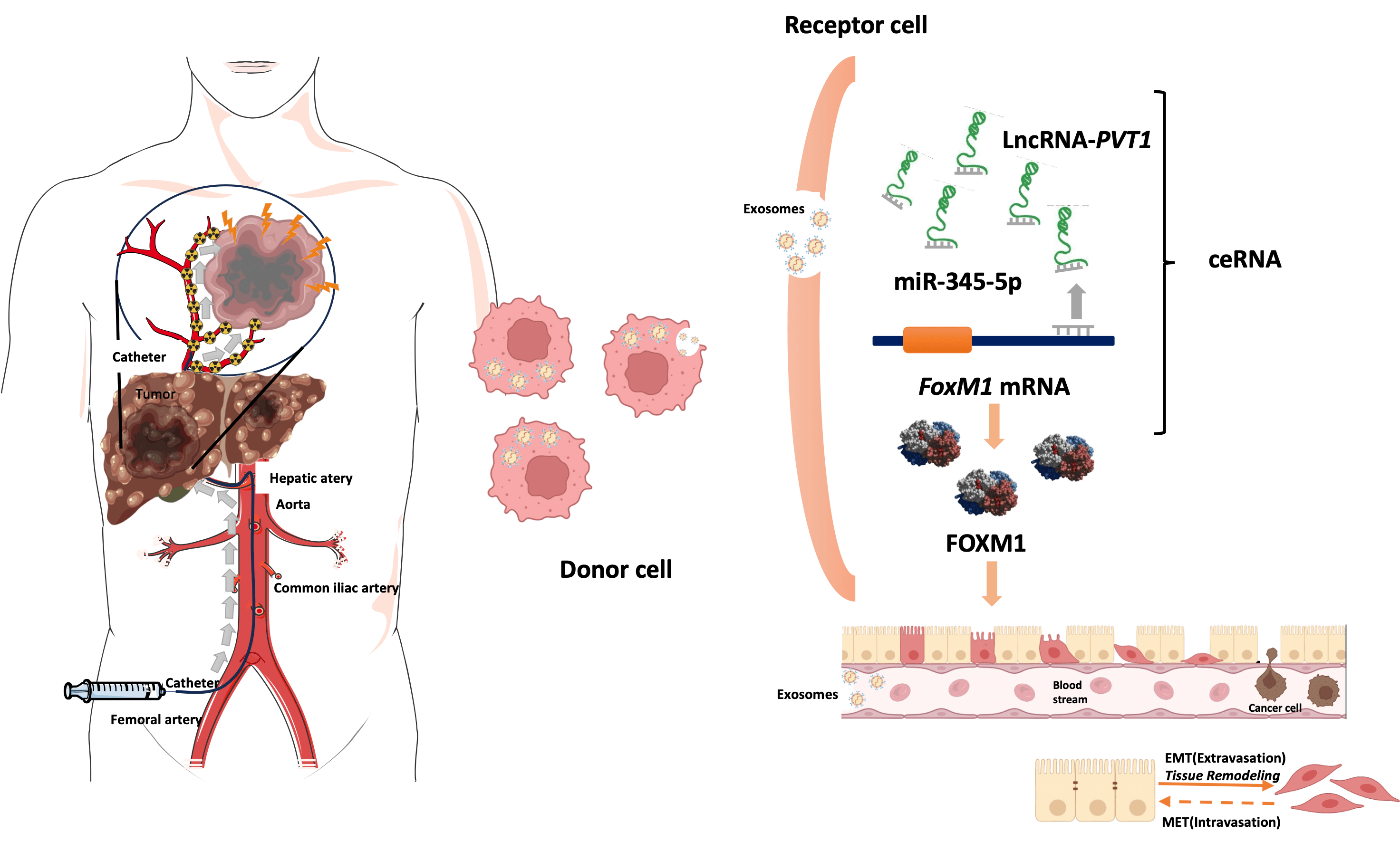
Objectives: Exosomal long noncoding RNAs (lncRNAs) might facilitate epithelial–mesenchymal transition (EMT) in liver cancer after transarterial chemoembolization (TACE), thereby enhancing tumor cell invasiveness and migration. This study investigated the prognostic role of plasma exosomal long noncoding RNA-plasmacytoma variant translocation 1 (lncRNA-PVT1) in TACE treated hepatocellular carcinoma (HCC). Methods: Plasma exosomal lncRNA-PVT1 was evaluated via qPCR before and after TACE. Hepatoma cell behavior was investigated in different HCC cell lines. A lncRNA-PVT1 plasmid was synthesized and overexpressed, and si-lncRNA PVT1 was transfected into poorly invasive cells to reveal its influence on cell characteristics. The lncRNA-PVT1–FoxM1 interaction was elucidated using a double-luciferase reporter gene assay. The effect of miRNA-345-5p on minimally invasive hepatoma cells was assessed. Three experimental groups were established: poorly invasive cells, poorly invasive cells co-cultured with exosomes from hypoxia-induced highly invasive cells, and poorly invasive cells co-cultured with normal hepatocyte exosomes. Liver cancer cells were subcutaneously inoculated into nude mice, with subsequent observations of weight, tumor formation, and tumor size. Results: We identified two lncRNAs (lncRNA-PVT1 and GAPLINC) associated with EMT in the hypoxic microenvironment of liver cancer. Cox multivariate regression analysis was used to establish a prognostic model distinguishing high- and low-risk groups. Hypoxia-induced HepG2 exosomes significantly promoted EMT in poorly invasive HCC cells. LncRNA-PVT1 overexpression and silencing altered E-cadherin, vimentin, and FoxM1 expression, cell proliferation, invasion, migration, and apoptosis. miR-345-5p directly targeted lncRNA-PVT1 and FoxM1, affecting downstream targets. In vivo, co-culturing poorly invasive hepatoma cells with exosomes from highly invasive cells increased tumor volumes, upregulated lncRNA-PVT1, FoxM1, Ki67, and MMP9 expression, and downregulated miR-345-5p expression. Conclusions: Plasma exosomal lncRNA-PVT1 expression is upregulated in highly invasive cells post-hypoxia, potentially serving as a biomarker for evaluating liver cancer prognosis after TACE. Through a miRNA-345-5p-mediated competing endogenous RNA mechanism, it promotes EMT in poorly invasive cells, likely contributing to recurrence and metastasis post-HCC interventional embolization.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools