 Open Access
Open Access
REVIEW
Exploring the mechanistic role of epidermal growth factor receptor activation in non-cancer kidney disease
1 Medical Course, College of Medicine, Catholic Kwandong University, Gangneung, 25601, Republic of Korea
2 Department of Anatomy, College of Medicine, Jeju National University, Jeju, 63243, Republic of Korea
3 Interdisciplinary Graduate Program in Advanced Convergence Technology & Science, Jeju National University, Jeju, 63243, Republic of Korea
* Corresponding Author: JINU KIM. Email:
BIOCELL 2025, 49(1), 79-92. https://doi.org/10.32604/biocell.2024.058340
Received 10 September 2024; Accepted 28 November 2024; Issue published 24 January 2025
Abstract
The epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein that plays a crucial role in signal transduction and cellular responses. This review explores the function of EGFR in kidney physiology and its implications for various kidney diseases. EGFR signaling is essential for kidney function and repair mechanisms, and its dysregulation significantly impacts both acute and chronic kidney conditions. The review discusses the normal distribution of EGFR in kidney tubular segments, the mechanism of its activation and inhibition, and the therapeutic potential of EGFR-targeting antagonists and ligands. Additionally, it explores the pathophysiological characteristics observed in rodent models of kidney diseases through pharmacological and genetic inhibition of EGFR, highlighting therapeutic challenges and limitations such as species differences, variability in disease models, and potential adverse effects. Overall, the findings underscore the multifaceted role of EGFR in kidney diseases, influencing inflammation, fibrosis, and tissue injury. This complex involvement suggests that targeting EGFR may be a beneficial therapeutic strategy for managing these conditions, potentially mitigating inflammation and fibrosis while promoting tissue repair.Keywords
Epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein composed of 1186 amino acids, first identified in 1986 [1,2]. As illustrated in Fig. 1, it consists of several domains: an extracellular domain (621 amino acids) with ligand-binding sites, a single hydrophobic transmembrane domain (23 amino acids) that anchors the receptor to the cell membrane, and an intracellular domain (542 amino acids) responsible for signaling [3,4]. The intracellular domain includes a juxtamembrane segment, a tyrosine kinase domain, and a COOH-terminal tail [5,6]. As depicted in Fig. 2, ligand binding to the extracellular domain of EGFR induces homodimerization or heterodimerization of the bound receptor [7,8]. This dimerization activates the intrinsic tyrosine domain, resulting in the autophosphorylation of other tyrosine kinase residues in the COOH-terminal tail [9,10]. Autophosphorylation generates docking sites for various intracellular signaling proteins, subsequently initiating multiple downstream signaling cascades [11,12]. Therefore, the dimerization and autophosphorylation of EGFR are crucial steps in its role in signal transduction and cellular response.
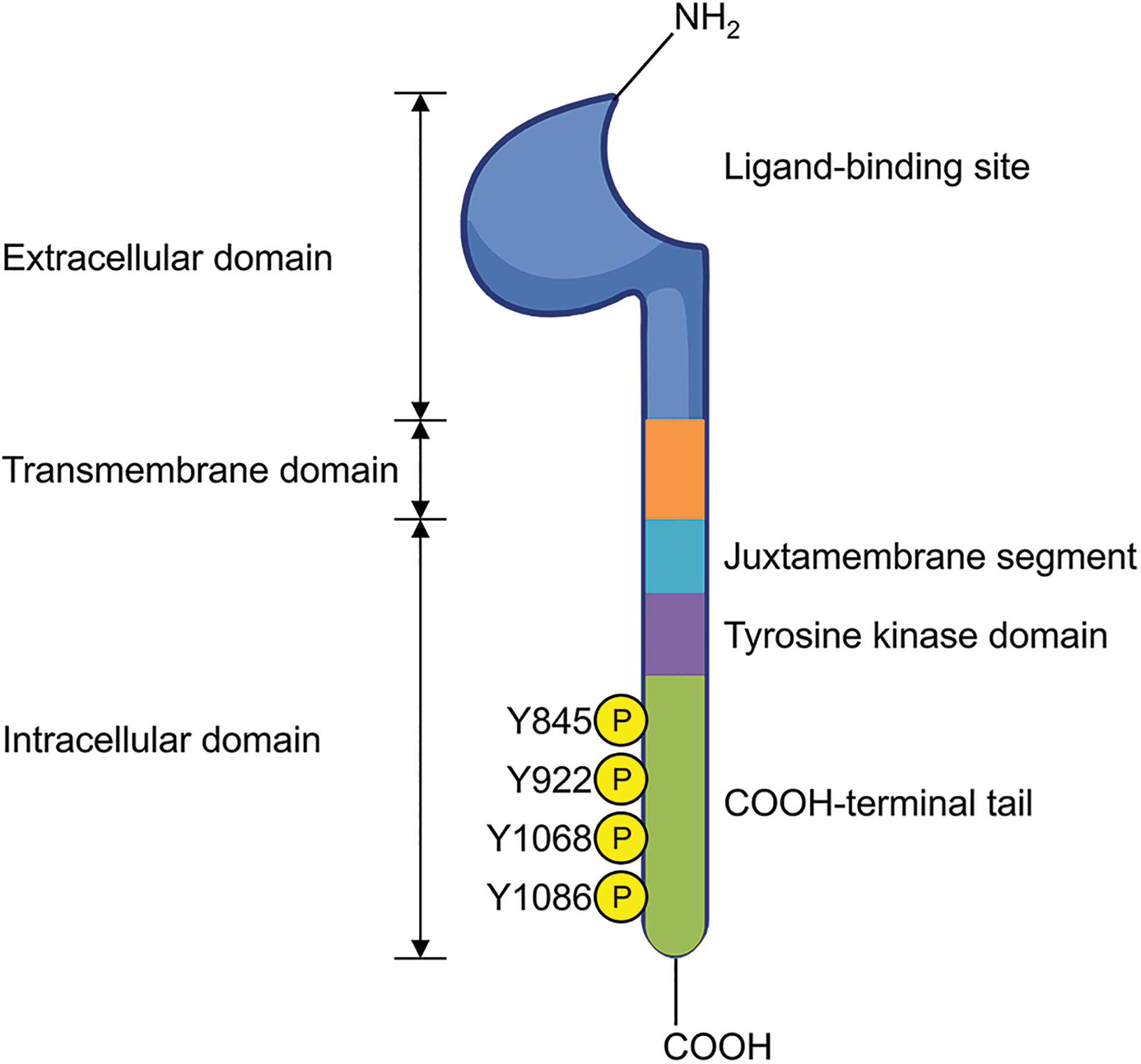
Figure 1: EGFR structure. The figure illustrates the extracellular domain, which contains the ligand-binding site; the transmembrane domain; and intracellular domain, which includes the juxtamembrane segment, the tyrosine kinase domain, and the COOH-terminal tail featuring autophosphorylated tyrosine residues. Created in BioRender.com.
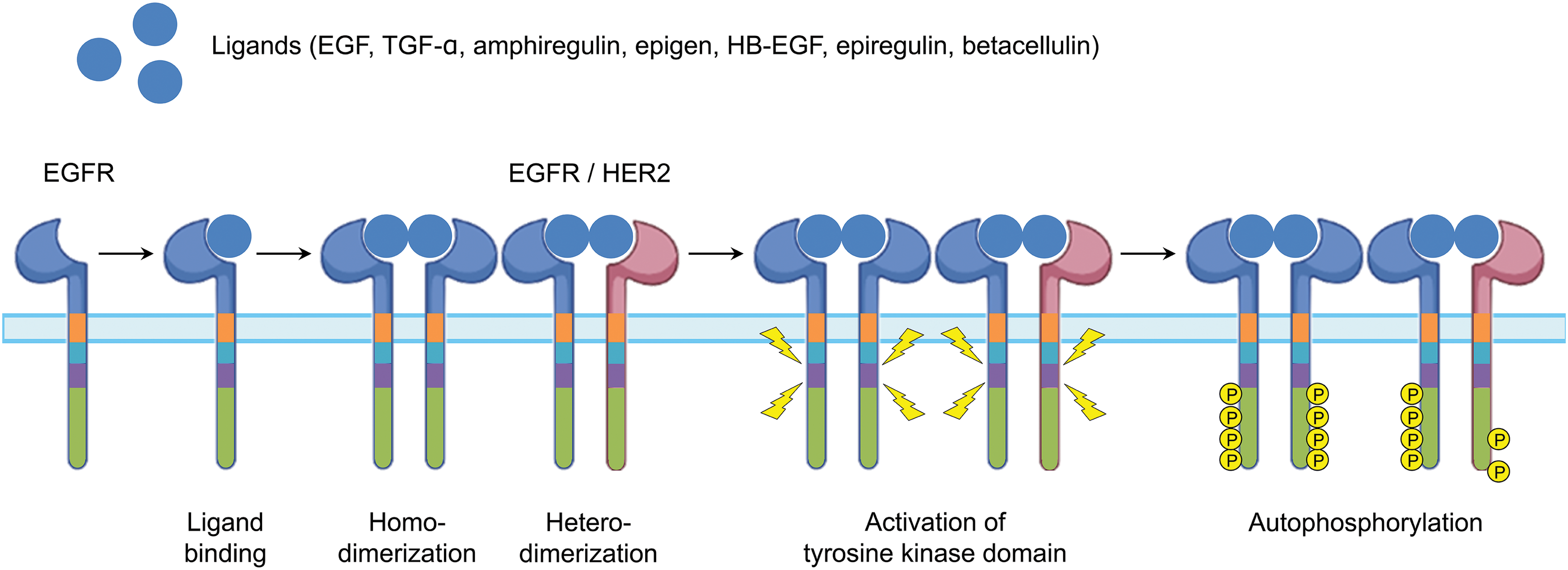
Figure 2: Flux of EGFR activation. Abbreviations: HB-EGF, heparin-binding EGF-like growth factor; HER2, human epidermal growth factor receptor 2; TGF-α, transforming growth factor-α. Created in BioRender.com.
Interest in the role of EGFR in kidney physiology has significantly increased in recent decades [13,14]. Numerous studies indicate that EGFR signaling is crucial for the progression of various kidney diseases [13,15]. This signaling pathway is central to regulating kidney function and the repair mechanisms essential for maintaining kidney health [14,16]. Dysregulation of EGFR has a profound impact on both acute and chronic kidney conditions [13,15]. This review aims to clarify the role of EGFR in kidney physiology and to introduce EGFR-targeting antagonists and ligands. Additionally, it explores the pathophysiological characteristics of EGFR observed in rodent models of various kidney diseases through pharmacological and genetic inhibition of EGFR.
Normal Distribution of EGRF in Kidney Tubular Segments
Distribution and activation of EGFR in kidney tubular segments
The kidney tubular apparatus in mammals comprises several distinct segments, each performing an indispensable function in kidney physiology [17,18]. These segments include the proximal tubule, loop of Henle, distal tubule, connecting tubule, and collecting duct, each characterized by specific structural and functional attributes [19,20]. The proximal tubule, which consists of convoluted and straight portions, features cuboidal epithelial cells with a brush border [21,22]. The loop of Henle includes the proximal tubule’s pars recta, descending thin limb, ascending thin limb, and thick ascending limb (TAL) [23,24]. The distal tubule consists of the TAL, macula densa, distal convoluted tubule (DCT), and connecting tubules [25,26]. Finally, the collecting duct, which comprises various segments, contains principal and intercalated cells.
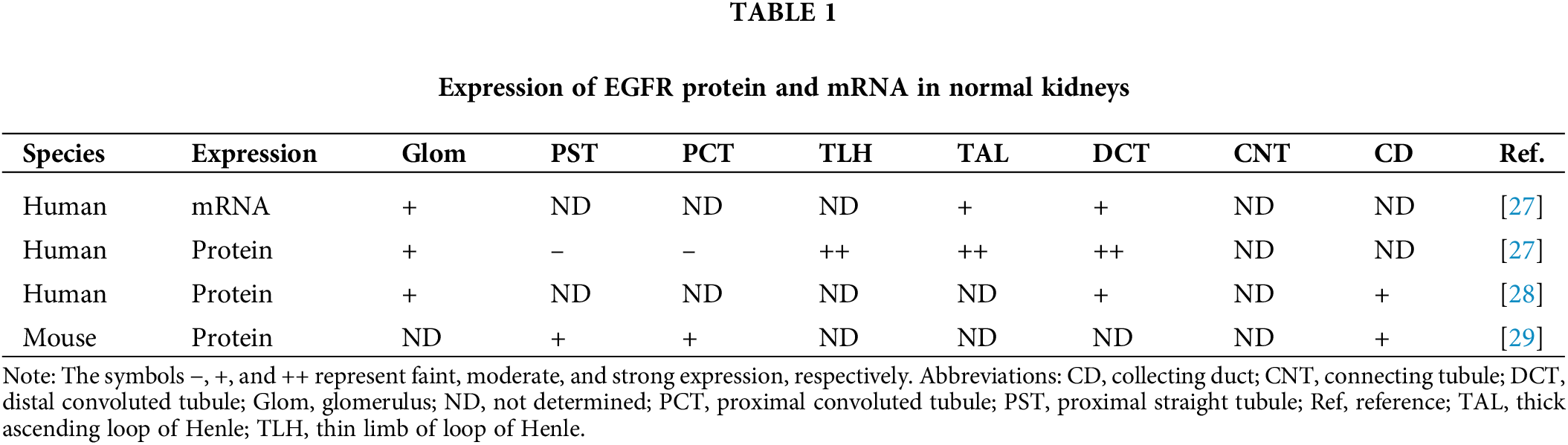
EGFR expression is observed in normal kidneys, where it is widely distributed across various segments, including proximal tubule, thin limb of the loop of Henle, TAL, DCT, and collecting duct, and glomerulus. Specifically, within the glomerulus, EGFR expression is found in podocytes, endothelial cells, mesangial cells, and along the glomerular basement membrane [27,28] (Table 1). Additionally, EGFR expression is present in medullary interstitial cells, peritubular capillaries, and arterioles in the kidneys [27,28]. In tubular epithelial cells, EGFR is predominantly localized to the basolateral membrane and the cytoplasm adjacent to the basal membrane [27,29]. Upon binding of a EGFR ligand, EGFR activation through tyrosine phosphorylation significantly increases at the basolateral membrane across all segments of the kidney tubules, including proximal tubules, where EGFR expression is faint under normal conditions [30–32]. Notably, EGFR activation is highest in proximal straight tubules, followed by proximal convoluted tubules, collecting ducts, and DCT [31,32].
EGFR Activation and Inhibition from Ligands to Inhibitors
The first ligand discovered for the EGFR was epidermal growth factor (EGF) [33,34]. This ligand was initially characterized by Nobel laureate Dr. Stanley Cohen and his colleagues, who identified it in an extract from the submaxillary gland [35,36]. In addition to EGF, several other ligands interact with EGFR, including transforming growth factor-α (TGF-α), amphiregulin, epigen, heparin-binding EGF-like growth factor (HB-EGF), epiregulin, and betacellulin [37,38]. These ligands can be categorized into two groups [39]: the first group, which includes EGF, TGF-α, amphiregulin, and epigen, binds specifically to EGFR, while the second group, comprising HB-EGF, epiregulin, and betacellulin, binds to both EGFR and human epidermal growth factor receptor 4 (HER4). Connective tissue growth factor (CTGF), recognized as a ligand for integrins and TGF-β receptors, can also bind to and activate EGFR [40,41]. Among these ligands, EGF, TGF-α, HB-EGF, and betacellulin are considered high-affinity ligands [42,43], whereas amphiregulin, epigen, and epiregulin are classified as low-affinity ligands [43,44].
All endogenous EGFR ligands, with the exception of CTGF, are initially expressed as type I transmembrane proteins. These ligands are then converted into their mature, soluble forms, which are released into the extracellular environment through proteolytic cleavage by metalloproteinases, particularly members of the a disintegrin and metalloproteinase (ADAM) family [45,46]. Most EGFR ligands are produced in the kidney. EGF is primarily found in the TAL and DCT, with immunoreactivity observed along the apical membrane and in the cytoplasm [27,47]. In the connecting tubule and cortical collecting duct, EGF exhibits membranous staining, particularly in intercalated cells, which display a distinctive octopus-like morphology [47]. TGF-α is predominantly localized in the proximal tubules of the kidney cortex [47,48], with additional presence in the DCT [48]. HB-EGF is mainly localized in the proximal tubule, especially in the S3 segment of the outer stripe of the outer medulla [49]. Amphiregulin is expected to be primarily expressed in the proximal and distal tubules, as demonstrated by its upregulation following kidney injuries [50,51], although detailed distribution along various tubular segments is limited. Epigen has been detected in mouse kidney tissues [52], epiregulin is expressed in primary cultured human glomerular mesangial cells [53], and betacellulin is expressed in bovine kidney epithelial cell [54]. However, there are no reports detailing the expression of these proteins along specific tubular segments.
Activation of the EGFR depends on its ligands, which promote the formation of either homo- or heterodimers at the cellular membrane, ultimately leading to receptor internalization. Upon ligand binding, EGFR can dimerize with another EGFR molecule (homodimerization) or with a different receptor of the human epidermal growth factor receptor (HER) family (heterodimerization) [7,8]. Although EGFR has also been shown to heterodimerize with receptors for hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1), and platelet-derived growth factor (PDGF) [55,56], the biologic significance of these interactions remains unclear. Following internalization, the dimerized receptors undergo autophosphorylation within their intracellular tyrosine kinase domains, generating binding sites for signaling molecules and initiating downstream intracellular signaling cascades [8,10]. Multiple post-translational modifications of EGFR, including methylation of the extracellular domain [57,58], phosphorylation on Serine and Threonine residues [57,59], SUMOylation [57,60], and ubiquitination [61,62], have been identified as regulatory factors that influence EGFR functionality and may serve as potential therapeutic targets to overcome drug-resistance mutations [63,64].
EGFR plays a crucial role in various signaling pathways that regulate cell growth, differentiation, and survival, particularly in kidney disease [30]. Following EGFR autophosphorylation, it activates downstream pathways such as mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), which promote cell proliferation, and phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), which enhances cell survival by inhibiting apoptosis [2,65]. Additionally, HER2, a member of the EGFR family, can form heterodimers with EGFR, intensifying signaling and contributing to kidney injury and fibrosis [30,66]. EGFR signaling is critical in kidney development and repair processes, promoting the proliferation of kidney epithelial cells and facilitating recovery following acute kidney injury (AKI) [30,67]. However, dysregulation of EGFR signaling is linked to chronic kidney disease (CKD), where it can exacerbate inflammation and fibrosis [13,68]. Consequently, targeting EGFR has been explored as a therapeutic strategy for various kidney diseases, particularly those associated with fibrosis. Dual targeting of EGFR and HER2 may offer potential benefits in conditions where both pathways are implicated.
Small molecules inhibit the activity of EGFR tyrosine kinase by binding to the ATP-binding site within the receptor’s intracellular domain [69]. EGFR tyrosine kinase inhibitors (TKIs) specifically target this domain of EGFR, competing with ATP for binding [69]. This inhibition prevents the activation of tyrosine kinase and the autophosphorylation of EGFR, thereby disrupting EGFR signal transduction pathways [69,70]. By preventing receptor phosphorylation, which is essential for the activation of downstream signaling pathways, TKIs effectively inhibit EGFR activation [70,71] (Table 2). As shown in, these inhibitors can selectively interfere with EGFR alone or with other human epidermal growth factor receptor (HER) family receptors in addition to EGFR. Numerous TKIs currently in clinical development have demonstrated potential antitumor activity. Furthermore, TKIs have shown protective effects against various kidney diseases in animal studies. These inhibitors can be further categorized based on whether their effects are reversible or irreversible [4,72].
Role of EGFR in Kidney Diseases
Animal models of kidney disease
Several studies have highlighted the role of EGFR in various animal models of kidney disease (Table 3), encompassing a wide range of conditions. These include ischemic kidney disease such as AKI and CKD induced by unilateral or bilateral kidney ischemia and reperfusion injury (IRI); septic AKI triggered by lipopolysaccharide or cecal ligation and puncture; metabolic kidney disease such as hyperuricemia, diabetic nephropathy, and obesity-induced nephropathy; inflammatory kidney disease such as glomerulonephritis, lupus nephritis, and inflammation induced by tumor necrosis factor-like weak inducer of apoptosis (TWEAK); and obstructive kidney disease such as unilateral ureteral obstruction (UUO).
EGFR inhibition in ischemic kidney diseases
IRI contributes to the development of AKI, characterized by a sudden decline in kidney function, tubular injury, and inflammation [99–101]. In cases where recovery is maladaptive, the initial AKI can progress to CKD due to chronic inflammation and tubulointerstitial fibrosis [86,102,103]. Bilateral kidney IRI induces kidney dysfunction and tubular injury; however, pharmacological and proximal tubule-specific inhibition of EGFR does not significantly affect kidney dysfunction and tubular injury in AKI induced by bilateral kidney IRI [79,86]. Conversely, unilateral kidney IRI exacerbates tubular injury and apoptosis in the tubules when EGFR is inhibited due to a point mutation in the Egfr gene (Waved-2 mutation) [85]. Pharmacological inhibition of EGFR has been demonstrated to reduce the pro-inflammatory response, as evidenced by decreased infiltration of macrophages and lymphocytes, as well as lower levels of pro-inflammatory mRNA expression following bilateral kidney IRI [86] and kidney transplantation [87]. Additionally, EGFR inhibition has been found to decrease IRI-induced activation of AKT and ERK [79,85], which may contribute to delayed recovery of kidney function and tubular morphology [79]. Generally, EGFR inhibition effectively reduces tubulointerstitial fibrosis following IRI and kidney transplantation [85–87].
Sepsis can lead to AKI through mechanisms such as hypotension, which impairs kidney perfusion and causes ischemia [104,105]. Additionally, the systemic inflammatory response in sepsis releases pro-inflammatory cytokines and mediators that can directly damage kidney tissues [104,106]. In mouse models of septic AKI induced by lipopolysaccharide or cecal ligation and puncture (CLP), studies have demonstrated that inhibiting EGFR, either pharmacologically or genetically, offers protection against the pro-inflammatory response [75,80]. This protective effect is evidenced by a reduction in macrophage infiltration, decreased pro-inflammatory mRNA expression [75,80]. This protection is associated with less severe kidney dysfunction, tubular injury [75], and mortality [80].
EGFR inhibition in metabolic kidney disease
Numerous animal studies have explored EGFR-targeted inhibition in diabetic nephropathy [82,92,93]. Both genetic and pharmacological inhibition of EGFR ultimately improve Type 2 diabetes by reducing blood glucose levels [92], but this effect is not observed in Type 1 diabetes [77,82,93]. In both types of diabetes, EGFR inhibition consistently reduces albuminuria [82,92,93], leading to the amelioration of diabetes-induced kidney dysfunction. In diabetic mice subjected to EGFR inhibition, the kidneys exhibit reduced histopathological injury in the tubules, decreased collagen deposition in the interstitial areas [82,92,93], and reduced infiltration of leukocytes, including macrophages [77,92] and T lymphocytes [92]. Glomeruli also show improvements, including reduced loss of podocytes [90,92,93], decreased cell cycle arrest [88], reduced macrophage infiltration [92], less mesangial expansion [93], and diminished glomerulosclerosis [77,92,93] during diabetic nephropathy. Interestingly, specific deficiencies of Egfr in podocytes [93] and proximal tubules [90] completely mitigate tubulointerstitial fibrosis. However, conflicting results exist regarding the effect of EGFR inhibition on tubular apoptosis [82,84,90]. Diabetic rats treated with PKI-166 exhibit increased apoptotic cell death in the tubules [84], while diabetic mice treated with AG-1478 show decreased apoptotic cell death in the tubules [82]. This controversy underscores the necessity for further research into the relationship between EGFR and apoptosis in diabetic kidneys. Treatment with gefitinib attenuates kidney dysfunction, histopathological injury in the tubules, and tubulointerstitial fibrosis induced by hyperuricemia from adenine and potassium oxonate [74]. Additionally, obesity-induced nephropathy is characterized by increased apoptosis and fibrosis, but treatment with AG-1478 effectively mitigates these symptoms [83].
EGFR inhibition in inflammatory kidney disease
In inflammatory kidney diseases, the role of EGFR has been primarily studied in models of glomerulonephritis and lupus nephritis [107,108]. Pharmacological inhibition of EGFR effectively enhances kidney function in cases of glomerulonephritis [73,78,94]. This inhibition also reduces crescent formation and macrophage infiltration in the glomeruli affected by glomerulonephritis [78,94]. Notably, specific deficiencies of Egfr in podocytes [78] and macrophages [73] protect against these symptoms during glomerulonephritis. In contrast, the effects of pharmacological EGFR inhibition in lupus nephritis models remain controversial. Treatment with erlotinib has been shown to reduce kidney dysfunction and tubulointerstitial fibrosis in lupus nephritis induced by Fcgr2b deficiency [95]. Conversely, treatment with lapatinib exacerbates kidney dysfunction and tubulointerstitial fibrosis in lupus nephritis induced by interferon-α administration [81]. Additionally, Egfr deficiency in CD4-positive T cells worsens glomerular and tubulointerstitial injuries in pristane-induced lupus nephritis [96]. On the other hand, treatment with erlotinib alleviates inflammation by decreasing macrophage and T cell infiltrations, as well as reducing the expression of cytokines and chemokines in kidneys affected by inflammation induced by TWEAK [97]. Further studies are necessary using animal models of lupus nephritis to gain a deeper understanding of the varying effects based on the type of disease model and the inhibition strategy employed.
EGFR inhibition in obstructive kidney disease
Obstructive kidney disease occurs when a blockage in the urinary tract impedes urine flow, leading to increased pressure in the kidneys [109,110]. Over time, this obstruction can damage the kidneys, potentially resulting in CKD [111,112]. UUO is a specific type of obstructive kidney disease characterized by the obstruction of one ureter [110,113,114]. UUO can be caused by various factors, including kidney stones, tumors, or congenital abnormalities [111,113]. This condition leads to increased collagen deposition and the expression of pro-fibrotic proteins, resulting in tubulointerstitial fibrosis [112,113]. However, EGFR inhibition through treatment with gefitinib, EGFR point mutations, or EGFR mimotopes has been shown to effectively reduce fibrosis [68,76,98]. Additionally, these interventions mitigate kidney inflammation, as indicated by reduced macrophage infiltration and lower levels of pro-inflammatory mRNA expression during UUO [68,76,98].
The EGFR plays a multifaceted role in kidney diseases, influencing both acute and chronic conditions through its effects on inflammation, fibrosis, and tissue injury (Fig. 3). In AKI, EGFR inhibition can either exacerbate or mitigate injury, depending on the type and severity of ischemia, while consistently reducing fibrosis and inflammation. In CKD, EGFR inhibition shows promise in alleviating symptoms of diabetic nephropathy and obesity-induced nephropathy, although results can vary based on specific conditions and the inhibitors used. Despite these advances, the effects of EGFR inhibition in inflammatory conditions like lupus nephritis and vascular diseases are mixed, suggesting that while targeting EGFR can be beneficial, its application must be carefully tailored to the specific disease context. Future research should focus on elucidating the mechanisms underlying these varied responses to optimize therapeutic strategies and enhance the efficacy of EGFR inhibition in managing kidney diseases.
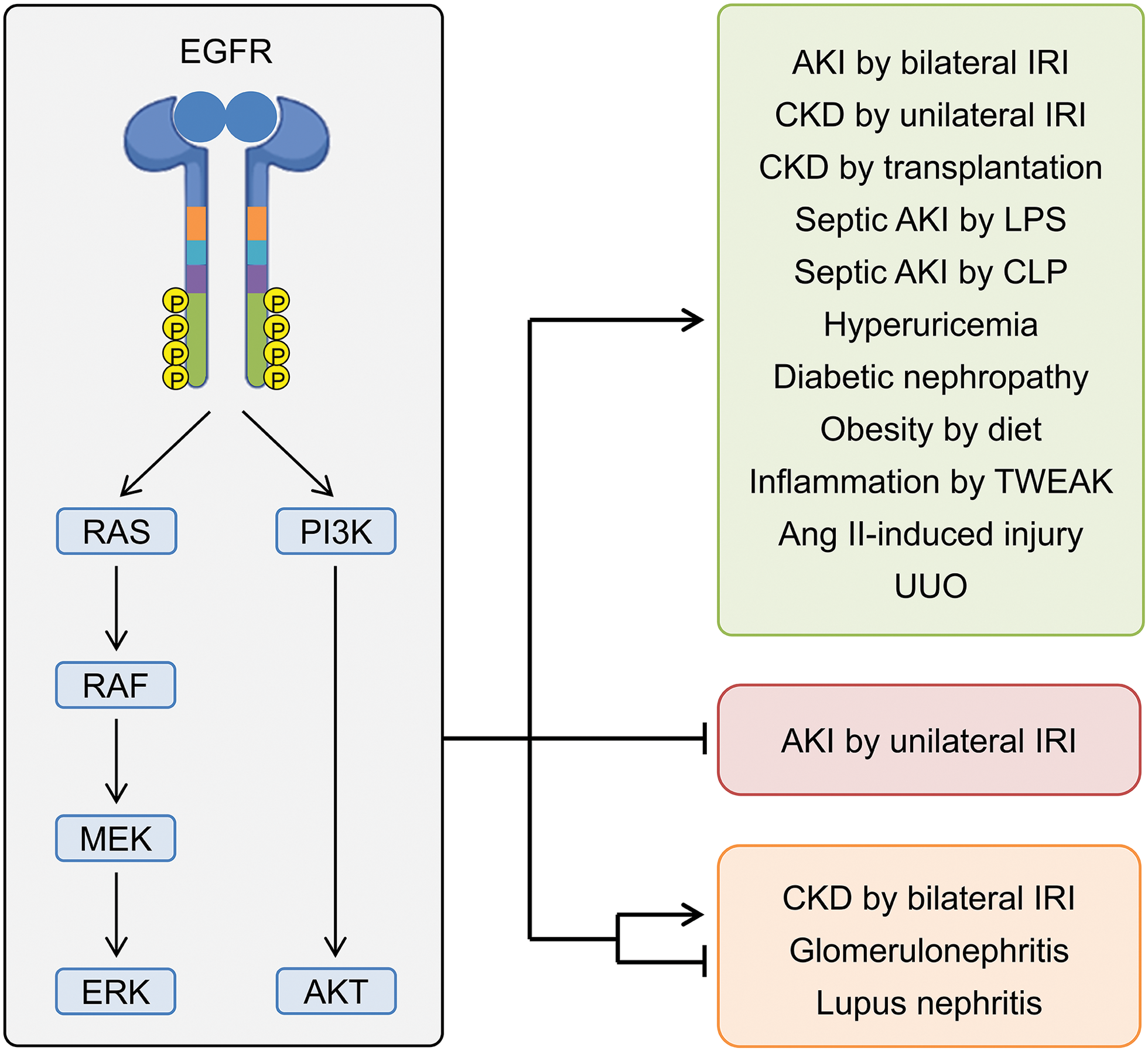
Figure 3: Outline of the roles of EGFR in rodent models of kidney disease. Abbreviations: AKI, acute kidney injury; AKT, protein kinase B; CKD, chronic kidney disease; CLP, cecal ligation, and puncture; ERK, extracellular signal-regulated kinase; IRI, ischemia and reperfusion injury; LPS, lipopolysaccharide; MEK, mitogen-activated protein kinase; PI3K, phosphoinositide 3-kinase; RAF, rapidly accelerated fibrosarcoma; RAS, rat sarcoma virus; TWEAK, tumor necrosis factor-like weak inducer of apoptosis; UUO, unilateral ureteral obstruction. Created in BioRender.com.
Acknowledgement: None.
Funding Statement: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Education (2021R1I1A3056002, to Jinu Kim; RS-2023-00274853, to Daeun MOON).
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: Jinu Kim, Daeun Moon; draft manuscript preparation: Ju-Yeon Lee, Daeun Moon, Jinu Kim; review and editing: Daeun Moon, Jinu Kim; visualization: Daeun Moon; supervision: Jinu Kim. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Lai KM, Lee WL. The roles of epidermal growth factor receptor in viral infections. Growth Factors. 2022;40(1–2):46–72. doi:10.1080/08977194.2022.2063123. [Google Scholar] [PubMed] [CrossRef]
2. Ramani S, Samant S, Manohar SM. The story of EGFR: from signaling pathways to a potent anticancer target. Future Med Chem. 2022;14(17):1267–88. doi:10.4155/fmc-2021-0343. [Google Scholar] [PubMed] [CrossRef]
3. Arkhipov A, Shan Y, Das R, Endres NF, Eastwood MP, Wemmer DE, et al. Architecture and membrane interactions of the EGF receptor. Cell. 2013;152(3):557–69. doi:10.1016/j.cell.2012.12.030. [Google Scholar] [PubMed] [CrossRef]
4. Chen J, Zeng F, Forrester SJ, Eguchi S, Zhang MZ, Harris RC. Expression and function of the epidermal growth factor receptor in physiology and disease. Physiol Rev. 2016;96(3):1025–69. doi:10.1152/physrev.00030.2015. [Google Scholar] [PubMed] [CrossRef]
5. Amelia T, Kartasasmita RE, Ohwada T, Tjahjono DH. Structural insight and development of EGFR tyrosine kinase inhibitors. Molecules. 2022;27(3):819. doi:10.3390/molecules27030819. [Google Scholar] [PubMed] [CrossRef]
6. Villalobo A. Regulation of ErbB receptors by the Ca2+ sensor protein calmodulin in cancer. Biomedicines. 2023;11(3):661. doi:10.3390/biomedicines11030661. [Google Scholar] [PubMed] [CrossRef]
7. Singh DR, King C, Salotto M, Hristova K. Revisiting a controversy: the effect of EGF on EGFR dimer stability. Biochim Biophys Acta Biomembr. 2020;1862(1):183015. doi:10.1016/j.bbamem.2019.07.003. [Google Scholar] [PubMed] [CrossRef]
8. Trenker R, Jura N. Receptor tyrosine kinase activation: from the ligand perspective. Curr Opin Cell Biol. 2020;63:174–85. doi:10.1016/j.ceb.2020.01.016. [Google Scholar] [PubMed] [CrossRef]
9. Wang D, Wang W, Song M, Xie Y, Kuang W, Yang P. Regulation of protein phosphorylation by PTPN2 and its small-molecule inhibitors/degraders as a potential disease treatment strategy. Eur J Med Chem. 2024;277(Suppl 3):116774. doi:10.1016/j.ejmech.2024.116774. [Google Scholar] [PubMed] [CrossRef]
10. Talukdar S, Emdad L, Das SK, Fisher PB. EGFR: an essential receptor tyrosine kinase-regulator of cancer stem cells. Adv Cancer Res. 2020;147:161–88. doi:10.1016/bs.acr.2020.04.003. [Google Scholar] [PubMed] [CrossRef]
11. Levantini E, Maroni G, Del Re M, Tenen DG. EGFR signaling pathway as therapeutic target in human cancers. Semin Cancer Biol. 2022;85(8):253–75. doi:10.1016/j.semcancer.2022.04.002. [Google Scholar] [PubMed] [CrossRef]
12. Thirukkumaran OM, Kluba M, Hofkens J, Mizuno H. Autophosphorylation of EGFR at Y954 Facilitated Homodimerization and Enhanced Downstream Signals. Biophys J. 2020;119(10):2127–37. doi:10.1016/j.bpj.2020.10.008. [Google Scholar] [PubMed] [CrossRef]
13. Harris RC. The epidermal growth factor receptor axis and kidney fibrosis. Curr Opin Nephrol Hypertens. 2021;30(3):275–9. doi:10.1097/MNH.0000000000000696. [Google Scholar] [PubMed] [CrossRef]
14. Gao L, Zhong X, Jin J, Li J, Meng XM. Potential targeted therapy and diagnosis based on novel insight into growth factors, receptors, and downstream effectors in acute kidney injury and acute kidney injury-chronic kidney disease progression. Signal Transduct Target Ther. 2020;5(1):9. doi:10.1038/s41392-020-0106-1. [Google Scholar] [PubMed] [CrossRef]
15. Sheng L, Bayliss G, Zhuang S. Epidermal growth factor receptor: a potential therapeutic target for diabetic kidney disease. Front Pharmacol. 2020;11:598910. doi:10.3389/fphar.2020.598910. [Google Scholar] [PubMed] [CrossRef]
16. Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16(5):269–88. doi:10.1038/s41581-019-0248-y. [Google Scholar] [PubMed] [CrossRef]
17. Balzer MS, Rohacs T, Susztak K. How many cell types are in the kidney and what do they do? Annu Rev Physiol. 2022;84(1):507–31. doi:10.1146/annurev-physiol-052521-121841. [Google Scholar] [PubMed] [CrossRef]
18. Tabibzadeh N, Satlin LM, Jain S, Morizane R. Navigating the kidney organoid: insights into assessment and enhancement of nephron function. Am J Physiol Renal Physiol. 2023;325(6):F695–706. doi:10.1152/ajprenal.00166.2023. [Google Scholar] [PubMed] [CrossRef]
19. Yousef Yengej FA, Pou Casellas C, Ammerlaan CME, Olde Hanhof CJA, Dilmen E, Beumer J, et al. Tubuloid differentiation to model the human distal nephron and collecting duct in health and disease. Cell Rep. 2024;43(1):113614. doi:10.1016/j.celrep.2023.113614. [Google Scholar] [PubMed] [CrossRef]
20. Dong C, Zhou J, Su X, He Z, Song Q, Song C, et al. Understanding formation processes of calcareous nephrolithiasis in renal interstitium and tubule lumen. J Cell Mol Med. 2024;28(7):e18235. doi:10.1111/jcmm.18235. [Google Scholar] [PubMed] [CrossRef]
21. Cong J, Chang SJ, Thomsen JS, Andreasen A, Chen X, Xing J, et al. Ultrastructural identification of developing proximal tubules based on three-dimensional reconstruction. Vet Med Sci. 2021;7(5):1989–98. doi:10.1002/vms3.558. [Google Scholar] [PubMed] [CrossRef]
22. Vanslambrouck JM, Wilson SB, Tan KS, Groenewegen E, Rudraraju R, Neil J, et al. Enhanced metanephric specification to functional proximal tubule enables toxicity screening and infectious disease modelling in kidney organoids. Nat Commun. 2022;13(1):5943. doi:10.1038/s41467-022-33623-z. [Google Scholar] [PubMed] [CrossRef]
23. Bankir L, Figueres L, Prot-Bertoye C, Bouby N, Crambert G, Pratt JH, et al. Medullary and cortical thick ascending limb: similarities and differences. Am J Physiol Renal Physiol. 2020;318(2):F422–F42. doi:10.1152/ajprenal.00261.2019. [Google Scholar] [PubMed] [CrossRef]
24. Bankir L, Crambert G, Vargas-Poussou R. The SLC6A18 transporter is most likely a Na-dependent glycine/urea antiporter responsible for urea secretion in the proximal straight tubule: influence of this urea secretion on glomerular filtration rate. Nephron. 2024:1–27. doi:10.1159/000539602. [Google Scholar] [PubMed] [CrossRef]
25. Castaneda-Bueno M, Ellison DH, Gamba G. Molecular mechanisms for the modulation of blood pressure and potassium homeostasis by the distal convoluted tubule. EMBO Mol Med. 2022;14(2):e14273. doi:10.15252/emmm.202114273. [Google Scholar] [PubMed] [CrossRef]
26. Ma YS, Deng SQ, Zhang P, Thomsen JS, Andreasen A, Chang SJ, et al. Identification of countercurrent tubule-vessel arrangements in the early development of mouse kidney based on immunohistochemistry and computer-assisted 3D visualization. PLoS One. 2024;19(8):e0307223. doi:10.1371/journal.pone.0307223. [Google Scholar] [PubMed] [CrossRef]
27. Gesualdo L, Di Paolo S, Calabro A, Milani S, Maiorano E, Ranieri E, et al. Expression of epidermal growth factor and its receptor in normal and diseased human kidney: an immunohistochemical and in situ hybridization study. Kidney Int. 1996;49(3):656–65. doi:10.1038/ki.1996.94. [Google Scholar] [PubMed] [CrossRef]
28. Nakopoulou L, Stefanaki K, Boletis J, Papadakis J, Kostakis A, Vosnides G, et al. Immunohistochemical study of epidermal growth factor receptor (EGFR) in various types of renal injury. Nephrol Dial Transplant. 1994;9(7):764–9. [Google Scholar] [PubMed]
29. Orellana SA, Sweeney WE, Neff CD, Avner ED. Epidermal growth factor receptor expression is abnormal in murine polycystic kidney. Kidney Int. 1995;47(2):490–9. doi:10.1038/ki.1995.62. [Google Scholar] [PubMed] [CrossRef]
30. Tawengi M, Al-Dali Y, Tawengi A, Benter IF, Akhtar S. Targeting the epidermal growth factor receptor (EGFR/ErbB) for the potential treatment of renal pathologies. Front Pharmacol. 2024;15:1394997. doi:10.3389/fphar.2024.1394997. [Google Scholar] [PubMed] [CrossRef]
31. Breyer MD, Redha R, Breyer JA. Segmental distribution of epidermal growth factor binding sites in rabbit nephron. Am J Physiol. 1990;259(4):F553–8. doi:10.1152/ajprenal.1990.259.4.F553. [Google Scholar] [PubMed] [CrossRef]
32. Behrens MT, Corbin AL, Hise MK. Epidermal growth factor receptor regulation in rat kidney: two models of renal growth. Am J Physiol. 1989;257(6):F1059–64. doi:10.1152/ajprenal.1989.257.6.F1059. [Google Scholar] [PubMed] [CrossRef]
33. Burgess AW. Regulation of signaling from the epidermal growth factor family. J Phys Chem B. 2022;126(39):7475–85. doi:10.1021/acs.jpcb.2c04156. [Google Scholar] [PubMed] [CrossRef]
34. Hajdu T, Varadi T, Rebenku I, Kovacs T, Szollosi J, Nagy P. Comprehensive model for epidermal growth factor receptor ligand binding involving conformational states of the extracellular and the kinase domains. Front Cell Dev Biol. 2020;8:776. doi:10.3389/fcell.2020.00776. [Google Scholar] [PubMed] [CrossRef]
35. Cohen S. Origins of growth factors: nGF and EGF. J Biol Chem. 2008;283(49):33793–7. doi:10.1074/jbc.X800008200. [Google Scholar] [PubMed] [CrossRef]
36. Brockhoff G. Shedding light on HER4 signaling in normal and malignant breast tissues. Cell Signal. 2022;97:110401. doi:10.1016/j.cellsig.2022.110401. [Google Scholar] [PubMed] [CrossRef]
37. Gonzalez L, Diaz ME, Miquet JG, Sotelo AI, Dominici FP. Growth hormone modulation of hepatic epidermal growth factor receptor signaling. Trends Endocrinol Metab. 2021;32(6):403–14. doi:10.1016/j.tem.2021.03.004. [Google Scholar] [PubMed] [CrossRef]
38. Hong CS, Sun EG, Choi JN, Kim DH, Kim JH, Ryu KH, et al. Fibroblast growth factor receptor 4 increases epidermal growth factor receptor (EGFR) signaling by inducing amphiregulin expression and attenuates response to EGFR inhibitors in colon cancer. Cancer Sci. 2020;111(9):3268–78. doi:10.1111/cas.14526. [Google Scholar] [PubMed] [CrossRef]
39. Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp Cell Res. 2009;315(4):602–10. doi:10.1016/j.yexcr.2008.08.005. [Google Scholar] [PubMed] [CrossRef]
40. Zaykov V, Chaqour B. The CCN2/CTGF interactome: an approach to understanding the versatility of CCN2/CTGF molecular activities. J Cell Commun Signal. 2021;15(4):567–80. doi:10.1007/s12079-021-00650-2. [Google Scholar] [PubMed] [CrossRef]
41. Shen YW, Zhou YD, Chen HZ, Luan X, Zhang WD. Targeting CTGF in cancer: an emerging therapeutic opportunity. Trends Cancer. 2021;7(6):511–24. doi:10.1016/j.trecan.2020.12.001. [Google Scholar] [PubMed] [CrossRef]
42. Odegard J, Sondresen JE, Aasrum M, Tveteraas IH, Guren TK, Christoffersen T, et al. Differential effects of epidermal growth factor (EGF) receptor ligands on receptor binding, downstream signalling pathways and DNA synthesis in hepatocytes. Growth Fact. 2017;35(6):239–48. doi:10.1080/08977194.2018.1453506. [Google Scholar] [PubMed] [CrossRef]
43. Boyles JS, Atwell S, Druzina Z, Heuer JG, Witcher DR. Structural basis of selectivity and neutralizing activity of a TGFα/epiregulin specific antibody. Protein Sci. 2016;25(11):2028–36. doi:10.1002/pro.3023. [Google Scholar] [PubMed] [CrossRef]
44. Kamentseva RS, Kharchenko MV, Gabdrahmanova GV, Kotov MA, Kosheverova VV, Kornilova ES. TGF-α and amphiregulin differently regulate endometrium-derived mesenchymal stromal/stem cells. Int J Mol Sci. 2023;24(17):13408. doi:10.3390/ijms241713408. [Google Scholar] [PubMed] [CrossRef]
45. Wilson JL, Kefaloyianni E, Stopfer L, Harrison C, Sabbisetti VS, Fraenkel E, et al. Functional genomics approach identifies novel signaling regulators of TGFα ectodomain shedding. Mol Cancer Res. 2018;16(1):147–61. doi:10.1158/1541-7786.MCR-17-0140. [Google Scholar] [PubMed] [CrossRef]
46. Soto-Gamez A, Chen D, Nabuurs AGE, Quax WJ, Demaria M, Boersma YL. A bispecific inhibitor of the EGFR/ADAM17 axis decreases cell proliferation and migration of EGFR-dependent cancer cells. Cancers. 2020;12(2):411. doi:10.3390/cancers12020411. [Google Scholar] [PubMed] [CrossRef]
47. Nouwen EJ, De Broe ME. EGF and TGF-alpha in the human kidney: identification of octopal cells in the collecting duct. Kidney Int. 1994;45(5):1510–21. doi:10.1038/ki.1994.198. [Google Scholar] [PubMed] [CrossRef]
48. Xian CJ. Expression of transforming growth factor-alpha mRNA and peptide in rodent kidneys. Histochem Cell Biol. 1999;111(6):467–75. doi:10.1007/s004180050383. [Google Scholar] [PubMed] [CrossRef]
49. Davis-Fleischer KM, Besner GE. Structure and function of heparin-binding EGF-like growth factor (HB-EGF). Front Biosci. 1998;3(4):d288–99. doi:10.2741/a241. [Google Scholar] [PubMed] [CrossRef]
50. Son SS, Hwang S, Park JH, Ko Y, Yun SI, Lee JH, et al. In vivo silencing of amphiregulin by a novel effective Self-Assembled-Micelle inhibitory RNA ameliorates renal fibrosis via inhibition of EGFR signals. Sci Rep. 2021;11(1):2191. doi:10.1038/s41598-021-81726-2. [Google Scholar] [PubMed] [CrossRef]
51. Wozniak J, Floege J, Ostendorf T, Ludwig A. Key metalloproteinase-mediated pathways in the kidney. Nat Rev Nephrol. 2021;17(8):513–27. doi:10.1038/s41581-021-00415-5. [Google Scholar] [PubMed] [CrossRef]
52. Strachan L, Murison JG, Prestidge RL, Sleeman MA, Watson JD, Kumble KD. Cloning and biological activity of epigen, a novel member of the epidermal growth factor superfamily. J Biol Chem. 2001;276(21):18265–71. doi:10.1074/jbc.M006935200. [Google Scholar] [PubMed] [CrossRef]
53. Mishra R, Leahy P, Simonson MS. Gene expression profiling reveals role for EGF-family ligands in mesangial cell proliferation. Am J Physiol Renal Physiol. 2002;283(5):F1151–9. doi:10.1152/ajprenal.00103.2002. [Google Scholar] [PubMed] [CrossRef]
54. Dunbar AJ, Priebe IK, Belford DA, Goddard C. Identification of betacellulin as a major peptide growth factor in milk: purification, characterization and molecular cloning of bovine betacellulin. Biochem J. 1999;344(3):713–21. doi:10.1042/bj3440713. [Google Scholar] [CrossRef]
55. Elshazly AM, Gewirtz DA. An overview of resistance to Human epidermal growth factor receptor 2 (Her2) targeted therapies in breast cancer. Cancer Drug Resist. 2022;5(2):472–86. doi:10.20517/cdr.2022.09. [Google Scholar] [PubMed] [CrossRef]
56. Ju F, Atyah MM, Horstmann N, Gul S, Vago R, Bruns CJ, et al. Characteristics of the cancer stem cell niche and therapeutic strategies. Stem Cell Res Ther. 2022;13(1):233. doi:10.1186/s13287-022-02904-1. [Google Scholar] [PubMed] [CrossRef]
57. He Q, Qu M, Bao H, Xu Y, Shen T, Tan D, et al. Multiple post-translational modifications ensure EGFR functionality: potential therapeutic targets to overcome its drug-resistance mutations. Cytokine Growth Factor Rev. 2023;70(Pt 2):41–53. doi:10.1016/j.cytogfr.2023.03.003. [Google Scholar] [PubMed] [CrossRef]
58. Fabrizio FP, Sparaneo A, Muscarella LA. Monitoring EGFR-lung cancer evolution: a possible beginning of a methylation era in TKI resistance prediction. Front Oncol. 2023;13:1137384. doi:10.3389/fonc.2023.1137384. [Google Scholar] [PubMed] [CrossRef]
59. Zhou Y, Sakurai H. New trend in ligand-induced EGFR trafficking: a dual-mode clathrin-mediated endocytosis model. J Proteomics. 2022;255(1999):104503. doi:10.1016/j.jprot.2022.104503. [Google Scholar] [PubMed] [CrossRef]
60. Sheng Z, Cao X, Deng YN, Zhao X, Liang S. SUMOylation of AnxA6 facilitates EGFR-PKCα complex formation to suppress epithelial cancer growth. Cell Commun Signal. 2023;21(1):189. doi:10.1186/s12964-023-01217-x. [Google Scholar] [PubMed] [CrossRef]
61. Zhu Q, Dong H, Bukhari AA, Zhao A, Li M, Sun Y, et al. HUWE1 promotes EGFR ubiquitination and degradation to protect against renal tubulointerstitial fibrosis. FASEB J. 2020;34(3):4591–601. doi:10.1096/fj.201902751R. [Google Scholar] [PubMed] [CrossRef]
62. Ray P, Raghunathan K, Ahsan A, Allam US, Shukla S, Basrur V, et al. Ubiquitin ligase SMURF2 enhances epidermal growth factor receptor stability and tyrosine-kinase inhibitor resistance. J Biol Chem. 2020;295(36):12661–73. doi:10.1074/jbc.RA120.013519. [Google Scholar] [PubMed] [CrossRef]
63. Zhao Y, Wang H, He C. Drug resistance of targeted therapy for advanced non-small cell lung cancer harbored EGFR mutation: from mechanism analysis to clinical strategy. J Cancer Res Clin Oncol. 2021;147(12):3653–64. doi:10.1007/s00432-021-03828-8. [Google Scholar] [PubMed] [CrossRef]
64. Sun R, Hou Z, Zhang Y, Jiang B. Drug resistance mechanisms and progress in the treatment of EGFR-mutated lung adenocarcinoma. Oncol Lett. 2022;24(5):408. doi:10.3892/ol.2022.13528. [Google Scholar] [PubMed] [CrossRef]
65. Li Q, Li Z, Luo T, Shi H. Targeting the PI3K/AKT/mTOR and RAF/MEK/ERK pathways for cancer therapy. Mol Biomed. 2022;3(1):47. doi:10.1186/s43556-022-00110-2. [Google Scholar] [PubMed] [CrossRef]
66. Li H, Shao F, Qian B, Sun Y, Huang Z, Ding Z, et al. Upregulation of HER2 in tubular epithelial cell drives fibroblast activation and renal fibrosis. Kidney Int. 2019;96(3):674–88. doi:10.1016/j.kint.2019.04.012. [Google Scholar] [PubMed] [CrossRef]
67. Chen J, You H, Li Y, Xu Y, He Q, Harris RC. EGF receptor-dependent YAP activation is important for renal recovery from AKI. J Am Soc Nephrol. 2018;29(9):2372–85. doi:10.1681/ASN.2017121272. [Google Scholar] [PubMed] [CrossRef]
68. Cao S, Pan Y, Terker AS, Arroyo Ornelas JP, Wang Y, Tang J, et al. Epidermal growth factor receptor activation is essential for kidney fibrosis development. Nat Commun. 2023;14(1):7357. doi:10.1038/s41467-023-43226-x. [Google Scholar] [PubMed] [CrossRef]
69. Roskoski R Jr. ErbB/HER protein-tyrosine kinases: structures and small molecule inhibitors. Pharmacol Res. 2014;87(10):42–59. doi:10.1016/j.phrs.2014.06.001. [Google Scholar] [PubMed] [CrossRef]
70. Takeuchi K, Ito F. EGF receptor in relation to tumor development: molecular basis of responsiveness of cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J. 2010;277(2):316–26. doi:10.1111/j.1742-4658.2009.07450.x. [Google Scholar] [PubMed] [CrossRef]
71. Liu Q, Yu S, Zhao W, Qin S, Chu Q, Wu K. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol Cancer. 2018;17(1):53. doi:10.1186/s12943-018-0793-1. [Google Scholar] [PubMed] [CrossRef]
72. Harskamp LR, Gansevoort RT, van Goor H, Meijer E. The epidermal growth factor receptor pathway in chronic kidney diseases. Nat Rev Nephrol. 2016;12(8):496–506. doi:10.1038/nrneph.2016.91. [Google Scholar] [PubMed] [CrossRef]
73. Tatsumoto N, Saito S, Rifkin IR, Bonegio RG, Leal DN, Sen GC, et al. EGF-receptor-dependent TLR7 signaling in macrophages promotes glomerular injury in crescentic glomerulonephritis. Lab Invest. 2023;103(9):100190. doi:10.1016/j.labinv.2023.100190. [Google Scholar] [PubMed] [CrossRef]
74. Liu N, Wang L, Yang T, Xiong C, Xu L, Shi Y, et al. EGF receptor inhibition alleviates hyperuricemic nephropathy. J Am Soc Nephrol. 2015;26(11):2716–29. doi:10.1681/ASN.2014080793. [Google Scholar] [PubMed] [CrossRef]
75. Xu X, Wang J, Yang R, Dong Z, Zhang D. Genetic or pharmacologic inhibition of EGFR ameliorates sepsis-induced AKI. Oncotarget. 2017;8(53):91577–92. doi:10.18632/oncotarget.21244. [Google Scholar] [PubMed] [CrossRef]
76. Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, et al. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol. 2012;23(5):854–67. doi:10.1681/ASN.2011050493. [Google Scholar] [PubMed] [CrossRef]
77. Zhang MZ, Wang Y, Paueksakon P, Harris RC. Epidermal growth factor receptor inhibition slows progression of diabetic nephropathy in association with a decrease in endoplasmic reticulum stress and an increase in autophagy. Diabetes. 2014;63(6):2063–72. doi:10.2337/db13-1279. [Google Scholar] [PubMed] [CrossRef]
78. Bollee G, Flamant M, Schordan S, Fligny C, Rumpel E, Milon M, et al. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med. 2011;17(10):1242–50. doi:10.1038/nm.2491. [Google Scholar] [PubMed] [CrossRef]
79. Chen J, Chen JK, Harris RC. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int. 2012;82(1):45–52. doi:10.1038/ki.2012.43. [Google Scholar] [PubMed] [CrossRef]
80. De S, Zhou H, DeSantis D, Croniger CM, Li X, Stark GR. Erlotinib protects against LPS-induced endotoxicity because TLR4 needs EGFR to signal. Proc Natl Acad Sci U S A. 2015;112(31):9680–5. doi:10.1073/pnas.1511794112. [Google Scholar] [PubMed] [CrossRef]
81. Gallo PM, Chain RW, Xu J, Whiteman LM, Palladino A, Caricchio R, et al. EGFR-ErbB2 dual kinase inhibitor lapatinib decreases autoantibody levels and worsens renal disease in Interferon α-accelerated murine lupus. Int Immunopharmacol. 2024;140(7):112692. doi:10.1016/j.intimp.2024.112692. [Google Scholar] [PubMed] [CrossRef]
82. Xu Z, Zhao Y, Zhong P, Wang J, Weng Q, Qian Y, et al. EGFR inhibition attenuates diabetic nephropathy through decreasing ROS and endoplasmic reticulum stress. Oncotarget. 2017;8(20):32655–67. doi:10.18632/oncotarget.15948. [Google Scholar] [PubMed] [CrossRef]
83. Fang Q, Zou C, Zhong P, Lin F, Li W, Wang L, et al. EGFR mediates hyperlipidemia-induced renal injury via regulating inflammation and oxidative stress: the detrimental role and mechanism of EGFR activation. Oncotarget. 2016;7(17):24361–73. doi:10.18632/oncotarget.8222. [Google Scholar] [PubMed] [CrossRef]
84. Wassef L, Kelly DJ, Gilbert RE. Epidermal growth factor receptor inhibition attenuates early kidney enlargement in experimental diabetes. Kidney Int. 2004;66(5):1805–14. doi:10.1111/j.1523-1755.2004.00955.x. [Google Scholar] [PubMed] [CrossRef]
85. Tang J, Liu N, Tolbert E, Ponnusamy M, Ma L, Gong R, et al. Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am J Pathol. 2013;183(1):160–72. doi:10.1016/j.ajpath.2013.04.005. [Google Scholar] [PubMed] [CrossRef]
86. Abdelmageed MM, Kefaloyianni E, Arthanarisami A, Komaru Y, Atkinson JJ, Herrlich A. TNF or EGFR inhibition equally block AKI-to-CKD transition: opportunities for etanercept treatment. Nephrol Dial Transplant. 2023;38(5):1139–50. doi:10.1093/ndt/gfac290. [Google Scholar] [PubMed] [CrossRef]
87. Rintala JM, Savikko J, Palin N, Rintala SE, Koskinen PK, von Willebrand E. Epidermal growth factor inhibition, a novel pathway to prevent chronic allograft injury. Transplantation. 2014;98(8):821–7. doi:10.1097/TP.0000000000000325. [Google Scholar] [PubMed] [CrossRef]
88. Advani A, Wiggins KJ, Cox AJ, Zhang Y, Gilbert RE, Kelly DJ. Inhibition of the epidermal growth factor receptor preserves podocytes and attenuates albuminuria in experimental diabetic nephropathy. Nephrology. 2011;16(6):573–81. doi:10.1111/j.1440-1797.2011.01451.x. [Google Scholar] [PubMed] [CrossRef]
89. Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, et al. EGFR signaling promotes TGFβ-dependent renal fibrosis. J Am Soc Nephrol. 2012;23(2):215–24. doi:10.1681/ASN.2011070645. [Google Scholar] [PubMed] [CrossRef]
90. Chen J, Chen JK, Harris RC. EGF receptor deletion in podocytes attenuates diabetic nephropathy. J Am Soc Nephrol. 2015;26(5):1115–25. doi:10.1681/ASN.2014020192. [Google Scholar] [PubMed] [CrossRef]
91. Chen J, Harris RC. Interaction of the EGF receptor and the hippo pathway in the diabetic kidney. J Am Soc Nephrol. 2016;27(6):1689–700. doi:10.1681/ASN.2015040415. [Google Scholar] [PubMed] [CrossRef]
92. Li Z, Li Y, Overstreet JM, Chung S, Niu A, Fan X, et al. Inhibition of epidermal growth factor receptor activation is associated with improved diabetic nephropathy and insulin resistance in type 2 diabetes. Diabetes. 2018;67(9):1847–57. doi:10.2337/db17-1513. [Google Scholar] [PubMed] [CrossRef]
93. Li Y, Pan Y, Cao S, Sasaki K, Wang Y, Niu A, et al. Podocyte EGFR inhibits autophagy through upregulation of rubicon in type 2 diabetic nephropathy. Diabetes. 2021;70(2):562–76. doi:10.2337/db20-0660. [Google Scholar] [PubMed] [CrossRef]
94. Rintala JM, Savikko J, Rintala SE, Palin N, Koskinen PK. Epidermal growth factor receptor inhibition with erlotinib ameliorates anti-Thy 1.1-induced experimental glomerulonephritis. J Nephrol. 2016;29(3):359–65. doi:10.1007/s40620-015-0233-x. [Google Scholar] [PubMed] [CrossRef]
95. Qing X, Chinenov Y, Redecha P, Madaio M, Roelofs JJ, Farber G, et al. iRhom2 promotes lupus nephritis through TNF-α and EGFR signaling. J Clin Invest. 2018;128(4):1397–412. doi:10.1172/JCI97650. [Google Scholar] [PubMed] [CrossRef]
96. Melderis S, Warkotsch MT, Dang J, Hagenstein J, Ehnold LI, Herrnstadt GR, et al. The amphiregulin/EGFR axis protects from lupus nephritis via downregulation of pathogenic CD4+ T helper cell responses. J Autoimmun. 2022;129(4):102829. doi:10.1016/j.jaut.2022.102829. [Google Scholar] [PubMed] [CrossRef]
97. Rayego-Mateos S, Morgado-Pascual JL, Sanz AB, Ramos AM, Eguchi S, Batlle D, et al. TWEAK transactivation of the epidermal growth factor receptor mediates renal inflammation. J Pathol. 2013;231(4):480–94. doi:10.1002/path.4250. [Google Scholar] [PubMed] [CrossRef]
98. Yang L, Yuan H, Yu Y, Yu N, Ling L, Niu J, et al. Epidermal growth factor receptor mimotope alleviates renal fibrosis in murine unilateral ureteral obstruction model. Clin Immunol. 2019;205(2014):57–64. doi:10.1016/j.clim.2019.05.014. [Google Scholar] [PubMed] [CrossRef]
99. Shiva N, Sharma N, Kulkarni YA, Mulay SR, Gaikwad AB. Renal ischemia/reperfusion injury: an insight on in vitro and in vivo models. Life Sci. 2020;256:117860. doi:10.1016/j.lfs.2020.117860. [Google Scholar] [PubMed] [CrossRef]
100. Moon D, Padanilam BJ, Park KM, Kim J. Loss of SAV1 in kidney proximal tubule induces maladaptive repair after ischemia and reperfusion injury. Int J Mol Sci. 2024;25(9):4610. doi:10.3390/ijms25094610. [Google Scholar] [PubMed] [CrossRef]
101. Moon D, Padanilam BJ, Jang HS, Kim J. 2-Mercaptoethanol protects against DNA double-strand breaks after kidney ischemia and reperfusion injury through GPX4 upregulation. Pharmacol Rep. 2022;74(5):1041–53. doi:10.1007/s43440-022-00403-x. [Google Scholar] [PubMed] [CrossRef]
102. Sato Y, Takahashi M, Yanagita M. Pathophysiology of AKI to CKD progression. Semin Nephrol. 2020;40(2):206–15. doi:10.1016/j.semnephrol.2020.01.011. [Google Scholar] [PubMed] [CrossRef]
103. Yu JB, Lee DS, Padanilam BJ, Kim J. Repeated administration of cisplatin transforms kidney fibroblasts through G2/M arrest and cellular senescence. Cells. 2022;11(21):3472. doi:10.3390/cells11213472. [Google Scholar] [PubMed] [CrossRef]
104. Wu Y, Li D, Wang H, Wan X. Protective effect of poria cocos polysaccharides on fecal peritonitis-induced sepsis in mice through inhibition of oxidative stress, inflammation, apoptosis, and reduction of treg cells. Front Microbiol. 2022;13:887949. doi:10.3389/fmicb.2022.887949. [Google Scholar] [PubMed] [CrossRef]
105. Manrique-Caballero CL, Del Rio-Pertuz G, Gomez H. Sepsis-associated acute kidney injury. Crit Care Clin. 2021;37(2):279–301. doi:10.1016/j.ccc.2020.11.010. [Google Scholar] [PubMed] [CrossRef]
106. Molema G, Zijlstra JG, van Meurs M, Kamps J. Renal microvascular endothelial cell responses in sepsis-induced acute kidney injury. Nat Rev Nephrol. 2022;18(2):95–112. doi:10.1038/s41581-021-00489-1. [Google Scholar] [PubMed] [CrossRef]
107. Lichtnekert J, Anders HJ. Lupus nephritis-related chronic kidney disease. Nat Rev Rheumatol. 2024;20(11):699–711. doi:10.1038/s41584-024-01158-w. [Google Scholar] [PubMed] [CrossRef]
108. Zhan HQ, Zhang X, Chen XL, Cheng L, Wang X. Application of nanotechnology in the treatment of glomerulonephritis: current status and future perspectives. J Nanobiotechnol. 2024;22(1):9. doi:10.1186/s12951-023-02257-8. [Google Scholar] [PubMed] [CrossRef]
109. Chen DQ, Chen L, Guo Y, Wu XQ, Zhao TT, Zhao HL, et al. Poricoic acid A suppresses renal fibroblast activation and interstitial fibrosis in UUO rats via upregulating Sirt3 and promoting beta-catenin K49 deacetylation. Acta Pharmacol Sin. 2023;44(5):1038–50. doi:10.1038/s41401-022-01026-x. [Google Scholar] [PubMed] [CrossRef]
110. Hammad FT. The long-term renal effects of short periods of unilateral ureteral obstruction. Int J Physiol Pathophysiol Pharmacol. 2022;14(2):60–72. [Google Scholar] [PubMed]
111. Reicherz A, Eltit F, Almutairi K, Mojtahedzadeh B, Herout R, Chew B, et al. Ureteral obstruction promotes ureteral inflammation and fibrosis. Eur Urol Focus. 2023;9(2):371–80. doi:10.1016/j.euf.2022.09.014. [Google Scholar] [PubMed] [CrossRef]
112. Nan QY, Piao SG, Jin JZ, Chung BH, Yang CW, Li C. Pathogenesis and management of renal fibrosis induced by unilateral ureteral obstruction. Kidney Res Clin Pract. 2024;43(5):586–99. doi:10.23876/j.krcp.23.156. [Google Scholar] [PubMed] [CrossRef]
113. Norregaard R, Mutsaers HAM, Frokiaer J, Kwon TH. Obstructive nephropathy and molecular pathophysiology of renal interstitial fibrosis. Physiol Rev. 2023;103(4):2827–72. doi:10.1152/physrev.00027.2022. [Google Scholar] [PubMed] [CrossRef]
114. Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol. 2013;24(2):229–42. doi:10.1681/ASN.2012070678. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF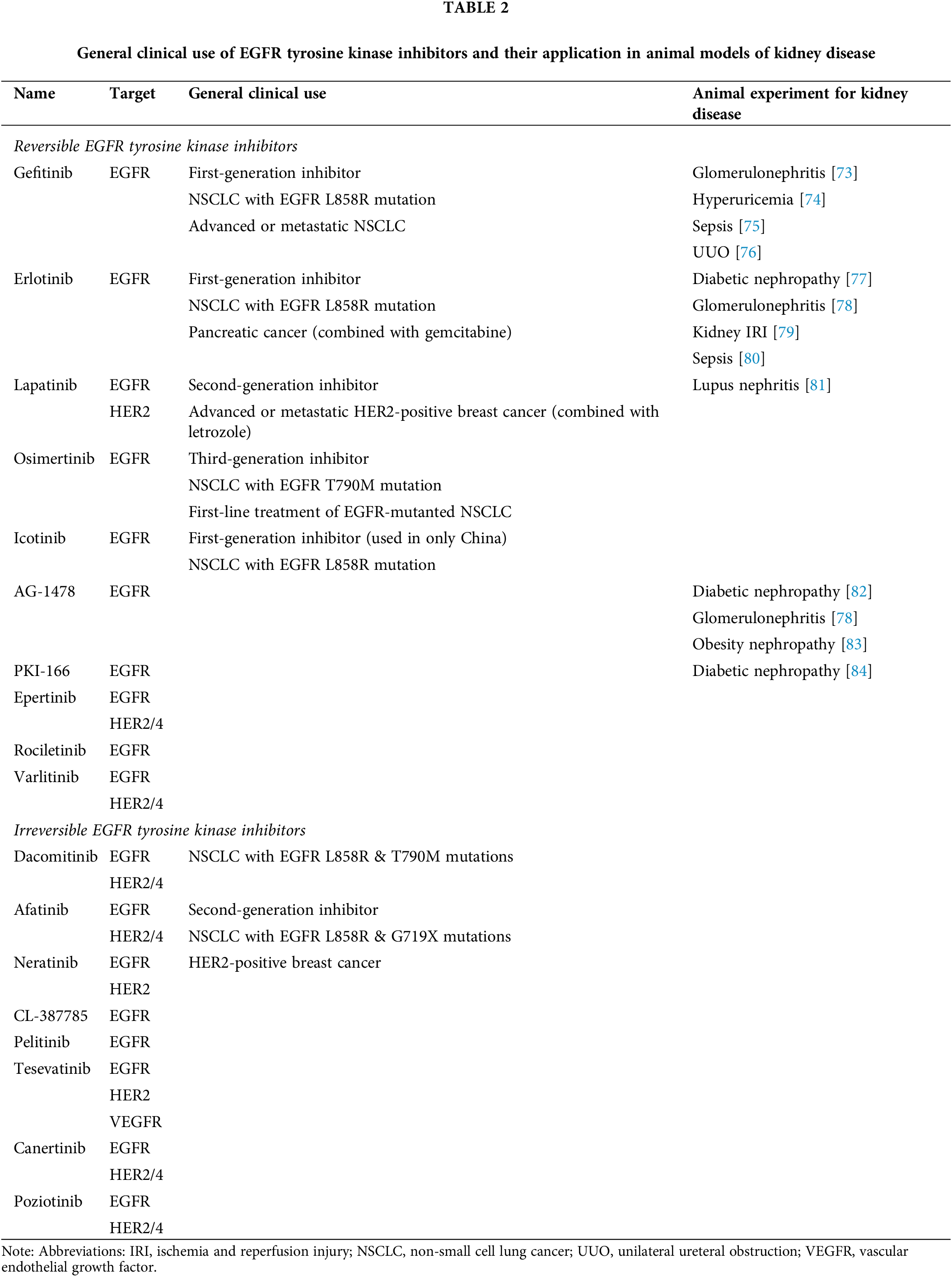

 Downloads
Downloads
 Citation Tools
Citation Tools