 Open Access
Open Access
ARTICLE
Analysis of Risk Factors for Central Venous Catheter-Associated Thrombosis in Children after Congenital Heart Surgery
1 Department of Cardiothoracic Surgery, Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China
2 School of Nursing, Shanghai Jiao Tong University, Shanghai, 200025, China
3 Intensive Care of Unit, Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China
4 Department of Nursing, Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China
* Corresponding Authors: Li Yuan. Email: ; Wenyi Luo. Email:
Congenital Heart Disease 2024, 19(6), 603-615. https://doi.org/10.32604/chd.2025.057681
Received 24 August 2024; Accepted 17 January 2025; Issue published 27 January 2025
Abstract
Objective: To investigate the status and influencing factors of central venous catheter-associated thrombosis in children after congenital heart surgery and to provide evidence for preventive measures. Methods: From January 2024 to March 2024, hospitalized children with central venous catheters (CVC) in the Shanghai Grade III Children’s Hospital intensive care unit were selected. Catheter-related thrombosis (CRT) was evaluated using bedside ultrasound technology combined with visible thrombus after CVC extraction, dividing the patients into the thrombus and non-thrombus groups. Univariate and LASSO regression analyses were used to analyze the factors influencing CRT in children after congenital heart surgery, and binary logistic regression was used to analyze the risk factors. Results: 229 children were included, of which 24 (10.48%) had CRT. Binary logistic regression analysis showed that time of vasoconstrictor use >one day, sedation and analgesia time, and flushing the CVC with saline were the risk factors for CRT in children after congenital heart surgery. Conclusion: The incidence of CRT is higher in children after congenital heart surgery. The medical staff can formulate targeted intervention measures based on the corresponding risk factors to reduce the incidence of CRT.Keywords
Nomenclature
| APTT | Activated partial thromboplastin time |
| BMI | Body mass index |
| CI | Confidence interval |
| CRT | Catheter-related thrombosis |
| CVC | Central venous catheter |
| ECMO | Extracorporeal membrane pulmonary oxygenation |
| FDP | Fibrin degradation product |
| ICU | Intensive-care unit |
| INR | International normalized ratio |
| LAC | Lactic acid value |
| LASSO | Least absolute shrinkage and selection operator |
| OR | Odds ratio |
| PCIS | Pediatric critical illness score |
| PLT | Platelet count |
| PT | Prothrombin time |
| SE | Standard error |
| VIS | Vasoactive inotrope score |
A central venous catheter (CVC) is a catheter whose tip is positioned within the proximal third of the superior vena cava, right atrium, or inferior vena cava [1]. Common sites for CVC insertion include the internal jugular, subclavian, and femoral veins [2]. The use of CVCs can prevent and reduce the pain and difficulty of repeated venous punctures in pediatric patients [3]. In cardiovascular surgery, CVC is used routinely as an important channel for administrating various vasoactive agents and for monitoring central venous pressure [4]. Catheter-related thrombosis (CRT) refers to the formation of a thrombus in the deep vein where the catheter is placed or in the adjacent venous drainage area after CVC implantation [5], and CRT is the most common noninfectious complication associated with CVC implantation [6–8]. The Children’s Hospital Acquired Thrombosis Consortium has found that 80% of hospital-acquired venous thromboembolism cases are related to CVC [9]. The incidence of CRT in children with congenital heart defects is 2.00%–22.65% [10–12]. While CRT is mainly asymptomatic, it can also present with symptoms such as inflammation or vascular obstruction [13,14]. Additionally, CRT may cause long-term complications, including post-thrombotic syndrome (PTS), adversely affect the prognosis of children, and can potentially lead to life-threatening conditions such as pulmonary embolism [14–16]. In addition, CRT can increase central venous pressure in children with congenital heart disease, leading to chylous effusions, which may complicate future surgeries and increase overall mortality [17]. Therefore, addressing the occurrence of CRT in children with congenital heart disease is crucial.
Current research has focused chiefly on the occurrence and risk factor analysis of venous thrombosis in children with congenital heart disease. Related studies have found several risk factors for thrombosis in this population, such as young age, history of catheterization, mechanical ventilation, and extracorporeal membrane pulmonary oxygenation (ECMO) [11,18,19]. Additionally, research has found that children with congenital or acquired heart disease undergoing cardiothoracic surgery are particularly prone to thromboembolic events [20]. However, few studies have paid specific attention to the occurrence and influencing factors of CRT in children after congenital heart surgery. This study aimed to investigate the incidence of CRT in children after congenital heart surgery and analyze the influencing factors to provide evidence for nurses to take implement appropriate preventive measures for reducing the incidence of CRT in children after congenital heart surgery.
This study is a single-center, cross-sectional prospective study.
Children with congenital heart disease in the intensive care unit of Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, were selected for this study from January 2024 to March 2024. The inclusion criteria were as follows: (1) age between 28 days and 18 years; (2) CVC retention time ≥48 h; and (3) all participants underwent cardiac surgery. The exclusion criteria were as follows: (1) death of the child; (2) withdrawal of treatment by the child or family members; and (3) preoperative history of catheterization, mechanical ventilation, or ECMO. This study was approved by the Ethics Committee of Shanghai Children’s Medical Center (SCMCIRB-K2023231-1) with the informed consent obtained from all survey subjects.
Based on the pre-systematic literature analysis and expert consultation, the researcher designed clinical data questionnaire, comprising six sections: (1) Patient-related factors, including age, gender, and ICU stays; (2) Disease-related factors, including whether the congenital heart disease staging is cyanotic or not, high-risk comorbidities or complications (common infections, liver function abnormalities, sepsis, organ hemorrhage, low cardiac output syndrome, cardiac arrest, inflammatory bowel disease), vasoactive inotrope score (VIS), pediatric critical illness score (PCIS), Caprini scale score, and nutritional status (that of children <6 years of age was calculated concerning the World Health Organization growth curve, and that of children ≥6 years of age was determined via calculation of body mass index [BMI]); (3) Drug-related factors, including use of parenteral nutrition, mannitol, and blood products, duration of glucocorticoid use, duration of vasoconstrictor use, and duration of sedation and analgesia; (4) Factors related to invasive treatment, including the use of ECMO, hemodialysis, cardiac catheterization intervention (either for diagnosis or treatment), duration of surgery, duration of extracorporeal circulation and duration of mechanical ventilation; (5) Laboratory test-related factors, including D-dimer concentration, fibrinogen concentration, activated partial thromboplastin time (APTT), prothrombin time (PT), international normalized ratio (INR), fibrin degradation product (FDP), lactic acid value (LAC), and platelet count (PLT); and (6) Catheter-related factors, including catheter type, number of catheter lumens, orientation of the CVC, vein used for CVC, and the type of fluid used for flushing and sealing the CVC.
The vasoactive drug score used in this study was VIS, proposed by Gaies et al. [21] in 2010, and the score was evaluated 24 h after surgery, except for children exposed to ECMO [22]. The PCIS is a scoring system used to assess the severity of diseases in children [23]. The tool includes 11 indicators such as heart rate, respiration, arterial blood oxygen partial pressure, blood pressure, and pH, where lower scores indicate a more serious condition. A lower score indicates a more severe condition [24]. Caprini’s thrombosis risk assessment table was put forward by American scholar Caprini [25], including patient-related factors, surgical factors, and clinical laboratory examination. Based on the total score, a higher score indicates a higher risk.
The data were collected by a trained researcher and checked by another researcher. The relevant data in the clinical data questionnaire were obtained by consulting electronic medical records and nursing records. The first ultrasound examination was conducted 48 to 72 h after CVC implantation. Subsequently, it was performed every three days until one of the following conditions arose: CRT, removal of the CVC in the intensive care unit (ICU), or transfer out of the ICU.
CRT was diagnosed by ultrasonography, which suggested the presence of an intravascular thrombus or a thrombus visible to the naked eye on the catheter after the removal of the CVC. After the child’s CVC was placed, ultrasound screening was performed by a nurse trained in intensive care ultrasound. In addition, sonographers familiar with the study’s objective guide its implementation. When an operator identified a positive case, the sonographers conducted a review to confirm the finding. The ultrasound diagnostic criteria for CRT were as follows [10,13,26,27]: (1) solid echoes were detected within the lumen of the CVC, catheter wall, or placed vein; (2) the lumen of the probe-pressurized vein could not be closed or could not be closed entirely; (3) the Color Doppler did not detect blood flow signals; and (4) the period-phase nature of the blood flow spectra was weakened or disappeared. CRT was judged positive if one of the above conditions was met.
SPSS 26.0 and R4.4.0 software were used for data analysis. Quantitative data conforming to normal distribution were presented as mean with standard deviation (X ± S), and Student’s t test was used for intergroup comparison. Quantitative data not conforming to normal distribution were presented as median with interquartile range (M [P25, P75]), and nonparametric tests were used for intergroup comparisons. Categorical data were expressed as frequency and percentage (%), and the chi-square test was used for intergroup comparisons. For variables that showed statistical significance in the univariate analysis, the minor absolute shrinkage and selection operator (LASSO) regression method was used for dimensionality reduction to screen the factors influencing CRT in children after congenital heart surgery. Then, binary logistic regression analysis was performed on the factors selected by LASSO. The differences were considered statistically significant at p < 0.05.
3.1 General Information and CRT Status
A total of 229 children were included in this study, with a median age of 23.0 (7.0, 56.5) months, 114 (49.8%) male and 115 (50.2%) female. Twenty-four (10.48%) patients developed CRT. The high incidence time of CRT occurs between 4–7 days after CVC placement. Detailed results are shown in Table 1.
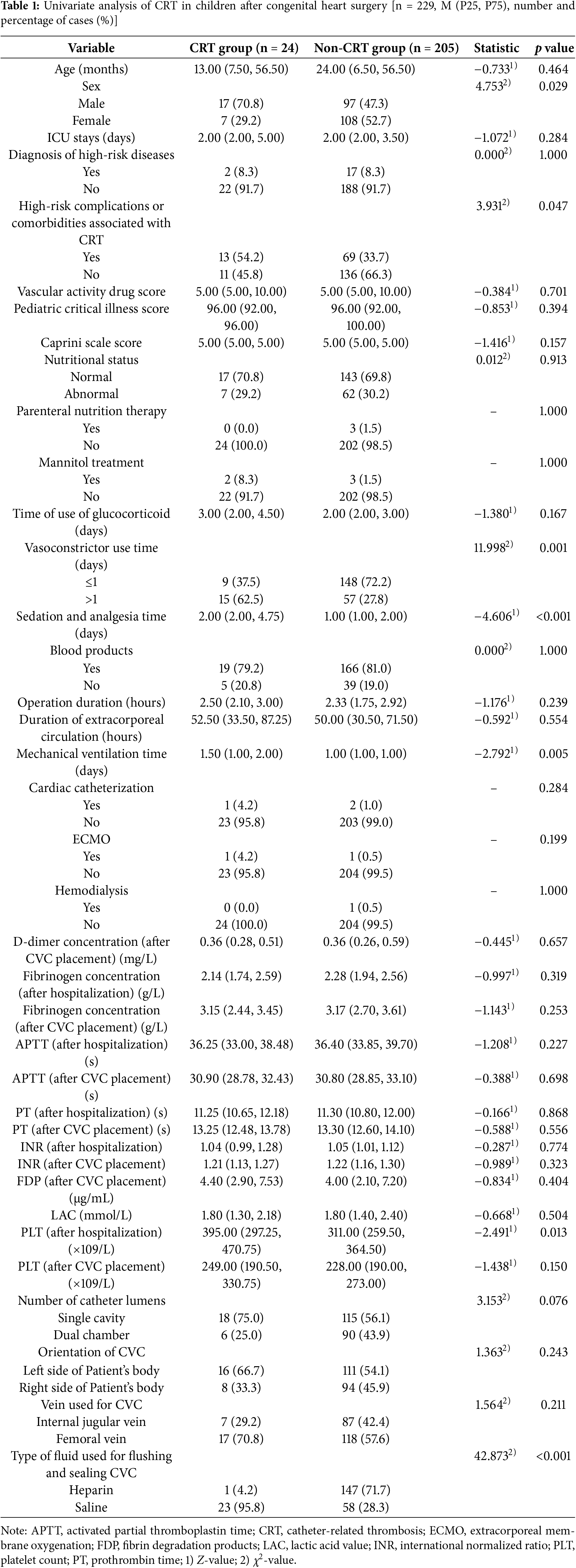
3.2 Univariate Analysis of CRT in Children after Congenital Heart Surgery
There were statistically significant differences (p < 0.05) between the CRT group and the non-CRT group in terms of gender, presence of high-risk comorbidities or complications, duration of vasoconstrictor use, duration of sedation and analgesia, duration of mechanical ventilation, PLT (after hospitalization), and the type of fluid used for flushing and sealing CVC. The detailed results are shown in Table 1.
3.3 LASSO Regression of CRT in Children after Congenital Heart Surgery
Seven variables with statistical significance in the univariate analysis were input to LASSO regression dimensionality reduction to extract the most important predictors and avoid overfitting. The LASSO model’s best parameter (lambda) was selected by minimum criteria using fivefold cross-validation. The minimum lambda value was selected as the standard error (SE) of the optimal value of the model (Figs. 1 and 2). LASSO regression results showed that gender, time of vasoconstrictor use, sedation and analgesia time, PLT (after hospitalization), and the type of fluid used for flushing and sealing CVC were essential factors affecting the development of CRT in children.
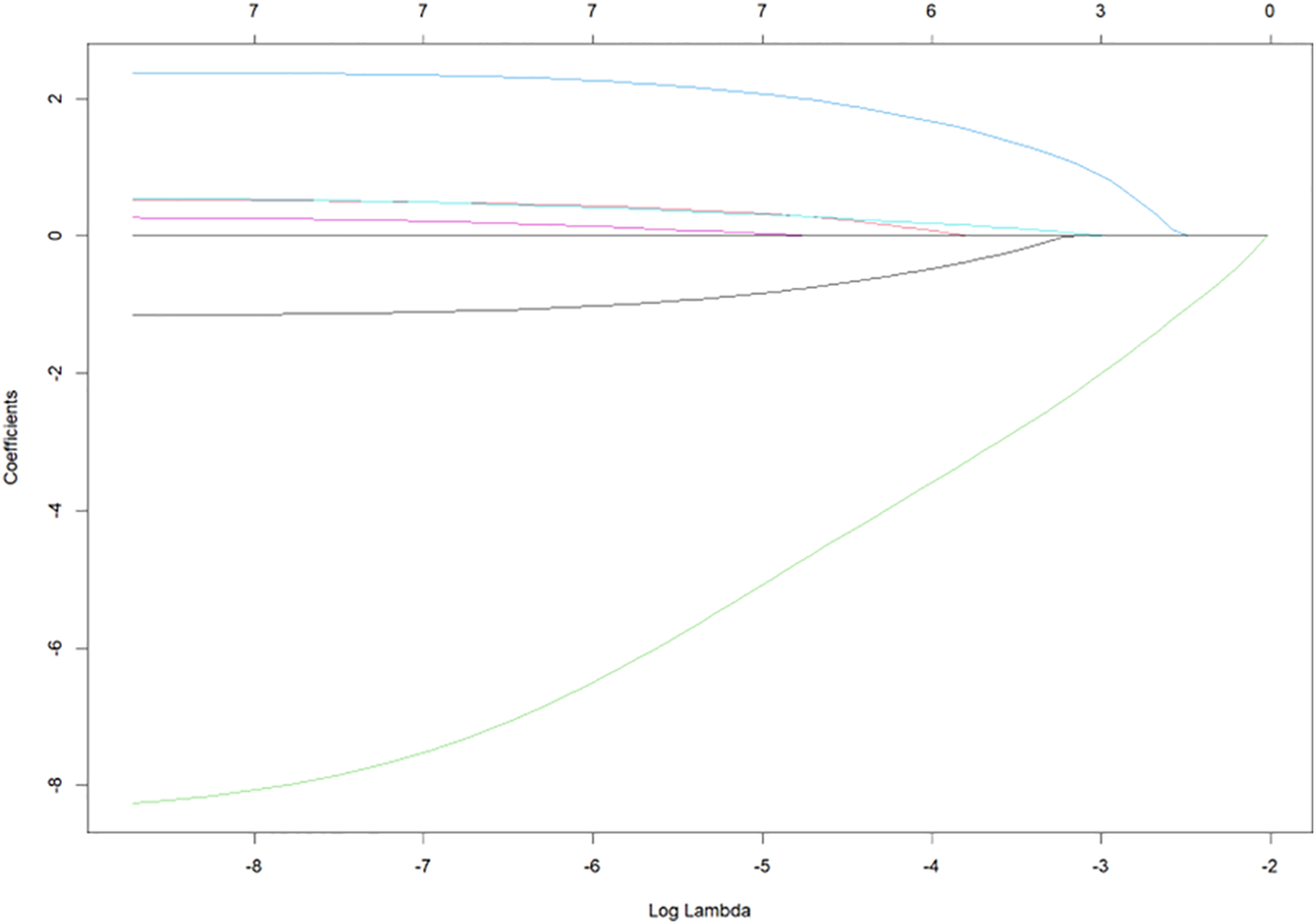
Figure 1: Optimal parameter (λ) selection in the least absolute shrinkage and selection operator (LASSO) model
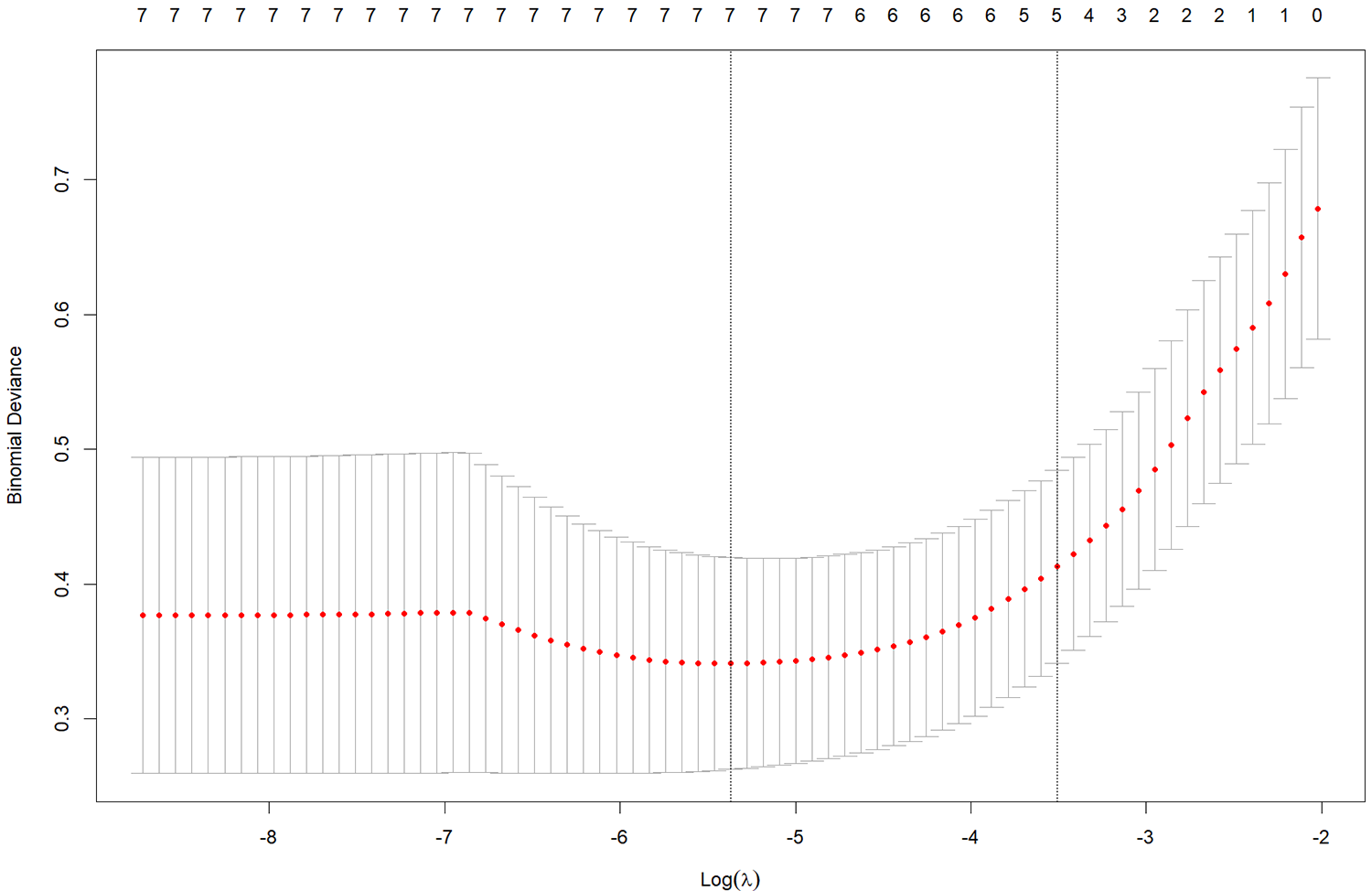
Figure 2: Distribution of the minor absolute shrinkage and selection operator (LASSO) coefficients for seven factors
3.4 Binary Logistic Regression Analysis of CRT in Children after Congenital Heart Surgery
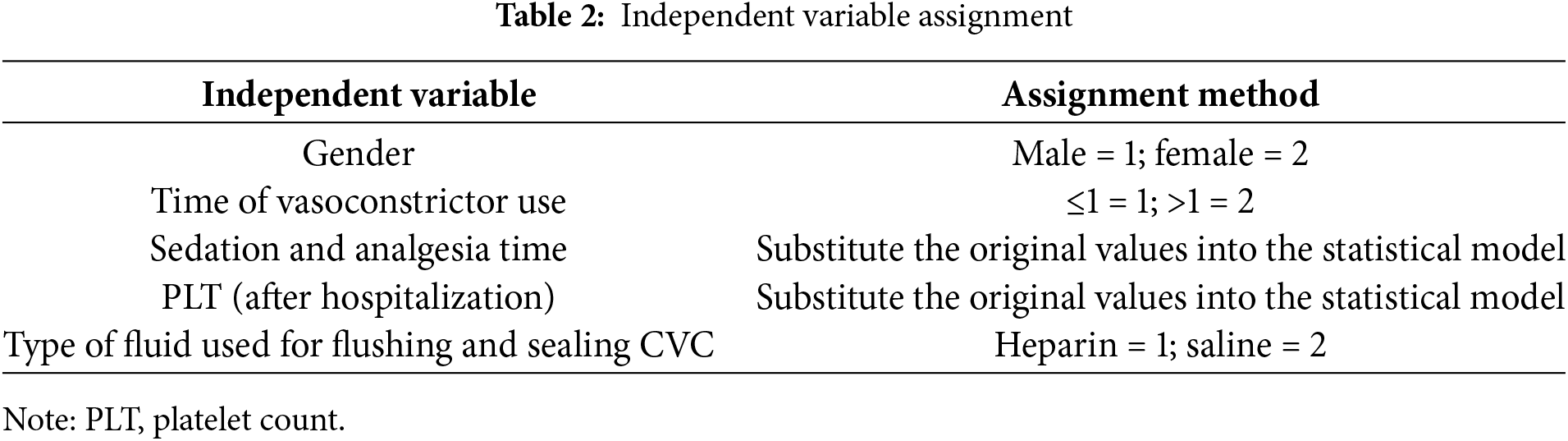
Binary logistic regression analysis was carried out with CRT as the dependent variable, and the five risk factors were screened out by LASSO regression as the independent variables. The backward method was selected. The assignment of the independent variables is shown in Table 2. The results showed that the time of using vasoconstrictors, sedation and analgesia, and flushing the CVC with normal saline were the independent risk factors for CRT (p < 0.05). The detailed results are shown in Table 3.
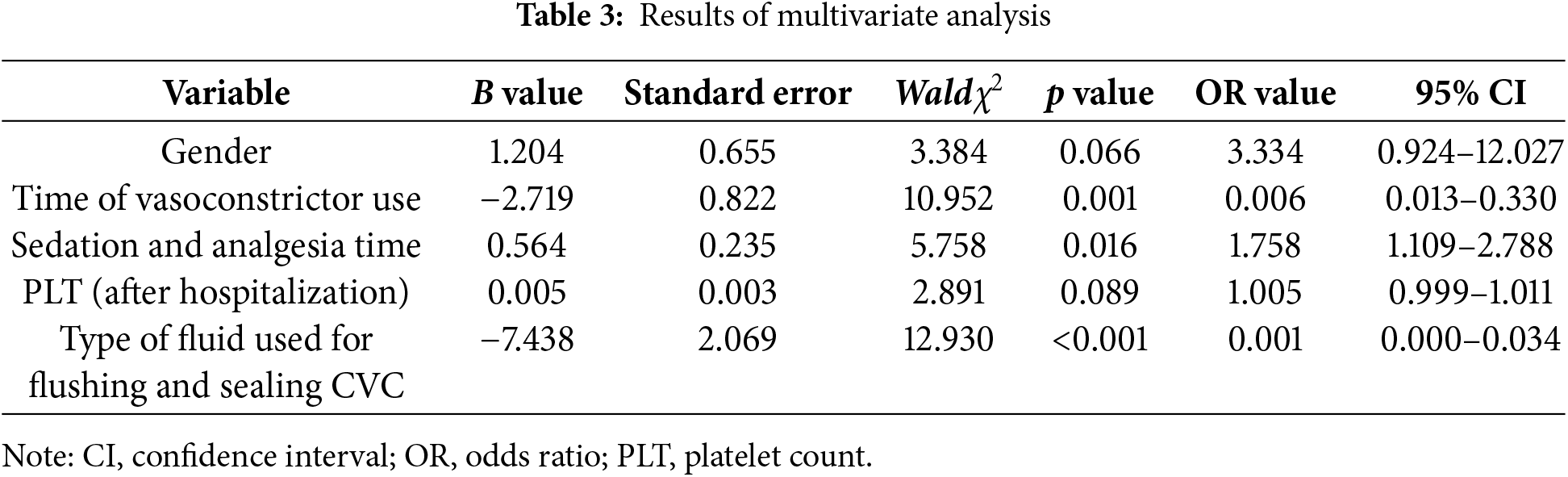
4.1 Higher Incidence of CRT in Children after Congenital Heart Surgery
In this study, 24 out of the 229 children after congenital heart surgery (10.48%) developed CRT, which is higher than the incidence of CRT in children with heart disease reported by DiPietro et al. [11] (2.0%). The reasons for this may be as follows. Children with congenital heart disease are generally young, their coagulation system is not fully developed, and the concentration of anticoagulant proteins in their bodies is lower than that in adults. Insufficient liver perfusion secondary to impaired cardiac function can also affect the synthesis of anticoagulant substances. In addition, during intraoperative extracorporeal circulation, thrombin production increases, and the blood exhibits a hypercoagulable state [28]. Although venography is the gold standard for detecting venous thrombosis, this examination is invasive and has limited practical application in clinical practice. For this reason, previous studies have utilized imaging methods such as ultrasound to detect venous thrombosis [29–31]. According to guidelines, ultrasound can be effectively used as a screening tool for venous thrombosis, and research has shown that it has a sensitivity of 88% and a specificity of 98%, indicating high detection accuracy [32]. In some cases, ultrasound examinations are performed only when there is a clinical suspicion of thrombosis, relying on the healthcare clinical judgment, which can result in missed diagnoses [29]. However, CRT is mainly asymptomatic, this study directly used Doppler ultrasound to evaluate and reduce omissions. It has been found that 8%–18% of children with CRT may develop post-thrombotic syndrome [33], which affects prognosis. Therefore, early identification of CRT risk and appropriate prevention measures are essential.
4.2 Longer Duration of Vasoconstrictor Use Is Associated with a Higher Risk of CRT in Children after Congenital Heart Surgery
This study showed that the use of vasoconstrictor drugs for a long time was a risk factor for CRT in children after congenital heart surgery, and the use of vasoconstrictor drugs has been reported as a risk factor for CRT in previous studies [34]. The vasoconstrictor drugs in this study were mainly epinephrine and norepinephrine, which can stimulate α receptors and cause systemic vasoconstriction, thereby narrowing the lumen, slowing down blood flow rate, and promoting CRT when platelets gather under the damaged endothelial tissue [35,36]. With the long-term use of such drugs, blood vessels continue to shrink, reducing blood flow and further promoting thrombosis. It has been found that vasoconstrictor drugs can reduce the activity of coagulation factor Xa in plasma, thereby reducing the effect of low-molecular-weight heparin sodium on preventing thrombosis [37]. However, other studies have shown no correlation between norepinephrine dose and anti-factor Xa activity in patients using low-molecular-weight heparin [38], and more high-quality studies are needed to explore the relationship between the two further. It is also worth noting that prolonged vasoconstrictor use typically indicates a more severe shock state, potentially reflecting more severe underlying heart disease or a more complex surgical procedure. Nursing staff should closely monitor the reaction after medication for children exposed to vasoconstrictor drugs to maintain good blood circulation. When the child’s circulation is stable, the staff should inform the doctor promptly to adjust the treatment plan and gradually reduce the use of vasoconstrictor drugs.
4.3 Longer Duration of Sedation and Analgesia Is Associated with a Higher Risk of CRT in Children after Congenital Heart Surgery
The present study showed that long duration of sedation and analgesia was a risk factor for CRT in children after congenital heart surgery, consistent with previous findings [10]. Sedative analgesics are needed early after surgery in children with congenital heart disease to reduce oxygen consumption, reduce the functional burden of various organs, and promote cardiac function and body recovery [39,40]. During sedation and analgesia, the child is in a state of immobilization and blood flow is slowed down. There is blood stasis in the vein, and increased platelet adhesion and aggregation in the intima of blood vessels favors thrombosis. Longer duration of sedation and analgesia is associated with a higher risk of CRT. While deep sedation may cause circulatory depression [41], children with prolonged bed rest and little or no physical activity are at an increased risk of thrombosis. Therefore, for children under long-term sedation and analgesic drugs, nurses need to control the pump speed and drug dosage strictly, as well as regularly assess the sedation and analgesia levels of the children. Then, according to the changes in the child’s condition, nurses must inform the doctor to adjust the drug dose promptly. If the patient’s condition permits, passive movement such as limb abduction and internal rotation should be performed appropriately.
4.4 Flushing and Sealing CVC with Heparin Reduces the Risk of CRT in Children after Congenital Heart Surgery
This study showed that children after congenital heart surgery who received heparin to flush the CVC had a lower risk of CRT. First, heparin is an anticoagulant with remarkable effects in preventing and treating thrombotic diseases [42]. However, excessive use of heparin can prolong blood clotting time, leading to an increased risk of bleeding. In addition, heparin induces thrombocytopenia, which leads to a decrease in platelet count and further elevating bleeding risk [43]. Children undergoing congenital heart surgery typically require extracorporeal circulation necessitating heparin before and after the surgery procedure. The dosage of heparin significantly affects coagulation function [44], making the concentration and dosage of heparin crucial. Unfortunately, in the absence of high-quality evidence, the existing guidelines and expert consensus recommend using normal saline to flush and seal CVC and do not recommend the routine use of CVC flushing and sealing fluids containing anticoagulants to prevent CRT [45]. “Expert Consensus on Central Venous Catheter Flushing and Sealing” points out that for patients with hypercoagulable blood, it is recommended first to use regular saline flushing and then use heparin saline sealing. Further high-quality studies are needed to explore the effects of different doses and concentrations of heparin on the formation of CRT.
However, some limitations should be acknowledged. First, the study was a single-center cross-sectional survey with a limited sample size. Second, the study monitored whether the children experienced CRT during ICU stays, which resulted in missed positive cases.
The incidence of CRT in children after congenital heart surgery is high, and it is affected by the duration of use of vasoconstrictor drugs, sedation and analgesia time, and flushing the CVC with saline. In the future, well-designed randomized controlled trials will be necessary to validate these results. Clinical staff should combine these risk factors to build a systematic and targeted CRT prevention program for children after congenital heart surgery and actively implement nursing preventive measures to reduce the risk of CRT.
Acknowledgement: We would like to thank the parents and subjects for participating in this study and the medical staff in the cardiac ICU for their contribution.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm their contribution to the paper: study conception and design: Wenyi Luo, Li Yuan, Zhuomin Xu; data collection: Zhimin Yang, Yueyue Zhang; analysis and interpretation of results: Hanfang Deng, Lin Chen, Wenlan Zhang; draft manuscript preparation: Hanfang Deng, Zhimin Yang. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval: This study was approved by the Ethics Committee of Shanghai Children’s Medical Center (SCMCIRB-K2023231-1) with the informed consent obtained from all survey subjects.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Xie W, Xu B, Lou X, Zhu J, Ye S. Development and validation of a nomogram for catheter-related thrombosis prediction in children with central venous catheter: a retrospective observational study. BMC Pediatr. 2024;24(1):1–7. doi:10.1186/s12887-024-05008-2. [Google Scholar] [PubMed] [CrossRef]
2. Vafek V, Skříšovská T, Kosinová M, Klabusayová E, Musilová T, Kramplová T, et al. Central venous catheter cannulation in pediatric anesthesia and intensive care: a prospective observational trial. Children. 2022;9(11):1611. doi:10.3390/children9111611. [Google Scholar] [PubMed] [CrossRef]
3. Li S, Luo Y, Deng J, Zeng J, Fan M, Wang T, et al. Risk factors for central venous catheter-related thrombosis in hospitalized children: a single-center a retrospective cohort study. Transl Pediatr. 2022;11(11):1840–51. doi:10.21037/tp-22-529. [Google Scholar] [PubMed] [CrossRef]
4. Phulli R, Arora P, Neema PK. Utility and futility of central venous catheterization. Ann Card Anaesth. 2021;24(3):378–80. doi:10.4103/aca.ACA_112_20. [Google Scholar] [PubMed] [CrossRef]
5. Elias A, Debourdeau P, Espitia O, Sevestre M-A, Girard P, Mahé I, et al. Central venous catheter associated upper extremity deep vein thrombosis in cancer patients: diagnosis and therapeutic management. Arch Cardiovasc Dis. 2024;117(1):72–83. doi:10.1016/j.acvd.2023.11.011. [Google Scholar] [PubMed] [CrossRef]
6. Rajasekhar A, Streiff MB. How I treat central venous access device-related upper extremity deep vein thrombosis. Blood. 2017;129(20):2727–36. doi:10.1182/blood-2016-08-693671. [Google Scholar] [PubMed] [CrossRef]
7. Linnemann, Lindhoff L. Risk factors, management and primary prevention of thrombotic complications related to the use of central venous catheters. Vasa. 2012;41(5):319–32. doi:10.1024/0301-1526/a000217. [Google Scholar] [PubMed] [CrossRef]
8. Yamashita T, Takamori A, Nakagawachi A, Tanigawa Y, Hamada Y, Aoki Y, et al. Early prophylaxis of central venous catheter-related thrombosis using 1% chlorhexidine gluconate and chlorhexidine-gel-impregnated dressings: a retrospective cohort study. Sci Rep. 2020;10(1):15952. doi:10.1038/s41598-020-72709-w. [Google Scholar] [PubMed] [CrossRef]
9. Clark HH, Ballester L, Whitworth H, Raffini L, Witmer C. Prevention of recurrent thrombotic events in children with central venous catheter-associated venous thrombosis. Blood. 2022;139(3):452–60. doi:10.1182/blood.2021013453. [Google Scholar] [PubMed] [CrossRef]
10. Jiang W, Jin C, Li S, Yang S, Yan C, Zhu J. Construction and validation of a risk prediction model for central venous catheter-associated deep venous thromboses in children with congenital heart disease after surgery. Chin J Nurs. 2022;57(18):2217–24. doi:10.3761/j.issn.0254-1769.2022.18.007. [Google Scholar] [CrossRef]
11. DiPietro LM, Gaies M, Banerjee M, Donohue JE, Zhang W, DeSena HC, et al. Central venous catheter utilization and complications in the pediatric cardiac ICU: a report from the pediatric cardiac critical care consortium (PC4). Pediatr Crit Care Med. 2020;21(8):729–37. doi:10.1097/pcc.0000000000002306. [Google Scholar] [PubMed] [CrossRef]
12. Steen EH, Lasa JJ, Nguyen TC, Keswani SG, Checchia PA, Anders MM. Central venous catheter-related deep vein thrombosis in the pediatric cardiac intensive care unit. J Surg Res. 2019;241:149–59. doi:10.1016/j.jss.2019.03.052. [Google Scholar] [PubMed] [CrossRef]
13. Gibb S, Engelhardt S, Von Dincklage F, Kuhn SO. Incidence and onset of central venous catheter-related thrombosis in critically ill surgical patients: a prospective observational single-center study. J Clin Anesth. 2024;97:111556. doi:10.1016/j.jclinane.2024.111556. [Google Scholar] [PubMed] [CrossRef]
14. Lasagni D, Nosadini M, Molinari AC, Saracco P, Pelizza MF, Piersigilli F, et al. Systemic catheter-related venous thromboembolism in children: data from the Italian registry of pediatric thrombosis. Front Pediatr. 2022;10:843643. doi:10.3389/fped.2022.843643. [Google Scholar] [PubMed] [CrossRef]
15. White D, Woller SC, Stevens SM, Collingridge DS, Chopra V, Fontaine GV. Comparative thrombosis risk of vascular access devices among critically ill medical patients. Thromb Res. 2018;172:54–60. doi:10.1016/j.thromres.2018.10.013. [Google Scholar] [PubMed] [CrossRef]
16. Gibson CD, Colvin MO, Park MJ, Lai Q, Lin J, Negassa A, et al. Prevalence and predictors of deep vein thrombosis in critically Ill medical patients who underwent diagnostic duplex ultrasonography. J Intensive Care Med. 2020;35(10):1062–6. doi:10.1177/0885066618813300. [Google Scholar] [PubMed] [CrossRef]
17. Swartz MF, Hutchinson DJ, Stauber SD, Taillie ER, Alfieris GM, Cholette JM. Enoxaparin reduces catheter-associated venous thrombosis after infant cardiac surgery. Ann Thorac Surg. 2022;114(3):881–8. doi:10.1016/j.athoracsur.2021.05.009. [Google Scholar] [PubMed] [CrossRef]
18. Tripathi S, Burkiewicz K, Gehlbach JA, Wang Y, Astle M. Catheter-associated deep vein thrombosis (CADVT) in a pediatric ICU: a retrospective case-control study. J Assoc Vasc Access. 2020;25(3):45–55. doi:10.2309/java-d-20-00012. [Google Scholar] [CrossRef]
19. Badheka AV, Hodge D, Ramesh S, Bloxham J, Espinoza E, Allareddy V, et al. Catheter related thrombosis in hospitalized infants: a neural network approach to predict risk factors. Thromb Res. 2021;200:34–40. doi:10.1016/j.thromres.2021.01.009. [Google Scholar] [PubMed] [CrossRef]
20. Atchison CM, Amankwah E, Wilhelm J, Arlikar S, Branchford BR, Stock A, et al. Risk factors for hospital-associated venous thromboembolism in critically ill children following cardiothoracic surgery or therapeutic cardiac catheterisation. Cardiol Young. 2018;28(2):234–42. doi:10.1017/S1047951117001755. [Google Scholar] [PubMed] [CrossRef]
21. Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234–8. doi:10.1097/PCC.0b013e3181b806fc. [Google Scholar] [PubMed] [CrossRef]
22. Alam S, Akunuri S, Jain A, Mazahir R, Hegde R. Vasoactive-ventilation-renal score in predicting outcome postcardiac surgery in children. Int J Crit Illn Inj Sci. 2018;8(3):143–8. doi:10.4103/IJCIIS.IJCIIS_1_18. [Google Scholar] [PubMed] [CrossRef]
23. Ge H, Zhang A, Teng Y, Hu L. Evaluation of the combined predictive value of multiple indicators based on diaphragmatic ultrasound using logistic regression and ROC curve in weaning from mechanical ventilation in pediatric patients. Front Pediatr. 2024;12:1344709. doi:10.3389/fped.2024.1344709. [Google Scholar] [PubMed] [CrossRef]
24. Pan T, Guo X, Yang D, Ding J, Chen C. Expression and significance of procalcitonin, leukotriene B4, serum amyloid A, and C-reactive protein in children with different types of pneumonia: an observational study. Medicine. 2024;103(19):e37817. doi:10.1097/MD.0000000000037817. [Google Scholar] [PubMed] [CrossRef]
25. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2–3):70–8. doi:10.1016/j.disamonth.2005.02.003. [Google Scholar] [PubMed] [CrossRef]
26. Zhang X, Chen H, Jing W, Pu L, Wu Z, Su X, et al. The clinical topography of peripherally inserted central catheter-related thrombosis in cancer patients: a prospective and longitudinal observational study based on ultrasound scans every two days. Thromb Res. 2023;229:232–42. doi:10.1016/j.thromres.2023.07.013. [Google Scholar] [PubMed] [CrossRef]
27. Wang N, Guo Z, Zhang Y, Wang J, Guo W, Wang J, et al. Risk factors analysis of central venous catheter-related thrombosis in critically ill patients and development of nomogram prediction model. Chin Crit Care Med. 2021;33(9):1047–51. doi:10.3760/cma.j.cn121430-20210712-01044. [Google Scholar] [CrossRef]
28. Silvey M, Brandão LR. Risk factors, prophylaxis, and treatment of venous thromboembolism in congenital heart disease patients. Front Pediatr. 2017;5:146. doi:10.3389/fped.2017.00146. [Google Scholar] [PubMed] [CrossRef]
29. Greiner J, Schrappe M, Claviez A, Zimmermann M, Niemeyer C, Kolb R, et al. THROMBOTECT—a randomized study comparing low molecular weight heparin, antithrombin and unfractionated heparin for thromboprophylaxis during induction therapy of acute lymphoblastic leukemia in children and adolescents. Haematologica. 2019;104(4):756–65. doi:10.3324/haematol.2018.194175. [Google Scholar] [PubMed] [CrossRef]
30. Shah PS, Kalyn A, Satodia P, Dunn MS, Parvez B, Daneman A, et al. A randomized, controlled trial of heparin versus placebo infusion to prolong the usability of peripherally placed percutaneous central venous catheters (PCVCs) in neonates: the HIP (Heparin Infusion for PCVC) study. Pediatrics. 2007;119(1):e284–91. doi:10.1542/peds.2006-0529. [Google Scholar] [PubMed] [CrossRef]
31. Tan Y, Sun X, Zhong J, Zou Y, Ren Y, Liu Y, et al. A randomized, controlled trial of continuous heparin infusion to prevent asymptomatic catheter-related thrombosis at discharge in infants after cardiac surgery: the CHIP-CRT trial. J Pediatr Hematol Oncol. 2024;46(6):e406–11. doi:10.1097/MPH.0000000000002905. [Google Scholar] [PubMed] [CrossRef]
32. Frankel HL, Kirkpatrick AW, Elbarbary M, Blaivas M, Desai H, Evans D, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically Ill patients-part I: general ultrasonography. Crit Care Med. 2015;43(11):2479–502. doi:10.1097/CCM.0000000000001216. [Google Scholar] [PubMed] [CrossRef]
33. Faustino E. Central venous catheter-associated deep venous thrombosis in critically ill children. Semin Thromb Hemost. 2018;44(1):52–6. doi:10.1055/s-0037-1603938. [Google Scholar] [PubMed] [CrossRef]
34. Reynolds PM, Van Matre ET, Wright GC, McQueen RB, Burnham EL, Ho PJM, et al. Evaluation of prophylactic heparin dosage strategies and risk factors for venous thromboembolism in the critically ill patient. Pharmacotherapy. 2019;39(3):232–41. doi:10.1002/phar.2212. [Google Scholar] [PubMed] [CrossRef]
35. Bachi K, Mani V, Jeyachandran D, Fayad ZA, Goldstein RZ, Alia-Klein N. Vascular disease in cocaine addiction. Atherosclerosis. 2017;262:154–62. doi:10.1016/j.atherosclerosis.2017.03.019. [Google Scholar] [PubMed] [CrossRef]
36. Tao W, Xie Y, Bao J, Ding W, Zhang Y, Hu X. Comparison of the effects of norepinephrine and phenylephrine on shivering and hypothermia in patients undergoing caesarean section under spinal anaesthesia at a tertiary hospital in China: a randomised, double-blind, controlled trial protocol. BMJ Open. 2024;14(7):e083202. doi:10.1136/bmjopen-2023-083202. [Google Scholar] [PubMed] [CrossRef]
37. Dörffler-Melly J, de Jonge E, de Pont A-C, Meijers J, Vroom MB, Büller HR, et al. Bioavailability of subcutaneous low-molecular-weight heparin to patients on vasopressors. Lancet. 2002;359(9309):849–50. doi:10.1016/S0140-6736(02)07920-5. [Google Scholar] [PubMed] [CrossRef]
38. Meenks SD, Foudraine NA, Broen K, Noble JLMLl, Janssen PKC. No effect of norepinephrine dose on anti-Xa activity in critically ill patients. Int J Clin Pharmacol Ther. 2020;58(4):223–9. doi:10.5414/CP203640. [Google Scholar] [PubMed] [CrossRef]
39. Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi:10.1097/CCM.0b013e3182783b72. [Google Scholar] [PubMed] [CrossRef]
40. Toklu HZ, Kwon O-S, Sakarya Y, Powers SK, Llinas K, Kirichenko N, et al. The effects of enalapril and losartan on mechanical ventilation-induced sympathoadrenal activation and oxidative stress in rats. J Surg Res. 2014;188(2):510–6. doi:10.1016/j.jss.2014.01.054. [Google Scholar] [PubMed] [CrossRef]
41. Subspecialty Group of Emergency Medicine. The Society of Pediatrics, Chinese Medical Association, Subspecialty Group of Pediatrics, The Society of Emergency Medicine, Chinese Medical Association, Society of Pediatric Critical Care, Chinese Medical Doctor Association. Experts’ consensus on sedation and analgesia for children in pediatric intensive care unit of China (2018). Chin J Pediatr. 2019;57(5):324–30. doi:10.3760/cma.j.issn.0578-1310.2019.05.002. [Google Scholar] [PubMed] [CrossRef]
42. Wu Y, Yin T, Jian G, Wan T, Zhou B. Cost-effectiveness analysis of direct oral anticoagulants versus low-molecular-weight heparin and no thromboprophylaxis in primary prevention of cancer-associated venous thromboembolism in China. Front Pharmacol. 2024;15:1373333. doi:10.3389/fphar.2024.1373333. [Google Scholar] [PubMed] [CrossRef]
43. Páramo JA. Microvascular thrombosis and clinical implications. Med Clin. 2021;156(12):609–14. doi:10.1016/j.medcli.2020.12.042. [Google Scholar] [PubMed] [CrossRef]
44. Haga T, Misaki Y, Sakaguchi T, Akamine Y. Factors affecting the discrepancy between coagulation times on extracorporeal circulation using unfractionated heparin in children and young adults. Clin Appl Thromb Hemost. 2024;30:682. doi:10.1177/10760296241252838. [Google Scholar] [PubMed] [CrossRef]
45. Gorski LA, Hadaway L, Hagle ME, Broadhurst D, Clare S, Kleidon T, et al. Infusion therapy standards of practice, 8th Edition. J Infus Nurs. 2021;44(1S Suppl 1):S1–S224. doi:10.1097/nan.0000000000000396. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools