 Open Access
Open Access
ARTICLE
Comparative assessment of antitumor effects between doxorubicin and mitochondria-targeted doxorubicin in combination with radiotherapy
1 Department of Pharmacy, The Second Affifiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, 310009, China
2 Taizhou Hospital of Zhejiang Province, Zhejiang University, Taizhou, 317099, China
3 Institute of Pharmaceutics, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, 310058, China
4 Department of Clinical Pharmacy, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310009, China
5 Zhejiang Provincial Key Laboratory for Drug Evaluation and Clinical Research, Hangzhou, 310003, China
6 Zhejiang Provincial Key Laboratory of Traditional Chinese Medicine for Clinical Evaluation and Translational Research, Hangzhou, 310003, China
* Corresponding Authors: WUPING SHUAI. Email: ; DONGHANG XU. Email:
# Co-first author: These authors contributed equally to this work
(This article belongs to the Special Issue: Recent Advances in Cancer Pharmacology)
Oncology Research 2025, 33(6), 1423-1436. https://doi.org/10.32604/or.2025.058997
Received 25 September 2024; Accepted 27 December 2024; Issue published 29 May 2025
Abstract
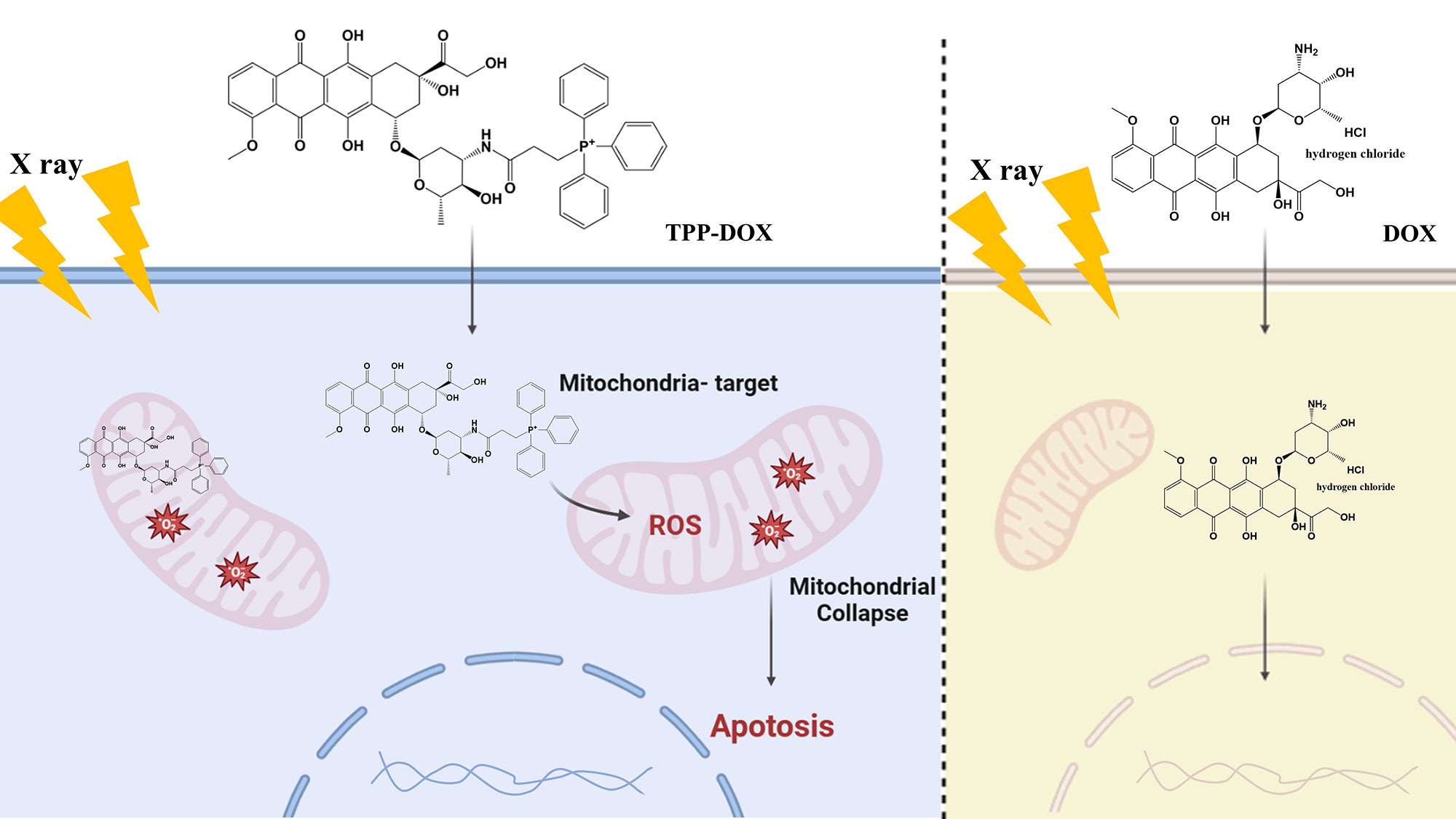
Objectives: Triphenylphosphine (TPP) and Doxorubicin (DOX) were conjugated to obtain Triphenylphosphine-Doxorubicin (TPP-DOX), which was applied in tumor cells for enhancement of DOX in mitochondria targeting. The study focused on investigating the anti-tumor effect of TPP-DOX in combination with radiotherapy throughout in vitro and in vivo studies. Methods: TPP-DOX was synthesized using the carbodiimide method. In vitro experiments were conducted with 4T1 cells (mouse breast cancer cell line) to assess apoptosis induction, mitochondrial targeting, reactive oxygen species (ROS) production, and mitochondrial membrane potential. The research evaluates the effects of TPP-DOX, DOX, and their combinations with radiotherapy. A nude mouse tumor heterograft model was established to investigate the synergistic effect of TPP-DOX and radiotherapy. Results: TPP-DOX was successfully synthesized and scrupulously verified. In vitro experiments showed that compared to DOX, TPP-DOX exhibited enhanced tumor cytotoxicity, improved cellular uptake in 4T1 cells, and increased apoptosis induction. Combined with radiotherapy, TPP-DOX promoted mitochondrial ROS production, reduced mitochondrial membrane potential, and amplified its anti-tumor effect. In vivo experiment confirmed that TPP-DOX combined with radiotherapy exhibited superior anti-tumor activity, promoted tumor tissue apoptosis, inhibited tumor angiogenesis, and showed a favorable in vivo safety profile. Conclusion: The study confirmed that when combined with radiotherapy, TPP-DOX promoted tumor cell apoptosis, and effectively enhanced the anti-tumor effect. In sensitive cells, TPP-DOX demonstrates comparable efficacy to DOX when combined with radiotherapy. TPP-DOX holds significant potential for a broader spectrum of applications and emerges as a valuable candidate for clinical application. These findings provide a promising and efficient therapeutic strategy for tumor treatment with improved efficacy and safety.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools