 Open Access
Open Access
ARTICLE
The Factors for Postoperative Peritoneal Dialysis Treatment in Infants after Congenital Cardiac Procedure
1 Department of Pediatric Intensive Care Unit, National Center for Cardiovascular Disease and Fuwai Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, 100037, China
* Corresponding Author: Xu Wang. Email:
# These authors contributed equally to this paper
Congenital Heart Disease 2024, 19(6), 617-626. https://doi.org/10.32604/chd.2025.058712
Received 19 September 2024; Accepted 16 January 2025; Issue published 27 January 2025
Abstract
Objectives: Fluid overload is common after congenital cardiac surgeries. This requires early application of peritoneal dialysis (PD) in infants to improve surgical outcomes. The objective of this study is to ascertain the factors correlated with the necessity for PD in infants, thereby informing the prophylactic placement of PD catheters intraoperatively. Methods: This was a single-center retrospective study. Infants aged three months or younger who underwent congenital cardiac procedures at the Fuwai Hospital between 2021 and 2022 were included. Patients with chronic renal failure or without RACHS-1 categories were excluded. Based on whether postoperative PD treatment was performed, the patients were classified into PD and non-PD groups. Preoperative and intraoperative parameters were compared between the groups to identify factors for postoperative PD treatment. Results: A total of 405 eligible patients were enrolled, with 35 and 370 patients allocated to the PD and non-PD groups. Multivariable analyses revealed that age (OR 0.563, 95% CI 0.348–0.911, p = 0.019), preoperative cystatin C level (OR 1.028, 95% CI 1.003–1.053, p = 0.03), cardiopulmonary bypass (CPB) minutes (OR 1.015; 95% CI 1.006–1.024, p = 0.001) and blood lactate level after weaning from CPB (OR 1.453, 95% CI 1.173–1.800, p = 0.001) were predictors for PD treatment. The ROC curve determined the following cutoff points for postoperative PD treatment: age less than 1 month, cystatin C level above 1.36 mg/L, CPB duration exceeding 130 min and blood lactate levels greater than 4 mmol/L. The multiple ROC curve demonstrated a sensitivity of 0.875, specificity of 0.826 and an area under the curve of 0.902. Conclusions: To facilitate the initiation of postoperative PD treatment, it is advised that intraoperative prophylactic PD catheterization be concurrently performed in patients presenting with all four identified factors.Keywords
Abbreviations
| PD | Peritoneal dialysis |
| CPB | Cardiopulmonary bypass |
Fluid overload is common in infants after congenital heart disease surgery, with its pathogenesis potentially associated with capillary leak syndrome, the critical preoperative condition, inflammatory response during cardiopulmonary bypass (CPB) and the presence of low cardiac output syndrome [1]. In addition, a bidirectional relationship exists between fluid overload and acute kidney injury, which can cause extended durations of postoperative mechanical ventilation, increased hospitalization periods and fatal outcomes [2]. Given the highly immature development of physiological systems, neonates and infants are particularly prone to experiencing fluid overload during surgical procedures, which may result in a poor prognosis and delayed recovery [3].
Renal replacement therapy and diuretics can be used to treat fluid overload [4]. The comparative efficacy of these approaches has been a focal point of research [5]. Renal replacement therapy is advocated for early implementation in pediatric patients with postoperative fluid overload, as recent studies have demonstrated its superiority over diuretic monotherapy in enhancing surgical outcomes. A single-center randomized trial involving 73 infants under 6 months of age following surgery for congenital heart disease was conducted by Kwiatkowski DM and colleagues. The infants were divided into two groups: 32 received furosemide and 41 received peritoneal dialysis (PD). The children in the PD group did not experience any related complications, but those in the furosemide treatment group had higher fluid overload, longer duration of ventilator and inotropic drug use and higher electrolyte disorder. According to the conclusion, PD should be actively employed in patients with postoperative acute renal injury and a high risk of fluid overload [6]. A single-center retrospective study of 48 children who required renal replacement treatment after cardiac or thoracic surgery was conducted by Hames DL and colleagues. Through multivariable analysis comparing the survival group (n = 12) with the death group (n = 36), it was observed that delayed initiation of renal replacement therapy after acute kidney injury correlated with increased mortality rates [7]. Pan and colleagues performed a retrospective propensity score-matched analysis involving 45 pairs (90 patients) of children who underwent PD treatment after congenital heart disease surgery. They found that patients in the early PD group (initiated within 6 h of ICU admission) experienced reduced durations of mechanical ventilation, lower vasoactive inotropic score, shorter treatment durations, and faster achievement of a negative fluid balance compared to those in the non-early PD group [8].
At present, PD and continuous renal replacement therapy are recognized as established renal replacement therapy modalities within the postoperative intensive care unit setting [9]. PD treatment is particularly advantageous in the context of infant and neonatal care, offering a reduced impact on circulation and eliminating the need for vascular access. However, the initiation of PD must be prompt, as the onset of peritoneal edema can significantly diminish the therapeutic benefits. The catheterization process for PD is an invasive surgical intervention that comes with inherent risks and potential complications [10].
The objective of this study is to delineate the factors for PD treatment in infants following surgical procedures and to utilize these factors to inform the prophylactic placement of PD catheters during surgery.
2.1 Inclusion and Exclusion Criteria for Patients
All pediatric patients who underwent surgery for congenital heart disease at the Fuwai Hospital between 2021 and 2022 were included in this study (n = 4776). Specifically, infants aged three months or younger were enrolled (n = 417). The study excluded certain patients: 1) debridement, thoracotomy for hemostasis, epicardial pacemaker insertion and other procedures not classified as RACHS-1 (n = 11); or 2) preoperative chronic renal failure (n = 1). After exclusions, 405 eligible patients were divided into two groups according to whether they underwent postoperative PD. Group 1 consisted of patients who received PD (n = 35), while Group 2 comprised those who did not (n = 370).
The medical records included whether the patient received PD treatment, where the PD catheter was placed (in the intensive care unit or operating room) and whether the patient received PD combined with continuous renal replacement therapy.
Preoperative data encompassed a range of demographic and clinical variables, including gender, age, premature birth, weight, preoperative ventilator support, emergency surgery, chromosomal abnormality, RACHS-1 categories, types of congenital heart disease or surgical methods. Additionally, laboratory parameters such as serum creatinine level, blood urea nitrogen level, and cystatin C level were recorded.
Intraoperative data included CPB time, aortic cross-clamp time, lactate level after CPB weaning, intraoperative urine output volume, left ventricular ejection fraction measured by transesophageal echocardiography and instances of delayed sternal closure.
Patient information was retrieved through a comprehensive search of the hospital’s database and electronic medical record system.
2.3 Indications and Treatment Procedures of PD Treatment
PD is an active treatment strategy at our center. Following postoperative measures to ensure circulatory stability (enhanced myocardial contractility, reduced ventricular afterload and arrhythmia control) for postoperative oliguria (urine output <1 mL/kg/h) with volume overload (increased central venous pressure with edema), intravenous diuretics and aminophylline therapy were then administered if the urine output is still unsatisfactory. If oliguria persists for more than four hours, PD treatment is initiated in the absence of contraindications [11]. For those with a PD catheter inserted intraoperatively, PD is commenced promptly. Otherwise, the PD treatment is initiated once the bedside catheter insertion by the surgical team is completed.
The procedure for PD catheter insertion in the intensive care unit is as follows: After induction of anesthesia, a median longitudinal or transverse incision of approximately 1–2 cm in length is made at a location 2–3 cm caudal to the umbilicus. Sequential dissection through the peritoneum, skin, and subcutaneous tissue is performed to gain access to the peritoneal cavity. The PD catheter is then positioned in the rectovesical pouch or pouch of Douglas, along the line of the pubic symphysis. Following catheter placement, the skin incision is closed in layers using sutures. The PD catheter is connected in a Y-configuration to the PD fluid input and output lines using a three-way connector. Finally, a small piece of gauze is applied to the wound site to prevent leakage and to secure the catheter against displacement.
The procedure for PD catheter insertion in the operating room is as follows: Before closing the chest, a small incision is made in the peritoneum horizontally through the median sternotomy, and a right-angle clamp is inserted through this incision, with the tip reaching 1.5 to 2.0 cm beside the umbilicus. The abdominal wall is then pushed outward from the abdominal cavity, and the skin is incised with a No. 11 surgical blade. Electrocautery is used to stop bleeding and the entire layer of the abdominal wall is cut open. This incision is small, just enough to accommodate the tip of the right-angle clamp; continuing to push the abdominal wall upward, ensuring a certain gap between the peritoneum and the intestines, a full-layer figure-eight suture is made around the abdominal wall incision using a No. 4 corner needle with silk thread, in preparation for fixing the PD catheter; the PD catheter is pulled into the abdominal cavity from outside through this abdominal wall incision, and is fixed with prepared silk thread; 6-0 Prolene is used to suture the peritoneum incision horizontally across the diaphragm.
In the conduct of PD, a 4.25% peritoneal dialysate solution is utilized. To this solution, 200 U/L of heparin is incorporated to prevent clotting, and the temperature is carefully adjusted to range between 37°C and 40°C prior to infusion. The standard volume for infusion is calculated at 10 mL per kilogram of body weight, with each dialysis cycle lasting for 60 min. As fluid retention improved, the duration of the dialysis cycle is extended, culminating in the discontinuation of PD following a 3-h cycle. When PD is found to be inadequate in alleviating the fluid overload, it is imperative to transition to continuous renal replacement therapy.
The contraindications of PD include the following: 1) Recent abdominal surgery within the past week; 2) Active peritonitis or necrotizing enteritis; 3) Uncorrected abdominal wall defects, including omphalocele and diaphragmatic hernia; 4) The presence of a ventriculoperitoneal shunt [12].
Statistical analysis was performed using the SPSS software (version 25.0; IBM Corporation, USA). The Shapiro-Wilk test was used to assess the conformity of the continuous variables to the normal distribution. Normal distribution was expressed as the mean plus or minus the standard deviation, whereas a non-normal distribution was expressed as the median accompanied by the interquartile range. Categorical variables are displayed in a numerical form, accompanied by their respective percentages. Missing values were estimated using expectation maximization. An independent t-test was used for normally distributed continuous variable analysis. Otherwise, the Wilcoxon rank-sum test was used for nonnormally distributed variables. Categorical variables were analyzed using the chi-squared test, correction for continuity, or Fisher’s exact test. Binary logistic regression was used to determine the correlation between variables and outcomes. The ROC curve was used to show the sensitivity, specificity, area under the curve (AUC), and cutoff point of the variables. Statistical significance was set at p < 0.05.
This study was approved by the Ethics Committee of the Fuwai Hospital (ID: 2022-1859). Because this was a retrospective study, the requirement for informed consent was waived. The ethical principles of the 1975 Declaration of Helsinki were followed in this study.
Among the 405 eligible patients, 37 underwent PD catheter insertion, 22 underwent dialysis catheter implantation in the operating room (2 patients who did not receive PD treatment were included in Group 2) and 15 underwent dialysis catheter implantation in intensive care unit. Group 1 consisted of 35 patients; Group 2 consisted of 370 patients. The types of congenital heart disease or surgical methods used for the patients are shown in Table 1.
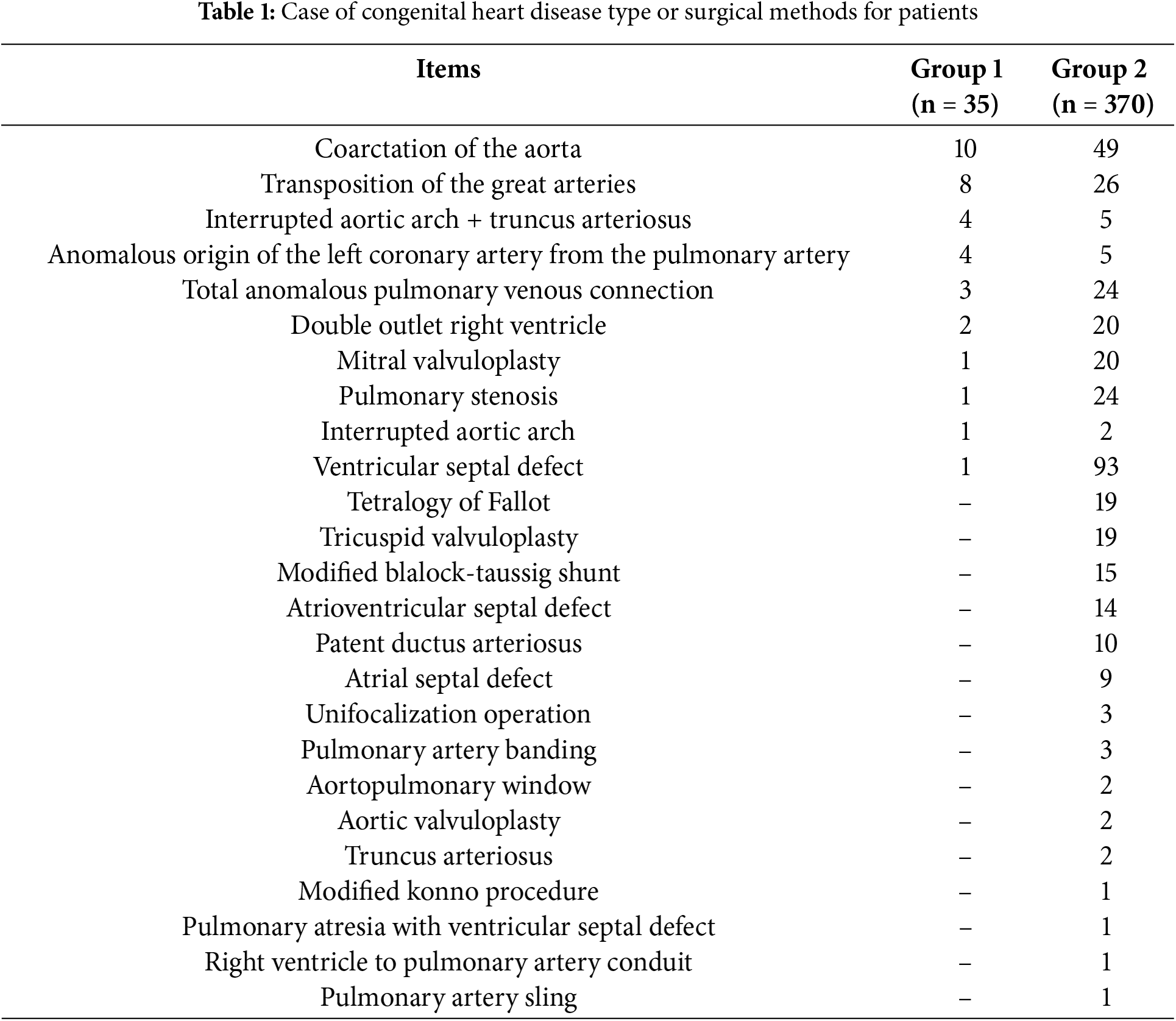
In this study, none of the patients were treated with PD because of contraindications. Two patients received a combined continuous renal replacement treatment because of the low efficacy of PD. No other complications were observed to be dialysis.
3.2 Univariable and Multivariable Results
The preoperative data of age, weight, preoperative creatinine, blood urea nitrogen, cystatin C, preoperative ventilator assistance, and RACHS-1 categories showed statistically significant differences (p < 0.05) between the two groups in univariate analysis. Table 2 displays the details of the preoperative data and statistical analyses of the two groups.
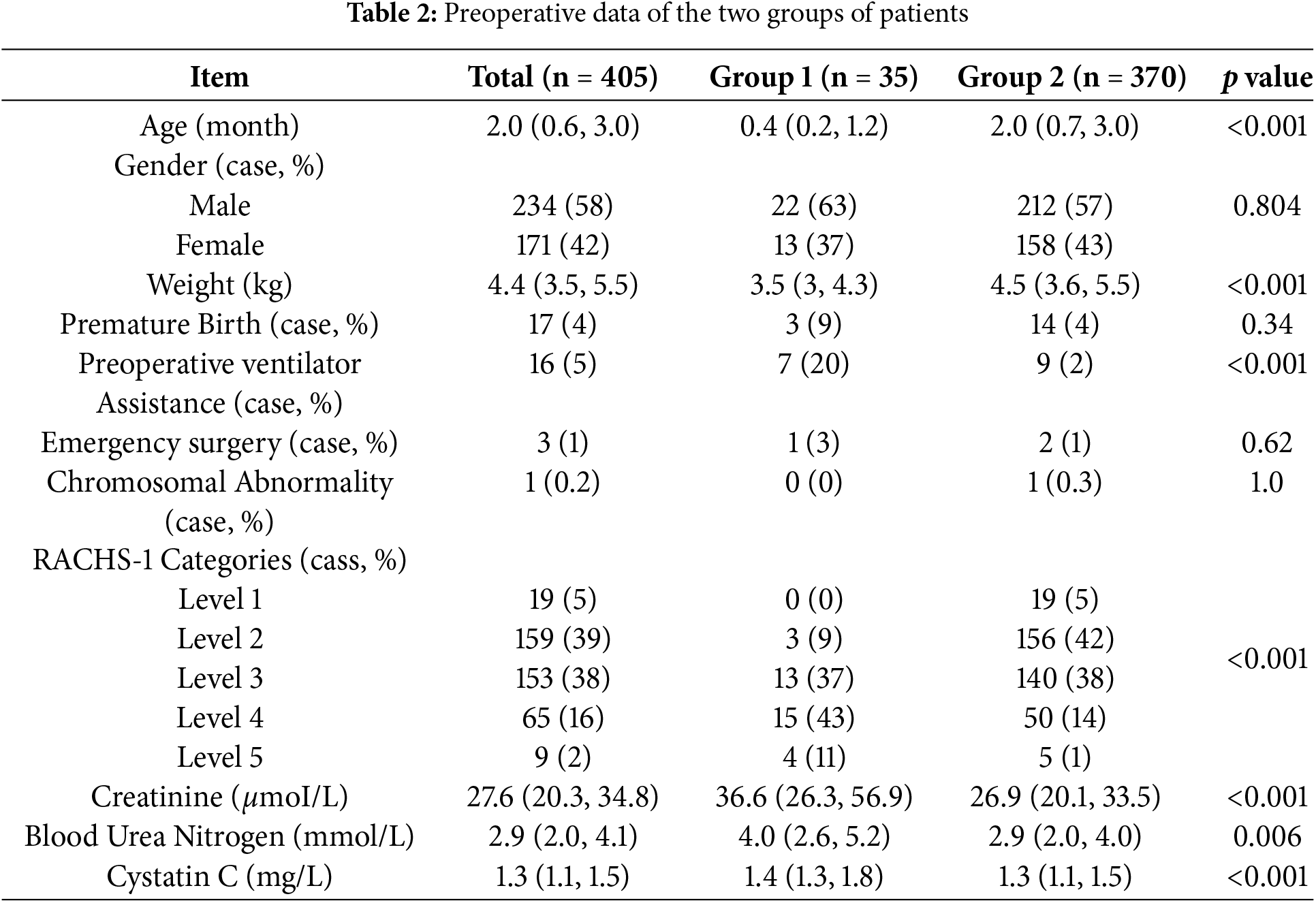
CPB time, aortic cross-clamp time, deep hypothermic circulatory arrest, lactate level after CPB weaning, intraoperative urine output volume and delayed sternal closure were significantly different between the two groups (p < 0.05). Details of the intraoperative data and statistical analyses of the two groups are shown in Table 3.
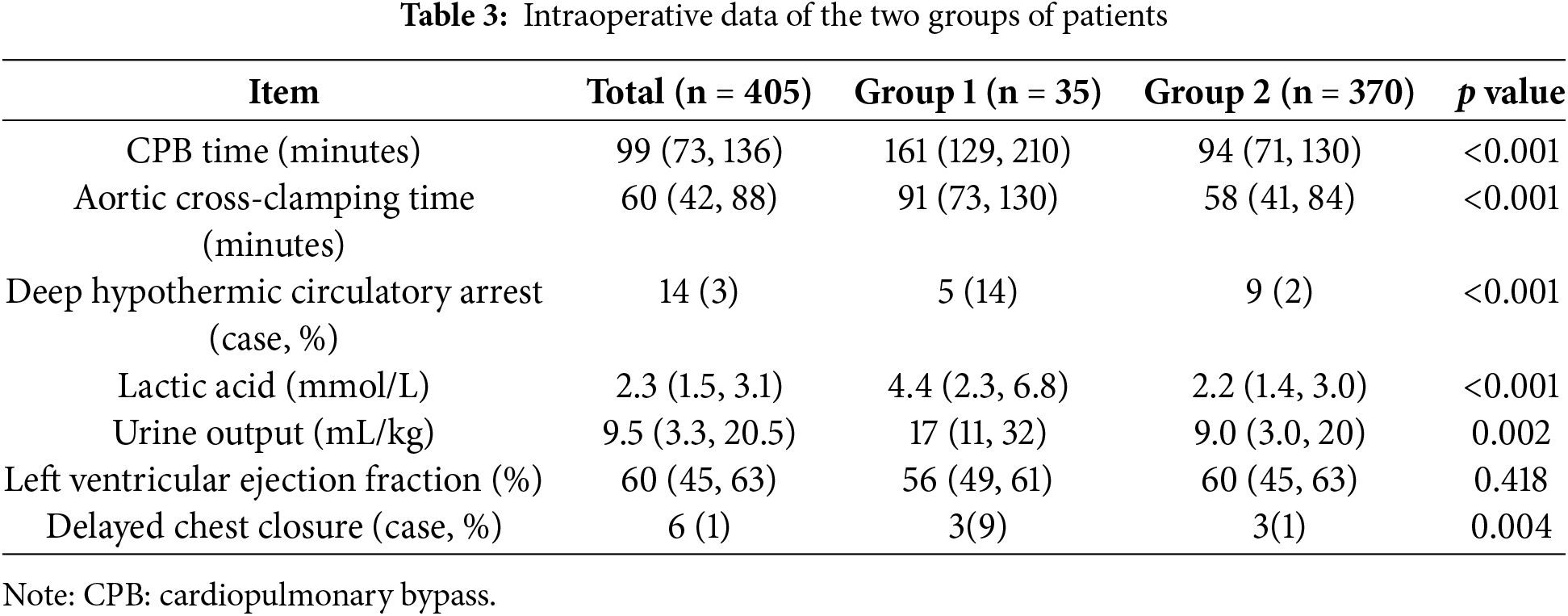
Indicators with statistical differences were analyzed using multivariate analysis. The results of logistic regression are presented in Table 4.
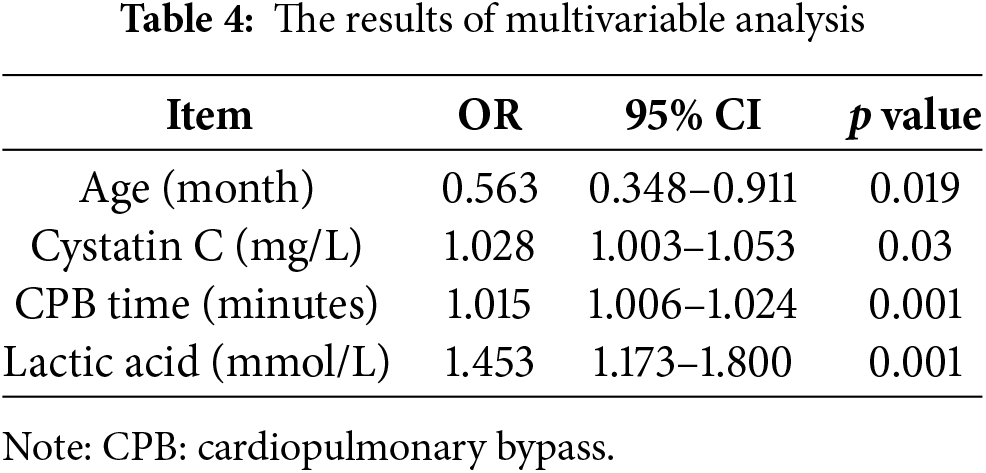
The ROC curve results showed that age less than 1 month (sensitivity 0.714, specificity 0.703, area under the curve 0.752), preoperative cystatin C higher than 1.36 mg/L (sensitivity 0.706, specificity 0.621, area under the curve 0.688), CPB time longer than 130 min (sensitivity 0.765, specificity 0.747, area under the curve 0.825) and lactate level exceeding 4 mmol/L after weaning from CPB (sensitivity 0.559, specificity 0.892, area under the curve 0.764) were the cutoff points for postoperative PD. The multivariate ROC curve analysis showed that the sensitivity, specificity, and area under the curve were 0.875, 0.826, and 0.902, respectively. The multivariate ROC curve is shown in Fig. 1.
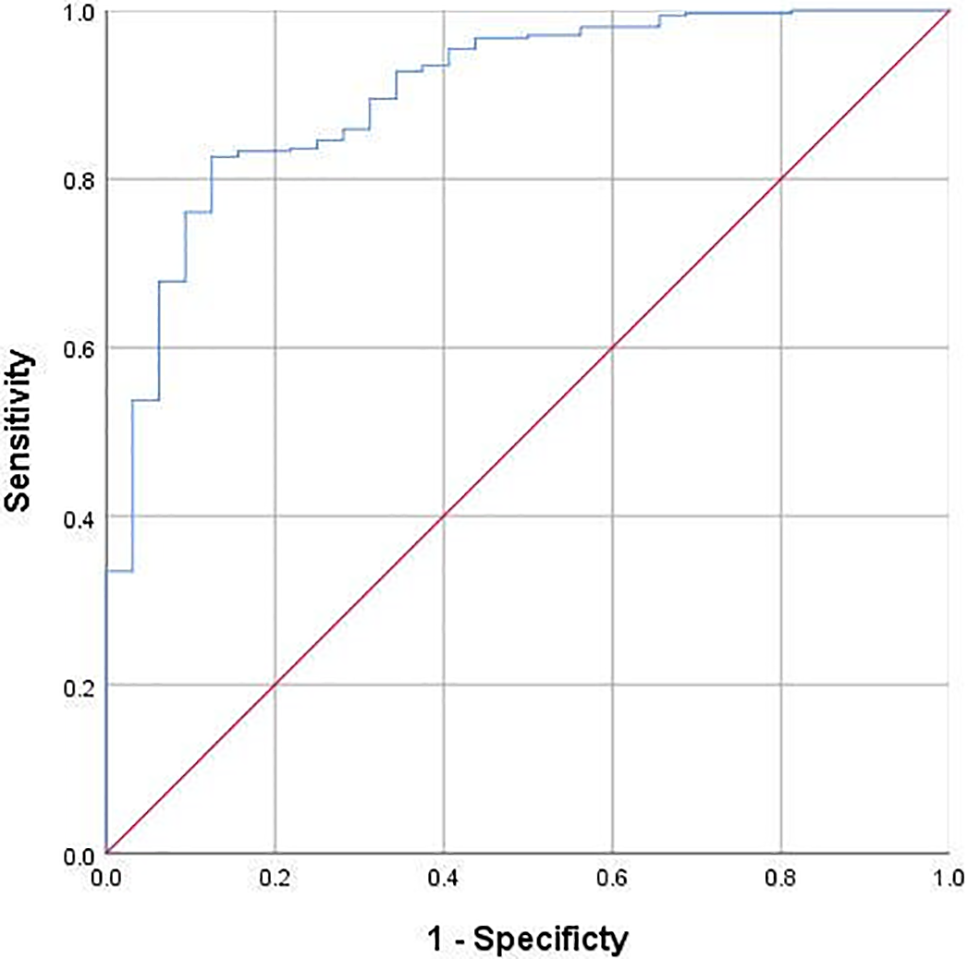
Figure 1: Multivariable ROC curve of age, cystatin C, cardiopulmonary bypass time and lactate in predicting postoperative peritoneal dialysis treatment (AUC = 0.875)
PD employs diffusion and ultrafiltration mechanisms to progressively and steadily eliminate excess fluid and solutes from the bloodstream. This process is facilitated by the semi-permeable nature of the peritoneal membrane. A significant advantage of PD is its minimal impact on hemodynamics and the absence of a requirement for arterial and venous access. Furthermore, infants and neonates, relative to adults, exhibit a larger peritoneal surface area in proportion to their body surface area, which enhances the efficacy of PD [13]. In the early postoperative period following cardiac surgery, the incomplete recovery of cardiac function, hemodynamic instability and challenges in establishing vascular access often preclude the initiation of early continuous renal replacement therapy in infants. Consequently, PD is frequently selected as the treatment of choice in clinical settings [14].
In the clinical management of patients experiencing fluid overload, early initiation of PD is advocated and is supported by research. If preoperative and intraoperative data can predict the need for postoperative PD, this information can be used to determine whether to prophylactically place PD catheters. However, there is currently a lack of direct evidence. In our study, we select preoperative and intraoperative data, drawing on both our clinical experience and the existing body of literature to find out possible predictors.
This study identified CPB as a factor for postoperative PD treatment. In pediatric patients with congenital heart disease, extended CPB duration is a well-established risk factor for acute kidney injury [15]. Our finding is corroborated by a previous study that identified prolonged CPB as a predictor of postoperative PD treatment in children [16]. The discrepancy between the 150-min threshold in that study and the 130-min threshold in ours is not contradictory; it reflects the different age groups studied. Their study included children under three years, while our cohort comprised infants less than three months old. The 130-min CPB duration threshold is more pertinent for predicting the need for postoperative PD in this younger and more vulnerable population.
An additional significant finding of our study is that newborn age (<1 month) is a factor for postoperative PD treatment. In the context of congenital cardiac disease, young age is a definitive risk factor for acute renal injury following surgical intervention [17]. The younger the patient, the less developed their organs are, and consequently, the higher the likelihood of multi-organ dysfunction, including renal dysfunction. A lot of studies have proved that neonates are particularly susceptible to acute renal injury, which escalates the risk of complications and mortality [18].
Cystatin C is an endogenous marker that reflects changes in glomerular filtration rate and is less susceptible to external influences, making it a reliable indicator of kidney function [19]. A study specifically focusing on newborns demonstrated that cystatin C is highly correlated with acute kidney injury in neonates, with a diagnostic threshold greater than 2.2 mg/L [20]. Our research indicates that preoperative cystatin C levels exceeding 1.36 mg/L are a significant predictor for postoperative PD treatment in children (normal range 0.51–1.09 mg/L). The cutoff points identified in these studies are not in conflict; they reflect the earlier initiation of renal replacement therapy in our center, leading to a lower cutoff point. It is important to emphasize that, due to cystatin C’s minimal influence by extrarenal factors, it is more suitable for application across different centers when assessing the same age group of patients. Further investigations are necessary to determine the applicability of these findings to children of varying ages.
Low cardiac output syndrome is a risk factor for the acute kidney injury following pediatric cardiac surgery [21]. Lactic acid, with a level of 4 mmol/L, serves as a marker for diagnosing low cardiac output syndrome [22]. Our study identified that elevated lactate level, specifically greater than 4 mmol/L after CPB weaning, is an independent risk factor for children who undergo postoperative PD treatment. This finding aligns with existing literature.
Congenital heart disease is occasionally associated with chromosomal abnormalities [23], which may influence the outcomes of cardiac surgery. However, only one patient in Group 2 was identified with a chromosomal abnormality (Williams syndrome) and statistical analysis revealed no significant difference between the two groups. Two factors may be responsible for this phenomenon. Firstly, prenatal screening in our country has improved significantly, leading to an increased rate of pregnancy termination for those with severe chromosomal abnormalities. Secondly, patients included in this study were too young (less than three months) to exhibit a noticeable developmental delay, which may have obscured the presence of chromosomal abnormalities.
Our study has several limitations. The relatively small number of patients constrained our ability to identify risk factors associated with poor efficacy of PD (combined with continuous renal replacement therapy). Therefore, further research with an expanded sample size is imperative to elucidate these risk factors. Given that this was a single-center study, the conclusions of our study may not be applicable to other centers. Consequently, future multi-center studies are essential to validate the applicability of our results across different clinical settings.
To facilitate the initiation of postoperative PD treatment, it is advisable to consider the prophylactic insertion of a PD catheter during surgery for congenital heart disease, provided that four specific criteria are concurrently satisfied: age less than 1 month, preoperative cystatin C above 1.36 mg/L, CPB time longer than 130 min and lactic acid level greater than 4 mmol/L after weaning from CPB.
Acknowledgement: None.
Funding Statement: This study was supported by the Artificial Intelligence and Information Application Foundation of Fuwai Hospital, Chinese Academy of Medical Sciences (grant number: 2024-AI17).
Author Contributions: Study conception and design: Xu Wang and Xiaofeng Wang; data collection: Xiaofeng Wang and Chenyu Li; analysis and interpretation of results: Chenyu Li, Xiaofeng Wang and Xu Wang; draft manuscript preparation: Xia Li, Chenyu Li and Zhongyuan Lu. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author, Xu Wang, upon reasonable request.
Ethics Approval: This study was reviewed and approved by the Ethics Committee of Fuwai Hospital (ID: 2022-1859. Due to the retrospective nature of the study, the requirement for informed consent was waived. The ethical principles outlined in the 1975 Helsinki Declaration were followed throughout the study.
Conflicts of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
1. Bellos I, Iliopoulos DC, Perrea DN. Association of postoperative fluid overload with adverse outcomes after congenital heart surgery: a systematic review and dose-response meta-analysis. Pediatr Nephrol. 2020 Jun;35(6):1109–19. doi:10.1007/s00467-020-04489-4. [Google Scholar] [CrossRef]
2. Murni IK, Djer MM, Yanuarso PB, Putra ST, Advani N, Rachmat J, et al. Outcome of pediatric cardiac surgery and predictors of major complication in a developing country. Ann Pediatr Cardiol. 2019 Jan–Apr;12(1):38–44. doi:10.4103/apc.APC_146_17. [Google Scholar] [CrossRef]
3. Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017 Nov;13(11):697–711. doi:10.1038/nrneph.2017.119. [Google Scholar] [CrossRef]
4. Messmer AS, Dill T, Müller M, Pfortmueller CA. Active fluid de-resuscitation in critically ill patients with septic shock: a systematic review and meta-analysis. Eur J Intern Med. 2023 Mar;109:89–96. doi:10.1016/j.ejim.2023.01.009. [Google Scholar] [CrossRef]
5. Flores S, Loomba RS, Elhoff JJ, Bronicki RA, Mery CM, Alsaied T, et al. Peritoneal dialysis vs diuretics in children after congenital heart surgery. Ann Thorac Surg. 2019 Sep;108(3):806–12. doi:10.1016/j.athoracsur.2019.03.066. [Google Scholar] [CrossRef]
6. Kwiatkowski DM, Goldstein SL, Cooper DS, Nelson DP, Morales DL, Krawczeski CD. Peritoneal dialysis vs furosemide for prevention of fluid overload in infants after cardiac surgery: a randomized clinical trial. JAMA Pediatr. 2017 Apr 1;171(4):357–64. doi:10.1001/jamapediatrics.2016.4538. [Google Scholar] [CrossRef]
7. Hames DL, Ferguson MA, Kaza AK, Rajagopal S, Thiagarajan RR, Teele SA, et al. Renal replacement therapy in the pediatric cardiac intensive care unit. J Thorac Cardiovasc Surg. 2019 Nov;158(5):1446–55. doi:10.1016/j.jtcvs.2019.06.061. [Google Scholar] [CrossRef]
8. Pan T, Li D, Li S, Yan J, Wang X. Early initiation of peritoneal dialysis improves postoperative recovery in children with right ventricular outflow tract obstructive lesions at high risk of fluid overload: a propensity score-matched analysis. Interact Cardiovasc Thorac Surg. 2018 Aug 1;27(2):250–6. doi:10.1093/icvts/ivy048. [Google Scholar] [CrossRef]
9. Niaz T, Stephens EH, Gleich SJ, Dearani JA, Johnson JN, Sas DJ, et al. Acute kidney injury and renal replacement therapy after Fontan operation. Am J Cardiol. 2021 Dec 15;161:84–94. doi:10.1016/j.amjcard.2021.08.056. [Google Scholar] [CrossRef]
10. Shrestha BM. Peritoneal dialysis or haemodialysis for kidney failure? JNMA J Nepal Med Assoc. 2018 Mar–Apr;56(210):556–7. [Google Scholar]
11. Kwan JR, Chong TT, Low GZ, Low GW, Htay H, Foo MW, et al. Outcomes following peritoneal dialysis catheter removal with reinsertion or permanent transfer to haemodialysis. J Vasc Access. 2019 May;20(1_suppl):60–4. doi:10.1177/1129729818773984. [Google Scholar] [CrossRef]
12. Teitelbaum. Peritoneal dialysis. N Engl J Med. 2021 Nov 4;385(19):1786–95. doi:10.1056/NEJMra2100152. [Google Scholar] [CrossRef]
13. Barhight MF, Soranno D, Faubel S, Gist KM. Fluid management with peritoneal dialysis after pediatric cardiac surgery. World J Pediatr Congenit Heart Surg. 2018 Nov;9(6):696–704. doi:10.1177/2150135118800699. [Google Scholar] [CrossRef]
14. Zhang L, Jin Y, Zhang F, Li H, Wu Q. Modified peritoneal dialysis for treatment of acute renal failure after complex congenital heart surgery in infants. Heart Surg Forum. 2018 Jun 25;21(4):E286–9. doi:10.1532/hsf.1915. [Google Scholar] [CrossRef]
15. Jing H, Liao M, Tang S, Lin S, Ye L, Zhong J, et al. Predicting the risk of acute kidney injury after cardiopulmonary bypass: development and assessment of a new predictive nomogram. BMC Anesthesiol. 2022 Dec 7;22(1):379. doi:10.1186/s12871-022-01925-w. [Google Scholar] [CrossRef]
16. Zheng J, Xiao Y, Chong M, Chen Y, Yao Y, Jin M, et al. The effect of cardiopulmonary bypass duration on renal injury after congenital heart surgery in infants and young children. Adv Clin Exp Med. 2013 Sep–Oct;22(5):693–8. [Google Scholar]
17. Zappitelli M, Parikh CR, Kaufman JS, Go AS, Kimmel PL, Hsu CY, et al. Acute kidney injury and risk of CKD and hypertension after pediatric cardiac surgery. Clin J Am Soc Nephrol. 2020 Oct 7;15(10):1403–12. doi:10.2215/CJN.00150120. [Google Scholar] [CrossRef]
18. Starr MC, Charlton JR, Guillet R, Reidy K, Tipple TE, Jetton JG, et al. Advances in neonatal acute kidney injury. Pediatrics. 2021 Nov;148(5):e2021051220. [Google Scholar]
19. Carrero JJ, Fu EL, Sang Y, Ballew S, Evans M, Elinder CG, et al. Discordances between creatinine- and cystatin C-based estimated GFR and adverse clinical outcomes in routine clinical practice. Am J Kidney Dis. 2023 Nov;82(5):534–42. doi:10.1053/j.ajkd.2023.04.002. [Google Scholar] [CrossRef]
20. Xu X, Nie S, Xu H, Liu B, Weng J, Chen C, et al. Detecting neonatal AKI by serum cystatin C. J Am Soc Nephrol. 2023 Jul 1;34(7):1253–63. doi:10.1681/ASN.0000000000000125. [Google Scholar] [CrossRef]
21. Mizuno T, Gist KM, Gao Z, Wempe MF, Alten J, Cooper DS, et al. Developmental pharmacokinetics and age-appropriate dosing design of milrinone in neonates and infants with acute kidney injury following cardiac surgery. Clin Pharmacokinet. 2019 Jun;58(6):793–803. doi:10.1007/s40262-018-0729-3. [Google Scholar] [CrossRef]
22. James C, Millar J, Horton S, Brizard C, Molesworth C, Butt W. Nitric oxide administration during paediatric cardiopulmonary bypass: a randomised controlled trial. Intensive Care Med. 2016 Nov;42(11):1744–52. doi:10.1007/s00134-016-4420-6. [Google Scholar] [CrossRef]
23. Wang H, Lin X, Lyu G, He S, Dong B, Yang Y. Chromosomal abnormalities in fetuses with congenital heart disease: a meta-analysis. Arch Gynecol Obstet. 2023 Sep;308(3):797–811. doi:10.1007/s00404-023-06910-3. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools