 Open Access
Open Access
REVIEW
The roles and regulation of Yes-associated protein 1 in stem cells
1 Hunan Normal University School of Medicine, Changsha, 410013, China
2 The Key Laboratory of Model Animals and Stem Cell Biology in Hunan Province, Changsha, 410013, China
3 The Manufacture-Based Learning Research Demonstration Center for Human Reproductive Health New Technology of Hunan Normal University, Changsha, 410013, China
* Corresponding Authors: CHUNYUN LI. Email: ; ZUPING HE. Email:
BIOCELL 2023, 47(1), 33-39. https://doi.org/10.32604/biocell.2023.023567
Received 03 May 2022; Accepted 19 July 2022; Issue published 26 September 2022
Abstract
Yes-associated protein 1 (YAP1) is a downstream effector of the Hippo signaling pathway, and it is involved in tumorigenesis, tissue repair, growth, and development. In this review, the biological roles and the mechanisms of YAP1 in mediating stem cell fate decisions are discussed, including cell proliferation, differentiation, and apoptosis. In general, YAP1 promotes the proliferation and differentiation of stem cells, including embryonic stem cells and adult stem cells. It inhibits apoptosis by binding to the transcription factors, e.g., transcriptional enhanced associate domain (TEAD), Smad, runt-related transcription factor 1/2, p73, p63, and Erb84, to maintain tissue homeostasis. The translocalization of YAP1 in cellular nuclei and the phosphorylation in the cytoplasm work as important and unusual events for the activation of YAP1. Moreover, YAP1 serves as the crosstalk for the Hippo pathway and other signaling pathways, including the Wnt and Notch pathways. It is highlighted in this review that YAP1 is an essential regulator for stem cells that have significant applications in regenerative medicine and reproductive medicine.Keywords
The YAP1 gene, which encodes the Yes-associated protein 1, is located on the human chromosome 11q13, and it was first discovered in Drosophila in 1994. YAP1 lacks DNA-binding domains and is thus unable to bind to DNA directly, and it is a transcriptional coactivator. Notably, YAP1 is an important effector of the Hippo signaling pathway, which comprises a series of conserved kinase cascades, including mammalian sterile 20-like kinase1/2 (MST1/2), Salvador family WW domain containing 1 (SAV1), large tumor suppressor kinase 1/2 (LATS1/2), Mps one binder 1(MOB1), and transcriptional coactivator with PDZ-binding motif (YAP1/TAZ). The Hippo signaling pathway can be activated by various factors, e.g., intercellular contact (Kim et al., 2011; Schlegelmilch et al., 2011; Varelas et al., 2010), inflammatory cytokines (Deng et al., 2018), cell polarity (Szymaniak et al., 2015), and the stimulation of G protein-coupled receptor (Yu et al., 2012). When the Hippo signaling pathway is activated, the interaction between MST1/2 and its adaptor protein SAV1 enhances the phosphorylation of MST1/2. The phosphorylated MST1/2 and SAV1 complex activate LATS1/2, which phosphorylates YAP1 in the cellular cytoplasm (Yu et al., 2015). Interestingly, the phosphorylated YAP1 is retained in the cytoplasm and binds to the 14-3-3 proteins, resulting in the ubiquitination and degradation of YAP1 (Hong and Guan, 2012). Conversely, when the signal pathway is blocked by some factors, e.g., epidermal growth factor (EGF) (Fan et al., 2013), YAP1 cannot be phosphorylated, and it thus enters into the nuclei to bind to transcriptional factors, e.g., TEAD, Smad, runt-related transcription factor 1/2 (RUNX1/2), p73, p63, and Erb84. The reduction of the phosphorylated YAP1 and the increase in YAP1 levels reflect the inhibition of the Hippo signaling pathway (Zhao et al., 2011). In the nuclei, the combination of YAP1 with the target genes plays essential roles in tumorigenesis, tissue homeostasis, cardiac development (Chen et al., 2020), and the fate decisions of stem cells, as we illustrated in Fig. 1.
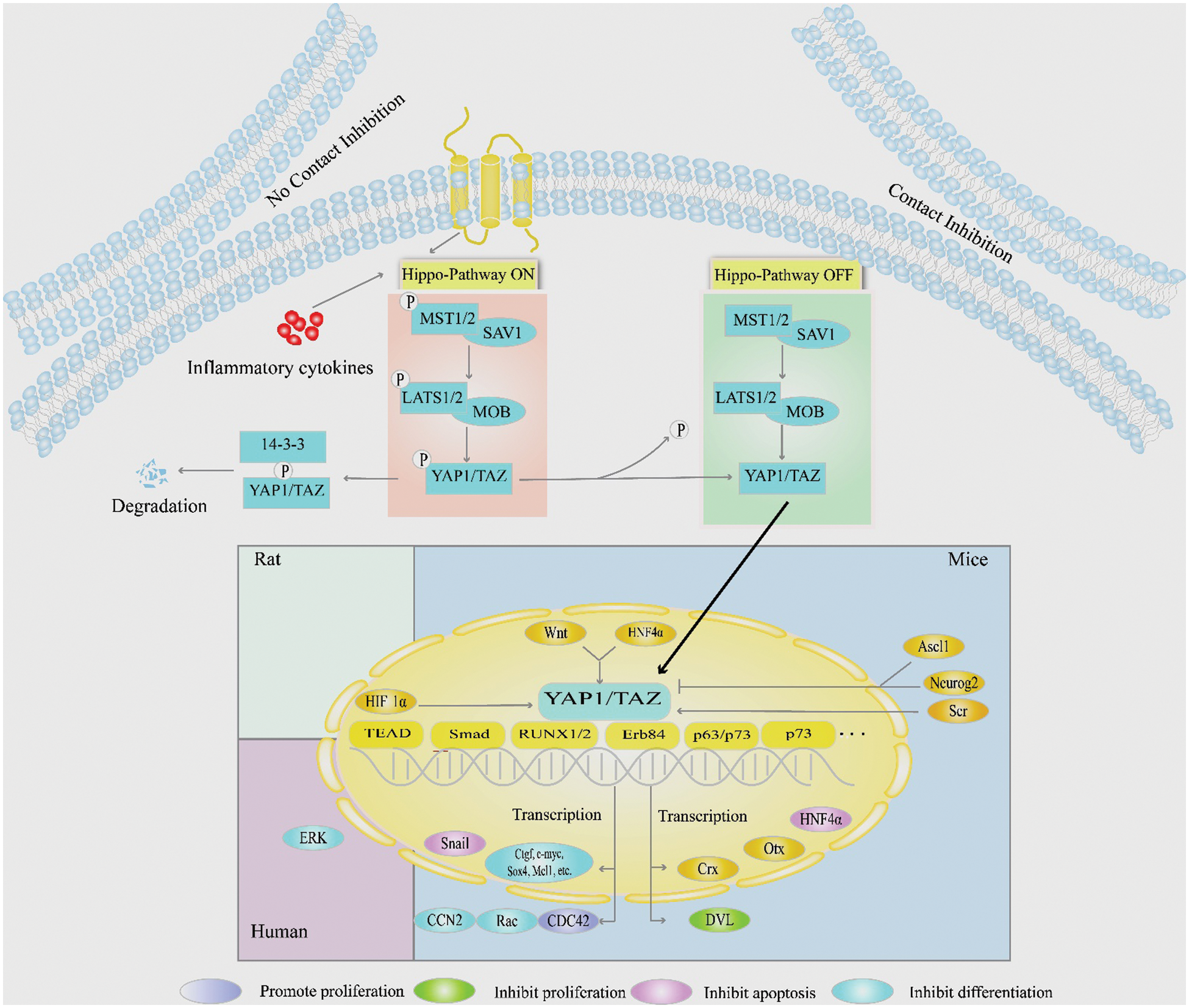
Figure 1: The role of YAP1 signaling pathways in regulating the fate decisions of stem cells. This schematic diagram shows the upstream and downstream regulators and signaling pathways of YAP1 in mediating the proliferation, differentiation, and apoptosis of various kinds of stem cells. YAP1 is phosphorylated in the cytoplasm of stem cells, whereas the dephosphorylated YAP1 localizes in the nuclei of these cells.
Stem cells are a type of cells with self-renewal and differentiation potential. Significantly, stem cells can replace the dead cells, repair the injured cells, and regulate immunity (Li and Clevers, 2010; Shi et al., 2012; Xiang et al., 2018). As such, stem cells have important applications in regenerative and reproductive medicine because of their great plasticity and differentiation potential. Under normal conditions, stem cells remain in a dormant state, with a few cells entering the proliferation cycle through asymmetric division (Li and Clevers, 2010). The stem cells undergo various fate decisions, including proliferation, differentiation, transdifferentiation, dedifferentiation, and apoptosis. The proliferation and differentiation maintain the dynamic balance of stem cells in the body. The microenvironment of stem cells, namely the niche, including extracellular and intracellular factors, is crucial for maintaining the fate determinations of stem cells (Vining and Mooney, 2017). As an intracellular transcriptional co-activator, YAP1 plays an important role in the regulation of stem cell fate determinations. For example, the deletion of YAP1 leads to the loss of the static and symmetric self-renewal ability of hematopoietic stem cells (Althoff et al., 2020). YAP1 controls the differentiation of mesenchymal stem cells (Guo et al., 2018), while it promotes the transition of the differentiated mammary, neuronal, and pancreatic exocrine cells to stem/progenitor cells (Panciera et al., 2016). In this review, we provide an overview of several types of stem cells which are regulated by YAP1. We focus on the functions and mechanisms of action of YAP1 in the fate determinations of stem cells, including the proliferation, differentiation, and apoptosis. This review will thus be helpful in providing insights into the molecular mechanisms of stem cell fate decisions, and significantly, it could offer important cells and targets for stem cell transplantation and gene therapy for a number of diseases.
Yes-associated protein 1 regulates stem cell proliferation
Yes-associated protein 1 promotes the proliferation of stem cells
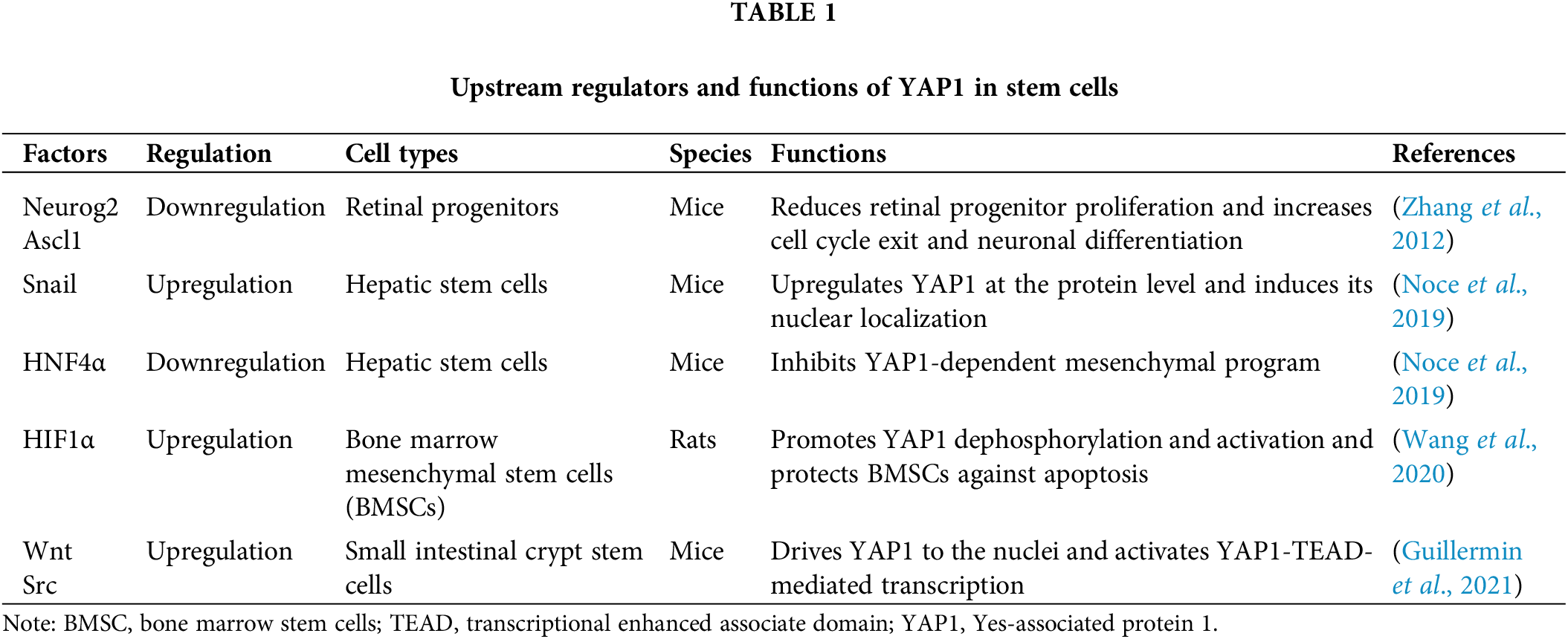
The canonical function of YAP1 promotes the proliferation of multiple types of stem cells. Hepatic stem cells regenerate themselves when the liver is injured. In most cases, YAP1 can promote the proliferation of hepatic stem cells. YAP1, together with TGFβ, stimulates hepatic stem cell proliferation to regenerate the damaged liver of mice undergoing partial hepatectomy (Oh et al., 2018). Furthermore, mechanical destruction of the murine ovary inhibits the ovarian Hippo signaling pathway, resulting in the increase in nuclear YAP1, CRY61, and CCN2 content, which promotes follicle growth and the generation of mature oocytes (Kawamura et al., 2013). The growth of follicles might be due to the activation of female germline stem cells by YAP1. In addition, Yap1-deficient retinal progenitors displayed the decrease in S-phase cells and altered cell cycle progression, as evidenced by YAP1-mediated enhancement of the proliferation of retinal progenitors (Kim et al., 2016). In porcine muscle stem cells, YAP1 promotes their proliferation at a high cell density (Liu et al., 2021). Yap1/Taz signaling in mesenchymal progenitor indirectly regulates the proliferation of muscle stem cells (Kaneshige et al., 2022). In the case of anemia, YAP1 stimulates the proliferation of erythroid progenitors by upregulating the expression of key enzymes involved in glutamine metabolism. This can be verified by the fact that erythroid progenitors fail to divide in the spleen for hematopoiesis when YAP1 is mutated (Grimm et al., 2019). Yap1 can also accelerate the proliferation of skin basal progenitor cells (Zhang et al., 2011), and it plays an essential role in stimulating cell proliferation in the development of the nervous system. The activation of YAP1 and its analog TAZ increases transcriptional activities and upregulates the expression of many genes associated with neural progenitor growth and division (Lavado et al., 2018). In neural stem cells of Drosophila, the loss of the Hippo signaling pathway leads to premature nuclear localization of Yorkie (equivalent to YAP1 in mammals), which increases the levels of microRNAs to trigger neural stem cell proliferation (Ding et al., 2016). In the chicken spine, Yap1-Tead increases the number of neural stem cells by enhancing the level of cyclin D1 (Cao et al., 2008). In mouse neural stem cells, YAP1 is an important downstream target of Notch, and it can rescue the inhibitory state of Notch signaling, thereby promoting cell proliferation (Li et al., 2012). Additionally, YAP1 enhances the proliferation of human periodontal ligament stem cells (h-PDLSC) (Jia et al., 2018). Collectively, these findings illustrate that YAP1 stimulates the division of numerous stem cells through the inhibition of the Hippo signaling pathway and regulation of the cell cycle and other factors.
Yes-associated protein 1 inhibits the division of stem cells
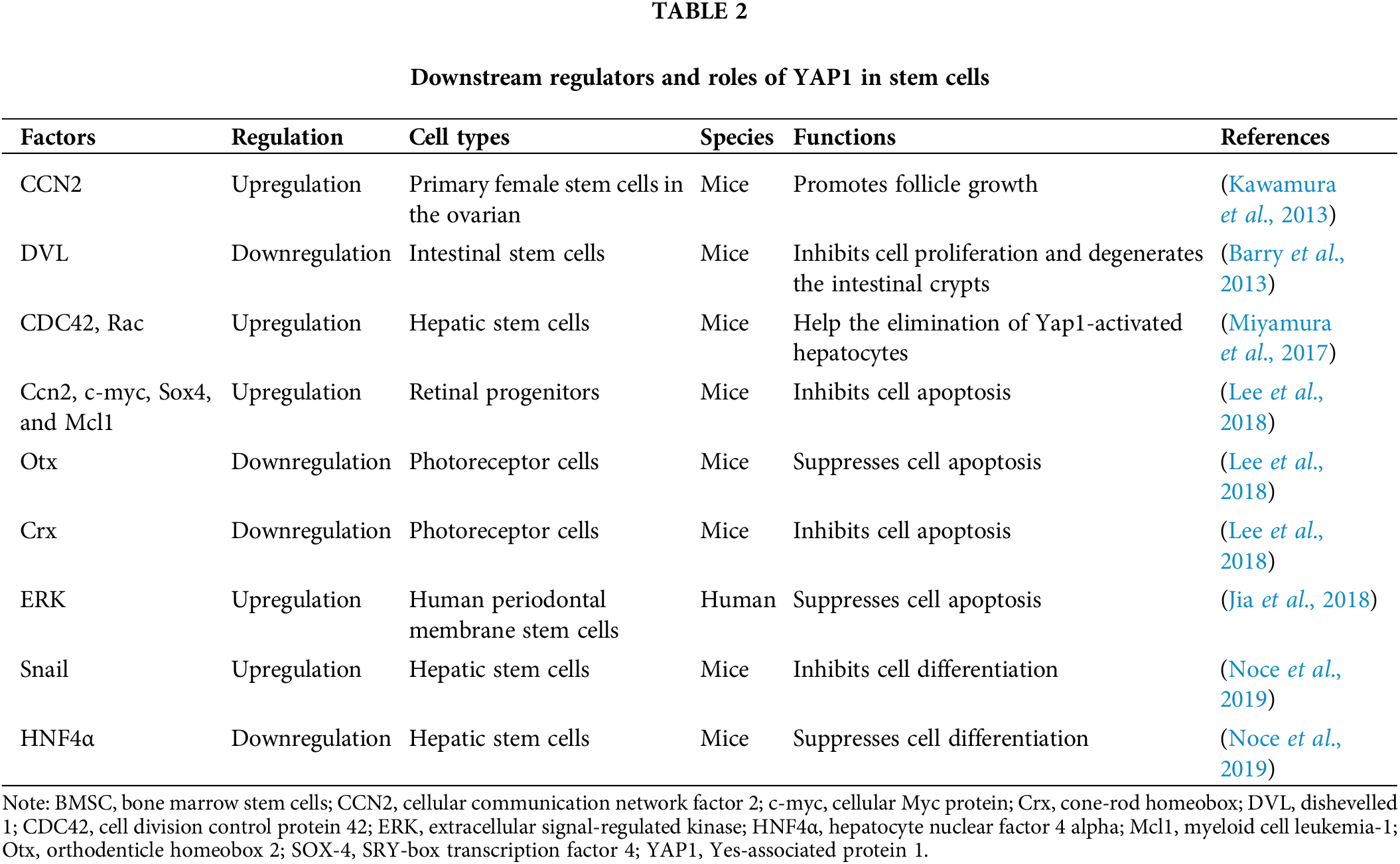
Interestingly, YAP1 assumes not only a powerful growth-inducing function for stem cells but also growth-suppressive biology. During the intestinal regeneration, YAP1 suppresses the Wnt signaling pathway by restricting the nuclear translocation of Dishevelled to inhibit the proliferation of intestinal stem cells and degenerate the intestinal crypts during regeneration. Additionally, deletion of Yap1 leads to Wnt hypersensitivity. This causes hyperplasia and expansion of intestinal stem cells and niche cells, which results in the formation of ectopic crypts and microadenomas (Barry et al., 2013). Considered together, YAP1 promotes the proliferation of most types of stem cells or progenitors, whereas it inhibits the division of certain stem cells.
Yes-associated protein 1 mediates the differentiation of stem cells
Numerous studies have demonstrated that YAP1 plays a significant role in regulating stem cell differentiation in many tissues. The skin is a powerful regenerative tissue, and its regeneration relies on the homeostasis of keratinocytes and basal epidermal cells. In 2011, it was shown that YAP1 is highly expressed in the nuclei of the single-layered basal epidermal progenitors, whereas its level becomes lower in the differentiated and mature keratinocytes. Yap1 can inhibit the differentiation of primary mouse keratinocytes. In this case, YAP1 translocates to the cytoplasm of the differentiating cells (Zhang et al., 2011) to be phosphorylated. Mechanistically, YAP1, together with TAZ, prevents the transformation of stem cells into progenitors through the interaction with TEAD, which inhibits the differentiation of keratinocytes and increases the lifespan of keratinocytes. YAP1 can be activated by cell stretching and low cell density, which further inhibits the downstream Notch signaling, thereby maintaining the stemness and undifferentiation of epidermal stem cells (Totaro et al., 2017). Similarly, YAP1/TAZ is activated by Kindlin-2 signaling to promote the differentiation of BMSCs into osteogenesis and adipocytes (Guo et al., 2018). YAP1 is essential for maintaining the differentiation of human epidermal stem cells (De Rosa et al., 2019). YAP1 also participates in the recovery of the retinal, intestinal, and injured liver via mediating stem cell differentiation. When Yap1 is ablated, retinal pigment epithelium transdifferentiates into retinal tissue (Kim et al., 2016). Additionally, YAP1 regulates the differentiation of muscle tissue, including skeletal muscle, smooth muscle, and myocardium. In skeletal muscle, the role of YAP1 changes with the stage of differentiation of stem cells. In the early phase of myoblast stem cell differentiation, YAP1 increases the rate of mitochondrial division by activating the expression of mitochondrial motility-related proteins, which provides sufficient energy for cell differentiation. YAP1 suppression reduces the expression of mitochondrial motility-related proteins and thus inhibits myoblast differentiation. On the contrary, in the process of late differentiation, YAP1 inhibits the differentiation of skeletal muscle stem cells (Sun et al., 2017). When MST1/2, the upstream of YAP1, is inhibited, YAP1 is phosphorylated to inhibit the differentiation of muscle satellite cells (Yang et al., 2022). Meanwhile, YAP1 activation by doxycycline can promote the differentiation of pig muscle stem cells at a high cell density (Liu et al., 2021). During osteogenesis, YAP1 is activated by tropomyosin-1 to maintain its nuclear localization and coaxes bone marrow mesenchymal stem cells (BMSCs) to differentiate into osteogenic cells (Brielle et al., 2021). Similarly, YAP1/TAZ is activated by Kindlin-2 signaling to promote the differentiation of BMSCs into osteogenesis and adipocytes (Guo et al., 2018). Meanwhile, YAP1 enhances the level of Snail and reduces hepatocyte nuclear factor 4 alpha (HNF4α) expression, which inhibits hepatic stem cell differentiation (Noce et al., 2019). Ectopic activation of Yap1 triggers intestinal stem cell switch from symmetric to asymmetric structures, which allows Paneth cell differentiation (Serra et al., 2019). Furthermore, YAP1 negatively regulates the differentiation of both absorptive and goblet cells in intestinal cells (Fallah and Beaulieu, 2020).
Notably, YAP1 assumes a dual role in regulating the differentiation of neural stem cells. On the one hand, YAP1 suppresses the differentiation of neural stem cells; on the other, Yap1-Tead reduces cyclin D1 expression to inhibit the differentiation of neural progenitor cells (Cao et al., 2008). This can be confirmed by the observation that inhibiting YAP1 along with TAZ promotes the differentiation of neural stem cells into mature functional neurons (Heng et al., 2020). It is consistent with the finding that inhibiting Yap1/Tead target genes in mouse embryonic neural stem cells contribute to the differentiation of premature neurons (Han et al., 2015). In pluripotent star stem cells, YAP1/TAZ is involved in maintaining cell differentiation and proliferation (Lorthongpanich et al., 2020). It is worth noting that YAP1, activated by tropomyosin-1 overexpression, maintains its nuclear localization and coaxes mesenchymal stem cell differentiation into osteogenic cells (Brielle et al., 2021).
In addition to the adult stem cells mentioned above, YAP1 silencing or depletion is required for the differentiation of pluripotent stem cells. YAP1 can be inactivated when embryonic stem cells (ESCs) differentiate, whereas the level of YAP1 expression increases when the induced pluripotent stem cells (iPSCs) are dividing. In mouse ESCs, Yap1 binds to the promoter of pluripotent genes, and it is essential for the pluripotency of mouse ESCs (Kaitsuka and Hakim, 2021). Knocking down Yap1 in mouse ESCs leads to the loss of pluripotency markers, e.g., OCT4 and SOX2, which facilitates the differentiation of these stem cells (Kaitsuka and Hakim, 2021). YAP1 deletion could further promote the differentiation of human ESCs into cardiomyocytes by inhibiting Wnt3 expression (Estaras et al., 2017), although the overexpression of YAP1 in human ESCs and iPSCs enhances the generation of naive PSCs (Qin et al., 2016). Therefore, the deficiency of YAP1 seems to be essential for the differentiation of pluripotent stem cells in humans and mice.
Yes-associated protein 1 controls the apoptosis of stem cells
Yes-associated protein 1 inhibits the apoptosis of stem cells
YAP1 is an inhibitor for apoptosis in many kinds of tissues. The mice with eye conditional knockout of Yap1 show more cleaved caspase 3-positive cells in the retina or retinal pigment epithelium-derived multilayered epithelium (Kim et al., 2016; Lee et al., 2018). The loss of Yap1 in the Nestin-expressing glial progenitors in mouse neonatal cerebellum enhances the apoptosis of later granule cell precursors (Yang and Joyner, 2019). Additionally, deficient YAP1 activity due to Yap1 conditional knockout leads to the apoptosis of crypt stem cells (Guillermin et al., 2021).
In human periodontal membrane stem cells, the expression levels of B-cell lymphoma-2 (Bcl-2) family members, e.g., Bak, Bid, and caspase-3, are decreased when YAP1 is overexpressed (Jia et al., 2018), suggesting that YAP1 suppresses the apoptosis in h-PDLSC by affecting the Bcl-2 family. Similarly, other factors indirectly affect YAP1 expression and cell apoptosis. In bone BMSCs, when Yap1 is activated, the mitochondrial apoptosis pathway promotes their apoptosis. In the hypoxia microenvironment, hypoxia-inducible factor 1-alpha (HIF1α) binds to the dephosphorylated Yap1 in the cell nuclei to form a complex, which transactivates the target genes responsible for the survival of BMSCs and thus inhibits the apoptosis (Wang et al., 2020). Likewise, the activation of YAP1 inhibits the apoptosis of h-PDLSC through extracellular signal-regulated kinase (ERK) and Bcl-2 signaling pathways (Jia et al., 2018).
Yes-associated protein 1 promotes apoptosis in stem cells
While in most cases, YAP1 inhibits the apoptosis of stem cells, it has a pro-apoptotic role in a specific environment, although it occurs less frequently in stem cells. Recurrent culture-acquired human pluripotent stem cells (hPSCs) display growth advantages over wild-type hPSCs (Price et al., 2021). YAP1 in the cytoplasm and f-actin redistribution promotes the apoptosis of wild-type hPSCs (Price et al., 2021). In neural progenitor cells, the high transcription of YAP1 inhibits differentiation and triggers replication stress and DNA damage, which results in cell apoptosis (Lavado et al., 2018). Together, these studies implicate the bi-directorial roles of YAP1 in either stimulation or suppression of apoptosis in stem cells under different conditions.
The upstream and downstream regulators of Yes-associated protein 1 in stem cells
YAP1 mediates decisions of stem cell fate through the upstream and downstream regulators. The expression of YAP1 is altered by multiple upstream cytoplasmic and nuclear factors. The cytoplasmic and nuclear factors include Ascl1, Neurog2 (Zhang et al., 2012), and Src family kinases (Guillermin et al., 2021), while the nuclear regulators include HNF4α (Noce et al., 2019), Wnt (Guillermin et al., 2021), and HIF1α (Wang et al., 2020). Downstream of YAP1, various factors are located in the nuclei or cytoplasm, e.g., HNF4α, Snail (Noce et al., 2019), Crx, and Otx, and various anti-apoptotic genes, including c-myc, Sox4, Mcl1, CDC42 (Lee et al., 2018), Rac, CCN2 (Miyamura et al., 2017), and DVL (Barry et al., 2013), that can be regulated by YAP1. Interestingly, YAP1 is bidirectionally regulated with Snail and HNF4α. YAP1 activation upregulates the Snail gene and downregulates the HNF4α gene, thereby inhibiting cell differentiation. Snail and HNF4α, in turn, affect YAP1 expression levels. Among the interaction, HNF4α has been shown to stably recruit at the YAP1 promoter and reduce its expression in liver cell lines and adult liver tissues (Noce et al., 2019). However, Snail inhibits the YAP1-dependent mesenchymal program to elevate YAP1 expression in hepatocytes (Noce et al., 2019). Thus, YAP1 and its upstream and downstream factors play essential roles in regulating the proliferation, differentiation, and apoptosis of certain types of stem cells; some of these factors and their functions and localization of the related factors are presented in Tables 1 and 2.
Conclusion and future perspectives
YAP1, as an important downstream effector of the Hippo signaling pathway, is involved in the regulation of stem cell fate determinations in various types of tissues, e.g., liver, cornea, skin, and intestine. In most cases, YAP1 promotes proliferation, inhibits apoptosis, and regulates the differentiation of stem cells. However, YAP1 mediates stem cell fate decisions with tissue specificity, which reflects the role of the Hippo-YAP1 signaling pathway in accurately regulating the size of organs and tissues. The precise regulatory mechanisms depend on the nuclear/cytoplasmic shuttling as well as the upstream and downstream factors of YAP1. The phosphorylation of YAP1 determines its location in the cytoplasm or the nuclei. Significantly, specific phosphorylation sites and post-transcriptional modifications, e.g., methylation, glycosylation, and ubiquitination, are important regulatory mechanisms that remain to be explored. Second, several classical signaling pathways, including Notch and Wnt, are involved in the biological process of YAP1 to regulate the fate of stem cells. In this review, we summarized the upstream and downstream factors of YAP1, e.g., Snail, HIF1α, HIF4α, and CCN2, especially the bidirectional functions of Snail and HIF4α in the regulation of YAP1. It would be interesting to identify other factors of YAP1 activity and their epigenetic interactions, including miRNAs, lncRNAs, circRNAs, and piRNAs, in controlling the fate determinations of stem cells. These studies would offer novel molecular mechanisms underlying stem cells proliferation, differentiation, and apoptosis and provide sufficient types of stem cells for translational medicine.
Author Contributions: Manuscript conception and design: ZH, CL, and QY; literature collection: QY, CL, WJ, and HG; manuscript preparation: QY, CL, WJ, and HG; manuscript revision & finalization: ZH. All authors approved the final version of the manuscript.
Ethics Approval: Not applicable.
Funding Statement: This work was supported by grants from the National Nature Science Foundation of China (32170862, 31872845), Major Scientific and Technological Projects for Collaborative Prevention and Control of Birth Defect in Hunan Province (2019SK1012), Key Grant of Research and Development in Hunan Province (2020DK2002), High-Level Talent Gathering Project in Hunan Province (2018RS3066), Natural Science Foundation of Hunan Province (2020JJ5383, 2021JJ40365), Health Commission Foundation of Hunan Province (202104052273, 202102050927), and Hunan Province College Student Research Learning and Innovative Experiment Project (S202010542084).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Althoff MJ, Nayak RC, Hegde S, Wellendorf AM, Bohan B et al. (2020). Yap1-Scribble polarization is required for hematopoietic stem cell division and fate. Blood 136: 1824–1836. DOI 10.1182/blood.2019004113. [Google Scholar] [CrossRef]
Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R et al. (2013). Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493: 106–110. DOI 10.1038/nature11693. [Google Scholar] [CrossRef]
Brielle S, Bavli D, Motzik A, Kan-Tor Y, Sun X, Kozulin C, Avni B, Ram O, Buxboim A (2021). Delineating the heterogeneity of matrix-directed differentiation toward soft and stiff tissue lineages via single-cell profiling. PNAS 118: e2016322118. DOI 10.1073/pnas.2016322118. [Google Scholar] [CrossRef]
Cao X, Pfaff SL, Gage FH (2008). YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes & Development 22: 3320–3334. DOI 10.1101/gad.1726608. [Google Scholar] [CrossRef]
Chen X, Li Y, Luo J, Hou N (2020). Molecular mechanism of hippo-YAP1/TAZ pathway in heart development, disease, and regeneration. Frontiers in Physiology 11: 389. DOI 10.3389/fphys.2020.00389. [Google Scholar] [CrossRef]
de Rosa L, Secone Seconetti A, de Santis G, Pellacani G, Hirsch T, Rothoeft T, Teig N, Pellegrini G, Bauer JW, de Luca M (2019). Laminin 332-dependent YAP dysregulation depletes epidermal stem cells in junctional epidermolysis bullosa. Cell Reports 27: 2036–2049.e2036. DOI 10.1016/j.celrep.2019.04.055. [Google Scholar] [CrossRef]
Deng Y, Lu J, Li W, Wu A, Zhang X, Tong W, Ho KK, Qin L, Song H, Mak KK (2018). Reciprocal inhibition of YAP/TAZ and NF-kappaB regulates osteoarthritic cartilage degradation. Nature Communication 9: 4564. DOI 10.1038/s41467-018-07022-2. [Google Scholar] [CrossRef]
Ding R, Weynans K, Bossing T, Barros CS, Berger C (2016). The Hippo signalling pathway maintains quiescence in Drosophila neural stem cells. Nature Communication 7: 10510. DOI 10.1038/ncomms10510. [Google Scholar] [CrossRef]
Estaras C, Hsu HT, Huang L, Jones KA (2017). YAP repression of the WNT3 gene controls hESC differentiation along the cardiac mesoderm lineage. Genes & Development 31: 2250–2263. DOI 10.1101/gad.307512.117. [Google Scholar] [CrossRef]
Fallah S, Beaulieu J-F (2020). The hippo pathway effector YAP1 regulates intestinal epithelial cell differentiation. Cells 9: 1895. DOI 10.3390/cells9081895. [Google Scholar] [CrossRef]
Fan R, Kim NG, Gumbiner BM (2013). Regulation of hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. PNAS 110: 2569–2574. DOI 10.1073/pnas.1216462110. [Google Scholar] [CrossRef]
Grimm L, Nakajima H, Chaudhury S, Bower NI, Okuda KS, Cox AG, Harvey NL, Koltowska K, Mochizuki N, Hogan BM (2019). Yap1 promotes sprouting and proliferation of lymphatic progenitors downstream of Vegfc in the zebrafish trunk. eLife 8: e42881. DOI 10.7554/eLife.42881. [Google Scholar] [CrossRef]
Guillermin O, Angelis N, Sidor CM, Ridgway R, Baulies A et al. (2021). Wnt and Src signals converge on YAP-TEAD to drive intestinal regeneration. EMBO Journal 40: e105770. DOI 10.15252/embj.2020105770. [Google Scholar] [CrossRef]
Guo L, Cai T, Chen K, Wang R, Wang J et al. (2018). Kindlin-2 regulates mesenchymal stem cell differentiation through control of YAP1/TAZ. Journal of Cell Biology 217: 1431–1451. DOI 10.1083/jcb.201612177. [Google Scholar] [CrossRef]
Han D, Byun SH, Park S, Kim J, Kim I, Ha S, Kwon M, Yoon K (2015). YAP/TAZ enhance mammalian embryonic neural stem cell characteristics in a Tead-dependent manner. Biochemical and Biophysical Research Communications 458: 110–116. DOI 10.1016/j.bbrc.2015.01.077. [Google Scholar] [CrossRef]
Heng BC, Zhang X, Aubel D, Bai Y, Li X, Wei Y, Fussenegger M, Deng X (2020). Role of YAP/TAZ in cell lineage fate determination and related signaling pathways. Frontiers in Cell and Developmental Biology 8: 735. DOI 10.3389/fcell.2020.00735. [Google Scholar] [CrossRef]
Hong W, Guan KL (2012). The YAP and TAZ transcription co-activators: Key downstream effectors of the mammalian Hippo pathway. Seminars in Cell & Developmental Biology 23: 785–793. DOI 10.1016/j.semcdb.2012.05.004. [Google Scholar] [CrossRef]
Jia L, Gu W, Zhang Y, Jiang B, Qiao X, Wen Y (2018). Activated yes-associated protein accelerates cell cycle, inhibits apoptosis, and delays senescence in human periodontal ligament stem cells. International Journal of Medical Sciences 15: 1241–1250. DOI 10.7150/ijms.25115. [Google Scholar] [CrossRef]
Kaitsuka T, Hakim F (2021). Response of pluripotent stem cells to environmental stress and its application for directed differentiation. Biology 10: 84. DOI 10.3390/biology10020084. [Google Scholar] [CrossRef]
Kaneshige A, Kaji T, Zhang L, Saito H, Nakamura A et al. (2022). Relayed signaling between mesenchymal progenitors and muscle stem cells ensures adaptive stem cell response to increased mechanical load. Cell Stem Cell 29: 265–280.e266. DOI 10.1016/j.stem.2021.11.003. [Google Scholar] [CrossRef]
Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y et al. (2013). Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. PNAS 110: 17474–17479. DOI 10.1073/pnas.1312830110. [Google Scholar] [CrossRef]
Kim JY, Park R, Lee J.H. J, Shin J, Nickas J, Kim S, Cho SH (2016). Yap is essential for retinal progenitor cell cycle progression and RPE cell fate acquisition in the developing mouse eye. Developmental Biology 419: 336–347. DOI 10.1016/j.ydbio.2016.09.001. [Google Scholar] [CrossRef]
Kim NG, Koh E, Chen X, Gumbiner BM (2011). E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. PNAS 108: 11930–11935. DOI 10.1073/pnas.1103345108. [Google Scholar] [CrossRef]
Lavado A, Park JY, Paré J, Finkelstein D, Pan H et al. (2018). The hippo pathway prevents YAP/TAZ-driven hypertranscription and controls neural progenitor number. Developmental Cell 47: 576–591.e578. DOI 10.1016/j.devcel.2018.09.021. [Google Scholar] [CrossRef]
Lee M, Goraya N, Kim S, Cho SH (2018). Hippo-yap signaling in ocular development and disease. Developmental Dynamics 247: 794–806. DOI 10.1002/dvdy.24628. [Google Scholar] [CrossRef]
Li L, Clevers H (2010). Coexistence of quiescent and active adult stem cells in mammals. Science 327: 542–545. DOI 10.1126/science.1180794. [Google Scholar] [CrossRef]
Li Y, Hibbs MA, Gard AL, Shylo NA, Yun K (2012). Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells 30: 741–752. DOI 10.1002/stem.1030. [Google Scholar] [CrossRef]
Liu Z, Lin L, Zhu H, Wu Z, Ding X, Hu R, Jiang Y, Tang C, Ding S, Guo R (2021). YAP promotes cell proliferation and stemness maintenance of porcine muscle stem cells under high-density condition. Cells 10: 3069. DOI 10.3390/cells10113069. [Google Scholar] [CrossRef]
Lorthongpanich C, Jiamvoraphong N, Klaihmon P, Lueangamornnara U, U-pratya Y, Laowtammathron C, Issaragrisil S (2020). Effect of YAP/TAZ on megakaryocyte differentiation and platelet production. Bioscience Reports 40: BSR20201780. DOI 10.1042/BSR20201780. [Google Scholar] [CrossRef]
Miyamura N, Hata S, Itoh T, Tanaka M, Nishio M et al. (2017). YAP determines the cell fate of injured mouse hepatocytes in vivo. Nature Communication 8: 16017. DOI 10.1038/ncomms16017. [Google Scholar] [CrossRef]
Noce V, Battistelli C, Cozzolino AM, Consalvi V, Cicchini C, Strippoli R, Tripodi M, Marchetti A, Amicone L (2019). YAP integrates the regulatory Snail/HNF4α circuitry controlling epithelial/hepatocyte differentiation. Cell Death & Disease 10: 768. DOI 10.1038/s41419-019-2000-8. [Google Scholar] [CrossRef]
Oh SH, Swiderska-Syn M, Jewell ML, Premont RT, Diehl AM (2018). Liver regeneration requires Yap1-TGFbeta-dependent epithelial-mesenchymal transition in hepatocytes. Journal of Hepatology 69: 359–367. DOI 10.1016/j.jhep.2018.05.008. [Google Scholar] [CrossRef]
Panciera T, Azzolin L, Fujimura A, Di Biagio D, Frasson C et al. (2016). Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell 19: 725–737. DOI 10.1016/j.stem.2016.08.009. [Google Scholar] [CrossRef]
Price CJ, Stavish D, Gokhale PJ, Stevenson BA, Sargeant S, Lacey J, Rodriguez TA, Barbaric I (2021). Genetically variant human pluripotent stem cells selectively eliminate wild-type counterparts through YAP-mediated cell competition. Developmental Cell 56: 2455–2470.E10. DOI 10.1016/j.devcel.2021.07.019. [Google Scholar] [CrossRef]
Qin H, Hejna M, Liu Y, Percharde M, Wossidlo M et al. (2016). YAP induces human naive pluripotency. Cell Reports 14: 2301–2312. DOI 10.1016/j.celrep.2016.02.036. [Google Scholar] [CrossRef]
Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR et al. (2011). Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell 144: 782–795. DOI 10.1016/j.cell.2011.02.031. [Google Scholar] [CrossRef]
Serra D, Mayr U, Boni A, Lukonin I, Rempfler M et al. (2019). Self-organization and symmetry breaking in intestinal organoid development. Nature 569: 66–72. DOI 10.1038/s41586-019-1146-y. [Google Scholar] [CrossRef]
Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G (2012). How mesenchymal stem cells interact with tissue immune responses. Trends in Immunology 33: 136–143. DOI 10.1016/j.it.2011.11.004. [Google Scholar] [CrossRef]
Sun C, De Mello V, Mohamed A, Ortuste Quiroga HP, Garcia-Munoz A et al. (2017). Common and distinctive functions of the hippo effectors Taz and Yap in skeletal muscle stem cell function. Stem Cells 35: 1958–1972. DOI 10.1002/stem.2652. [Google Scholar] [CrossRef]
Szymaniak AD, Mahoney JE, Cardoso WV, Varelas X (2015). Crumbs3-mediated polarity directs airway epithelial cell fate through the hippo pathway effector Yap. Developmental Cell 34: 283–296. DOI 10.1016/j.devcel.2015.06.020. [Google Scholar] [CrossRef]
Totaro A, Castellan M, Battilana G, Zanconato F, Azzolin L, Giulitti S, Cordenonsi M, Piccolo S (2017). YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nature Communication 8: 15206. DOI 10.1038/ncomms15206. [Google Scholar] [CrossRef]
Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL (2010). The crumbs complex couples cell density sensing to hippo-dependent control of the TGF-beta-SMAD pathway. Developmental Cell 19: 831–844. DOI 10.1016/j.devcel.2010.11.012. [Google Scholar] [CrossRef]
Vining KH, Mooney DJ (2017). Mechanical forces direct stem cell behaviour in development and regeneration. Nature Reviews: Molecular Cell Biology 18: 728–742. DOI 10.1038/nrm.2017.108. [Google Scholar] [CrossRef]
Wang Z, Cui M, Qu Y, He R, Wu W, Lin H, Shao Z (2020). Hypoxia protects rat bone marrow mesenchymal stem cells against compression-induced apoptosis in the degenerative disc microenvironment through activation of the HIF-1α/YAP signaling pathway. Stem Cells and Development 29: 1309–1319. DOI 10.1089/scd.2020.0061. [Google Scholar] [CrossRef]
Xiang Q, Liao Y, Chao H, Huang W, Liu J, Chen H, Hong D, Zou Z, Xiang AP, Li W (2018). ISL1 overexpression enhances the survival of transplanted human mesenchymal stem cells in a murine myocardial infarction model. Stem Cell Research & Therapy 9: 51. DOI 10.1186/s13287-018-0803-7. [Google Scholar] [CrossRef]
Yang J, Wang K, An Y, Wu R, Li J, Wang H, Dong Y (2022). Mst1/2 is necessary for satellite cell differentiation to promote muscle regeneration. Stem Cells 40: 74–87. DOI 10.1093/stmcls/sxab010. [Google Scholar] [CrossRef]
Yang Z, Joyner AL (2019). YAP1 is involved in replenishment of granule cell precursors following injury to the neonatal cerebellum. Developmental Biology 455: 458–472. DOI 10.1016/j.ydbio.2019.07.018. [Google Scholar] [CrossRef]
Yu FX, Zhao B, Guan KL (2015). Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163: 811–828. DOI 10.1016/j.cell.2015.10.044. [Google Scholar] [CrossRef]
Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I et al. (2012). Regulation of the hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150: 780–791. DOI 10.1016/j.cell.2012.06.037. [Google Scholar] [CrossRef]
Zhang H, Pasolli HA, Fuchs E (2011). Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. PNAS 108: 2270–2275. DOI 10.1073/pnas.1019603108. [Google Scholar] [CrossRef]
Zhang H, Deo M, Thompson RC, Uhler MD, Turner DL (2012). Negative regulation of Yap during neuronal differentiation. Developmental Biology 361: 103–115. DOI 10.1016/j.ydbio.2011.10.017. [Google Scholar] [CrossRef]
Zhao B, Tumaneng K, Guan KL (2011). The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nature Cell Biology 13: 877–883. DOI 10.1038/ncb2303. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools