 Open Access
Open Access
ARTICLE
Numerical Simulation of Blood Flow Dynamics in a Stenosed Artery Enhanced by Copper and Alumina Nanoparticles
1 Department of Applied Mathematics, M. J. P. Rohilkhand University, Bareilly, 243006, India
2 Fakulti Teknologi dan Kejuruteraan Mekanikal, Universiti Teknikal Malaysia, Melaka, 76100, Malaysia
3 Forecasting and Engineering Technology Analysis (FETA) Research Group, Universiti Teknikal Malaysia, Melaka, 76100, Malaysia
4 Faculty of Engineering, Kuwait College of Science and Technology, Doha, 35004, Kuwait
* Corresponding Author: Nurul Amira Zainal. Email:
Computer Modeling in Engineering & Sciences 2025, 142(2), 1839-1864. https://doi.org/10.32604/cmes.2024.056661
Received 27 July 2024; Accepted 13 November 2024; Issue published 27 January 2025
Abstract
Nanotechnology holds immense importance in the biomedical field due to its ability to revolutionize healthcare on a molecular scale. Motivated by the imperative of enhancing patient outcomes, a comprehensive numerical simulation study on the dynamics of blood flow in a stenosed artery, focusing on the effects of copper and alumina nanoparticles, is conducted. The study employs a 2-dimensional Newtonian blood flow model infused with copper and alumina nanoparticles, considering the influence of a magnetic field, thermal radiation, and various flow parameters. The governing differential equations are first non-dimensionalized to facilitate analysis and subsequently solved using the 4th order collocation method, bvp4c module in MATLAB. This approach obtains velocity and temperature profiles, revealing the impact of relevant parameters crucial in the biomedical field. The findings of this study underscore the significance of understanding blood flow dynamics in stenosed arteries and the potential benefits of utilizing copper and alumina nanoparticles in treatment strategies. The incorporation of nanoparticles introduces novel avenues for enhancing therapeutic interventions, particularly in mitigating the effects of stenosis. The elucidation of velocity and temperature profiles provides valuable insights into the behavior of blood flow under different conditions, thereby informing the development of targeted biomedical applications. The arterial curvature flow parameter influences temperature profiles, with increased parameters promoting more efficient heat dissipation. The elevated values of Prandtl number and thermal radiation parameter showcase the diminished temperature profiles, indicating stronger dominance of momentum diffusion over thermal diffusion and radiative heat transfer mechanism. Sensitivity analysis of the pertinent physical parameters reveals that the Prandtl number has the most significant impact on blood flow dynamics. A statistical analysis of the present results and existing literature has also been included in the study. Overall, this research contributes to advancing our understanding of vascular health and lays the groundwork for innovative approaches in stenosis treatment and related biomedical fields.Keywords
Nanotechnology, the manipulation of matter at the nanoscale, has revolutionized numerous industries and scientific fields, offering unprecedented control over materials and their properties. One area where nanotechnology has made significant strides is in the development of nanofluids. Nanofluids, engineered by dispersing nanoparticles into conventional fluids, exhibit unique and enhanced properties compared to their base fluids. These properties include improved thermal conductivity, heat transfer efficiency, and rheological behavior [1]. The importance of nanofluids lies in their wide-ranging applications across diverse fields, including thermal management systems, energy generation, biomedical devices, and environmental remediation. By harnessing the remarkable characteristics of nanofluids, researchers and engineers can develop innovative solutions to address pressing challenges and propel technological advancements in various sectors [2–4].
Nanofluids, colloidal suspensions of nanoparticles in a base fluid, represent a cutting-edge frontier in fluid dynamics research, offering remarkable properties with diverse applications. Their significance spans various domains, from enhancing heat transfer efficiency in industrial processes to improving lubrication in mechanical systems. However, perhaps most compelling is their burgeoning role in biomedicine. Within this realm, nanofluids exhibit tremendous promise, particularly in studying blood flow dynamics and the pathology of diseased arteries. Copper and alumina nanoparticles, in particular, have emerged as pivotal components in this research landscape. Their interactions with blood components and vascular walls can profoundly influence flow behavior, offering insights into cardiovascular diseases such as atherosclerosis. Jerka et al. [5] delved deeply into the detailed workings outlined in deciphering endothelial cell migration. Zuberi et al. [6] investigated the interplay of Brownian motion and thermophoresis on blood-based Casson nanofluid dynamics on a non-linearly stretching sheet, contributing to understanding fluid mechanics in biomedical contexts. This study provides valuable insights for applications in heat transfer and biomedical engineering. Fig. 1 represents the blood flowing in the human artery.
Figure 1: Blood flowing in the human artery
Stenosis is the abnormal narrowing of a blood vessel, which poses a significant threat to cardiovascular health, often leading to restricted blood flow and increased risk of serious complications such as heart attacks and strokes. Stenotic arteries, in particular, have narrowed due to various factors, including atherosclerosis, inflammation, or congenital defects. The treatment of stenosis necessitates innovative approaches, including the use of nanoparticles [7,8]. In biomedical research, a diverse array of authors have delved into the exploration of blood flow dynamics within stenotic arteries through the lens of nanoparticles [9–11]. Each author’s selection of nanoparticles for their investigations reflects a nuanced approach tailored to specific research objectives. Some authors, such as Ali et al. [12], Karmakar et al. [13], Shahzad et al. [14], Muthtamilselvan et al. [15], etc., opt for gold nanoparticles leveraging their unique optical properties for precise imaging and tracking within the intricate vascular network. Others favor [16,17] iron oxide and aluminum oxide nanoparticles, capitalizing on their magnetic properties to enable targeted delivery and manipulation within the constricted confines of stenotic arteries. Titanium dioxide nanoparticles also find their proponents, with their biocompatibility and photocatalytic capabilities offering intriguing possibilities for therapeutic interventions [18]. Through meticulous experimentation and analysis, these authors collectively contribute to a deeper understanding of the complex interplay between nanoparticles and blood flow dynamics within stenotic arteries, paving the way for innovative diagnostic and therapeutic strategies in vascular medicine [19–22]. Infusing copper and alumina nanoparticles into blood flow under a stenosed artery represents a novel and promising approach in addressing the complex dynamics of cardiovascular diseases. Within the confined space of a stenotic artery, blood flow is often disrupted, leading to adverse effects on vascular health. Copper and alumina nanoparticles offer unique advantages in this context [23]. Their presence within the bloodstream can modulate key physiological processes, including inflammation, oxidative stress, and endothelial dysfunction, all of which are implicated in the pathogenesis of stenosis. Moreover, these nanoparticles possess inherent properties that enable them to target specific sites within the arterial wall, facilitating localized therapeutic effects while minimizing systemic side effects [24]. By harnessing the synergistic potential of copper and alumina nanoparticles, researchers aim to develop innovative therapies that alleviate the symptoms of stenosis and promote arterial healing and restore normal blood flow, thereby improving patient outcomes and quality of life. This interdisciplinary approach holds promise for revolutionizing cardiovascular disease management and heralding a new era in precision medicine. Also, the aggregation of nanoparticles in the base fluid is a matter of concern as it can influence the effective viscosity, reduce the surface area available for interaction, and potentially alter the intended therapeutic effects. It may also impact the rheological properties of blood, deviating from the assumed Newtonian behavior. Moreover, their biocompatibility and tunable characteristics enable tailored interventions that minimize adverse effects and maximize therapeutic efficacy. As such, the integration of copper and alumina nanoparticles into treatment modalities for stenosis represents a promising avenue for advancing cardiovascular medicine and improving patient outcomes. Gandhi et al. [25] discussed the applications of the KKL (Koo-Kleinstreuer-Li) model for blood flow in stenotic permeable arteries. Numerous authors have also engaged in discussions surrounding blood flow dynamics within stenosed arteries using hybrid nanoparticles [26–29]. Poonam et al. [30] explored the concept of hybrid nanoparticles for blood flowing through a curve-shaped artery having stenosis. Khanduri et al. [31] explained the concept of hematocrit-dependent viscosity and the Hall effect for blood flow in stenosed arteries with hybrid nanoparticles. A numerical as well as analytical solution to heat transfer problems with mild stenosis has been a part of research by Jalili et al. [32]. The behavior of blood with nanoparticles in narrow arteries has been discussed by the authors Hussain et al. [33]. This emerging area of research amalgamates the distinctive attributes of different nanoparticle types to create multifunctional platforms tailored for enhanced diagnostics and therapeutics. Scholars delve into the synergistic effects of combining various nanoparticle materials, such as gold-silver hybrids or silica-iron oxide hybrids, to exploit complementary properties for improved imaging resolution, targeted drug delivery, and precise monitoring of hemodynamic parameters within stenotic arteries [34,35]. Through their collaborative efforts, these authors shed light on the potential of hybrid nanoparticle systems to revolutionize our understanding and management of vascular diseases, offering promising avenues for developing next-generation biomedical technologies [36–39].
Abbas et al. [40] conducted a computational investigation of the hydrodynamic behavior of copper and alumina nanofluid mixtures in water, analyzing their flow characteristics over a vertical wedge to advance the understanding of such fluids in engineering applications. Tripathi et al. [41] demonstrated how hybrid nanoparticles enhance blood flow regulation, improving the efficiency of therapeutic treatments in arterial systems. Similarly, Basha et al. [42] investigated the behavior of Au-Cu/magneto-bio-hybrid nanofluids in stenosed arteries, emphasizing their impact on hemodynamic parameters. Das et al. [43] further outlined the effects of hybrid nanoparticles on blood flow in inclined stenosed arteries under the influence of magnetic fields, showing a marked improvement in heat and mass transfer. Hussain et al. [44] focused on clinical applications, studying the interaction of hybrid nanoparticles and magnetic fields to manage thrombosis in multiple stenosed arteries. Additionally, Karmakar et al. [45] explored how tri-hybrid nanoadditives improve non-Newtonian magnetized blood flow through a charged artery. These studies collectively underscore the potential of hybrid nanoparticles to revolutionize treatments in various medical conditions involving compromised blood flow [46–48]. Furthermore, Verma [49] analyzed and simulated nanofluid flow using MATLAB, highlighting how these fluids can be effectively modeled for complex flow scenarios. Das et al. [50] examined the impact of Hall and ion slip currents on electromagnetic blood flow in hybrid nanoparticle-infused fluids, showing their influence on peristaltic motion through an endoscope. Arif et al. [51] extended this work by analyzing heat transfer in Casson tri-hybrid nanofluids, emphasizing the biomedical potential of nanoparticles with varying shapes. Nazar et al. [52] explored the irreversibility of ternary nanofluid flow in inclined arteries using Caputo-Fabrizio fractional derivatives, further expanding the understanding of such systems. Additionally, Abo-Elkhair et al. [53] studied the magnetic force effects on peristaltic transport of AuCu hybrid nanofluids at moderate Reynolds numbers, providing insights into the practical applications of these hybrid fluids in arterial flow. Recent studies have expanded the understanding of nanofluids and their applications in biomedical and thermal engineering. Also, Shah et al. [54] investigated the dynamics of time-dependent cross nanofluids on a melting surface influenced by cubic autocatalysis, providing new insights into the behavior of such fluids in thermal systems. Kabeel et al. [55] reviewed the effects of magnetic fields on flow and heat transfer in liquids, emphasizing both the current knowledge and future potential of these applications. Alghamdi et al. [56] explored magnetohydrodynamic (MHD) hybrid nanofluid flow, particularly in the context of medication delivery through a blood artery, showcasing the therapeutic potential of these fluids. Shabbir et al. [57] studied entropy generation in ternary nanofluid flow through atherosclerotic vessels under periodic body acceleration, highlighting the implications for medical treatments involving nanoparticle-laden fluids. Das et al. [58] examined electromagnetic hybrid nano-blood pumping through an endoscope in the presence of blood clotting and hall and ion slip currents, demonstrating how these mechanisms can be controlled to improve medical outcomes. These works reflect the growing body of research exploring the use of nanofluids in both thermal and biomedical applications [59–62]. Moreover, Li et al. [63] introduced a novel machine learning approach for estimating blood flow dynamics factors in eccentric stenotic arteries, demonstrating its potential to improve accuracy and efficiency in computational hemodynamics models. Here is a two-line citation incorporating both references: The significance of partial slip due to lateral velocity and viscous dissipation in blood-gold Carreau nanofluids has been analyzed through numerical solutions of partial differential equations [64]. Additionally, a partitioning method for finite element approximation in fluid-plate interaction systems has been explored to improve computational efficiency [65]. A recent study by Zuberi et al. [66] investigated the intricate hemodynamic behavior of Sisko model blood, enriched with gold and silver nanoparticles, flowing through a stenosed artery featuring porous walls, providing key insights into the thermal and flow characteristics under complex biological conditions. The investigation of blood flow dynamics in a stenosed artery with the infusion of copper and alumina nanoparticles presents a groundbreaking avenue in cardiovascular research. This study explores the intricate interplay of thermal radiation, magnetic field, and arterial wall curvature, which significantly influence blood flow behavior and arterial health. The conditions modeled in this study represent primarily in vitro scenarios, where the parameters such as nanoparticle concentration, magnetic fields, and fluid properties are controlled to investigate the fundamental dynamics of blood-nanoparticle interaction within a stenosed artery. The use of a simplified two-dimensional Newtonian fluid model and constant thermal and flow parameters aligns with the controlled environment typically seen in vitro setups. The originality of this research lies in bridging the existing gap in understanding the combined effects of nanoparticles and external stimuli on blood flow within stenotic arteries. Previous studies have often focused on individual aspects, neglecting the holistic perspective essential for comprehensive insights into disease progression and treatment. By elucidating the complex interactions among nanoparticles, thermal radiation, magnetic field, and arterial wall curvature, this research promises to fill this critical void and pave the way for more effective therapeutic strategies. The future scope of this research in biomedicine is vast, with potential applications ranging from targeted drug delivery to precise interventions for stenosis. Doctors can leverage the present in vitro findings to tailor treatments based on patient-specific characteristics, optimizing outcomes and minimizing risks associated with conventional approaches. Ultimately, this integrative approach holds the potential to revolutionize the management of stenotic artery diseases, offering personalized solutions that address the underlying pathophysiology while enhancing patient care and well-being.
Consider that the flow of blood is incompressible and two-dimensional which follows the characteristics of uniform Newtonian fluid within a stenosed artery of length
the width of the stenosed region,
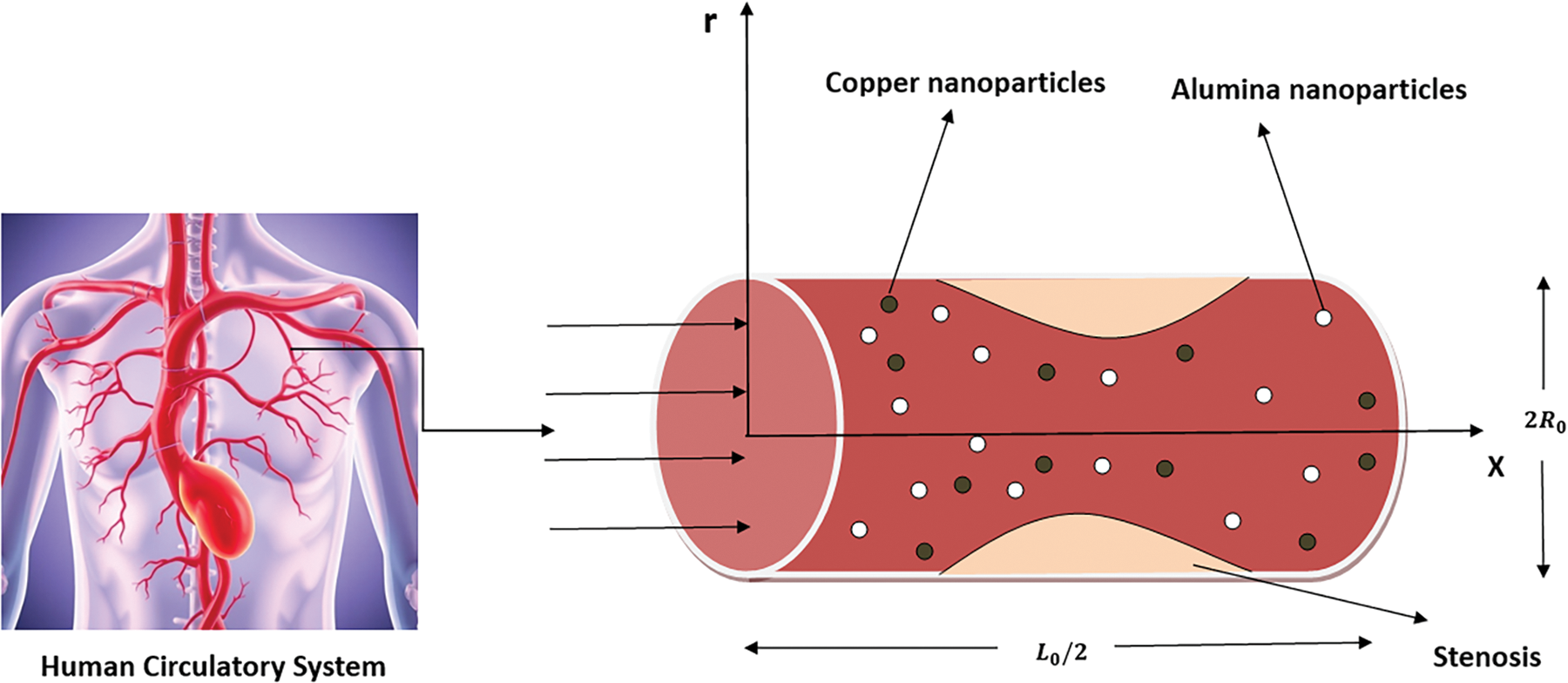
Figure 2: Physical sketch of the stenosed artery with copper and alumina nanoparticles
The term
The thermal attributes of nanofluids as described by Ahmad et al. [1] are employed in this research and are given by
The equation of continuity embedded in Eq. (2) is satisfied trivially by considering
where
Using Eq. (8) and taking into account the subsequent dimensionless parameters
The equation presented in (3) and (4) change into
Considering the dimensionless parameters outlined in Eq. (9), the particular boundary conditions specified in Eq. (5) are altered to
The dimensionless flow parameters that directly influence the flow of blood in stenotic arteries, which arose as a consequence of the non-dimensionalization of partial differential equations are encapsulated in Table 1.
The important parameters, namely the heat transfer coefficient (Nusselt number,
Here, the drag force
By integrating dimensionless parameters as outlined in Eq. (9), Eq. (14) assumes the following structure:
The complexity of the modeled problem necessitated the utilization of an advanced numerical technique, the bvp4c module in MATLAB, since while dealing with intricate boundary value problems that involve nonlinearities, stiff equations, or complex boundary conditions, conventional solvers struggle to produce accurate results efficiently. In such cases, the bvp4c solver shines by offering robust solutions with high accuracy and stability. Its versatility allows it to handle various problems across various domains, providing researchers and engineers with a powerful tool to model and analyze complex phenomena effectively. The importance of the bvp4c module lies in its ability to overcome the challenges posed by complex boundary value problems, enabling users to gain valuable insights and make informed decisions in fields ranging from engineering and physics to biology and finance.
In the current problem, the non-linear coupled ordinary differential equations (Eqs. (10) and (11)), along with their boundary conditions (Eq. (13)), are solved numerically using the shooting technique implemented with the bvp4c solver, the built-in function in the computational tool MATLAB. At this stage, the higher-order system of equations is converted into a first-order system as follows:
where
Initial guesses are required for the bvp4c solver, which iteratively refines the solution by adjusting step sizes until the desired precision is achieved. The choice of initial guess and boundary layer thickness depends on the parameter values used. A tolerance of
4 Simulation Results and Discussion
The current paper conducts an extensive numerical simulation investigation into the behavior of blood flow in narrowed arteries, with a specific focus on how copper and alumina nanoparticles affect this dynamic. The research utilizes a 2D model of Newtonian blood flow infused with copper and alumina nanoparticles, taking into account the influence of factors such as a magnetic field, thermal radiation, and curvature flow parameters. To facilitate analysis, the governing differential equations are initially normalized and then solved using the
Figs. 3–12 are presented to offer insights into the fluid dynamics of copper and alumina nanoparticles suspended in blood flowing through a cosine-shaped stenotic artery. Figs. 3–5 specifically delve into the effect of nanoparticle volume fraction, curvature flow parameter, and magnetic parameter on the velocity profiles of Cu/blood and Al2O3/blood nanofluids. These figures provide valuable information on how these parameters influence the velocity distribution within the artery. Figs. 6–10 further explore the impacts of the volume fraction of nanoparticles, curvature flow parameter, magnetic parameter, Prandtl number, and thermal radiation parameter on the temperature profiles of Cu/blood and Al2O3/blood nanofluids. These figures elucidate the temperature distribution within the artery, considering various influencing factors. Figs. 11 and 12 highlight the influence of the volume fraction of nanoparticles on the skin friction coefficient and Nusselt number for different values of the curvature flow parameter. The skin friction coefficient and Nusselt number are crucial parameters in understanding the heat and momentum transfer characteristics within the artery. The analysis of these figures will result in comprehensive insights into how changes in nanoparticle volume fraction affect these key parameters, providing valuable guidance for biomedical applications and treatment strategies.
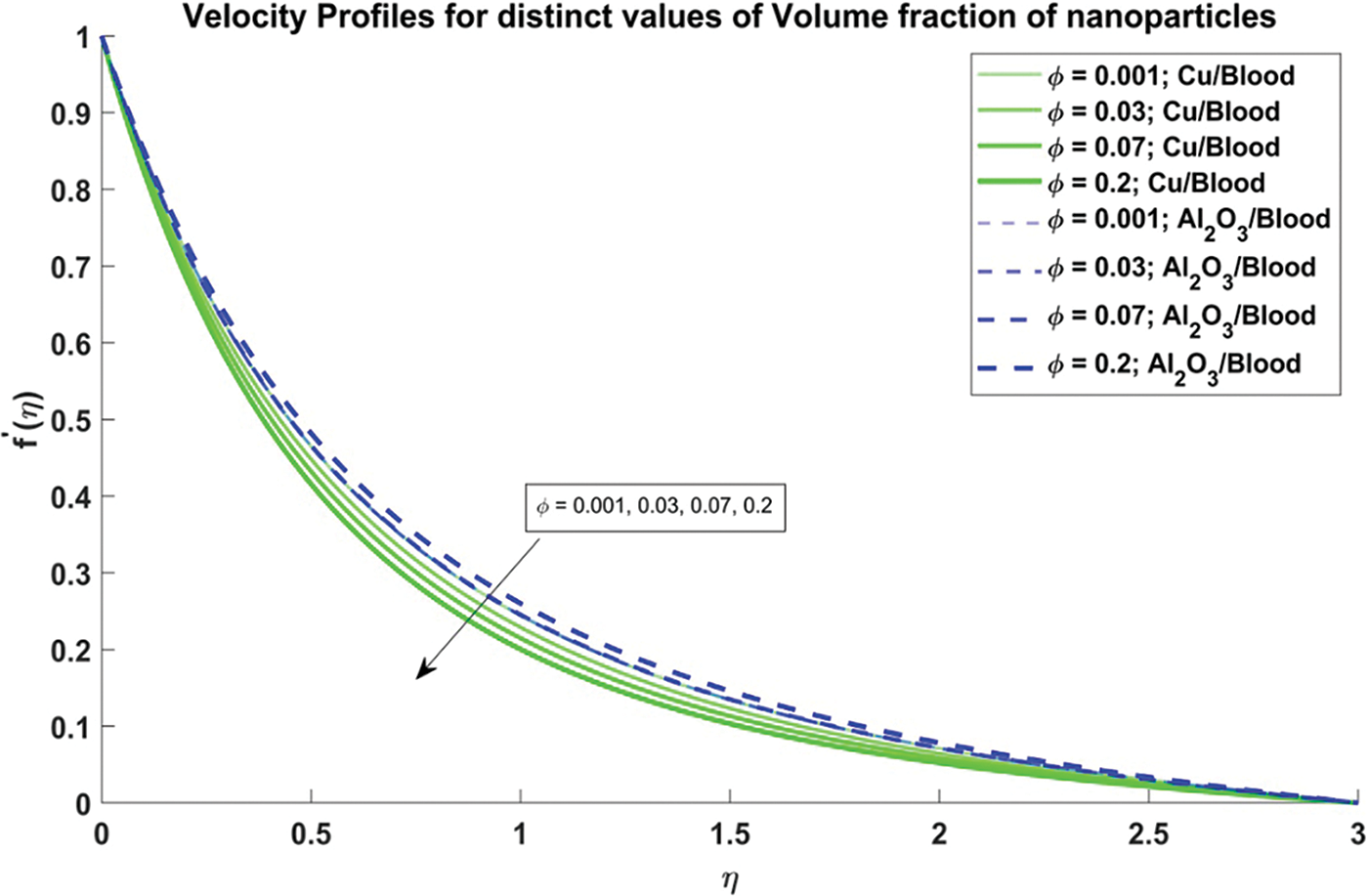
Figure 3: Velocity profiles for distinct values of Volume fraction of nanoparticles
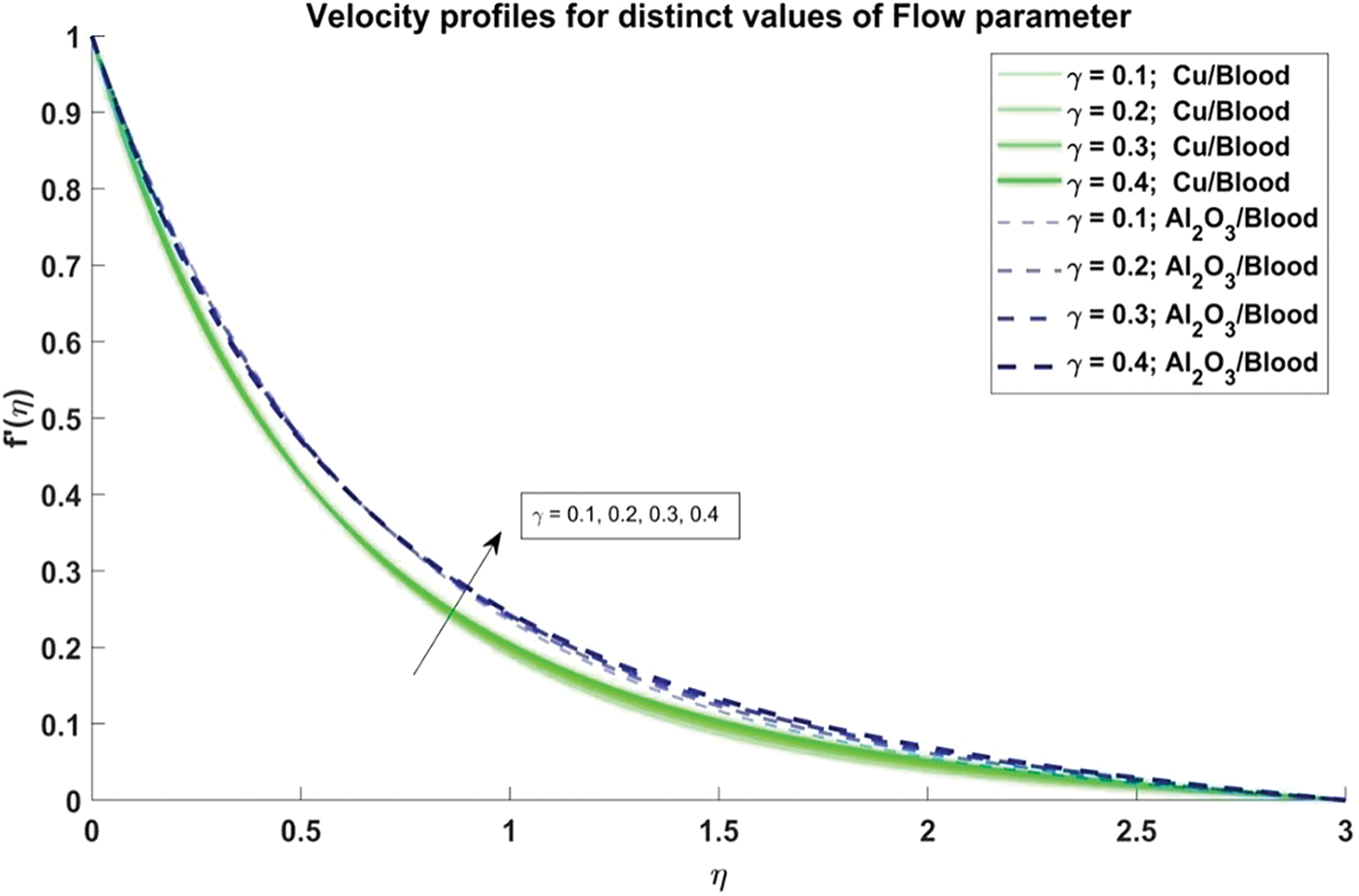
Figure 4: Velocity profiles for distinct values of Curvature flow paramete
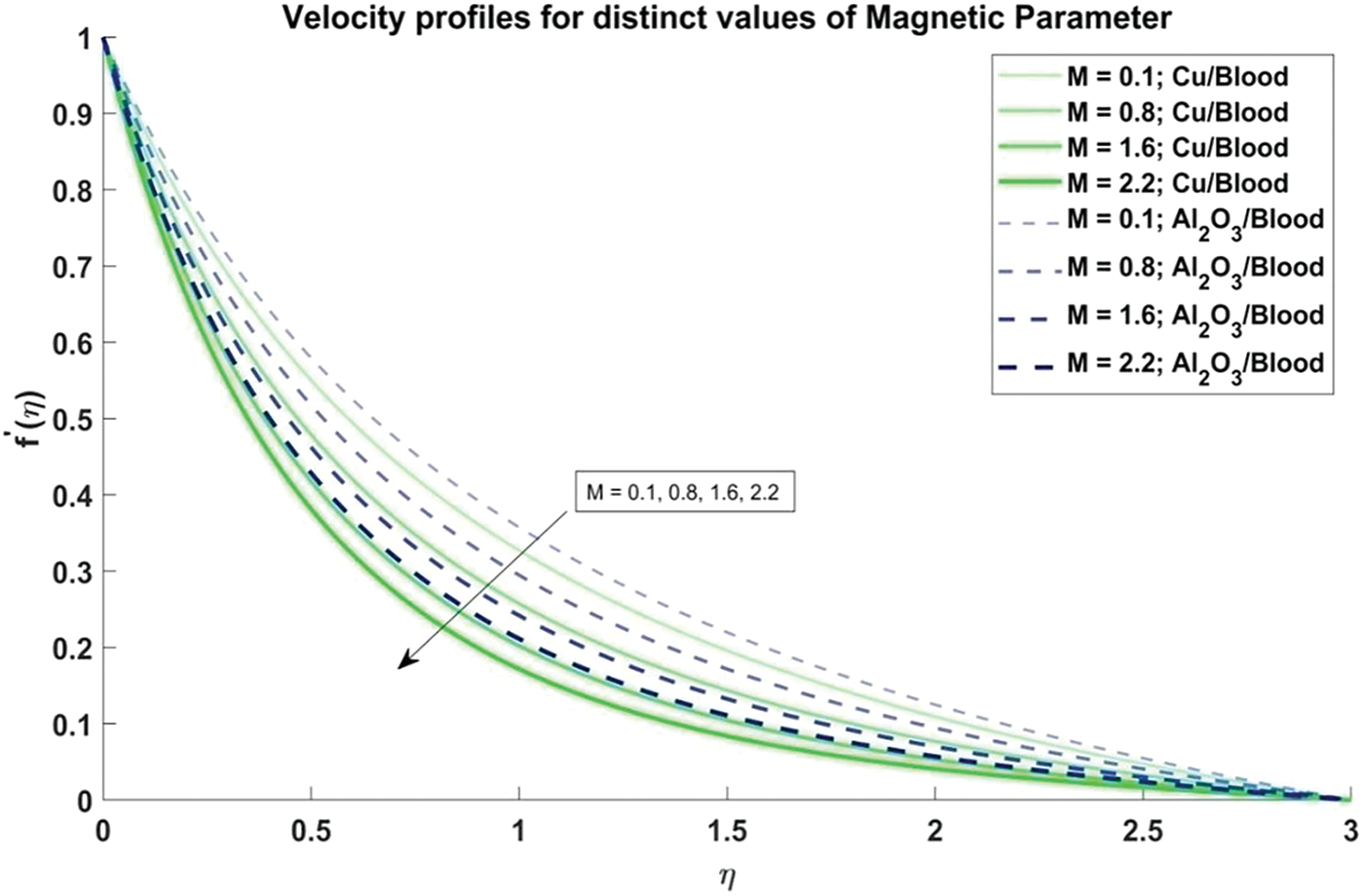
Figure 5: Velocity profiles for distinct values of Magnetic parameter
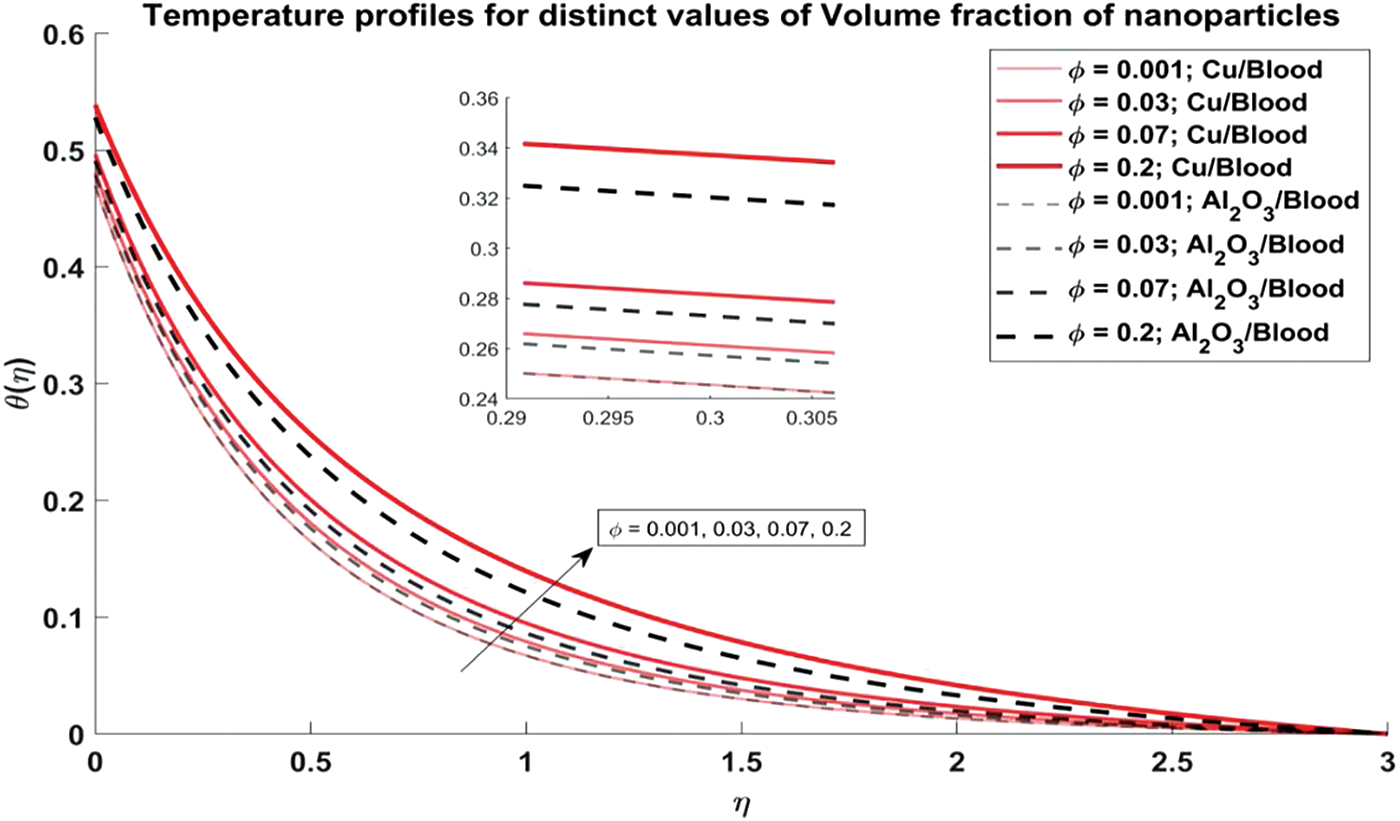
Figure 6: Temperature profiles for distinct values of Volume fraction of nanoparticles
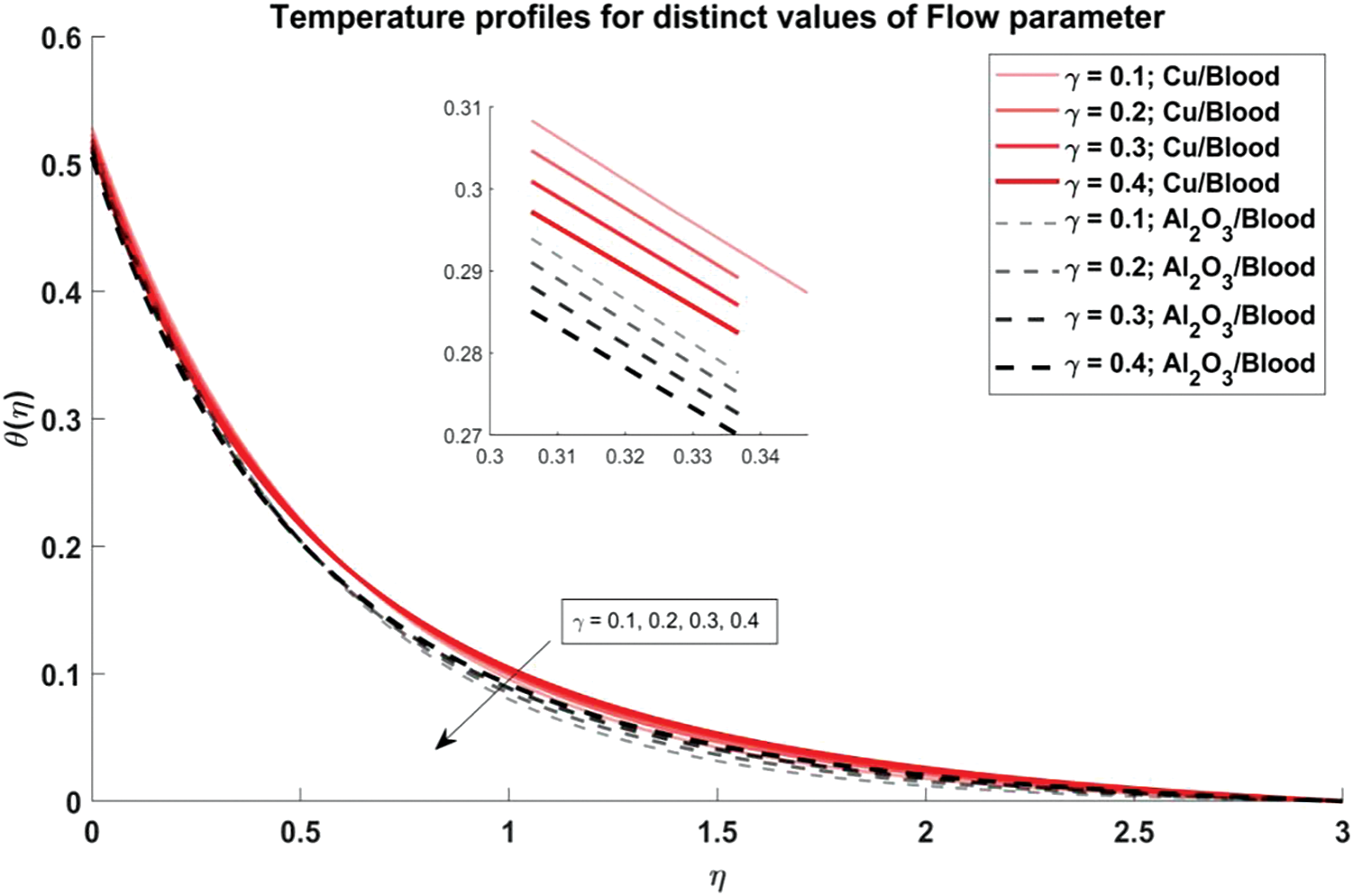
Figure 7: Temperature profiles for distinct values of Curvature flow parameter
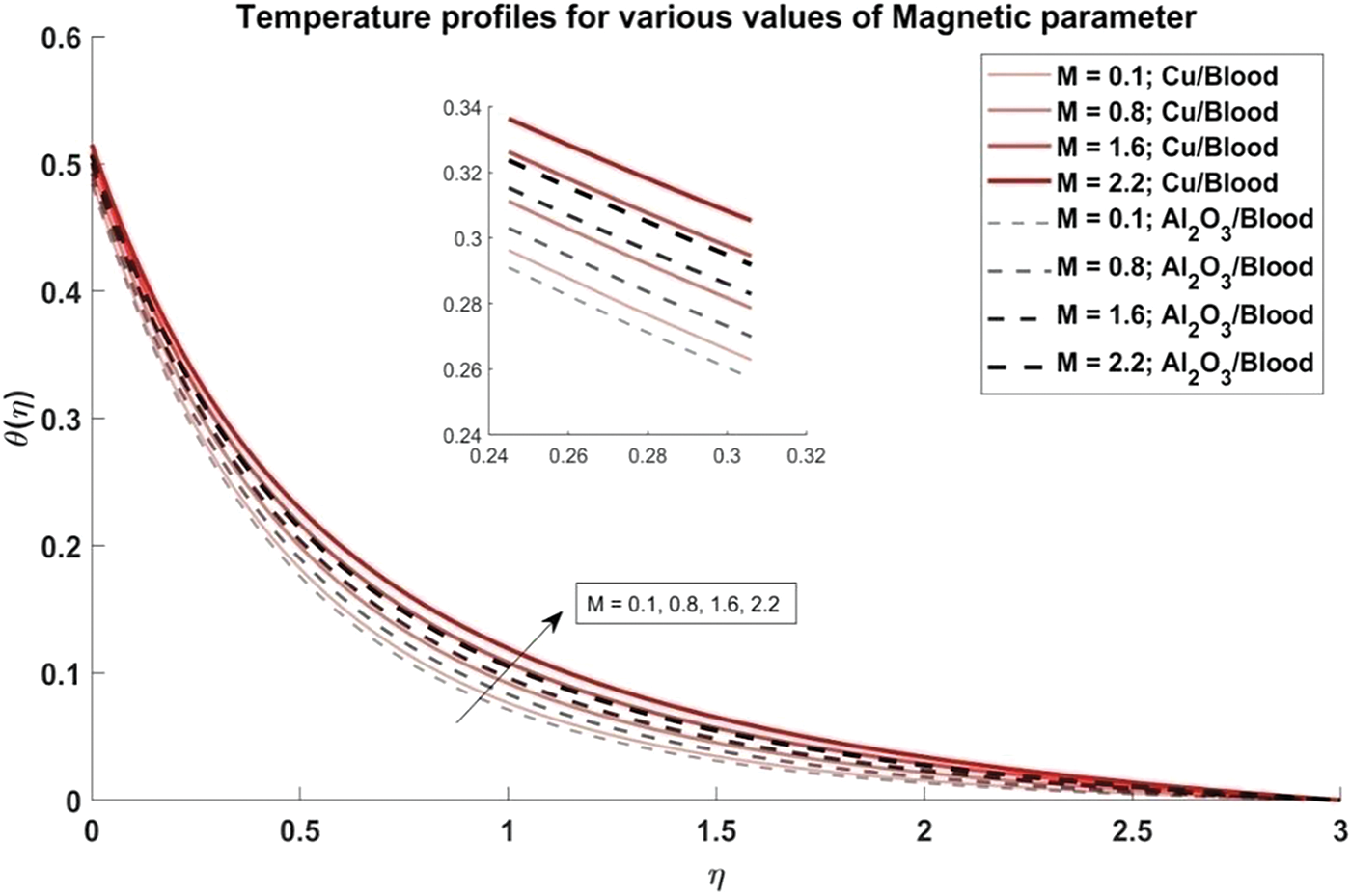
Figure 8: Temperature profiles for distinct values of Magnetic parameter
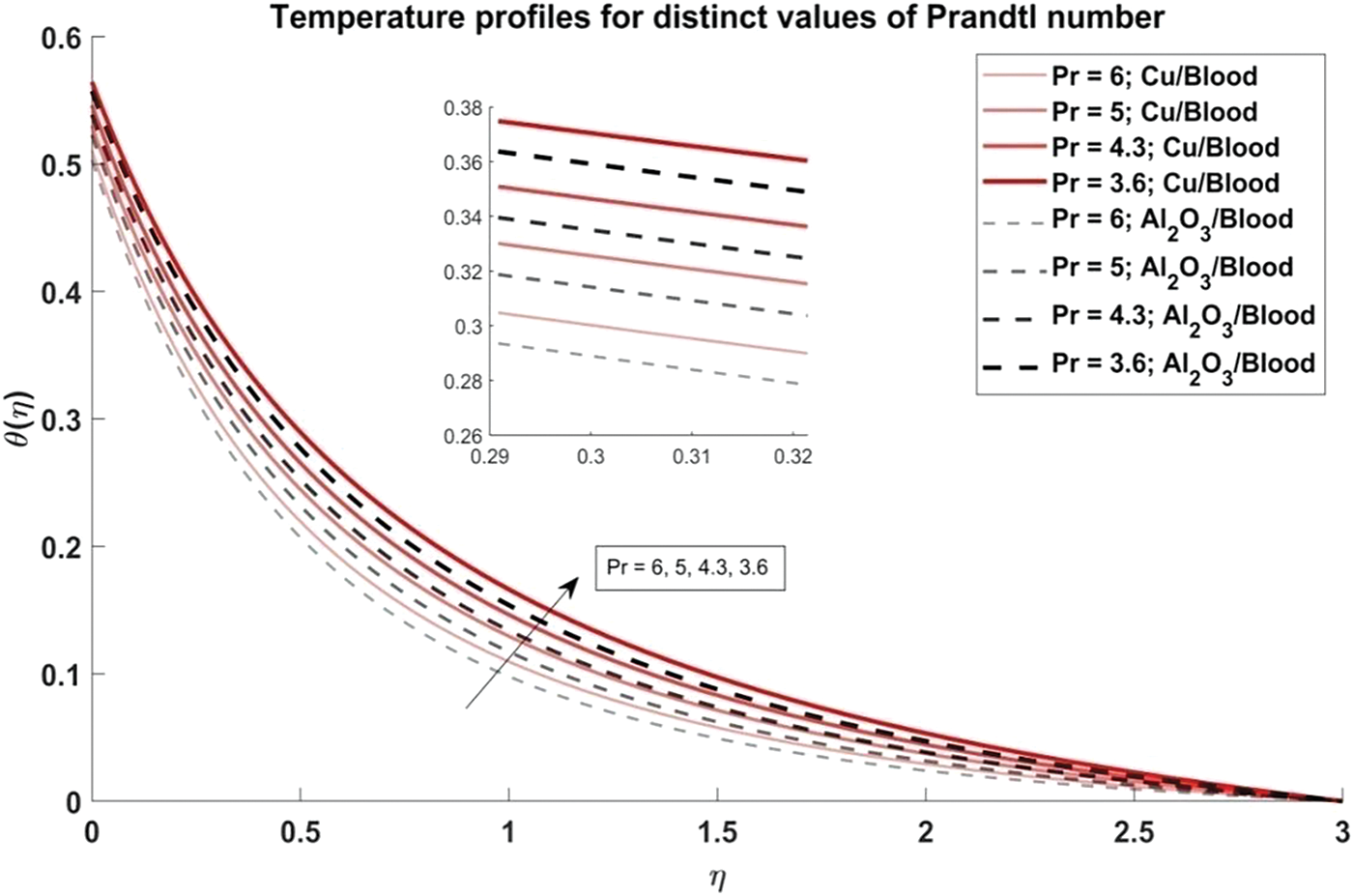
Figure 9: Temperature profiles for distinct values of Prandtl number
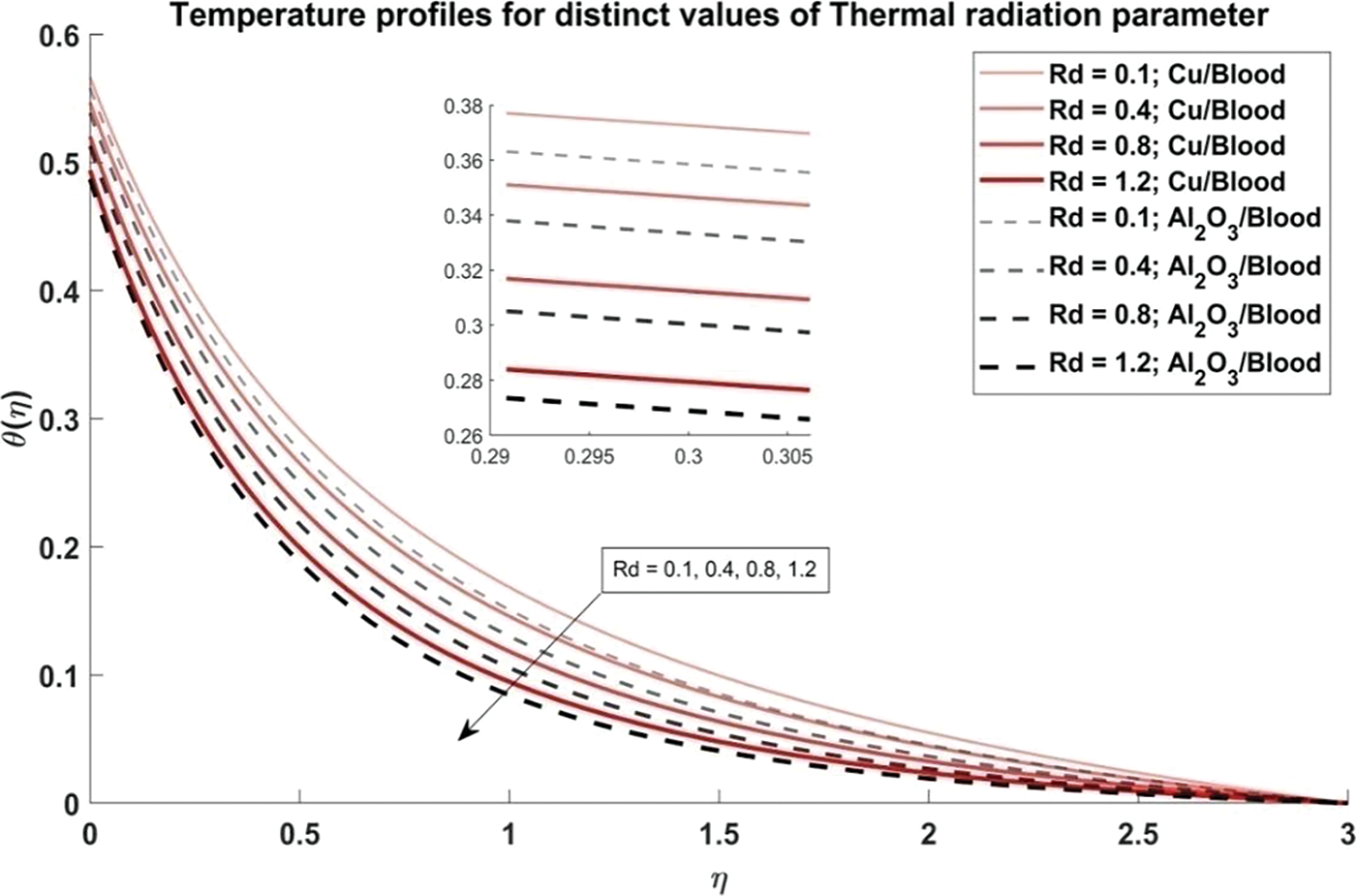
Figure 10: Temperature profiles for distinct values of Thermal radiation parameter
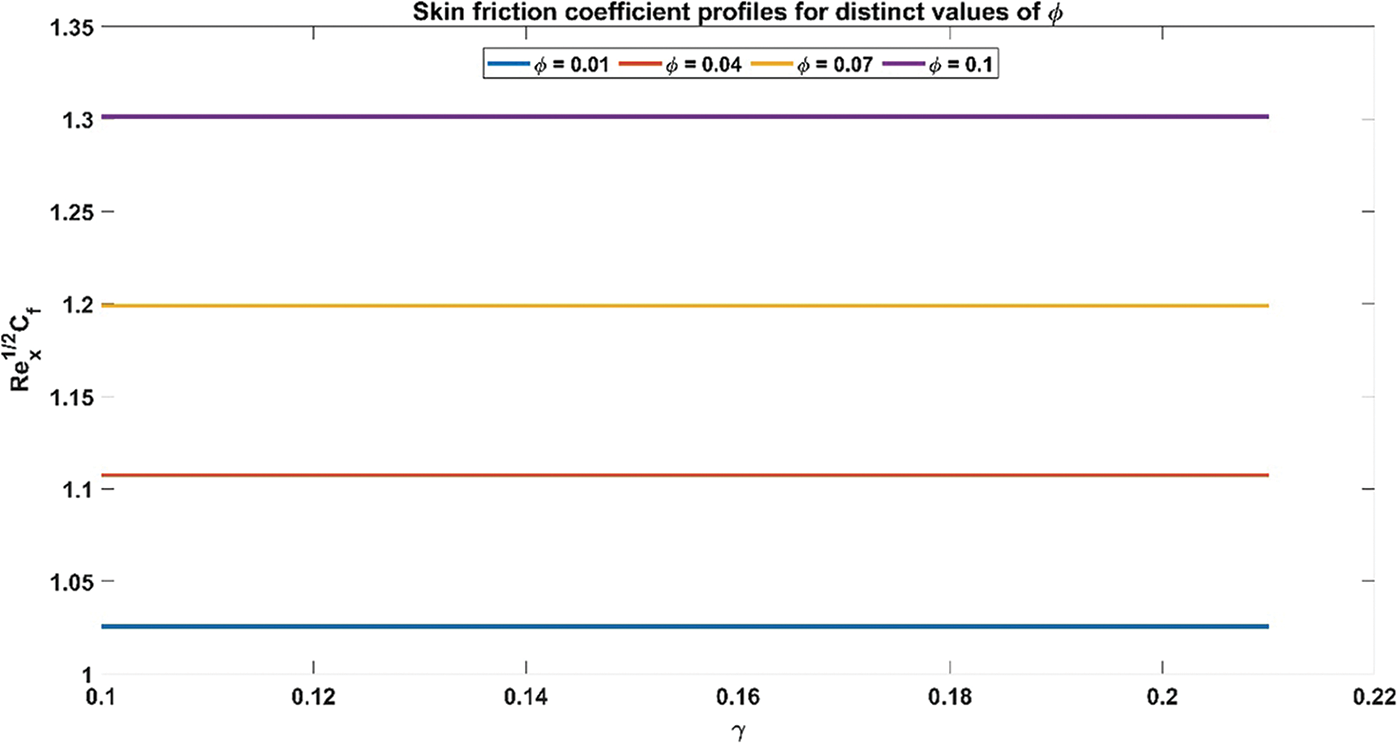
Figure 11: Skin friction coefficient profiles for distinct values of Volume fraction of nanoparticles
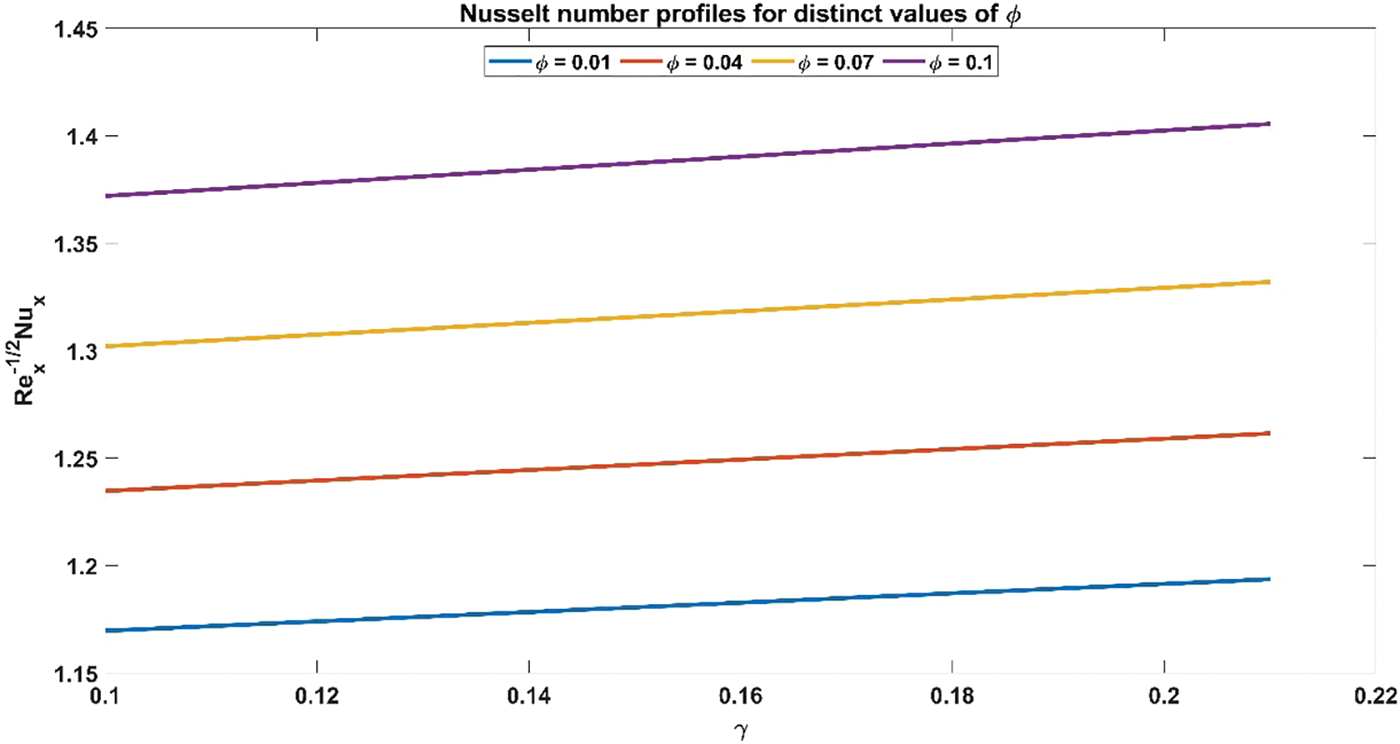
Figure 12: Nusselt number profiles for distinct values of Volume fraction of nanoparticles
In Fig. 3, the influence of the volume fraction of nanoparticles, both copper and alumina, on the velocity profiles of the nanofluid is elucidated. Notably, the velocity profiles exhibit a decreasing trend as the volume fraction of nanoparticles increases. This observation holds significant physical significance as it is reflecting the augmented viscosity and density of the nanofluid due to the presence of nanoparticles. The decrease in velocity indicates enhanced resistance to flow within the artery, a critical consideration in understanding blood flow dynamics in stenosed arteries. Moreover, it is noteworthy that the velocity profiles of Al2O3/blood exhibit higher velocities as compared to Cu/blood. This disparity underscores the varying impacts of different nanoparticles on fluid dynamics, with Al2O3 nanoparticles seemingly exerting a more pronounced effect on enhancing flow velocities. Such insights are invaluable for designing efficient therapeutic strategies and biomedical interventions targeting improved blood flow in stenosed arteries. The effect of the curvature flow parameter on the velocity profiles of the nanofluid is illustrated in Fig. 4. As the arterial curvature flow parameter increases, the velocity profiles demonstrate a corresponding increase. This trend can be attributed to the geometric configuration of the artery; as curvature increases, the centrifugal force acting on the fluid becomes more pronounced, leading to higher velocities along the curved sections. The physical reason behind this observation lies in the principle of fluid dynamics, where curved geometries induce variations in pressure and velocity distributions due to changes in flow direction and acceleration. Additionally, it’s notable that the velocity profiles of Al2O3/blood exhibit higher velocities compared to Cu/blood. This variation can be ascribed to variances in the rheological characteristics of the nanofluids, where alumina nanoparticles might create lower resistance to flow compared to copper nanoparticles, resulting in increased velocities.
In Fig. 5, the influence of the magnetic parameter on the velocity profiles of the nanofluid is investigated. It is observed that as the magnetic parameter increases, the velocity profiles exhibit a corresponding decrease. This phenomenon can be attributed to the magnetic field’s effect on the fluid, which tends to impede flow motion, leading to reduced velocities. The physical reason behind this trend lies in the interaction between the magnetic field and the conducting fluid, which induces Lorentz forces that act against the flow direction. Moreover, it is observed that the velocity profiles of Al2O3/blood demonstrate higher velocities compared to Cu/blood.
Fig. 6 explores the trend of the volume fraction of both copper and alumina nanoparticles on the temperature profiles of the nanofluids. It is observed that as the volume fraction of nanoparticles increases, the temperature profiles demonstrate a corresponding increase. This trend can be attributed to the higher thermal conductivity of the nanoparticles compared to the blood, leading to enhanced heat transfer within the nanofluid. The physical reason behind this phenomenon lies in the increased surface area available for heat exchange as more nanoparticles are introduced into the fluid, resulting in elevated temperatures. Also, it is noted that the temperature profiles of Cu/blood exhibit higher temperatures compared to Al2O3/blood. This difference can be attributed to the higher thermal conductivity of copper nanoparticles compared to alumina nanoparticles, allowing for more efficient heat transfer and thus higher temperatures in the Cu/blood nanofluid. In Fig. 7, the influence of the arterial curvature flow parameter on the temperature profiles of the nanofluid is examined. It is observed that as the arterial curvature flow parameter increases, the temperature profiles exhibit a corresponding decrease. This trend can be attributed to the geometrical effect of increased curvature, which promotes more efficient heat dissipation from the fluid to the surrounding environment. The main reason behind this eventuality is the increased surface area available for heat transfer as the curvature of the artery intensifies, leading to enhanced convective cooling. Furthermore, it is worth mentioning that the temperature profiles of Cu/blood exhibit elevated temperatures in comparison to those of Al2O3/blood due to the greater thermal conductivity of copper nanoparticles as compared to alumina nanoparticles. In Fig. 8, the influence of the magnetic parameter on the temperature profiles of the nanofluid is investigated. It is observed that as the magnetic parameter increases, the temperature profiles demonstrate a corresponding rise. This can be attributed to the magnetic field’s effect on the nanofluid, which leads to increased thermal agitation and subsequently elevated temperatures. The physics behind this trend lies in the interaction between the magnetic field and the nanoparticles, which induces greater energy dissipation and heat generation within the fluid. Additionally, it is noteworthy that the temperature profiles of Cu/blood exhibit higher temperatures compared to Al2O3/blood. In Fig. 9, the manifestation of the Prandtl number’s influence on the temperature profiles of the nanofluid is delineated. Upon scrutiny, it is discerned that an augmentation in the Prandtl number is met with a concomitant diminution in the temperature profiles. This decrease in temperature with increasing values of Prandtl number is also validated with noted reducing values of Nusselt number as evident from Table 3. This phenomenon finds its roots in the Prandtl number’s nuanced impact on the equilibrium between thermal diffusion and momentum diffusion within the fluid medium. Notably, elevated Prandtl numbers signify a heightened predominance of momentum diffusion relative to thermal diffusion. As a consequence, this engenders a reduction in the thickness of the thermal boundary layer and a deceleration in temperature gradients across the fluidic domain. Furthermore, it merits attention that the temperature profiles of Cu/blood evince superior temperatures when juxtaposed with those of Al2O3/blood. Within the confines of Fig. 10, the portrayal of the thermal radiation parameter’s influence on the temperature profiles of the nanofluid is delineated. It is discerned that the temperature profiles exhibit a diminishing trend in tandem with escalating values of the thermal radiation parameter. This observable trend is grounded in the inherent physical rationale underlying the augmentation of the thermal radiation parameter, wherein heightened values engender a more pronounced dominance of radiative heat transfer mechanisms over conductive and convective counterparts. This also agrees with the Nusselt number values obtained in Table 3. Consequently, this engenders a suppression in temperature gradients across the fluidic medium. Moreover, it merits acknowledgment that the temperature profiles pertaining to Cu/blood manifest elevated temperatures when contrasted with those associated with Al2O3/blood. This discernible contrast can be ascribed to the superior thermal conductivity exhibited by copper nanoparticles compared to their alumina counterparts, thus facilitating heightened heat transfer rates and ensuing higher temperatures within the Cu/blood nanofluid.
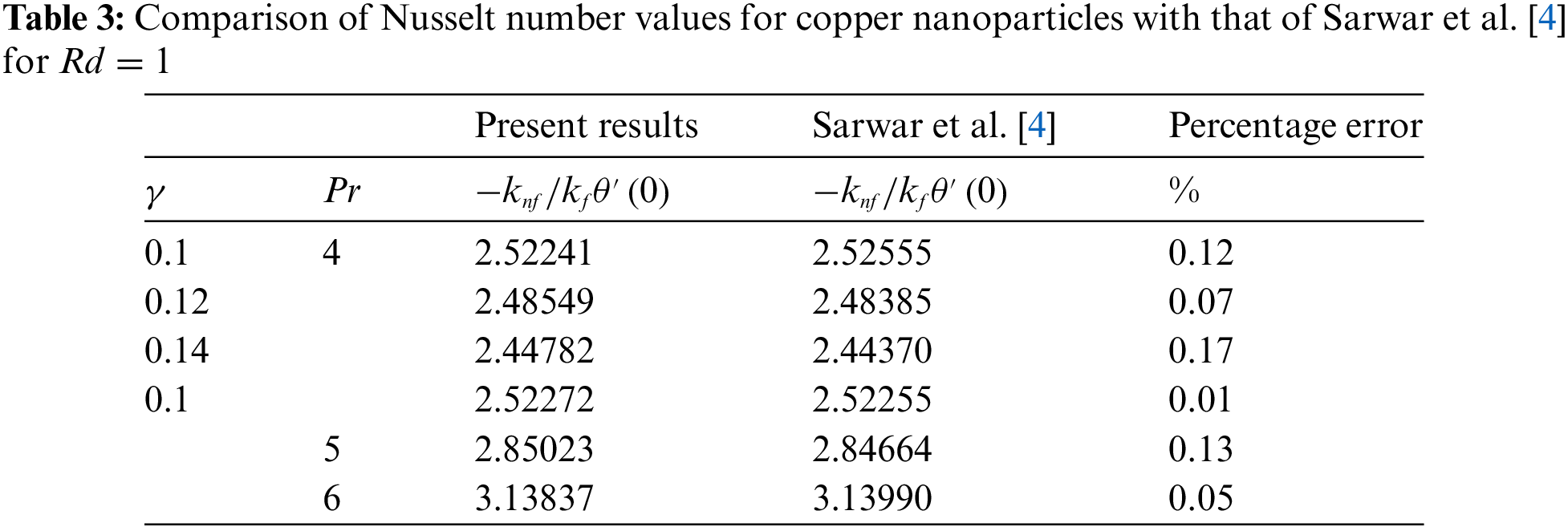
Fig. 11 illustrates the influence of nanoparticle volume fraction on the relationship between skin friction coefficient and curvature flow parameter. The skin friction coefficient profiles exhibit an ascending trajectory as the volume fraction of nanoparticles increases. This observed trend is underpinned by the inherent physical rationale governing the augmentation of the volume fraction of nanoparticles, wherein heightened values entail an increase in the concentration of nanoparticles within the nanofluid medium. Consequently, this leads to an intensified interaction between the nanoparticles and the fluid, resulting in elevated levels of skin friction. Fig. 12 provides a visual representation of the interplay between the volume fraction of nanoparticles and the Nusselt number concerning the curvature flow parameter. It is perceptible that the Nusselt number profiles ascend in tandem with escalating values of the volume fraction of nanoparticles. This observed correlation is rooted in the fundamental physical principle governing the augmentation of nanoparticle volume fraction, whereby higher concentrations thereof within the nanofluid medium foster an intensified convective heat transfer mechanism. Consequently, this leads to an amplification in the Nusselt number, reflecting enhanced heat transfer rates facilitated by the increased presence of nanoparticles within the fluid.
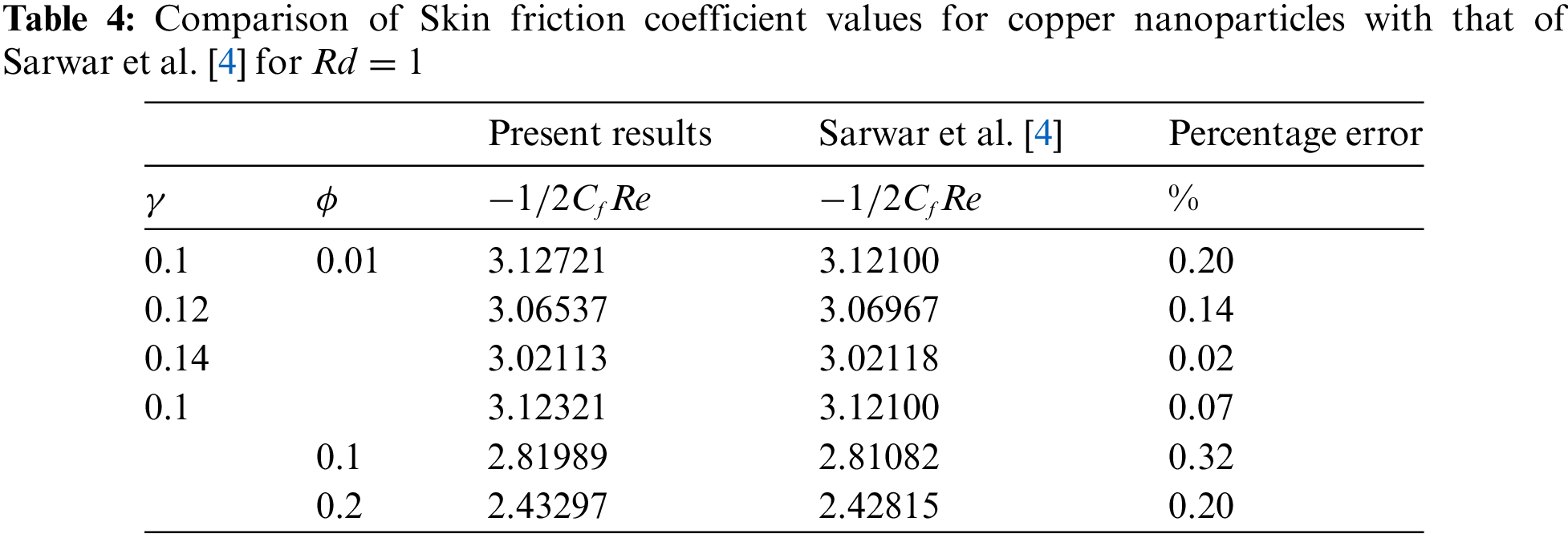
In evaluating the present results of Nusselt number values and skin friction coefficient for copper nanoparticles, a benchmarking comparison with the findings of Sarwar et al. [4] reveals a notable consistency. A statistical analysis is also carried out to find the percentage error between the present results and the results obtained by Sarwar et al. [4]. The errors found in the Nusselt number and skin friction coefficient are explored in Tables 3 and 4. These low percentage errors between the present study and the benchmarked results validate the current numerical method and the model’s accuracy in predicting heat transfer and skin friction coefficients. Hence, the achieved outcomes exhibit a commendable agreement with the previously established data, suggesting a robust validation of the methodology and findings of the current study. This alignment with Sarwar et al. [4] results underscores the reliability and accuracy of the present investigation’s approach in analyzing the convective heat transfer and fluid flow characteristics of copper nanoparticles. Such congruence bolsters confidence in the results’ applicability and relevance, further enriching the scientific discourse surrounding nanofluid dynamics and thermal transport phenomena.
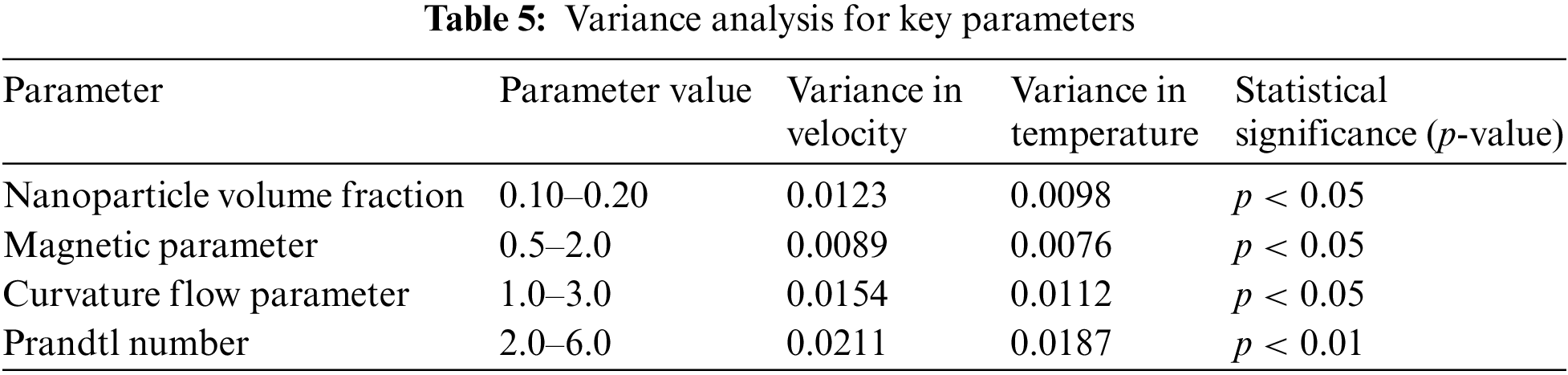
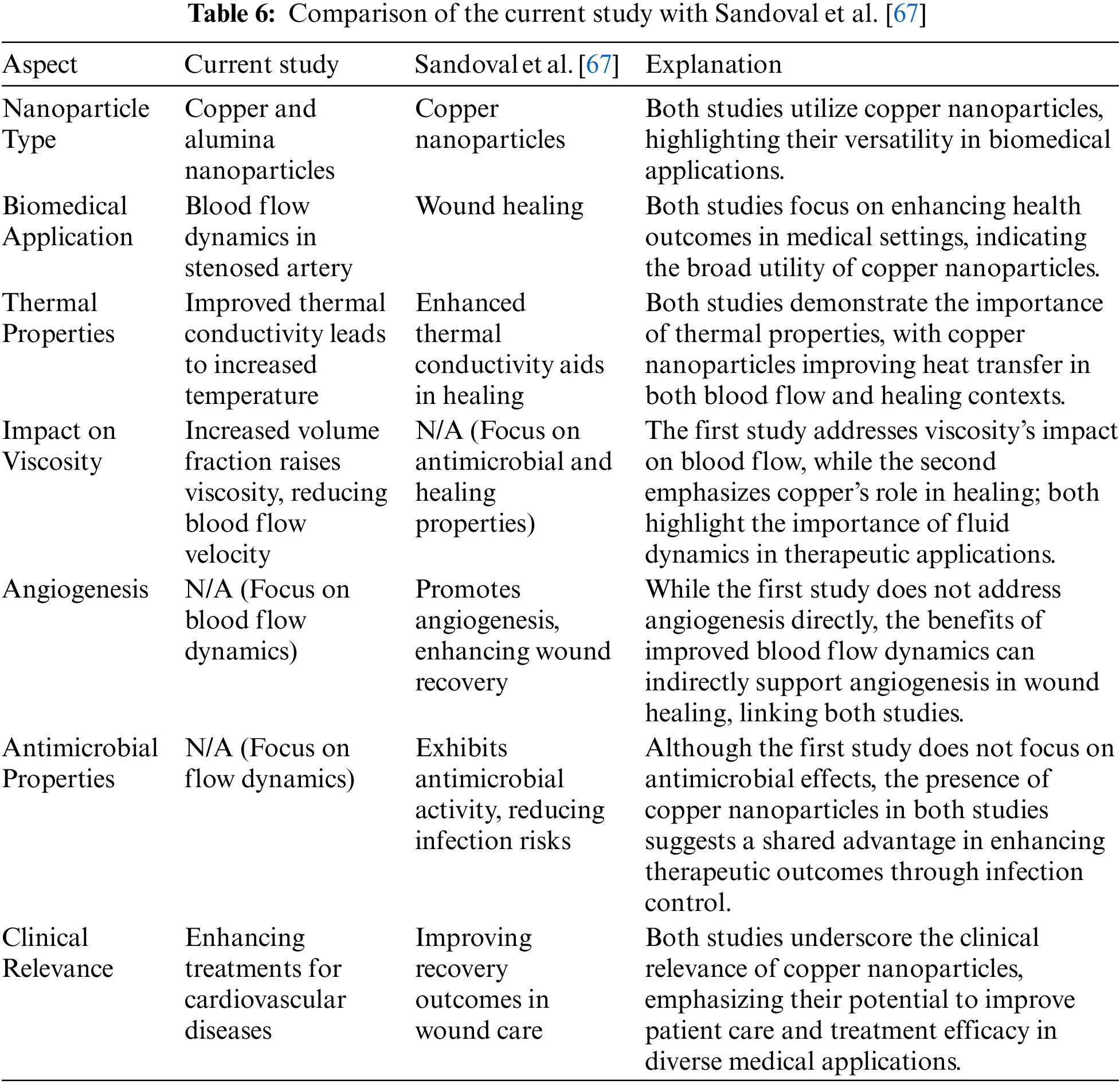
Furthermore, Table 5 represents the variance analysis of key parameters on velocity and temperature profiles. The p-values indicate that variations in these parameters have a statistically significant effect on the blood-nanoparticle flow, with the Prandtl number and curvature flow parameter showing the highest significance. Also, Table 6 illustrates the key similarities between the current study and the experimental study done by Sandoval et al. [67], showcasing how copper nanoparticles serve crucial roles in both blood flow dynamics and wound healing. Despite their different focuses, the studies share common themes, including the therapeutic potential of copper nanoparticles, their beneficial thermal properties, and their implications for improving patient outcomes in various biomedical contexts.
Sensitivity analysis is essential to understand how variations in different flow parameters impact blood flow dynamics. In the current study, parameters such as the volume fraction of nanoparticles (
The sensitivity analysis was conducted by varying each key parameter by ±20% and ±10% from their base values. The results indicate how changes in these parameters influence outputs such as velocity (
• A 20% increase in
• An increase in
• Increasing
• A significant impact on temperature and velocity profiles was observed, with increases in
• An increase in
The sensitivity analysis reveals that the Prandtl number
5 Conclusions and Future Scope
In this study, we have focused on the Newtonian properties of blood, and the solution to the discussed problem is obtained through numerical method employing the bvp4c solver. The nanofluid is modeled as a combination of copper/alumina and blood. The effects of various parameters on the temperature and velocity of blood are examined through graphical representations and tabular data. Mathematical modeling and numerical solutions are crucial for predicting the onset of atherosclerosis, and our analysis suggests that nanoparticle-based techniques could offer promising therapeutic interventions against arterial diseases. Therefore, this study elucidates key aspects relevant to biomedical applications. Key findings include:
1. An increase in the volume fraction of copper and alumina nanoparticles as well as magnetic parameter leads to a decrease in velocity profiles due to augmented viscosity and density of the nanofluid and the complex interplay of Lorentz force.
2. Higher arterial curvature flow parameter resulted in increased velocity profiles attributed to intensified centrifugal forces along curved sections.
3. The increase in nanoparticle volume fraction of copper and alumina corresponds to elevated temperatures facilitated by enhanced heat transfer. Also, the magnetic parameter influences temperature profiles, with higher parameters leading to increased thermal agitation and subsequently elevated temperatures.
4. The arterial curvature flow parameter influences temperature profiles, with increased parameter promoting more efficient heat dissipation.
5. The large values of the Prandtl number resulted in diminished temperature profiles, highlighting the nuanced impact on thermal diffusion and momentum diffusion equilibrium. Additionally, thermal radiation parameter exhibit a diminishing trend in temperature profiles, showcasing the dominance of the radiative heat transfer mechanism.
6. A sensitivity analysis of the relevant parameters revealed that the Prandtl number has the most significant impact on blood flow dynamics.
7. This comprehensive analysis sheds light on the complex relationship between various parameters and their impacts on blood flow dynamics, offering valuable insights for biomedical interventions and treatment strategies.
5.2 Limitations and Future Scope
Despite the significant advancement in the study of Cu/blood and Al2O3/blood nanofluid flow in biomedical applications, several limitations persist. While simulations provide valuable insights, real-world biological complexities such as varying arterial geometries, blood composition, and patient-specific conditions are often oversimplified. Additionally, the long-term biocompatibility and potential side effects of nanoparticles, particularly in human systems, remain underexplored, with studies focusing on short-term impacts. The interaction between nanoparticles and blood components, including proteins and cells, is not fully understood, which raises concerns about toxicity and immune responses. Furthermore, the current study predominantly focuses on steady or simplified flow conditions, whereas in reality, blood flow is highly dynamic, pulsatile, and influenced by various physiological factors. Also, the simulation assumes rigid arterial walls, neglecting the fluid-structure interaction (FSI) between the blood flow and the arterial wall. In reality, arterial walls are elastic and deform under pressure, which significantly affects the blood flow dynamics, especially in stenosed arteries. This simplification reduces the accuracy of the model, particularly for pulsatile or high-pressure flows.
Additionally, there is immense potential for expanding the applications of nanofluids in personalized medicine, particularly in targeted drug delivery and minimally invasive procedures. Future research should focus on incorporating more realistic, patient-specific models and expanding clinical trials to validate theoretical findings. Moreover, the integration of machine learning and artificial intelligence could enhance the prediction and optimization of nanofluid behavior in complex biological environments.
Further in-depth studies are essential to assess the long-term safety and biocompatibility of various nanoparticles in medical applications. Collaborative efforts between computational scientists, biologists, and medical professionals are critical in bridging the gap between theoretical research and clinical application. This approach would ensure that nanofluid-based treatments are both effective and safe for broader use in cardiovascular diseases and other biomedical applications. Additionally, future research should incorporate fluid-structure interaction (FSI) models that account for the elasticity of arterial walls, thereby improving the accuracy of simulated results and better representing real-world blood flow conditions in the human arterial system. Moreover, the current study does not address the potential aggregation of nanoparticles within the bloodstream, a factor that could significantly influence both their therapeutic effectiveness and interaction with blood flow. Addressing these aspects in future research would provide a more comprehensive and applicable model for medical applications.
Acknowledgement: The authors wish to extend their profound gratitude to Universiti Teknikal Malaysia Melaka (UTeM) for the unconditional support that enabled this research to be conducted. Also, the authors would like to express sincere thanks to all contributors whose hard work, knowledge, and teamwork were crucial to the accomplishment of this research.
Funding Statement: This research was funded by Universiti Teknikal Malaysia Melaka and Ministry of Higher Education (MoHE) Malaysia, grant number FRGS/1/2024/FTKM/F00586.
Author Contributions: The authors confirm their contribution to the paper as follows: Study conception and design: Haris Alam Zuberi, Madan Lal, Nurul Amira Zainal; Data collection: Haris Alam Zuberi, Madan Lal, Amol Singh, Nurul Amira Zainal, Ali J. Chamkha; Analysis and interpretation of results: Haris Alam Zuberi, Madan Lal, Nurul Amira Zainal, Ali J. Chamkha; Draft manuscript preparation: Haris Alam Zuberi, Nurul Amira Zainal, Amol Singh. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Ahmed A, Nadeem S. The study of (Cu,TiO2,Al2O3) nanoparticles as antimicrobials of blood flow through diseased arteries. J Mol Liq. 2016;216:615–23. doi:10.1016/j.molliq.2016.01.059. [Google Scholar] [CrossRef]
2. Manchi R, Ponalagusamy R. Modeling of pulsatile EMHD flow of Au-blood in an inclined porous tapered atherosclerotic vessel under periodic body acceleration. Arch Appl Mech. 2021;91(7):3421–47. doi:10.1007/s00419-021-01974-6. [Google Scholar] [CrossRef]
3. Wajihah SA, Sankar D, Nagar AK. Effects of catheter, stenosis and thrombosis in non-Newtonian blood flow through narrow arteries with clinical applications: a mathematical model. Int J Appl Comput Math. 2022;8(3):136. doi:10.1007/s40819-022-01335-z. [Google Scholar] [CrossRef]
4. Sarwar L, Hussain A, Fernandez-Gamiz U, Akbar S, Rehman A, Sherif ESM. Thermal enhancement and numerical solution of blood nanofluid flow through stenotic artery. Sci Rep. 2022;12(1):17419. doi:10.1038/s41598-022-20267-8. [Google Scholar] [PubMed] [CrossRef]
5. Jerka D, Bonowicz K, Piekarska K, Gokyer S, Derici US, Hindy OA, et al. Unraveling endothelial cell migration: insights into fundamental forces, inflammation, biomaterial applications, and tissue regeneration strategies. ACS Appl Bio Mater. 2024;7(4):2054–69. [Google Scholar]
6. Zuberi HA, Lal M, Verma S, Zainal NA. Computational investigation of brownian motion and thermophoresis effect on blood-based casson nanofluid on a non-linearly stretching sheet. J Adv Res Numer Heat Transfer. 2024;18(1):49a67. [Google Scholar]
7. Akbar NS. A new thermal conductivity model with shaped factor ferromagnetism nanoparticles study for the blood flow in non-tapered stenosed arteries. IEEE Trans Nanobiosci. 2015;14(7):780–9. doi:10.1109/TNB.2015.2462755. [Google Scholar] [PubMed] [CrossRef]
8. Mousavi SM, Darzi AAR, ali Akbari O, Toghraie D, Marzban A. Numerical study of biomagnetic fluid flow in a duct with a constriction affected by a magnetic field. J Magn Magn Mater. 2019;473:42–50. doi:10.1016/j.jmmm.2018.10.043. [Google Scholar] [CrossRef]
9. Jamil DF, Roslan R, Abdulhameed M, Che-Him N, Sufahani S, Mohamad M, et al. Unsteady blood flow with nanoparticles through stenosed arteries in the presence of periodic body acceleration. J Phys: Conf Ser. 2018;995:012032. doi:10.1088/1742-6596/995/1/012032. [Google Scholar] [CrossRef]
10. Vasu B, Dubey A, Beg OA, Gorla RSR. Micropolar pulsatile blood flow conveying nanoparticles in a stenotic tapered artery: non-Newtonian pharmacodynamic simulation. Comput Biol Med. 2020;126:104025. doi:10.1016/j.compbiomed.2020.104025. [Google Scholar] [PubMed] [CrossRef]
11. Yan SR, Zarringhalam M, Toghraie D, Foong LK, Talebizadehsardari P. Numerical investigation of non-Newtonian blood flow within an artery with cone shape of stenosis in various stenosis angles. Comput Methods Programs Biomed. 2020;192:105434. doi:10.1016/j.cmpb.2020.105434. [Google Scholar] [PubMed] [CrossRef]
12. Ali A, Das S, Muhammad T. Dynamics of blood conveying copper, gold, and titania nanoparticles through the diverging/converging ciliary micro-vessel: further analysis of ternary-hybrid nanofluid. J Mol Liq. 2023;390:122959. doi:10.1016/j.molliq.2023.122959. [Google Scholar] [CrossRef]
13. Karmakar P, Das S. Electro-blood circulation fusing gold and alumina nanoparticles in a diverging fatty artery. BioNanoScience. 2023;13(2):541–63. [Google Scholar]
14. Shahzad F, Jamshed W, Aslam F, Bashir R, Tag El Din ESM, Khalifa HAEW, et al. MHD pulsatile flow of blood-based silver and gold nanoparticles between two concentric cylinders. Symmetry. 2022;14(11):2254. doi:10.3390/sym14112254. [Google Scholar] [CrossRef]
15. Muthtamilselvan M, Gifteena Hingis Y. Flow characteristics of gold nanoparticles and microorganisms in a multistenotic artery treated with a catheter. Aust J Mech Eng. 2023;1–16. doi:10.1080/14484846.2023.2290335. [Google Scholar] [CrossRef]
16. Gandhi R, Sharma B, Al-Mdallal QM, Mittal H. Entropy generation and shape effects analysis of hybrid nanoparticles (Cu-Al2O3/blood) mediated blood flow through a time-variant multistenotic artery. Int J Thermofluids. 2023;18:100336. doi:10.1016/j.ijft.2023.100336. [Google Scholar] [CrossRef]
17. Das S, Karmakar P, Ali A. Simulation for bloodstream conveying bi-nanoparticles in an endoscopic canal with blood clot under intense electromagnetic force. Wave Random Complex. 2023;1–38. doi:10.1080/17455030.2023.2198036. [Google Scholar] [CrossRef]
18. Shojaie Chahregh H, Dinarvand S. TiO2-Ag/blood hybrid nanofluid flow through an artery with applications of drug delivery and blood circulation in the respiratory system. Int J Numer Methods Heat Fluid Flow. 2020;30(11):4775–96. doi:10.1108/HFF-10-2019-0732. [Google Scholar] [CrossRef]
19. Alhussain ZA. A comprehensive study of thermal conductivity models with metallic and nonmetallic nanoparticles in the blood flow through a regular catheter in multi-stenosed artery. Appl Nanosci. 2022;12(12):4033–45. doi:10.1007/s13204-022-02622-3. [Google Scholar] [CrossRef]
20. Elsaid EM, Sayed A, Abdel-wahed MS. Electromagnetohydrodynamic unsteady blood flow with ternary nanoparticles in a vertical irregular peristaltic flow: an exact treatment. J Therm Anal Calorim. 2023;148(24):14163–81. doi:10.1007/s10973-023-12598-z. [Google Scholar] [CrossRef]
21. Ali A, Bukhari Z, Umar M, Ismail MA, Abbas Z. Cu and Cu-SWCNT nanoparticlesa suspension in pulsatile casson fluid flow via darcy-forchheimer porous channel with compliant walls: a prospective model for blood flow in stenosed arteries. Int J Mol Sci. 2021;22(12):6494. doi:10.3390/ijms22126494. [Google Scholar] [PubMed] [CrossRef]
22. Sharma BK, Khanduri U, Mishra NK, Albaijan I, Perez LM. Entropy generation optimization for the electroosmotic MHD fluid flow over the curved stenosis artery in the presence of thrombosis. Sci Rep. 2023;13(1):15441. doi:10.1038/s41598-023-42540-0. [Google Scholar] [PubMed] [CrossRef]
23. Chandra P, Das R. A hybrid RSA-IPA optimizer for designing an artificial neural network to study the Jeffery-Hamel blood flow with copper nanoparticles: application to stenotic tapering artery. Results Eng. 2023;20:101542. doi:10.1016/j.rineng.2023.101542. [Google Scholar] [CrossRef]
24. Lin Y, Lin R, Lin HB, Shen S. Nanomedicine-based drug delivery strategies for the treatment of atherosclerosis. Med Drug Discov. 2024;22:100189. [Google Scholar]
25. Gandhi R, Sharma BK, Mishra NK, Al-Mdallal QM. Computer simulations of EMHD casson nanofluid flow of blood through an irregular stenotic permeable artery: application of Koo- Kleinstreuer-Li correlations. Nanomater. 2023;13(4):652. doi:10.3390/nano13040652. [Google Scholar] [PubMed] [CrossRef]
26. Hafed ZS, Arafa AA, Hussein SA, Ahmed SE, Morsy Z. Bioconvective blood flow of tetra composition nanofluids passing through a stenotic artery with Arrhenius energy. Numer Heat Transf B: Fundamentals. 2023;1–24. doi:10.1080/10407790.2023.2289505. [Google Scholar] [CrossRef]
27. Qayyum M, Afzal S, Saeed ST, Akgul A, Riaz MB. Unsteady hybrid nanofluid (Cu-UO2/blood) with chemical reaction and non-linear thermal radiation through convective boundaries: an application to bio-medicine. Heliyon. 2023;9(6):e16578. doi:10.1016/j.heliyon.2023.e16578. [Google Scholar] [PubMed] [CrossRef]
28. Ali A, Bukhari Z, Shahzadi G, Abbas Z, Umar M. Numerical simulation of the thermally developed pulsatile flow of a hybrid nanofluid in a constricted channel. Energies. 2021;14(9):2410. doi:10.3390/en14092410. [Google Scholar] [CrossRef]
29. Vaidya H, Prasad KV, Tripathi D, Choudhari R, Hanumantha, Ahmad H. Viscoplastic hybrid nanofluids flow through vertical stenosed artery. BioNanoScience. 2023;13(4):2348–70. doi:10.1007/s12668-023-01213-y. [Google Scholar] [CrossRef]
30. Poonam, Sharma BK. Mathematical analysis of hybrid nanoparticles (Au–Al2O3) on MHD blood flow through a curved artery with stenosis and aneurysm using hematocrit-dependent viscosity. In: Nonlinear dynamics and applications. Cham: Springer; 2022. p. 407–19. [Google Scholar]
31. Khanduri U, Sharma B. Mathematical analysis of hall effect and hematocrit dependent viscosity on Au/GO-blood hybrid nanofluid flow through a stenosed catheterized artery with thrombosis. In: International Workshop of Mathematical Modelling, Applied Analysis and Computation, 2024; Springer; vol. l7, p. 121–37. [Google Scholar]
32. Jalili P, Sadeghi Ghahare A, Jalili B, Domiri Ganji D. Analytical and numerical investigation of thermal distribution for hybrid nanofluid through an oblique artery with mild stenosis. SN Appl Sci. 2023;5(4):95. doi:10.1007/s42452-023-05312-z. [Google Scholar] [CrossRef]
33. Hussain A, Sarwar L, Rehman A, Al Mdallal Q, Almaliki AH, El-Shafay A. Mathematical analysis of hybrid mediated blood flow in stenosis narrow arteries. Sci Rep. 2022;12(1):12704. doi:10.1038/s41598-022-15117-6. [Google Scholar] [PubMed] [CrossRef]
34. Manchi R, Ponalagusamy R. Pulsatile flow of EMHD micropolar hybrid nanofluid in a porous bifurcated artery with an overlapping stenosis in the presence of body acceleration and joule heating. Braz J Phys. 2022;52(2):52. doi:10.1007/s13538-022-01061-3. [Google Scholar] [CrossRef]
35. Bagheri E, Ansari L, Abnous K, Taghdisi SM, Charbgoo F, Ramezani M, et al. Silica based hybrid materials for drug delivery and bioimaging. J Control Release. 2018;277:57–76. doi:10.1016/j.jconrel.2018.03.014. [Google Scholar] [PubMed] [CrossRef]
36. Ketchate CGN, Kapen PT, Fokwa D, Tchuen G. Stability analysis of non-Newtonian blood flow conveying hybrid magnetic nanoparticles as target drug delivery in presence of inclined magnetic field and thermal radiation: application to therapy of cancer. Inform Med Unlocked. 2021;27:100800. doi:10.1016/j.imu.2021.100800. [Google Scholar] [CrossRef]
37. Khan MS, Mei S, Shabnam, Fernandez-Gamiz U, Noeiaghdam S, Khan A. Numerical simulation of a time-dependent electroviscous and hybrid nanofluid with Darcy-Forchheimer effect between squeezing plates. Nanomater. 2022;12(5):876. doi:10.3390/nano12050876. [Google Scholar] [PubMed] [CrossRef]
38. Khazayinejad M, Hafezi M, Dabir B. Peristaltic transport of biological graphene-blood nanofluid considering inclined magnetic field and thermal radiation in a porous media. Powder Technol. 2021;384:452–65. doi:10.1016/j.powtec.2021.02.036. [Google Scholar] [CrossRef]
39. Das S, Karmakar P, Ali A. Electrothermal blood streaming conveying hybridized nanoparticles in a non-uniform endoscopic conduit. Med Biol Eng Comput. 2022;60(11):3125–51. doi:10.1007/s11517-022-02650-9. [Google Scholar] [PubMed] [CrossRef]
40. Abbas N, Nadeem S, Saleem A. Computational analysis of water based Cu-Al2O3/H2O-flow over a vertical wedge. Adv Mech Eng. 2020;12(11):1687814020968322. doi:10.1177/1687814020968322. [Google Scholar] [CrossRef]
41. Tripathi J, Vasu B, Subba Reddy Gorla R, Chamkha AJ, Murthy P, Anwar Beg O. Blood flow mediated hybrid nanoparticles in human arterial system: recent research, development and applications. J Nanofluids. 2021;10(1):1–30. doi:10.1166/jon.2021.1769. [Google Scholar] [CrossRef]
42. Basha HT, Rajagopal K, Ahammad NA, Sathish S, Gunakala SR. Finite difference computation of Au-Cu/magneto-bio-hybrid nanofluid flow in an inclined uneven stenosis artery. Complexity. 2022;2022:1–18. [Google Scholar]
43. Das S, Pal T, Jana R. Outlining impact of hybrid composition of nanoparticles suspended in blood flowing in an inclined stenosed artery under magnetic field orientation. BioNanoScience. 2021;11:99–115. doi:10.1007/s12668-020-00809-y. [Google Scholar] [CrossRef]
44. Hussain A, Dar MNR, Cheema WK, Han Y, Kanwal R. Clinical symbiosis of hybrid nanoparticles and induced magnetic field on heat and mass transfer in multiple stenosed artery with erratic thrombosis. Sci Rep. 2023;13(1):15588. doi:10.1038/s41598-023-42795-7. [Google Scholar] [PubMed] [CrossRef]
45. Karmakar P, Das S. Modeling non-Newtonian magnetized blood circulation with tri- nanoadditives in a charged artery. J Computat Sci. 2023;70:102031. doi:10.1016/j.jocs.2023.102031. [Google Scholar] [CrossRef]
46. Khanduri U, Sharma BK, Sharma M, Mishra NK, Saleem N. Sensitivity analysis of electroosmotic magnetohydrodynamics fluid flow through the curved stenosis artery with thrombosis by response surface optimization. Alex Eng J. 2023;75:1–27. doi:10.1016/j.aej.2023.05.054. [Google Scholar] [CrossRef]
47. Bhatti M, Abdelsalam SI. Scientific breakdown of a ferromagnetic nanofluid in hemodynamics: enhanced therapeutic approach. Math Model Nat Phenom. 2022;17:44. doi:10.1051/mmnp/2022045. [Google Scholar] [CrossRef]
48. Karmakar P, Ali A, Das S. Circulation of blood loaded with trihybrid nanoparticles via electroosmotic pumping in an eccentric endoscopic arterial canal. Int Commun Heat Mass Transf. 2023;141:106593. doi:10.1016/j.icheatmasstransfer.2022.106593. [Google Scholar] [CrossRef]
49. Verma R. Analysis and simulation of nano fluids flow using Matlab. National Institute of Technology: India; 2018. [Google Scholar]
50. Das S, Barman B, Jana R, Makinde O. Hall and ion slip currentsa impact on electromagnetic blood flow conveying hybrid nanoparticles through an endoscope with peristaltic waves. BioNanoScience. 2021;11(3):770–92. [Google Scholar]
51. Arif M, Di Persio L, Kumam P, Watthayu W, Akgül A. Heat transfer analysis of fractional model of couple stress Casson tri-hybrid nanofluid using dissimilar shape nanoparticles in blood with biomedical applications. Sci Rep. 2023;13(1):4596. doi:10.1038/s41598-022-25127-z. [Google Scholar] [PubMed] [CrossRef]
52. Nazar T, Shabbir M. Irreversibility analysis in the ternary nanofluid flow through an inclined artery via Caputo-Fabrizio fractional derivatives. Results Phys. 2023;53:106992. doi:10.1016/j.rinp.2023.106992. [Google Scholar] [CrossRef]
53. Abo-Elkhair R, Bhatti M, Mekheimer KS. Magnetic force effects on peristaltic transport of hybrid bio- nanofluid (AuCu nanoparticles) with moderate Reynolds number: an expanding horizon. Int Commun Heat Mass Transf. 2021;123:105228. doi:10.1016/j.icheatmasstransfer.2021.105228. [Google Scholar] [CrossRef]
54. Shah SZH, Ayub A, Sabir Z, Adel W, Shah NA, Yook SJ. Insight into the dynamics of time dependent cross nanofluid on a melting surface subject to cubic autocatalysis. Case Stud Therm Eng. 2021;27:101227. doi:10.1016/j.csite.2021.101227. [Google Scholar] [CrossRef]
55. Kabeel A, El-Said EM, Dafea S. A review of magnetic field effects on flow and heat transfer in liquids: present status and future potential for studies and applications. Renew Sustain Energ Rev. 2015;45:830–7. doi:10.1016/j.rser.2015.02.029. [Google Scholar] [CrossRef]
56. Alghamdi W, Alsubie A, Kumam P, Saeed A, Gul T. MHD hybrid nanofluid flow comprising the medication through a blood artery. Sci Rep. 2021;11(1):11621. doi:10.1038/s41598-021-91183-6. [Google Scholar] [PubMed] [CrossRef]
57. Shabbir M, Nazar T. Entropy generation in the ternary nanofluid flow through an atherosclerotic vessel under periodic body acceleration. Alex Eng J. 2023;84:301–15. doi:10.1016/j.aej.2023.10.048. [Google Scholar] [CrossRef]
58. Das S, Pal T, Jana R. Electromagnetic hybrid nano-blood pumping via peristalsis through an endoscope having blood clotting in presence of hall and ion slip currents. BioNanoScience. 2021;11(3):848–70. doi:10.1007/s12668-021-00853-2. [Google Scholar] [CrossRef]
59. Paul P, Das S. Electro-pumping paradigm of non-Newtonian blood with tetra-hybrid nanoparticles infusion in a ciliated artery. Chin J Phys. 2024;87:195–231. doi:10.1016/j.cjph.2023.12.008. [Google Scholar] [CrossRef]
60. Ansari MS, Otegbeye O, Trivedi M, Goqo S. Magnetohydrodynamic bio-convective Casson nanofluid flow: a numerical simulation by paired quasilinearisation. J Appl Computat Mech. 2020;7(4):2024–39. doi:10.22055/JACM.2020.31205.1839. [Google Scholar] [CrossRef]
61. Kang H, Jasim SA, Sedeh SN, Hekmatifar M, Toghraie D, Suksatan W, et al. Heat transfer and hemodynamic analysis of systolic and diastolic hypertension on abdominal aortic thrombosis. Case Stud Therm Eng. 2022;30:101738. doi:10.1016/j.csite.2021.101738. [Google Scholar] [CrossRef]
62. Maranna T, Sachhin S, Mahabaleshwar U, Hatami M. Impact of Navieras slip and MHD on laminar boundary layer flow with heat transfer for non-Newtonian nanofluid over a porous media. Sci Rep. 2023;13(1):12634. doi:10.1038/s41598-023-39153-y. [Google Scholar] [PubMed] [CrossRef]
63. Li Y, Wan D, Hu D, Li C. A novel approach for estimating blood flow dynamics factors of eccentric stenotic arteries based on ML. Eng Anal Bound Elem. 2024;163:175–85. doi:10.1016/j.enganabound.2024.03.003. [Google Scholar] [CrossRef]
64. Koriko OK, Adegbie KS, Shah NA, Animasaun IL, Olotu MA. Numerical solutions of the partial differential equations for investigating the significance of partial slip due to lateral velocity and viscous dissipation: the case of blood-gold Carreau nanofluid and dusty fluid. Numer Methods Partial Differ Equ. 2024;40(2):e22754. doi:10.1002/num.v40.2. [Google Scholar] [CrossRef]
65. Geredeli PG, Kunwar H, Lee H. Partitioning method for the finite element approximation of a 3D fluid-2D plate interaction system. Numer Methods Partial Differ Equ. 2024;40(6):e23132. doi:10.1002/num.v40.6. [Google Scholar] [CrossRef]
66. Zuberi HA, Lal M, Deo S, Saxena A, Bég OA, Kuharat S, et al. Computational hemodynamics of Sisko-blood doped with gold and silver nanoparticles in a stenosed artery with porous walls. Numer Heat Transf A: Appl. 2024 (In Press). [Google Scholar]
67. Sandoval C, Rios G, Sepulveda N, Salvo J, Souza-Mello V, Farias J. Effectiveness of copper nanoparticles in wound healing process using in vivo and in vitro studies: a systematic review. Pharmaceutics. 2022;14(9):1838. doi:10.3390/pharmaceutics14091838. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools