 Open Access
Open Access
ARTICLE
SMC1A served as a potential therapeutic target to regulate malignant phenotypes of cervical cancer
Department of Gynecology, Hebei General Hospital, Shijiazhuang, 050051, China
* Corresponding Author: CONGWEI DAI. Email:
BIOCELL 2023, 47(11), 2471-2484. https://doi.org/10.32604/biocell.2023.029617
Received 28 February 2023; Accepted 26 July 2023; Issue published 27 November 2023
Abstract
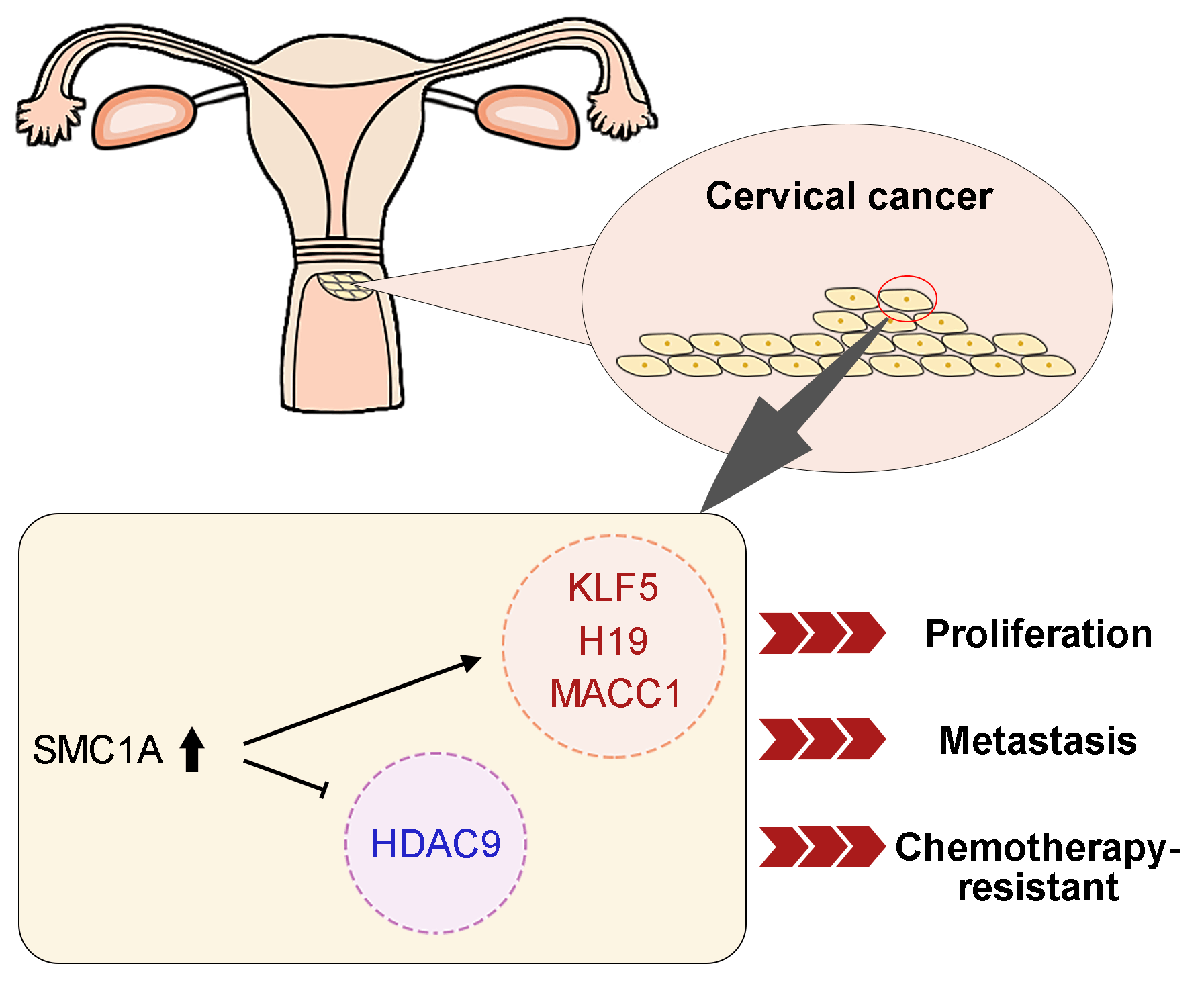
Introduction: Structural maintenance of chromosome 1A (SMC1A) is a crucial compound of the cohesin complex. It has been reported to regulate the epithelial-mesenchymal transition (EMT) process in multiple cancers. Objectives: The present study aims to further clarify the role of SMC1A in cervical cancer. Methods: We analyzed data from four datasets and confirmed that SMC1A showed high expression in cervical cancer samples and was related to poor prognosis of patients with cervical cancer. Cell proliferation of SiHa and C-33A with knockdown of SMC1A was assessed using CCK-8 and colony formation assay. The migration and invasion were estimated by wound healing assay and Transwell assay separately. The effect of SMC1A on the chemosensitivity of cisplatin and paclitaxel in cervical cancer cells was detected by flow cytometry assay. Results: Results of the immunohistochemistry (IHC) assay confirmed that the expression of SMC1A was increased in tumor tissues. The cell viability was remarkably suppressed in SiHa and C-33A by knocking down the expression of SMC1A. The increase of E-cadherin expression and decrease of N-cadherin and Snail expression verified that inhibition of SMC1A suppressed the EMT process of cervical cancer cells. Further, cell migration, and invasion were significantly repressed by the absence of SMC1A. Cisplatin and paclitaxel are effective chemotherapeutic agents used in the treatment of cervical cancer. Silencing of SMC1A remarkably promoted the apoptosis induced by cisplatin and paclitaxel, revealing that the chemotherapy resistance to cisplatin and paclitaxel in cervical cancer could reduce by knocking down SMC1A. Further, metastasis associated with colon cancer 1 (MACC1) was identified as the downstream factor of SMC1A. Its upregulation reversed the proliferation and the EMT process induced by SMC1A silencing. Conclusion: Therefore, our study concluded that SMC1A serves as a therapeutic molecular target to regulate the malignant phenotypes of cervical cancer.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools