 Open Access
Open Access
CASE REPORT
Case Report: Laubry-Pezzi Syndrome: Confronting the Lethal Nexus of Life-Threatening Complications in Resource Constrained Settings
1 Department of Internal Medicine, Usmanu Danfodiyo University Teaching Hospital, Sokoto, 23270, Nigeria
2 Cardiovascular Division, Aster Prime Hospitals, Hyderabad, 500038, India
3 Department of Internal Medicine, Federal Medical Center Nguru, Nguru, 630261, Yobe, Nigeria
* Corresponding Author: Hayatu Uma. Email:
Congenital Heart Disease 2024, 19(6), 635-645. https://doi.org/10.32604/chd.2025.056641
Received 27 July 2024; Accepted 16 January 2025; Issue published 27 January 2025
Abstract
Laubry-Pezzi syndrome (L-PS) is a rare congenital heart disease characterized by a ventricular septal defect (VSD) and aortic valve prolapse. These cardiac lesions predispose individuals to infective endocarditis (IE), a life-threatening complication, especially in resource-constrained settings. A 17-year-old male presented with a three-week history of fever and headache, and a one-week history of abdominal pain, vomiting, and diarrhea. On presentation, he appeared toxic, was febrile, tachypneic, tachycardic, and blood pressure of 120/30 mmHg, and heart sounds were S1, S2. Abdominal examination revealed generalized tenderness. A provisional diagnosis of typhoid sepsis with intestinal perforation was considered. However, a thorough clinical evaluation led to the definitive diagnosis of L-PS complicated by right-sided IE, sepsis, acute kidney injury, acute heart failure, and haemoptysis. Despite significant improvement with appropriate antibiotics and adjunctive therapy, the patient’s inability to afford surgical correction and subsequent non-adherence to medical advice he resorted to traditional medicine this led to readmission for heart failure. This case highlights the diagnostic and treatment challenges associated with L-PS with life-threatening complications, particularly in resource-constrained settings, where it may be misdiagnosed as common infections like typhoid sepsis with intestinal perforation. It emphasizes the importance of a comprehensive clinical approach, including a detailed history, meticulous physical examination, and targeted investigations, to ensure accurate diagnosis and timely intervention in patients with L-PS and its life-threatening complications.Keywords
Abbreviations
| LP-S | Laubry-Pezzi syndrome |
| RSIE | Right-sided infective endocarditis |
| VSD | Ventricular septal defect |
| HF | Heart failure |
| IE | Infective endocarditis |
| AKI | Acute kidney injury |
Laubry-Pezzi syndrome is a rare genetic disorder characterized by features such as congenital heart defects defined by VSD and aortic valve prolapse with incompetence [1], distinct facial features and short stature [2,3]. It is caused by mutations in the TGFBR1 gene, which is involved in the development of connective tissue and cardiovascular structures [2,3]. This syndrome is extremely rare, with only a handful of cases reported in literature. Due to its rarity, precise epidemiological data are limited. Cases have been identified in various populations, suggesting that it may not be confined to a specific ethnic group [2,3].
The cardiac lesions associated with this syndrome heighten the risk of infective endocarditis (IE) [4,5], a serious life-threatening condition that can lead to severe complications such as sepsis, septic shock, septic encephalopathy, acute kidney injury (AKI), multiple organ dysfunction syndrome, heart failure, septic pulmonary/systemic embolism, cardioembolic stroke, and acute abdomen. Prompt identification and surgical repair of cardiac lesions in L-PS can help prevent the development of IE and its associated catastrophic complications and dismal prognosis.
The literature reports few case studies of L-PS with left-sided infective endocarditis (IE) [4,5], but there is a notable lack of documented case studies of L-PS with right-sided infective endocarditis (RSIE), sepsis, acute kidney injury (AKI), hemoptysis, and recurrent heart failure in literature to the extent of our search. Clinically, L-PS can present asymptomatic or symptomatic with severe complications, posing significant diagnostic and management challenges, especially in resource-limited settings. Factors such as delayed presentation, limited diagnostic tools, lack of effective antibiotics, and insufficiently trained specialists contribute to poor outcomes. To Diagnose L-PS and its associated life-threatening complications requires thorough history-taking, meticulous physical examination, and echocardiography, which are essential for a definitive diagnosis.
Currently, there is no universal consensus on the specific cure for L-PS; treatment is symptomatic and supportive. Management typically involves: (1) Early detection and surgical closure of VSD and repair of aortic valve prolapse is of paramount importance to prevent devastating complications and dismal prognosis that may be associated with this syndrome; (2) Growth Hormone therapy in cases of short stature; (3) Close monitoring to address any arising complications from the syndrome. As research continues, there may be advancements in gene therapy or targeted treatments, but these are still in the experimental stages.
We present a rare case of L-PS with life-threatening complications RSIE, sepsis, AKI, hemoptysis and acute left ventricular HF, who achieved a favorable clinical outcome with medical therapy, but could not undergo surgical treatment due to financial constrained. A common observation in most patients of resource constrained settings with only watchful waiting.
Herein, a 17-year-old male college student arrived at our emergency department with a three-week history of high-grade continuous fever, generalized throbbing headaches, body aches, and fatigue. One week before his visit, he began experiencing recurrent colicky abdominal pain, which was accompanied by postprandial vomiting and diarrhea that was non-mucoid and non-bloody, occurring 2–3 times a day for three days. He reported no associated symptoms like constipation, dysuria, increased urinary frequency, urethral discharge, dyspnea, cough, chest pain, neck pain and stiffness, and irrational talk or behavior. He stated he had been in good health before the onset of his febrile illness. He cannot ascertain antecedent history of rheumatic fever or preferential squatting during childhood and no history of intravenous (IV) drug abuse. He sourced his drinking water from a piped supply, occasionally ate without washing his hands, frequented food vendors, and used a pit latrine. A week after his fever began, he received IV medications at home and was later hospitalized for a week at a public facility, but his condition did not improve.
At presentation, he was acutely ill-looking, febrile (T = 39.2°C), not pale, anicteric, acyanosed with SPO2 of 97%, respiratory rate was 26 cycle/min, and no pedal edema. Pulse rate was 126/min with blood pressure of 120/30 mmHg, Jugular venous pressure not raised, Apex normal, Heart sounds were S1 and S2 with pericardial friction, Chest was clinically clear, Abdomen: generalized tenderness and Central nervous system examination was unremarkable. A, provisional diagnosis of typhoid sepsis to rule out intestinal perforation was entertained with differential diagnosis of severe malaria. He was administered ceftriaxone, metronidazole, and artesunate. Blood samples were taken for relevant investigations as indicated in (Table 1a). Abdomino-pelvic ultrasound revealed kidneys normal in size, position and outline, however the echogenicity is greater than that of liver and spleen. Hepatobiliary system, gall bladder, pancreas, para-aortic area and bowel loops sonographic findings were within normal limits. The spleen was enlarged with uniform echogenicity. No ascites or lymphadenopathy. Sonographic conclusion was mild splenomegaly and increase renal echogenicity cause.
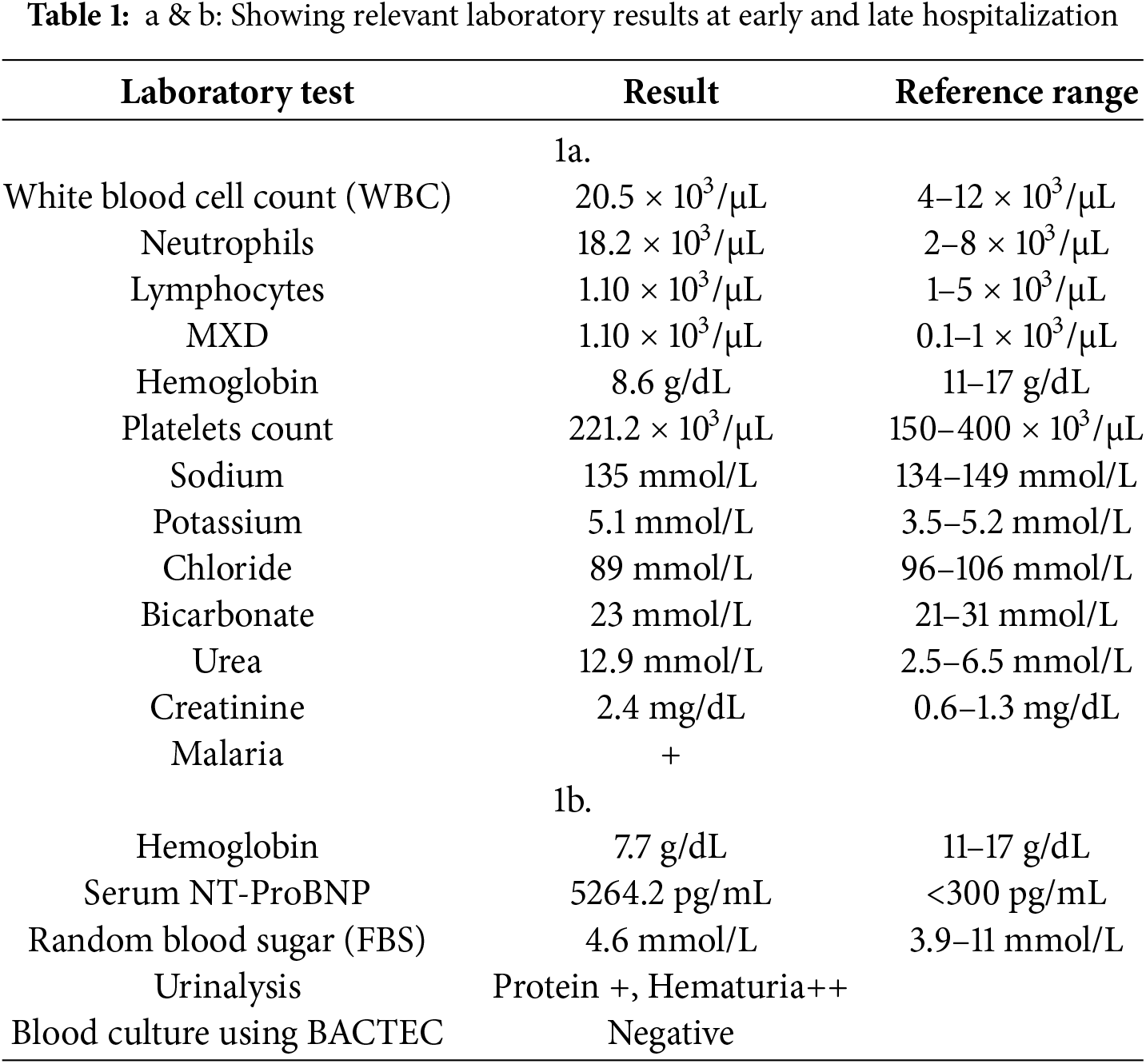
Further review and meticulous physical examination and investigations revealed confused and restless patient, febrile (T = 40°C), pale, acyanosed, RR: 38 breaths/min, SPO2 90%, pulse rate 122/min, blood pressure of 120/40 mmHg, Jugular venous pressure not raised, displaced apex and hyperdynamic, heart sound: S1, S2 and S3 gallop, PSM grade 3/6 loudest at 4th left intercostals space lateral to sternal border, tricuspid PSM grade 1/6 epigastrium, aortic diastolic murmur grade 2/4, bi-basal fine crackles. a provisional diagnosis of congenital heart disease (VSD) with aortic incompetence complicated by infective endocarditis, sepsis, AKI and acute left ventricular HF was entertained with differential diagnosis of Rheumatic heart disease (RHD) with complications. He was started on oxygen therapy and switched from generic ceftriaxone to the branded ceftriaxone (Rocephin). Metronidazole was stopped and gentamicin added adjusted for renal function, he also received furosemide along with conservative renal therapy and close monitoring. Additional relevant investigations were requested which include (1) Laboratory test as indicated in (Table 1b) (2) Electrocardiogram (ECG) which showed sinus tachycardia with heart rate of 115 beat per min (Fig. 1). (3) Chest X-ray (CXR): Showed cardiomegaly of biventricular configuration with cardiothoracic ratio of 0.61, prominent pulmonary vasculature, upward blood diversion and blunted left costophrenic angle (Fig. 2). (4) Transthoracic echocardiography (TTE): revealed interventricular septal thickness of 9.4 mm and left ventricular posterior wall thickness of 13.5 mm. Moderately dilated left ventricle (LV) with internal diameter of 66.4 mm, mildly dilated left atrial with internal of diameter 44.1 mm, mildly dilated right ventricle with internal diameter of 43.9 mm, moderately dilated right atrium with internal diameter of 53.2 mm, Peri-membranous VSD measured 5.3 mm in size with color Doppler demonstrating left to right shunt (Fig. 3A,B). Aortic valve prolapse of the non-coronary cusp 4.5 mm from the plane and severe aortic regurgitation [Width of vena contracta of 7.4 mm and regurgitant jet width/LV out flow tract diameter ratio of 2.1 cm/2.6 cm of 0.80 (80%)] (Fig. 4A,B), large oscillating echogenic mass attached to tricuspid valve leaflet was noted (Fig. 5) and (Supplementary Materials Video S1). LV Ejection fraction (EF) was 64.7% on M-mode guided 2-D with fractional shortening of 36.3% and normal RV systolic function (TAPSE: 20.4 mm). LV end-diastolic volume index was 135.8 mL/m2 (Severely abnormal), while LV end-systolic volume index was 47.9 mL/m2 (Severely abnormal) and LV mass index (LVMI) was 209.3 g/m2 (Severely abnormal). Other heart valves appeared normal in motion and morphology. Moderate mitral regurgitation, severe tricuspid regurgitation and mild pulmonary regurgitation were noted. Pulmonary arterial systolic pressure at rest was 52.2 mmHg.
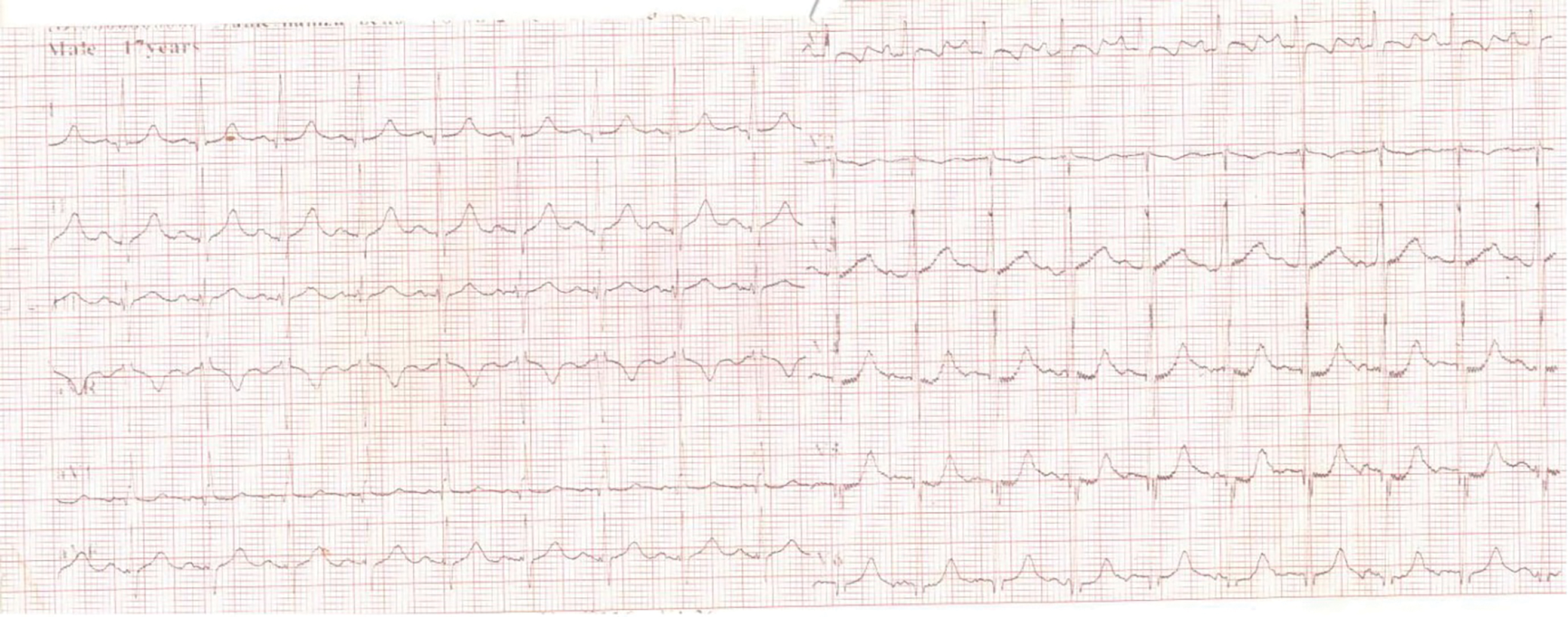
Figure 1: ECG showing sinus tachycardia
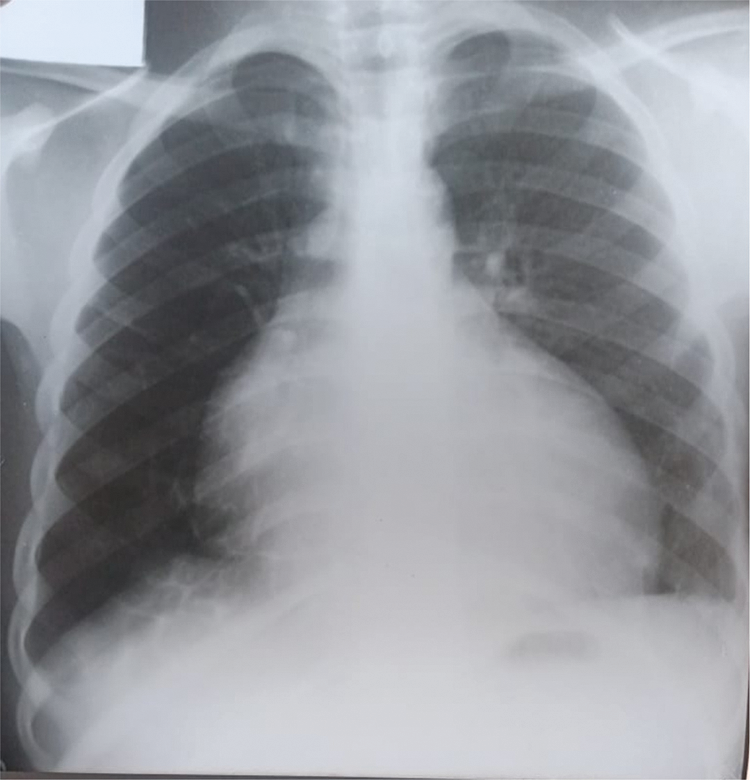
Figure 2: CXR showing cardiomegaly of biventricular configuration, pulmonary vascular engorgement, upwards blood diversion and blunted left costophrenic angle
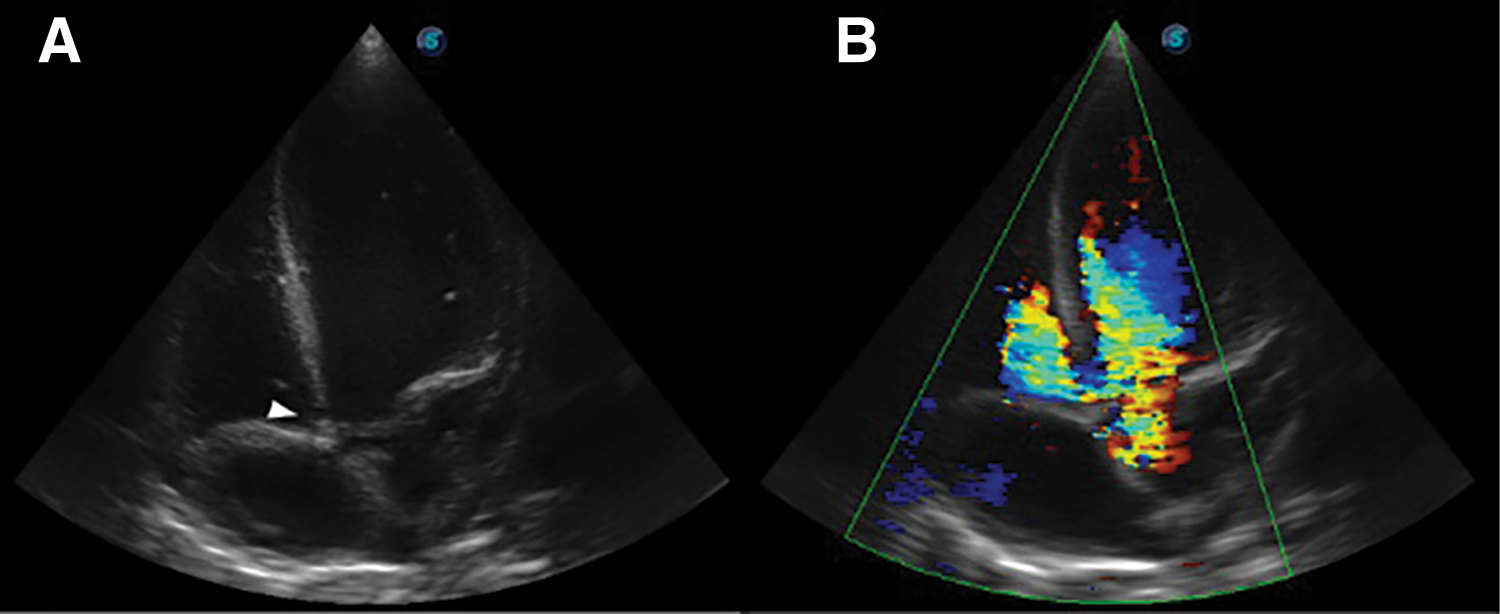
Figure 3: TTE apical 5-chamber view, (A): Arrow head showing Peri-membranous VSD and (B): Color Doppler demonstrating left to right shunt
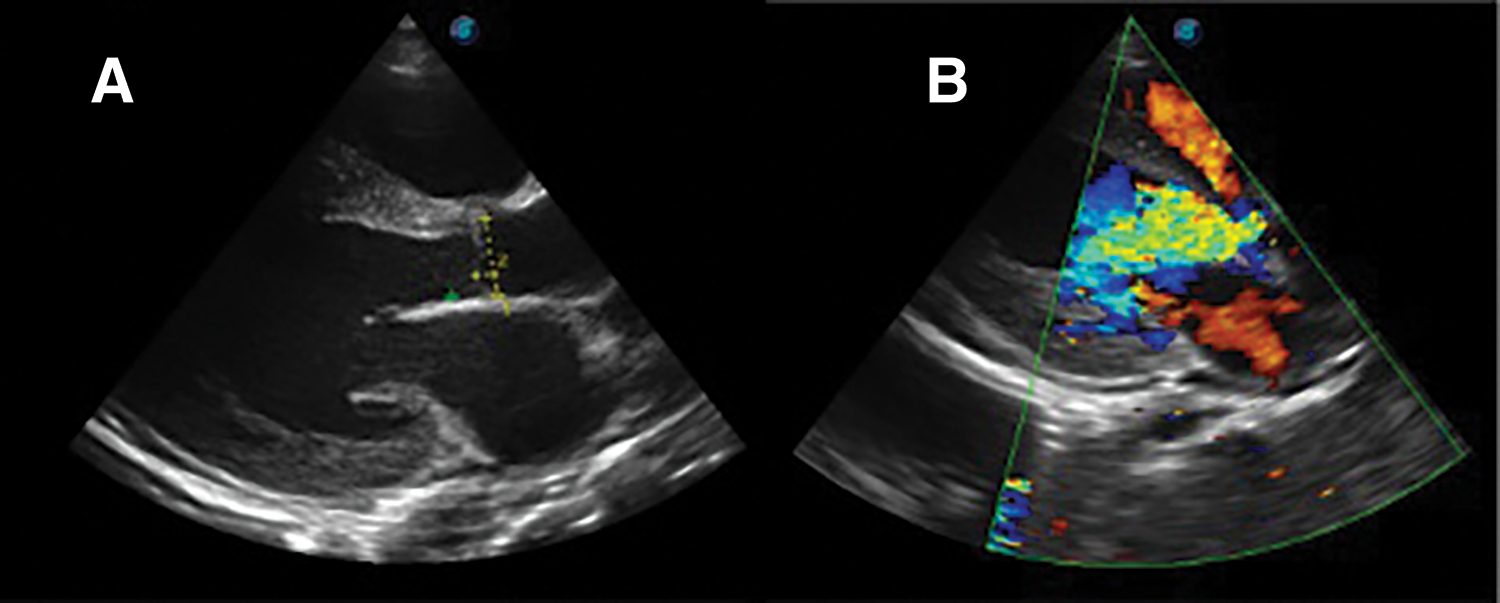
Figure 4: TTE parasternal long axis view, (A): showing aortic valve prolapse of non-coronary cusp and (B): color doppler demonstrating aortic regurgitation
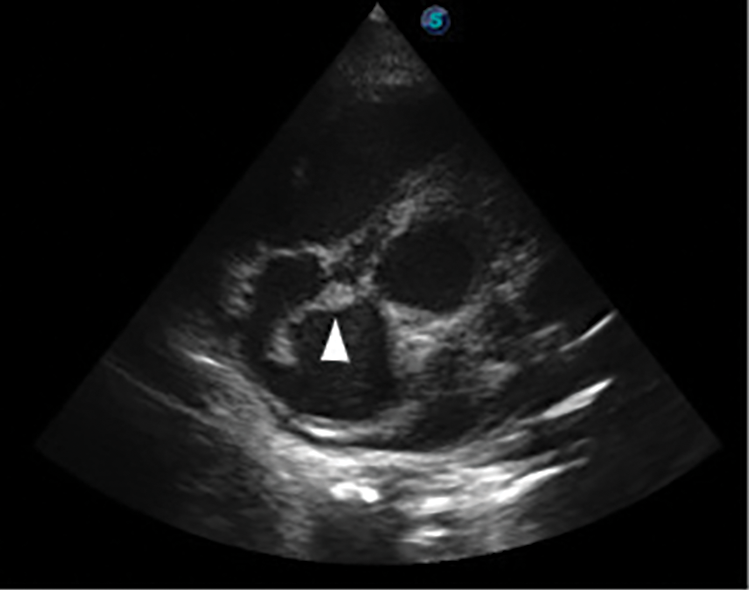
Figure 5: Transthoracic echocardiography (TTE) parasternal short axis view, arrow head showing large tricuspid valve vegetation
A, definitive diagnosis of Laubry-Pezzi syndrome complicated by right-sided infective endocarditis, sepsis, AKI and heart failure was made. Despite antibiotics and adjunctive therapy fever persisted, and a few days later he developed an episode of chest pain with associated hemoptysis with estimated blood loss of 200 mL. He was transfused with 2 pints of blood on account of anemia. Rocephin was stopped and vancomycin renal dose and rifampicin were commenced while gentamycin renal adjusted dose sustained along with regular electrolyte, urea and creatinine, and conservative renal therapy. The patient showed significant improvement after three weeks of parenteral antibiotics and rifampicin. Following this, parenteral antibiotics were discontinued, and he was prescribed oral Augmentin while continuing rifampicin. He received counseling on surgical options for cardiac lesions, IE prophylaxis, medication adherence, and clinic follow-up appointments. After a 28-day hospital stay, he was discharged home asymptomatic with normal renal function with PCV of 36% and on oral antibiotics (Augmentin and Rifampicin). However, after two clinic follow-up visits, he stopped attending and resorted to traditional medicine (intake of traditional herbal concoction), leading to his readmission 3 months after discharge from the hospital with heart failure symptoms dyspnea on exertion, easy fatigue, cough productive of whitish frothy sputum, PND. However, no associated fever, leg and abdominal swelling, and easy satiety. He was administered oxygen, frusemide, spironolactone, captopril and subsequently blocker carvedilol, he achieved remarkable improvement.
Co-occurring right-sided infective endocarditis, sepsis, AKI, hemoptysis and acute HF in L-PS is a life-threatening medical emergency that requires prompt recognition and rapid institution of appropriate management to avert dismal prognosis. In literature we found few reported case studies of L-PS with infective endocarditis [4,5]. But not L-PS with RSIE, sepsis, AKI, acute HF and hemoptysis to the extent of our search.
The case study highlights several distinctions from previously reported case studies of L-PS with infective endocarditis [4,5]; (1) The index case exhibited multiple complications, including right-sided infective endocarditis (RSIE), sepsis, acute kidney injury (AKI), haemoptysis, and recurrent heart failure, whereas previous cases typically presented with IE alone [4,5]; (2) Unlike earlier case reports, which likely presents with left-sided infective endocarditis [4,5], this index case presented with RSIE; (3) Previous case reports documented cultured organisms causing infective endocarditis [4,5], while the index case had negative blood cultures, possibly due to prior antibiotics treatment or the methods used for culturing; (4) The prolapsed aortic valve cusp in this case was the non-coronary cusp, differing probably from earlier reported case studies [4,5]; (5) Unlike previous reported cases that underwent surgical correction of cardiac lesions [4,5], the index case has not yet received surgical correction due to financial constrained. In summary, this index case presents a unique combination of life-threatening complications and characteristics that set it apart from previously reported case studies of L-PS with infective endocarditis.
Although, the provisional diagnosis of typhoid sepsis, to rule out viscus perforation, was considered for this index case at early presentation. This provisional diagnosis is in line and is reasonably supported by (1) Typhoid fever often causes persistent fever even with antibiotic treatment in this region of the world; (2) The patient’s presenting symptoms and abdominal examination finding of generalized abdominal tenderness, are highly suggestive of typhoid perforation in our setting; (3) Previous studies have shown that infective endocarditis can complicate typhoid fever, particularly in patients with pre-existing structural heart disease [6–8]; (4) The abdominal examination findings of generalized abdominal tenderness reinforced the concern for typhoid perforation, a serious complication of enteric fever. However, an abdominopelvic ultrasound ruled out this dreadful complication.
The abdominal pain and generalized tenderness in this case may stem from complications associated with infective endocarditis (IE). These complications can arise from paradoxical septic embolism, potentially leading to visceral infarction, rupture, or mycotic aneurysms, all of which can cause abdominal pain or an acute abdomen as reported in previous literature [9–11]. However, the likelihood of these complications due to RSIE is low, as the phenomenon of paradoxical embolism from right-sided IE (RSIE) is rare except in cases of ASD with transient reversal of blood flow that can reverse the shunt or ASD with Eisenmenger’s syndrome, or VSD with Eisenmenger’s syndrome [12], none of which were present in this index case. So, the possible explanations for the abdominal symptoms in this index case include: (1) Co-occurring left-sided IE with abdominal septic embolism, though there’s no echocardiographic evidence to support this; bearing in mind that TTE is less sensitive in detecting vegetation less than 2 mm compared to Transesophageal echocardiography (TEE) (2) Sepsis related to IE, which could explain the abdominal pain and diarrhea noted in this index case; (3) Typhoid fever, which could present with abdominal symptoms and may be complicated by IE, particularly in the context of Laubry-Pezzi syndrome. In conclusion, the abdominal pain and generalized tenderness in this index case may be linked to complications of infective endocarditis, such as septic embolism or sepsis leading to abdominal symptoms.
The definitive diagnosis of L-PS with right-sided infective endocarditis (RSIE), sepsis, acute kidney injury (AKI), hemoptysis and acute heart failure (HF) was made in this index case. Key echocardiographic findings included a ventricular septal defect (VSD) and aortic valve prolapse with aortic regurgitation, which are characteristic of L-PS. While the definite diagnosis of RSIE was supported by the presence of echocardiographic evidence of tricuspid valve vegetation, underlying L-PS cardiac lesions (VSD and Aortic valve prolapse with regurgitation), fever with temperature of 40°C, and vascular phenomenon (septic lung infarction with hemoptysis/paradoxical septic embolization causing abdominal visceral infarction presenting with pain). These observations fulfilled the modified Duke’s criteria for definite infective endocarditis, despite negative blood cultures. Uniquely, this case is distinguished by the occurrence of right-sided endocarditis (RSIE), which contrasts with typical previously reported cases of L-PS that presented with left-sided infective endocarditis [4,5]. Additionally, the usual risk factor for RSIE, intravenous drug abuse, was ruled out in this index case.
Infective endocarditis in congenital heart defects like VSD or valvular heart disease like aortic valve prolapse with regurgitation or TR, arises from complex interplay of abnormal blood flow and microbial exposure. This process can be summarized as follows; (1) In conditions like VSD, AR or TR, abnormal blood flow creates turbulence that injures the endocardium, exposing underlying tissues and leading to thrombus formation [13,14]; (2) The damaged endocardium can form sterile vegetations, which are aggregates of platelets, fibrin, and bacteria, serving as sites for bacterial colonization [13,14]; (3) Bacteraemia through various means, including dental work, skin infections, intravenous drug use, or abdominal infections. In patients with congenital heart defects, even brief episodes of bacteraemia can result in infection due to the vulnerable cardiac surfaces [13,14]; (4) Once bacteria attach to the vegetations, they multiply, creating infected vegetations that can obstruct blood flow and further damage the endocardium [13,14]; (5) The body’s immune system responds to the infection, causing inflammation and additional harm to the heart valves or endocardium [13,14]; (6) If left untreated, IE can lead to severe complications, including systemic emboli, valvular dysfunction, heart failure, and sepsis with associated life-threatening complications, often requiring prompt medical or surgical intervention. In summary, IE in congenital heart disease, AR or TR is a complex interplay of hemodynamic changes, immune response and infection risk, leading to significant cardiovascular complications to prevent IE and it associated complications in these group of patients closed monitoring and prophylaxis are crucial [13,14].
In this index case, the potential factors, that likely contributed to the development of RSIE include: (1) Underlying congenital heart defect VSD, which poses a higher risk for infective endocarditis compared to an atrial septal defect [15]; (2) Hemodynamic changes left-to-right shunt likely causes increased pressure and turbulence flow in the right side of the heart, as evidenced by elevated pulmonary arterial systolic pressure of 52.2 mmHg; (3) Affected valve is tricuspid valve with significant regurgitation makes this regurgitant valve more prone to IE compared to stenotic valve [15]; (4) The index case had received intravenous medications both at home and in a public hospital prior to presentation, where aseptic techniques may have been compromised a potential risk factor for RSIE as documented by Previous research by Divya Rabindranath et al. [16], highlights that IV drug use and catheter-related infections are known risks for RSIE, especially in those with underlying structural heart disease. However, since the IV medications were administered after the onset of a febrile illness, they are considered less likely to be a direct predisposing factor of RSIE in this index case, even-though infections at IV sites can still occur alongside other febrile illnesses. In summary, the combination of a congenital heart defect, hemodynamic changes, significant tricuspid regurgitation, and patient immune response all contribute to the development of RSIE [13–16], in this index case.
The diagnosis of sepsis in this index case was established using both the systemic inflammatory response syndrome (SIRS) and the sequential organ failure assessment (SOFA) diagnostic criteria [17,18]. Notably, splenomegaly was observed, which supports the diagnosis of sepsis or infective endocarditis (IE). Regarding complication acute kidney injury (AKI), it was considered to be sepsis-induced AKI rather than type 1 cardio-renal syndrome, since the patient presented with fever and renal dysfunction before developing acute left ventricular heart failure (HF). Given the patient’s underlying structural heart disease, sepsis was likely the precipitant for the heart failure, although anemia could also be a possible precipitant of HF but is unlikely in this index case since anemia developed after the onset of symptoms of acute HF.
Haemoptysis in RSIE typically arises from several interconnected mechanisms like in this index, and these mechanisms include: (1) Infective vegetations on the right heart valves (typically the tricuspid valve) can break off and embolize (septic embolization) to the pulmonary vasculature and lungs, leading to infarction, pneumonia or abscess and haemorrhage manifesting as haemoptysis [19], this is likely the mechanism in our index case; but less likely due to lung abscess in our index case who has no clinical and radiologic findings in keeping with lung abscess even-though he has coarse crepitation on examination likely in keeping with pneumonia or infarction (2) Localized infection from septic emboli or sepsis can weaken pulmonary blood vessels, contributing to rupture and subsequent haemoptysis [19], this another possible mechanism in this index case; (3) Inflammatory response associated with RSIE can lead to coagulopathy, increasing the risk of bleeding in the pulmonary vasculature [19]; not likely in this index case with no prank coagulopathy (4) Septic emboli or the systemic effects of infection can predispose the lungs to secondary infections, further damaging lung tissue and contributing to haemoptysis [19], a probable mechanism in this index case. Overall, the combination of embolic events and direct infection/inflammation in the lungs leads to the clinical manifestation of haemoptysis.
The major management challenges in this index case included a persistent fever that did not respond to some antibiotics and antimalarial treatment, difficulties in accessing effective antibiotics like Rocephin and Vancomycin, and the high costs associated with them. Additionally, the patient had associated acute kidney injury (AKI) and financial constrained, with many investigations and treatments being partially funded by the medical team. These factors, along with late presentation and diagnosis and multiple complications, put the patient at higher risk management challenges, morbidity and mortality often seen in resource-constrained settings. Despite these challenges, the patient had a favourable clinical outcome.
The index case highlights critical learning points for managing patients with L-PS with associated complications in resource-limited settings, which include (1) Young patients with persistent high-grade fever not responding to standard treatments should have a thorough evaluation, including history-taking, physical exams, and focused echocardiography, to check for congenital or rheumatic heart diseases with IE; (2) In young patients, the presence of a pansystolic murmur from a ventricular septal defect (VSD) should lead to further auscultation for an aortic diastolic murmur, which could suggest L-PS; (3) Improving patient outcomes for infective endocarditis and sepsis depends on making effective antibiotics accessible, affordable, and readily available; (4) Patients with L-PS and infective endocarditis face increased risks of severe complications due to delays in diagnosis, financial constrained, and a lack of access to effective antibiotics.
The case faced several limitations in management that include (1) Transesophageal echocardiography (TEE), which is more sensitive than transthoracic echocardiography (TTE) for detecting small valvular and mural vegetations <2 mm, was not performed. This omission is significant given the absence of physical evidence of left-sided IE on TTE (2) The negative blood culture results may have been affected by previous antibiotic treatment or the presence of deep-seated microbial organisms within vegetations, and probably culture techniques used (3) The management of the case was significantly hindered by financial limitations, which affected the procurement of necessary antibiotics, surgical interventions for cardiac lesions, and essential diagnostic imaging, such as a Chest CT, to identify septic pulmonary and abdominal embolic complications.
The uniqueness of this index case was the masquerading clinical presentation of L-PS and the associated life-threatening complications and emergencies: RSIE, sepsis, AKI, hemoptysis and acute HF. The case highlights the effectiveness of thorough history taking, prompt recognition, meticulous physical examinations, focused investigations and multidisciplinary care approach, which led to a favorable clinical outcome. Notably, our literature review found no documented case study of such a combination of severe complications arising from L-PS, making this case particularly noteworthy.
In conclusion, our case highlights the critical importance of thorough history-taking, meticulous physical examinations, and focused investigations such as echocardiography in young patient with L-PS presenting with persistent high-grade fever unresponsive to antibiotics or antimalarial treatment. This approach is essential for ruling out serious cardiac diseases like congenital L-PS or rheumatic heart disease with complications infective endocarditis, which can lead to life-threatening emergencies if not swiftly diagnosed and treated appropriately. Early identification is key to improving patient outcomes and preventing a dismal prognosis.
Acknowledgement: This case study would not have been possible without adequate information provided by the patient and his family. We are grateful to all.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: (I) Study conception and design: Hayatu Umar, Abdulaziz Aminu, Femi Akindotun Akintomide; (II) Data collection: Hayatu Umar, Femi Akindotun Akintomide, Abduaziz Aminu, Adamu Mohammad, Aisha Aminu Lawal (III) Analysis and interpretation of results: Hayatu Umar, Abdulaziz Aminu, Raghu Cherukupalli, Femi Akindotun Akintomide, Abdul Habu, Aisha Aminu Lawal; (IV) Draft manuscript preparation: Hayatu Umar, Abdulaziz Aminu, Raghu Cherukupalli, Femi Akindotun Akintomide, Abdul Habu, Adamu Mohammad, Aisha Aminu Lawal. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author, HU, upon reasonable request.
Informed Consent: The authors obtained patient and his parents written consent. In the form he and his parent consented for his clinical information and images to be published in a journal. The patient and his parent understand that his name and initials will not be published in a journal.
Ethics Approval: Ethical approval for this case study was obtained from institutional ethics committee of Usmanu Danfodiyo University Teaching Hospital, Sokoto. Nigeria. Reference number: UDUTH/HREC/2024/1435.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/chd.2025.056641, Video S1: Transthoracic echocardiography (TTE); apical four chamber view, video showing large mobile tricuspid valve vegetation.
References
1. Laubry C, Pezzi C. Treatise on congenital heart diseases. Paris: JB Bailliere; 1921. Quoted by Laubry C, Routier D, Soulie P. The blows of Roger’s disease. Rev Med Paris. 1933;50:439–48. doi:10.1097/MS9.0000000000000254. [Google Scholar] [CrossRef]
2. Charfo MB, Ettagmouti Y, Mahoungou Mackonia NM, Arouss S, Drighil AN. Laubry-Pezzi syndrome: three case reports and review of the literature. Ann Med Surg. 2023;85(5):1843–7. doi:10.1097/MS9.0000000000000254. [Google Scholar] [CrossRef]
3. Yasuhara J, Garg V. Genetics of congenital heart disease: a narrative review of recent advances and clinical implications. Transl Pediatr. 2021;10(9):2366–86. doi:10.21037/tp-21-297. [Google Scholar] [CrossRef]
4. Choukrani H, Hamine Y, Bennani G, Drighil A, Azzouzi L, Habbal R. Infective endocarditis revealing Laubry Pezzi syndrome: a rare case report. Asian J Cardiol Res. 2023;6(1):240–5. doi:10.1016/j.rec.2015.100. [Google Scholar] [CrossRef]
5. Kraiem S, Chehaibi N, Lahidheb D, Sfaxi A, Fennira S, Ben Ameur Y et al. Infectious enterococcal endocarditis associated with Laubry and Pezzi syndrome. Tunis Med. 2000;78(1):66–9. doi:10.1016/j.rec.2015.100. [Google Scholar] [CrossRef]
6. Khan GQ, Kadri SM, Hassan G, Shahid IT, Gazanfar A, Kak M, et al. Salmonella typhi endocarditis: a case report. J Clin Pathol. 2003;56(10):801–2. doi:10.1136/jcp.56.10.801. [Google Scholar] [CrossRef]
7. Cheng WL, Li CW, Li MC, Lee NY, Lee CC, Ko WC. Salmonella infective endocarditis. J Microbiol Immunol Infect. 2016;49(3):313–20. doi:10.1016/j.jmii.2015.02.659. [Google Scholar] [CrossRef]
8. Robson C, O’Sullivan MVN, Sivagnanam S. Salmonella enterica Serovar Typhi: an unusual cause of infective endocarditis. Trop Med Infect Dis. 2018;3(1):35. doi:10.3390/tropicalmed3010035. [Google Scholar] [CrossRef]
9. Hatab H, Wahab F, El-Mahy H. Endocarditis presenting as acute abdomen. BMJ Case Rep. 2010;2010:bcr0720092060. doi:10.1136/bcr.07.2009.2060. [Google Scholar] [CrossRef]
10. Vergne R, Selland B, Gobel FL, Hall WH. Rupture of the spleen in infective endocarditis. Arch Intern Med. 1975;135(9):1265–7. doi:10.1001/archinte.1975.00330090137017. [Google Scholar] [CrossRef]
11. Chang CY, Gan YL, Radhakrishnan AP, Ong ELC. Acute abdomen revealed Streptococcus gordonii infective endocarditis with systemic embolism. Oxf Med Case Rep. 2022;2022(1):omab145. doi:10.1093/omcr/omab145. [Google Scholar] [CrossRef]
12. Hakman EN, Cowling KM. Paradoxical embolism. Treasure Island, FL, USA: StatPearls Publishing; 2024. [Google Scholar]
13. Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG Jr, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the international collaboration on endocarditis-prospective cohort study. Arch Intern Med. 2009;169(5):463–73. doi:10.1001/archinternmed.2008.603. [Google Scholar] [CrossRef]
14. Moscatelli S, Leo I, Bianco F, Surkova E, Pezel T, Donald NA. The role of multimodality imaging in patients with congenital heart disease and infective endocarditis. Diagnostic. 2023;13(24):3638. doi:10.3390/diagnostics13243638. [Google Scholar] [CrossRef]
15. Uma DS. Comprehensive cardiology. New Delhi, India: Jaypee; 2008. [Google Scholar]
16. Divya Rabindranath DR, Rabindranath Eswaran RE, Khan AA, Nishat Afroz NA, Senthil P, Asfia Sultan AS. Intravenous cannula site infection presenting as endocarditis—a preventable complication. Int J Curr Microbiol App Sci. 2015;(Spec No 1):75–80. [Google Scholar]
17. Chakraborty RK, Burns B. Systemic inflammatory response syndrome. Treasure Island, FL, USA: StatPearls Publishing LLC; 2024. [Google Scholar]
18. Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on sepsis-related problems of the European society of intensive care medicine. Crit Care Med. 1998;26(11):1793–800. doi:10.1097/00003246-199811000-00016. [Google Scholar] [CrossRef]
19. Bui JT, Schranz AJ. Pulmonary complications observed in patients with infective endocarditis with and without injection drug use: an analysis of the national inpatient sample. PLoS One. 2021;16(9):e0256757. doi:10.1371/journal.pone.0256757. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools