 Open Access
Open Access
ARTICLE
Valtrate exerts anticancer effects on gastric cancer AGS cells by regulating reactive oxygen species-mediated signaling pathways
1 Department of Biochemistry and Molecular Biology, College of Life Science and Technology, Heilongjiang Bayi Agricultural University, Daqing, 163319, China
2 Hemodialysis Center, Daqing Oilfield General Hospital, Daqing, 163001, China
3 Department of Food Science and Engineering, College of Food Science, Heilongjiang Bayi Agricultural University, Daqing, 163319, China
4 Agricultural Products and Processed Products Supervision and Testing Center, Ministry of Agriculture, Daqing, 163319, China
5 National Coarse Cereals Engineering Research Center, Daqing, 163319, China
* Corresponding Authors: CHANG WANG. Email: ; CHENGHAO JIN. Email:
# These authors contributed equally to this work
BIOCELL 2024, 48(2), 313-325. https://doi.org/10.32604/biocell.2023.043474
Received 03 July 2023; Accepted 16 October 2023; Issue published 23 February 2024
Abstract
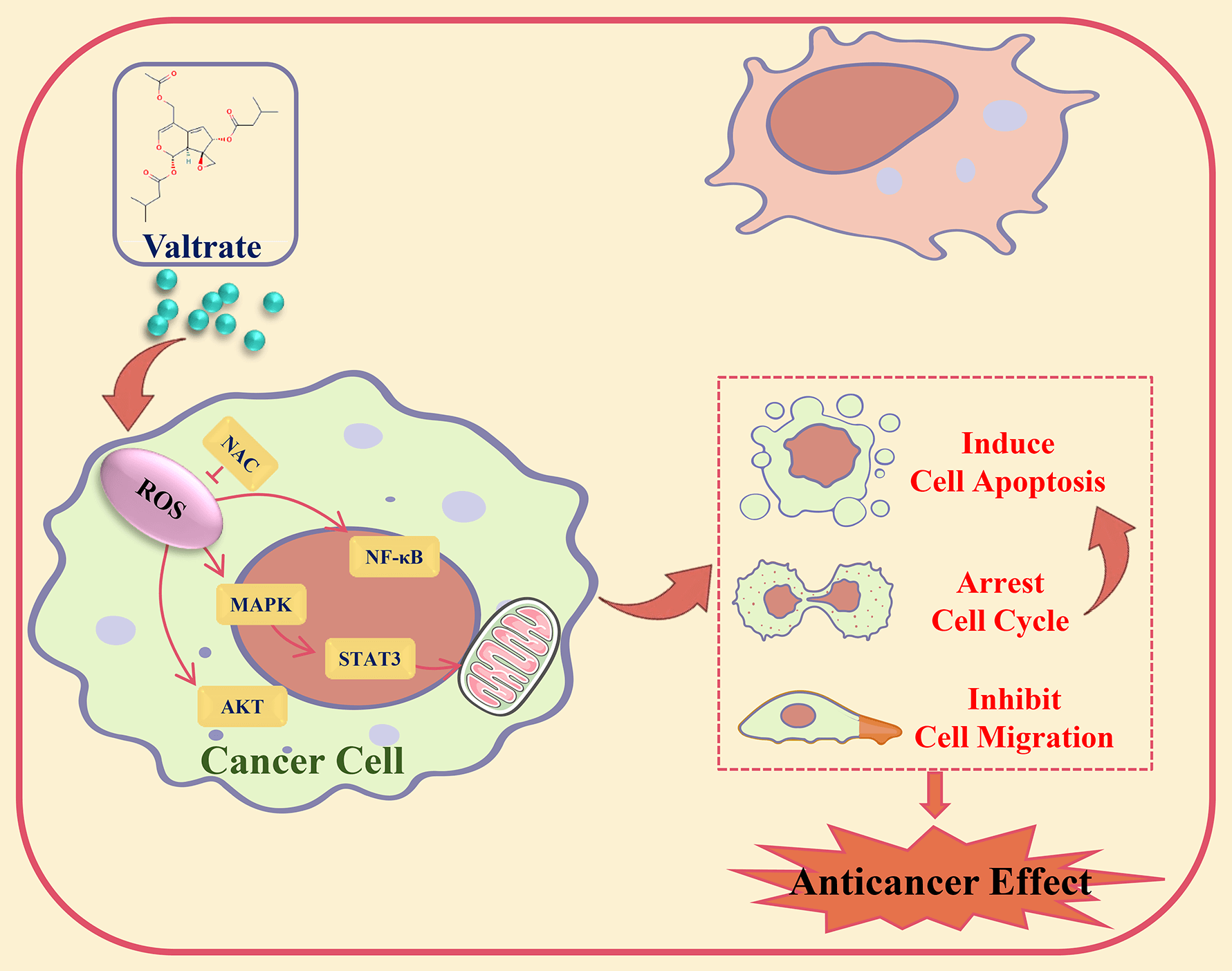
Background: Valtrate (Val) was extracted from the Valeriana jatamansi Jones plant, had good antitumor activity. However, its precise molecular mechanism in cancer cells was still unclear. This study investigated the effect of Val on gastric cancer (GC) cells and its potential molecular mechanism. Methods: Cell viability was examined by CCK-8 assay. Cell cycle, apoptosis, and Reactive oxygen species (ROS) level were analyzed by flow cytometry. The migration effect of Val on AGS cells was analyzed by transwell and wound-healing assay. The expression levels of proteins were analyzed by western blot. Results: The cell viability assay results demonstrated that Val significantly decreased GC cell viability. Apoptosis assay results revealed that Val induced mitochondria-dependent apoptosis through the Bad/Bcl-2/cyto-c/cle-casp-3/cle-PARP pathways. Further exploration found that Val induced apoptosis through increasing the expression of phosphorylated p38 mitogen-activated protein kinase (p-p38), phosphorylated c-Jun N-terminal kinase (p-JNK), and Inhibitor kappa B alpha (IκB-α) proteins and decreasing the expression of phosphorylated extracellular signal-regulated kinase (p-ERK), phosphorylated signal transducer and activator of transcription 3 (p-STAT3), and nuclear factor kappa-B (NF-κB) proteins; these expression levels of proteins were reversed by mitogen-activated protein kinase (MAPK) inhibitor. Furthermore, Val induced G2/M phase arrest in AGS cells through downregulating the expression of phosphorylated protein kinase B (p-AKT). Moreover, Val induced inhibition of AGS cell migration through downregulating the expression of p-GSK-3β and β-catenin. In addition, Val promoted the ROS accumulation of AGS cells. Further investigation found that Val-induced apoptosis, arrested the cell cycle, and inhibited cell migration, and that its signaling pathways related to protein expressions were reversed by the ROS scavenger, N-acetyl-L-cysteine. Conclusion: Val induced apoptosis, arrested the cell cycle, and inhibited migration by ROS-mediated MAPK/STAT3/NF-κB, AKT/Cyclin B/CDK1/2, and GSK-3β/β-catenin signaling pathways in AGS cells.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools