 Open Access
Open Access
ARTICLE
Differences at diagnosis between long-term survivors and not long-term survivors in metastatic renal cell carcinoma initially treated with TKI
Department of Urology and Nephrology, Virgen del Rocío University Hospital, Seville, 41013, Spain
* Corresponding Author: Miguel Ángel Gómez-Luque. Email:
Canadian Journal of Urology 2025, 32(2), 101-109. https://doi.org/10.32604/cju.2025.063073
Received 04 January 2025; Accepted 28 March 2025; Issue published 30 April 2025
Abstract
Introduction: In recent years, significant advancements in the treatment of metastatic renal cell carcinoma (mRCC) have notably extended overall survival (OS) times, particularly with the introduction of tyrosine kinase inhibitors (TKIs) and combination immunotherapy. However, survival outcomes in mRCC remain highly variable. Materials and Methods: This study retrospectively analyzed clinical and demographic factors at diagnosis in patients treated for mRCC to identify predictors of long-term survival (defined as OS ≥ 48 months). Patients were categorized into long-term survivors (LTS) and non-long-term survivors (nLTS). Results: The analysis revealed that factors such as better Karnofsky Performance Status (KPS), normal baseline laboratory values (e.g., hemoglobin, calcium), and the presence of lung-only metastases were significantly associated with longer survival. Conversely, comorbid conditions like hypertension and dyslipidemia, poorer KPS, and certain adverse laboratory findings were more common in the nLTS group. Conclusion: These findings underscore the importance of baseline prognostic factors in predicting survival outcomes and emphasize the need for personalized treatment strategies in mRCC.Keywords
In the past decades, many important changes in the treatment for metastatic renal cell carcinoma (mRCC) have been established. This fundamental change in therapies have improved the overall survival (OS) of these patients from a little over 12 months in the cytokine therapy era, around 48 months during the time of vascular endothelial growth factor (VEGF) tyrosine kinase inhibitors to conclude in nowadays, with an OS of 4 to 5 years with combination immunotherapy treatments.1,2
Nevertheless, it is of general knowledge that the disease course of mRCC is widely variable between patients. As a result, many studies have tried to identify disease and patients’ characteristics that can predict prognosis. Several clinical and laboratory factors have been identified as important prognostic factors, resulting in prognostic models to estimate survival in these patients.3 There are several prognostic models, such as the Memorial Sloan-Kettering Cancer Center (MSKCC) model or the French model, developed during the cytokine therapy era; the Cleveland Clinic Foundation (CCF) and International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) models, developed during the VEGF-targeted therapy era; or the International Kidney Cancer Working Group (IKCWG) model, validated in both cytokine and tyrosine kinase therapy settings. Both IMDC and MSKCC models are utilized in landmark clinical trials for modern immuno-oncology (IO) combination therapies, such as CHECKMATE-214,4 KEYNOTE-4265,6 and CHECKMATE-9ER.7,8
In spite of the great effort and accuracy of these models to predict survival in mRCC patients, some of them manage to escape their prognosis, becoming long-term survivors (LTS), defined as patients with an overall survival (OS) of 48 months or more.9 To predict which patient will be LTS, baseline prognostic criteria are key. However, despite the advances in prognostic models, there is still a need for a better understanding of the factors that contribute to long-term survival in mRCC. This study aims to address this gap by exploring key clinical and demographic factors at the time of diagnosis of patients with mRCC that may serve as indicators of long-term survival, contributing to improved prognostic accuracy and personalized patient care strategies.
To explore key clinical and demographic factors at the time of diagnosis of patients with mRCC that may serve as indicators of long-term survival, contributing to improving prognostic accuracy and personalized patient care strategies. Specifically, this study aims to extend beyond existing prognostic models by: a) Investigating the impact of comorbidities such as hypertension and dyslipidemia on treatment outcomes in mRCC patients treated with first-line TKI therapy; b) Incorporating patient-reported outcomes (PROs), specifically the FKSI-19 questionnaire, to assess health-related quality of life (HRQoL) and its potential prognostic value; and c) Providing insights into prognostic factors in a real-world clinical setting, which may differ from those identified in highly selected clinical trial populations.
A retrospective, observational analysis was performed in 2023, including patients who initiated treatment for mRCC at the Virgen del Rocío University Hospital, Seville, Spain, between 2014 and 2019. Baseline characteristics were gathered, including demographics, laboratory values, clinical findings, and punctuation in questionnaires in the form of Patient Reported Outcomes (PROs); in our case, we always used NCCN-FKSI 19. Patients were divided into two groups: those with an OS equal to or greater than 48 months were assigned to the long-term survivor (LTS) group, and those with a shorter OS were assigned to the no-long-term survivor (nLTS) group. In our study, there were no patients with less than 48 months of follow-up. Therefore, all patients categorized as nLTS had a definitive outcome with a follow-up period of at least 48 months.
Patients were selected based on the following inclusion and exclusion criteria: being 18 years or older, diagnosed with mRCC, having no prior systemic treatment for mRCC, being able to understand and complete the FKSI-19 questionnaire independently, and having completed the FKSI-19 questionnaire before treatment initiation. Patients were excluded if they declined participation in the study. A total of 78 patients met the inclusion criteria, of whom 7 were excluded due to incomplete FKSI-19 questionnaires (less than 15 items answered), resulting in a final sample size of 71 patients. Ultimately, 25 patients were classified as LTS and 46 as nLTS. Figure 1 shows a flow diagram depicting the screening process and the inclusion and exclusion criteria.

FIGURE 1. Flow diagram showing the selection process of the patients included in the study
Bivariate analyses were performed for each variable with the appropriate statistical test using SPSS v23.0, and results were collected and analyzed. While we acknowledge that multivariable models are often employed in prognostic studies, we opted not to conduct multivariable analysis in this study primarily due to the small sample size, which may limit the statistical power and stability of such models. Additionally, the exploratory nature of our research focuses on identifying potential prognostic factors rather than building definitive prediction models. The Kolmogorov-Smirnov test was used to define which quantitative variables followed a normal distribution in both groups. Statistically significant differences were found when performing the appropriate statistical test. For quantitative variables following a normal distribution, a Student’s T was carried out, considering a statistically significant difference when the p-value was less than 0.05. When quantitative variables did not follow a normal distribution, we used the Mann-Whitney U test, considering a statistically significant difference when the p-value was less than 0.05, using asymptotic significance. For qualitative variables, we used a chi-square test, considering a statistically significant difference when the p-value was less than 0.05, using a unilateral significance.
Later, to identify independent predictors of long-term survival, a multivariable logistic regression analysis was performed. Variables with a p-value < 0.05 in the bivariate analysis were considered for inclusion in the model. The model was built using a stepwise approach, starting with the variable showing the strongest association with the outcome in the bivariate analysis. Variables were added to the model sequentially based on their statistical significance and contribution to model fit. The final model included variables with a p-value < 0.05 and demonstrated good overall fit.
71 patients initiated treatment for mRCC in our unit between 2014 and 2019. All of them (100%) received a vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor (TKI) as first-line treatment. They were subsequently divided into two groups according to their OS at the time of performing the study (2023): 46 patients (64.8%) were assigned to the nLTS group, while 25 patients (35.2%) were assigned to the LTS group. A descriptive analysis of each variable for each group is shown in the following tables. Tables 1 and 2 are the descriptive analyses of the quantitative variables for non-long-term survivors (nLTS) and long-term survivors (LTS), respectively. Table 3 shows the descriptive analysis for the qualitative variables in both groups.

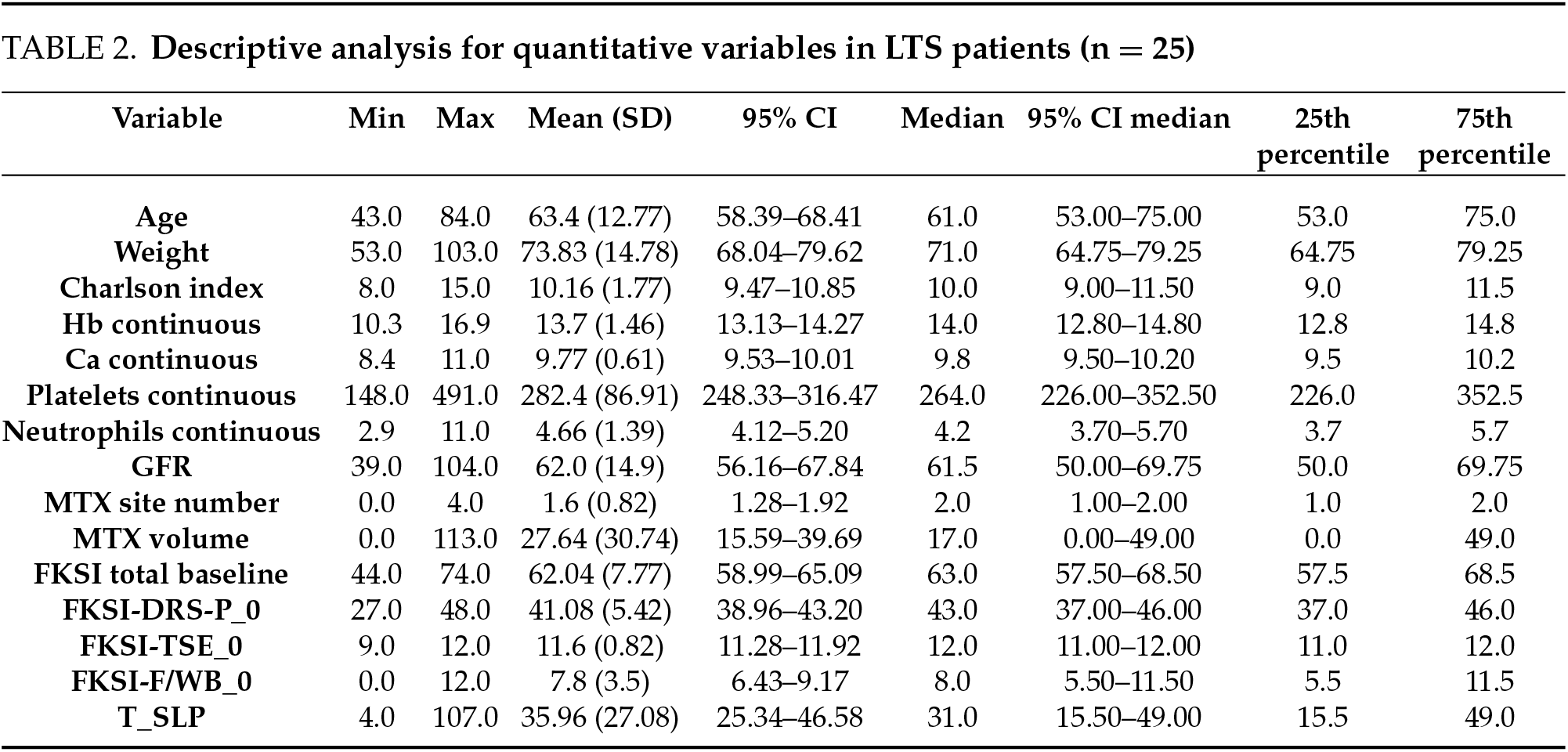

This study’s comprehensive analysis unveiled several statistically significant differences that underscore the complex interplay between patient-specific factors and oncological outcomes in metastatic renal cell carcinoma (mRCC). Our findings highlight the prognostic implications of various clinical and laboratory parameters, which appear to affect long-term survival.
The characteristics of our patient cohort are comparable to those described in real-world mRCC populations.10 The median age in our study aligns with the median age reported in a study of patients receiving first-line systemic therapy for mRCC. Additionally, the proportion of male patients in our cohort is consistent with the gender distribution observed in the broader mRCC population. Furthermore, the distribution of ECOG performance status and the prevalence of clear cell histology in our study are comparable to those reported in larger studies. These similarities suggest that our patient cohort shares key demographic and clinical features with the broader mRCC population, supporting the representativeness of our study. However, we acknowledge that the single-center design may limit the generalizability of our findings to other settings.
The statistically significant differences identified within the baseline Functional Assessment of Cancer Therapy-Kidney Symptom Index scores point towards a differentiated impact on patients’ quality of life, a critical yet often underappreciated aspect of cancer treatment regimens. Specifically, higher baseline total FKSI, DRS-P FKSI, F/WB FKSI, and TSE FKSI scores have been linked to improved overall patient well-being, suggesting that these could potentially serve as indicators for treatment efficacy and patient resilience in mRCC. These findings are supported by another study performed in our unit, which proposed that the baseline FKSI 19 score had prognostic potential, suggesting that this base score could replace the clinician’s subjective determination of PS.11 Other studies performed in the context of clinical trials, such as CheckMate 214, also found a correlation between OS and baseline FKSI-19 score.12 HRQoL measuring is well established in clinical trials but not in daily practice. We think this information is critical because each patient with mRCC should receive the most informed and effective care possible, with a focus not just on survival but on the quality of life and functional status as well.
Moreover, our progression-free survival times showcase a pronounced divergence, with long-term survivors demonstrating a mean PFS time substantially exceeding that of non-LTS. This divergence emphasizes not only the potential for long-term disease control with targeted therapies but also the need to further understand the biological underpinnings that contribute to such disparate patient trajectories. Various studies have researched the impact of diverse treatments in PFS throughout the different eras of treatment.13,14 Thus, underlining how important it is to be able to predict the difference in PFS between different subgroups of patients. The first line of treatment is of utmost importance in mRCC patients, with several studies underlining the differences between the OS when comparing different treatments. Here lies one of the weaknesses of our research, with all patients receiving TKIs as the first line of treatment, which is far from the standard of care currently.
In examining patient demographics and clinical features, we observed a higher incidence of hypertension and dyslipidaemia in the non-LTS group compared to the LTS group. While these comorbidities are often prevalent in the general population, their association with poorer outcomes in mRCC prompts consideration of comprehensive management strategies that encompass these systemic health concerns. It is important to establish the difference between hypertension prior to treatment initiation and treatment-induced hypertension. In a paper published by Liu et al., 15 the predictive value of TKIs-induced hypertension in patients with mRCC was assessed, concluding that this hypertension might be a predictor of better prognosis, especially when using sunitinib or pazopanib. This conclusion, to which the authors arrived via meta-analysis, clarified the conflicting evidence previously existing about TKIs-induced hypertension.16–18 We could not find any consistent data about hypertension at the time of diagnosis and its possible prognostic power. In the case of dyslipidaemia, it is described in the literature that the use of statins may improve treatment outcomes in patients receiving first-line sunitinib.19 We could not find any consistent data either about dyslipidaemia at the time of diagnosis and its prognostic implications. We are, however, inclined to think that patients who suffer from one or both of these pathologies might suffer from other comorbidities, which could worsen the prognosis of this group of patients.
The Karnofsky Performance Status (KPS), a dependable measure of functional impairment, showed statistically significant differences between the LTS and non-LTS cohorts. The overwhelming majority of LTS had a KPS higher than 80, reinforcing the value of functional status as a prognostic factor for treatment tolerance and survival. As it is already established in the MSKCC prognostic factor model, a KSP less than 80% was a predictor of poor prognosis, and even an independent predictor of short survival20 as it may reflect, in combination with other factors from the MSKCC model, tumour burden, aggressive tumour biology, and/or paraneoplastic processes.21,22
Concerning laboratory abnormalities, low haemoglobin levels were markedly more prevalent in the non-LTS group. This finding aligns with existing literature suggesting a correlation between anaemia and unfavorable cancer prognoses, potentially due to underlying mechanisms like tumour hypoxia. Being anaemic, one of the items in the IMDC prognostic model, patients who show low haemoglobin levels are more often classified as intermediate or poor risk group, which would be responsible for a shorter overall survival, as seen in previous literature.23
Notably, the absence of hypercalcemia, thrombopenia, and neutrophilia in the LTS group may indicate a less aggressive disease phenotype or a better overall health status, both of which could contribute to enhanced responsiveness to targeted therapies. The significant gap in incidence of these parameters between the two groups points to their potential utility in refining prognostic models and tailoring treatment approaches.
Furthermore, the Heng risk classification, which stratifies patients based on key prognostic indicators, corroborated the role of these criteria in predicting outcomes. The preponderance of LTS with intermediate risk scores underscores the nuanced nature of risk assessment in mRCC and the importance of considering a spectrum of factors beyond traditional high-risk categorization.
Nephrectomy status emerged as a particularly impactful variable, with all long-term survivors having undergone this surgical procedure, in stark contrast to less than three-fifths of the non-LTS group. This complements existing evidence suggesting a survival benefit with nephrectomy in the context of metastatic disease and highlights the procedure’s potential role in enhancing treatment efficacy. However, in this case, we might be facing a selection bias, as patients belonging to the LTS group are the ones with better prognostic factors, and thus they are the most eligible patients to undergo cytoreductive nephrectomy (CN). Moreover, CN has been widely questioned in the last few years with the publication of trials such as CARMENA,24 which showed that sunitinib alone was noninferior to CN followed by sunitinib, with level 1 evidence. However, as some literature already points out,25 in this study only intermediate and poor-risk patients were included, making it impossible to generalize the results of this study to the population of our study.
We also observed differences in the distribution of metastases, with variations in the presence of metastatic lesions across different anatomical locations. For instance, lung metastases are the most common in mRCC, and were notably prevalent among LTS in our study. This site of metastases carries one of the best prognoses of all visceral sites.26 Moreover, prognostic models based on nomograms have been developed for lung metastatic RCC, which accurately predict OS in these patients.27 Other locations of metastases, such as bone and liver, offer a much worse prognosis. For bone metastases, their emergence usually indicates an advanced disease stage. Moreover, their osteolytic property augmented the incidence of skeletal-related events (SRE), such as pathological fracture and spinal compression,28 thus increasing mortality and reducing the median survival time.29,30 Liver metastases also offer a very poor prognosis, being the location of metastasis in which a solitary lesion offers the lowest overall survival rate.31 When hepatic metastases are present in mRCC, metastatic disease is usually widespread, which limits the pool of patients suitable for local treatment such as hepatectomy.32
To further refine our understanding of the factors influencing long-term survival, we conducted a multivariable logistic regression analysis. This approach allowed us to identify independent predictors of long-term survival while controlling for the influence of other variables. Our final model revealed that dyslipidemia (OR = 0.147, p = 0.025) and anemia (OR = 0.220, p = 0.036) were significantly associated with lower odds of achieving long-term survival. These findings suggest that addressing these specific factors could potentially enhance our prediction of long-term outcomes in patients with mRCC.
Our statistical analysis, while providing valuable insights, has limitations inherent to its methodology and data source. The multivariable logistic regression, despite controlling for multiple variables, operates under the assumption of linearity and independence between predictors, which might not fully capture the complex interplay in biological systems. Additionally, our model’s generalizability is constrained by the sample size and the specific characteristics of our patient cohort, potentially limiting its applicability to broader populations. Furthermore, the retrospective nature of our study introduces the possibility of unmeasured confounding factors and biases related to data collection and patient selection, which could influence the observed associations. These limitations underscore the need for cautious interpretation of our findings and emphasize the importance of future prospective studies with larger, more diverse cohorts to validate and expand upon our results.
Our study has several limitations that warrant consideration. Firstly, the sample size is relatively small and the study was conducted at a single center, which may limit the generalizability of our findings to other populations. Secondly, all patients in our study received first-line TKIs, which may introduce bias and limit the applicability of our findings to current treatment paradigms where combination therapies are often favored. However, we believe that our study still provides valuable insights into prognostic factors that may remain relevant regardless of the treatment regimen used.
Despite these limitations, our findings offer valuable insights into the prognostic factors associated with long-term survival in mRCC. Our results are consistent with key prognostic factors identified in established models like MSKCC and IMDC, such as KPS, anemia, and Heng risk group. Additionally, our study suggests the potential value of incorporating comorbidities and patient-reported outcomes, such as FKSI-19 scores, into existing prognostic models.
Future research should aim to validate our findings in larger, more diverse populations and investigate the impact of comorbidities and PROs in a more comprehensive manner. Our findings could be integrated into existing prognostic tools by adding FKSI-19 scores and specific comorbidities to these models. Additionally, our results can generate hypotheses for prospective research, such as studies evaluating the impact of early interventions targeting specific comorbidities or those designed to improve HRQoL in mRCC patients.
Notwithstanding the limitations mentioned above, our study contributes to a deeper understanding of the factors affecting long-term survival in mRCC. By identifying potential prognostic factors and suggesting ways to incorporate them into existing models, our findings can help clinicians better predict outcomes and personalize treatment strategies for their patients. Moreover, our results highlight the importance of considering HRQoL and the impact of comorbidities in the management of mRCC, emphasizing a more holistic approach to patient care.
Our study highlights the impact of various factors on long-term survival in patients with metastatic renal cell carcinoma (mRCC) initially treated with tyrosine kinase inhibitors (TKIs). We found that a lower Karnofsky performance status (KPS < 80), bone or liver metastases, and certain laboratory abnormalities (hypercalcemia, thrombocytopenia, neutrophilia) are associated with a lower likelihood of long-term survival. Conversely, a good quality of life (high FKSI score) and lung-only metastases appear to be associated with better oncological outcomes. Multivariable analysis revealed dyslipidemia and anemia as independent predictors of decreased long-term survival, suggesting potential targets for improving outcomes in mRCC patients. While our findings offer valuable insights into prognostic factors in mRCC, it is essential to acknowledge the limitations of our study, particularly the small sample size and potential for unmeasured confounding. Further research with larger and more diverse cohorts is needed to validate these findings and refine our understanding of long-term survival in mRCC.
Acknowledgement
The authors would like to express their gratitude to the staff of the unit, the entire medical team at Virgen del Rocío University Hospital, and the patients who participated in this study.
Funding Statement
The authors received no specific funding for this study.
Author Contributions
Conceptualization, Miguel Ángel Gómez-Luque, Guillermo Lendínez-Cano; Methodology, Miguel Ángel Gómez-Luque, Guillermo Lendínez-Cano, Carmen Belén Congregado-Ruiz, Ignacio Osman-García; Investigation and Data Curation, Miguel Ángel Gómez-Luque, Guillermo Lendínez-Cano, Carmen Belén Congregado-Ruiz, Ignacio Osman-García; Writing—Original Draft Preparation, Miguel Ángel Gómez-Luque; Writing—Review & Editing, Miguel Ángel Gómez-Luque, Guillermo Lendínez-Cano, Carmen Belén Congregado-Ruiz, Ignacio Osman-García, Rafael Antonio Medina-López; Supervision, Rafael Antonio Medina-López. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
The data supporting the findings of this study are stored at the Virgen del Rocío University Hospital and are available from the corresponding author upon reasonable request.
Ethics Approval
This study was conducted in accordance with the Declaration of Helsinki. Due to the retrospective nature of the research, which utilized clinical data collected during routine patient care, formal approval from the institutional ethics committee was not required according to institutional guidelines and/or national regulations for this type of study. The requirement for specific patient informed consent for this retrospective analysis was waived. Patient data confidentiality was maintained throughout the study.
Conflicts of Interest
The authors declare no conflicts of interest to report regarding the present study.
References
1. Motzer RJ, Tannir NM, McDermott DF et al. 661P conditional survival and 5-year follow-up in CheckMate 214: first-line nivolumab + ipilimumab (N+I) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). Ann Oncol 2021;32:S685–S687. doi:10.1016/j.annonc.2021.08.057. [Google Scholar] [CrossRef]
2. Motzer RJ, Hutson TE, Tomczak P et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27(22):3584–3590. doi:10.1200/JCO.2008.20.1293. [Google Scholar] [PubMed] [CrossRef]
3. Lemelin A, Takemura K, Heng DYC, Ernst MS. Prognostic models in metastatic renal cell carcinoma. Hematol Oncol Clin North Am 2023;37(5):925–935. doi:10.1016/j.hoc.2023.04.016. [Google Scholar] [PubMed] [CrossRef]
4. Escudier B, Motzer RJ, Tannir NM et al. Efficacy of nivolumab plus ipilimumab according to number of IMDC risk factors in CheckMate 214. Eur Urol 2020;77(4):449–453. doi:10.1016/j.eururo.2019.10.025. [Google Scholar] [PubMed] [CrossRef]
5. Rini BI, Plimack ER, Stus V et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med 2019;380(12):1116–1127. doi:10.1056/NEJMoa1816714. [Google Scholar] [PubMed] [CrossRef]
6. Plimack ER, Powles T, Stus V et al. Pembrolizumab plus axitinib versus sunitinib as first-line treatment of advanced renal cell carcinoma: 43-month follow-up of the phase 3 KEYNOTE-426 study. Eur Urol 2023;84(5):449–454. doi:10.1016/j.eururo.2023.06.006. [Google Scholar] [PubMed] [CrossRef]
7. Choueiri TK, Powles T, Burotto M et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med 2021;384(9):829–841. doi:10.1056/NEJMoa2026982. [Google Scholar] [PubMed] [CrossRef]
8. Motzer RJ, Powles T, Burotto M et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ERlong-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol 2022;23(7):888–898. doi:10.1016/S1470-2045(22)00290-X. [Google Scholar] [PubMed] [CrossRef]
9. Fay AP, Xie WL, Lee JL et al. Characteristics of long-term and short-term survivors of metastatic renal cell carcinoma treated with targeted therapies: results from the international mRCC database consortium. Clin Genitourin Cancer 2015;13(2):150–155. doi:10.1016/j.clgc.2014.09.003. [Google Scholar] [PubMed] [CrossRef]
10. Shah NJ, Sura SD, Shinde R et al. Real-world treatment patterns and clinical outcomes for metastatic renal cell carcinoma in the current treatment era. Eur Urol Open Sci 2023;49:110–118. doi:10.1016/j.euros.2022.12.015. [Google Scholar] [PubMed] [CrossRef]
11. Lendínez-Cano G, Vilches-Arenas Á, Congregado-Ruíz B, Medina-López R. Patient’s self-reported quality of life as a prognostic factor in metastatic renal cell carcinoma initially treated with TKI: nomogram proposal. World J Urol 2024;42(1):267. doi:10.1007/s00345-024-04972-9. [Google Scholar] [PubMed] [CrossRef]
12. Cella D, Choueiri TK, Hamilton M et al. The relationship between health-related quality of life and overall survival in patients with advanced renal cell carcinoma in CheckMate 214. Oncologist 2024;29(6):511–518. doi:10.1093/oncolo/oyae003. [Google Scholar] [PubMed] [CrossRef]
13. Choueiri TK, Hessel C, Halabi S et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (alliance A031203 CABOSUN randomised trialprogression-free survival by independent review and overall survival update. Eur J Cancer 2018;94(2):115–125. doi:10.1016/j.ejca.2018.02.012. [Google Scholar] [PubMed] [CrossRef]
14. Motzer RJ, Hutson TE, Cella D et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. New Engl J Med 2013;369(8):722–731. doi:10.1056/NEJMoa1303989. [Google Scholar] [PubMed] [CrossRef]
15. Liu Y, Zhou L, Chen Y et al. Hypertension as a prognostic factor in metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: a systematic review and meta-analysis. BMC Urol 2019;19(1):1–13. doi:10.1186/s12894-019-0481-5. [Google Scholar] [PubMed] [CrossRef]
16. Szmit S, Langiewicz P, Żłnierek J et al. Hypertension as a predictive factor for survival outcomes in patients with metastatic renal cell carcinoma treated with sunitinib after progression on cytokines. Kidney Blood Press Res 2012;35(1):18–25. doi:10.1159/000329933. [Google Scholar] [PubMed] [CrossRef]
17. Goldstein D, Rosenberg JE, Figlin RA et al. Is change in blood pressure a biomarker of pazopanib and sunitinib efficacy in advanced/metastatic renal cell carcinoma? Eur J Cancer 2016;53:96–104. doi:10.1016/j.ejca.2015.10.006. [Google Scholar] [PubMed] [CrossRef]
18. Fukuda H, Kondo T, Iida S, Takagi T, Tanabe K. Treatment-related deterioration of renal function is associated with the antitumor efficacy of sunitinib in patients with metastatic renal cell carcinoma. Urol Oncol: Semin Orig Investigat 2016;34(8):338.e1–338.e9. doi:10.1016/j.urolonc.2016.03.010. [Google Scholar] [PubMed] [CrossRef]
19. Boegemann M, Schlack K, Rink M et al. Effect of comorbidities/comedications on sunitinib outcomes for metastatic renal cell carcinoma: the STAR-TOR registry. Future Oncol 2020;16(35):2939–2948. doi:10.2217/fon-2020-0548. [Google Scholar] [PubMed] [CrossRef]
20. Heng DYC, Xie W, Regan MM et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27(34):5794–5799. doi:10.1200/JCO.2008.21.4809. [Google Scholar] [PubMed] [CrossRef]
21. Mekhail TM, Abou-Jawde RM, BouMerhi G et al. Validation and extension of the memorial sloan-kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2005;23(4):832–841. doi:10.1200/JCO.2005.05.179. [Google Scholar] [PubMed] [CrossRef]
22. Kim HL, Belldegrun AS, Freitas DG et al. Paraneoplastic signs and symptoms of renal cell carcinoma: implications for prognosis. J Urol 2003;170(5):1742–1746. doi:10.1097/01.ju.0000092764.81308.6a. [Google Scholar] [PubMed] [CrossRef]
23. Mizuno R, Yasumizu Y, Tanaka N et al. Anemia in patients ≥ 75 years with metastatic clear cell renal cell carcinoma: an important poor prognostic factor in the international metastatic renal cell carcinoma database consortium model. BMC Urol 2024;24(1):1–8. doi:10.1186/s12894-024-01403-0. [Google Scholar] [PubMed] [CrossRef]
24. Méjean A, Ravaud A, Thezenas S et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. New Engl J Med 2018;379(5):417–427. doi:10.1056/NEJMoa1803675. [Google Scholar] [PubMed] [CrossRef]
25. Soares A, Maia MC, Vidigal F, Marques Monteiro FS. Cytoreductive nephrectomy for metastatic renal cell carcinoma: how to apply new evidence in clinical practice. Oncology 2020;98(1):1–9. doi:10.1159/000502778. [Google Scholar] [PubMed] [CrossRef]
26. Abdel-Rahman O. Clinical correlates and prognostic value of different metastatic sites in metastatic renal cell carcinoma. Future Oncol 2017;13(22):1967–1980. doi:10.2217/fon-2017-0175. [Google Scholar] [PubMed] [CrossRef]
27. Mao W, Fu Z, Wang K, Wu J, Xu B, Chen M. Prognostic nomogram for patients with lung metastatic renal cell carcinoma: a seer-based study. Ann Palliat Med 2021;10(3):2791–2804. doi:10.21037/apm-20-1488. [Google Scholar] [PubMed] [CrossRef]
28. Zekri J, Ahmed N, Coleman R, Hancock B. The skeletal metastatic complications of renal cell carcinoma. Int J Oncol 2001;19(2):379–382. doi:10.3892/ijo.19.2.379. [Google Scholar] [PubMed] [CrossRef]
29. Santini D, Procopio G, Porta C et al. Natural history of malignant bone disease in renal cancer: final results of an Italian bone metastasis survey. PLoS One 2013;8(12):e83026. doi:10.1371/journal.pone.0083026. [Google Scholar] [PubMed] [CrossRef]
30. Huang Z, Du Y, Zhang X, Liu H, Liu S, Xu T. Clear cell renal cell carcinoma bone metastasis: what should be considered in prognostic evaluation. Eur J Surg Oncol 2019;45(7):1246–1252. doi:10.1016/j.ejso.2019.01.221. [Google Scholar] [PubMed] [CrossRef]
31. Tappero S, Barletta F, Piccinelli ML et al. Survival of patients with clear cell renal carcinoma according to number and location of organ-specific metastatic sites. Urol Oncol: Semin Orig Investig 2024;42(1):22.e23–22.e31. doi:10.1016/j.urolonc.2023.08.014. [Google Scholar] [PubMed] [CrossRef]
32. Grimes NG, Devlin JM, Dunne DFJ et al. A systematic review of the role of hepatectomy in the management of metastatic renal cell carcinoma. Eur J Surg Oncol 2014;40(12):1622–1628. doi:10.1016/j.ejso.2014.08.472. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools