 Open Access
Open Access
REVIEW
Tetralogy of Fallot: Anatomy, Physiology, and Outcomes
1 Department of Cardiovascular and Thoracic Surgery, Children’s Mercy, Kansas City, MO, 64108, USA
2 Department of Pediatric Cardiovascular Surgery, Arkansas Children’s Hospital, Little Rock, AR, 72202, USA
3 Department of Pediatric Cardiology, Arkansas Children’s Hospital, Little Rock, AR, 72202, USA
4 Department of Pediatric Cardiothoracic Surgery, Medical College of Georgia, Augusta, GA, 30912, USA
* Corresponding Author: Edo Bedzra. Email:
Congenital Heart Disease 2024, 19(6), 541-562. https://doi.org/10.32604/chd.2025.059788
Received 17 October 2024; Accepted 17 January 2025; Issue published 27 January 2025
Abstract
Since the first identification of Tetralogy of Fallot in 1671, consisting of a combination of anatomical defects including biventricular origin of the aorta, maligned ventricular septal defect, overriding aorta, and narrowing or atresia of the pulmonary outflow tract. The first successful operation consisted of a shunt between the left subclavian artery and pulmonary artery. Following this palliative procedure, complete repair is performed once the patient reaches indicative criteria. Since the first attempts at surgical palliation and repair, techniques and outcomes have improved drastically. Definitive repair of Tetralogy of Fallot consists of a multi-patch closure of any Ventricular Septal Defect along with clearance of any muscular obstructions of the Right Ventricular Outflow Tract and reconstruction of the outflow tract. Current results of Tetralogy of Fallot palliation yield excellent long and short-term results with 5-year freedom from reintervention of 90%. The iterative improvement of repair techniques has greatly reduced intraoperative and postoperative complications. Future innovations such as increased use of percutaneous repair methods and additional data on the benefits of primary repair as opposed to staged palliation will continue to improve patient outcomes.Keywords
Abbreviation
| Ao | Aorta |
| AVSD | Atrioventricular Septal Defect |
| ASD | Atrial Septal Defect |
| HR | Hazard Ratio |
| MAPCAs | Major Aorta-pulmonary Collateral Arteries |
| PA | Pulmonary Artery |
| PFO | Patent Foramen Ovale |
| RV | Right Ventricle |
| RVOT | Right Ventricular Outflow Tract |
| STS | Society of Thoracic Surgeons |
| TOF | Tetralogy of Fallot |
| VSD | Ventricular Septal Defect |
The constellation of findings that would come to be called Tetralogy of Fallot was first described by the Danish monk, Niels Stenson in 1671. However, it is to Ettienne-Louis Arthur Fallot that mainstream credit is given for his publication on la maladie bleue describing the anatomic underpinning of the defect which now carries his name. The tetrad that underpins this diagnosis includes right ventricular hypertrophy, a Ventricular Septal Defect (VSD), overriding aorta and right ventricular outflow tract obstruction as seen in Fig. 1 [1,2]. It would take more than another half century for the first successful surgical palliation.
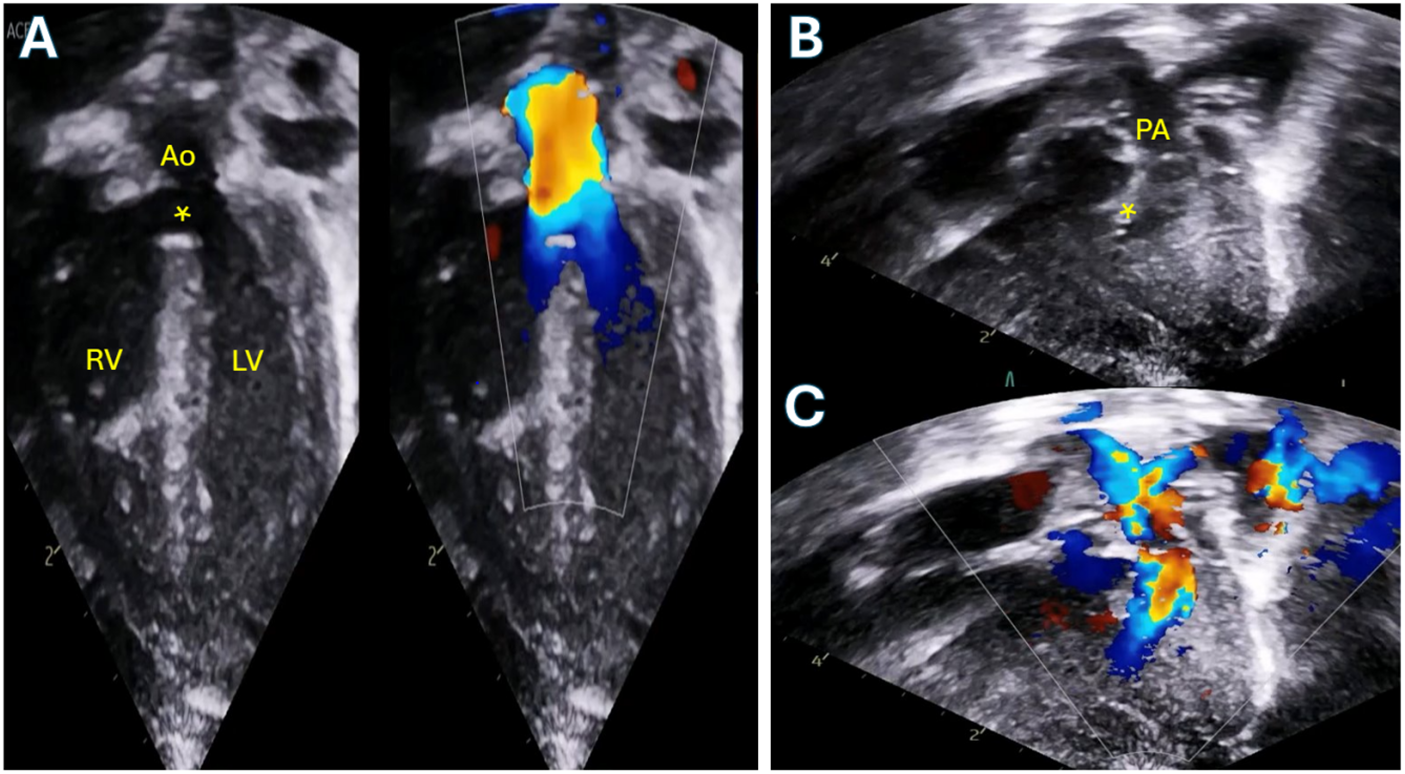
Figure 1: Transthoracic echocardiogram of a neonate with Tetralogy of Fallot with moderate pulmonary valve stenosis. A: Apical 5-chamber view demonstrating the large ventricular septal defect (asterisk) and overriding aorta. B: 2D modified subcostal view demonstrating the anteriorly deviated conal septum with sub-pulmonary crowding. C: Color Doppler of the same view demonstrating flow acceleration beginning below the level of the pulmonary valve. RV: Right ventricle; LV: Left ventricle; PA: Pulmonary artery
In 1944, after suggestions from Helen Taussig and laboratory work by Vivien Thomas (who also guided him through the first procedure) to develop the procedure, Alfred Blalock performed the first aortopulmonary shunt that successfully palliated the ‘blue baby syndrome’. The Blalock-Thomas-Taussig between the subclavian artery and the pulmonary artery was followed by an anastomosis between the descending aorta and left pulmonary artery that became known as the Potts shunt. In addition to these developments, the Waterston shunt between the ascending aorta and right pulmonary artery, and more recently, the central shunt were also developed in aid in the treatment of Tetralogy of Fallot. In the interim, Sellors et al. and Brock et al. would describe what became known as the Brock procedure which consisted of a pulmonary valvotomy and RVOT dilation through a right ventriculotomy without the use of cardiopulmonary support [3,4]. 10 years after Taussig, Blalock and Thomas’ success, Lillehei et al. performed the first successful intracardiac repair using cross-circulation [5]. Kirklin at the Mayo Clinic followed a year later with repair using a cardiopulmonary bypass [6].
The International Working Group for Mapping and Coding of Nomenclatures for Pediatric and Congenital Cardiac Disease defined Tetralogy of Fallot as a group of malformations with biventricular atrioventricular alignments or connections characterized by anterosuperior deviation of the conal or outlet septum or its fibrous remnant, narrowing or atresia of the pulmonary outflow, a maligned ventricular septal defect, and biventricular origin of the aorta. Hearts with Tetralogy of Fallot will always have a ventricular septal defect, narrowing or atresia of the pulmonary outflow, and an overriding aorta. Hearts with Tetralogy of Fallot will most often have right ventricular hypertrophy [6]. This can be seen in Fig. 2. It constitutes 10% of congenital heart disease and is the most common cyanotic congenital heart disease. It has a prevalence of 340–421 affected per million live births worldwide [7,8].
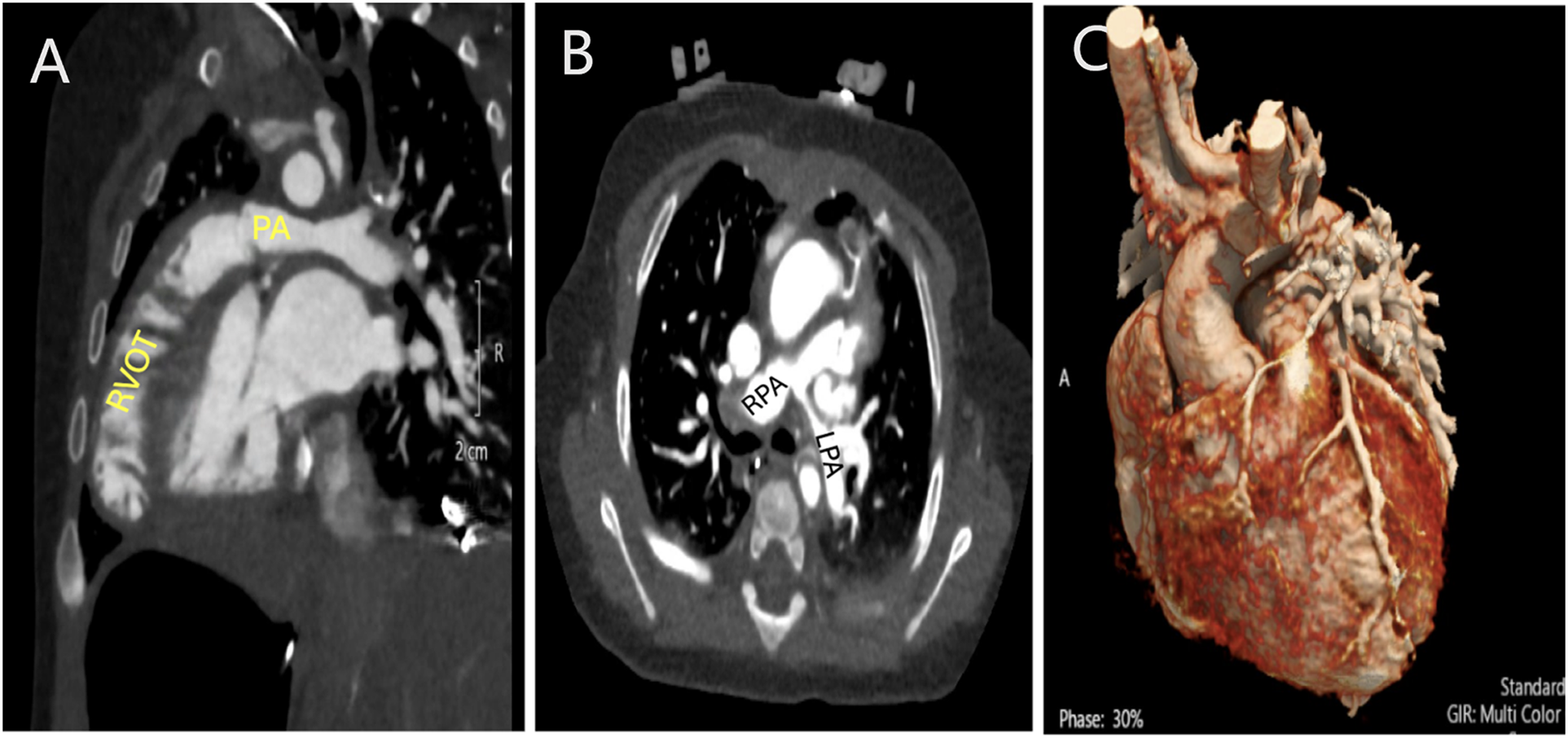
Figure 2: Computed tomography angiogram (CTA) in a patient with Tetralogy of Fallot and severe pulmonary stenosis. A: Sagittal view showing the severe sub-pulmonary right ventricular outflow tract obstruction. B: Axial view showing the branch pulmonary arteries with hypoplasia of the left pulmonary artery. C: 3D rendering demonstrating no coronary artery crossing the right ventricular outflow tract (RVOT). PA: Pulmonary artery; RPA: Right pulmonary artery. LPA: Left pulmonary artery
Many attempts have been made at finding a unifying underlying abnormality responsible for the anomalies found in a tetralogy heart. Van Praagh et al. postulated underdevelopment of the subpulmonary infundibulum [9]. However, incremental work by Anderson et al. suggested first that anterocephalad deviation of the outlet septum and, subsequently, an associated malformation of the septoparietal trabeculations producing right ventricular outflow tract obstruction. Malalignment between the septomarginal trabecular and infundibular septum results in a “ventricular septal defect” with overriding of the aortic valve. Due to this, the VSD is perpendicular to the plane of the septum. It is usually a large non-restrictive defect with fibrous continuity between the aortic and tricuspid valves and thus accurately defined as a perimembranous defect. It results in equalization of ventricular pressures [1,10,11]. In less than 5% of cases, there are additional muscular VSDs. The aortic valve is posterior and to the right of the pulmonary valve in majority of cases. However, in 35% of cases, it is side by side and to the right of the pulmonary valve. The consensus definition suggests there must be <100% aortic override for a defect to be considered Tetralogy of Fallot. However, there are others who argue that exclusively right ventricular support of the aorta, a double outlet right ventricle variation, should not preclude the designation of tetralogy [12].
The pulmonary valve is bicuspid in greater than half of patients and close to two thirds have raphes in an anteroposterior orientation. The valve is tricuspid in a third of cases. The pulmonary annulus is often hypoplastic and the leaflets are generally dysplastic and thickened with commissural fusion leading to stenosis. Tetralogy of Fallot may be accompanied by branch pulmonary stenosis, especially at the insertion of the ductus arteriosus into the left pulmonary artery [13–15].
There is frequently an atrial septal defect present, a right aortic arch in 15%–25% of patients, absent pulmonary valve in 3%, and atrioventricular septal defect as seen in Fig. 3, usually of the Rastelli C type in 3%. These are discussed in subsequent sections. Tetralogy of Fallot with pulmonary atresia, grouped with pulmonary atresia-VSD by the STS and seen in 7% of cases, is beyond the scope of this review. In about 3%–5% of patients, the left anterior descending artery originates from the right coronary artery and crosses the RVOT just inferior to the pulmonary annulus [16,17]. Tetralogy of Fallot is usually non-syndromic. However, in about 20% of patients, the defect is associated with genetic syndromes. About 12% have chromosomal anomalies including Trisomy 21 which is found in 4%–13% of patients, del 22q11 found in a further 8%–15%, VACTERL and Alagille syndrome. Syndromic patients had worse outcomes after surgical repair, particularly when they had hypoplastic central pulmonary arteries [18–20].
Figure 3: Transthoracic echocardiogram showing Tetralogy of Fallot with atrioventricular septal defect
3 Clinical Presentation and Diagnosis
In this era of prenatal screening for congenital heart disease, Tetralogy of Fallot is frequently diagnosed before birth, with recent cases being prenatally diagnosed 70% of the time [21]. Short- and long-term plans can then be made for the management of patients after birth. However, in many cases, it is still diagnosed after birth. The presenting sign is usually cyanosis. Physical exam findings include pallor on the milder end and cyanosis of the mucus membranes on the more severe end. Due to equalization of pressures in the ventricles, it is difficult, if not impossible to auscultate a VSD murmur on physical examination. However, a systolic ejection murmur from pulmonary stenosis is often present.
Most patients present within 3–6 months of life when cyanosis is discovered at well baby visits or when they develop cyanotic, so called, “tet spells”. Patients who present earlier than this usually have fixed hypoplastic pulmonary annuli or valves leading to right to left shunting. The degree of right to left shunting is directly correlated with the degree of cyanosis and distress. With the right substrate, Tetralogy of Fallot patients can have profound cyanotic episodes. These episodes are accompanied by extreme desaturation, agitation and rapid shallow breathing. They can result in limpness and loss of consciousness. Babies and infants assume the knee to chest position in an attempt to increase systemic resistance and pulmonary blood flow. Tet spells are believed to be caused by infundibular muscle spasm resulting in dynamic right ventricular outflow tract obstruction and right to left shunting. They are unusual in patients with absent valve syndrome. The murmur of pulmonary stenosis softens or disappears during spells.
In the older child, exertional dyspnea is the main presenting symptom. As infundibular stenosis worsens, exertional dyspnea and cyanosis increases in severity. To change relative resistances, children assume the characteristic squatting position. In the rest of the world, there are still large numbers of Tetralogy of Fallot patients who arrive to adulthood unrepaired. These patients present with heart failure, atrial and ventricular arrhythmias, and infective endocarditis [22].
Findings on diagnostic testing are indicative of right ventricular hypertrophy which leads to an elevated cardiac apex, an enlarged aorta, a right sided arch in a quarter of patients and a small main PA. Electrocardiography, an example of which is shown in Fig. 4, shows RV hypertrophy and right axis deviation. Oligemia of the lung fields and a classic boot shaped appearance of the heart are seen on chest x-ray. In the past, cardiac catheterization was a staple of diagnosis. However, this modality has little utility in current identification of tetralogy and is only deployed for delineation of pulmonary and aortopulmonary collateral anatomy in the management of patients with tetralogy, pulmonary atresia and major aortopulmonary collaterals.
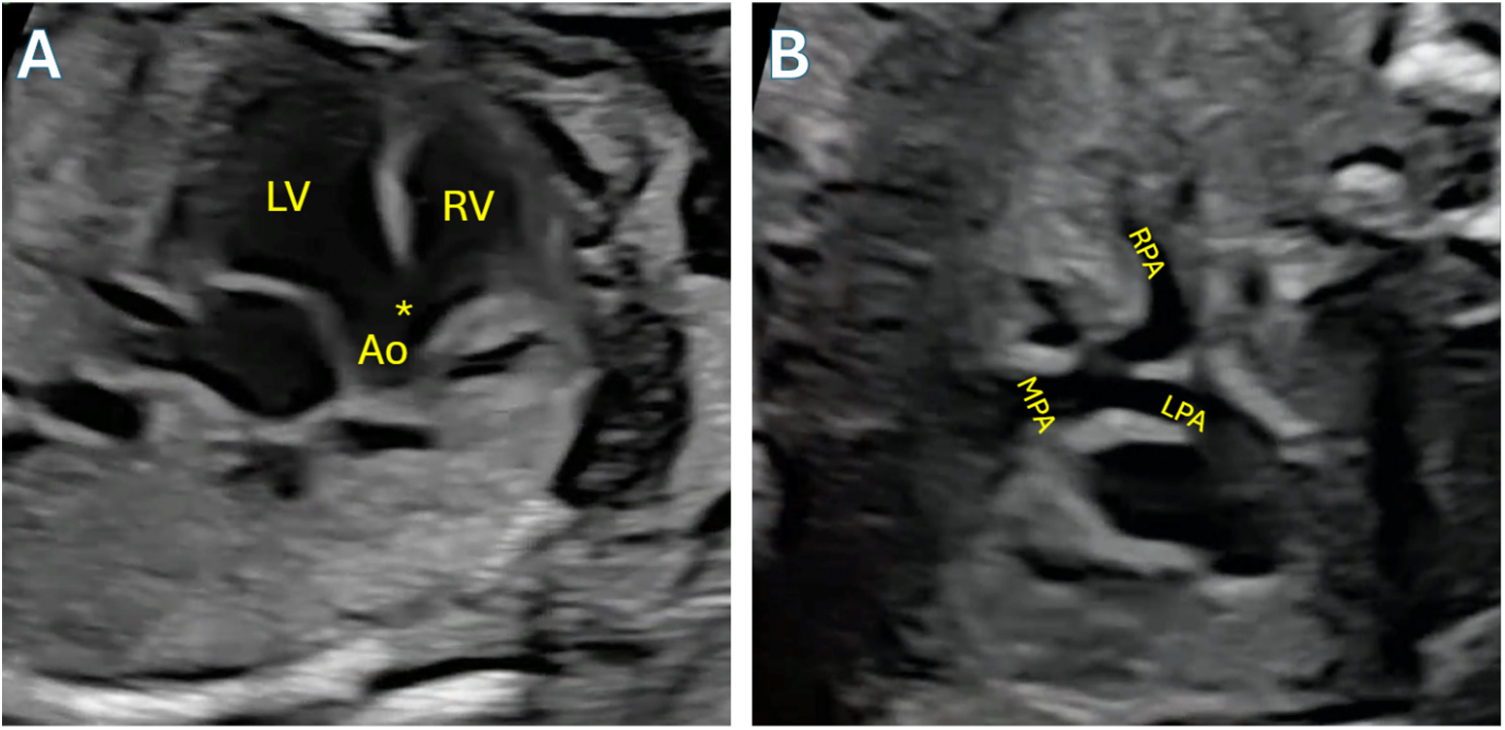
Figure 4: Fetal echocardiogram demonstrating Tetralogy of Fallot. A: Angling anteriorly from 4-chamber view to demonstrate the large VSD (asterisk) and overriding aorta (Ao). B: 3-vessel trachea view demonstrating size discrepancy between aorta and pulmonary artery, a hallmark feature of TOF, and confluent mildly hypoplastic branch pulmonary arteries. LV: Left ventricle; RV: Right ventricle; LPA: left pulmonary artery; RPA: Right pulmonary artery; MPA: Main pulmonary artery
Echocardiography is now the mainstay of diagnosis. High resolution echocardiography is employed to identify and characterize the number and size of VSDs, the subvalvar, valvar, and supravalvar anatomy. This anatomy includes the muscle bundles creating obstruction of the RVOT, pulmonary arterial anatomy, and coronary anatomy including branches crossing the infundibulum. CT angiography has evolved significantly and 2D and 3D CT can be performed in limited circumstances to further characterize coronary, pulmonary and AP collateral anatomy. MRI has no role in the management of unrepaired Tetralogy of Fallot. However, it has played an increasing role in the long term follow up of repaired tetralogy patients to determine RV volumes, pulmonary insufficiency and other indices and is now the standard for guideline-based reintervention on patients with repaired tetralogy [23,24].
The medical management of unrepaired Tetralogy of Fallot is mostly limited to emergency situations. In the neonatal period, prostaglandin E1 infusion to reopen a closed ductus can be an important step in the resuscitation of a cyanotic, ductal dependent child. An example of an opened ductus can be seen in Fig. 5. Once past the neonatal stage with acceptable systemic saturations, most specialists recommend careful monitoring in the outpatient setting until the patient is ready for surgical repair. In the presence of a tet spell, the physiological underpinning of management is improving pulmonary blood flow. This is done by manipulating systemic and pulmonary resistance, increasing stroke volume, and decreasing the dynamic RVOT obstruction.
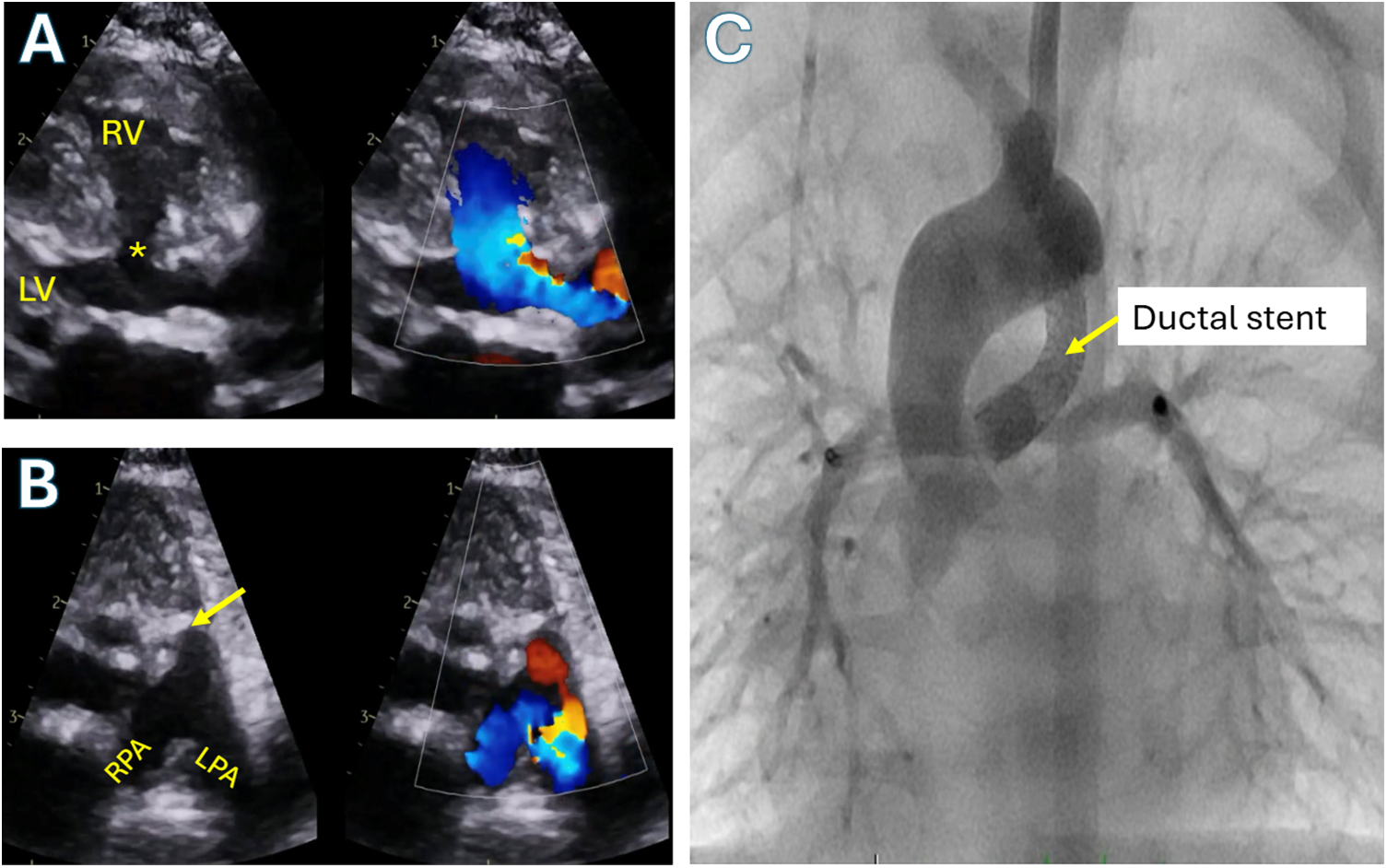
Figure 5: Tetralogy of Fallot with pulmonary atresia and confluent branch pulmonary arteries supplied by ductus arteriosus. A: Color compare transthoracic echocardiographic parasternal long axis view showing the large ventricular septal defect (asterisk) and overriding aorta. B: Color compare transthoracic echocardiographic parasternal short axis showing atretic pulmonary valve (arrow) with branch pulmonary arteries supplied by a ductus arteriosus (red flow). C: Anterior-posterior angiogram in the aorta demonstrating a stented patent ductus arteriosus supplying flow into the branch pulmonary arteries in the same patient. RV: Right ventricle; LV: Left ventricle; RPA: Right pulmonary artery; LPA: Left pulmonary artery
The first step is to sedate the agitated child to decrease systemic oxygen demand. Morphine, the mainstay of sedation, is additionally thought to mitigate infundibular spasm. Concurrently, oxygen is administered to the patient both to improve pulmonary venous saturation and decrease pulmonary vascular resistance. Stroke volume is augmented by fluid boluses that increase ventricular preload, and by beta blockade, usually with propranolol. The latter decreases chronotropy and thus infundibular spasm and increases diastolic filling time by decreasing heart rate. The final step in medical management is administration of a peripheral vasoconstrictor such as phenylephrine which raises systemic vascular resistance and decreases the right to left shunt at the VSD thus improving oxygenation [25,26]. An adjunctive measure is to place the child in the knee to chest position.
The frequency and severity of tet spells in the child determine next management steps which include outpatient management with beta blockade for tet spell prevention in stable patients, surgical or interventional palliation in patients who are unstable but high risk for complete repair, and complete repair of patients at low surgical risk. It is worth noting that the preoperative use of propranolol results in increased inotropic and pacing requirements postoperatively. However, this does not seem to have any impact on total hospital stay, morbidity or mortality [27].
4.2 Indications for Intervention
Increasing hypoxemia with systemic saturations below 75%–80% is the usual indication for intervention in the tetralogy patient, frequent or severe tet spells is another. However, most patients do not require any intervention in the neonatal stage as their systemic oxygen saturations are acceptable. Whatever the case, regardless of symptoms, most recommend complete repair by age 1 and in many institutions, repair is completed between ages 3 and 6 months.
In adequately sized infants, complete repair is the preferred path when indications are met and adequate pulmonary arterial tree exists. In the absence of the latter, many palliative options exist to allow central pulmonary arterial growth prior to repair. However, controversy arises when patients present in the neonatal stage or early infancy cyanotic. The prevailing management in previous eras was a palliative procedure, usually, a systemic to pulmonary artery shunt with staged repair. However, this obligates a period of relative cyanosis which may affect neurodevelopmental outcomes. In addition, shunts could distort pulmonary arteries, affecting development and retain the obstructive RVOT leading to maladaptive RV hypertrophy.
With increasing dexterity at neonatal repair, tetralogy repair in the neonatal period has been advocated, and performed at certain centers with low mortality comparable to shunted patients. This was achieved at the compromise of higher rate of repeat interventions, longer initial hospital and ICU stays, and high rates of transannular patch use for repairs in the neonatal groups nearing 100% [28,29]. It is with this high rate of transannular patching that proponents of staged repair and increased postoperative complications in the neonatal group contend. They argue that the long-term effects of free pulmonary insufficiency caused by transannular patching and long ventriculotomies include arrhythmias, sudden death, right ventricular dilation, and dysfunction [30–33]. To this group, it is preferable to substitute a brief period of cyanosis and a second hospitalization with reoperative sternotomy for the improved comorbidity profile and probability of pulmonary valvular salvage and growth in some series [34].
It does not appear that this decision will get any clearer soon. Two recent reports present conflicting results. Smith et al. looking at the long-term outcomes of tetralogy repair found increased early and late mortality risks with staged repair, and non-valve sparing repair (HR of 2.68 and 3.76, respectively). Savla et al. on the other hand examined 2363 patients who underwent staged or complete repair in the neonatal period and found significantly higher risks of mortality at all time points within 2 years of repair in the complete repair group [35,36]. The choice of approach is likely to be determined by center experience and resource availability. Although high volume centers may be able to perform complete neonatal repair with excellent results, perhaps the better part of valor for smaller centers and the developing world is to adopt staged palliation which has equally and perhaps better outcomes.
Cyanotic TOF patients can undergo a neonatal repair if their birth weight >4 kg and imaging show good pulmonary arteries (PAs). Symptomatic neonates with high-risk features such as prematurity, low birth weight or small PAs are managed with palliative or staged approach. Palliation intervention options include modified Blalock-Thomas-Taussig (BTT) shunts, ductal stenting, or right ventricular outflow tract (RVOT) stent palliation followed by surgical repair. Complete surgical repair involves a ventricular septal defect (VSD) patch with either the transannular patch approach, a valvular conduit, or an annulus-preserving repair. In asymptomatic TOF neonates’ surgery is preferred at 3–6 months of age (Fig. 6).
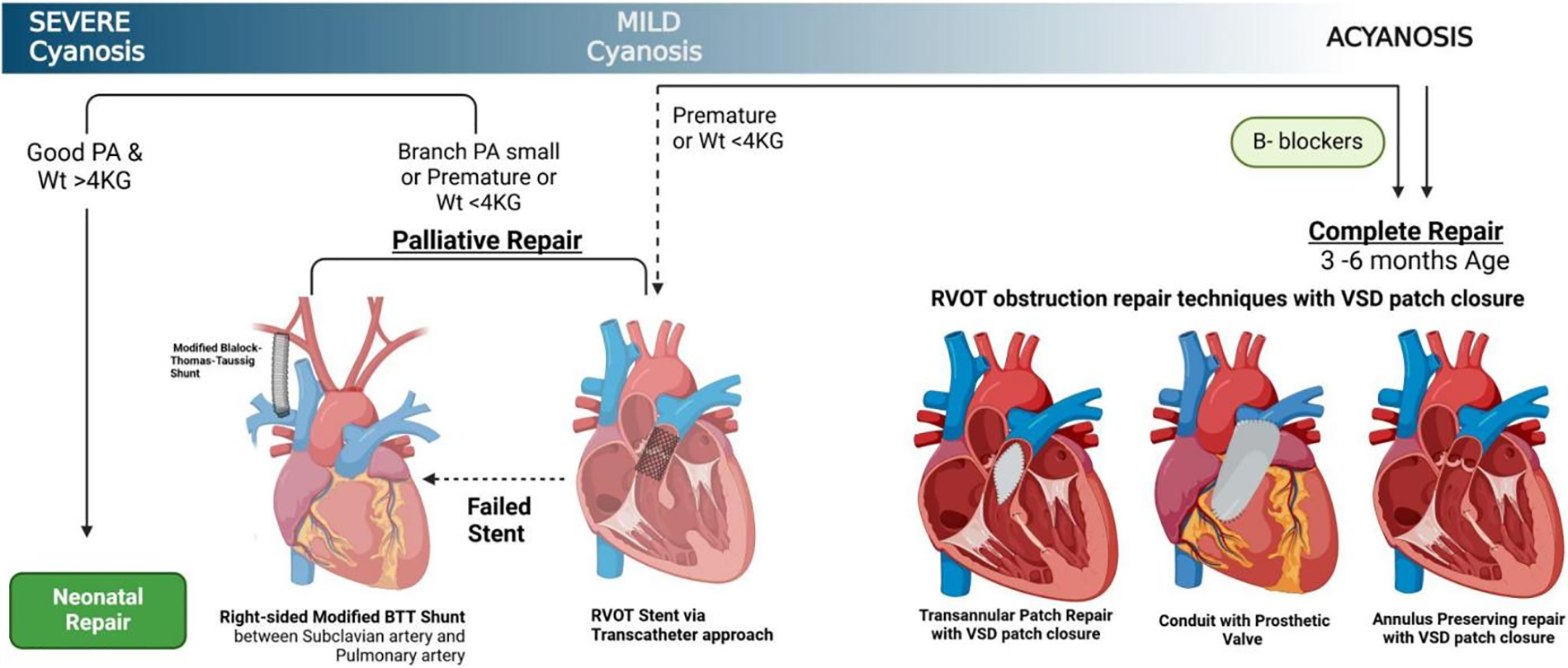
Figure 6: A schematic representation of Tetralogy of Fallot surgical management
4.3 Palliative Repair: Surgery vs. Stent
Several palliative repair options exist for the management of Tetralogy of Fallot. The intent of these options is to provide consistent pulmonary blood flow, encourage central pulmonary arterial growth and allow for somatic growth prior to complete repair. The most commonly employed shunt is the modified Blalock-Thomas-Taussig shunt sewn between the innominate artery and ipsilateral pulmonary artery. Central shunts between the ascending aorta and the main pulmonary artery are increasing in popularity due to their relative ease of creation, though adoption is likely limited by the frequent need for cardiopulmonary bypass. In infrequent situations, a transannular patch is performed to allow prograde flow with the obvious downside of sacrificing the pulmonary valves [37].
Nonsurgical palliation has gained in popularity in the current era with transcatheter pulmonary valvotomy, right ventricular outflow tract and ductal stenting obviating the need for sternotomy or surgery in higher risk patients [38,39]. RVOT stenting allows antegrade flow without the deleterious effects of systemic steal and branch pulmonary arterial distortion that can occur with systemic to pulmonary artery shunting or ductal stenting. However, it does prevent any option of saving the pulmonary valve and lines the RVOT with significant metal burden that may make subsequent surgery challenging and prolong operative times [40]. There is at least one report suggesting better branch PA growth with RVOT stenting when compared to surgical shunting [41]. When compared to complete repair early in life, however, adjusted analysis showed no difference in branch PA growth or catheter interventions. There was also no significant difference in mortality, pulmonary insufficiency, or somatic growth [42]. It seems reasonable that particularly in symptomatic low weight babies with small branch pulmonary arteries, endovascular stenting is a viable option for palliation [43]. In addition to RVOT stenting, ductal stenting also plays an important role in the managing Tetralogy of Fallot patients. Early reports of ductal stenting reported poor outcomes with high mortality and complication rates; however, continuous improvement of technology and techniques have led to dramatically improved outcomes [44,45]. Currently, ductal stenting has superior outcomes when compared to surgical shunt placement in both length of hospital stays and mortality but is also associated with higher rates of reintervention [46].
Deliberate preoperative planning is necessary for the management of the patient with Tetralogy of Fallot. This is because a relatively stable patient can rapidly decompensate with profound desaturation and spells at any point of the operative course prior to institution of cardiopulmonary bypass and complete repair. It is essential that the patient’s fluids, ventilation and oxygenation be optimized and vasopressors remain at hand to ensure systemic and pulmonary blood flow are balanced. The greatest risk of decompensation arises at induction. Alternatives to inhalational agents, which can cause extensive vasodilation, are preferred. Ketamine is a useful induction agent in this instance. However, decompensation can also occur with dissection and manipulation of the pulmonary arteries. Therefore, we generally recommend early institution of bypass in tetralogy patients. In patients with prior shunts, it is important to obtain control of the shunt and occlude it on initiation of bypass.
The goals of surgical repair include creating an unobstructed right ventricular outflow tract, closing the ventricular septal defect, and augmenting the pulmonary arteries where necessary. It is our practice to perform intraoperative transesophageal echocardiogram before and after repair. Important findings to note are character and extent of RVOT obstruction, pulmonary annular size, coronary branching pattern, additional muscular VSDs, and biventricular function. After median sternotomy and resection of thymus, if present, external cardiac inspection is performed evaluating the main PA and infundibulum. Any crossing coronary arterial branch that may preclude infundibular incision is noted. Systemic heparin is then administered, and cardiopulmonary bypass initiated through an aortic and bicaval venous configuration. Cooling is initiated with a goal temperature of 32 degrees Celsius. The main pulmonary artery is then dissected free of the aorta and the branch pulmonary arteries dissected for a short segment to allow occlusion with aneurysm clips. The cavae are snared and antegrade cardioplegia administered. Topical cooling with ice slush can be used to provide further myocardial protection.
A right atriotomy is then performed and a left ventricular vent placed across the frequently present PFO. Intracardiac inspection is performed to identify the VSD in its perimembranous position posterior to the anteroseptal commissure of the tricuspid valve. It is important to examine the full extent of the VSD, particularly its anterio-superior extent prior to any attempts at repair as this is the most common area to leave residual VSDs. The RVOT is then inspected, and any obvious obstructing muscle bundles incised. Our practice is to probe trabeculations with a right angle to ensure they are entirely within the RVOT before incising with a 15-blade. This protects against exiting the RV free wall or creating muscular VSDs. All obstructing bundles must be incised or resected. However, caution is to be used on the anterior limb of the septal band and the conus where the VSD patch is to be anchored as incisions into the endocardium weaken the muscle. Care must also be taken when incising muscles on the free wall to ensure the RV free wall is not thinned too aggressively, and importantly, epicardial coronary arteries are not incised as this can be catastrophic.
In many cases, RV muscle bundle resection can be achieved entirely through the tricuspid valve and complete repair performed without a right ventricular incision or with limited incision into the infundibulum. However, when this is insufficient to relieve obstruction, an incision is made in the infundibulum just inferior to the pulmonary valve. This incision is extended below the level of the sub-pulmonary conus and further muscle resection performed as necessary to create an unobstructed pathway. The pulmonary valve is then sized to determine adequacy of the valve for a full cardiac output. Generally, a pulmonary valve z-score greater than −2.5 is considered adequate for valve sparing repair provided the valve leaflets are free and mobile [14,47,48]. Gentle dilation of the pulmonary valve and annulus can be performed with graded Hagar dilators. Valvotomy can also be performed with incision of fused commissures. If necessary, a counter incision is made on the main PA to perform valvotomy and augment the PA. If the valve cannot be spared, the ventricular incision is carried across the valve into the pulmonary artery.
Attention is then turned to closure of the VSD. It is perfectly acceptable to close the VSD through the ventricular incision and we do this frequently. This is the classic approach to repair and provides good visualization of the VSD. It generally requires a longer ventriculotomy leading to concerns about immediate postoperative diastolic dysfunction and long term right ventricular dysfunction and arrhythmia burden. However, the transatrial approach also presents challenges in visualization, postoperative tricuspid valvar dysfunction, and recurrent RVOT obstruction. There have been conflicting data on complications and morbidity after either approach but there is no mortality difference on long term follow up. The main study finding superiority of the transatrial approach was in an older cohort of patients [49–52]. Regardless, the trend is towards a transatrial approach to minimize extent of ventriculotomy.
For this approach, three atrial retraction stitches are placed, one at the superior apex, one just off the inferior apex of the anterior edge and one just anterior to the crista terminalis. Stay sutures are then placed in the tricuspid valve leaflets as needed to expose the VSD. Generally, a stay suture is required in each of the anterior and septal leaflets and at the anteroseptal commissure. Chordae tendineae guarding the VSD are retracted. A vein retractor can provide additional exposure. The size of the VSD is measured and a patch of bovine pericardium is brought to the table and shaped accordingly. The patch is placed in running fashion. A suture of appropriate size, 5–0 or 6–0 is chosen with a curved needle. Suturing is begun at or just inferior to the junction of the limbs of the septal band and taken anterosuperiorly with each bite exposing the next. Care is taken to ensure the bites are close to the aortic annulus to prevent any residual VSDs across trabeculations. At the superior aspect when aortic and tricuspid continuity is observed, the needle is passed through the tricuspid leaflet. The inferior edge of the VSD is then run in continuous fashion taking thin bites remote from the edge of the defect to avoid conduction tissue. At junction of the septal leaflet and ventricular septum remote from the VSD edge, the needle is reversed through the patch, septal muscle and externalized on the atrial side of the leaflet. Using a strip of pericardium as pledget and only sewing in valve tissue to avoid the AV nodal tissue the septal leaflet is then attached to the patch using a horizontal mattress running technique until the anterosuperior edge of the previously sewn patch is reached. The suture is tied to complete the VSD repair.
The right ventricular outflow is then reconstructed as needed with a transannular patch, isolated infundibular patch, or infundibular and main pulmonary arterial patches. In cases where a crossing coronary artery precludes patch enlargement of the RVOT, an RV to PA conduit is often required although there have been innovations in valveless augmentation utilizing an anterior pulmonary arterial flap. The tricuspid valve is then tested. If there is significant regurgitation, an anteroseptal commissuroplasty is usually all that is required to repair the valve. The patient is rewarmed, deairing maneuvers are performed and the PFO closed primarily. In younger patients with long ventriculotomies, consideration should be made to leaving a partial PFO of 3–4 mm to enable right to left shunting in the presence of RV diastolic dysfunction. The heart is reperfused, ventilation resumed, and cardiopulmonary bypass weaned in standard fashion.
A completion transesophageal echocardiogram is performed to examine residual lesions. Residual VSDs greater than 2–3 mm should be closed as these are unlikely to close in follow up of patients with tetralogy repair [53]. In the presence of optimized pulmonary ventilation and medically optimized pulmonary vascular resistance and equivocal lesions such as residual obstruction, a right ventricular to left ventricular (RV/LV) systolic pressure ratio of greater than 2/3 is generally an indication for further intervention which may include muscle bundle resection, transannular patch creation, extension of right ventriculotomy or patching of discrete branch pulmonary stenosis. In the absence of any addressable lesion, it can usually be safely assumed in the infant that pulmonary vascular reactivity or dynamic obstruction are the etiologies of increased ventricular pressure. The patient is transferred to the intensive care unit for further management. In the absence of fixed obstruction, these patients develop significant improvement in right ventricular pressure and have no long-term mortality effects [54,55]. A pre- and postoperative TEE can be seen in Fig. 7.
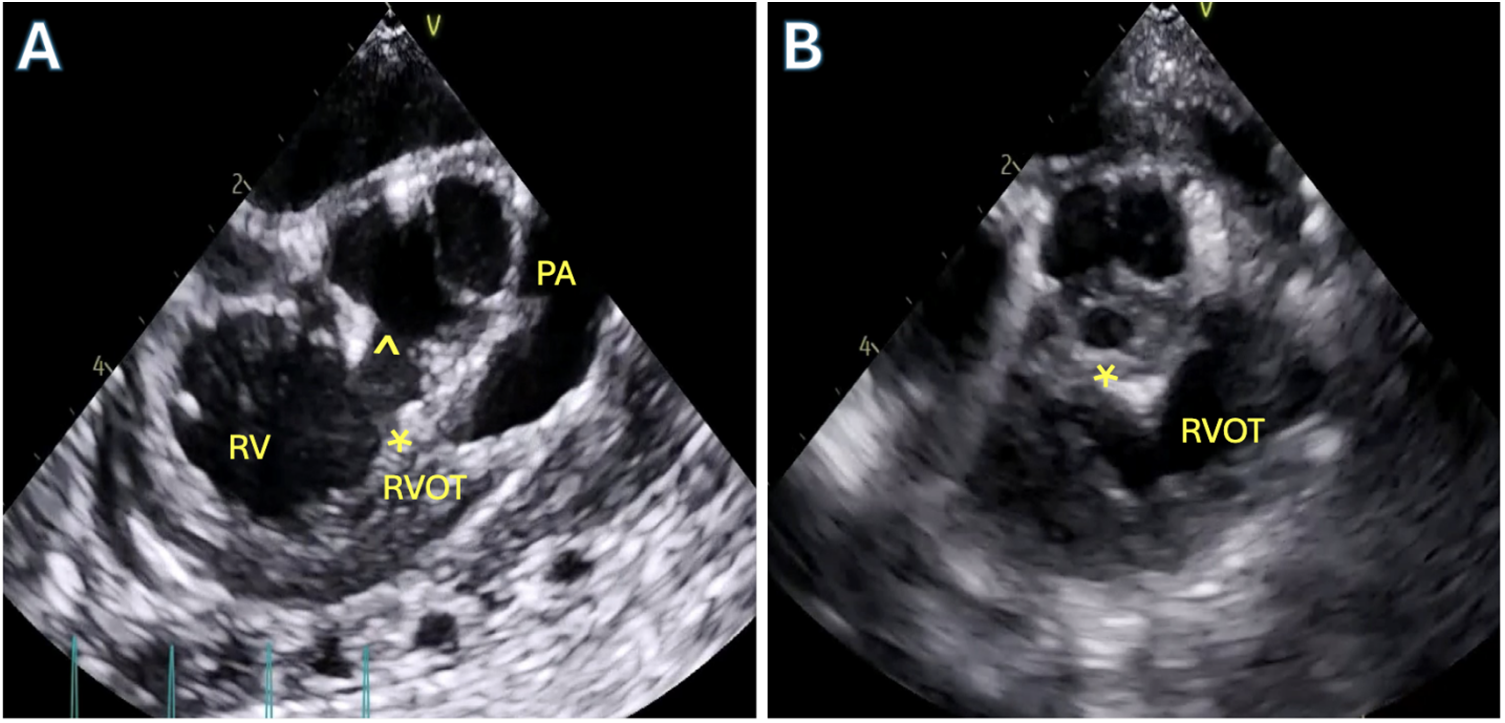
Figure 7: Transesophageal echocardiography (TEE) in a patient with Tetralogy of Fallot. A: Pre-operative TEE mid-esophageal right ventricular (RV) outflow tract view showing the anteriorly deviated conal septum (asterisk) with the large ventricular septal defect (VSD,^) and severe sub-pulmonary right ventricular outflow tract obstruction. B: Post-operative TEE after complete repair demonstrating patch closure of VSD (asterisk) and unobstructed right ventricular outflow tract (RVOT)
With improvements in surgical technique and perioperative management, Tetralogy of Fallot repair is now performed with excellent outcomes. In the current era, operative mortality is low at 0%–5%. A recent meta-analysis of 143 papers comprising 21,427 patients yielded a pooled operative mortality of 2.84% and a long-term mortality risk of 0.42% per year. Freedom from reintervention was 90% at 5 years with RVOT reintervention rate of 12% at 10 years (4.2% for PI, 7.6% for RVOT obstruction) [56]. This is consistent with the Society of Thoracic Surgeons discharge mortality of 1.3% over the study period of 2002–2007 investigated by Al Habib et al. They also found neonatal primary repair mortality of 7.8% and neonatal palliation mortality of 6.2% [37]. Conflicting data exist regarding staged and complete repair with recent large population studies showing statistically and clinically significant survival benefits to both primary repair and staged palliation. Other predictors of mortality include non-valve sparing operation, repair in earlier eras and presence of a genetic abnormality [35,36].
Both transatrial/transpulmonary and transventricular approaches to repair yield similar outcomes. However, there is significantly higher rate of reoperation for RVOT obstruction in the transatrial/transpulmonary group when compared to the transventricular approach with 10 years freedom from reoperation from RVOTO of 98% vs. 75%. RVOTO is the most common indication for reoperation in all patients after Tetralogy of Fallot repair. Significant pulmonary regurgitation was found in greater than 40% of patients with transventricular approach. However, at 10 years, less than 5% of these patients met indications for intervention. Early evaluation of myocardial function showed improved wall motion and greater ejection fraction in the transatrial group [49,57].
In longitudinal studies of patients after Tetralogy of Fallot, 4%–15% had ventricular tachycardia with 2% rate of sudden cardiac death. The etiology is thought to be the reentry circuits in the surgical ventriculotomy scars and further scarring from right ventricular dilation. Patients with transannular patches and moderate or severe PI had higher risk of ventricular tachycardia and sudden death than those without. Other predictors of VT and sudden death included older age at repair and QRS > 180 ms. In contradistinction, tricuspid regurgitation was the most common lesion in patients with atrial arrhythmias [58,59]. However, another study from Japan found a ventricular tachycardia risk of 3% in patients with transatrial approach to tetralogy repair [60].
Despite the focus on ventricular tachycardia, atrial arrhythmias, present in 20% of patients, are the major causes of morbidity in patients after repair. Junctional ectopic tachycardia (JET) is the most common arrhythmia after tetralogy repair with a higher prevalence in the transatrial/transpulmonary approach. Incidence as high as 12% has been reported. JET can be managed with cooling, electrolyte replacement and, in the failure of these therapies, amiodarone bolus and infusion. Excessive traction on the right atrium and sinoatrial node during repair is a possible etiology of JET. Late arrhythmias are uncommon earlier than 10 postoperative years and include atrial fibrillation and flutter. However, when they do occur, they are independent predictors of death. Ablation has been increasingly performed in patients and is shown to improve functional outcomes. However, there is no significant long term follow up of these patients [61–63].
Long term complications of Tetralogy of Fallot repair include right ventricular dilation and dysfunction from chronic volume overload, ascending aortic dilation, and ventricular dysrhythmias. Aortic root dilation has been reported in as high as 30% of patients after repair of Tetralogy of Fallot with a higher prevalence in patients with absent pulmonary valves. Progressive dilation is associated with male sex, right aortic arch, and longer interval from palliation to repair. The last factor is consistent with the thinking that progressive aortic root dilation is exacerbated by volume overload from shunted physiology. Aortic regurgitation can be a sequela of root dilation in Tetralogy of Fallot with pulmonary stenosis; however, it is usually mild. Aortic dissections have been reported but are rare. When aortic dilation is discovered, monitoring is warranted, and repair performed when indications for aortic replacement are met according to guidelines [64–66].
Pulmonary valve replacement is the most common late indication for reoperation on Tetralogy of Fallot patients after repair with reported risk of PVR of 0.8% per year [67]. The combination of chronic pulmonary stenosis and volume overload leads to right ventricular maladaptation manifesting as dilation, hypertrophy, and dysfunction which is exacerbated by secondary tricuspid regurgitation. With pulmonary valve replacement there is potential for ventricular remodeling and improvement in heart failure symptoms. However, no patients achieved normal RV end-diastolic volume if their preoperative global RV end-diastolic volume index before PVR was >160–170 mL/m2 or RV end-systolic volume index was >82–85 mL/m2 [68,69]. A large meta-analysis found decreases in QRS duration, improvement in left ventricular function and symptoms. Paradoxically, there was no obvious improvement in right ventricular function [70].
Pulmonary valve replacement is recommended for relief of symptoms not otherwise explained in patients with repaired tetralogy and moderate or greater pulmonary regurgitation, ventricular enlargement, or dysfunction, in conjunction with other cardiac surgical procedures and in addition to arrhythmia management for ventricular tachycardia. Any two of mild or moderate RV or LV systolic dysfunction, severe RV dilation with RV end-diastolic volume index before PVR >160 mL/m2 or RV end-systolic volume index >80 mL/m2 or RV end diastolic volume greater than 2 times LV end diastolic volume, right ventricular systolic pressure greater than two-thirds systemic pressure, and progressive reduction in exercise tolerance. Before patients undergo intervention, catheterization or imaging is warranted to delineate coronary anatomy [24].
Surgical management in the absence of a right ventricular to pulmonary artery conduit involves placement of a prosthetic valve in the pulmonary annulus with cardiopulmonary bypass. Techniques involve circumferential running stitch and interrupted mattress stitches with the valve tilted in the plane of the pulmonary artery. In many instances, an RVOT patch is required to complete the anterior third of the valve annulus. This patch is also used to enlarge the RVOT and main PA. Additional procedures such as branch pulmonary arterial augmentation are performed at this time. Since valve replacement has not been shown to decrease risk of ventricular arrhythmias or sudden cardiac death, tachyarrhythmia ablation and ICD insertion are performed as indicated.
In recent years, endovascular, transcatheter pulmonary valve replacement has been developed and used to good effect in management of post repair patients with RV to PA conduits. The technique was introduced by Bonhoeffer and there has since been the development of many valves including the Melody Valve from Medtronic with a post market freedom from valve reintervention and explantation of 76% and 92%, respectively, and the SAPIEN XT Valve (Edwards Lifesciences) with freedom from reintervention of 94% at 3 years. These valves are available for RVOT diameter >16 mm. The maximum valve diameter on the market is 29 mm. Additional devices include the Medtronic Harmony Valve and the Edwards Lifesciences SAPIEN S3 and Alterra Prestent. This Prestent can be used to accommodate a larger (>30 mm) RVOT diameter then preexisting devices and broadens the population that can be treated with transcatheter valves. Complications from transcatheter valve replacements include an up to 30% stent fracture risk, 5% risk of coronary compression and of endocarditis of 2% per patient year [71–73].
Tetralogy with absent pulmonary valve is a rare form of Tetralogy (<5% of cases) in which a pulmonary annular ring creates fixed obstruction to right ventricular outflow. It is believed that the absence of a valve leads to back-and-forth flow from the right ventricle into the pulmonary artery leading to aneurysmal dilation of the main PA and branch pulmonary arteries sometimes extending to the hilum as seen in Fig. 8. Z-scores of the arteries are frequently greater than +2 or 3. The pulmonary vessels show a wide spectrum of anatomical variation. In milder cases, a patent Ductus Arteriosus (PDA) is present alongside well-developed pulmonary arteries. However, in most cases no PDA is present resulting in diminutive or missing vessels with Pulmonary circulation supported via major aorto-pulmonary collateral arteries (MAPCAs) originating from the aorta as seen in Fig. 9. Although the right ventricle is hypertrophied there is usually no dynamic sub-annular obstruction as the infundibulum frequently is dilated without stenosis. Associated abnormalities include tracheo- and bronchomalacia leading to respiratory abnormalities including hyper inflated lungs and air trapping [74]. Patients present in two groups, those with early symptoms, and those without. Early presenters have cyanosis mostly driven by respiratory distress and regularly need mechanical ventilation and urgent surgery. In some instances, mechanical ventilation can be augmented (or avoided) with placing the baby prone to relieve obstruction.
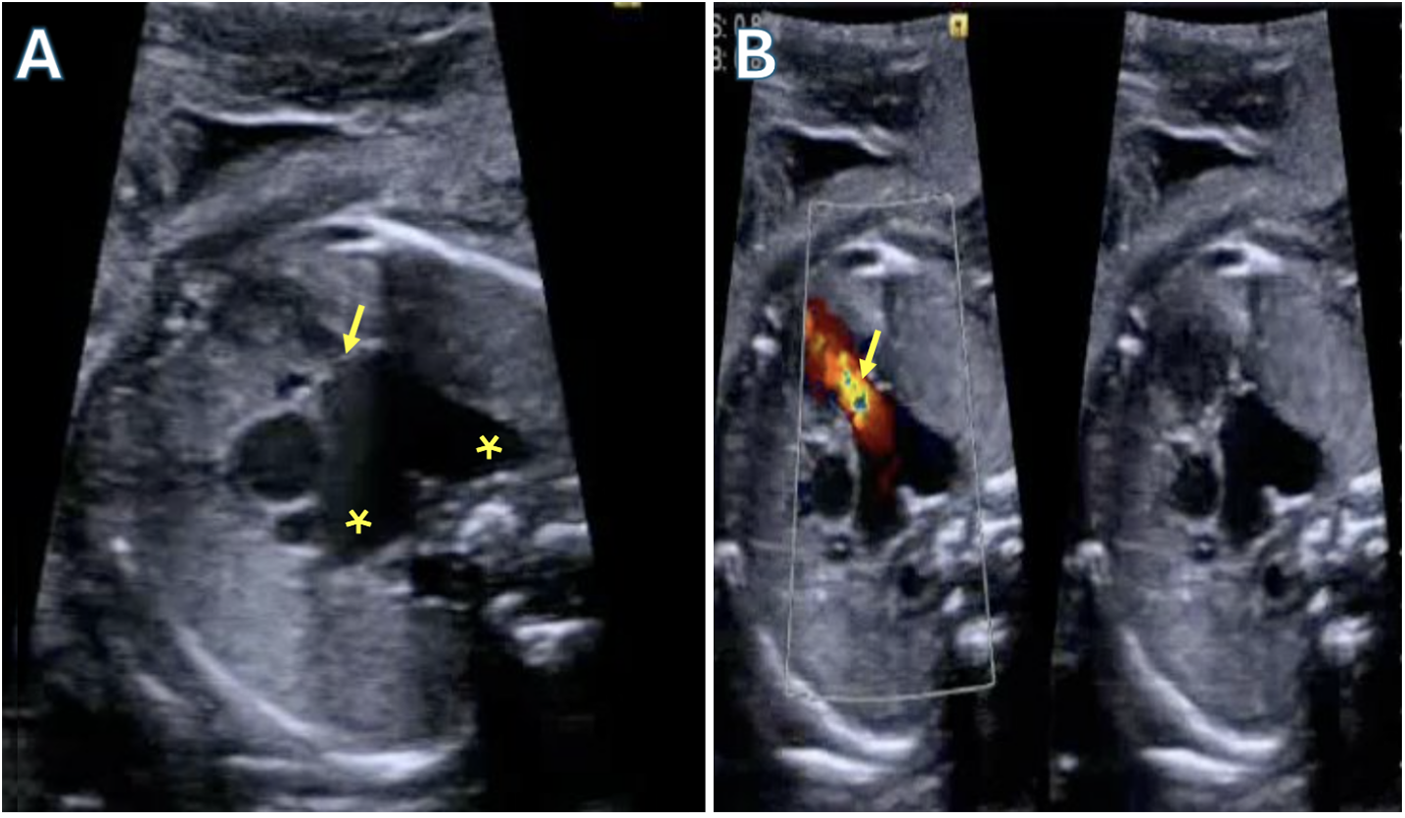
Figure 8: Fetal echocardiogram demonstrating Tetralogy of Fallot with absent pulmonary valves. A: Severe dilated branch pulmonary arteries (asterisks) with a rudimentary pulmonary valve (arrow). B: Color compare showing severe regurgitation through the rudimentary pulmonary valve (arrow)
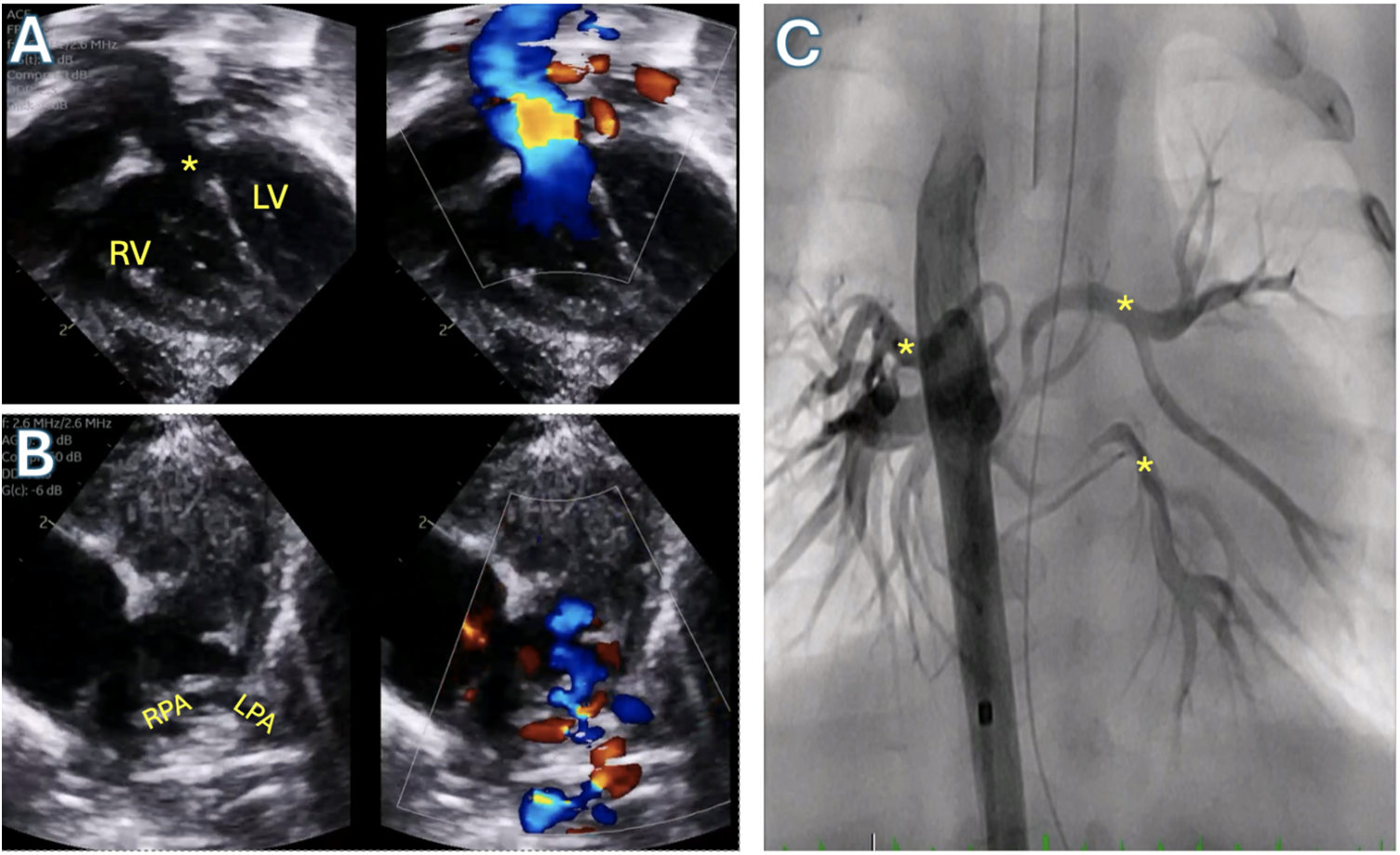
Figure 9: Tetralogy of Fallot with pulmonary atresia and pulmonary blood flow supplied by major aortopulmonary collateral arteries (MAPCAs). A: Transthoracic echocardiogram subcostal long axis view demonstrating large ventricular septal defect (asterisk) and overriding aorta. B: Transthoracic echocardiogram parasternal short axis view demonstrating pulmonary atresia with absent pulmonary artery segment and severely hypoplastic branch pulmonary arteries supplied by MAPCAs. C: Angiogram in the descending aorta demonstrating the MAPCAs (asterisks). RV: Right ventricle; LV: Left ventricle; RPA: Right pulmonary artery; LPA: Left pulmonary artery
Late presenters are managed like patients with Tetralogy of Fallot and pulmonary stenosis and are fully repaired before one year of age. This invariably involves closure of the VSD. The approach to management of the pulmonary arteries however varies significantly. With mild respiratory symptoms and mild aneurysmal dilation of the pulmonary arteries, transannular patching has been described to relieve pulmonary obstruction while others have inserted competent pulmonary valves. However, when there is severe aneurysmal dilation, bronchial compression and severe respiratory symptoms, efforts to reduce the size of the PAs and relieve compression are warranted. The most extensive approach is resection of the central pulmonary arteries and replacement with a branched pulmonary artery homograft extending to each hilum. Others perform anterior resection of the branch pulmonary arteries and posterior plication to stiffen the tissue in proximity to the airway to decrease arterial size. Homograft replacement of the main PA is then performed. These latter approaches consign the patient to short term reoperative surgery to replace the conduit [75,76]. As a last resort, a Lecompte maneuver can be performed to translocate the PAs from the airways.
Operative mortality in the current era is less than 5% with good mid- and long-term outcomes of 80%–95% survival at 5 and 10 years. Freedom from RVOT reoperation varies by age at repair and surgical technique with younger age at surgery correlating with higher rates of reoperation. Even so, freedom from reoperation at 10 years averages 70%–90% [77–80].
TOF/AVSD is found in less than 5% of patients with Tetralogy of Fallot and is associated with Trisomy 21 in the majority of patients [81–84]. The Rastelli C AVSD with no attachment of the superior bridging leaflet to the septal crest is most frequently associated with Tetralogy of Fallot. Every attempt should be made to screen for this additional lesion on preoperative echocardiography as it changes management significantly. The ventricular septal defect in this case is large, extending to the inlet with anterior malalignment of the septum requiring a large, asymmetric patch for closure. The former feature increases the risk of biventricular outflow tract obstruction while the latter makes visualization of the anterosuperior aspect of the VSD from the right atrium exceedingly difficult often necessitating a ventriculotomy. Pulmonary valves are frequently dysplastic [85]. Perhaps because of this constellation of features, however, presentation in neonatal or early infancy with cyanosis is rare.
Different approaches have been suggested for the surgical management of the TOF/AVSD including transatrial-transpulmonary repair with minimal infundibular incision, transannular patch insertion, combined right atrial and right ventricular approach, and a double patch vs. single patch closure of the AV septal defect [83,86,87]. The debate on definitive one stage repair vs. staged repair after systemic to pulmonary artery shunting in tetralogy patients extends to this anatomic subtype as well. While some reports [86,88] found significantly worse outcomes, including ICU stays, ventilator support, inotropic scores, and incidence of reoperation, in patients undergoing shunt palliation prior to repair when compared to definitive primary repair, primary repair was performed in an older cohort of patients when compared to shunted patients. The driver of worse outcomes is thought to be volume overloading of the ventricles from increased pulmonary flow with surgical shunting. Others found equivalent outcomes between the two management paradigms [83,89]. However, both camps conclude that primary repair is the preferable approach. Further, a meta-analysis showed no mortality differences but found increased RVOT interventions in patients who underwent staged repair [84]. A recent single center study found no differences in mortality or reoperative risks in their staged vs. primary repair patients at 10 years postoperatively [90]. However, a limitation of these studies is that consideration is not made of patients who died while awaiting definitive repair instead of being shunted. As in other tetralogy, patients with small pulmonary arteries and cyanosis at an early age may benefit from early shunting [89].
Operative mortality has improved in the current era to less than 5% with 5-year survival of >80%–90% in most series and freedom from reoperation greater than 70%. Indications for reoperation include Atrial Ventricular Valve regurgitation, recurrent RVOT obstruction and pulmonary valve replacement. Patients with right ventriculotomies experienced higher rates of arrhythmias [84,88,91].
The current standard-of-care has provided excellent results with low mortality through both primary repair and staged palliation repair. Current guidelines based upon the AATS consensus statement have given a significantly clarified ideal operative period [92,93]. The development of clear guidelines in the timing of intervention is a major advancement that will reduce institutional variance in treatment and improve outcomes. In addition to more evidence driven decision making in deciding between staged palliation and early definitive repair, percutaneous options for repairing parts of the malformation and assisting in palliation will continue to prove as a vital tool in treating Tetralogy of Fallot. These lower risk treatments serve not only the pediatric population but are also indispensable in the treatment of the adult Tetralogy of Fallot population with use in transcatheter pulmonary valve replacements as well as in the stenting of conduits to reverse the development of stenosis [94]. In addition to advancements in treatment options, advancements in the early screening and diagnosis of Tetralogy of Fallot will allow clinicians more time and information to aid in decision making, including new understanding in variations in anatomic variations in patients with Tetralogy of Fallot that may lead to higher rates of fetal detection [95]. These screening programs are also vital for ensuring that cases of TOF do not go undiagnosed and receive the treatment needed. The care of adults with Tetralogy of Fallot requires a specialized approach as this population can offer unique challenges to clinicians, with varied techniques needed for accurate assessment of hypertrophic conditions and new predictive indicators for long term complications such as the development of arrhythmias [96,97]. Developments in the understanding of the basic science have also been underway, including insights into the gene regulation differences in control patients compared to those with Tetralogy of Fallot [98]. The combination of improved techniques and technology to treat patients, higher rates of prenatal diagnosis, and an advancement in the basic science involved in Tetralogy of Fallot will hopefully lead to a continuation in the improvement of outcomes that have been experienced in recent years.
Anatomy: Tetralogy of Fallot includes right ventricular hypertrophy, a ventricular septal defect (VSD), overriding aorta and right ventricular outflow tract obstruction. In addition to these common findings, variations consisting of atrial septal defects, atrioventricular defects, absent pulmonary valves, or MAPCAs are also commonly found.
Diagnosis: Prenatal Echocardiography is the main modality of diagnosis. Many cases diagnosed after birth present with cyanosis in the neonatal period “Blue Baby syndrome” presenting with Tet spells. Echocardiography is the primary diagnostic method with CT angiography for surgical approach.
Medical Management: Management consists of administration of Prostaglandin E1 in ductal dependent children followed by careful outpatient monitoring until surgical intervention is available.
Surgical Intervention: Surgical intervention consists of either a staged palliative to repair approach or a primary complete repair. Palliation is performed in patients who are poor candidates for primary repair, and frequently includes percutaneous placement of RVOT stents.
Outcomes: Patients undergoing surgical intervention for complete repair experiences favorable outcomes with freedom from reintervention rates of 70%–90% depending on age at intervention and specific anatomical variation. Common complications include dilation and dysfunction of the Right Ventricle along with arrhythmias.
Acknowledgement: None.
Funding Statement: Taufiek Konrad Rajab’s research is supported by the National Institutes of Health/National Heart, Lung, and Blood Institute grant R41 HL169059, the American Association for Thoracic Surgery, the Brett Boyer Foundation, the Saving tiny Heart Society, the Emerson Rose Heart Foundation, philanthropy from Senator Campbell and the Arkansas Children’s Research Institute. Edo Bedzra’s work is supported with funding by Children’s Mercy Kansas City Ward Family Heart Center.
Author Contributions: Edo Bedzra—Supervision, Original draft and Revisions, Herra Javed—Revisions and Images, Eli Contorno—Revisions and Images, Amna Qasim—Images and Revisions, James St. Louis—Original Draft and Revision, Taufiek Konrad Rajab—Supervision, Original Draft, Figures, and Revisions.
Availability of Data and Materials: No original data was utilized in the creation of this manuscript.
Ethics Approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Harley HRS. What is fallot’s tetralogy? Am Heart J. 1961;62(6):729–34. [Google Scholar] [PubMed]
2. Lev M, Eckner FA. The pathologic anatomy of Tetralogy of Fallot and its variations. Dis Chest. 1964;45(3):251–61. doi:10.1378/chest.45.3.251. [Google Scholar] [PubMed] [CrossRef]
3. Brock RC. Pulmonary valvulotomy for congenital pulmonary stenosis. Br Med J. 1948 Jun 12;1(4562):1121–6. doi:10.1136/bmj.1.4562.1121. [Google Scholar] [CrossRef]
4. Holmes Sellors T. Surgery of pulmonary stenosis; a case in which the pulmonary valve was successfully divided. Lancet. 1948;1(6513):988–9. doi:10.1016/S0140-6736(48)90615-1. [Google Scholar] [CrossRef]
5. Lillehei CW, Varco RL, Cohen M, Warden HE, Gott VL, de Wall RA, et al. The first open heart corrections of Tetralogy of Fallot: a 26–31 year follow-up of 106 patients. Ann Surg. 1986;204(4):490. doi:10.1097/00000658-198610000-00017. [Google Scholar] [PubMed] [CrossRef]
6. Béland MJ, Franklin RCG, Jacobs JP, Tchervenkov CI, Aiello VD, Colan SD, et al. Update from the international working group for mapping and coding of nomenclatures for paediatric and congenital heart disease. Cardiol Young. 2004;14(2):225–9. doi:10.1017/S1047951104002239. [Google Scholar] [PubMed] [CrossRef]
7. Van der Linde D, Konings EEM, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJM, et al. Birth prevalence of congenital heart disease worldwide. J Am Coll Cardiol. 2011 Nov 15;58(21):2241. doi:10.1016/j.jacc.2011.08.025. [Google Scholar] [PubMed] [CrossRef]
8. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002 Jun 19;39(12):1890. doi:10.1016/s0735-1097(02)01886-7. [Google Scholar] [PubMed] [CrossRef]
9. Van Praagh R, Van Praagh S, Nebesar RA, Muster AJ, Sinha SN, Paul MH. Tetralogy of Fallot: underdevelopment of the pulmonary infundibulum and its sequelae. Am J Cardiol. 1970;26(1):25–33. doi:10.1016/0002-9149(70)90754-X. [Google Scholar] [PubMed] [CrossRef]
10. Anderson RH, Tynan M. Tetralogy of Fallot—a centennial review. Int J Cardiol. 1988;21(3):219–32. doi:10.1016/0167-5273(88)90100-3. [Google Scholar] [PubMed] [CrossRef]
11. Anderson RH, Jacobs ML. The anatomy of Tetralogy of Fallot with pulmonary stenosis. Cardiol Young. 2008;18(S3):12–21. doi:10.1017/S1047951108003259. [Google Scholar] [PubMed] [CrossRef]
12. Khan SM, Drury NE, Stickley J, Barron DJ, Brawn WJ, Jones TJ, et al. Tetralogy of Fallot: morphological variations and implications for surgical repair. Eur J Cardiothorac Surg. 2019 Jan 16;56(1):101–9. [Google Scholar]
13. Altrichter PM, Olson LJ, Edwards WD, Puga FJ, Danielson GK. Surgical pathology of the pulmonary valve: a study of 116 cases spanning 15 years. Mayo Clin Proc. 1989 Nov 1;64(11):1352–60. [Google Scholar]
14. Sinha R, Gooty V, Jang S, Dodge-Khatami A, Salazar J. Validity of pulmonary valve z-scores in predicting valve-sparing tetralogy repairs-systematic review dagger. Child Basel Switz. 2019 May 4;6(5). doi:10.3390/children6050067. [Google Scholar] [PubMed] [CrossRef]
15. Chacko BR, Chiramel GK, Vimala LR, Manuel DA, Joseph E, Reka K. Spectrum of pulmonary valve morphology and its relationship to pulmonary trunk in Tetralogy of Fallot. Indian J Radiol Imaging. 2017;27(1):65–9. [Google Scholar] [PubMed]
16. Suzuki A, Ho SY, Anderson RH, Deanfield JE. Further morphologic studies on Tetralogy of Fallot, with particular emphasis on the prevalence and structure of the membranous flap. J Thorac Cardiovasc Surg. 1990 Mar;99(3):528–35. doi:10.1016/S0022-5223(19)36984-3. [Google Scholar] [CrossRef]
17. Chiariello L, Meyer J, Wukasch DC, Hallman GL, Cooley DA. Intracardiac repair of Tetralogy of Fallot. Five-year review of 403 patients. J Thorac Cardiovasc Surg. 1975 Sep;70(3):529–35. doi:10.1016/S0022-5223(19)40329-2. [Google Scholar] [CrossRef]
18. Digilio MC, Marino B, Giannotti A, Toscano A, Dallapiccola B. Recurrence risk figures for isolated Tetralogy of Fallot after screening for 22q11 microdeletion. J Med Genet. 1997 Mar;34(3):188–90. doi:10.1136/jmg.34.3.188. [Google Scholar] [PubMed] [CrossRef]
19. Michielon G, Marino B, Formigari R, Gargiulo G, Picchio F, Digilio MC, et al. Genetic syndromes and outcome after surgical correction of Tetralogy of Fallot. Ann Thorac Surg. 2006 Mar;81(3):968–75. doi:10.1016/j.athoracsur.2005.09.033. [Google Scholar] [PubMed] [CrossRef]
20. Morgenthau A, Frishman WH. Genetic origins of Tetralogy of Fallot. Cardiol Rev. 2018 Apr;26(2):86–92. doi:10.1097/CRD.0000000000000170. [Google Scholar] [PubMed] [CrossRef]
21. Zhao Y, Abuhamad A, Fleenor J, Guo Y, Zhang W, Cao D, et al. Prenatal and postnatal survival of fetal Tetralogy of Fallot. J Ultrasound Med. 2016 May 1;35(5):905–15. doi:10.7863/ultra.15.04055. [Google Scholar] [CrossRef]
22. Yang M-C, Chiu S-N, Wang J-K, Lu C-W, Lin M-T, Chen C-A, et al. Natural and unnatural history of Tetralogy of Fallot repaired during adolescence and adulthood. Heart Vessels. 2012 Jan 1;27(1):65–70. doi:10.1007/s00380-011-0119-3. [Google Scholar] [PubMed] [CrossRef]
23. Lapierre C, Dubois J, Rypens F, Raboisson M-J, Déry J. Tetralogy of Fallot: preoperative assessment with MR and CT imaging. Imaging Congenit Card Dis Fetus Adult. 2016 May 1;97(5):531–41. [Google Scholar]
24. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. 2018 AHA/ACC guideline for the management of adults with Congenital Heart Disease. J Am Coll Cardiol. 2019 Apr;73(12):e81–192. doi:10.1016/j.jacc.2018.08.1028. [Google Scholar] [CrossRef]
25. Shaddy RE, Viney J, Judd VE, McGough EC. Continuous intravenous phenylephrine infusion for treatment of hypoxemic spells in Tetralogy of Fallot. J Pediatr. 1989 Mar;114(3):468–70. doi:10.1016/s0022-3476(89)80574-8. [Google Scholar] [PubMed] [CrossRef]
26. Tanaka K, Kitahata H, Kawahito S, Nozaki J, Tomiyama Y, Oshita S. Phenylephrine increases pulmonary blood flow in children with Tetralogy of Fallot. Can J Anaesth J Can Anesth. 2003 Nov;50(9):926–9. doi:10.1007/BF03018741. [Google Scholar] [PubMed] [CrossRef]
27. Graham EM, Bandisode VM, Bradley SM, Crawford FAJ, Simsic JM, Atz AM. Effect of preoperative use of propranolol on postoperative outcome in patients with Tetralogy of Fallot. Am J Cardiol. 2008 Mar 1;101(5):693–5. doi:10.1016/j.amjcard.2007.10.033. [Google Scholar] [PubMed] [CrossRef]
28. Kanter KR, Kogon BE, Kirshbom PM, Carlock PR. Symptomatic neonatal Tetralogy of Fallot: repair or shunt? Ann Thorac Surg. 2010 Mar;89(3):858–63. doi:10.1016/j.athoracsur.2009.12.060. [Google Scholar] [PubMed] [CrossRef]
29. Hirsch JC, Mosca RS, Bove EL. Complete repair of Tetralogy of Fallot in the neonate: results in the modern era. Ann Surg. 2000 Oct;232(4):508–14. doi:10.1097/00000658-200010000-00006. [Google Scholar] [PubMed] [CrossRef]
30. Bacha EA, Scheule AM, Zurakowski D, Erickson LC, Hung J, Lang P, et al. Long-term results after early primary repair of Tetralogy of Fallot. J Thorac Cardiovasc Surg. 2001 Jul;122(1):154–61. doi:10.1067/mtc.2001.115156. [Google Scholar] [PubMed] [CrossRef]
31. Kirklin JK, Kirklin JW, Blackstone EH, Milano A, Pacifico AD. Effect of transannular patching on outcome after repair of Tetralogy of Fallot. Ann Thorac Surg. 1989 Dec;48(6):783–91. doi:10.1016/0003-4975(89)90671-1. [Google Scholar] [PubMed] [CrossRef]
32. Yang S, Wen L, Tao S, Gu J, Han J, Yao J, et al. Impact of timing on in-patient outcomes of complete repair of Tetralogy of Fallot in infancy: an analysis of the United States National inpatient 2005–2011 database. BMC Cardiovasc Disord. 2019 Feb 26;19(1):46. doi:10.1186/s12872-019-0999-1. [Google Scholar] [PubMed] [CrossRef]
33. Ylitalo P, Nieminen H, Pitkanen OM, Jokinen E, Sairanen H. Need of transannular patch in Tetralogy of Fallot surgery carries a higher risk of reoperation but has no impact on late survival: results of Fallot repair in Finland. Eur J Cardio-Thorac Surg off J Eur Assoc Cardio-Thorac Surg. 2015 Jul;48(1):91–7. doi:10.1093/ejcts/ezu401. [Google Scholar] [PubMed] [CrossRef]
34. Nakashima K, Itatani K, Oka N, Kitamura T, Horai T, Hari Y, et al. Pulmonary annulus growth after the modified Blalock-Taussig shunt in Tetralogy of Fallot. Ann Thorac Surg. 2014 Sep;98(3):934–40. doi:10.1016/j.athoracsur.2014.04.083. [Google Scholar] [PubMed] [CrossRef]
35. Smith CA, McCracken C, Thomas AS, Spector LG, St Louis JD, Oster ME, et al. Long-term outcomes of Tetralogy of Fallot: a study from the pediatric cardiac care consortium. JAMA Cardiol. 2019 Jan 1;4(1):34–41. doi:10.1001/jamacardio.2018.4255. [Google Scholar] [PubMed] [CrossRef]
36. Savla JJ, Faerber JA, Huang Y-SV, Zaoutis T, Goldmuntz E, Kawut SM, et al. 2-year outcomes after complete or staged procedure for Tetralogy of Fallot in neonates. J Am Coll Cardiol. 2019 Sep 24;74(12):1570–9. doi:10.1016/j.jacc.2019.05.057. [Google Scholar] [PubMed] [CrossRef]
37. Al Habib HF, Jacobs JP, Mavroudis C, Tchervenkov CI, O’Brien SM, Mohammadi S, et al. Contemporary patterns of management of Tetralogy of Fallot: data from the society of thoracic surgeons database. Ann Thorac Surg. 2010 Sep 1;90(3):813–20. doi:10.1016/j.athoracsur.2010.03.110. [Google Scholar] [PubMed] [CrossRef]
38. Alwi M. Stenting the ductus arteriosus: case selection, technique and possible complications. Ann Pediatr Cardiol. 2008 Jan;1(1):38–45. doi:10.4103/0974-2069.41054. [Google Scholar] [PubMed] [CrossRef]
39. Moore JW. PDA stenting for ductal-dependent cyanotic Congenital Heart Disease: history and view from 10,000 feet. Pediatric Cardiol. 2024;71:1–6. doi:10.1007/s00246-024-03737-w. [Google Scholar] [PubMed] [CrossRef]
40. Barron DJ, Ramchandani B, Murala J, Stumper O, de Giovanni JV, Jones TJ, et al. Surgery following primary right ventricular outflow tract stenting for Fallot’s tetralogy and variants: rehabilitation of small pulmonary arteries. Eur J Cardiothorac Surg. 2013 Oct;44(4):656–62. doi:10.1093/ejcts/ezt188. [Google Scholar] [PubMed] [CrossRef]
41. Quandt D, Ramchandani B, Stickley J, Mehta C, Bhole V, Barron DJ, et al. Stenting of the right ventricular outflow tract promotes better pulmonary arterial growth compared with modified blalock-taussig shunt palliation in Tetralogy of Fallot-Type Lesions. JACC Cardiovasc Interv. 2017 Sep 11;10(17):1774–84. doi:10.1016/j.jcin.2017.06.023. [Google Scholar] [PubMed] [CrossRef]
42. Wilder TJ, Van Arsdell GS, Benson L, Pham-Hung E, Gritti M, Page A, et al. Young infants with severe Tetralogy of Fallot: early primary surgery versus transcatheter palliation. J Thorac Cardiovasc Surg. 2017 Nov;154(5):1692–700.e2. doi:10.1016/j.jtcvs.2017.05.042. [Google Scholar] [PubMed] [CrossRef]
43. Bigdelian H, Ghaderian M, Sedighi M. Surgical repair of Tetralogy of Fallot following primary palliation: right ventricular outflow track stenting versus modified Blalock-Taussig shunt. Indian Heart J. 2018;70(Suppl 3):S394–8. [Google Scholar] [PubMed]
44. Gibbs JL, Rothman MT, Rees MR, Parsons JM, Blackburn ME, Ruiz CE. Stenting of the arterial duct: a new approach to palliation for pulmonary atresia. Br Heart J. 1992;67(3):240–5. doi:10.1136/hrt.67.3.240. [Google Scholar] [PubMed] [CrossRef]
45. Aggarwal V, Petit CJ, Glatz AC, Goldstein BH, Qureshi AM. Stenting of the ductus arteriosus for ductal-dependent pulmonary blood flow-current techniques and procedural considerations. Congenit Heart Dis. 2019;14(1):110–5. doi:10.1111/chd.12709. [Google Scholar] [PubMed] [CrossRef]
46. Bauser-Heaton H, Price K, Weber R, El-Said H. Stenting of the patent ductus arteriosus: a meta-analysis and literature review. J Soc Cardiovasc Angiogr Interv. 2022;1(6):100392. doi:10.1016/j.jscai.2022.100392. [Google Scholar] [PubMed] [CrossRef]
47. Hofferberth SC, Nathan M, Marx GR, Lu M, Sleeper LA, Marshall AC, et al. Valve-sparing repair with intraoperative balloon dilation in Tetralogy of Fallot: midterm results and therapeutic implications. J Thorac Cardiovasc Surg. 2018 Mar;155(3):1163–73.e4. doi:10.1016/j.jtcvs.2017.08.147. [Google Scholar] [PubMed] [CrossRef]
48. Lozano-Balseiro M, Garcia-Vieites M, Martinez-Bendayan I, Garcia-Hernandez I, Cuenca-Castillo JJ, Rueda-Nunez F, et al. Valve-sparing Tetralogy of Fallot repair with intraoperative dilation of the pulmonary valve. mid-term results. Semin Thorac Cardiovasc Surg. 2019;31(4):828–34. doi:10.1053/j.semtcvs.2019.04.007. [Google Scholar] [PubMed] [CrossRef]
49. Alexiou C, Chen Q, Galogavrou M, Gnanapragasam J, Salmon AP, Keeton BR, et al. Repair of Tetralogy of Fallot in infancy with a transventricular or a transatrial approach. Eur J Cardiothorac Surg. 2002 Aug 1;22(2):174–83. doi:10.1016/s1010-7940(02)00295-6. [Google Scholar] [PubMed] [CrossRef]
50. Karl TR, Sano S, Pornviliwan S, Mee RB. Tetralogy of Fallot: favorable outcome of nonneonatal transatrial, transpulmonary repair☆. Ann Thorac Surg. 1992 Nov;54(5):903–7. doi:10.1016/0003-4975(92)90646-l. [Google Scholar] [PubMed] [CrossRef]
51. Padalino MA, Vida VL, Stellin G. Transatrial-transpulmonary repair of Tetralogy of Fallot. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2009:48–53. doi:10.1053/j.pcsu.2009.01.005. [Google Scholar] [PubMed] [CrossRef]
52. Ungerleider RM. Invited letter concerning: right ventricular function after transatrial versus transventricular repair of Tetralogy of Fallot. J Thorac Cardiovasc Surg. 1992 Oct;104(4):1173–4. doi:10.1111/jocs.13236. [Google Scholar] [CrossRef]
53. Dodge-Khatami A, Knirsch W, Tomaske M, Prêtre R, Bettex D, Rousson V, et al. Spontaneous closure of small residual ventricular septal defects after surgical repair. Ann Thorac Surg. 2007 Mar;83(3):902–5. doi:10.1016/j.athoracsur.2006.09.086. [Google Scholar] [PubMed] [CrossRef]
54. Kaushal SK, Radhakrishanan S, Dagar KS, Iyer PU, Girotra S, Shrivastava S, et al. Significant intraoperative right ventricular outflow gradients after repair for Tetralogy of Fallot: to revise or not to revise? Ann Thorac Surg. 1999;68(5):1705–12. doi:10.1016/s0003-4975(99)01069-3. [Google Scholar] [PubMed] [CrossRef]
55. Boni L, García E, Galletti L, Pérez A, Herrera D, Ramos V, et al. Current strategies in Tetralogy of Fallot repair: pulmonary valve sparing and evolution of right ventricle/left ventricle pressures ratio☆. Eur J Cardiothorac Surg. 2009 May 1;35(5):885–90. doi:10.1016/j.ejcts.2009.01.016. [Google Scholar] [PubMed] [CrossRef]
56. Romeo JLR, Etnel JRG, Takkenberg JJM, Roos-Hesselink JW, Helbing WA, Van de Woestijne P, et al. Outcome after surgical repair of Tetralogy of Fallot: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2022;159(1):220–36.e8. doi:10.1016/j.jtcvs.2019.08.127. [Google Scholar] [PubMed] [CrossRef]
57. Miura T, Nakano S, Shimazaki Y, Kobayashi J, Hirose H, Sano T, et al. Evaluation of right ventricular function by regional wall motion analysis in patients after correction of Tetralogy of Fallot. Comparison of transventricular and nontransventricular repairs. J Thorac Cardiovasc Surg. 1992 Oct;104(4):917–23. [Google Scholar] [PubMed]
58. Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of Tetralogy of Fallot: a multicentre study. Lancet Lond Engl. 2000 Sep 16;356(9234):975–81. doi:10.1016/S0140-6736(00)02714-8. [Google Scholar] [PubMed] [CrossRef]
59. Bassareo PP, Mercuro G. QRS complex enlargement as a predictor of ventricular arrhythmias in patients affected by surgically treated Tetralogy of Fallot: a comprehensive literature review and historical overview. ISRN Cardiol. 2013;2013:782508. doi:10.1155/2013/782508. [Google Scholar] [PubMed] [CrossRef]
60. Hamada H, Terai M, Jibiki T, Nakamura T, Gatzoulis MA, Niwa K. Influence of early repair of Tetralogy of Fallot without an outflow patch on late arrhythmias and sudden death: a 27-year follow-up study following a uniform surgical approach. Cardiol Young. 2002 Jul;12(4):345–51. doi:10.1017/s1047951100012944. [Google Scholar] [PubMed] [CrossRef]
61. Stewart RD, Backer CL, Young L, Mavroudis C. Tetralogy of Fallot: results of a pulmonary valve-sparing strategy. Ann Thorac Surg. 2005 Oct;80(4):1431–9. doi:10.1016/j.athoracsur.2005.04.016. [Google Scholar] [PubMed] [CrossRef]
62. Valente AM, Gauvreau K, Assenza GE, Babu-Narayan SV, Schreier J, Gatzoulis MA, et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired Tetralogy of Fallot enrolled in the INDICATOR cohort. Heart Br Card Soc. 2014 Feb;100(3):247–53. doi:10.1136/heartjnl-2013-304958. [Google Scholar] [PubMed] [CrossRef]
63. Ezzat VA, Ryan MJ, O’Leary J, Ariti C, Deanfield J, Pandya B, et al. Radiofrequency ablation of atrial tachyarrhythmias in adults with Tetralogy of Fallot—Predictors of success and outcome. Cardiol Young. 2017 Mar;27(2):284–93. doi:10.1017/S1047951116000482. [Google Scholar] [PubMed] [CrossRef]
64. Cruz C, Pinho T, Ribeiro V, Dias CC, Silva Cardoso J, Maciel MJ. Aortic dilatation after Tetralogy of Fallot repair: a ghost from the past or a problem in the future? Rev Port De Cardiol. 2018 Jul;37(7):549–57. doi:10.1016/j.repc.2017.10.014. [Google Scholar] [PubMed] [CrossRef]
65. Kawasaki Y, Takajo D, Gupta P, Aggarwal S. Higher left ventricular stroke volume is associated with aortic dilatation in repaired Tetralogy of Fallot patients. Cardiol Young. 2024;105:1–6. doi:10.1017/S1047951124026842. [Google Scholar] [PubMed] [CrossRef]
66. Frischhertz BP, Shamszad P, Pedroza C, Milewicz DM, Morris SA. Thoracic aortic dissection and rupture in conotruncal cardiac defects: a population-based study. Int J Cardiol. 2015 Apr 1;184:521–7. doi:10.1016/j.jacc.2013.04.107. [Google Scholar] [CrossRef]
67. Hickey EJ, Veldtman G, Bradley TJ, Gengsakul A, Manlhiot C, Williams WG, et al. Late risk of outcomes for adults with repaired Tetralogy of Fallot from an inception cohort spanning four decades. Eur J Cardiothorac Surg. 2009 Jan 1;35(1):156–64. doi:10.1016/j.ejcts.2008.06.050. [Google Scholar] [PubMed] [CrossRef]
68. Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after Tetralogy of Fallot repair. Am J Cardiol. 2005 Mar;95(6):779–82. doi:10.1016/j.amjcard.2004.11.037. [Google Scholar] [PubMed] [CrossRef]
69. Oosterhof T, Van Straten A, Vliegen HW, Meijboom FJ, Van Dijk APJ, Spijkerboer AM, et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected Tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007 Jul 31;116(5):545–51. doi:10.1161/CIRCULATIONAHA.106.659664. [Google Scholar] [PubMed] [CrossRef]
70. Cavalcanti PEF, Sá MPBO, Santos CA, Esmeraldo IM, de Escobar RR, de Menezes AM, et al. Pulmonary valve replacement after operative repair of Tetralogy of Fallot: meta-analysis and meta-regression of 3118 patients from 48 studies. J Am Coll Cardiol. 2013 Dec 10;62(23):2227–43. doi:10.1016/j.jacc.2013.04.107. [Google Scholar] [PubMed] [CrossRef]
71. Shahanavaz S, Qureshi AM, Levi DS, Boudjemline Y, Peng LF, Martin MH, et al. Transcatheter pulmonary valve replacement with the melody valve in small diameter expandable right ventricular outflow tract conduits. JACC Cardiovasc Interv. 2018 Mar 26;11(6):554. doi:10.1016/j.jcin.2018.01.239. [Google Scholar] [PubMed] [CrossRef]
72. Ghawi H, Kenny D, Hijazi ZM. Transcatheter pulmonary valve replacement. Cardiol Ther. 2012 Dec;1(1):5. doi:10.1007/s40119-012-0005-9. [Google Scholar] [PubMed] [CrossRef]
73. Balzer D. Pulmonary valve replacement for Tetralogy of Fallot. Methodist DeBakey Cardiovasc J. 2019;15(2):122–32. doi:10.14797/mdcj-15-2-122. [Google Scholar] [PubMed] [CrossRef]
74. Lakier JB, Stanger P, Heymann MA, Hoffman JI, Rudolph AM. Tetralogy of Fallot with absent pulmonary valve: natural history and hemodynamic considerations. Circulation. 1974;50(1):167–75. doi:10.1161/01.cir.50.1.167. [Google Scholar] [PubMed] [CrossRef]
75. Welke KF, Ungerleider RM. Repair of Tetralogy of Fallot with absent pulmonary valve syndrome. Oper Tech Thorac Cardiovasc Surg. 2007 Mar 1;12(1):25–35. doi:10.1053/j.semtcvs.2019.05.022. [Google Scholar] [PubMed] [CrossRef]
76. Kreutzer C, Schlichter A, Kreutzer G. Tetralogy of Fallot with absent pulmonary valve: a surgical technique for complete repair. J Thorac Cardiovasc Surg. 1999 Jan 1;117(1):192–4. doi:10.1016/s0022-5223(99)70488-5. [Google Scholar] [PubMed] [CrossRef]
77. Chen JM, Glickstein JS, Margossian R, Mercando ML, Hellenbrand WE, Mosca RS, et al. Superior outcomes for repair in infants and neonates with Tetralogy of Fallot with absent pulmonary valve syndrome. J Thorac Cardiovasc Surg. 2006 Nov 1;132(5):1099–104. doi:10.1016/j.jtcvs.2006.05.049. [Google Scholar] [PubMed] [CrossRef]
78. Hew CC, Daebritz SH, Zurakowski D, del Nido PI, Mayer JE, Jonas RA. Valved homograft replacement of aneurysmal pulmonary arteries for severely symptomatic absent pulmonary valve syndrome. Ann Thorac Surg. 2002 Jun 1;73(6):1778–85. doi:10.1016/s0003-4975(02)03511-7. [Google Scholar] [PubMed] [CrossRef]
79. Talwar S, Divya A, Choudhary SK, Gupta SK, Ramakriahnan S, Kothari SS, et al. Mid-term results of correction of Tetralogy of Fallot with absent pulmonary valve. Indian Heart J. 2017 Nov 1;69(6):767–71. doi:10.1016/j.ihj.2017.04.009. [Google Scholar] [PubMed] [CrossRef]
80. Yong MS, Yim D, Brizard CP, Robertson T, Bullock A, d’Udekem Y, et al. Long-term outcomes of patients with absent pulmonary valve syndrome: 38 years of experience. Ann Thorac Surg. 2014 May;97(5):1671–7. doi:10.1016/j.athoracsur.2014.01.035. [Google Scholar] [PubMed] [CrossRef]
81. Nguyen HH, Jay PY. A single misstep in cardiac development explains the co-occurrence of Tetralogy of Fallot and complete atrioventricular septal defect in down syndrome. J Pediatr. 2014 Jul;165(1):194–6. doi:10.1016/j.jpeds.2014.02.065. [Google Scholar] [PubMed] [CrossRef]
82. Karl TR. Atrioventricular septal defect with Tetralogy of Fallot or double-outlet right ventricle: Surgical considerations. Semin Thorac Cardiovasc Surg. 1997 Jan;9(1):26–34. [Google Scholar] [PubMed]
83. Shuhaiber JH, Robinson B, Gauvreau K, Breitbart R, Mayer JE, Del Nido PJ, et al. Outcome after repair of atrioventricular septal defect with Tetralogy of Fallot. J Thorac Cardiovasc Surg. 2012 Feb;143(2):338–43. doi:10.1016/j.jtcvs.2011.05.031. [Google Scholar] [PubMed] [CrossRef]
84. Lenko E, Kulyabin Y, Zubritskiy A, Gorbatykh Y, Naberukhin Y, Nichay N, et al. Influence of staged repair and primary repair on outcomes in patients with complete atrioventricular septal defect and Tetralogy of Fallot: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2018 Jan 1;26(1):98–105. doi:10.1093/icvts/ivx267. [Google Scholar] [PubMed] [CrossRef]
85. Bastos P, de Leval M, Macartney F, Stark J. Correction of type C atrioventricular canal associated with Tetralogy of Fallot. Thorax. 1978 Oct;33(5):646–8. doi:10.1136/thx.33.5.646. [Google Scholar] [PubMed] [CrossRef]
86. Najm HK, Van Arsdell GS, Watzka S, Hornberger L, Coles JG, Williams WG. Primary repair is superior to initial palliation in children with atrioventricular septal defect and Tetralogy of Fallot. J Thorac Cardiovasc Surg. 1998 Dec;116(6):905–13. doi:10.1016/S0022-5223(98)70040-6. [Google Scholar] [PubMed] [CrossRef]
87. Henmi S, Ryan JA, Mehta R, Haverty MC, Hovis IW, Puente BN, et al. A uniform strategy of primary repair of Tetralogy of Fallot: transventricular approach results in low reoperation rate in the first decade. J Thorac Cardiovasc Surg. 2023;166(6):1731–8.e3. doi:10.1016/j.jtcvs.2023.05.036. [Google Scholar] [PubMed] [CrossRef]
88. Callahan CP, Argo MB, McCrindle BW, Barron DJ, Jegatheeswaran A, Honjo O, et al. Early outcomes for management of atrioventricular septal defect-Tetralogy of Fallot in the last decade: a congenital heart surgeons’ society study. World J Pediatr Congenit Heart Surg. 2024:21501351241293158. doi:10.1177/21501351241293158. [Google Scholar] [PubMed] [CrossRef]
89. Alhawri KA, Mcmahon CJ, Alrih MM, Alzein Y, Khan AA, Mohammed SK, et al. Atrioventricular septal defect and Tetralogy of Fallot—a single tertiary center experience: a retrospective review. Ann Pediatr Cardiol. 2019 Aug;12(2):103–9. doi:10.4103/apc.APC_87_18. [Google Scholar] [PubMed] [CrossRef]
90. Vitanova K, Cleuziou J, Schreiber C, Gunther T, Pabst Von Ohain J, Horer J, et al. Long-term outcome of patients with complete atrioventricular septal defect combined with the Tetralogy of Fallot: staged repair is not inferior to primary repair. Ann Thorac Surg. 2017 Mar;103(3):876–80. doi:10.1016/j.athoracsur.2016.07.038. [Google Scholar] [PubMed] [CrossRef]
91. Krieger EV, Zeppenfeld K, de Witt ES, Duarte VE, Egbe AC, Haeffele C, et al. Arrhythmias in repaired Tetralogy of Fallot: a scientific statement from the american heart association. Circ Arrhythm Electrophysiol. 2022;15(11):e000084. doi:10.1161/HAE.000000000000008492. [Google Scholar] [CrossRef]
92. Miller JR, Stephens EH, Goldstone AB, Glatz AC, Kane L, Van Arsdell GS, et al. The American Association for Thoracic Surgery (AATS) 2022 expert consensus document: management of infants and neonates with tetralogy of fallot. J Thorac Cardiovasc Surg. 2023;165(1):221–50. doi:10.1016/j.jtcvs.2022.07.025. [Google Scholar] [PubMed] [CrossRef]
93. Al Mosa A, Bernier PL, Tchervenkov CI. Considerations in timing of surgical repair in Tetralogy of Fallot. CJC Pediatr Congenit Heart Dis. 2023;2(6):361–7. doi:10.1016/j.cjcpc.2023.10.006. [Google Scholar] [PubMed] [CrossRef]
94. Flores-Umanzor E, Alshehri B, Keshvara R, Wilson W, Osten M, Benson L, et al. Transcatheter-based interventions for Tetralogy of Fallot across all age groups. JACC Cardiovasc Interv. 2024;17(9):1079–90. doi:10.1016/j.jcin.2024.02.009. [Google Scholar] [PubMed] [CrossRef]
95. De Vore GR, Satou GM, Afshar Y, Harake D, Sklansky M. Evaluation of fetal cardiac size and shape: a new screening tool to identify fetuses at risk for Tetralogy of Fallot. J Ultrasound Med: off J Am Inst Ultrasound Med. 2021;40(12):2537–48. doi:10.1002/jum.15639. [Google Scholar] [PubMed] [CrossRef]
96. Brar S, Kang M, Sodhi A, Deyell MW, Laksman Z, Andrade JG, et al. Correlation of ECG and cardiac MRI for assessment of ventricular hypertrophy and dilatation in adults with repaired Tetralogy of Fallot. Int J Cardiol Congenit Heart Dis. 2024;16:100508. doi:10.1016/j.ijcchd.2024.100508. [Google Scholar] [PubMed] [CrossRef]
97. Kangvanskol W, Chungsomprasong P, Sanwong Y, Nakyen S, Vijarnsorn C, Patharateeranart K, et al. Myocardial strain analysis by cardiac magnetic resonance associated with arrhythmias in repaired Tetralogy of Fallot patients. BMC Med Imaging. 2024;24(1):328. doi:10.1186/s12880-024-01514-y. [Google Scholar] [PubMed] [CrossRef]
98. Voskamp SM, Hammonds MA, Knapp TM, Pekmezian AL, Hadley D, Nelson JS, et al. Meta-analysis reveals differential gene expression in Tetralogy of Fallot versus controls. Birth Defects Res. 2024;116(1):e2293. doi:10.1002/bdr2.2293. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools