 Open Access
Open Access
ARTICLE
Early Cardiac Catheterizations within 30 Days Post Congenital Heart Surgery in Children
1
Pediatric Heart Centre–Division of Pediatric Cardiology, University Children’s Hospital Zurich, Zurich, Switzerland
2
Department Neonatology and Pediatric Intensive Care, University Children’s Hospital Zurich, Zurich, Switzerland
3
Department of Congenital Cardiothoracic Surgery, University Children’s Hospital Zurich, Zurich, Switzerland
4
Children’s Research Centre, Zurich, Switzerland, University Children’s Hospital Zurich, Zurich, Switzerland
5
University of Zurich, Zurich, Switzerland
* Corresponding Author: Daniel Quandt. Email:
# These two authors share the first authorship of this manuscript
Congenital Heart Disease 2023, 18(1), 79-95. https://doi.org/10.32604/chd.2022.022401
Received 09 March 2022; Accepted 27 May 2022; Issue published 09 January 2023
Abstract
Background: This study set out to assess the indications, feasibility, safety, and outcome of early cardiac catheterizations (CC) within 30 days after congenital heart surgery (CHS) in children. Methods and Results: This is a retrospective, single-center case review study of all CC within 30 days after CHS between 1/2010-12/2020. A total of 317 (138 diagnostic, 179 interventional) CC were performed in 245 patients at a median of 4 days (IQR 13) after CHS. The median age was 3 months (IQR 6), and body weight was 5 kg (IQR 4). A total of 194 (61.2%) CC were performed in patients with univentricular hearts. CC revealed significant pathologies leading to early redo-surgery in 37 patients (12%). The transcatheter interventions primarily were needed in patients after cavo-pulmonary connection (n = 69%, 21.8%), right ventricle to pulmonary artery conduit (n = 39%, 12.3%), and Norwood-I surgery (n = 34%, 10.7%) presenting with hypoxemia, prolonged postoperative course, and suspected arterial stenosis on echocardiography. The clinical impact of an early postoperative transcatheter intervention for the following clinical course was high in most cases. There were nine (2.8%) major and 20 (6.3%) minor intra-procedural complications. Risk factor analysis revealed no difference for the occurrence of complications for patients’ age, weight, and time from initial CHS, underlying uni- vs. biventricular heart disease, or ECMO. Conclusion: Early CC within 30 days after CHS in children can be performed safely with a high diagnostic and therapeutic value. The rate of complications is low, while the therapeutic consequence is relevant.Keywords
Cardiac catheterizations (CC) in the early postoperative period after congenital heart surgery (CHS) are proclaimed to be high-risk procedures associated with an elevated rate of morbidity and mortality [1–5]. This is due to early postoperative hemodynamic instability and vulnerability, especially in young patients and children. Therefore, CCs during the early postoperative period are often avoided or postponed. Nevertheless, unsatisfying outcomes after congenital heart surgery will in some cases necessitate re-operations or transcatheter interventions in the immediate postoperative period [1–9]. Yet, early cardiac transcatheter interventions may be the safest procedural option, especially in critically ill children in whom re-operations on cardiopulmonary bypass in quick succession are deemed to be of even greater risk [6–9]. Considering the limited data available in the literature [1–9], we propose to evaluate indications, feasibility, safety, and outcome of early CC within 30 days after CHS in children at our institution over an 11-year period. During the assessed period (from 2010 to 2020), the interventional lead and team of the CC laboratory with its standards, know-how, and technical options remained unchanged.
2.1 Study Design and Patient Selection
This is a retrospective, single-center case study. All consecutive patients undergoing an early postoperative CC within 30 days after CHS between 01/2010 and 12/2020 at our institution were included. Demographic data, underlying congenital heart disease, preceding congenital cardiac surgery, treatment prior to CC (i.e., ventilation status, cardiac medication), and indications for CC were analyzed. Pre-procedural and intra-procedural details of the CC (e.g., the status of inotropic support), vascular access used, procedural time and fluoroscopy time, peri-procedural complications and post-interventional outcomes including mortality were recorded and analyzed. Analysis was stratified according to the preceding cardiac surgical procedure. Consecutive procedures after diagnostic and interventional catheterizations were registered. To categorize the different CC procedures, the “procedure type risk category” (PTRC) published by Bergersen et al. [10] was used, as well as their “definitions for adverse event severity” [10]. Analysis of adverse events (AEs) and their relation to procedure type risk categories was performed.
2.2 Cardiac Catheterization Procedures
Percutaneous CCs were performed under general anesthesia at our institution. Patients received 100 IU/kg bolus of heparin intravenously if they were not anticoagulated prior to CC; otherwise, peri-procedural anticoagulation management was monitored, and titrated measuring activated clotting time (ACT). Standard intravenous antibiotic prophylaxis (cephalosporin) was given if foreign body material was implanted. Biplane angiography was used in all interventional trans-catheteral procedures.
Continuous data are presented as median (IQR) and categorical data are expressed as counts and percentages (%). For normally distributed continuous variables, Levene’s test for equality of variance was used to analyze if the variability in the two groups was significantly different and group comparison was performed using two-sample t-tests. Wilcoxon rank analyses were used for group comparisons of non-normally distributed continuous variables. Ordinal, nominal, and dichotomic variables were evaluated with contingency tables and compared with chi-square-tests analyses Significance testing was 2-sided with the significance level set at p < 0.05. Risk factor analysis calculation was used to evaluate the correlation between the number of AEs and corresponding PTRCs.
The study design fulfills the guidelines of the declaration of Helsinki regarding ethical principles for medical research involving human subjects. The study was approved by the ethical committee of the Kanton Zurich, Switzerland (ID PB_2016-00311).
A total of 317 (138 diagnostic, 179 interventional) postoperative CCs were performed in 245 patients during January 2010 and December 2020. There was a total of 3247 CHS within this period, giving a rate of early postoperative CC of 9.7%. The study includes a median follow-up period of 1.7 years (IQR 2.9) after the postoperative CC. Preceding cardiac surgeries and cardiac anatomies are detailed in Table 1.
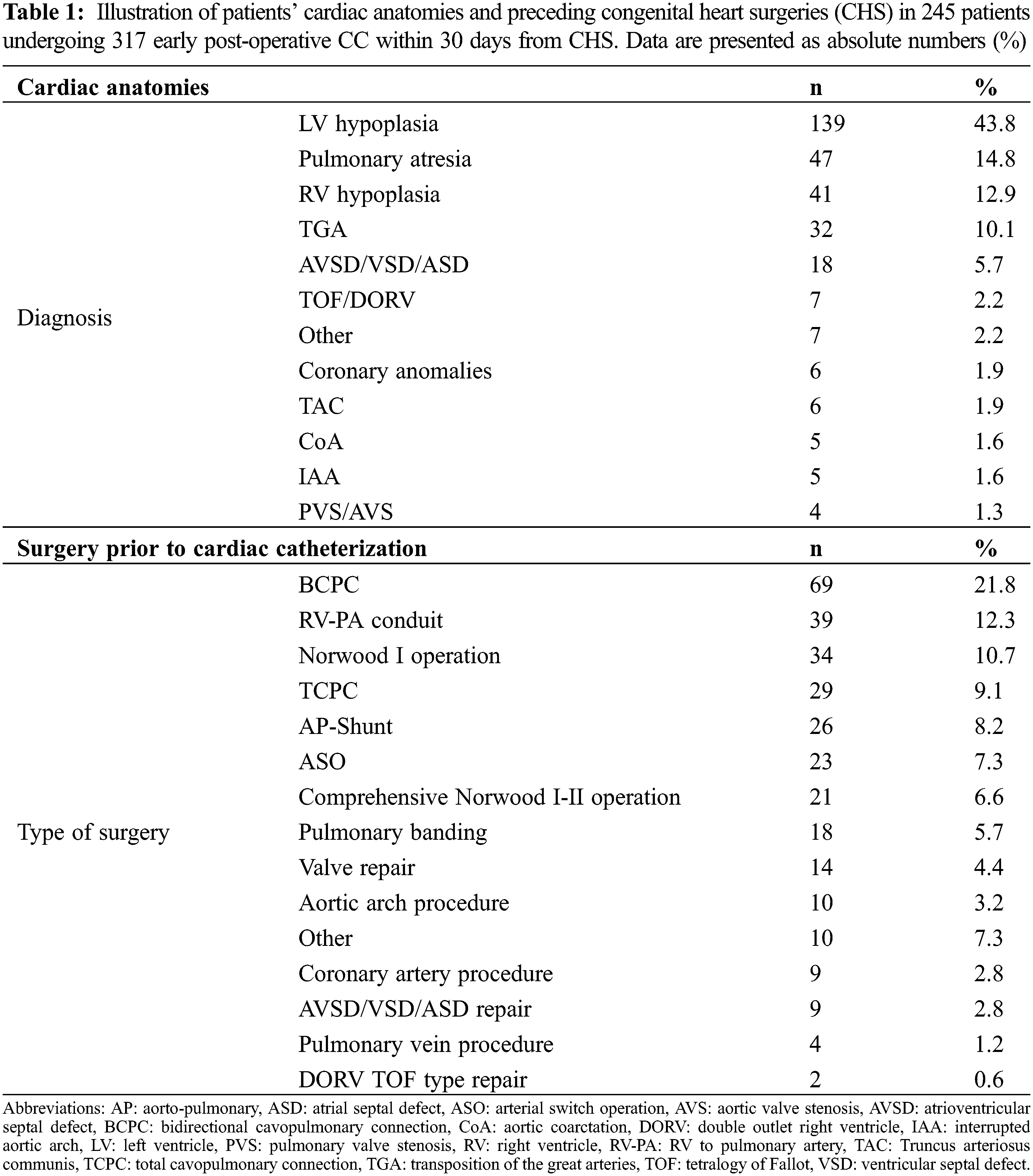
A total of 194 procedures (61.2%) were performed in patients with univentricular heart disease. Median age at catheterization was 3 months (IQR 6); median weight was 5 kg (IQR 4); sex was male in 190 (59.9%) and CC were performed at a median of 4 days (IQR 13) after CHS. Indications to perform early CC are detailed in Table 2.
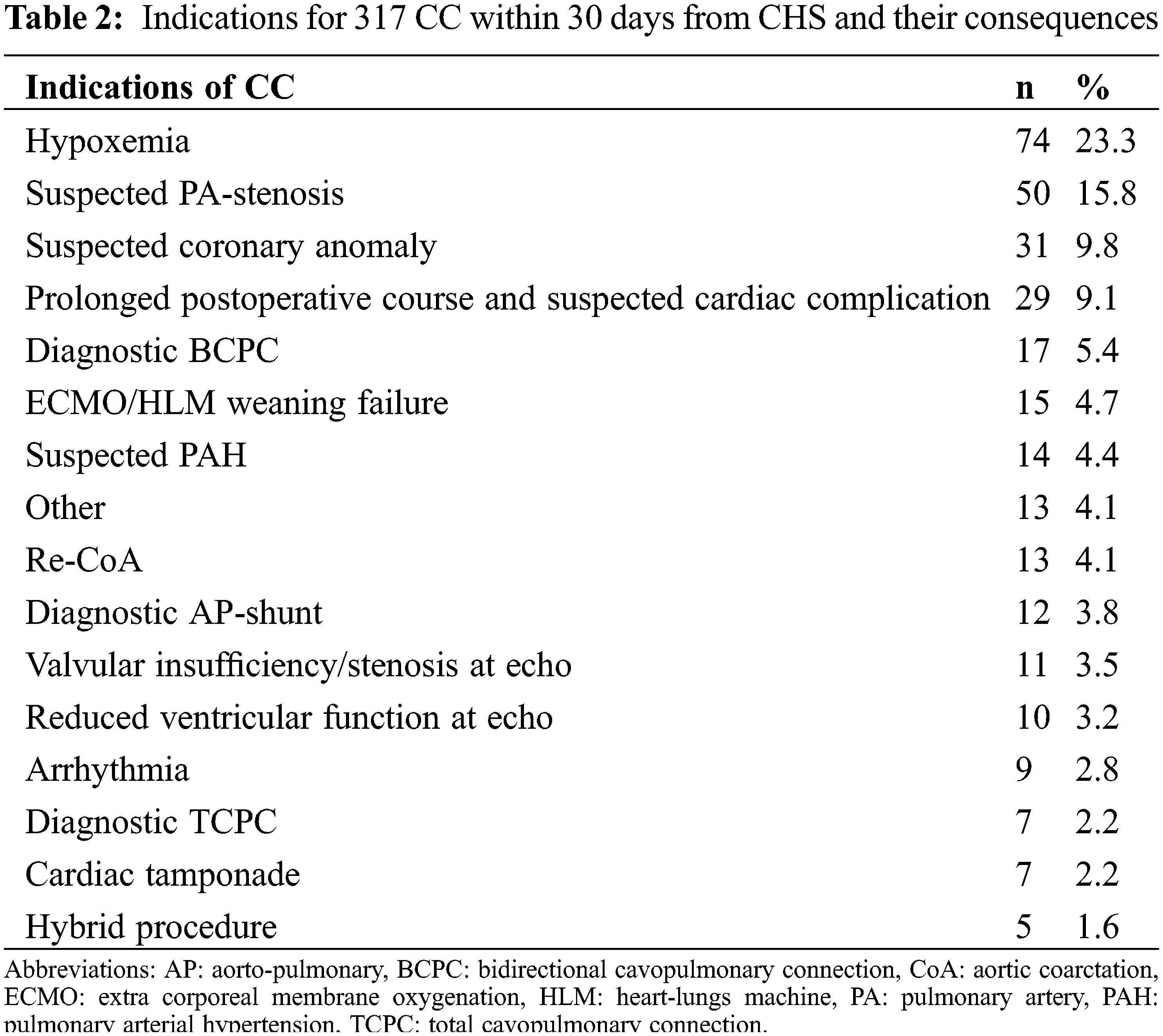
Hypoxaemia, suspected pulmonary artery stenosis, prolonged postoperative course, suspected coronary artery abnormalities, diagnostic after BCPC and ECMO/HLM weaning failure were the most frequent.
3.1 Technical Details of all Cardiac Catheterizations
A total of 196 (61.8%) patients were ventilated prior to CC; 150 (47.3%) CC were performed under pre-existing inotropic support, whilst 41 (8%) CC were performed under extracorporeal mechanical support (ECMO). The median PTRC score (5) was 4 (IQR 2) for all CCs. Vascular venous access (femoral or jugular) was the preferred access site (n = 120%, 37.9%), followed by combined venous and arterial access (n = 137%, 37.9%), and arterial access (n = 55%, 17.4%). Twenty-nine procedures were performed using other access sites (e.g., ECMO cannulas or hybrid procedures), where vascular access for the procedure was surgically provided by the cardiac surgeon. Median procedure time was 61 min (IQR 65) with a radiography screening time of 19 min (IQR 53).
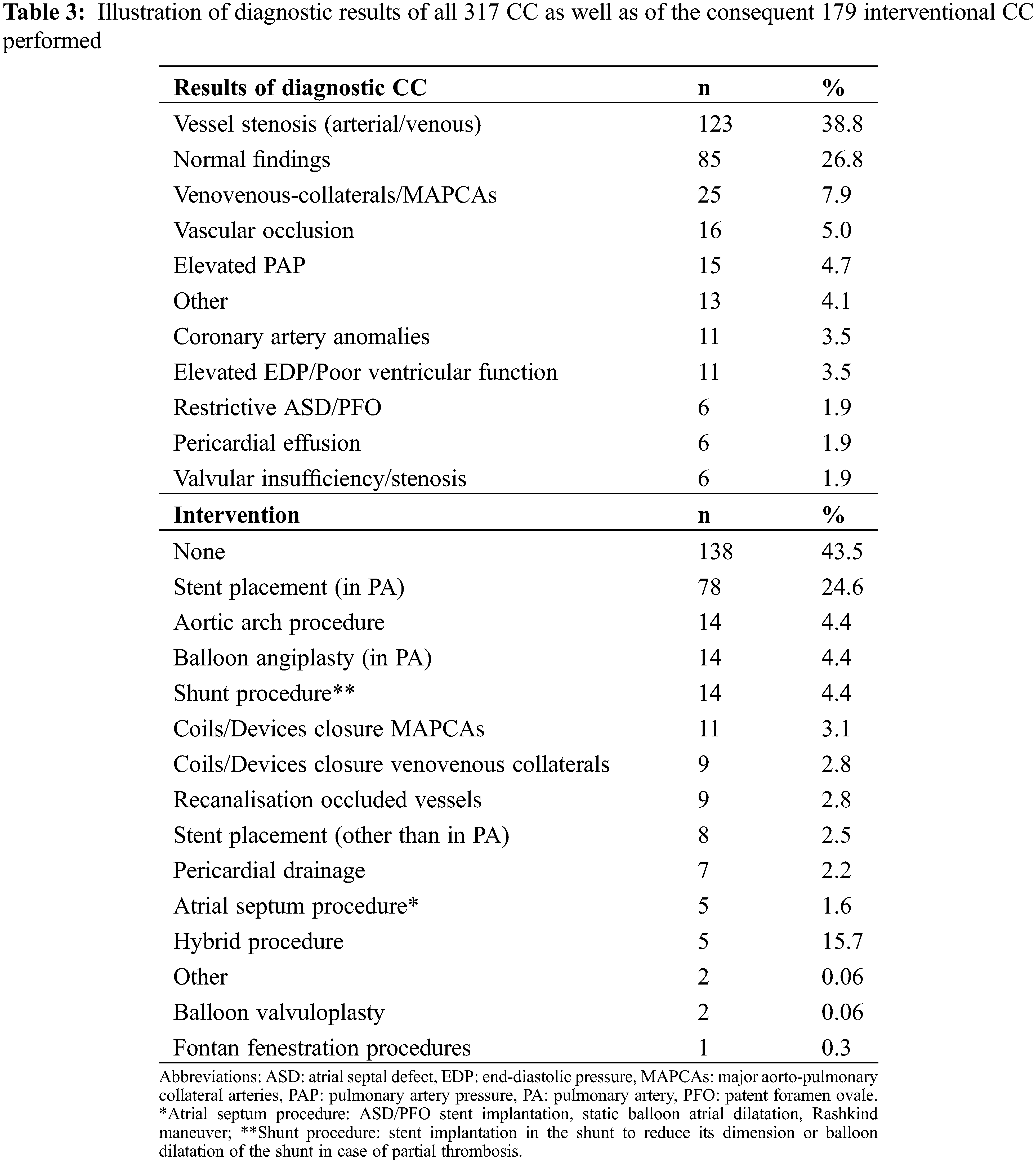
3.2 Results of Diagnostic Cardiac Catheterization and Interventions Performed
Diagnostic procedures revealed significant pathologies leading to early redo-surgery in 37/317 procedures (11.7%), while 179 (56%) underwent a catheter intervention. Overall immediate technical success rate (e.g., successful stent or device placement, release of stenosis, successful collateral occlusion) for catheter interventions was 93.5%. Table 3 summarizes the intra-interventional findings and interventions performed.
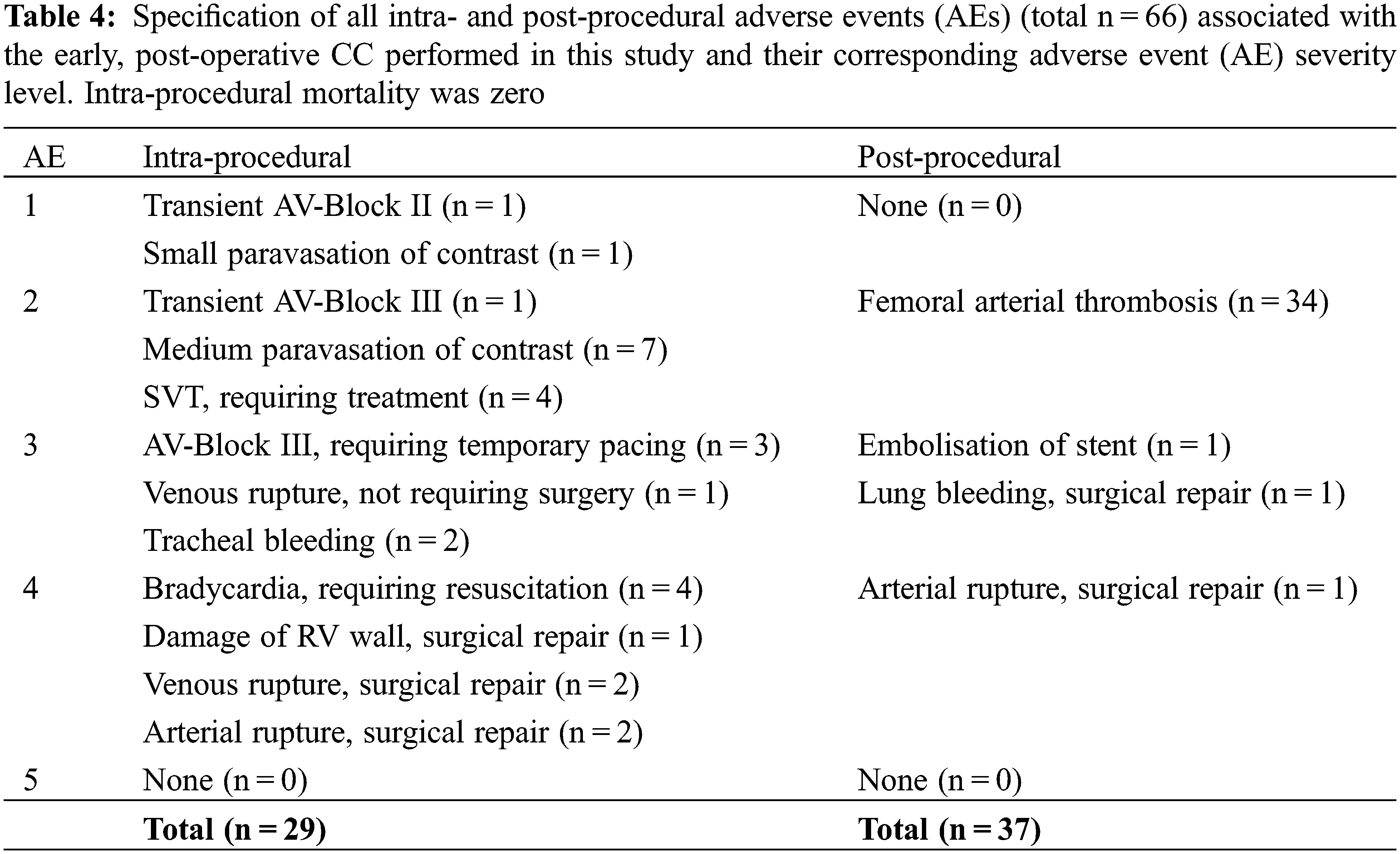
Intra- and post-procedural complications (AEs) were scored as suggested by Bergersen et al. [10] with a severity level for AEs ranging from (1-None to 5-Catastrophic). There was a total of 29 (9.1%) intra-procedural and 37 (11.6%) post-procedural adverse events. Most of these events were minor (AE severity level ≤3), while 9 (2.8%) intra-procedural events were major (AE severity level ≥4). All procedure related minor and major AEs and severity levels are detailed in Table 4.
In relation to the number of CC performed in each PTRC, the relative risk for the occurrence of any intra- or post-procedural complication was the highest in procedure type risk category 3 with 40%, followed by PRTC 4 with 30%, PTRC 2 with 17%, and 0% of PRTC 1.
Incidence of adverse events was not influenced by patients’ weight (p = 0.1), patients’ age (p = 0.26), time from initial surgery to early CC (p = 0.08), ICU-time after CC (p = 0.09), interval from CC to death (p = 0.6), mechanical ventilation prior to CC (p = 0.9), CC on ECMO (p = 0.1), inotropic support prior to CC (p = 0.09) or underlying uni- versus biventricular heart disease (p = 0.71). Procedure time was shorter in patients without AEs (p < 0.01).
3.4 Impact and Outcome of Early Post-Operative Cardiac Catheterizations
To assess the potential influence on the clinical outcome of the performed early post-operative CC interventions, different clinical parameters were evaluated. Clinical improvement of heart failure (e.g., reduced work of breathing, improved capillary refill, etc.) was noticed within 24 h after early CC in 87/179 (49%) interventional CC. Reduction of oxygen supplementation (within 24 h from CC) was documented in 71/179 (40%), reduction of inotropic support in 15/179 (8%), and reduction in the amount of pleural drainage in 4/179 (2%) patients was observed.
In 36/317 (11.4%) procedures diagnostic or interventional CC led to establishment of a new medication post procedure. These included anti-pulmonary hypertensive drug medication in 23/36 (64%), further anti-congestive heart failure medication (e.g., milrinone or ACE inhibitors) in 6/36 (16%), and intensified anticoagulation treatment due to suspected thrombus formation in 7/36 (20%) cases.
3.5 Intra-Procedural Aspects of Diagnostic and Transcatheter Procedures Related to Preceding CHS
The CC interventions were analyzed stratified by the type of surgery performed for all groups with n > 20, namely right ventricle to pulmonary artery (RV-PA) conduit, arterial switch operation (ASO), total cavopulmonary connection (TCPC), bidirectional cavopulmonary connection (BCPC), Norwood operation and AP-Shunt (aorto-pulmonary shunt) surgery.
Figs. 1–6 show all clinical indications, which led the team to decide for a diagnostic CC and the resulting interventions that followed the diagnostic CC findings.
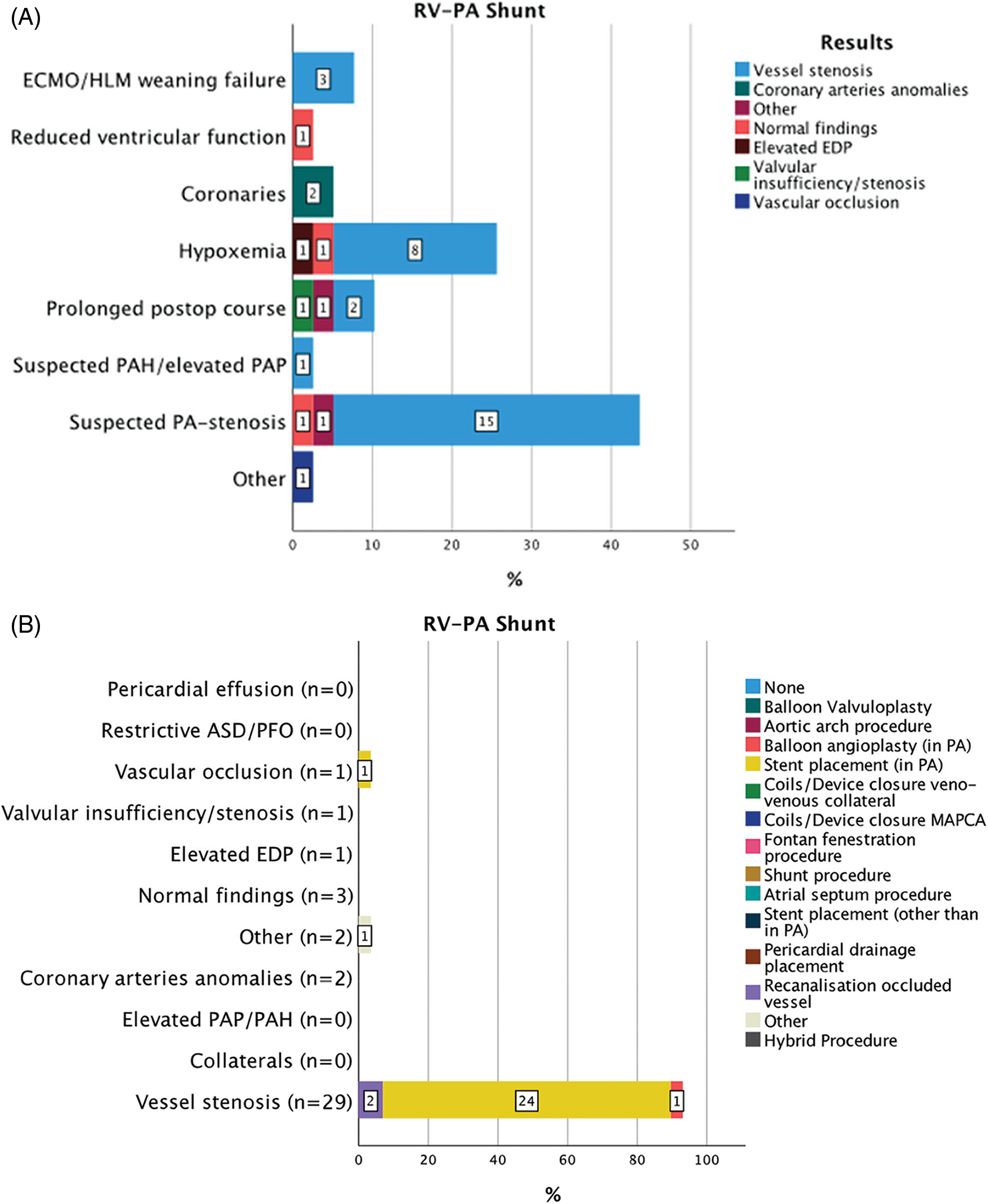
Figure 1: Indications for 39 CC within 30 days from CHS in patients with RV-PA shunt (A). Results of diagnostic CC and consequent interventions (B)
Abbreviations: RV-PA: right ventricle to pulmonary artery, ECMO: extra corporeal membrane oxygenation, HLM: heart-lungs machine, PAP: pulmonary artery pressure, PAH: pulmonary arterial hypertension, PA: pulmonary artery, ASD: atrial septal defect, PFO: patent foramen ovale, EDP: end-diastolic pressure, MAPCAs: major aorto-pulmonary collateral arteries, CoA: aortic coarctation, BCPC: bidirectional cavopulmonary connection, TCPC: total cavopulmonary connection, AP: aorto-pulmonary.
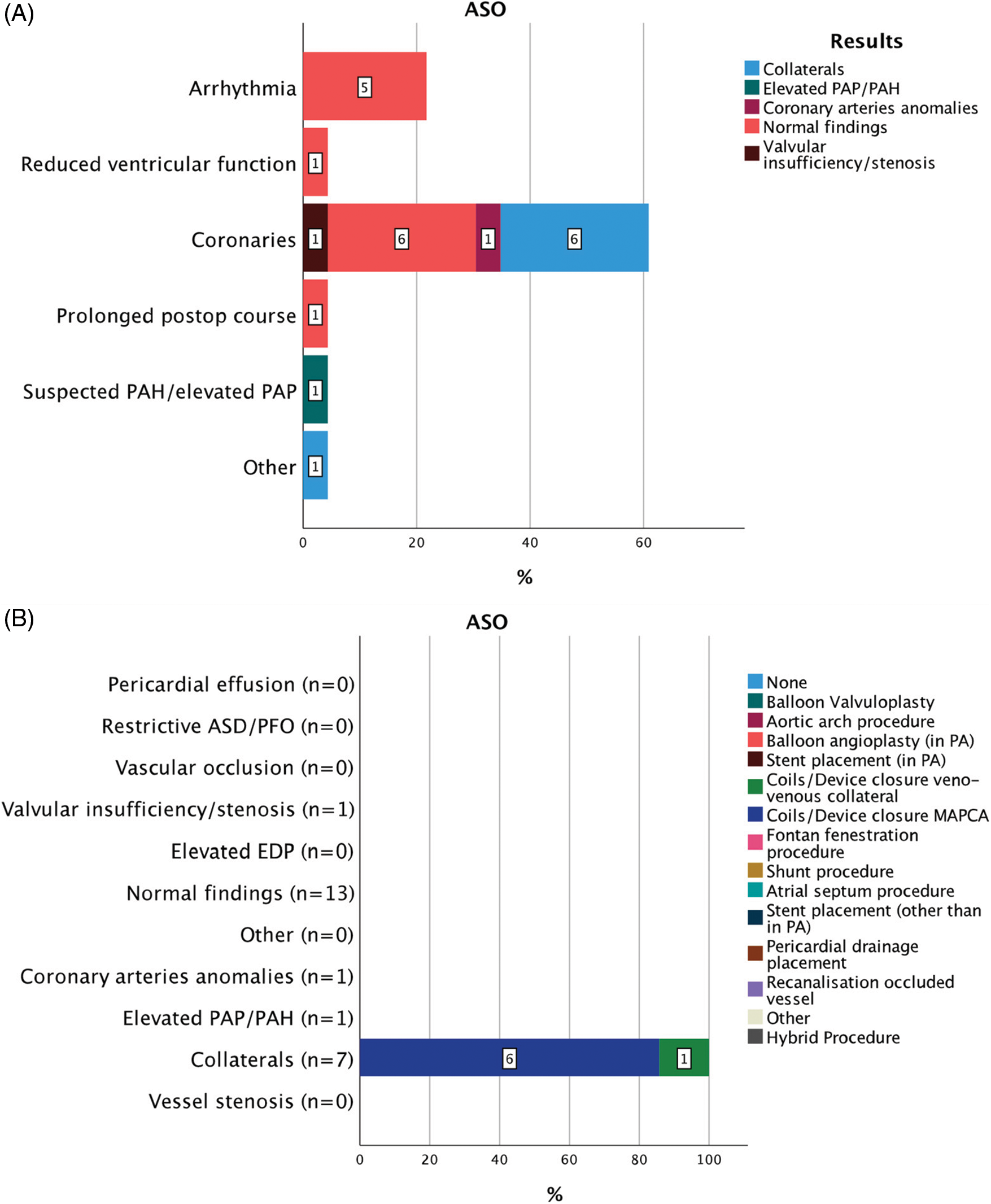
Figure 2: Indications for 23 CC within 30 days from CHS in patients after ASO (A). Results of diagnostic CC and consequent interventions (B)
Abbreviations: ASO: aortic switch operation, PAP: pulmonary artery pressure, PAH: pulmonary arterial hypertension, PA: pulmonary artery, ASD: atrial septal defect, PFO: patent foramen ovale, EDP: end-diastolic pressure, MAPCAs: major aorto-pulmonary collateral arteries, CoA: aortic coarctation.
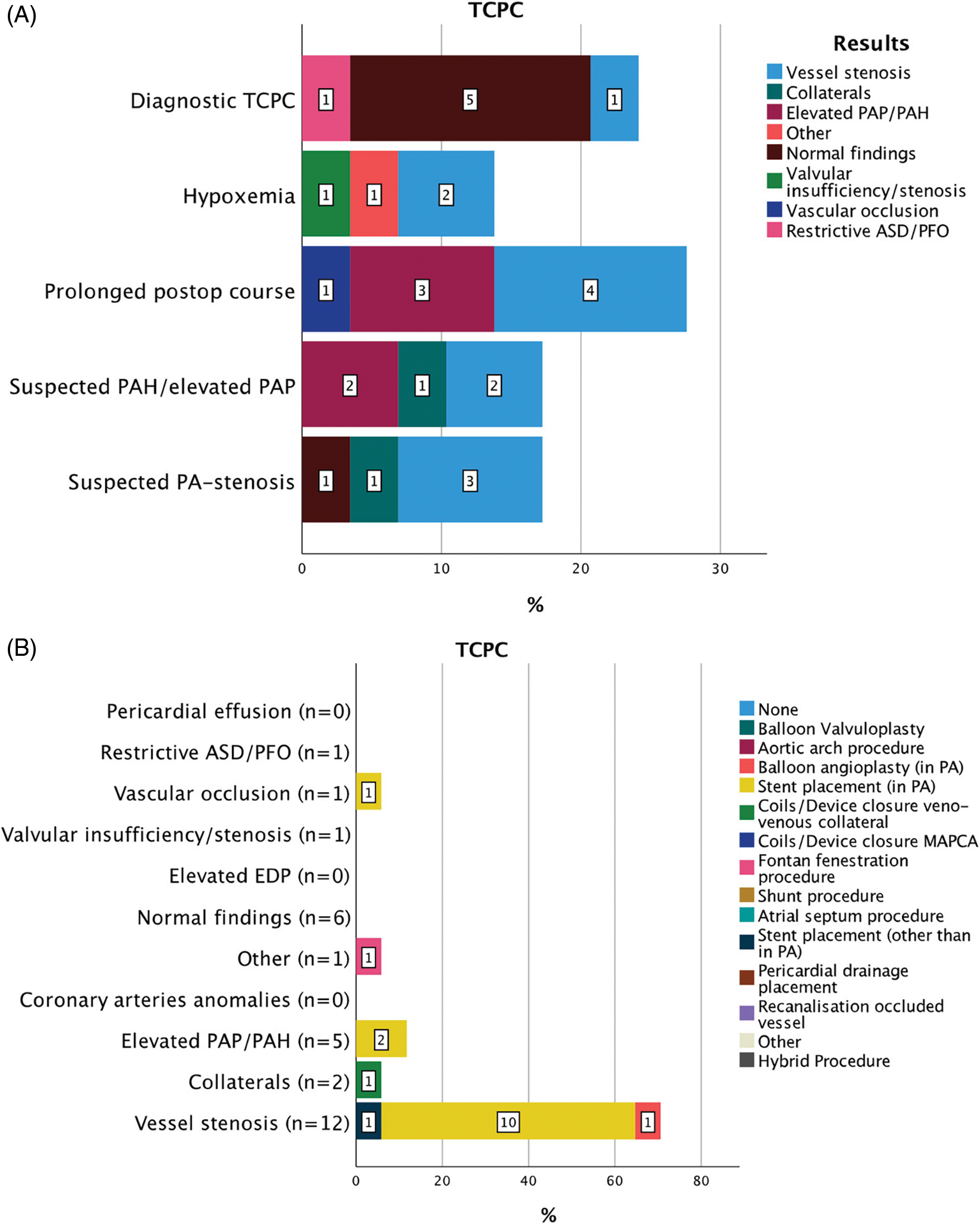
Figure 3: Indications for 29 CC within 30 days from CHS in patients after TCPC (A). Results of diagnostic CC and consequent interventions (B)
Abbreviations: TCPC: total cavopulmonary connection, PAP: pulmonary artery pressure, PAH: pulmonary arterial hypertension, PA: pulmonary artery, ASD: atrial septal defect, PFO: patent foramen ovale, EDP: end-diastolic pressure, MAPCAs: major aorto-pulmonary collateral arteries
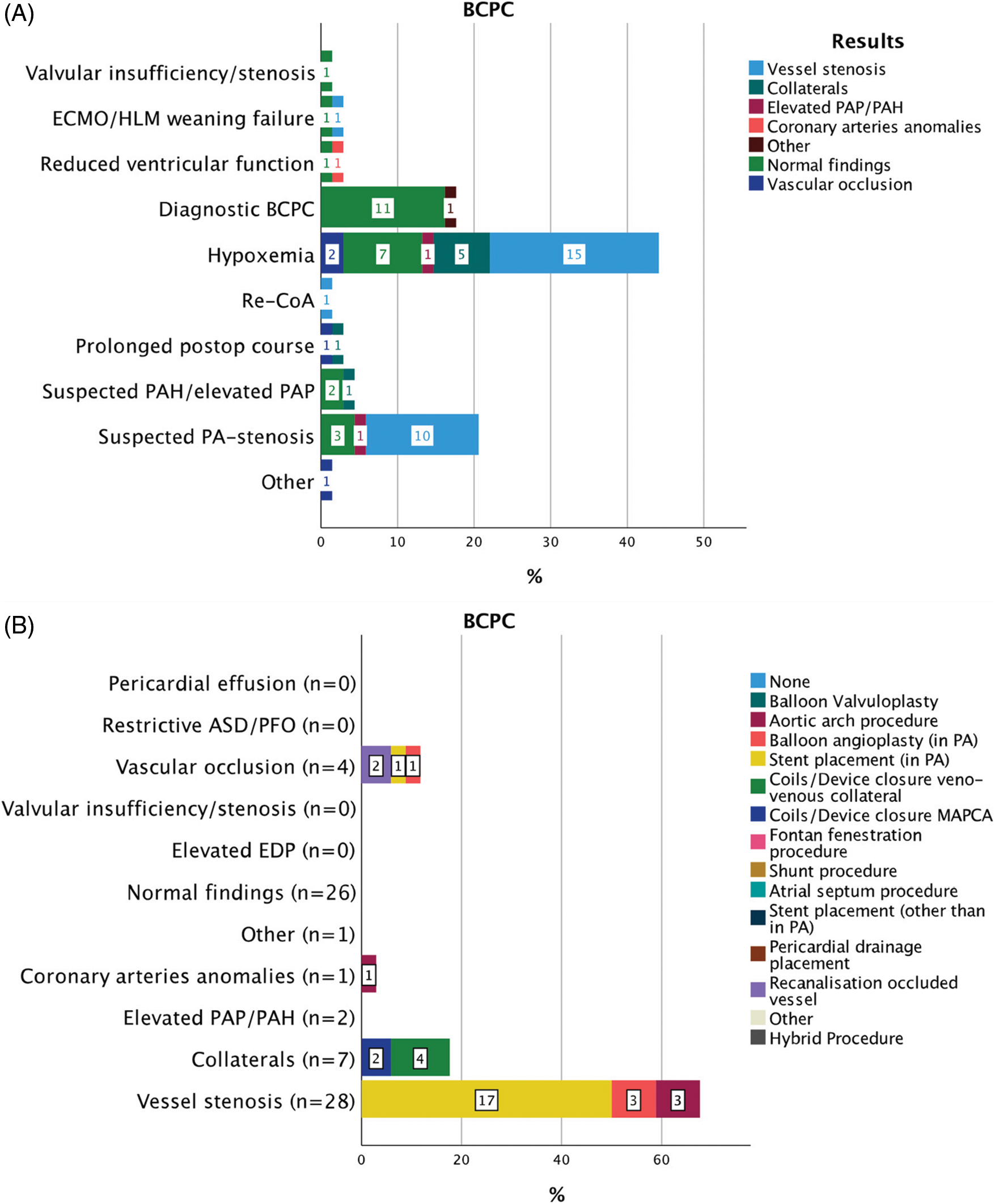
Figure 4: Indications for 69 CC within 30 days from CHS in patients after BCPC (A). Results of diagnostic CC and consequent interventions (B)
Abbreviations: BCPC: bidirectional cavopulmonary connection, ECMO: extra corporeal membrane oxygenation, HLM: heart-lungs machine, PAP: pulmonary artery pressure, PAH: pulmonary arterial hypertension, PA: pulmonary artery, ASD: atrial septal defect, PFO: patent foramen ovale, EDP: end-diastolic pressure, MAPCAs: major aorto-pulmonary collateral arteries, CoA: aortic coarctation.
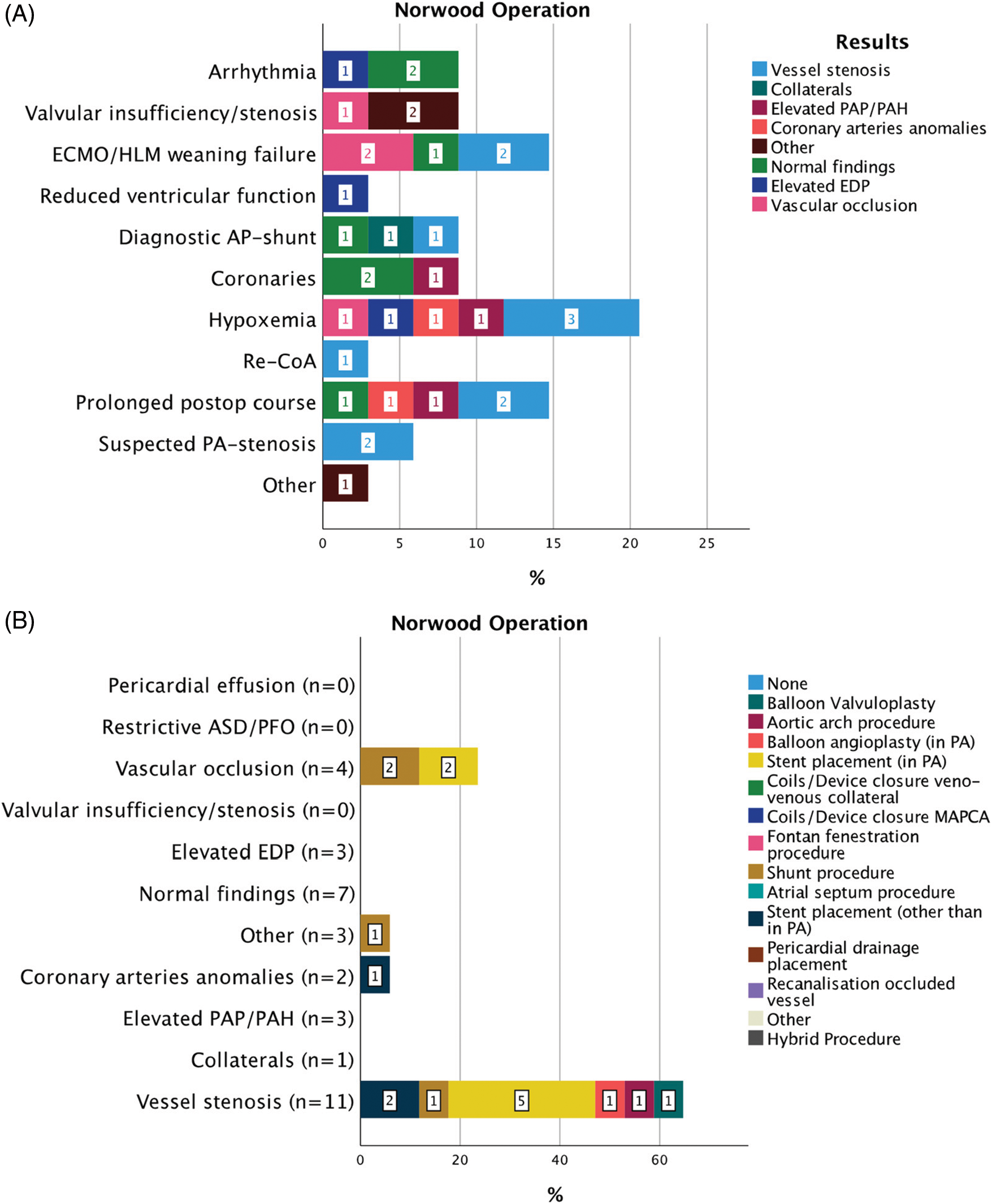
Figure 5: Indications for 34 CC within 30 days from CHS in patients after Norwood operation (A). Results of diagnostic CC and consequent interventions (B)
Abbreviations: ECMO: extra corporeal membrane oxygenation, HLM: heart-lungs machine, PAP: pulmonary artery pressure, PAH: pulmonary arterial hypertension, PA: pulmonary artery, ASD: atrial septal defect, PFO: patent foramen ovale, EDP: end-diastolic pressure, MAPCAs: major aorto-pulmonary collateral arteries, CoA: aortic coarctation.
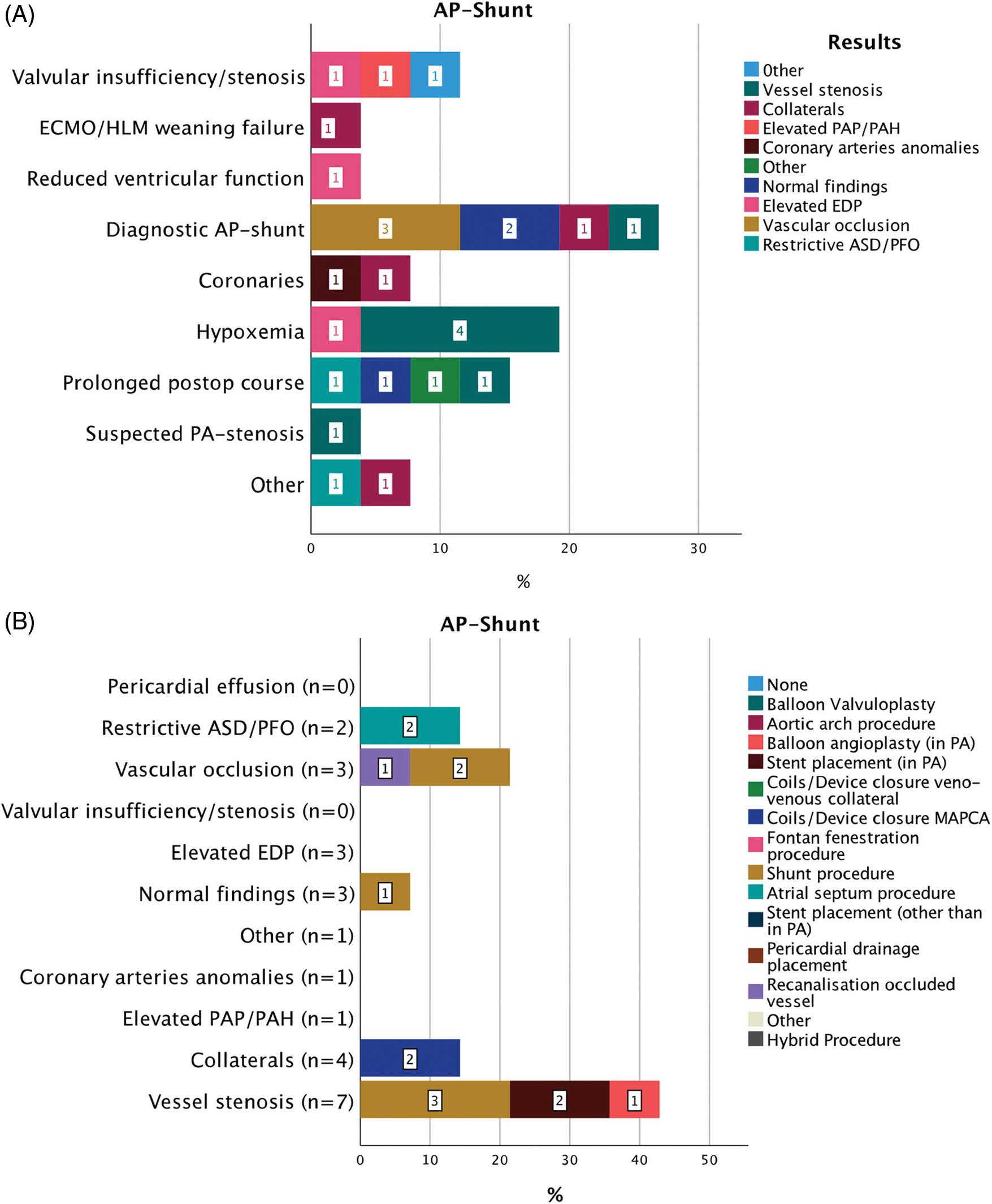
Figure 6: Indications for 34 CC within 30 days from CHS in patients after AP-shunt placement (A). Results of diagnostic CC and consequent interventions (B)
Abbreviations: AP: aorto-pulmonary, ECMO: extra corporeal membrane oxygenation, HLM: heart-lungs machine, PAP: pulmonary artery pressure, PAH: pulmonary arterial hypertension, PA: pulmonary artery, ASD: atrial septal defect, PFO: patent foramen ovale, EDP: end-diastolic pressure, MAPCAs: major aorto-pulmonary collateral arteries.
A total of 39 CC after RV-PA conduit were performed at a median of 2 (9) (IQR) days after CHS (Fig. 1). Suspected PA-stenosis 17/39 (43.5%), hypoxemia 10/39 (25.6%), and prolonged postoperative course 4/39 (10.2%) were the most frequent indications for CC. In these indication subgroups, vessel stenosis was found in 15/17 (88.2%), 8/10 (80%) and 2/4 (50%) cases, respectively. After diagnostic CC a total of 29/39 (74.3%) interventional, 1/39 (2.6%) hybrid, and 1/39 (2.6%) surgical procedures were performed. The most common intervention involved stent implantation for a stenotic vessel in 24/29 (82.7%) cases. Clinical improvement resulted in 23/29 (79.3%) patients. All interventions were successful. Median time to the next intervention, either transcatheter or surgically, was 70 (IQR 194) days.
Twenty-three CCs after ASO were performed at a median of 15 (IQR 9) days from CHS (Fig. 2). Suspected anomalies of the coronary arteries were the main indication for CC 14/23 (60.1%) followed by arrhythmias 5/23 (21.7%). In case of suspected postoperative coronary artery abnormality residual aortopulmonary collateral arteries (MAPCAs) were present in 6/14 (42.9%) while a coronary anomaly was found only once 1/14 (7.1%). All patients presenting with arrhythmia (5/5%, 100%) had normal coronary artery findings. Following the diagnostic procedures, a total of 7/23 (30.4%) interventions and 1/23 (4.3%) surgery were performed. All interventions were coil occlusions of significant residual MAPCAs or veno-venous collaterals. Clinical improvement was seen in 4/7 (57.1%) patients. All interventions were successful. Interval to next intervention (either surgical or interventional) was 17 (IQR 170) days.
In 29 cases a CC after TCPC was performed, 2 (IQR 12) days after CHS. Indications for CC after TCPC were diverse (Fig. 3). Prolonged postoperative course in 8/29 (27.5%), elective diagnostic angiography in 7/29 (24.1%), suspected pulmonary artery stenosis or suspected pulmonary arterial hypertension/elevated pulmonary resistance or both in 5/29 (17.2%) and hypoxemia in 4/29 (13.7%) lead to the decision for a CC. Overall, vessel stenosis was the most frequent diagnostic finding in 12/29 (41.4%) cases, in case of prolonged postoperative course in 4/8 (50%), suspected PA-stenosis in 3/5 (60%), and hypoxemia in 2/4 (50%) cases. Postoperative diagnostic angiography without clinical suspicion showed normal results in 5/7 (71.4%) patients. According to the diagnostic findings, 17/29 (58.6%) CC interventions and 2/29 (6.9%) surgical procedures followed. Overall, stent placement in the pulmonary arteries was the most frequent intervention, in 10/12 (83.3%) cases of suspected vessel stenosis and in 2/5 (40%) cases of elevated PAP. All interventions were successful. A relevant clinical improvement was seen in 14/17 (82.3%) cases. After the catheter procedure the interval to the next surgical or interventional procedure was median 262 (IQR 315) days.
A total of 69 CCs 2 (IQR 9) days after BCPC were performed (Fig. 4). Hypoxemia (30/69; 43.5%) and suspected PA-stenosis (14/69; 20.2%) were frequent indications for CC. Hypoxemia was associated with vessel stenosis in 15/30 (50%) and veno-venous collaterals in 5/30 (16.6%); suspected PA-stenosis with evident vessel stenosis in 10/14 (71.4%). After diagnostic CC, 34/69 (49.3%) interventions and 2/69 (28.9%) surgical procedures were performed. Vessel stenosis was associated with the highest rate of intervention 23/28 (82.2%), including stent placement in the pulmonary arteries in 17/23 (74%).
After diagnostic CC, 34/69 (49.3%) interventions and 2/69 (2.9%) surgeries were performed. A subsequent clinical improvement was seen in 25/34 (73.5%) patients. In two cases the interventional procedure was unsuccessful. Procedure-free interval after CC was 85 (IQR 510) days in this group of patients.
Thirty-four cases of CC followed 6.5 (IQR 11.5) days after Norwood operation (Fig. 5). Clinical indications for CC involved many categories, but the most frequent were hypoxemia in 7/34 (20.4%); failure to wean ECMO therapy in 5/34 (14.7%); and prolonged postoperative course in 5/34 (14.7%) cases. Overall, vessel stenosis was the most frequent result of CC 11/34 (32.3%) followed by normal findings in 7/34 (20.6%) and vascular occlusion in 4/34 (11.7%) cases. Therapeutic consequences of CC were catheter interventions in 17/34 (50%) and a surgical approach in 6/34 (17.6%) of these patients. Vessel stenosis and vascular occlusion were treated with stent placement in 5/11 (45.5%) and 2/4 (50%) of patients. In two cases the interventional procedure was unsuccessful and therefore was followed by surgery. Clinical improvement was described in 11/17 (64.7%) patients. Interval to the next intervention was median 12 (IQR 11) days.
A total of 26 CC after AP-shunt surgery were performed (Fig. 6). Time from CHS was 8.5 (IQR 14.5) days. Diagnostic angiography of the AP-shunt (7/26; 26.9%); hypoxemia (5/26; 19.3%) and prolonged postoperative course (3/26; 11.5%), were main indications for CC. Diagnostic angiography of the AP-shunt revealed AP-shunt occlusion or stenosis in 4/7 (57.1%), while in patients with hypoxemia normal findings were found in 4/5 (80%). A total of 14/26 (53.8%) interventions and 2/26 hybrid procedures were performed subsequently. In three cases interventional procedures were unsuccessful and surgery was planned. In case of vessel stenosis or AP-shunt occlusion a surgical redo-shunt procedure was usually performed. A relevant clinical improvement was seen in 10/14 (71%) cases. Procedure-free interval after CC was 59 (IQR 87) days.
There is a variety of different possible post-operative problems or residual lesions after CHS and some surgical outcomes after CHS in children need further, early and often invasive diagnostic investigation or interventional transcatheter treatment [11–13]. The threshold to perform further investigations, including invasive CC studies should be low, if significant hemodynamic or clinical problems are observed in the early post-operative period. A benefit of CC is the correct delineation of the anatomy and physiology, which allows for effective therapy; thus, a substantial number of patients undergo reoperation based on the findings during CC. Furthermore, medical, and interventional treatments performed will have a positive effect, optimizing postoperative therapy of these patients.
Earlier studies of post-operative CC in children [1–9] mainly described the feasibility and safety of early post-operative CC, but to our knowledge did not focus on indications and likeliness for interventional treatment according to the previously performed surgery. As to the diverse surgical strategies, different underlying anatomies, and different preceding surgeries it is imminent, that the assessed study population is heterogeneous. Therefore, we aimed to analyze a period of 11-year, where (at least) the interventional lead and team of the CC laboratory with its standards and interventional philosophy have been stable.
In case of CHS with preceding RV-PA conduit surgery, suspected pulmonary artery (PA) stenosis or hypoxia seem valid indications for a CC, since this could be resolved with an intervention in more than 70% of the cases and up to 80% of the patients showed a significant clinical benefit after the intervention. On the other hand, angiographic visualizations of coronary arteries after ASO and/or arrhythmias mostly confirmed a good postoperative result without the need for transcatheter interventional treatment. In 6 patients after ASO, where the indication for CC was coronary angiography, the performed interventions (“consequences”) were coil occlusions of significant residual MAPCAs, which can mimic coronary undersupply due to diastolic runoff [14].
Interestingly, approximately 61% of interventions were performed in the group of single ventricle physiology patients at different stages of their palliation. This is despite the fact, that it is institutional practice to perform CC prior to BCPC and TCPC. In the patients after TCPC the rate of interventions following diagnostic CC was around 50% and mostly involved treatment of vessel stenosis (pulmonary artery) associated with prolonged postoperative course. Nevertheless, when a CC intervention was performed its clinical impact was high. Interestingly, diagnostic angiography without any clinical problem post TCPC surgery showed normal results in most cases. The cohort after BCPC reflects the largest group in our study and involves 69 patients. In this group, there were solid indications for CC, such as hypoxemia or suspected PA-stenosis on echocardiography, which were associated with vessel stenosis and with a very high rate of interventions as well as a significant clinical impact after the catheter procedure.
The patients after a Norwood procedure had various indications for CC and a lower interventional rate compared to the other groups. Independently from the indication for CC, vessel stenosis or shunt occlusion were the most frequent findings, but the associated clinical impact of their treatment was lower than in other groups. Lastly, the patients after AP-shunt showed, that suspected shunt dysfunction is associated with a high and urgent need of reintervention, which lead to a clinical benefit in more than 70% of the cases.
Our study showed that these early high-risk CC interventions mostly can be performed safely and target-oriented, with no intra-procedural mortality. There was a total of 29 (9.1%) intra-procedural and 37 (11.6%) post-procedural adverse events. Most of these events were minor complications, while only 9 (2.8%) intra-procedural events stratified as major complications [10]. These occurrence rates of AEs are comparable to reported rates in normal, elective CC procedures in children in the literature [15,16]. Regarding this, early post-operative CC should not be postponed. Nevertheless, these interventions should be considered high-risk and procedural preparation and a multi-disciplinary approach with good surgical and intensive care back-up are essential.
This is a retrospective, single center analysis and therefore limitations regarding relatively small numbers of patients which limits subgroup analysis and risk factor analysis are imminent to this study. The gold standard of a prospective, randomized control study is ethically not applicable in this population, and we think that the results of our study are important to justify CC in the early post-operative period, although they are considered to be high risk interventions. Nevertheless, the lack of a control group especially limits the interpretation of the impact of these procedures on outcome and clinical improvement of these patients in the early post-operative period.
Cardiac catheterizations in the early postoperative period after CHS can be performed safely and should not be withheld from patients after CHS. Hypoxemia suspected arterial stenosis and prolonged postoperative course are solid indications for CC after RV-PA shunt, TCPC and BCPC. After ASO a CC only sporadically results in an intervention. Suspected AP-shunt dysfunction had a high probability for the necessity of an interventional transcatheter treatment.
Authorship: Quandt Daniel & Callegari Alessia: Design of the study, Data collection/analysis, Drafting/approval of article; Niesse Oliver: Design of the study, Data collection, Critical revision/approval of article; Christmann Martin, Meinhold Anke, Dave Hitendu & Kretschmar Oliver: Data collection, Critical revision/approval of article; Knirsch Walter: Interpretation of data, Critical revision/approval of article.
Data Sharing: The authors confirm that the data supporting the findings of this study are available within the article. Supplementary data that support the findings of this study are available from the corresponding author, DQ, upon reasonable request.
Funding Statement: All authors have no financial relationships relevant to this article to disclose.
Conflicts of Interest: All authors have no potential conflicts of interest to disclose.
Reference
1. Zahn, E. M., Dobrolet, N. C., Nykanen, D. G., Ojito, J., Hannan, R. L. et al. (2004). Interventional catheterization performed in the early postoperative period after congenital heart surgery in children. Journal of the American College of Cardiology, 43(7), 1264–1269. DOI 10.1016/j.jacc.2003.10.051. [Google Scholar] [CrossRef]
2. Nicholson, G. T., Kim, D. W., Vincent, R. N., Kogon, B. E., Miller, B. E. (2014). Cardiac catheterization in the early post-operative period after congenital cardiac surgery. JACC: Cardiovascular Interventions, 7(12), 1437–1443. DOI 10.1016/j.jcin.2014.06.022. [Google Scholar] [CrossRef]
3. Siehr, S. L., Martin, M. H., Axelrod, D., Efron, B., Peng, L. et al. (2014). Outcomes following cardiac catheterization after congenital heart surgery. Catheterization and Cardiovascular Interventions, 84(4), 622–628. DOI 10.1002/ccd.25490. [Google Scholar] [CrossRef]
4. Asoh, K., Hickey, E., Dorostkar, P. C., Chaturvedi, R., Arsdell, G. V. et al. (2009). Outcomes of emergent cardiac catheterization following pediatric cardiac surgery. Catheterization and Cardiovascular Interventions, 73(7), 933–940. DOI 10.1002/ccd.21919. [Google Scholar] [CrossRef]
5. Rosales, A. M., Lock, J. E., Perry, S. B., Geggel, R. L. (2002). Interventional catheterization management of perioperative peripheral pulmonary stenosis: Balloon angioplasty or endovascular stenting. Catheterization and Cardiovascular Interventions, 56(2), 272–277. DOI 10.1002/(ISSN)1522-726X. [Google Scholar] [CrossRef]
6. Bahaidarah, S., Al-Ata, J., Abdelmohsen, G., Alkhushi, N., Abdelsalam, M. et al. (2020). Cardiac catheterization addressing early post-operative complications in congenital heart surgery–A single-center experience. The Egyptian Heart Journal, 72(1), 83. DOI 10.1186/s43044-020-00117-6. [Google Scholar] [CrossRef]
7. Eraso-Díaz Del Castillo, A. M., Escobar-Díaz, M. C., Lince Varela, R., Díaz Medina, L. H., Cañas Arenas, E. M. (2019). Catheterization performed in the early postoperative period after congenital heart surgery in children. Pediatric Cardiology, 40(4), 827–833. DOI 10.1007/s00246-019-02078-3. [Google Scholar] [CrossRef]
8. Kasar, T., Cansaran Tanidir, I., Ozturk, E., Kafali, C., Sahin, M. et al. (2018). Cardiac catheterization in the early post-operative period after congenital heart surgery. Acta Cardiologica Sinica, 34(6), 481–487. [Google Scholar]
9. Kojima, T., Imamura, T., Osada, Y., Muraji, S., Nakano, M. et al. (2018). Efficacy of catheter interventions in the early and very early postoperative period after CHD operation. Cardiology in the Young, 28(12), 1426–1430. DOI 10.1017/S1047951118001452. [Google Scholar] [CrossRef]
10. Bergersen, L., Gauvreau, K., Foerster, S. R., Marshall, A. C., McElhinney, D. B. et al. (2011). Catheterization for congenital heart disease adjustment for risk method (CHARM). JACC: Cardiovascular Interventions, 4(9), 1037–1046. DOI 10.1016/j.jcin.2011.05.021. [Google Scholar] [CrossRef]
11. Bhole, V., Wright, J. G. C., de Giovanni, J. V., Dhillon, R., Miller, P. A. et al. (2011). Transcatheter interventions in the early postoperative period after the fontan procedure. Catheterization and Cardiovascular Interventions, 77(1), 92–98. DOI 10.1002/ccd.22667. [Google Scholar] [CrossRef]
12. Lynch, W., Boekholdt, S. M., Hazekamp, M. G., de Winter, R. J., Koolbergen, D. R. (2015). Hybrid branch pulmonary artery stent placement in adults with congenital heart disease. Interactive Cardiovascular and Thoracic Surgery, 20(4), 499–503. DOI 10.1093/icvts/ivu435. [Google Scholar] [CrossRef]
13. Nicholson, G. T., Kim, D. W., Vincent, R. N., Petit, C. J. (2015). Transcatheter interventions across fresh suture lines in infants and children: An 8-year experience. Catheterization and Cardiovascular Interventions, 86(2), 271–277. DOI 10.1002/ccd.25908. [Google Scholar] [CrossRef]
14. Wipf, A., Christmann, M., Navarini-Meury, S., Dave, H., Quandt, D. et al. (2018). Aortopulmonary collaterals in neonates with d-transposition of the great arteries-clinical significance early after arterial switch operation. International Journal of Cardiology, 258, 237–242. DOI 10.1016/j.ijcard.2018.01.132. [Google Scholar] [CrossRef]
15. Chaudhry-Waterman, N., Coombs, S., Porras, D., Holzer, R., Bergersen, L. (2014). Developing tools to measure quality in congenital catheterization and interventions: The congenital cardiac catheterization project on outcomes (C3PO). Methodist DeBakey Cardiovascular Journal, 10(2), 63–67. DOI 10.14797/mdcj-10-2-63. [Google Scholar] [CrossRef]
16. Bergersen, L., Marshall, A., Gauvreau, K., Beekman, R., Hirsch, R. et al. (2010). Adverse event rates in congenital cardiac catheterization-a multi-center experience. Catheterization and Cardiovascular Interventions, 75(3), 389–400. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools