 Open Access
Open Access
REVIEW
Drosophila melanogaster as an indispensable model to decipher the mode of action of neurotoxic compounds
1 Neural Developmental Biology Lab, Department of Life Science, NIT Rourkela, Rourkela, 769008, India
2 Centre for Nanomaterials, NIT Rourkela, Rourkela, 769008, India
* Corresponding Author: Monalisa Mishra,
(This article belongs to the Special Issue: Cellular and Molecular Toxicology in Reproductive and Developmental Biology)
BIOCELL 2023, 47(1), 51-69. https://doi.org/10.32604/biocell.2022.023392
Received 24 April 2022; Accepted 21 June 2022; Issue published 26 September 2022
Abstract
Exposure to some toxic compounds causes structural and behavioral anomalies associated with the neurons in the later stage of life. Those toxic compounds are termed as a neurotoxicant, which can be a physical factor, a toxin, an infection, radiation, or maybe a drug. The incongruities caused due to a neurotoxicant further depend on the toxicity of the compound. More importantly, the neurotoxicity of the compound is associated with the concentration and the time point of exposure. The neurodevelopmental defect appears depending on the toxicity of the compound. A neurodevelopmental defect may be associated with a delay in developmental time, defective growth, structural abnormality of many organs, including sensory organs, behavioral abnormalities, or death in the fetus stage. Numerous model organisms are employed to assess the effect of neurotoxicants. The current review summarizes several methods used to check the effect of neurotoxicant and their effect using the model organism Drosophila melanogaster.Keywords
Neurotoxicity is an anatomical, morphological, physiological, biochemical as well as behavioral abnormality (Coyle et al., 1976) that occurs during the development of the embryo to the fetus and can cause deformities in the adult. The causative agent for neurotoxicity is termed as a neurotoxicant. A neurotoxicant can be physical (thermal), electromagnetic (Wang et al., 2007), magnetic (Ho et al., 1992), ultrasound (Williams and Casanova, 2013), X-ray, or a chemical (Wang et al., 2007) factor. Neurotoxins or neurotoxicants alter the activity of the nervous system in such a way that it causes reversible or irreversible damage to nervous tissue (Cunha-Oliveira et al., 2008). With damaged neurons, the ability of transmission and processing of information in the central and peripheral nervous systems is hampered to a certain extent. A neurotoxicant may cause mutation in the organism by interfering with mitosis and resulting in altered chromosome numbers, nucleic acid synthesis, and function. Often a neurotoxicant causes deficiencies in precursors, substrates, enzymes, and other prerequisites necessary for the normal biochemical and metabolic function of the cell. Thus, it may alter the property of the cell, causing osmotic imbalance and altering the cell membrane, molecular, and biochemical composition (Wilson, 1968). Different potential neurotoxicants like infection and drugs have dissimilar effects on the developmental pattern. Many model organisms, including Drosophila melanogaster are used to identify a potential neurotoxicant (Daston, 2011; Mishra and Barik, 2018). Barik and Mishra reported in their review that nanoparticles act as a behavioral teratogen in the model organism D. melanogaster (Barik and Mishra, 2019). At low doses, some of the teratogens affect the behavior of the animal and thus act as a neurotoxicant (Coyle et al., 1976). Since the behavior of an organism originates from the nervous system, a toxicant at a lower dose can cause neurotoxicity, which appears as neurodegeneration in the later part of life. D. melanogaster is well studied to identify behavioral abnormalities in several stages of development (Mishra and Barik, 2018).
Drosophila melanogaster as a Model to Study Neurotoxicity
D. melanogaster is widely used to identify innumerable toxicants which can cause teratogenicity and neurotoxicity. The physiology and genetic similarity with higher vertebrates (Abnoos et al., 2013), short life cycle, low rearing cost, completely sequenced genome, and availability of gene-editing tools (Affleck and Walker, 2008) make it an ideal model to analyze the neurotoxicity and teratogenicity of innumerable compounds. Furthermore, the developmental pathways of D. melanogaster and humans have a significant similarity. D. melanogaster is also used for the study of glycobiology since the O-linked glycosylation of its cells share similarity with that of mammalian cells (Hagen et al., 2008). Some of the common pathways include the insulin pathway (McClure et al., 2011), the mitogen-activated protein kinase (MAPK) signaling pathway (Abnoos et al., 2013), the Notch signaling pathway (Alattia et al., 2011), folate metabolism (Affleck and Walker, 2008), fatty acid metabolism (Logan-Garbisch et al., 2015), the dopaminergic pathway (Bainton et al., 2000), and Wnt, TGFβ, Hedgehog, EGF, and cytokine pathways. Besides the signaling pathways, various channels (Sharma, 2004), which are useful in identifying pathway-specific interactions of different chemicals (including transient receptor channel, TRP), are also conserved between D. melanogaster and human being. More importantly, the transport, uptake, and efflux of many metals are conserved (Calap-Quintana et al., 2017). Altogether, D. melanogaster makes an ideal model for identifying neurotoxicants.
Factors Affecting Neurotoxicity
The dose of any compound plays a key role in determining its toxicity (Podratz et al., 2011; Sadler et al., 1988; Sudmeier et al., 2015). A compound at a low dose for a shorter period to an embryo/fetus may cause a neuronal abnormality, while chronic exposure to that drug may cause lethality. Lower doses of a toxic compound affect the nervous system and thus act as a neurotoxicant.
D. melanogaster development includes stages like an embryo, larva, pupa, and adult (Fig. 1), which help in understanding the influence of myriad compounds on several organs, including the nervous system (Rand, 2010). There are several modes via which a neurotoxicant can be introduced to different developmental stages of D. melanogaster (Fig. 2). The maternal feeding method is widely used as a common mode of exposure (Fig. 2A). During maternal feeding, the exact dose of the compound consumed, metabolized, and absorbed determines the toxicity (Rand et al., 2010). For this method, the neurotoxicant of interest is mixed in food media or with diluted yeast paste for a long and short exposure (Pandey and Nichols, 2011). Often the neurotoxicants are injected into the embryo (Fig. 2B) or adults so that they are circulated to the hemolymph of the whole organism, and their toxicity can be checked (Dzitoyeva et al., 2003). Embryos are also exposed by the permeabilization method for the exposure of the neurotoxicant (Rand et al., 2010). However, due to the presence of a vitelline membrane, sometimes, direct incubation of embryos with the compound of interest fails (Rand, 2010). Thus, the vitelline membrane of the embryo is also taken out (Sabat et al., 2015) before it is exposed to the toxicants (Fig. 2C). The larvae are also injected into the neurotoxicant to study the effect (Fig. 2D). Larvae are preferred for the feeding experiments because they are a rapacious eater. Neurotoxicants like ethanol and cocaine, which are used in the form of vapor (McClung and Hirsh, 1998; Moore et al., 1998), are soaked in filter paper in the saturated form (Nichols et al., 2012) to expose the flies or larvae (Fig. 2E). Often drugs are exposed to the nerve cord of decapitated flies to see the effect (Torres and Horowitz, 1998). A number of times the drugs in introduced via glass microcapillary. This method allows the precise measurement of the drugs consumed by a single or group of flies (Ja et al., 2007). This method is popularly known as the capillary feeder assay.

Figure 1: Developmental cycle of Drosophila melanogaster. The developmental stages include egg, larva, pupae, and adult. Each stage occurs at a particular time point, which does not change unless there is some internal or external stressor.

Figure 2: Mode of exposure of neurotoxicant to Drosophila melanogaster. Any developmental stage of Drosophila can be used for exposure to neurotoxicants. (A) Adult flies are exposed to the neurotoxicant via oral mode, (B) The embryos are exposed via injection, (C) Exposure to the neurotoxicant by membrane permeabilization method, (D) Exposure of larvae to the neurotoxicant by microinjection or feeding method, and (E) Various stages exposed to volatile neurotoxicants.
Besides mode, the developmental stage in which the compound is administered also plays a central role in determining its toxicity. Thus, the same compound administered at different developmental stages shows a different result. Why do different stages respond differently to the same compound? In the embryonic stage, the fate of various cells gets determined and leads to the formation of the organ, including neurons and pathfinding for axons (Pandey and Nichols, 2011). The development of central, peripheral, motor, and interneurons occurs in the embryonic stage (Bossing et al., 1996; Brewster and Bodmer, 1996; Schmid et al., 1999). This allows a fly embryo to be used in numerous neurotoxicology studies (Rand et al., 2010). In the larval stage, the nervous system originates from the embryonic stage and differentiates as the ventral neurectoderm. The larva has fully functional circuits with conventional synapse like a neuromuscular junction, enabling it to perform motor and sensory functions. Using the nervous system, the larvae can detect food, chemical, temperature, light, and sound, as reviewed by Mishra and Barik (Mishra and Barik, 2018). The larva possesses many undifferentiated precursor organs known as imaginal discs, which later transform into adult structures during the pupa stage (Pandey and Nichols, 2011). The pupal stage is important in a developing D. melanogaster, as neuronal remodeling and new patterns of synaptogenesis occur in this period (Rand et al., 2010), which subsequently form a healthy adult nervous system capable of performing normal functions. If a chemical interferes with any of the above-mentioned developmental stages, it alters the structure and functioning of several organs, including sensory organs (Bournias-Vardiabasis and Teplitz, 1982; Bournias-Vardiabasis et al., 1983; Mellerick and Liu, 2004).
Absorption, Distribution, and Metabolism of Drugs
The absorption, distribution, and metabolism of a drug depends on the route of administration (Fig. 2). The drug enters into different parts of the body through the route of administration. It is slowly eliminated from the body by absorption, distribution, metabolism, and excretion (Pappus and Mishra, 2018). During the stay within the body, the drug may undergo a structural change through a process known as biotransformation (Baars, 1980). Often the release of the drug takes place in the air, and this step is known as transportation. The absorption of a drug depends on its physiochemical properties and the route of its entry into the circulatory system. A drug can be transported via transmembrane transport by active or passive or dynamic transport (Stewart, 2002). Passive transportation takes place from higher to lower concentrations by means of diffusion. Active transportation takes place from lower to higher concentration using a carrier protein. Macromolecules get transported via the membrane by pinocytosis or exocytosis.
Drugs are administered into the body by (1) the digestive tract, (2) injection, or (3) transdermal delivery. Drugs that enter through the digestive tract get absorbed via epithelial cells of the gastrointestinal tract via passive transport (Pappus and Mishra, 2018; Sahu et al., 2022). Once the drug is absorbed, it mixes with the hemolymph. The villi present in the small intestine increase the surface area of absorption (Sahu et al., 2022). Drug absorption depends on the pH, absorption, and drug dissolution. High pH facilitates the absorption of alkaline drugs, whereas low pH promotes the absorption of acidic drugs (Pappus and Mishra, 2018). The interaction of drugs with fat soluble compounds affects absorption.
Drugs injected into the body are absorbed faster than those that are orally administered. On being injected, water-soluble drugs diffuse from the site of injection to different parts of the body (Mayer et al., 2009). Drugs administered through injection are fully absorbed. The absorption is directly proportional to the blood flow in the area.
Administration of drugs via the skin, smearing, and spraying is known as transdermal drug delivery (Panonnummal et al., 2021). In this method, drugs enter into the body through the cuticle and enter into the hemolymph via passive diffusion. Fat-soluble drugs are absorbed easily via the epidermis. A drug entering through the olfactory organ reaches the trachea, where it is absorbed via passive diffusion to reach the blood vessel (Scholl et al., 2021). From the blood vessel, it reaches the central nervous system (CNS). Large molecular weight drugs have a lesser chance of absorption, and peptides and circular proteins are absorbed faster than linear ones.
Once the drug is absorbed, it is transported to different parts of the tissue and organs. The distribution of the drug occurs in an uneven manner. The binding of the drug to the plasma protein is dependent on the pH and physicochemical properties of the drug (Pandey and Nicholas, 2011). The unbound drug binds to the intracellular component of the hemolymph for its distribution. The drug is distributed unevenly in different parts of the body.
The drug metabolism depends on the drug-metabolizing enzyme present in the hemolymph and the tissue (Misra et al., 2011). Drugs are metabolized in the digestive system, nephrocyte, trachea, skin, and CNS. Some of the drugs are metabolized by the bacteria present within the body. Some drugs bind to the enzymes present in the digestive system, thus reducing the bioavailability. Often the drugs undergo reduction, hydrolysis, acetylation, and dealkylation after binding to the microbial flora (Guengerich, 2001). Drug metabolism results in phase I and Phase II reaction. Phase I includes metabolism resulting in carbonyl, carboxyl, sulfhydryl, and amino groups. The oxidation includes oxidation of sulfur, nitrogen, amino, and desulfurization. Cytochrome p450 is the most critical enzyme in the phase I metabolism of drugs (Chung et al., 2009). In phase II reaction, the metabolite covalently binds to glucuronic acid, sulfuric acid, glycin, and glutathione. Genetic factors regulate the drug metabolism, and the non-genetic factors include age, sex, nutrition, and temperature (Rand, 2010). Drug metabolism is affected by different developmental stages. In the early developmental stages, the drug may cause toxicity. With age, metabolic enzymes and endogenous cofactors get reduced. Drugs are excreted from the body by passive or active transport with respect to concentration from the hemolymph.
Methods to Check Neurotoxicity
A neurotoxicant can alter the morphology of the neurons as well as functions or behaviors associated with it. The behavior of an animal is regulated by many sensory organs (Hirsch et al., 2012) and the neurons associated with it (Markow and Gottesman, 1993). During development, a network of genes cross-talk with each other and forms the nervous system, which regulates the physiology and behavioral pattern of an animal. If the stressor becomes uncontrollable and reoccurs for a longer period, then it causes chronic stress. Chronic stress results in allostatic load and initiates molecular changes within the key regulator of the nervous system, i.e., the brain (Min and Condron, 2005). Thus a neurotoxicant may cause developmental instability in the CNS, alter the symmetry of neuronal numbers, chemistry, or connections (Markow and Gottesman, 1993), and can cause behavioral changes in any species in later developmental stages (Alves-Pimenta et al., 2018). Also, any contact with toxins during development affects the development of the nervous and endocrine systems resulting in behavioral defects in adults (Hirsch et al., 2012). Parameters like developmental cycle, morphological parameters of different developmental stages, immunohistochemistry and histology, biochemical assay, and several behavioral assays are used to assess neurotoxicity.
The developmental cycle of D. melanogaster is used widely to check the toxic effect of a neurotoxicant or teratogen (Bianchini et al., 2018; Li and Bi, 2018). Many developmental time points like the hatching of eggs to larva, a transition of larvae to pupae, pupation time, hatching of pupae to eclosion of adult flies, and their survivability or life span are already known (Fig. 1). Developmental time points are noted after the exposure of neurotoxicants and compared with the control flies. Alteration in the time point of any of these stages is considered a defect caused due to neurotoxicants (Bianchini et al., 2018). Besides time, the number of animals affected at the developmental stages due to the effect of a neurotoxicant is also calculated (Figs. 3A–3E). The hatching of fewer larvae suggests the death of the embryos (Fig. 3C). The dead embryos can be imaged under light and scanning electron microscope to identify the phenotypic defect (Bianchini et al., 2018; Rand et al., 2014). Similarly, if the death occurs during the larval stage, then the dead larvae (Fig. 3D) can be checked under light and scanning electron microscope to identify the phenotypic defect (Affleck et al., 2006b; Rand et al., 2014). Death may also occur in the pupa stage (Fig. 3E). The dead pupa appears black, indicating incomplete pupation (Fig. 3E), and in such cases, fewer flies are hatched (Rand et al., 2014). The dead pupa is dissected and compared with the control pupa to check the developmental defect due to the neurotoxicant. Thus, the analysis of the number of animals in all the stages gives a clear picture of the effect of the neurotoxicant in the development of D. melanogaster (Fig. 3).

Figure 3: Toxicity evaluation of neurotoxicants using developmental cycle. (A) Quantification of death of adult flies after feeding the neurotoxicants, (B) Reduction of the adult flies number after feeding the neurotoxicant from first instar larval stage onwards, (C) Quantification of dead embryos after exposure to the neurotoxicant, (D) Quantification of dead larvae after exposure to the neurotoxicant, and (E) Quantification of dead pupa formed from larvae exposed to the neurotoxicant.
Morphometric validation of various stages
A neurotoxicant can alter the size of the various developmental stages (described in the above section). The teratogen-induced morphometric developmental defect is reported for many chemicals (Lynch et al., 1991; Schuler et al., 1982). Those strategies can be adopted to check the toxic effect of neurotoxicants. Some of the neurotoxicants even produce more than one phenotypic defect. To check the abnormality of the embryo, it is collected and imaged under both light and scanning electron microscopes. Defective regions can be assessed using Image J for any kind of morphological defect. Similarly, the larvae are collected after the treatment of neurotoxicants and imaged under a stereomicroscope. From the image, the length, breadth, diameter, and area of the larvae are measured using Image J software. Similarly, in case of any phenotypic abnormality in the adult fly, an image of that region is taken and subjected to quantification to deduce a value. An increase or decrease in the size of the larva or adult flies can be measured through its weight for quantification purposes.
Immunohistochemistry and histology
A teratogen can alter the cell cycle and cell division. To check the effect of a teratogen on the cell division and cell cycle, apoptosis is checked in various tissues. If the teratogen is administered orally, the midgut of the larvae is analyzed for apoptosis (Priyadarsini et al., 2020). Double staining of Caspase and DAPI/Hoescht can provide information about apoptosis in various tissues. Hoescht/DAPI staining detects the nuclei shape within the tissue (Fig. 4A). Apoptotic nuclei are smaller in size in comparison to the normal nuclei. From the shape and size of the nuclei, the apoptotic condition of the cell can be determined (Fig. 4A). DAPI binds to DNA, and Caspase binds to apoptotic cells. The TUNEL (TdT-mediated dUTP-biotin nick end labeling) assay is also used to check the apoptosis in the cell (Vasudevan and Ryoo, 2016). Several studies also use acridine orange and propidium iodide to detect cell death (Sahu and Mishra, 2020b; Vasudevan and Ryoo, 2016). Imaginal discs (eye and wing) of the larvae are stained with acridine orange to detect the cell death caused by neurotoxicants (Figs. 4B and 4C). Newly eclosed flies were examined under the stereomicroscope. After detecting the defect, they are analyzed under the scanning electron microscope for visualization at a higher resolution. The defective organ of the adult stage is analyzed in a developmental time window to detect the role of cell death and cell cycle in the formation of a phenotypic defect (Mishra et al., 2010; Sahu and Mishra, 2020a). To check the internal defect, the defective tissue of organs is fixed for histological analyses. The sections are cut, stained, and imaged under the microscope for phenotypic analysis. For the analysis of fine structural defects, the tissues are fixed and analyzed under a transmission electron microscope. Any abnormality at the organelle level can be detected after the analysis of the sample under a transmission electron microscope (Moreira et al., 2010).

Figure 4: Apoptosis detection in Drosophila melanogaster tissues using histological staining. (A) Drawing of control gut (Left one) and a gut exposed towards a neurotoxicant orally (Right; note the difference between the size and number of nuclei present in the left and right side), (B) Eye imaginal disc drawing of a control (left) and after treatment with the neurotoxicant (right), and (C) Wing imaginal disc drawing of a control (left) and neurotoxicant treated one (right).
Biochemical assays are used to detect the neurotoxicity of an unknown compound. Some of the common endpoints of measurements are (1) glutathione content, (2) glutathione-S-transferase, (3) lipid peroxidation, (4) protein carbonylation, (5) acetylcholinesterase (AchE) activity, (6) monoamine oxidase, and (7) caspase-9 and caspase-3 activity. Glutathione content after the treatment of neurotoxicants can be studied using Ellman’s Reagent (Jollow et al., 1974). Depletion of glutathione results in the death of the neurons (White and Cappai, 2003) because it is an essential neuronal antioxidant necessary to detoxify free radicals and prevent oxidative stress (Bains and Shaw, 1997). Glutathione-S-transferase (GST) is a major detoxifying defense enzyme of the antioxidant enzyme system (Dasari et al., 2018). GSTs activity is measured at 340 nm from the end product of the reaction. The lipid peroxidation assay measures the level of stress within the body. Stress can be estimated following the protocol of Mishra and Acharya (Mishra and Acharya, 2004) by quantifying the end product of the reaction, i.e., thiobarbituric acid (TBARs) at 535 nm. More TBARs content leads to apoptosis-induced neuronal cell death. The protein carbonyl content can be estimated following Hawkins et al. (2009). Carbonylation alters the protein functions and leads to several intermolecular aggregates and crosslinks that degrade the intracellular proteases. Thus, the accumulation of carbonylated protein content leads to several CNS disorders. Acetylcholinesterase is an essential neurotransmitter. The activity of AchE directly defines the effects of neurotoxicants on the nervous system. AchE activity can be estimated at 412 nm following Ellman and Courtney (1961). Monoamine oxidase (MAO) plays a significant role in the bioactivation of neurotoxic analogs (Heikkila et al., 1988). Although the MAO homolog is not present in the fly (Roelofs and van Haastert, 2001), its activity has been reported by several authors (Chaudhuri et al., 2007, Wang et al., 2011). Interestingly, drugs which can inhibit the activity of MAO can also inhibit the activity of flies (Yellman et al., 1997). Recently, the activity of MAO was measured from the fly head (Oyeniran et al., 2021). Lemons can protect from Alzheihmer’s disease by decreasing the MAO activity (Oyeleye et al., 2021). Capsaicin can also reduce the MAO activity in the Parkinson’s model of D. melanogaster (Siddique et al., 2018). MAO can be quantified following Mcewen (McEwen, 1965). Caspase-9 (Dronc) and Caspase-3 (Drice) reveal the neurotoxicity leading to neuronal cell death and are quantified by detecting the chromophore p-nitroanilide at 405 nm (Shakya and Siddique, 2018).
Behavioral assays associated with developmental stages
Behavioral assays are well studied to check the effect of a neurotoxicant or teratogen. A neurotoxicant/teratogen can modify the behavior of an organism by altering the expression of genes and neurotransmitters associated with it (Barik and Mishra, 2019; Moore et al., 1998; Nichols et al., 2012). The alteration of the nervous system in developmental time offers D. melanogaster as a model to study behaviors associated with the nervous system (Dhar et al., 2020b; Rand, 2010). The exposure of the embryonic stage to a neurotoxicant can interrupt the development of the nervous system and the glia associated with it (Rand et al., 2010). Those embryos later have a defective nervous system. (Bianchini et al., 2018; Rand et al., 2010). A functional nervous system makes the fly respond toward light (Hardie, 2012), odors (Montell, 2009), sound, tastants (Montell, 2009; Weiss et al., 2011), humidity (Liu et al., 2007), temperature (McKemy, 2007), and gravity (Inagaki et al., 2010; Kamikouchi et al., 2009). These responses are used to check the functionality of the nervous system by doing numerous behavioral assays in adult flies (Fig. 5). The assay is choice to detect the response towards the light (Lilly and Carlson, 1990), vision (Gerber et al., 2004), smell (Shaver et al., 1998), heat (Liu et al., 2003), and taste (Heimbeck et al., 1999). Third instar larvae are used to check for foraging behavior (Pereira et al., 1995; Sokolowski et al., 1997), light sensing ability (Busto et al., 1999), and the coordination of neuromuscular junction (Fig. 6A). The larval response towards light and determines the functionality of circadian rhythm (Luna et al., 2013). Larvae can detect the right type of food using olfaction (Kim et al., 2015), this behaviour is called feeding behavior (Fig. 7B). For this assay, yeast paste is kept at the center of the Petri plate, and the time taken by the larvae to reach the area is calculated (Min and Condron, 2005). The time taken by the control and the treatment larva is calculated and compared for the abnormality. Self-righting behavior is associated with the functioning of mechanosensory organs. The self-right test is used to keep the animal maintained at its right position. For this, the first instar larvae are placed towards the ventral side up (Fig. 6C). The time taken by the larva to turn to its original position is monitored. This behavior was recently reviewed by Dhar et al. (2020b), who used third instar larva to detect their ability to differentiate between heat and cold (Fig. 6D). To respond to the cold temperature, the larval body contracts (Turner et al., 2016) by activating class III (CIII) multicentric neurons (Turner et al., 2016). The touch-sensitive assay is used to detect the larval response towards touch by gently touching the thoracic segment (Fig. 6E) (Caldwell et al., 2003). Similarly, the larval body has numerous channels to detect the sound. To detect the sound sensing ability of the larva, sound avoidance behavioral assay is used in the third instar larvae (Dhar et al., 2020a, 2020b). If there is any defect in the channel, the larvae do not respond to the sound properly. Late third instar larvae can climb the wall of the vial to form pupae (Fig. 6F). The height climbed by the untreated and treated larvae is calculated. Larvae with defective motor neurons are unable to climb high either due to defects in the morphology or in the signaling molecule (Bianchini et al., 2018; Lozinsky et al., 2012; Lozinsky et al., 2013). Thus a defect in the sensory organ can be screened. Larvae also choose the right place for pupation for their survival. This performance is called pupation site preference (PSP) behavior (Sameoto and Miller, 1968). A larva having a defective nervous system shows altered PSP behavior in response to epileptic drugs (Beltramí et al., 2012). Temporal evaluation is also considered one of the parameters to measure behavioral teratogenesis. For this evaluation, flies are released into food-containing vials and allowed to lay eggs. After 24 h, the number of larvae are counted. Next, the formation of second and third instar larvae was checked with time. The time taken to form the first pupa and the number of pupae are counted. Similarly, the hatching time of the first fly and the number of flies hatched are counted after metamorphosis (Bianchini et al., 2018). Adult flies hatched after neurotoxic compound treatment are checked for their climbing ability against gravity (Fig. 6D). Dopamine, octopamine, tyramine, and serotonin are associated with locomotory behavior (Pendleton et al., 2000; Sombati and Hoyle, 1984; Saraswati et al., 2004; Silva et al., 2014). Several functions of the antennae, including balancing, have been reviewed by Bokolia and Mishra (Bokolia and Mishra, 2015). A defective antennae make the fly positively geotaxis. Thus, the climbing assay quantifies the neuronal defects and aging in D. melanogaster (symptoms of Parkinson’s disease) (Feany and Bender, 2000; Shaltiel-Karyo et al., 2010). Similarly, the aversive phototaxic suppression (APS) assay (Le Bourg and Buecher, 2002; Seugnet et al., 2009) is used to study Alzheimer’s, Parkinson’s, and Huntington’s diseases in the D. melanogaster model to enumerate the deviations in locomotion, learning, and memory (Ali et al., 2011). Dopamine is involved in memory formation (Berry et al., 2012) and octopamine is involved in both learning and memory. Serotonin is responsible for long-term memory (Sitaraman et al., 2015; Scheunemann et al., 2018), and gamma-aminobutyric acid (GABA) convert sleep into memory consolidation (Haynes et al., 2015). Adult flies also respond towards starvation and desiccation (Hoffmann and Harshman, 1999). Any anomaly after neurotoxicant treatment can amend this behavior, pointing to a defect in the nervous system. The larval photosensitive pattern is analyzed under a regulated light-dark cycle, and the locomotion is monitored. A neurotoxicant may alter the 24-h light–dark cycle by altering the sleep cycle (Hirsch et al., 2012). D. melanogaster Activity Monitors (DAMs) is widely used to track the locomotor activity of adult flies. Dopamine is involved in sleep and arousal (van Swinderen and Andretic, 2011), acetylcholine regulate sleep promotion (Aso et al., 2014), and glutamate (Guo et al., 2016) and serotonin (Liu et al., 2019) regulate the sleep.

Figure 5: Behavioral assays in adult flies. (A) Light-dark choice assay, (B) Heat or cold sensitivity assay, (C) Y-maze device for odor test, and (D) Climbing assay.
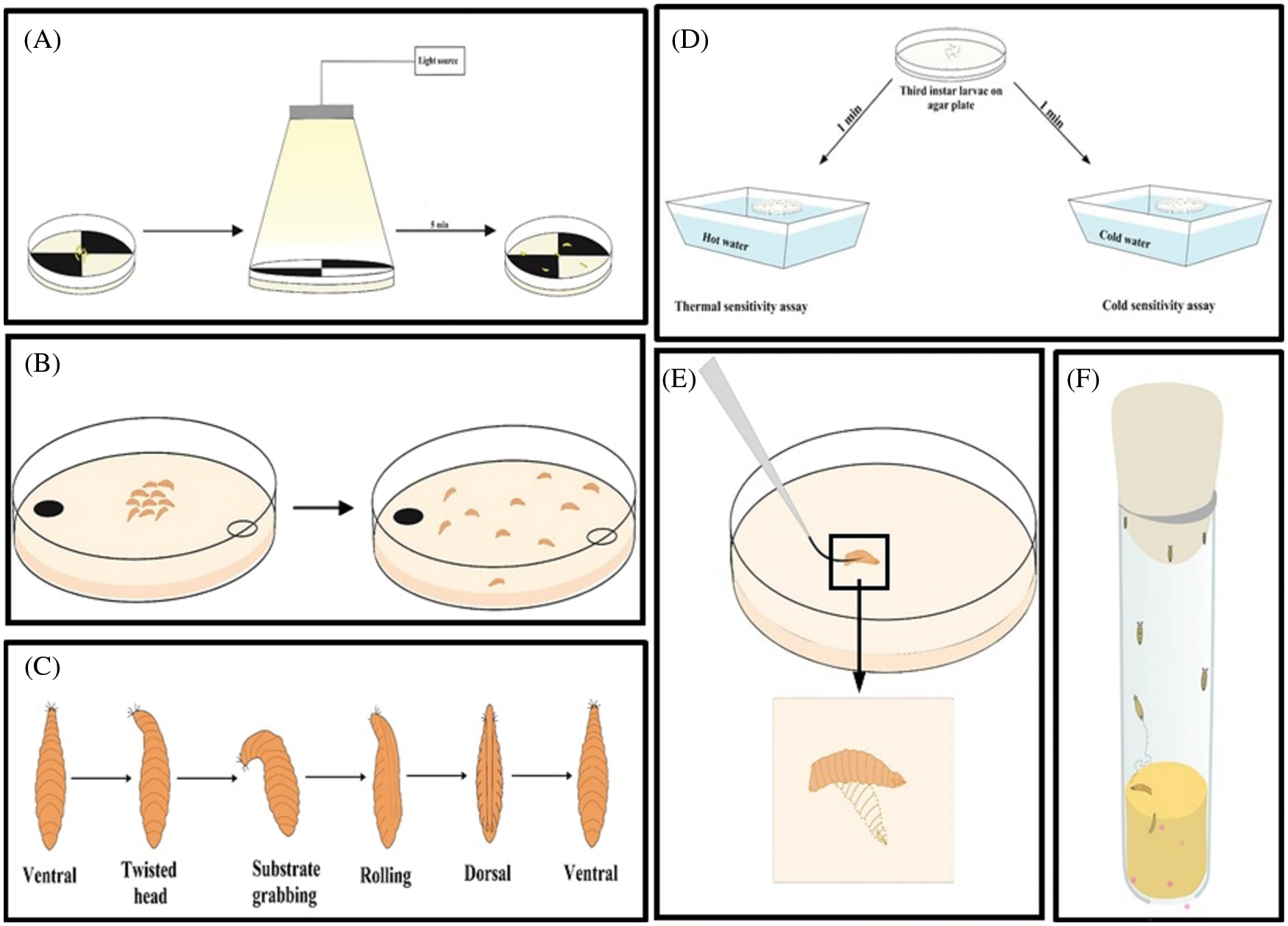
Figure 6: Behavioral assays in larvae. (A) Light-dark choice assay, (B) Choice assay for odor test, (C) Self-righting behavior, (D) Heat or cold sensitivity assay, (E) Touch response in larvae, and (F) Pupation site preference behavior of the late third instar larvae.

Figure 7: Complex behavior in adult flies after treatment of neurotoxicant. (A) Grooming behavior (B) Steps involved in mating behavior.
Some of the D. melanogaster behaviors need the signal from many sensory organs, as recently reviewed by (Sahu et al., 2020). They are courtship, grooming, aggression, social avoidance, and predator fear behavior (Fig. 7). These behaviors depend on the signal from the eye, hearing and the chemosensory organ (Nichols et al., 2012). Many neurotransmitters are also involved in this process. Male courtship behavior is associated with dopamine and tyramine (Huang et al., 2016). Octopamine regulates both male and female courtship behavior (Zhou et al., 2012; Rezával et al., 2014). Courtship behavior follows particular steps before mating (Spieth, 1974); this behavior is used to check the functionality of neurons (Nichols et al., 2012). Any anomaly in the mating step indicates defective neurons. Defective courtship behavior is seen as a fly model of Parkinson’s disease (Shaltiel-Karyo et al., 2012). Similarly, grooming behavior helps the fly to keep itself clean. A defective mechanosensory organ resulted in faulty grooming behavior keeping the fly dirty (Sahu and Mishra, 2020a). Aggressive behavior helps the animal to find its food and partner, and protect the territory (Dhar et al., 2020a; Sahu et al., 2020). Aggressive behavior is associated with the environment, and (Dhar et al., 2020a) the level of serotonin and dopamine also regulates aggression (Alekseyenko et al., 2013). A defective aggressive behavior is associated with serotonin and the dysfunction of the antennae (Dhar et al., 2020a). When exhibiting social avoidance behavior, flies move away from a stress signal released by the stressed fly. The stress signal released by the flies is known as dS (Suh et al., 2004). For this assay, flies are placed in a T-maje device. Flies have a chance to avoid dSO present in the vial and a fresh vial. This experiment is carried out at 25°C–30°C, with even light and more than 30% humidity. After one minute of introduction to the T-maje device, flies present in both the chambers are counted. This experiment is repeated for three independent data sets and a T-test is carried out to compare the result (Fernandez et al., 2014). D. melanogaster perform different behavior in presence of a predator (Parigi et al., 2019). The behaviors include (1) lifting of the abdomen, (2) flying (moving in space using wings), (3) jumping (instantaneous movement between points without the use of the wing), (4) pausing (inactive period, similar to stopping; the duration lasts for less than one second, (5) turn orient 180° without changing the position, (6) wing display (lifting of wing without singing or vibration), (7) grooming (rolling of legs over different parts of the body while in a stationary stage), (8) walking (movement through space), (9) running (rapid movements of the body), (10) stopping (immobile for more than one second), and (11) retreating (walking in reverse direction while encountred with a predator). The predatory response behavior varies with species and different stages of development.
Neurotoxicants Tested Using Drosophila melanogaster
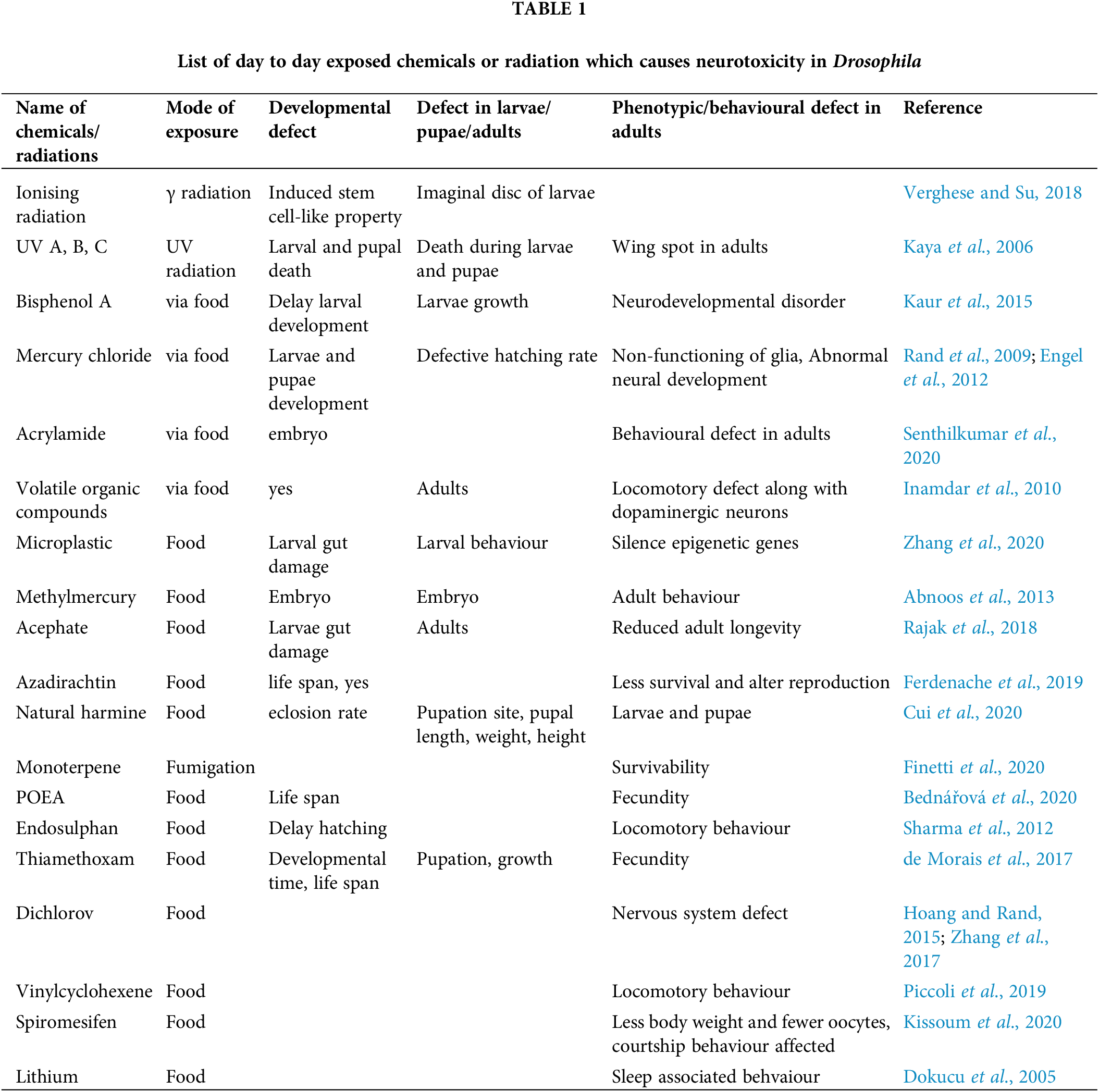
D. melanogaster is an effective model to investigate the neurotoxicity of many chemicals and physical parameters. Several factors like UV, gamma radiation, numerous drugs, pesticide, fungicide, herbicides, water pollutants, and air pollutants also show neurotoxicity and teratogenicity (Abnoos et al., 2013; Bednářová et al., 2020; Cui et al., 2020; de Morais et al., 2017; Dokucu et al., 2005; Ferdenache et al., 2019; Finetti et al., 2020; Hoang and Rand, 2015; Inamdar and Bennett, 2014; Kaur et al., 2015; Kaya et al., 2006; Kissoum et al., 2020; Piccoli et al., 2019; Rajak et al., 2018; Rand et al., 2009; Senthilkumar et al., 2020; Sharma et al., 2012; Verghese and Su, 2018; Zhang et al., 2020; Zhang et al., 2017). The neurotoxic or teratogenic effects of those compounds are summerized in Table 1. Many neurotoxicants alter the redox activity of essential metals necessary to maintain brain functions and homeostasis. The redox activity of the metals induces oxidative stress and impairs the nervous system (Sharma et al., 2014). Heavy metal toxicity can also cause deafness and loss of vision. We next discuss the compounds which are exclusively tested for their neurotoxic or teratogenic effect in the fly model.
D. melanogaster model is used to study ethanol-induced developmental and behavioral defects. Prenatal exposure to ethanol causes reduced viability, delay in developmental time, and smaller size of the hatched flies due to less cell division (McClure et al., 2011). Ethanol vapor affects the behavior of D. melanogaster and makes the fly hyperactive, disoriented, and uncoordinated (Cohan and Hoffmann, 1986). Ethanol can target receptors and channels like voltage-gated channels, NMDA, serotonin, and GABA (Diamond and Gordon, 1997). Some of the behavioral defects in D. melanogaster are dependent on dopamine (Bainton et al., 2000). Among signaling pathways, the insulin pathway is largely affected (reduced insulin receptor and D. melanogaster insulin-like peptide (DILP)) due to the action of mediating developmental and behavioral defects (McClure et al., 2011). DILP regulates the growth, reproduction, longevity, metabolism of carbohydrates and fat (Géminard et al., 2009).
MTX, a commonly used drug in chemotherapy, is also checked for its teratogenic effect using D. melanogaster (Affleck and Walker, 2008). MTX inhibits the dihydrofolate reductase in D. melanogaster (Affleck et al., 2006a) and in humans, affects the folate metabolism, which is mediated by dihydrofolate reductase (Affleck and Walker, 2008).
MTX can cause irreversible defects in gestation and embryogenesis (Affleck et al., 2006a) in D. melanogaster, and malformed eye (photoreceptor organ), wing, bristle (mechanoreceptor organ), and the curvature of appendage are some of the developmental defects caused due to MTX (Affleck et al., 2006a).
Volatile organic compounds (Fungal VOCs)
Exposure of VOCs to flies causes defective coordination during rapid mobility, restlessness, and frequent fall during jumping near the vial. Later, the flies become sluggish in movement or suffer from bradykinesia after the exposure to the toxicant for 12–18 h. This behavior shows similarity with the fly model for Parkinson’s disease when dopamine gets affected (Inamdar et al., 2010). Low concentrations of fungal VOCs in adult D. melanogaster results in the locomotory defect by altering the activity of dopaminergic neurons (Inamdar et al., 2010). Industrial VOC exposure increases the production of reactive oxygen species (Bayil et al., 2008; Singh et al., 2009) which can induce lipid peroxidation with the production of toxic products (Inamdar et al., 2010).
Exposure to AED during the first trimester causes anatomical abnormalities, whereas exposure during the third trimester causes behavioral anomalies in D. melanogaster. AEDs such as phenytoin, valproic acid (VPA), and carbamazepine alter the genotoxicity of D. melanogaster in a dose-dependent manner (Yüksel et al., 2010). Mating propensity, larval PSP, and climbing ability are largely affected after treatment with AEDs (Harini, 2016). AEDs are reported to act via the GABA channel, which along with dopamine, regulates the female receptivity during courtship and mating (Gayathri and Harini, 2012). VPA reduces the copulation duration, whereas pentylenetetrazole amends the climbing speed of adult flies. The larva changes the PSP concerning different AEDs. PSP has an important role in pre-adult development as per the survivability of pupae (hence adult D.melanogaster) is concerned (Harini, 2016). A high dose of phenytoin resulted in death in all the developmental stages of D. melanogaster (Gayathri and Harini, 2013).
It is a potent psychostimulant in D. melanogaster. A low dose of cocaine can make flies hyperactive, uncontrollable, and continuous grooming behavior as a defective behavioral endpoint. A locomotory defect such as circling behavior is seen among flies (Schafer, 2002). A high dose of cocaine was shown to induce irregular activity, shock, and complete immobility (akinesia) in D. melanogaster (Bainton et al., 2000). Repeated exposure of flies to cocaine increases the behavioral response (sensitization) (Schafer, 2002) and male flies show more sensitivity towards cocaine than females. Alteration of dopamine levels can change the grooming and locomotive behaviors of D. melanogaster (Torres and Horowitz, 1998).
The effect of nicotine is confined to the CNS in D. melanogaster since acetylcholine receptors for nicotine in insects are found only in the nervous system (Gundelfinger, 1992; Restifo and White, 1990). Nicotine elicits similar behavioral defects as cocaine. Volatilized nicotine exposure can make flies hyperactive and induce occasional movements, resulting in grooming, hypokinesis, and akinesia in the maximum treated individuals, along with impaired negative geotaxis movement (Bainton et al., 2000; Heberlein et al., 2009). Interestingly, flies treated with both cocaine and nicotine simultaneously show impaired climbing ability in a dose-dependent manner (Bainton et al., 2000).
Oral ingestion of hydrogen peroxide (0.1%–2%) as well as the injection of hydrogen peroxide (1%) can increase the locomotory activity of adult flies. Injected hydrogen peroxide mixed with the hemolymph thus causes an alteration of the activity of the fly. Superoxide dismutase (SOD), a hydrogen peroxide-producing enzyme, also increases fly activity. Injection of hydrogen peroxide and expression of SOD resulted in abnormal walking movement in flies (Grover et al., 2009).
Physical stress (thermal and magnetic field)
Environmental stress, such as thermal stress, can disrupt the CNS (Wang et al., 2007). The mushroom body (MB) is associated with the integration of sensory and associated center of the insect brain, which is further responsible for memory and conditioned behaviors (Davis, 2005). Thermal exposure of larva and pupa during development can change the MB by reducing intrinsic Kenyon cells and the number of neurons by affecting the associative odor learning in adults (Bahrndorff et al., 2016; Wang et al., 2007). Similarly, brief exposure to a weak static magnetic field during the early period of development greatly affects the cuticular pattern of D. melanogaster larva (Ho et al., 1992) and the negative geotaxis behavior of adults (Fedele et al., 2014). The hatching rate of the larva decreases due to weak static magnetic fields (Ramirez et al., 1983).
Elements depicting neurotoxicity
Elements are essential for several biological activities in an organism, and their roles are conserved between D. melanogaster and higher vertebrates (Calap-Quintana et al., 2017). The human body utilizes metals from food. The body adjusts to the deficiency or excess of metals at the cellular level by altering the metabolic pathways. In the fly, the metals are metabolised using the secretory pathways. If the concentration of the metal exceeds the tolerance level within the body for any reason, it causes neurotoxicity or teratogenicity in flies. We have listed a few elements which are validated as teratogen using D. melanogaster.
Hg is an environmental pollutant and teratogen in D. melanogaster. Hg increases the larva to pupa transition time, and reduces the size of the larva, pupae, and hatching rate of pupae. It affects the phosphorylation of various kinases such as MAPK, extracellular signal-regulated kinase (ERK), and c-JUN N-terminal kinase (JNK). In D. melanogaster, ERK regulates cell growth and differentiation (Posser et al., 2009), whereas JNK is responsible for cell cytoskeleton and cell formation (Pereira et al., 2011). The toxic effects of Hg include non-functioning of the nervous system and glia (Rand et al., 2009), polarity loss in follicular cells (Baffet et al., 2009), and differentiation defect of nerve and muscle cell (Bournias-Vardiabasis et al., 1990). Abnormal cell differentiation during metamorphosis was also observed in the latter study. More importantly, Hg inhibits the notch pathway and thus disrupts normal neural development (Engel et al., 2012). The inhibition of the notch pathway reduces the hatching rate of the larva (Abnoos et al., 2013).
Pb2+ can change the developmental neuronal plasticity in D. melanogaster (Jin et al., 2005). Ca2+ contributes to synaptic development at different steps, such as guiding the growth cone (Jin et al., 2005), the formation of the synapse (Xu et al., 2009), elimination, and stabilization (Lohmann and Bonhoeffer, 2008; Pratt et al., 2003). Pb2+ affects Ca2+ binding proteins (Hirsch et al., 2012) and causes abnormal larval neuromuscular junction and mitochondria with defective ATP synthesis (Flora et al., 2008). Less ATP delays pupal development (Hirsch et al., 2012). Recently it was found that behavioral abnormality induced by Pb2+ in flies is by altering the microbiota (Sun et al., 2020).
Chromium exists in various forms like Cr(III)Cl3, K2Cr(VI)O4, and K2Cr(VI)2O7, and its toxicity was checked using D. melanogaster. Wing spots are seen in the adults when the larvae were fed with Cr (VI) salt (Katz et al., 2001; Yeşilada, 2001). Feeding CrCl3 does not cause any wing spots in adults. [Cr(pic)3], another chromium (III) compound was fed at a concentration of 200–600 μg per day (Hepburn et al., 2003). In the offspring, it causes a delay in development and death during the process of development (Hepburn et al., 2003). [Cr(pic)3] does not cause any phenotypic or behavioral, or survival defect when fed to the adult flies. [Cr(pic)3] does not cause defects genetically but interferes with the metabolic pathways (Hepburn et al., 2003).
Ni exposure is known to exert many epidemiological and mutagenic effects on human beings. The Ni effect was investigated using D. melanogaster. Two different Ni compounds (NiCl2 and NiSO4) were employed to check the toxic effect of Ni (Carmona et al., 2011). The genotoxic potential of two Ni-compounds was assessed using D. melanogaster using the wing-spot assay and comet assay in the hemolymph. Single- and double-strand DNA breaks were detected after Ni exposure. The frequencies of the wing spot do not increase significantly. However, NiSO4 can significantly induce DNA damage, as evidenced by comet assay (Carmona et al., 2011).
Wild type (Canton S) flies were grown on Al mixed food media (20–240 mg/kg). Larvae grown in Al-supplemented food have defective walking behavior. The locomotory activity decreases with the increasing concentration of alumina. The number of pupae and imago count decreases after the treatment of alumina (Kijak et al., 2014). The adult flies hatched after alumina treatment have defective sleep cycle and arrhythmic behavior. The toxicity of alumina depends on concentration and time of exposure. Alumina further decreases the life span of the flies. At 120 mg/kg, the male life span, as well as locomotor activity, were found to be increased (Kijak et al., 2014).
Dietary intake of several concentrations of Zn (0.1 to 1000 ppm) was checked by mixing it with the fly food (Al-Momani and Massadeh, 2005). Third instar larvae grown on Zn-added food did not exhibit altered growth and development in the first generation up to 500 ppm. At 500 ppm, 75% of pupa and adult death occurred. At 1000 ppm, survival percentages were further significantly reduced (35%) for pupa and adult stages. For the second generation, survival, growth, and development remain unaltered up to 100 ppm; however, at 500 ppm, a significant reduction in these activities was seen (Al-Momani and Massadeh, 2005). Zn upregulates the expression of metallothionein, and thus serves as an antioxidant. Zn can prevent apoptosis and thus prevent oxidative damage in the brain (Kocatürk et al., 1996). Oral supplementation of ZnCl2 at a concentration of 4 mM to parkin mutant flies (park25/25) increased the eclosion frequency and life span of the adult (Saini and Schaffner, 2010). The same concentration of ZnCl2 decreases the life span of the adults and alters the feeding behavior.
Cu has a role in antioxidant mechanisms, the formation of pigments, and neurotransmitters. It is used in many fungicides. The teratogenic effect of Cu was checked using D. melanogaster. Several concentrations (40 to 320 mgL−1) of CuSO4 were mixed with the fly food (Ding and Wang, 2006) and fed to the first instar larvae. At low concentration, the larval growth rate was slow; with the increasing concentration of Cu, the larval body length decreased (Ding and Wang, 2006). At 320 mgL−1 concentration, the larval body length decreased, and they took 11 days to reach the pupae stage compared to 5 days for control flies (Ding and Wang, 2006).
CoCl2 was administered orally and the adults hatched from the pupae were analyzed for morphological defects. Trans-heterozygous larvae for the multiple wing hairs (mwh) and flare (flr) were used in this study. The wing spots were observed in the wing after treatment with CoCl2. CoCl2 can induce both small and large spots in the wings (Ogawa et al., 1994). In mwh/TM3 flies, CoCl2 could not induce large spots in the wings due to suppressed mitotic crossing-over. To induce large spots, mwh/flr system clones were generated.
Mn is an essential element but can induce toxicity if it exceeds the limit. Several concentrations of MnCl2 were supplemented to the food medium (at 0.1, 0.5, or 1 mM)(Ternes et al., 2014). MnCl2 treatment was given throughout the development from the egg to the adult stage. Significantly enhanced locomotor activity was observed at 0.5 and 1 mM of Mn. These concentrations can induce reactive oxygen species within the body. At 1 mM, Mn can increase the mRNA expression level of catalase, superoxide dismutase, and Hsp83 without altering the activity. Mn can increase the activity of thioredoxin reductase and GST (Ternes et al., 2014).
Li affects the phosphoinositide signaling pathway and thus distresses neuronal function (Schafer, 2002). Alteration of the inositol polyphosphate pool can cause defects in synaptic function and plasticity (Acharya et al., 1998; de Camilli et al., 1996). Li2CO3 is a well-known sedative in psychiatric literature. Li has a role in aging, and at a moderate dose, it has a beneficial effect on longevity. Li treatment affects male mating success and female fecundity (Matsagas et al., 2009). However, chronic treatment with a low to moderate dose of lithium chloride does not alter the lifespan of D. melanogaster (Zhu et al., 2015). Affymetrix Genome Arrays from the Li-treated head of mRNA of D. melanogaster suggest an alteration of 12 genes associated with amino acid metabolism and functioning of the nervous system (Kasuya et al., 2009).
Diverse biological, chemical or physical agents that we come across in our day-to-day life may be toxic to our neurons and are referred to as neurotoxicants. The toxin released by these neurotoxicants is known as a neurotoxin. These neurotoxins, upon accumulation, can alter the functioning of the nervous system by altering the electrical and chemical transmission. The toxic effect of these neurotoxicants can be assayed using D. melanogaster as the model organism. D. melanogaster and vertebrate central and peripheral nervous systems share similarities in their functioning. Thus, the toxicity of the neurotoxicants observed using D. melanogaster cannot be neglected. The neurotoxicant effect varies with respect to age, time, and mode of administration. Different modes of administration and their effect on the metabolism of neurotoxicants are described in this paper. The deleterious effect of the neurotoxicant with respect to age and dose can be checked using different developmental stages of D. melanogaster. Innumerable well-studied behavioral assays help us to check the effect of neurotoxicant in a dose and time-dependent manner. Stress-related enzymes, which change with respect to the neurotoxicant, can be estimated by numerous biochemical assays reviewed in this paper. The effect of the neurotoxicant on the developmental pattern or timing can also be checked by comparing the developmental cycle. The neurotoxin may cause neuronal degeneration by inducing apoptosis. The apoptotic cell death can be checked easily in many tissues using easy histochemical staining methods described in this review. Change in morphology is a robust assay to detect the effect of the neurotoxicant. The morphometric analysis explained in this paper will help to check the effect of neurotoxicants on developmental stages. Thus this paper summarizes the multidimensional approaches to screening the neurotoxicity of any unknown compounds.
Acknowledgement: MM Lab is supported by SERB/EMR/2017/003054, BT/PR21857/NNT/28/1238/2017, and Odisha DBT 3325/ST(BIO)-02/2017.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: Monalisa Mishra, Figs. 1–4 drwn by Punyatoya Panda, Figs. 5–7 drawn by Bedanta Kumar Barik. The Table 1 is made with the help of Amrita Mondal, Mrutunjaya Panda help in the write up of Biochemical estimation. MM data collection, analysis and interpretation, draft manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: Not applicable.
Funding Statement: This work is not supported by any funding agency.
Conflicts of Interest: The authors declare no conflict of interest.
References
Abnoos H, Fereidoni M, Mahdavi-Shahri N, Haddad F, Jalal R (2013). Developmental study of mercury effects on the fruit fly (Drosophila melanogaster). Interdisciplinary Toxicology 6: 34–40. DOI 10.2478/intox-2013-0007. [Google Scholar] [CrossRef]
Acharya JK, Labarca P, Delgado R, Jalink K, Zuker CS (1998). Synaptic defects and compensatory regulation of inositol metabolism in inositol polyphosphate 1-phosphatase mutants. Neuron 20: 1219–1229. DOI 10.1016/S0896-6273(00)80502-4. [Google Scholar] [CrossRef]
Affleck JG, Al-Batayneh KM, Neumann K, Cole SP, Walker VK (2006a). Drosophila dihydrofolate reductase mutations confer antifolate resistance to mammalian cells. European Journal of Pharmacology 529: 71–78. DOI 10.1016/j.ejphar.2005.10.054. [Google Scholar] [CrossRef]
Affleck JG, Neumann K, Wong L, Walker VK (2006b). The effects of methotrexate on Drosophila development, female fecundity, and gene expression. Toxicological Sciences 89: 495–503. DOI 10.1093/toxsci/kfj036. [Google Scholar] [CrossRef]
Affleck JG, Walker VK (2008). A role for Drosophila in understanding drug-induced cytotoxicity and teratogenesis. Cytotechnology 57: 1–9. DOI 10.1007/s10616-008-9124-5. [Google Scholar] [CrossRef]
Al-Momani FA, Massadeh AM (2005). Effect of different heavy-metal concentrations on Drosophila melanogaster larval growth and development. Biological Trace Element Research 108: 271–277. DOI 10.1385/BTER:108:1-3:271. [Google Scholar] [CrossRef]
Alattia JR, Kuraishi T, Dimitrov M, Chang I, Lemaitre B, Fraering PC (2011). Mercury is a direct and potent γ-secretase inhibitor affecting Notch processing and development in Drosophila. The FASEB Journal 25: 2287–2295. DOI 10.1096/fj.10-174078. [Google Scholar] [CrossRef]
Ali YO, Escala W, Ruan K, Zhai RG (2011). Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. Journal of Visualized Experiments 11. DOI 10.3791/2504 JoVE. [Google Scholar] [CrossRef]
Alekseyenko OV, Chan YB, Li R, Kravitz EA (2013). Single dopaminergic neurons that modulate aggression in Drosophila. PNAS 110: 6151–6156. DOI 10.1073/pnas.1303446110. [Google Scholar] [CrossRef]
Alves-Pimenta S, Colaço B, Oliveira PA, Venâncio C (2018). Biological Concerns on the Selection of Animal Models for Teratogenic Testing, Teratogenicity Testing, pp. 61–93. Switzerland: Springer. [Google Scholar]
Aso Y, Sitaraman D, Ichinose T, Kaun KR, Vogt K et al. (2014). Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. Elife 3: e04580. DOI 10.7554/eLife.04580. [Google Scholar] [CrossRef]
Baars AJ (1980). Biotransformation of xenobiotics in Drosophila melanogaster and its relevance for mutagenicity testing. Drug Metabolism Reviews 11: 191–221. DOI 10.3109/03602538008994025. [Google Scholar] [CrossRef]
Baffet A, Benoit B, Gourhand V, Heichette C, Chretien D, Guichet A (2009). 08-P013 Mercury (Drosophila Tubulin Binding Cofactor B) controls cell polarity through the stabilisation of the microtubule network. Mechanisms of Development 126: S147–S148. DOI 10.1016/j.mod.2009.06.320. [Google Scholar] [CrossRef]
Bahrndorff S, Gertsen S, Pertoldi C, Kristensen TN (2016). Investigating thermal acclimation effects before and after a cold shock in Drosophila melanogaster using behavioural assays. Biological Journal of the Linnean Society 117: 241–251. DOI 10.1111/bij.12659. [Google Scholar] [CrossRef]
Bains JS, Shaw CA (1997). Neurodegenerative disorders in humans: The role of glutathione in oxidative stress-mediated neuronal death. Brain Research Reviews 25: 335–358. DOI 10.1016/S0165-0173(97)00045-3. [Google Scholar] [CrossRef]
Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U (2000). Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Current Biology 10: 187–194. DOI 10.1016/S0960-9822(00)00336-5. [Google Scholar] [CrossRef]
Barik BK, Mishra M (2019). Nanoparticles as a potential teratogen: A lesson learnt from fruit fly. Nanotoxicology 13: 258–284. DOI 10.1080/17435390.2018.1530393. [Google Scholar] [CrossRef]
Bayil S, Cicek H, Cimenci I, Hazar M (2008). How volatile organic compounds affect free radical and antioxidant enzyme activity in textile workers. Archives of Industrial Hygiene and Toxicology 59: 283–287. DOI 10.2478/10004-1254-59-2008-1918. [Google Scholar] [CrossRef]
Bednářová A, Kropf M, Krishnan N (2020). The surfactant polyethoxylated tallowamine (POEA) reduces lifespan and inhibits fecundity in Drosophila melanogaster-In vivo and in vitro study. Ecotoxicology and Environmental Safety 188: 109883. DOI 10.1016/j.ecoenv.2019.109883. [Google Scholar] [CrossRef]
Beltramí M, Medina-Muñoz MC, Del Pino F, Ferveur JF, Godoy-Herrera R (2012). Chemical cues influence pupation behavior of Drosophila simulans and Drosophila buzzatii in nature and in the laboratory. PLoS One 7: e39393. DOI 10.1371/journal.pone.0039393. [Google Scholar] [CrossRef]
Bianchini MC, Portela JL, Puntel RL, Ávila DS (2018). Cellular Responses in Drosophila melanogaster Following Teratogen Exposure, Teratogenicity Testing, pp. 243–276. New York, NY: Springer, Humana Press. [Google Scholar]
Berry JA, Cervantes-Sandoval I, Nicholas EP, Davis RL (2012). Dopamine is required for learning and forgetting in Drosophila. Neuron 74: 530–542. DOI 10.1016/j.neuron.2012.04.007. [Google Scholar] [CrossRef]
Bokolia NP, Mishra M (2015). Hearing molecules, mechanism and transportation: Modeled in Drosophila melanogaster. Developmental Neurobiology 75: 109–130. DOI 10.1002/dneu.22221. [Google Scholar] [CrossRef]
Bossing T, Udolph G, Doe CQ, Technau GM (1996). The embryonic central nervous system lineages of Drosophila melanogaster: I. Neuroblast lineages derived from the ventral half of the neuroectoderm. Developmental Biology 179: 41–64. DOI 10.1006/dbio.1996.0240. [Google Scholar] [CrossRef]
Bournias-Vardiabasis N, Buzin C, Flores J (1990). Differential expression of heat shock proteins in Drosophila embryonic cells following metal ion exposure. Experimental Cell Research 189: 177–182. DOI 10.1016/0014-4827(90)90233-Z. [Google Scholar] [CrossRef]
Bournias-Vardiabasis N, Teplitz RL (1982). Use of Drosophila embryo cell cultures as an in vitro teratogen assay. Teratogenesis, Carcinogenesis, and Mutagenesis 2: 333–341. DOI 10.1002/(ISSN)1520-6866. [Google Scholar] [CrossRef]
Bournias-Vardiabasis N, Teplitz RL, Chernoff GF, Seecof RL (1983). Detection of teratogens in the Drosophila embryonic cell culture test: Assay of 100 chemicals. Teratology 28: 109–122. DOI 10.1002/(ISSN)1096-9926. [Google Scholar] [CrossRef]
Brewster R, Bodmer R (1996). Cell lineage analysis of the Drosophila peripheral nervous system. Genesis 18: 50–60. DOI 10.1002/(SICI)1520-6408(1996)18:1<50::AID-DVG6>3.0.CO;2-0. [Google Scholar] [CrossRef]
Busto M, Iyengar B, Campos AR (1999). Genetic dissection of behavior: Modulation of locomotion by light in the Drosophila melanogaster larva requires genetically distinct visual system functions. Journal of Neuroscience 19: 3337–3344. DOI 10.1523/JNEUROSCI.19-09-03337.1999. [Google Scholar] [CrossRef]
Calap-Quintana P, González-Fernández J, Sebastiá-Ortega N, Llorens JV, Moltó MD (2017). Drosophila melanogaster models of metal-related human diseases and metal toxicity. International Journal of Molecular Sciences 18: 1456. DOI 10.3390/ijms18071456. [Google Scholar] [CrossRef]
Caldwell JC, Miller MM, Wing S, Soll DR, Eberl DF (2003). Dynamic analysis of larval locomotion in Drosophila chordotonal organ mutants. PNAS 100: 16053–16058. DOI 10.1073/pnas.2535546100. [Google Scholar] [CrossRef]
Carmona ER, Creus A, Marcos R (2011). Genotoxic effects of two nickel-compounds in somatic cells of Drosophila melanogaster. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 718: 33–37. DOI 10.1016/j.mrgentox.2010.10.008. [Google Scholar] [CrossRef]
Chaudhuri A, Bowling K, Funderburk C, Lawal H, Inamdar A, Wang Z, O’Donnell JM (2007). Interaction of genetic and environmental factors in a Drosophila parkinsonism model. Journal of Neurosci 27: 2457–2467. DOI 10.1523/JNEUROSCI.4239-06.2007. [Google Scholar] [CrossRef]
Chung H, Sztal T, Pasricha S, Sridhar M, Batterham P, Daborn PJ (2009). Characterization of Drosophila melanogaster cytochrome P450 genes. PNAS 106: 5731–5736. DOI 10.1073/pnas.0812141106. [Google Scholar] [CrossRef]
Cohan FM, Hoffmann AA (1986). Genetic divergence under uniform selection. II. Different responses to selection for knockdown resistance to ethanol among Drosophila melanogaster populations and their replicate lines. Genetics 114: 145–164. DOI 10.1093/genetics/114.1.145. [Google Scholar] [CrossRef]
Coyle I, Wayner M, Singer G (1976). Behavioral teratogenesis: A critical evaluation. Pharmacology Biochemistry and Behavior 4: 191–200. DOI 10.1016/0091-3057(76)90014-9. [Google Scholar] [CrossRef]
Cunha-Oliveira T, Rego AC, Oliveira CR (2008). Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain Research Reviews 58: 192–208. [Google Scholar]
Cui G, Yuan H, Jiang Z, Zhang J, Sun Z, Zhong G (2020). Natural harmine negatively regulates the developmental signaling network of Drosophila melanogaster (Drosophilidae: Diptera) in vivo. Ecotoxicology and Environmental Safety 190: 110134. DOI 10.1016/j.ecoenv.2019.110134. [Google Scholar] [CrossRef]
Dasari S, Ganjayi MS, Meriga B (2018). Glutathione S-transferase is a good biomarker in acrylamide induced neurotoxicity and genotoxicity. Interdisciplinary Toxicology 11: 115–121. DOI 10.2478/intox-2018-0007. [Google Scholar] [CrossRef]
Daston GP (2011). Laboratory models and their role in assessing teratogenesis. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. Wiley Online Library 157: 183–187. DOI 10.1002/ajmg.c.30312. [Google Scholar] [CrossRef]
Davis RL (2005). Olfactory memory formation in Drosophila: From molecular to systems neuroscience. Annual Review of Neuroscience 28: 275–302. DOI 10.1146/annurev.neuro.28.061604.135651. [Google Scholar] [CrossRef]
de Camilli P, Emr SD, McPherson PS, Novick P (1996). Phosphoinositides as regulators in membrane traffic. Science 271: 1533–1538. DOI 10.1126/science.271.5255.1533. [Google Scholar] [CrossRef]
de Morais CR, Carvalho SM, Naves MPC, Araujo G, de Rezende AAA, Bonetti AM, Spanó MA (2017). Mutagenic, recombinogenic and carcinogenic potential of thiamethoxam insecticide and formulated product in somatic cells of Drosophila melanogaster. Chemosphere 187: 163–172. DOI 10.1016/j.chemosphere.2017.08.108. [Google Scholar] [CrossRef]
Dhar G, Bag J, Mishra M (2020a). Environmental cue affects the hearing-related behaviors of Drosophila melanogaster by targeting the redox pathways. Environmental Science and Pollution Research 27: 1–14. DOI 10.1007/s11356-020-09141-0. [Google Scholar] [CrossRef]
Dhar G, Mukherjee S, Nayak N, Sahu S, Bag J, Rout R, Mishra M (2020b). Various Behavioural Assays to Detect the Neuronal Abnormality in Flies, Fundamental Approaches to Screen Abnormalities in Drosophila, pp. 223–251. Springer Nature Switzerland: Springer. [Google Scholar]
Diamond I, Gordon AS (1997). Cellular and molecular neuroscience of alcoholism. Physiological Reviews 77: 1–20. DOI 10.1152/physrev.1997.77.1.1. [Google Scholar] [CrossRef]
Ding L, Wang Y (2006). Effect of copper on the development, protein and esterase isozymes of Drosophila melanogaster. Integrative Zoology 1: 73–77. DOI 10.1111/j.1749-4877.2006.00017.x. [Google Scholar] [CrossRef]
Dokucu ME, Yu L, Taghert PH (2005). Lithium-and valproate-induced alterations in circadian locomotor behavior in Drosophila. Neuropsychopharmacology 30: 2216–2224. DOI 10.1038/sj.npp.1300764. [Google Scholar] [CrossRef]
Dzitoyeva S, Dimitrijevic N, Manev H (2003). γ-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: Adult RNA interference and pharmacological evidence. PNAS 100: 5485–5490. DOI 10.1073/pnas.0830111100. [Google Scholar] [CrossRef]
Ellman G, Courtney K, Andres Jr V, Featherstone RM (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology 7: 88–90, IN1, 91–95. DOI 10.1016/0006-2952(61)90145-9. [Google Scholar] [CrossRef]
Engel G, Delwig A, Rand M (2012). The effects of methylmercury on Notch signaling during embryonic neural development in Drosophila melanogaster. Toxicology in Vitro 26: 485–492. DOI 10.1016/j.tiv.2011.12.014. [Google Scholar] [CrossRef]
Feany MB, Bender WW (2000). A Drosophila model of Parkinson’s disease. Nature 404: 394–398. DOI 10.1038/35006074. [Google Scholar] [CrossRef]
Fedele G, Green EW, Rosato E, Kyriacou CP (2014). An electromagnetic field disrupts negative geotaxis in Drosophila via a CRY-dependent pathway. Nature Communications 5: 1–6. DOI 10.1038/ncomms5391. [Google Scholar] [CrossRef]
Ferdenache M, Bezzar-Bendjazia R, Marion-Poll F, Kilani-Morakchi S (2019). Transgenerational effects from single larval exposure to azadirachtin on life history and behavior traits of Drosophila melanogaster. Scientific Reports 9: 1–12. DOI 10.1038/s41598-019-53474-x. [Google Scholar] [CrossRef]
Fernandez RW, Nurilov M, Feliciano O, McDonald IS, Simon AF (2014). Straightforward assay for quantification of social avoidance in Drosophila melanogaster. JoVE Journal 94: e52011. DOI 10.3791/52011. [Google Scholar] [CrossRef]
Finetti L, Ferrari F, Caló G, Cassanelli S, de Bastiani M, Civolani S, Bernacchia G (2020). Modulation of Drosophila suzukii type 1 tyramine receptor (DsTAR1) by monoterpenes: A potential new target for next generation biopesticides. Pestic Biochem Physiol 165: 104549. DOI 10.1016/j.pestbp.2020.02.015. [Google Scholar] [CrossRef]
Flora S, Mittal M, Mehta A (2008). Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian Journal of Medical Research 128: 501–523. [Google Scholar]
Gayathri D, Harini B (2012). Adverse effect of valproic acid on mating behaviour and fertility in Drosophila melanogaster. Bioscan 7: 31–34. [Google Scholar]
Gayathri D, Harini B (2013). Effect of phenytoin on development and life-history traits of Drosophila melanogaster. Current Science: 508–514. [Google Scholar]
Géminard C, Rulifson EJ, Léopold P (2009). Remote control of insulin secretion by fat cells in Drosophila. Cell Metabolism 10: 199–207. DOI 10.1016/j.cmet.2009.08.002. [Google Scholar] [CrossRef]
Gerber B, Scherer S, Neuser K, Michels B, Hendel T, Stocker RF, Heisenberg M (2004). Visual learning in individually assayed Drosophila larvae. Journal of Experimental Biology 207: 179–188. DOI 10.1242/jeb.00718. [Google Scholar] [CrossRef]
Grover D, Ford D, Brown C, Hoe N, Erdem A, Tavaré S, Tower J (2009). Hydrogen peroxide stimulates activity and alters behavior in Drosophila melanogaster. PLoS One 4: e7580. DOI 10.1371/journal.pone.0007580. [Google Scholar] [CrossRef]
Gundelfinger ED (1992). How complex is the nicotinic receptor system of insects? Trends in Neurosciences 15: 206–211. DOI 10.1016/0166-2236(92)90035-7. [Google Scholar] [CrossRef]
Guengerich FP (2001). Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chemical Research in Toxicology 14: 611–650. DOI 10.1021/tx0002583. [Google Scholar] [CrossRef]
Guo F, Yu J, Jung HJ, Abruzzi KC, Luo W, Griffith LC, Rosbash M (2016). Circadian neuron feedback controls the Drosophila sleep-activity profile. Nature 536: 292–297. DOI 10.1038/nature19097. [Google Scholar] [CrossRef]
Hagen KGT, Zhang L, Tian E, Zhang Y (2008). Glycobiology on the fly: Developmental and mechanistic insights from Drosophila. Glycobiology 19: 102–111. [Google Scholar]
Hardie RC (2012). Phototransduction mechanisms in Drosophila microvillar photoreceptors. Wiley Interdisciplinary Reviews: Membrane Transport and Signaling 1: 162–187. DOI 10.1002/wmts.20. [Google Scholar] [CrossRef]
Harini B (2016). Climbing responses of few species of Drosophila on exposure to different aniti epileptic drugs. International Journal of Current Research 8: 25075–25079. [Google Scholar]
Hawkins CL, Morgan PE, Davies MJ (2009). Quantification of protein modification by oxidants. Free Radical Biology and Medicine 46: 965–988. DOI 10.1016/j.freeradbiomed.2009.01.007. [Google Scholar] [CrossRef]
Haynes PR, Christmann BL, Griffith LC (2015). A single pair of neurons links sleep to memory consolidation in Drosophila melanogaster. Elife 4: e03868. DOI 10.7554/eLife.03868. [Google Scholar] [CrossRef]
Heberlein U, Tsai LTY, Kapfhamer D, Lasek AW (2009). Drosophila, a genetic model system to study cocaine-related behaviors: A review with focus on LIM-only proteins. Neuropharmacology 56: 97–106. DOI 10.1016/j.neuropharm.2008.07.023. [Google Scholar] [CrossRef]
Heikkila RE, Kindt MV, Sonsalla PK, Giovanni A, Youngster SK, McKeown KA, Singer TP (1988). Importance of monoamine oxidase A in the bioactivation of neurotoxic analogs of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. PNAS 85: 6172–6176. DOI 10.1073/pnas.85.16.6172. [Google Scholar] [CrossRef]
Heimbeck G, Bugnon V, Gendre N, Häberlin C, Stocker RF (1999). Smell and taste perception in Drosophila melanogaster larva: Toxin expression studies in chemosensory neurons. Journal of Neuroscience 19: 6599–6609. DOI 10.1523/JNEUROSCI.19-15-06599.1999. [Google Scholar] [CrossRef]
Hepburn DD, Xiao J, Bindom S, Vincent JB, O’Donnell J (2003). Nutritional supplement chromium picolinate causes sterility and lethal mutations in Drosophila melanogaster. PNAS 100: 3766–3771. DOI 10.1073/pnas.0636646100. [Google Scholar] [CrossRef]
Hirsch HV, Lnenicka G, Possidente D, Possidente B, Garfinkel MD, Wang L, Lu X, Ruden DM (2012). Drosophila melanogaster as a model for lead neurotoxicology and toxicogenomics research. Frontiers in Genetics 3: 68. DOI 10.3389/fgene.2012.00068. [Google Scholar] [CrossRef]
Ho MW, Stone TA, Jerman I, Bolton J, Bolton H, Goodwin B, Saunders P, Robertson F (1992). Brief exposures to weak static magnetic field during early embryogenesis cause cuticular pattern abnormalities in Drosophila larvae. Physics in Medicine and Biology 37: 1171–1179. DOI 10.1088/0031-9155/37/5/011. [Google Scholar] [CrossRef]
Hoang TC, Rand GM (2015). Acute toxicity and risk assessment of permethrin, naled, and dichlorvos to larval butterflies via ingestion of contaminated foliage. Chemosphere 120: 714–721. DOI 10.1016/j.chemosphere.2014.10.040. [Google Scholar] [CrossRef]
Hoffmann AA, Harshman LG (1999). Desiccation and starvation resistance in Drosophila: Patterns of variation at the species, population and intrapopulation levels. Heredity 83: 637–643. DOI 10.1046/j.1365-2540.1999.00649.x. [Google Scholar] [CrossRef]
Huang J, Liu W, Qi YX, Luo J, Montell C (2016). Neuromodulation of courtship drive through tyramine-responsive neurons in the Drosophila brain. Current Biology 26: 2246–2256. [Google Scholar]
Inagaki HK, Kamikouchi A, Ito K (2010). Methods for quantifying simple gravity sensing in Drosophila melanogaster. Nature Protocols 5: 20–25. DOI 10.1038/nprot.2009.196. [Google Scholar] [CrossRef]
Inamdar AA, Bennett JW (2014). A common fungal volatile organic compound induces a nitric oxide mediated inflammatory response in Drosophila melanogaster. Scientific Reports 4: 1–9. DOI 10.1093/toxsci/kfq222. [Google Scholar] [CrossRef]
Inamdar AA, Masurekar P, Bennett JW (2010). Neurotoxicity of fungal volatile organic compounds in Drosophila melanogaster. Toxicological Sciences 117: 418–426. DOI 10.1093/toxsci/kfq222. [Google Scholar] [CrossRef]
Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S (2007). Prandiology of Drosophila and the CAFE assay. PNAS 104: 8253–8256. DOI 10.1073/pnas.0702726104. [Google Scholar] [CrossRef]
Jin M, Guan CB, Jiang YA, Chen G, Zhao CT, Cui K, Song YQ, Wu CP, Poo MM, Yuan XB (2005). Ca2+-dependent regulation of Rho GTPases triggers turning of nerve growth cones. Journal of Neuroscience 25: 2338–2347. DOI 10.1523/JNEUROSCI.4889-04.2005. [Google Scholar] [CrossRef]
Jollow D, Mitchell J, Zampaglione Na, Gillette J (1974). Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11: 151–169. DOI 10.1159/000136485. [Google Scholar] [CrossRef]
Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Göpfert MC, Ito K (2009). The neural basis of Drosophila gravity-sensing and hearing. Nature 458: 165–171. DOI 10.1038/nature07810. [Google Scholar] [CrossRef]
Kasuya J, Kaas G, Kitamoto T (2009). Effects of lithium chloride on the gene expression profiles in Drosophila heads. Neuroscience Research 64: 413–420. DOI 10.1016/j.neures.2009.04.015. [Google Scholar] [CrossRef]
Katz AJ, Chiu A, Beaubier J, Shi X (2001). Combining Drosophila melanogaster somatic-mutation-recombination and electron-spin-resonance-spectroscopy data to interpret epidemiologic observations on chromium carcinogenicity. Molecular and Cellular Biochemistry 222: 61–68. DOI 10.1023/A:1017959222379. [Google Scholar] [CrossRef]
Kaur K, Simon AF, Chauhan V, Chauhan A (2015). Effect of bisphenol A on Drosophila melanogaster behavior—A new model for the studies on neurodevelopmental disorders. Behavioural Brain Research 284: 77–84. DOI 10.1016/j.bbr.2015.02.001. [Google Scholar] [CrossRef]
Kaya B, Kocaoğlu S, Demir E (2006). Analysis of UV-stimulated recombination in the Drosophila SMART assay. Environmental and Molecular Mutagenesis 47: 357–361. DOI 10.1002/(ISSN)1098-2280. [Google Scholar] [CrossRef]
Kijak E, Rosato E, Knapczyk K, Pyza E (2014). Drosophila melanogaster as a model system of aluminum toxicity and aging. Insect Science 21: 189–202. DOI 10.1111/1744-7917.12017. [Google Scholar] [CrossRef]
Kim H, Choi MS, Kang K, Kwon JY (2015). Behavioral analysis of bitter taste perception in Drosophila larvae. Chemical Senses 41: 85–94. DOI 10.1093/chemse/bjv061. [Google Scholar] [CrossRef]
Kissoum N, Bensafi-Gheraibia H, Hamida Z, Soltani N (2020). Evaluation of the pesticide Oberon on a model organism Drosophila melanogaster via topical toxicity test on biochemical and reproductive parameters. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 228: 108666. DOI 10.1016/j.cbpc.2019.108666. [Google Scholar] [CrossRef]
Kocatürk S, Kocatürk P, Kayas GÖ, Mutluer N (1996). Antioxidant defence system in a patient with cerebrovascular accident. Journal of International Medical Research 24: 376–380. DOI 10.1177/030006059602400410. [Google Scholar] [CrossRef]
Le Bourg É., Buecher C (2002). Learned suppression of photopositive tendencies in Drosophila melanogaster. Learning & Behavior 30: 330–341. DOI 10.3758/BF03195958. [Google Scholar] [CrossRef]
Li D, Bi X (2018). Effect of Teratogens on Development of Drosophila melanogaster, Teratogenicity Testing, pp. 233–241. Springer. DOI 10.1007/978-1-4939-7883-0_12. [Google Scholar] [CrossRef]
Lilly M, Carlson J (1990). Smellblind: A gene required for Drosophila olfaction. Genetics 124: 293–302. DOI 10.1093/genetics/124.2.293. [Google Scholar] [CrossRef]
Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, Kim C, Welsh MJ (2007). Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature 450: 294–298. DOI 10.1038/nature06223. [Google Scholar] [CrossRef]
Liu L, Yermolaieva O, Johnson WA, Abboud FM, Welsh MJ (2003). Identification and function of thermosensory neurons in Drosophila larvae. Nature Neuroscience 6: 267–273. DOI 10.1038/nn1009. [Google Scholar] [CrossRef]
Liu C, Meng Z, Wiggin TD, Yu J, Reed ML, Guo F, Zhang Y, Rosbash M, Griffith LC (2019). A serotonin-modulated circuit controls sleep architecture to regulate cognitive function independent of total sleep in Drosophila. Current Biology 29: 3635–3646.e5. DOI 10.1016/j.cub.2019.08.079. [Google Scholar] [CrossRef]
Logan-Garbisch T, Bortolazzo A, Luu P, Ford A, Do D, Khodabakhshi P, French RL (2015). Developmental ethanol exposure leads to dysregulation of lipid metabolism and oxidative stress in Drosophila. G3: Genes, Genomes, Genetics 5: 49–59. DOI 10.1534/g3.114.015040. [Google Scholar] [CrossRef]
Lohmann C, Bonhoeffer T (2008). A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron 59: 253–260. DOI 10.1016/j.neuron.2008.05.025. [Google Scholar] [CrossRef]
Lozinsky OV, Lushchak OV, Storey JM, Storey KB, Lushchak VI (2012). Sodium nitroprusside toxicity in Drosophila melanogaster: Delayed pupation, reduced adult emergence, and induced oxidative/nitrosative stress in eclosed flies. Archives of Insect Biochemistry and Physiology 80: 166–185. DOI 10.1002/arch.21033. [Google Scholar] [CrossRef]
Lozinsky OV, Lushchak V, Kryshchuk NI, Shchypanska NY, Riabkina AH, Skarbek SV, Maksymiv IV, Storey JM, Storey KB, Lushchak VI (2013). S-nitrosoglutathione-induced toxicity in Drosophila melanogaster: Delayed pupation and induced mild oxidative/nitrosative stress in eclosed flies. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 164: 162–170. DOI 10.1016/j.cbpa.2012.08.006. [Google Scholar] [CrossRef]
Luna AJF, von Essen AM, Widmer YF, Sprecher SG (2013). Light preference assay to study innate and circadian regulated photobehavior in Drosophila larvae. Journal of Visualized Experiments 20: e50237. [Google Scholar]
Lynch DW, Schuler RL, Hood RD, Davis DG (1991). Evaluation of Drosophila for screening developmental toxicants: test results with eighteen chemicals and presentation of a new Drosophila bioassay. Teratogenesis, Carcinogenesis, and Mutagenesis 11: 147–173. DOI 10.1002/(ISSN)1520-6866. [Google Scholar] [CrossRef]
Markow TA, Gottesman II (1993). Behavioral phenodeviance: A Lerneresque conjecture. Genetica 89: 297–305. DOI 10.1007/BF02424522. [Google Scholar] [CrossRef]
Matsagas K, Lim DB, Horwitz M, Rizza CL, Mueller LD, Villeponteau B, Rose MR (2009). Long-term functional side-effects of stimulants and sedatives in Drosophila melanogaster. PLoS One 4: e6578. DOI 10.1371/journal.pone.0006578. [Google Scholar] [CrossRef]
Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ (2009). Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila. Journal of Neuroscience 29: 3538–3550. DOI 10.1523/JNEUROSCI.5564-08.2009. [Google Scholar] [CrossRef]
McClung C, Hirsh J (1998). Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Current Biology 8: 109–112. DOI 10.1016/S0960-9822(98)70041-7. [Google Scholar] [CrossRef]
McClure KD, French RL, Heberlein U (2011). A Drosophila model for fetal alcohol syndrome disorders: Role for the insulin pathway. Disease Models & Mechanisms 4: 335–346. DOI 10.1242/dmm.006411. [Google Scholar] [CrossRef]
McEwen CM (1965). Human plasma monoamine oxidase II. Kinetic studies. Journal of Biological Chemistry 240: 2011–2018. DOI 10.1016/S0021-9258(18)97418-1. [Google Scholar] [CrossRef]
McKemy DD (2007). Temperature sensing across species. Pflügers Archiv-European Journal of Physiology 454: 777–791. DOI 10.1007/s00424-006-0199-6. [Google Scholar] [CrossRef]
Mellerick DM, Liu H (2004). Methanol exposure interferes with morphological cell movements in the Drosophila embryo and causes increased apoptosis in the CNS. Developmental Neurobiology 60: 308–318. DOI 10.1002/(ISSN)1097-4695. [Google Scholar] [CrossRef]
Min VA, Condron BG (2005). An assay of behavioral plasticity in Drosophila larvae. Journal of Neuroscience Methods 145: 63–72. DOI 10.1016/j.jneumeth.2004.11.022. [Google Scholar] [CrossRef]
Misra JR, Horner MA, Lam G, Thummel CS (2011). Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes & Development 25: 1796–1806. DOI 10.1101/gad.17280911. [Google Scholar] [CrossRef]
Mishra M, Acharya UR (2004). Protective action of vitamins on the spermatogenesis in lead-treated Swiss mice. Journal of Trace Elements in Medicine and Biology 18: 173–178. DOI 10.1016/j.jtemb.2004.03.007. [Google Scholar] [CrossRef]
Mishra M, Barik BK (2018). Behavioral Teratogenesis in Drosophila melanogaster, Teratogenicity Testing, pp. 277–298. New York: Springer. [Google Scholar]
Mishra M, Oke A, Lebel C, McDonald EC, Plummer Z, Cook TA, Zelhof AC (2010). Pph13 and orthodenticle define a dual regulatory pathway for photoreceptor cell morphogenesis and function. Development 137: 2895–2904. DOI 10.1242/dev.051722. [Google Scholar] [CrossRef]
Montell C (2009). A taste of the Drosophila gustatory receptors. Current Opinion in Neurobiology 19: 345–353. DOI 10.1016/j.conb.2009.07.001. [Google Scholar] [CrossRef]
Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U (1998). Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell 93: 997–1007. DOI 10.1016/s0092-8674(00)81205-2. [Google Scholar] [CrossRef]
Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G (2010). Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1802: 2–10. DOI 10.1016/j.bbadis.2009.10.006. [Google Scholar] [CrossRef]
Nicholas CD, Becnel J, Pandey UB (2012). Methods to assay Drosophila behavior. JoVE Journal 7: e3795. DOI 10.3791/3795 (2012). [Google Scholar]
Ogawa HI, Shibahara T, Iwata H, Okada T, Tsuruta S, Kakimoto K, Sakata K, Kato Y, Ryo H, Itoh T (1994). Genotoxic activities in vivo of cobaltous chloride and other metal chlorides as assayed in the Drosophila wing spot test. Mutation Research/Genetic Toxicology 320: 133–140. DOI 10.1016/0165-1218(94)90065-5. [Google Scholar] [CrossRef]
Oyeniran, Olubukola H, Adedayo O, Ademiluyi, Ganiyu Oboh (2021). Comparative study of the phenolic profile, antioxidant properties, and inhibitory effects of Moringa (Moringa oleifera Lam.) and Almond (Terminalia catappa Linn.) leaves on acetylcholinesterase and monoamine oxidase activities in the head region of Fruitfly (Drosophila melanogaster Meigen) in vitro. Journal of Food Biochemistry 45: e13401. DOI 10.1111/jfbc.13401. [Google Scholar] [CrossRef]
Oyeleye SI, Ogunsuyi OB, Adedeji V, Olatunde D, Oboh G (2021). Citrus spp. essential oils improve behavioural pattern, repressed cholinesterases and monoamine oxidase activities, and production of reactive species in fruit fly (Drosophila melanogaster) model of Alzheimer’s Disease. Journal of Food Biochemistry 45: e13558. DOI 10.1111/jfbc.13558. [Google Scholar] [CrossRef]
Pandey UB, Nicholas CD (2011). Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacological Reviews 63: 411–436. DOI 10.1124/pr.110.003293. [Google Scholar] [CrossRef]
Panonnummal R, Kumar VS, Jayakumar R, Sabitha M (2021). Application of chitosan and its derivatives in transdermal drug delivery. In: Chitosan for Biomaterials IV, pp. 411–446. Switzerland: Springer Cham. [Google Scholar]
Pappus SA, Mishra M (2018). A Drosophila model to decipher the toxicity of nanoparticles taken through oral routes. In: Cellular and Molecular Toxicology of Nanoparticles, pp. 311–322. [Google Scholar]
Parigi A, Porter C, Cermak M, Pitchers WR, Dworkin I (2019). The behavioral repertoire of Drosophila melanogaster in the presence of two predator species that differ in hunting mode. PLoS One 14: e0216860. DOI 10.1371/journal.pone.0216860. [Google Scholar] [CrossRef]
Pendleton RG, Rasheed A, Hillman R (2000). Effects of adrenergic agents on locomotor behavior and reproductive development in Drosophila. Drug Development Research 50: 142–146. DOI 10.1002/(ISSN)1098-2299. [Google Scholar] [CrossRef]
Pereira AM, Tudor C, Kanger JS, Subramaniam V, Martin-Blanco E (2011). Integrin-dependent activation of the JNK signaling pathway by mechanical stress. PLoS One 6: e26182. DOI 10.1371/journal.pone.0026182. [Google Scholar] [CrossRef]
Pereira HS, MacDonald DE, Hilliker AJ, Sokolowski MB (1995). Chaser (Csra new gene affecting larval foraging behavior in Drosophila melanogaster. Genetics 141: 263–270. DOI 10.1093/genetics/141.1.263. [Google Scholar] [CrossRef]
Piccoli BC, Segatto ALA, Oliveira CS, da Silva FDA, Aschner M, da Rocha JBT (2019). Simultaneous exposure to vinylcyclohexene and methylmercury in Drosophila melanogaster: Biochemical and molecular analyses. BMC Pharmacology and Toxicology 20: 83. DOI 10.1186/s40360-019-0356-0. [Google Scholar] [CrossRef]
Podratz JL, Staff NP, Froemel D, Wallner A, Wabnig F, Bieber AJ, Tang A, Windebank AJ (2011). Drosophila melanogaster: A new model to study cisplatin-induced neurotoxicity. Neurobiology of Disease 43: 330–337. DOI 10.1016/j.nbd.2011.03.022. [Google Scholar] [CrossRef]
Posser T, Franco JL, Bobrovskaya L, Leal RB, Dickson PW, Dunkley PR (2009). Manganese induces sustained Ser40 phosphorylation and activation of tyrosine hydroxylase in PC12 cells. Journal of Neurochemistry 110: 848–856. DOI 10.1111/j.1471-4159.2009.06185.x. [Google Scholar] [CrossRef]
Pratt KG, Watt AJ, Griffith LC, Nelson SB, Turrigiano GG (2003). Activity-dependent remodeling of presynaptic inputs by postsynaptic expression of activated CaMKII. Neuron 39: 269–281. DOI 10.1016/S0896-6273(03)00422-7. [Google Scholar] [CrossRef]
Priyadarsini S, Mukherjee S, Mishra M (2020). Methodology to Detect the Abnormality of Drosophila Gut by Various Staining Techniques, Fundamental Approaches to Screen Abnormalities in Drosophila, pp. 51–64. New York: Springer. [Google Scholar]
Rajak P, Khatun S, Dutta M, Mandi M, Roy S (2018). Chronic exposure to acephate triggers ROS-mediated injuries at organismal and sub-organismal levels of Drosophila melanogaster. Toxicology Research 7: 874–887. DOI 10.1039/C8TX00052B. [Google Scholar] [CrossRef]
Ramirez E, Monteagudo JL, Garcia-Gracia M, Delgado JM (1983). Oviposition and development of Drosophila modified by magnetic fields. Bioelectromagnetics 4: 315–326. DOI 10.1002/(ISSN)1521-186X. [Google Scholar] [CrossRef]
Rand MD (2010). Drosophotoxicology: The growing potential for Drosophila in neurotoxicology. Neurotoxicology and Teratology 32: 74–83. DOI 10.1016/j.ntt.2009.06.004. [Google Scholar] [CrossRef]
Rand MD, Dao JC, Clason TA (2009). Methylmercury disruption of embryonic neural development in Drosophila. Neurotoxicology 30: 794–802. DOI 10.1016/j.neuro.2009.04.006. [Google Scholar] [CrossRef]
Rand MD, Kearney AL, Dao J, Clason T (2010). Permeabilization of Drosophila embryos for introduction of small molecules. Insect Biochemistry and Molecular Biology 40: 792–804. DOI 10.1016/j.ibmb.2010.07.007. [Google Scholar] [CrossRef]
Rand MD, Montgomery SL, Prince L, Vorojeikina D (2014). Developmental toxicity assays using the Drosophila model. Current Protocols in Toxicology 59: 1.12.1–1.12.20. DOI 10.1002/0471140856.tx0112s59. [Google Scholar] [CrossRef]
Restifo LL, White K (1990). Molecular and genetic approaches to neurotransmitter and neuromodulator systems in Drosophila. Advances in Insect Physiology 22: 115–219. DOI 10.1016/S0065-2806(08)60006-5. [Google Scholar] [CrossRef]
Rezával C, Nojima T, Neville MC, Lin AC, Goodwin SF (2014). Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Current Biology 24: 725–730. [Google Scholar]
Roelofs J, van Haastert PJ (2001). Genes lost during evolution. Nature 411: 1013–1014. DOI 10.1038/35082627. [Google Scholar] [CrossRef]
Sabat D, Johnson E, Abhinay A, Jayabalan R, Mishra M (2015). A protocol to generate germ free Drosophila for microbial interaction studies. Advanced Techniques in Biology & Medicine 1: 1764–2379. DOI 10.1002/cptx.52. [Google Scholar] [CrossRef]
Sadler T, Shum L, Warner C, Smith MK (1988). The role of pharmacokinetics in determining the response of rodent embryos to teratogens in whole-embryo culture. Toxicology in Vitro 2: 175–180. DOI 10.1016/0887-2333(88)90005-7. [Google Scholar] [CrossRef]
Sahu S, Dhar G, Mishra M (2020). Methods to Detect the Complex Behaviours in Drosophila, Fundamental Approaches to Screen Abnormalities in Drosophila, pp. 253–265. New York: Springer. [Google Scholar]
Sahu S, Mishra M (2020a). Hydroxyapatite nanoparticle causes sensory organ defects by targeting the retromer complex in Drosophila melanogaster. NanoImpact 19: 100237. DOI 10.1016/j.impact.2020.100237. [Google Scholar] [CrossRef]
Sahu S, Mishra M (2020b). Simple Histochemical Methods to Detect Cell Death in the Eye-Antennae Imaginal Disc of Drosophila, Fundamental Approaches to Screen Abnormalities in Drosophila, pp. 77–86. New York: Springer. [Google Scholar]
Sahu S, Jaysingh P, Mishra M (2022). As an in vivo model for the investigation of Host-Microbiota interaction. In: Prebiotics, Probiotics and Nutraceuticals, pp. 275–300. Singapore: Springer. [Google Scholar]
Saini N, Schaffner W (2010). Zinc supplement greatly improves the condition of parkin mutant Drosophila. Biological Chemistry 391: 513–518. DOI 10.1515/bc.2010.052. [Google Scholar] [CrossRef]
Sameoto D, Miller RS (1968). Selection of pupation site by Drosophila melanogaster and D. simulans. Ecology 49: 177–180. DOI 10.2307/1933580. [Google Scholar] [CrossRef]
Saraswati S, Fox LE, Soll DR, Wu CF (2004). Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. Journal of Neurobiology 58: 425–441. DOI 10.1002/(ISSN)1097-4695. [Google Scholar] [CrossRef]
Schafer WR (2002). Neuropsychopharmacology of worms and flies. Neuropsychopharmacology: The Fifth Generation of Progress. American College of Neuropsychopharmacology. [Google Scholar]
Schmid A, Chiba A, Doe CQ (1999). Clonal analysis of Drosophila embryonic neuroblasts: Neural cell types, axon projections and muscle targets. Development 126: 4653–4689. DOI 10.1242/dev.126.21.4653. [Google Scholar] [CrossRef]
Scholl A, Ndoja I, Jiang L (2021). Drosophila trachea as a novel model of COPD. International Journal of Molecular Sciences 22: 12730. DOI 10.3390/ijms222312730. [Google Scholar] [CrossRef]
Schuler RL, Hardin BD, Niemeier RW (1982). Drosophila as a tool for the rapid assessment of chemicals for teratogenicity. Teratogenesis, Carcinogenesis, and Mutagenesis 2: 293–301. DOI 10.1002/(ISSN)1520-6866. [Google Scholar] [CrossRef]
Scheunemann L, Plaçais PY, Dromard Y, Schwärzel M, Preat T (2018). Dunce phosphodiesterase acts as a checkpoint for Drosophila long-term memory in a pair of serotonergic neurons. Neuron 98: 350–365. DOI 10.1016/j.neuron.2018.03.032. [Google Scholar] [CrossRef]
Senthilkumar S, Raveendran R, Madhusoodhan S, Sundar M, Shankar S, Sharma S, Sundararajan V, Dan P, Mohideen SS (2020). Developmental and behavioural toxicity induced by acrylamide exposure and amelioration using phytochemicals in Drosophila melanogaster. Journal of Hazardous Materials 394: 122533. DOI 10.1016/j.jhazmat.2020.122533. [Google Scholar] [CrossRef]
Seugnet L, Suzuki Y, Stidd R, Shaw P (2009). Aversive phototaxic suppression: Evaluation of a short-term memory assay in Drosophila melanogaster. Genes, Brain and Behavior 8: 377–389. DOI 10.1111/j.1601-183X.2009.00483.x. [Google Scholar] [CrossRef]
Shakya B, Siddique YH (2018). Exploring the neurotoxicity and changes in life cycle parameters of Drosophila melanogaster exposed to arecoline. The Journal of Basic and Applied Zoology 79: 1–11. DOI 10.1186/s41936-018-0057-z. [Google Scholar] [CrossRef]
Shaltiel-Karyo R, Davidi D, Menuchin Y, Frenkel-Pinter M, Marcus-Kalish M, Ringo J, Gazit E, Segal D (2012). A novel, sensitive assay for behavioral defects in Parkinson’s disease model Drosophila. Parkinson’s Disease 2012: 1–6. DOI 10.1155/2012/697564. [Google Scholar] [CrossRef]
Shaltiel-Karyo R, Frenkel-Pinter M, Egoz-Matia N, Frydman-Marom A, Shalev DE, Segal D, Gazit E (2010). Inhibiting α-synuclein oligomerization by stable cell-penetrating β-synuclein fragments recovers phenotype of Parkinson’s disease model flies. PLoS One 5: e13863. DOI 10.1371/journal.pone.0013863. [Google Scholar] [CrossRef]
Sharma A (2004). Procedure for Screening of Neuroactive Substance and the Associated Neural Plasticity. US Patent, Google Patents. [Google Scholar]
Sharma A, Mishra M, Shukla A, Kumar R, Abdin M, Chowdhuri DK (2012). Organochlorine pesticide, endosulfan induced cellular and organismal response in Drosophila melanogaster. Journal of Hazardous Materials 221: 275–287. DOI 10.1016/j.jhazmat.2012.04.045. [Google Scholar] [CrossRef]
Sharma B, Singh S, Siddiqi NJ (2014). Biomedical implications of heavy metals induced imbalances in redox systems. BioMed Research International. [Google Scholar]
Shaver S, Varnam C, Hilliker A, Sokolowski M (1998). The foraging gene affects adult but not larval olfactory-related behavior in Drosophila melanogaster. Behavioural Brain Research 95: 23–29. DOI 10.1016/S0166-4328(97)00206-4. [Google Scholar] [CrossRef]
Siddique YH, Naz F, Jyoti S (2018). Effect of capsaicin on the oxidative stress and dopamine content in the transgenic Drosophila model of Parkinson’s disease. Acta Biologica Hungarica 69: 115–124. DOI 10.1556/018.69.2018.2.1. [Google Scholar] [CrossRef]
Sitaraman D, Aso Y, Jin X, Chen N, Felix M, Rubin GM, Nitabach MN (2015). Propagation of homeostatic sleep signals by segregated synaptic microcircuits of the Drosophila mushroom body. Current Biology 25: 2915–2927. DOI 10.1016/j.cub.2015.09.017. [Google Scholar] [CrossRef]
Silva B, Goles NI, Varas R, Campusano JM (2014). Serotonin receptors expressed in Drosophila mushroom bodies differentially modulate larval locomotion. PLoS One 9: e89641. DOI 10.1371/journal.pone.0089641. [Google Scholar] [CrossRef]
Singh MP, Reddy MK, Mathur N, Saxena D, Chowdhuri DK (2009). Induction of hsp70, hsp60, hsp83 and hsp26 and oxidative stress markers in benzene, toluene and xylene exposed Drosophila melanogaster: Role of ROS generation. Toxicology and Applied Pharmacology 235: 226–243. DOI 10.1016/j.taap.2008.12.002. [Google Scholar] [CrossRef]
Sokolowski MB, Pereira HS, Hughes K (1997). Evolution of foraging behavior in Drosophila by density-dependent selection. PNAS 94: 7373–7377. DOI 10.1073/pnas.94.14.7373. [Google Scholar] [CrossRef]
Sombati S, Hoyle G (1984). Generation of specific behaviors in a locust by local release into neuropil of the natural neuromodulator octopamine. Journal of Neurobiology 15: 481–506. DOI 10.1002/(ISSN)1097-4695. [Google Scholar] [CrossRef]
Spieth HT (1974). Courtship behavior in Drosophila. Annual Review of Entomology 19: 385–405. DOI 10.1146/annurev.en.19.010174.002125. [Google Scholar] [CrossRef]
Stewart BA (2002). Membrane trafficking in Drosophila wing and eye development. Seminars in Cell & Developmental Biology, vol. 13, pp. 91–97. Academic Press. DOI 10.1016/s1084-9521(02)00013-7. [Google Scholar] [CrossRef]
Sudmeier LJ, Howard SP, Ganetzky B (2015). A Drosophila model to investigate the neurotoxic side effects of radiation exposure. Disease Models & Mechanisms 8: 669–677. DOI 10.1242/dmm.019786. [Google Scholar] [CrossRef]
Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ (2004). A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 431: 854–859. DOI 10.1038/nature02980. [Google Scholar] [CrossRef]
Sun Y, Tang Y, Xu X, Hu K, Zhang Z, Zhang Y, Yi Z, Zhu Q, Xu R, Zhang Y (2020). Lead exposure results in defective behavior as well as alteration of gut microbiota composition in flies and their offsprings. International Journal of Developmental Neuroscience 80: 699–708. DOI 10.1002/jdn.10067. [Google Scholar] [CrossRef]
Ternes APL, Zemolin AP, da Cruz LC, da Silva GF, Saidelles APF, de Paula MT, Wagner C, Golombieski RM, de Moraes Flores É.M, Picoloto RS (2014). Drosophila melanogaster—an embryonic model for studying behavioral and biochemical effects of manganese exposure. EXCLI Journal 13: 1239. [Google Scholar]
Torres G, Horowitz JM (1998). Activating properties of cocaine and cocaethylene in a behavioral preparation of Drosophila melanogaster. Synapse 29: 148–161. DOI 10.1002/(ISSN)1098-2396. [Google Scholar] [CrossRef]
Turner HN, Armengol K, Patel AA, Himmel NJ, Sullivan L, Iyer SC, Bhattacharya S, Iyer EPR, Landry C, Galko MJ (2016). The TRP channels Pkd2, NompC, and Trpm act in cold-sensing neurons to mediate unique aversive behaviors to noxious cold in Drosophila. Current Biology 26: 3116–3128. DOI 10.1016/j.cub.2016.09.038. [Google Scholar] [CrossRef]
van Swinderen B, Andretic R (2011). Dopamine in Drosophila: Setting arousal thresholds in a miniature brain. Proceedings of the Royal Society B: Biological Sciences 278: 906–913. DOI 10.1098/rspb.2010.2564. [Google Scholar] [CrossRef]
Vasudevan D, Ryoo HD (2016). Detection of Cell Death in Drosophila Tissues, Programmed Cell Death, pp. 131–144. New York: Springer, Humana Press. [Google Scholar]
Verghese S, Su TT (2018). Ionizing radiation induces stem cell-like properties in a caspase-dependent manner in Drosophila. PLoS Genetics 14: e1007659. DOI 10.1371/journal.pgen.1007659. [Google Scholar] [CrossRef]
Wang X, Green DS, Roberts SP, de Belle JS (2007). Thermal disruption of mushroom body development and odor learning in Drosophila. PLoS One 2: e1125. DOI 10.1371/journal.pone.0001125. [Google Scholar] [CrossRef]
Wang Z, Ferdousy F, Lawal H, Huang Z, Daigle JG, Izevbaye I, Doherty O, Thomas J, Stathakis DG, O’Donnell JM (2011). Catecholamines up integrates dopamine synthesis and synaptic trafficking. Journal of Neurochemistry 119: 1294–1305. DOI 10.1111/j.1471-4159.2011.07517.x. [Google Scholar] [CrossRef]
Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR (2011). The molecular and cellular basis of bitter taste in Drosophila. Neuron 69: 258–272. DOI 10.1016/j.neuron.2011.01.001. [Google Scholar] [CrossRef]
White AR, Cappai R (2003). Neurotoxicity from glutathione depletion is dependent on extracellular trace copper. Journal of Neuroscience Research 71: 889–897. DOI 10.1002/(ISSN)1097-4547. [Google Scholar] [CrossRef]
Williams E, Casanova M (2013). Reassessment of teratogenic risk from antenatal ultrasound. Translational Neuroscience 4: 81–87. DOI 10.2478/s13380-013-0112-7. [Google Scholar] [CrossRef]
Wilson J (1968). Introduction: Problems of teratogenic testing. In: Fink BR (ed.Toxicity of Anesthetics, pp. 259–268. Baltimore, Md: Williams and Williams. [Google Scholar]
Xu F, Hennessy DA, Lee TK, Syed NI (2009). Trophic factor-induced intracellular calcium oscillations are required for the expression of postsynaptic acetylcholine receptors during synapse formation between Lymnaea neurons. Journal of Neuroscience 29: 2167–2176. DOI 10.1523/JNEUROSCI.4682-08.2009. [Google Scholar] [CrossRef]
Yellman C, Tao H, He B, Hirsh J (1997). Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. PNAS 94: 4131–4136. DOI 10.1073/pnas.94.8.4131. [Google Scholar] [CrossRef]
Yeşilada E (2001). Genotoxicity testing of some metals in the Drosophila wing somatic mutation and recombination test. Bulletin of Environmental Contamination and Toxicology 66: 464–469. DOI 10.1007/s001280029. [Google Scholar] [CrossRef]
Yüksel M, Sarıkaya R, Bostanci N (2010). Genotoxic evaluation of antiepileptic drugs by Drosophila somatic mutation and recombination test. Food and Chemical Toxicology 48: 2682–2687. DOI 10.1016/j.fct.2010.06.040. [Google Scholar] [CrossRef]
Zhang Y, Wolosker MB, Zhao Y, Ren H, Lemos B (2020). Exposure to microplastics cause gut damage, locomotor dysfunction, epigenetic silencing, and aggravate cadmium (Cd) toxicity in Drosophila. Science of the Total Environment 744: 140979. DOI 10.1016/j.scitotenv.2020.140979. [Google Scholar] [CrossRef]
Zhang Y, You J, Zhou Y, Zhang P, Zhang Z (2017). The effect of dichlorvos on control of Drosophila and its safety evaluation under different application methods. Environmental Science and Pollution Research 24: 22940–22947. DOI 10.1007/s11356-017-9879-3. [Google Scholar] [CrossRef]
Zhou C, Huang H, Kim SM, Lin H, Meng X, Han KA, Chiang AS, Wang JW, Jiao R, Rao Y (2012). Molecular genetic analysis of sexual rejection: Roles of octopamine and its receptor OAMB in Drosophila courtship conditioning. Journal of Neuroscience 32: 14281–14287. [Google Scholar]
Zhu F, Li Q, Zhang F, Sun X, Cai G, Zhang W, Chen X (2015). Chronic lithium treatment diminishes the female advantage in lifespan in Drosophila melanogaster. Clinical and Experimental Pharmacology and Physiology 42: 617–621. DOI 10.1111/1440-1681.12393. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools