 Open Access
Open Access
ARTICLE
Right Axillary Thoracotomy vs. Median Sternotomy for Repair of Congenital Heart Defects in Infants and Children
1 Division of Pediatric and Adult Congenital Cardiac Surgery, Department of Surgery, Maria Fareri Children’s Hospital, Westchester Medical Center, New York Medical College, Valhalla, NY 10595, USA
2 Department of Cardiothoracic Surgery, Faculty of Medicine, Alexandria University, Alexandria, 21321, Egypt
3 Division of Pediatric Critical Care, M Health Fairview Health System, Masonic Children’s Hospital, Minneapolis, MN 55454, USA
4 Division of Pediatric Anesthesia, M Health Fairview Health System, Masonic Children’s Hospital, Minneapolis, MN 55454, USA
5 Division of Pediatric Critical Care Medicine, Department of Pediatrics, Masonic Children’s Hospital, University of Minnesota, Minneapolis, MN 55454, USA
6 Department of Cardiothoracic Surgery, Port Said University, Port Said, 42523, Egypt
7 Department of Anesthesiology, University of Minnesota, Minneapolis, MN 55454, USA
* Corresponding Author: Sameh M. Said. Email:
Congenital Heart Disease 2024, 19(6), 563-575. https://doi.org/10.32604/chd.2025.061819
Received 04 December 2024; Accepted 20 January 2025; Issue published 27 January 2025
Abstract
Objective: Vertical right thoracotomy (VRAT) has become an alternative to sternotomy for the repair of non-complex congenital heart defects in our infants and children. Summary Background Data: Limited data exists on the comparison of the two approaches. Methods: The present study consisted of two groups; Group I: (sternotomy; 33 patients) and Group II: (VRAT; 35 patients). We compared the two groups on operative data, hours of invasive lines, narcotics used, length of stay, and total variable cost of stay. Results: The most frequent procedures were atrial and ventricular septal defect closure (25 patients, 75.8% in Group I) and (14 patients, 40% in Group II). The average age and weight were 33.43 ± 53 months, and 14.7 ± 16.9 kg for Group I, and 75.3 ± 60.2 months and 24.9 ± 18 kg for Group II, respectively, (p < 0.001). We found no differences in aortic cross-clamp/bypass times between groups (p = 0.39 and 0.42, respectively). The use of narcotics was not significantly different between the two study groups (p = 0.37) as was the total variable cost (p = 0.115). Group II had a lower time without invasive lines (p < 0.001). In Group II the total length of stay was significantly less as well (p < 0.001). Conclusions: VRAT is a useful technique for repairing a wide range of heart defects and does not result in prolonged cardiopulmonary bypass or aortic cross-clamp times. Although total opioid use and total cost of stay are no different as with sternotomy, the shortened duration of invasive line use, and shorter length of stay make this approach worthy of consideration.Keywords
Abbreviations
| ASD | Atrial septal defect |
| AXC | Aortic cross clamp |
| CHB | Complete heart block |
| CHDs | Congenital heart defects |
| CPB | Cardiopulmonary bypass |
| ICU | Intensive care unit |
| IRB | Institutional review board |
| MS | Median sternotomy |
| NIRS | Near infra-red spectroscopy |
| PAPVC | Partial anomalous pulmonary venous connection |
| VRAT | Vertical right axillary thoracotomy |
| VSD | Ventricular septal defect |
Median sternotomy has traditionally been the preferred approach for the repair of congenital heart defects (CHDs) in the pediatric population. Optimal exposure of all mediastinal structures and safe cannulation for the initiation and conduct of cardiopulmonary bypass (CPB) are essential for a safe and successful repair of CHDs and, while it is anticipated that many of these repairs will be achieved with high success rate and negligible morbidity and reoperation/reintervention rate [1], recent era has witnessed an increased demand for alternative approaches to sternotomy to avoid/minimize visible scars that can leave life-long psychological burden on the child and their parents, in addition to avoiding sternal deformities, a serious complication in infants and young children [2]. While anterior/anterolateral thoracotomy [3] and mini/partial sternotomy [4] approaches have been adopted at times, these approaches have not gained wide popularity due to concerns of possible musculoskeletal/sternal deformity, visible scar, and occasional breast disfigurement especially in prepubescent girls [5].
The emergence of right axillary thoracotomy and its wide-spread adoption for repair of a wide array of CHDs in infants and children [6] has gained global acceptance due to its proven cosmetic superiority and comparable surgical outcomes reported by many institutions [7]. It is a muscle-sparing mini-thoracotomy incision placed underneath the right arm that allows quick recovery and rapid return to normal activity, which makes it very popular among patients and their parents. Concerns with the later approach have been mainly related to the exposure provided, the range of CHDs that can be repaired, possible musculoskeletal deformities from the thoracotomy in growing children and if breast disfigurement in young girls can still occur. Limited true comparisons of right axillary thoracotomy vs. sternotomy in the pediatric population is limited in the literature [8–11].
We have previously reported our early experience and lessons learned from vertical right axillary thoracotomy [12]. Since then, we have adapted the vertical right axillary thoracotomy (VRAT) as our standard approach for all simple heart defects in the pediatric age group and in the current study, we compare the outcomes between the VRAT and sternotomy in children.
This study was conducted in accordance with the Declaration of Helsinki principles. The current study was approved by the University of Minnesota Institutional Review Board (IRB) (#00014940), Date of Approval: 2/16/2022. Individual patient’s consent was not required and was waived by the IRB as the present study was retrospective in nature, involved analyses with minimal-to-no risk, and utilized medical records. Data were collected in a retrospective fashion by reviewing electronic medical records looking at preoperative evaluations, operative reports, hospital course and follow-up visits.
2.1 Patients’ Characteristics (Table 1)
The current study is a single-center study. Inclusion criteria were patients less than 18 years of age, and weight >4.5 kg for the VRAT group with simple heart defects (septal defects, partial anomalous pulmonary venous connections that can be approached through the right chest, simple mitral valve pathology). Those with CHDs >18 years of age, weight <4.5 kg (for VRAT), and more complex heart defects were excluded. Only those who received the VRAT approach were operated upon by the same surgeon. Two groups are included in the current study: Group I with 33 patients who underwent median sternotomy (MS) and Group II with 35 patients who underwent VRAT. Operative data, postoperative outcomes, perioperative invasive lines (PIV) use, postoperative narcotic use, length of stay and variable cost of hospitalization were compared between both groups (Table 1 provides the baseline characteristics of the patients).
All patients were subjected to general endotracheal anesthesia and the use of bilateral cerebral and somatic near infra-red spectroscopy (NIRS) is our standard. Routine lung isolation for VRAT cases was not performed and for the majority of infants and young children, the right lung is easily retracted, and once pericardial stay sutures are placed, the lung is typically out of the way. In older children and teenagers, an endobronchial blocker was used to isolate the right lung.
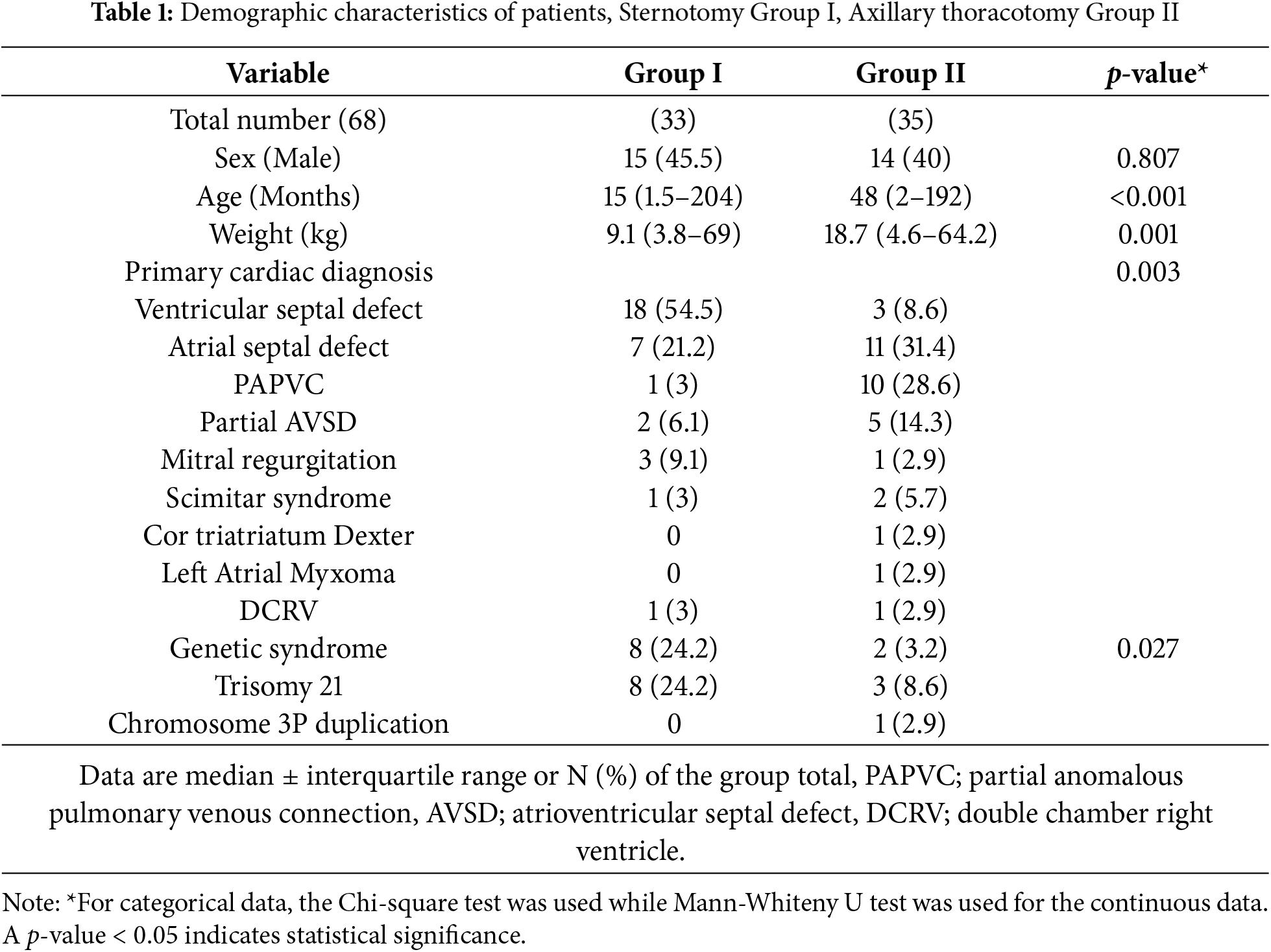
In those undergoing VRAT, routine erector spinae or paravertebral block is performed (Fig. 1A).
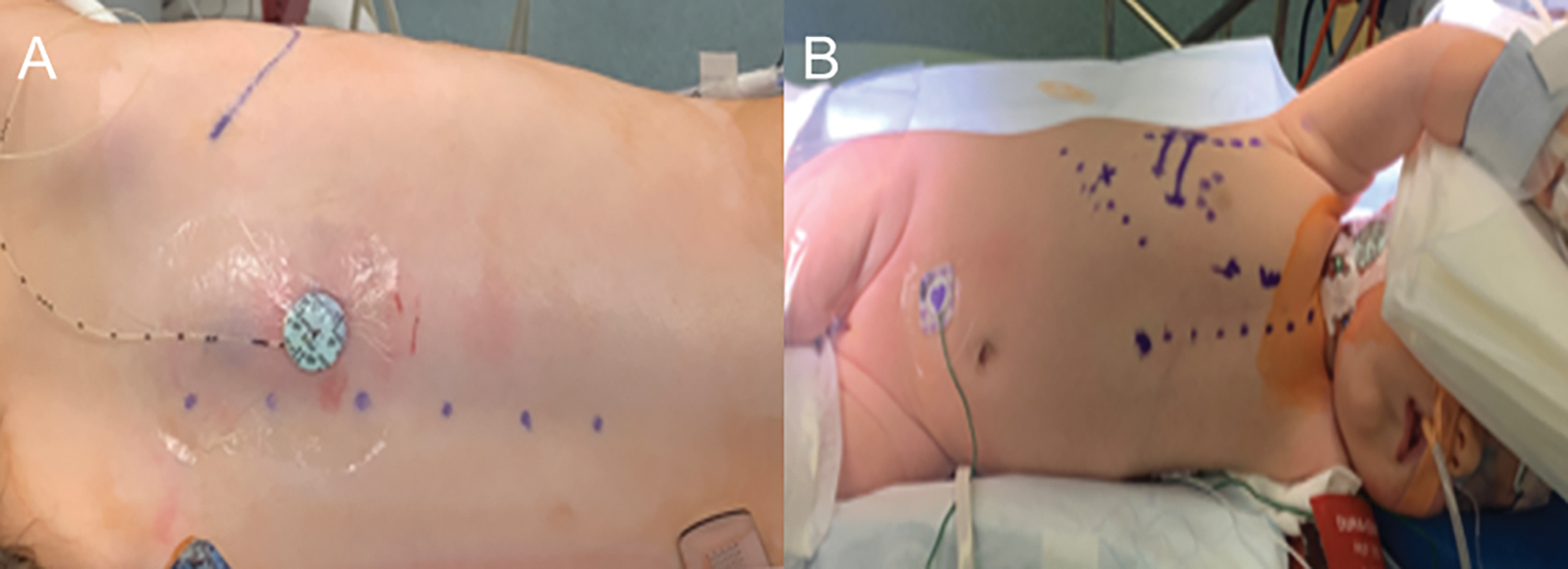
Figure 1: (A & B): Intraoperative photos showing: (A) erector spinae catheter in place for managing postoperative pain, and (B) the patient is positioned in the modified left lateral decubitus position with the right arm above the shoulder. All markings were done in the supine position to ensure accurate location of the axillary incision
For VRAT cases, the incision is marked while the patient is supine prior to definitive positioning. The patient is then positioned into a modified left lateral decubitus with the right arm abducted to expose the axillary region (Fig. 1B). The right groin vessels are marked in anticipation of potential need for groin canulation in patients >45 kg.
The technique for VRAT has been described previously [12]. In summary, the incision is vertical and is created in line with the right mid-axillary line and is typically measures between 5 and 6 cm long and runs approximately from the second to the fifth ribs. Mobilization of the skin and subcutaneous tissues is then performed using electrocautery, creating generous flaps for retraction. Special caution should be exercised when creating these flaps to ensure that the nerve to serratus anterior muscle (long thoracic nerve) does not get injured due to its close position to the serratus anterior muscle. The right chest is subsequently accessed below the third or the fourth ribs, and this depends on the type of heart defect being repaired. For atrial septal defects (ASDs) and pulmonary venous anomalies, repair through the third intercostal space is easily achievable, whereas the fourth intercostal space is suitable for repairing ventricular septal defects (VSDs). Two chest retractors are usually placed perpendicular to each other to provide satisfactory exposure. In teenagers and larger patients, a specially designed retractor for minimally invasive surgery and an adequate size soft tissue retractor can be used. The pericardium is exposed once the right lung is retracted and is incised anterior to the right phrenic nerve. Standard aortic and right atrial/bicaval cannulation is used to initiate CPB. The right femoral vessels were cannulated in one patient.
We have used standard instruments, including the standard aortic cross clamp for infants and young children as the heart and the aorta are much closer proximity to the chest wall than with older children and teenagers. In teenagers and older children, the “Flex Clamp V. Mueller Cosgrove, (McKesson, Irving, TX®)” has been used as it provides the needed length and flexibility to cross clamp the aorta and at the same time can be directed out of the surgical field. In none of our patients, we have used an additional incision to apply the cross clamp.
The same surgical principles applied via MS for repair are followed during VRAT. Prior to weaning off CPB, temporary pacing wires can be placed if needed. Once satisfactory repair is confirmed by transesophageal echocardiogram, all cannulae are removed and protamine is administered. The edges of the pericardium is approximated with a few interrupted sutures. A single drainage tube is usually sufficient. The incision is then closed in layers and the patient is extubated at the end of the procedure.
Data was fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY, USA: IBM Corp.). Categorical data were expressed as numbers and percentages. The Chi-square test was performed for comparison between two groups. Alternatively, the Fisher exact correction test was applied when more than 20% of the cells had an expected count less than 5. Continuous data were tested for normality using the Kolmogorov–Smirnov test. Quantitative data were expressed as range (minimum and maximum), mean, standard deviation, and median for quantitative variables that were not normally distributed. The Mann–Whitney test was used to compare two groups. The significance of the obtained results was judged at the 5% level.
3.1 Baseline Characteristics (Table 1)
In Group I, the mean age and weight were 33.43 ± 53 months, and 14.7 ± 16.9 kg, respectively; while in Group II, the mean age and weight were 75.3 ± 60.2 months and 24.9 ± 18 kg, respectively, (p < 0.001). Defects that were approached through MS (total of 33 defects) included 18 patients (54.5%) with VSDs, seven patients (21.2%) with ASDs, mitral regurgitation in three (9.1%), partial atrioventricular septal defects (PAVSDs) in two (6.1%), while partial anomalous pulmonary venous connections (PAPVCs), double chambered right ventricle (DCRV), and scimitar syndrome occurred in one patient (3%) each. In terms of heart defects that were corrected via VRAT (Total 35 defects), these included 11 patients (31.4%) with ASDs, PAPVCs in 10 (28.6%), PAVSDs in five (14.3%), VSDs in three (8.6%), two patients (5.7%) with scimitar syndrome, while mitral regurgitation, cor triatriatum dexter, left atrial myxoma and DCRV, occurred in one patient (2.9%) each.
With gaining more experience with the VRAT approach, we were able to offer the approach to patients at substantially lower weights (the smallest patient in the current cohort was a 4.6 kg infant with cor triatriatum dexter, Fig. 2A).
Figure 2: (A–D): Intraoperative photos of a few patients who underwent right axillary thoracotomy showing (A) resection of a cor triatriatum dexter, (B) fenestrated atrial septum with multiple secundum septal defects, (C) repair of a membranous ventricular septal defect, and (D) patch closure of secundum atrial septal defect
3.2 Preoperative Interventions and Operative Procedures
In the VRAT group: four patients (11.4%) underwent unsuccessful attempts at transcatheter closure of secundum ASDs in three, and in the fourth, the patient developed sinus node dysfunction as a result of placing a large device. This last patient underwent VRAT with explantation of the device and repairing the defect which led to subsequent recovery of the sinus node function. Catheter ablation of supraventricular arrhythmias was performed in one patient prior to the closure of the ASD.
Procedures performed through MS included patch closure of ASDs and VSDs (25 patients; 75.8%), mitral valve repair in three (9.1%), repair of PAPVCs including scimitar syndrome in two (6.1%), repair of PAVSDs in another two (6.1%), and repair of DCRV in one (3%). Through VRAT, procedures performed included repair of PAPVCs in 12 patients (34.2%, six intra-atrial baffle, four Warden, and two 2-patch repair for scimitar; Fig. 3A–D), patch closure of ASDs in 11 patients (31.4%; Fig. 2B,D), repair of PAVSDs in five (14.3%), VSDs patch closure in three (8.6%; Fig. 2C), while cor triatriatum resection, repair of DCRV, resection of left atrial myxoma, and mitral valve repair was performed in one patient (2.9%) each. Right femoral vessels cannulation was performed only in one patient (62 kg).
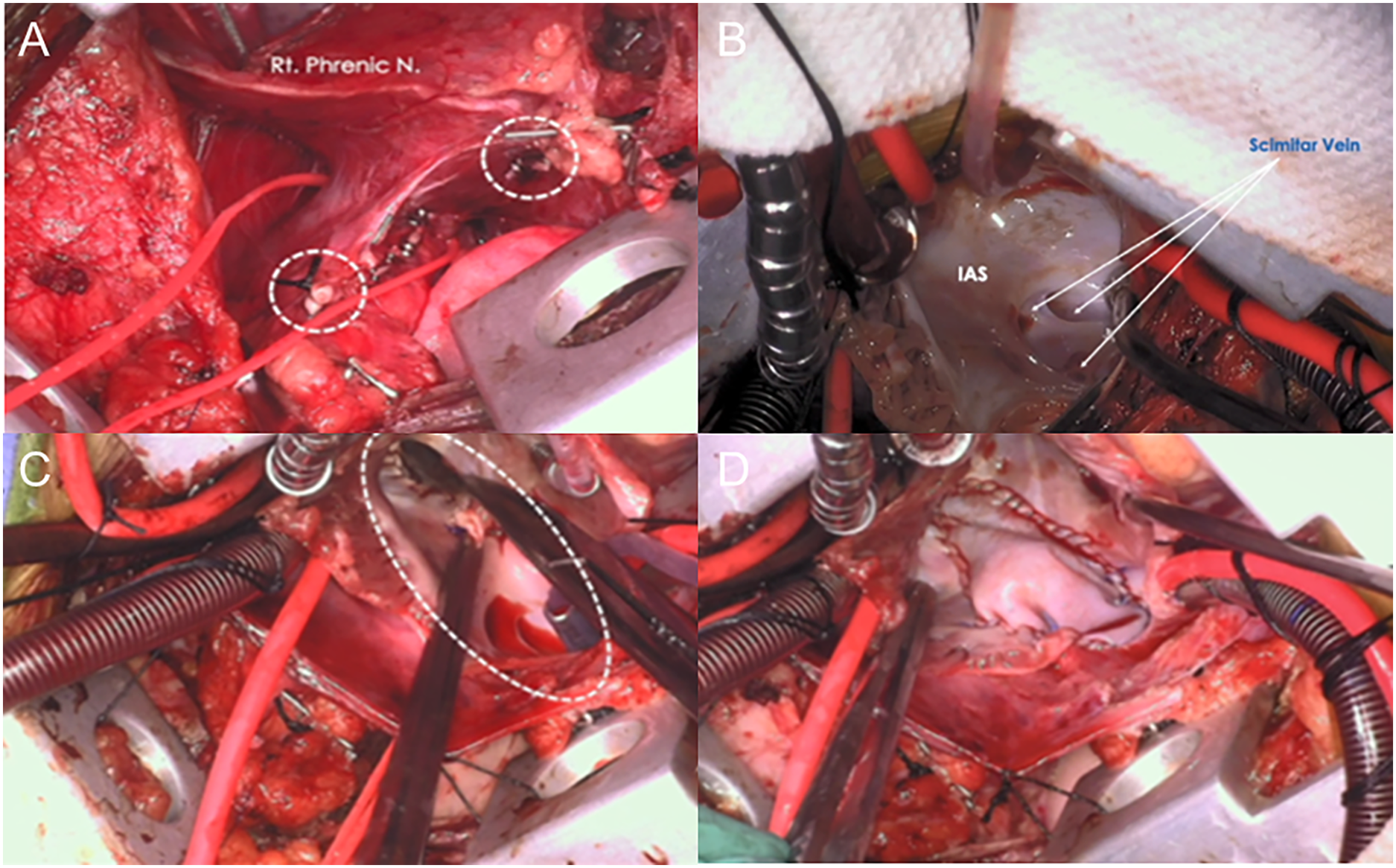
Figure 3: (A–D): Repair of a scimitar syndrome via thoracotomy: (A) the scimitar vein (red vessel loop), and the aorto-systemic collaterals (white circles) are seen, (B) right atriotomy, (C) an atrial septal defect is created, and (D) the intra-atrial baffle is completed. Rt.: right; N: nerve; IAS: interatrial septum
A list of the surgical procedures that were performed is provided in Table 2.
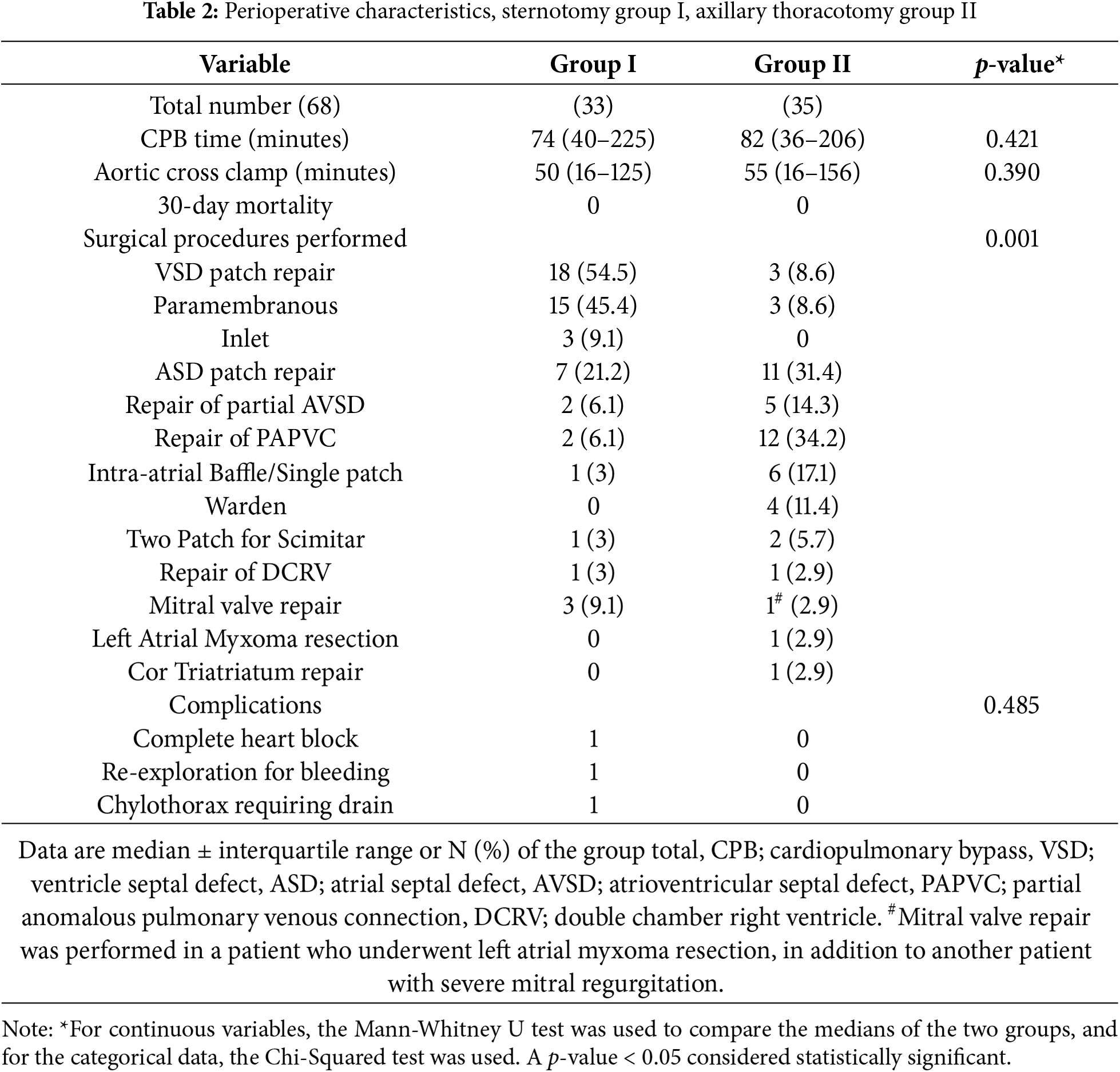
There was no early mortality in either group. There was no conversion to MS in the VRAT group. All patients were extubated in the operating room and followed an enhanced recovery protocol in both groups. The aortic cross clamp (AXC) and CPB times did not differ significantly between the two groups (51.2 ± 24.6 and 79.8 ± 37.3 min in Group I and 58.1 ± 31.5 and 88.1 ± 43 min in Group II, (p = 0.39 and 0.42), respectively).
One patient in each group underwent early reoperation due to postoperative bleeding. One patient in the MS cohort developed complete heart block (CHB) after VSD closure and had an epicardial permanent pacemaker implanted. A child with trisomy 21 syndrome, who underwent membranous VSD repair via MS, developed a chylous effusion but responded to conservative management. Two patients in the VRAT group underwent a second run of CPB to close residual defects.
In the VRAT group, six patients (17.1%) experienced post-pericardiotomy pericarditis manifested as pericardial rub, and ST segment elevation on electrocardiogram but no effusion This was managed with nonsteroidal anti-inflammatory drugs and in one patient colchicine was used with resolution of symptoms. Transient junctional rhythm occurred in four patients (11.4%) and resolved in three of them prior to hospital discharge. Pneumothorax occurred in one patient after removal of the chest drain and resolved with insertion of a pig-tail catheter.
No significant difference in the total postoperative opioid use between the two groups (p = 0.37). The duration of postoeprative invasive lines use in Group II was significantly shorter than in Group I (p < 0.001 and p = 0.004 for the arterial and central lines, respectively).
The intensive care unit (ICU) length of stay was significantly shorter in Group II (p = 0.0072) (Fig. 4A), and so as the total length of hospitalization (Group II: 81.1 ± 43 h vs. Group I: 175.3 ± 293.3 h; p = 0.0001) (Fig. 4B). Of note, all patients who underwent VRAT were discharged directly from the intensive care unit (ICU) due to their brief length of stay. As for the total variable cost for the stay, there was no statistically significant difference between the two groups (p = 0.115).
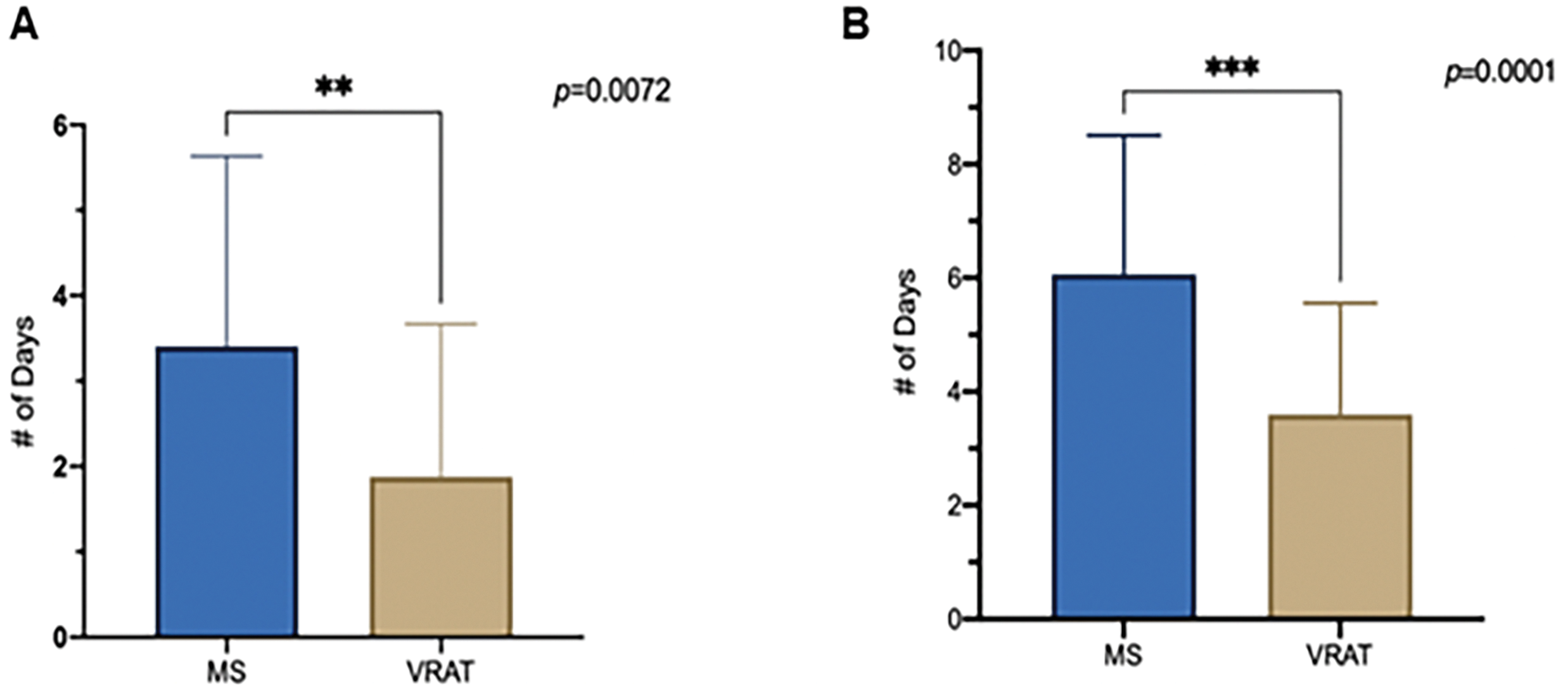
Figure 4: (A), Intensive care unit length of stay, (B), Total hospitalization length of stay, (MS) Median Stemotomy vs. Vertical Right Axillary Thoracotomy (VRAT)
Follow-up was complete in all patients with a mean follow-up of 30 ± 5 months and was complete in all patients. This entailed routine clinic visits a week after hospital discharge, phone calls/clinic visit at 1, 3, and 6 months and then yearly thereafter.
No late mortality. A small seroma occurred in one patient in the VRAT group, and it resolved spontaneously. Junctional rhythm persisted in one patient who underwent an epicardial pacemaker placement almost five months after her initial surgery. In the MS group, one patient with scimitar syndrome experienced pulmonary venous obstruction and required repeat operation after one year.
Patients/parents were satisfied with the cosmetic aspect of the incision (Fig. 5) and the quick return to full activities.
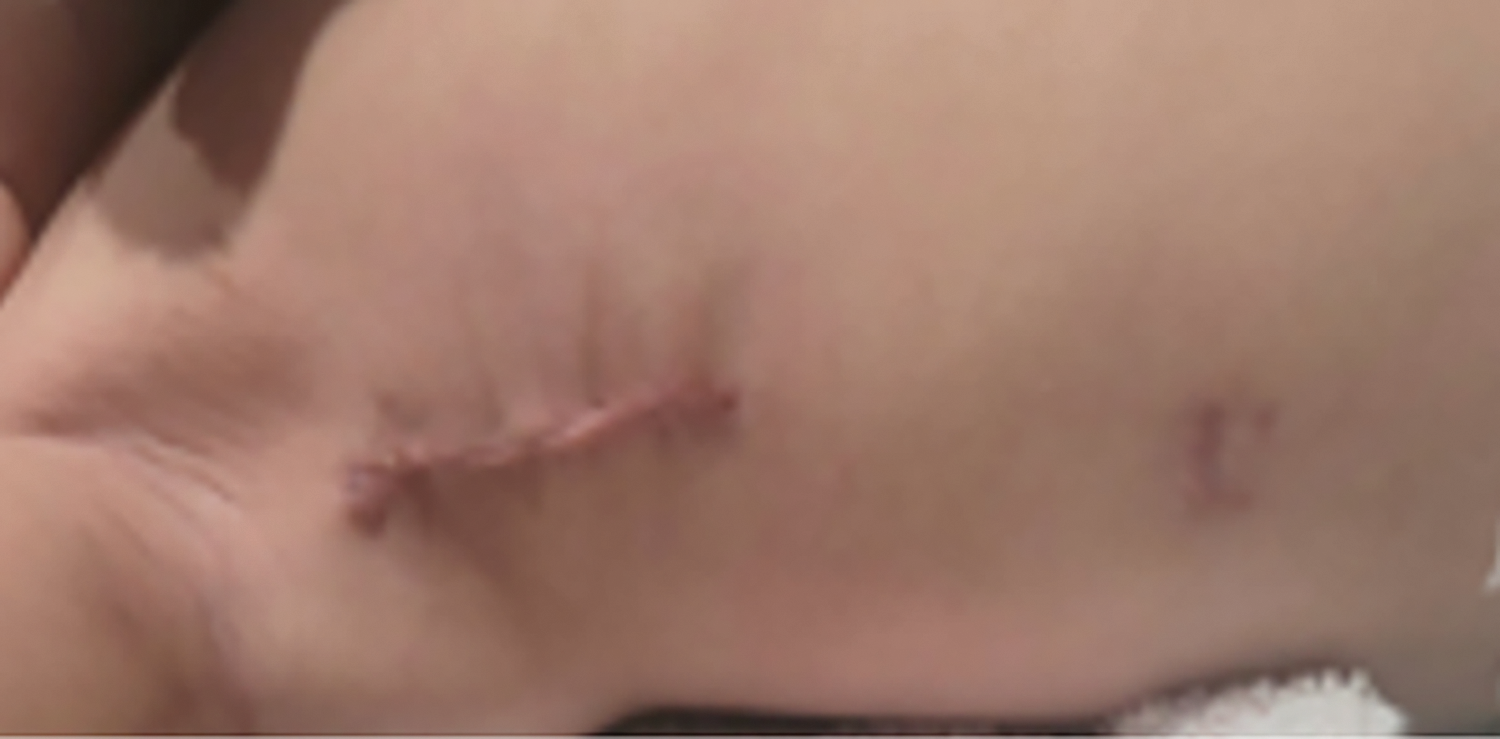
Figure 5: Patient’s photo showing a well-healed right thoracotomy incision only two weeks after surgical repair of membranous ventricular septal defect
3.6 Echocardiographic Follow-Up
Subsequent echocardiographic studies revealed satisfactory repairs in both groups with no significant residual lesions. One patient in the VRAT group had a small residual ventricular level shunt but did not require repeat operation. In other patients, all pulmonary (but one in the MS group) and systemic venous anatomical pathways were patent without significant gradients.
Unlike its adult counterparts, the advances in minimally invasive/minimal access approaches for pediatric cardiac surgery have lagged [13]. Although alternative surgical approaches to MS like anterolateral (mini-) thoracotomy, and ministernotmy have been employed for several decades by many surgeons/centers [14], they have many drawbacks in the pediatric population and in our view, are not aesthetically superior to MS. In fact, in the study by Bleiziffer and colleagues, right anterolateral thoracotomy when performed in prepubescent girls was tied to late asymmetric breast development [5].
The axillary approach for repairing a wide variety of CHDs have been previously reported [15,16]. This approach has several advantages as previously shown by Dodge-Khatami and colleagues [2]. This includes superior cosmesis due to the hidden incision under the right arm, and by being vertical, it lies away from any developing breast tissue, therefore the risk of future breast disfigurement is quite low [17]. This incision allows rapid recovery of the arm/shoulder movements and subsequent rapid return to full activity due to its muscle-sparing nature. The chance of future skeletal deformities, especially scoliosis, is also low as there is no rib cutting and the spreading of the ribs is minimal. The VRAT approach was in fact, popularized by thoracic surgeons several decades ago for the same reasons [18].
We used the VRAT approach initially for closure of secundum ASDs, but as we gained more experience, we tackled other defects such as pulmonary venous anomalous connections [19], VSDs, atrioventricular septal defects, and resection of certain cardiac tumors such as atrial myxomas [20] Our previous study highlighted our initial experience with this approach. But now, a true comparison with MS is warranted.
To minimize variability, and ensure a fair comparison between the two approaches, only patients undergoing simple CHDs were included in the present study. In our experience, many of the patients with those defects who fall into STAT Categories I and II are amenable to repair of these defects via the VRAT approach, with excellent outcomes and minimal morbidities, comparable to MS.
While the two groups of patients in the current study seem to have a vast age and weight different, that was part of our initial experience with the VRAT approach, with time and experience in VRAT, our inclusion criteria broadened and now includes patients as young as 2 months of age and 4.5 kg in weight. In the present study, we maintained the same techniques of repairs in both groups. Central cannulation was always our goal with the exception of one patient who needed a groin cannulation in the VRAT group due to her weight (62 kg) and body habitus, where the aorta was further away from the chest wall. Dave et al. [16] performed repair of various CHDs with a 4–5 cm incision parallel to the intercostal space. In the vast majority of patients, iliac vessels cannulation was used with the youngest patient being 12 kg. They also utilized ventricular fibrillation during ASD and PAPVC repairs. In their series, one neurologic was noted where left hemiparesis ensued. We have crossed clamp the aorta in all our patients to minimize any risks related to inadequate de-airing or use of fibrillatory arrest. While it frees up the operative field and can make the incision even smaller, we have not favored peripheral cannulation. We think that, particularly in infants/young children, all phases of the procedure can be conducted safely through the same chest incision.
There was no mortality or major complication in either group which speaks about the safety of VRAT. Despite the beliefs that minimally invasive approaches could or may be associated with longer CPB/AXC times, this was not the case in the current study where there was no difference between the two groups. The VRAT procedure can be safely conducted, and the repair is performed in a similar manner to MS [21]. The outcomes and postoperative recovery were compared between VRAT and MS in 59 and 77 patients respectively in the study by Hu et al. [22]. The pump time and the duration of postoperative mechanical ventilation were similar between both groups. Nevertheless, the operative time, incision length, postoperative cheat tubes drainage and length of hospital stay were significantly shorter in the VRAT group. The authors concluded that VRAT is as safe as conventional MS with no increased morbidities, in addition to its superiority in terms of the hidden incision, and postoperative rapid recovery.
Another important finding in the present study was the significantly shorter duration of use of invasive lines as well as the significantly shorter duration of hospital stay in the VRAT group compared to MS. This is a major benefit of the use of VRAT where it truly represents an enhanced recovery approach for children after heart surgery. All patients were extubated in the operating room in both groups, which is our routine in simple defects regardless of the approach, but the ability of patients in one group (VRAT) to leave the hospital in such a short of a time frame, speaks to the merits of this technique. This has future implications for the timing of surgery and the go-to strategy for many of these patients with simple CHDs. We believe the VRAT approach may alter the balance of transcatheter device closure for ASDs and surgery as the gap between these two modalities with respect to cosmesis and length of stay appears to be closing significantly.
Our postoperative pain management has evolved over time as we gained more experience with VRAT. Our anesthesia team has placed intra-operatively and left in-situ erector spinae/paravertebral regional anesthesia catheter to manage incisional pain during the postoperative period. In the present study, narcotics use postoperatively was found to be the same between the two groups. This can be interpreted as thoracotomy is not associated with worse pain compared to MS as perceived by many. Having ensured safety of the VRAT approach, we began to view postoperative analgesia in these patients in a different way. We have gone away from using regional analgesia catheters and we use a combination of non-steroidal anti-inflammatory drugs with minimal/no narcotic use postoperatively in addition to caudal block with morphine and erector spinae block intraoperatively. This will be the subject of a future study.
The quality of the repair and the technique were the same as they would be via MS. This was one of our goals during the transition from MS to VRAT. We do believe that in any minimally invasive approach, it is essential not to jeopardize or alter the technique of repair for the sake of a more cosmetically pleasing incision. In the current study, two patients required a second run of bypass to close residual defects. The first patient was a teenager with a large ASD and had undergone transcatheter ablation of supraventricular arrhythmia 48 h before. The patch partially dehisced at its inferior margin and needed to be reinforced through a second pump run. We question whether we should have waited a little longer before performing the surgery to allow the tissues to heal and gain enough strength after the ablation. In a second patient with DORV, the residual defect occurred at the superior margin of the VSD patch and was close to the aortic valve. We do believe these cases may be better served with a standard MS when there is a need to create a long intraventricular tunnel. Overall, no patient required repeat operation in the VRAT group to correct any residual or recurrent defects.
In the series by Dave et al. [16], three patients required a second run of bypass. Five had residual shunts noted on echocardiography, but the repair was felt to be potentially definitive. We have one patient who is being followed for a small ventricular-level shunt.
All patients/parents in the VRAT group were satisfied with the incision and the cosmetic superiority of the VRAT.
We have demonstrated in the current study that the advantages of VRAT extend beyond the cosmetic superiority and includes significantly shorter duration of invasive lines use and shorter length of hospital stay while maintaining safety and surgical outcomes.
In conclusion, VRAT is a safe and effective alternative to MS for repairing several heart defects in infants and children. It is associated with superior cosmetic outcomes; significantly shorter duration of use of invasive line, and reduced hospital length of stay and is not associated with increased cardiopulmonary bypass or cross clamp times.
The current study may represent the basis for multiple future studies related to the VRAT approach. Several questions remain that will require larger studies with more patients to answer. Further studies will also evaluate the postoperative narcotic use for pain management in those undergoing sternotomy compared to VRAT, postoperative transfusion of blood and blood products between the two groups, and longer follow-up is required to determine the esthetic properties of the incision especially in young females and the effect on musculoskeletal/breast development.
The current study is limited by its retrospective nature and the small number of patients in each group, but it was important to compare this earlier experience with VRAT vs. sternotomy to answer several pressing questions related to safety and early outcomes.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Sameh M. Said conceptualized and designed the study, drafted the initial manuscript, and critically reviewed and revised the manuscript. Kristin C. Greathouse, Christina McCarthy, Megan Khan, and Molly Hagen designed the data collection instruments, collected data, and critically reviewed and revised the manuscript. Nicholas Brown, and Sacha Kumar critically reviewed and revised the manuscript. Mahmoud I. Salem carried out the initial analyses, and critically reviewed and revised the manuscript. James Flaherty critically reviewed and revised the manuscript. Yasin Essa critically reviewed and revised the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All relevant data are provided in the current manuscript, and any additional information can be provided upon request to the corresponding author.
Ethics Approval: This study was performed in line with the principles of the Declaration of Helsinki. Institutional Review Board (IRB) at the University of Minnesota approved the current study (#00014940), Date of Approval: 2/16/2022. An individual patient consent was waived by the IRB due to the retrospective nature of the study and the minimal-to-no risk involved in chart reviews.
Conflicts of Interest: The first author (Sameh M. Said) is a Consultant for Artivion but no financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
References
1. European Congenital Heart Surgeons Association Congenital Database. Specific procedures harvest, 1999–2022. [cited 2025 Jan 19]. Available from: http://www.echsacongenitaldb.org. [Google Scholar]
2. Dodge-Khatami J, Dodge-Khatami A. Advantages of a mini right axillary thoracotomy for congenital heart defect repair in children. Cardiol Young. 2022;32(2):276–81. doi:10.1017/S1047951121001979. [Google Scholar] [PubMed] [CrossRef]
3. Rosengart TK, Stark JF. Repair of atrial septal defect through a right thoracotomy. Ann Thorac Surg. 1993;55(5):1138–40. doi:10.1016/0003-4975(93)90020-I. [Google Scholar] [PubMed] [CrossRef]
4. Seipelt RG, Popov A, Danner B, Paul T, Tirilomis T, Schoendube FA, et al. Minimally invasive partial inferior sternotomy for congenital heart defects in children. J Cardiovasc Surg. 2010;51(6):929–33. [Google Scholar]
5. Bleiziffer S, Schreiber C, Burgkart R, Regenfelder F, Kostolny M, Libera P, et al. The influence of right anterolateral thoracotomy in prepubescent female patients on late breast development and on the incidence of scoliosis. J Thorac Cardiovasc Surg. 2004;127(5):1474–80. doi:10.1016/j.jtcvs.2003.11.033. [Google Scholar] [PubMed] [CrossRef]
6. Silva LD, Silva JP, Turquetto ALR, Franchi SM, Cascudo CM, Castro RM, et al. Horizontal right axillary minithoracotomy: aesthetic and effective option for atrial and ventricular septal defect repair in infants and toddlers. Rev Bras Cir Cardiovasc. 2014;29(2):123–30. doi:10.5935/1678-9741.20140028. [Google Scholar] [PubMed] [CrossRef]
7. Yan L, Zhou ZC, Li HP, Lin M, Wang HT, Zhao ZW, et al. Right vertical infra-axillary mini-incision for repair of simple congenital heart defects: a matched-pair analysis. Eur J Cardiothorac Surg. 2013;43(1):136–41. doi:10.1093/ejcts/ezs280. [Google Scholar] [PubMed] [CrossRef]
8. Li F, Cheng T, Yan M, Li T, Zhang T, Huang Y, et al. Analysis of the therapeutic effect of right mid-axillary approach in the surgical treatment of ASD and VSD in children. J Cardiothorac Surg. 2024;19(1):587. doi:10.1186/s13019-024-03105-y. [Google Scholar] [PubMed] [CrossRef]
9. Lo Rito M, Brindicci YCM, Moscatiello M, Varrica A, Reali M, Saracino A, et al. Minimally invasive surgery for simple Congenital Heart Defects: preserving aesthetics without jeopardizing patient safety. J Cardiovasc Dev Dis. 2023;10(11):452. doi:10.3390/jcdd10110452. [Google Scholar] [PubMed] [CrossRef]
10. Yang X, Hu Y, Dong J, Huang P, Luo J, Yang G, et al. Right vertical axillary incision for atrial septal defect: a propensity score matched study. J Cardiothorac Surg. 2022;17(1):256. doi:10.1186/s13019-022-01999-0. [Google Scholar] [PubMed] [CrossRef]
11. Luo ZR, Chen Q, Yu LL, Chen LW, Huang ZY. Comparative study between surgical repair of Atrial Septal Defect via median sternotomy, right submammary thoracotomy, and right vertical infra-axillary thoracotomy. Braz J Cardiovasc Surg. 2020;35(3):285–90. doi:10.21470/1678-9741-2019-0096. [Google Scholar] [PubMed] [CrossRef]
12. Said SM, Greathouse KC, McCarthy CM, Brown N, Kumar S, Salem MI, et al. Safety and efficacy of right axillary thoracotomy for repair of Congenital Heart Defects in children. World J Pediatr Congenit Heart Surg. 2023;14(1):47–54. doi:10.1177/21501351221127283. [Google Scholar] [PubMed] [CrossRef]
13. Dodge-Khatami J, Dodge-Khatami A, Nguyen TD, Rüffer A. Minimal invasive approaches for pediatric&congenital heart surgery: safe, reproducible, more cosmetic than through sternotomy, and here to stay. Transl Pediatr. 2023;12(9):1744–52. doi:10.21037/tp-23-282. [Google Scholar] [PubMed] [CrossRef]
14. Liu H, Wang Z, Xia J, Hu R, Wu Z, Hu X, et al. Evaluation of different minimally invasive techniques in surgical treatment for Ventricular Septal Defect. Heart Lung Circ. 2018;27(3):365–70. doi:10.1016/j.hlc.2017.01.014. [Google Scholar] [PubMed] [CrossRef]
15. An K, Li S, Yan J, Wang X, Hua Z. Minimal right vertical infra-axillary incision for repair of Congenital Heart Defects. Ann Thorac Surg. 2022;113(3):896–902. doi:10.1016/j.athoracsur.2021.01.052. [Google Scholar] [PubMed] [CrossRef]
16. Dave HH, Comber M, Solinger T, Bettex D, Dodge-Khatami A, Prêtre R. Mid-term results of right axillary incision for the repair of a wide range of congenital cardiac defects. Eur J Cardiothorac Surg. 2009;35(5):864–9. doi:10.1016/j.ejcts.2009.01.022. [Google Scholar] [PubMed] [CrossRef]
17. Schreiber C, Bleiziffer S, Kostolny M, Hörer J, Eicken A, Holper K, et al. Minimally invasive midaxillary muscle sparing thoracotomy for atrial septal defect closure in prepubescent patients. Ann Thorac Surg. 2005;80(2):673–6. doi:10.1016/j.athoracsur.2005.03.020. [Google Scholar] [PubMed] [CrossRef]
18. Baeza OR, Foster ED. Vertical axillary thoracotomy: a functional and cosmetically appealing incision. Ann Thorac Surg. 1976;22(3):287–8. doi:10.1016/S0003-4975(10)64918-1. [Google Scholar] [PubMed] [CrossRef]
19. Said SM. Vertical right axillary thoracotomy for a modified Warden procedure with a descending thoracic aortic homograft: tips and pitfalls. Multimed Man Cardiothorac Surg. 2021;2021. doi:10.1510/mmcts.2021.070. [Google Scholar] [PubMed] [CrossRef]
20. Trager LE, Miranda C, Kloesel B, Said SM. Resection of a large left atrial myxoma combined with mitral valve repair via a vertical right axillary thoracotomy in a pediatric patient. Multimed Man Cardiothorac Surg. 2021;2021. doi:10.1510/mmcts.2021.081. [Google Scholar] [PubMed] [CrossRef]
21. Tran DM, Tran VQ, Nguyen MT, Mai DD, Doan AV, Hoang ST, et al. Minimally invasive surgical repair of simple Congenital Heart Defects using the right vertical infra-axillary thoracotomy approach. Innovations. 2024;19(5):520–25. [Google Scholar] [PubMed]
22. Hu CX, Tan J, Chen S, Ding H, Xu ZW. Comparison of clinical outcomes and postoperative recovery between two open heart surgeries: minimally invasive right subaxillary vertical thoracomy and traditional median sternotomy. Asian Pac J Trop Med. 2014;7(8):625–9. doi:10.1016/S1995-7645(14)60105-X. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools