 Open Access
Open Access
REVIEW
Surgical Ablation in Congenital Heart Disease: Advances in Techniques and Clinical Outcomes
1 Department of Cardiology, Erasmus Medical Center, Rotterdam, 3015GD, The Netherlands
2 Department of Cardiology, UT Southwestern Medical Center, Dallas, TX 75390, USA
3 Department of Cardiothoracic Surgery, Erasmus Medical Center, Rotterdam, 3015GD, The Netherlands
* Corresponding Author: Natasja M. S. de Groot. Email:
# Both authors contributed equally to this manuscript
Congenital Heart Disease 2024, 19(6), 577-592. https://doi.org/10.32604/chd.2025.062129
Received 11 December 2024; Accepted 20 January 2025; Issue published 27 January 2025
Abstract
Surgical ablation (SA) has become an essential rhythm-control strategy for managing tachyarrhythmias in patients with congenital heart disease. Atrial tachyarrhythmias, such as atrial flutter and atrial fibrillation, are prevalent in congenital heart disease, affecting up to 50% of patients, and pose significant risks, including increased morbidity and mortality. Ventricular tachyarrhythmias, though less common, can lead to sudden cardiac death, particularly in conditions like Tetralogy of Fallot. Prior studies suggested that SA for tachyarrhythmias in patients with congenital heart disease offers significant benefits, including superior long-term rhythm control compared to catheter ablation (CA). Atrial tachyarrhythmia burden is effectively reduced by SA, such as the Cox-Maze IV procedure, thereby also improving the quality of life. Ablation strategies targeting critical isthmuses in the ventricles, such as between the tricuspid valve annulus and right ventricular outflow tract scar in patients with Tetralogy of Fallot, are effective in managing ventricular tachyarrhythmias. However, SA is associated with procedural risks, such as sinus node dysfunction and atrioventricular conduction block. Despite these risks, SA is recommended for congenital heart disease patients with symptomatic tachyarrhythmias unresponsive to drug therapy and CA, and it is increasingly considered as a prophylactic procedure in high-risk patients. Emerging innovative technologies, including enhanced mapping- and hybrid ablation techniques, are poised to further improve SA outcomes. The goal of this review is to assess the efficacy, outcomes, and risks of SA in congenital heart disease patients, compare the outcomes of SA with CA, and evaluate the risk-benefit ratio of (prophylactic) arrhythmia surgery. In summary, SA offers significant benefits, including long-term arrhythmia control and improved quality of life, particularly in patients in whom CA is unfeasible or has failed. Although SA is currently underutilized in clinical practice, the potential as a comprehensive treatment for specific patients with congenital heart disease underscores the importance of a patient tailored approach.Graphic Abstract
Keywords
Abbreviations
| CHD | Congenital Heart Disease |
| AFL | Atrial flutter |
| AF | Atrial fibrillation |
| ToF | Tetralogy of Fallot |
| CA | Catheter ablation |
| SA | Surgical ablation |
| VT | Ventricular tachyarrhythmia |
| AP | Accessory pathway |
| EA | Ebstein’s Anomaly |
| LA | Left atrium |
| RA | Right atrium |
| ASD | Atrial septal defect |
Over the past three decades, the life expectancy of patients with congenital heart disease (CHD) has increased due to advances in surgical techniques and clinical care [1]. Throughout life, CHD patients are at high risk for redo-surgery, not only for residual lesions, but also for acquired cardiovascular diseases such as coronary artery disease. The prevalence of both atrial and ventricular tachyarrhythmias in CHD patients undergoing redo-surgery may be considerable. As demonstrated in Fig. 1, atrial tachyarrhythmias, including atrial flutter (AFL) and atrial fibrillation (AF), occur in up to 60% of the patients with CHD [2] and are associated with significant morbidity and mortality [3]. In CHD patients, AF arises at a relatively younger age compared to patients without CHD and progresses more rapidly [4]. Ventricular tachyarrhythmias, although less common, can significantly increase the risk of sudden death in conditions like Tetralogy of Fallot (ToF). Overall, approximately 20% to 25% of late mortality in adult CHD patients is attributable to sudden cardiac events resulting from ventricular tachyarrhythmias (VTs) [5].
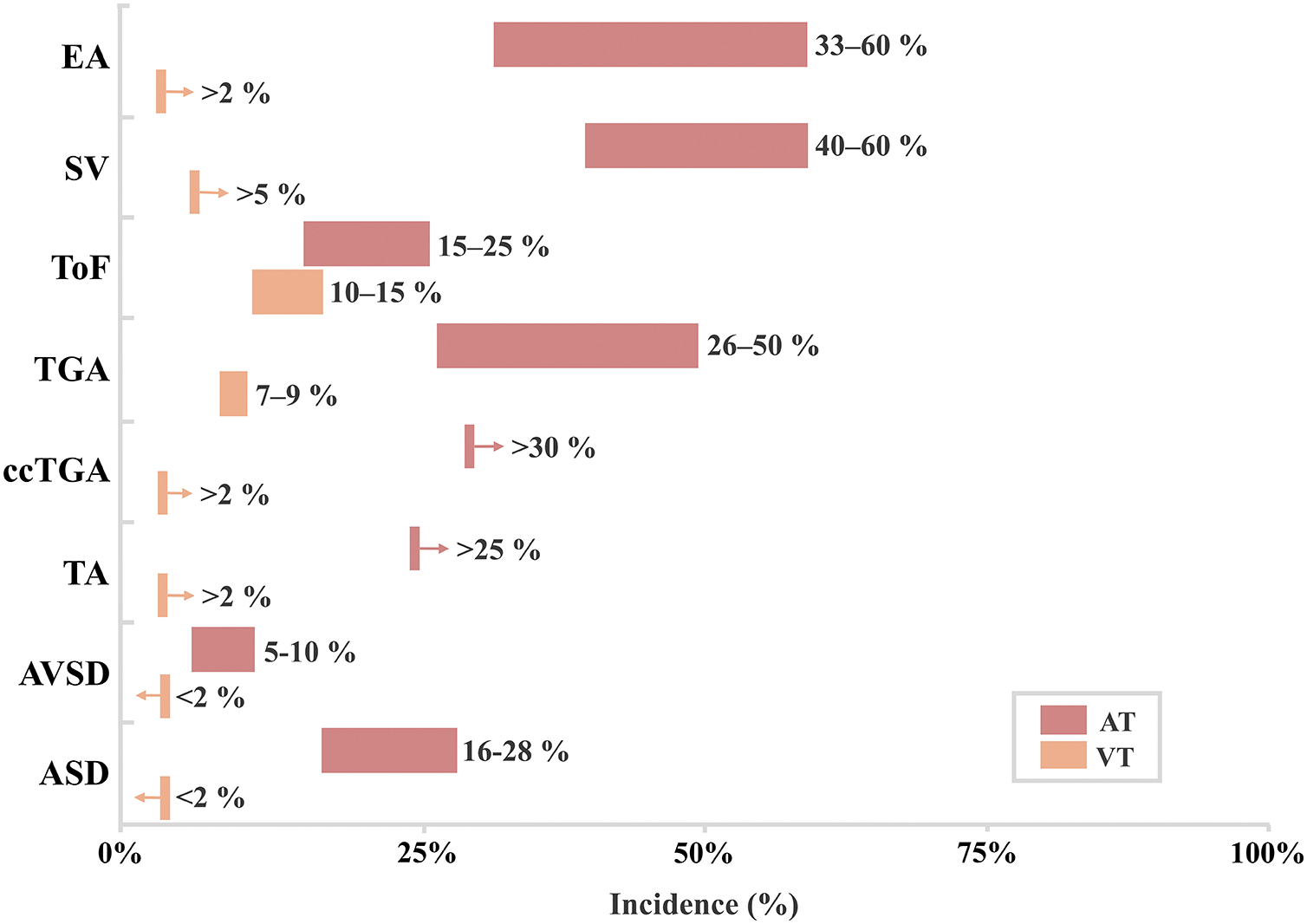
Figure 1: Incidence of tachyarrhythmias in patients with adult congenital heart disease [3,4]. AT = Atrial tachyarrhythmia, VT = Ventricular tachyarrhythmia, EA = Ebstein anomaly, SV = Single ventricle, ToF = Tetralogy of Fallot, TGA = Transposition of the great arteries, atrial switch, ccTGA = Congenitally corrected transposition of the great arteries, TA = Truncus arteriosus, AVSD = Atrioventricular septal defect, ASD = Atrial septal defect
Technological and procedural advancements have transformed catheter ablation (CA) and surgical ablation (SA) into well-established rhythm-control therapies for tachyarrhythmias [6]. The effectiveness of CA in CHD patients is hampered by altered anatomy and complex arrhythmogenic substrates, induced by atrial volume or pressure overload, surgical scars and prosthetic materials. [2,5,7,8].
If CA fails in achieving rhythm-control, SA becomes an important treatment modality. Current guidelines endorse SA for patients with symptomatic tachyarrhythmias who are undergoing concomitant surgical repair and have tachyarrhythmia refractory to both antiarrhythmic drugs and CA [9,10].
Beyond treating existing tachyarrhythmias, there is growing interest in the potential for prophylactic SA during surgical cardiac repair. Currently, limited knowledge is available on (1) how to identify patients at risk for tachyarrhythmias, (2) what the outcome of prophylactic SA for specific CHD and tachyarrhythmia substrates are, and (3) what the overall risk-benefit ratio of incorporating prophylactic SA into surgical cardiac repair is [2].
The role of SA in tachyarrhythmia management has evolved significantly since its early applications. In 1968, Sealy and colleagues performed the first successful SA of a manifest accessory pathway (AP) [11]. Subsequent advancements in SA included epicardial and endocardial approaches to treat tachyarrhythmias such as atrioventricular reentrant tachycardia, atrioventricular nodal reentrant tachycardia, and VT. The Cox-Maze procedure, introduced in the late 1980s, was a pivotal advancement for treating AFL and AF. Initially performed using a ‘cut-and-sew’ technique, the procedure strategically created surgical atrial lesions to disrupt abnormal electrical circuits and prevent AF [12]. Over time, the Cox-Maze procedure evolved into multiple variations employing energy-based techniques like cryothermal or radiofrequency energy to enhance safety and effectiviness. These surgical variations highlight the continuous improvement of SA as a crucial treatment modality for managing tachyarrhythmias, particularly in patients with CHD [2,13–15].
The aim of this review is to critically discuss (1) the indications for surgical ablation, (2) which surgical ablation techniques are suitable for specific atrial tachyarrhythmias, (3) atrial tachyarrhythmias associated with specific CHD, (4) long-term outcomes of SA with CA in patients with various CHD and (5) the risk-benefit ratio of prophylactic arrhythmia surgery.
A comprehensive literature search was conducted using Embase, PubMed, Scopus, and Google Scholar to identify relevant articles published before August 2024. The search included keywords such as “surgical ablation”, “congenital heart disease”, “children”, “pediatric”, “supraventricular tachycardia”, “ventricular tachycardia”, and “arrhythmia”. Articles were selected based on their relevance, focusing on studies that reported the use of SA in both pediatric and adult populations with CHD. Only published articles with full text available online in English were included. Studies were excluded from analysis if they did not include CHD patients or solely focused on CA. Given that the introduction of the Cox-Maze procedure marked a pivotal change in SA techniques, studies published prior to 1989 were also excluded from analysis. No formal quality assessment was performed. A total of 24 articles were included in this review. Two independent reviewers reviewed the articles and extracted the data (Fig. 2).
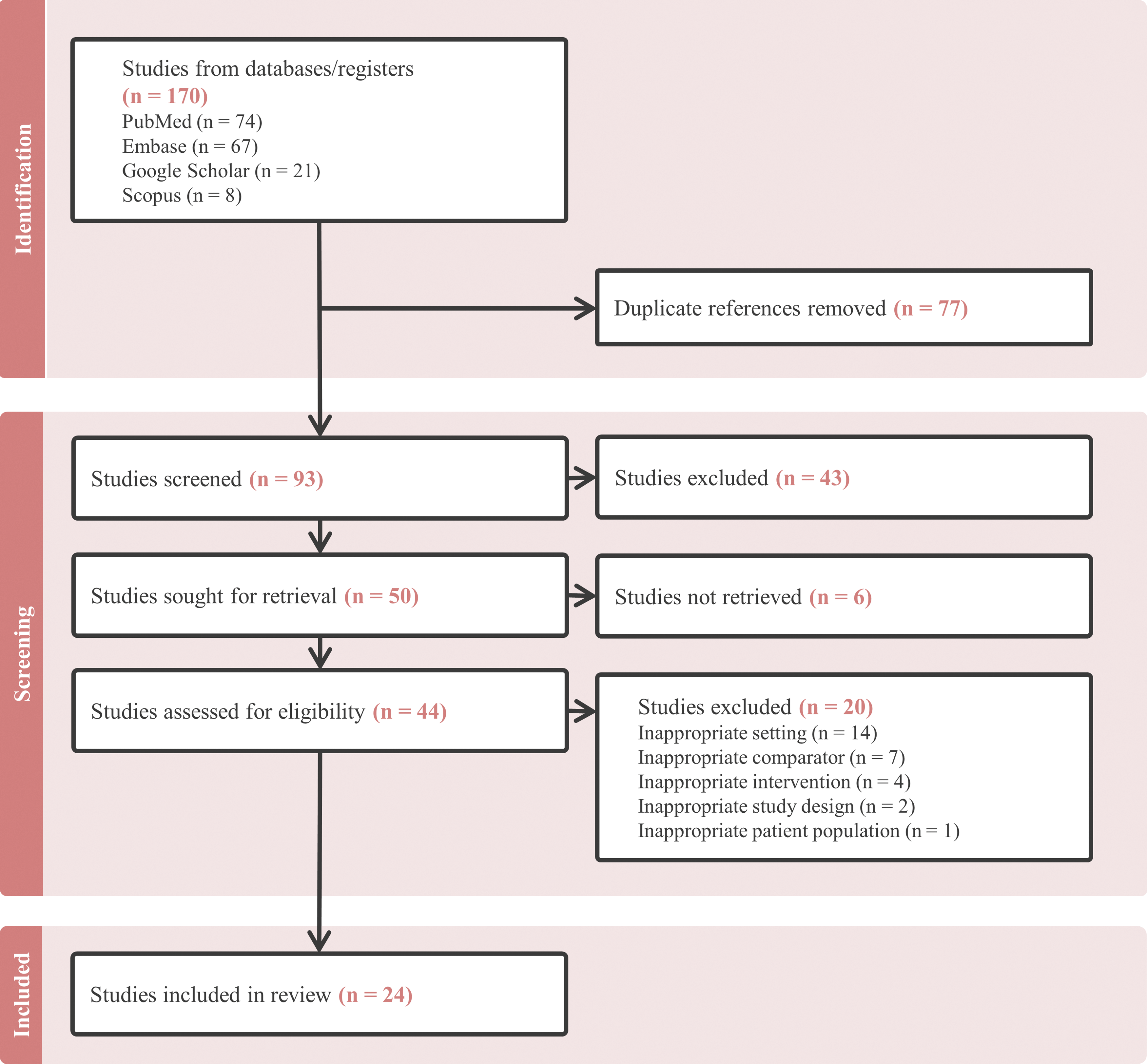
Figure 2: Flowchart of study selection, screening, in- and exclusion criteria
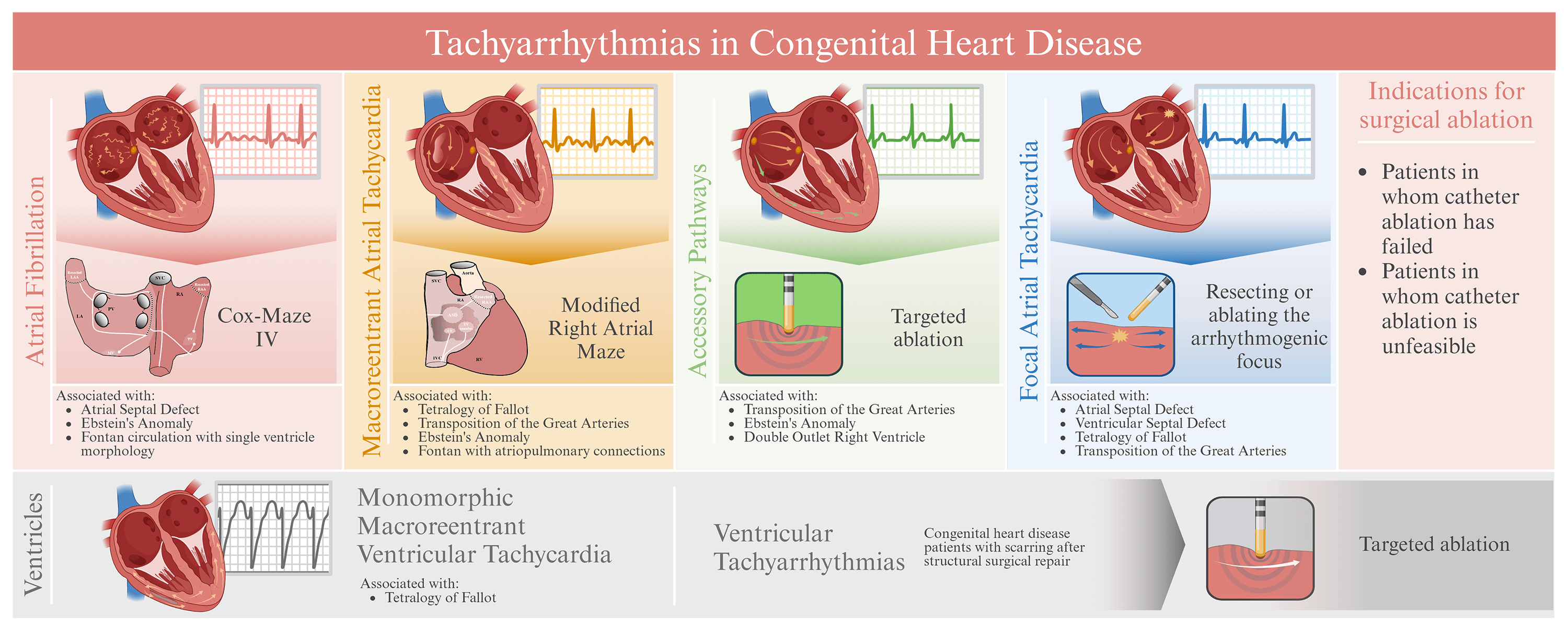
SA is typically considered for CHD patients when factors like complex anatomy, multiple tachyarrhythmia substrates, patient age and size, or limited cardiac access make CA less effective or unfeasible [15]. Especially in very young patients, CA can be technically challenging and carries higher risks of complications such as heart block or coronary artery injury [15]. Furthermore, SA is useful in conditions like Ebstein’s Anomaly (EA), ToF, and other complex CHDs where tachyarrhythmias are a significant concern. The Cox-Maze procedure has proven to be particularly effective for treating AF in patients with complex CHD [15].
The decision to perform SA depends on the patient’s tachyarrhythmia severity, the specific congenital defect, and the associated risks [14,15]. On the other hand, preventive concomitant SA is performed when there is a known predisposition to future tachyarrhythmias caused by tissue damage including surgical scarring [5].
Successful outcomes of SA depend on thorough pre-operative assessments, including identifying APs and unveiling any inducible atrial tachyarrhythmias to lower the risk of postoperative tachyarrhythmias. When atrial tachyarrhythmias have a high probability of persisting despite hemodynamic improvement after surgical repair, integrating SA into the surgical procedure increases the likelihood of arrhythmia free survival.
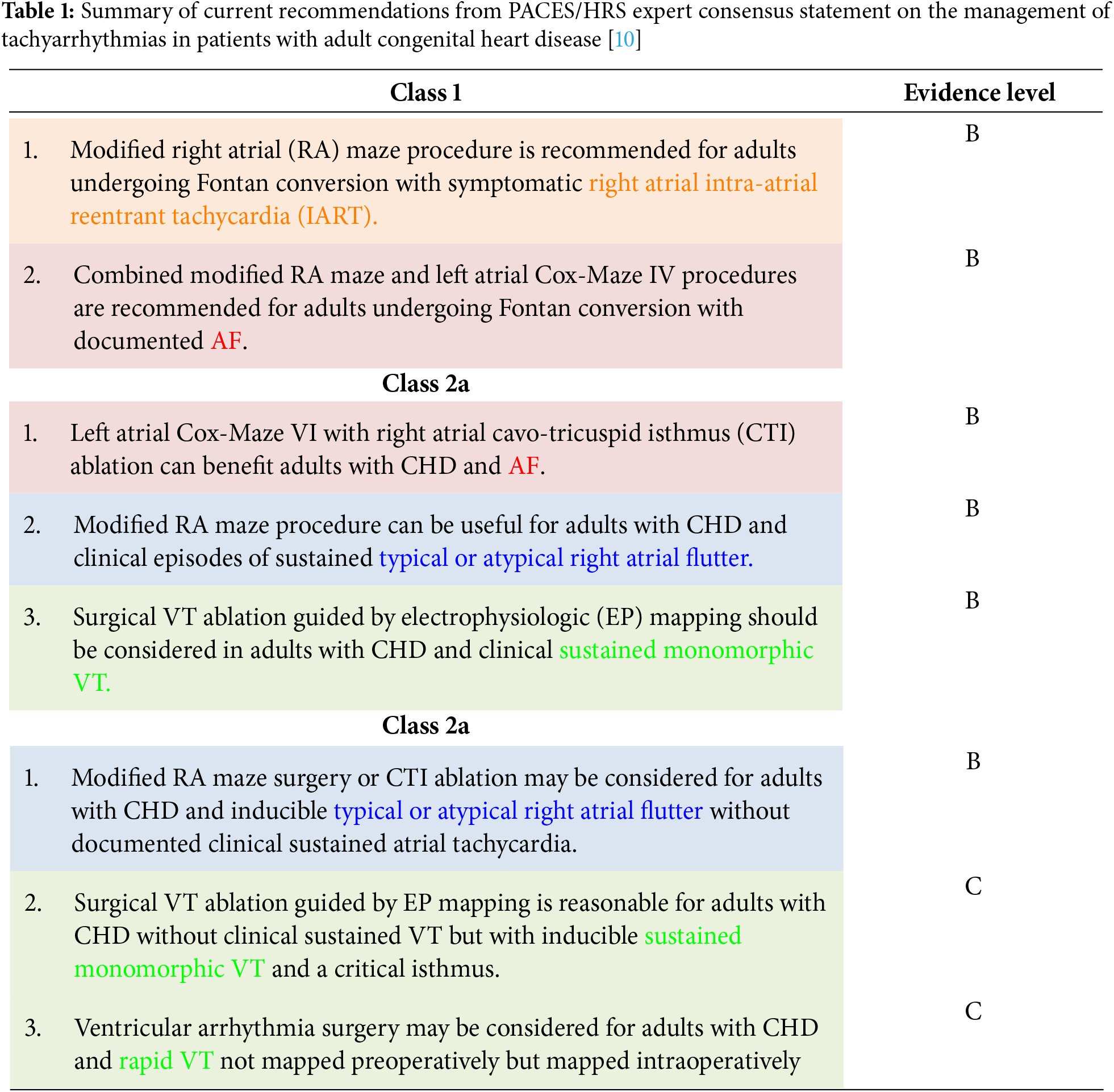
The current recommendations for SA in adult patients with CHD are summarized in Table 1 [2,10]. These recommendations highlight the strategic use of SA alongside cardiac surgeries, tailored to individual risks and anatomical considerations to improve outcomes in CHD patients.
4 Contraindications for SA in CHD
Contraindications for SA include severe comorbidities that increase surgical risks such as advanced heart failure or significant pulmonary hypertension [14]. Furthermore, the location of the ablation target can make SA significantly challenging or infeasible. For instance, APs of the left atrial (LA) free wall are notoriously difficult to reach surgically, especially in infants and small children [16].
Prophylactic arrhythmia surgery is not recommended for adults with CHD who face high surgical mortality risk due to ventricular dysfunction or major comorbidities, especially when extended cardiopulmonary bypass or cross-clamp times could worsen outcomes [2]. Similarly, empiric VT surgery is not advised for adults with CHD lacking clinical or inducible sustained VTs [5,9].
Careful patient selection and a thorough risk-benefit assessment are crucial when considering SA in CHD patients, especially in patients with complex CHD.
5 Ablation Strategies for Atrial Tachyarrhythmias
5.1 Macroreentrant Atrial Tachycardia
Macroreentrant atrial tachycardias are common in the CHD population, especially in patients with atrial dilation or prior atrial surgeries such as patients with EA, ToF, transposition of the great arteries corrected by a Senning or Mustard procedure, or Fontan patients with atriopulmonary connections. These reentrant tachyarrhythmias involve circuits that require slow conduction areas and anatomical barriers, like the inferior vena cava, coronary sinus, tricuspid valve annulus. In the modified right atrial (RA) maze procedure, techniques like cut-and-sew, radiofrequency, or cryothermal energy are used to create transmural lesions that block conduction through the slow conduction areas [17].
The modified RA maze, as described by Deal and colleagues, encompasses resection of the right atrial appendage, inferomedial cavotricuspid isthmus ablation, resection of part of the RA anterior wall, ablation lesion from the atrial septal defect (ASD) across the crista terminalis to the lateral RA wall and a lesion from the superior edge of the ASD to the base of the resected right atrial appendage (Fig. 3) [18]. Deal and colleagues showed that performing a modified RA maze was more successful compared to inferomedial RA cryoablation when treating atrial macroreentrant tachycardia in postoperative Fontan patients. This comparison showed a 7% rate of inducible tachyarrhythmia during postoperative electrophysiological study in the modified RA maze group, as opposed to 62% with isolated inferomedial RA cryoablation [17,18].
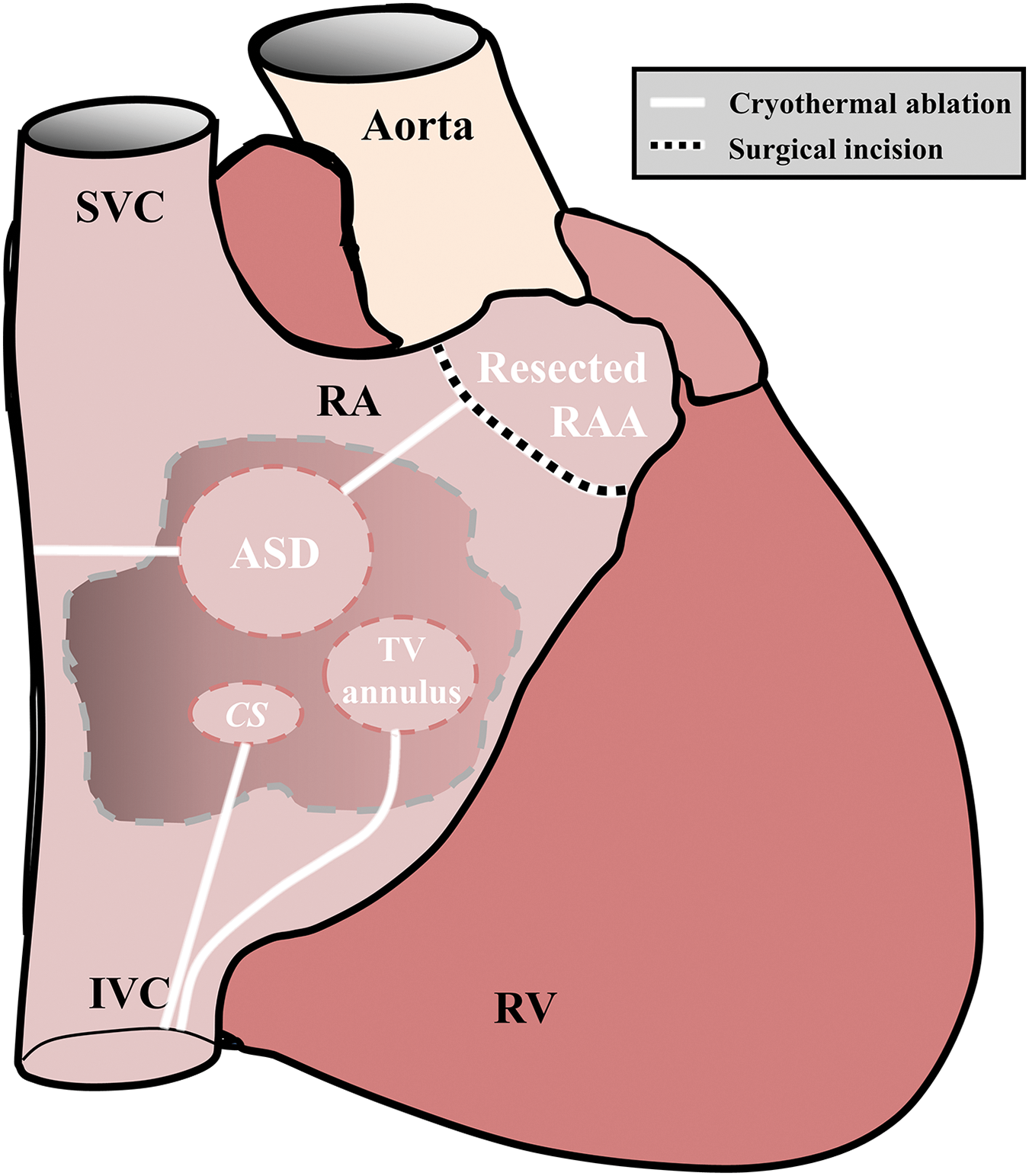
Figure 3: Modified right atrial Maze procedure. RA = right atrium, SVC = superior vena cava, IVC = inferior vena cava, CS = coronary sinus, TV = tricuspid valve, RAA = right atrial appendage, RV = right ventricle
Modifications to the right atrial maze are often necessary in CHD patients to account for anatomical variations, such as juxtaposed atrial appendages, atrial isomerisms, anomalous pulmonary veins, separately draining hepatic veins, ASDs, and scar tissue from prior surgeries [17]. The possibility of multiple reentrant circuits in the atria lead to high recurrence rates, if all of the reentrant circuits are not ablated successfully [17].
Patients with CHD and atrial dilatation, a history of significant atrial-level surgery, or both, are at the highest risk of developing macroreentrant tachycardia [17]. These atrial tachyarrhythmia often originate from the right atrium, in which chronic volume overload often induces atrial remodeling [17,19]. It is therefore assumed that the modified RA maze procedure is the preferred approach in CHD patients with macroreentrant atrial tachycardia.
Due to disorganized atrial excitation and loss of atrial transport function, AF can lead to serious hemodynamic consequences. This negative effect may be more pronounced in patients with CHD due to pre-existing structural abnormalities and reduced ventricular function.
It is generally assumed that trigger-driven AF originates mainly from the pulmonary veins. Whether this is also case for patients with CHD, is debatable. Patients with CHD may have a pre-existing arrhythmogenic substrate, resulting from structural remodeling of atrial tissue which is further enhanced by right atrial dilatation [20].
In addition, when AF has progressed from a trigger-driven to a substrate mediated tachyarrhythmia, it is unknown what the preferential locations and features of the AF-related arrhythmogenic substrate are. It is most likely that substrates vary considerably between patients, depending on the type of CHD and surgical procedures. Consequently, exclusive right-sided ablation strategies, such as the RA Maze, may be less effective in CHD patients [17]. This underscores the potential need for a bi-atrial approach for treatment AF in this population.
The Cox-Maze IV is designed to address AF utilizing a bi-atrial approach (Fig. 4). Key elements in this procedure include encircling pulmonary vein ablation, right and left atrial appendage amputation and creating linear lesions in the right and left atrium, ensuring comprehensive disruption of potential reentrant circuits.
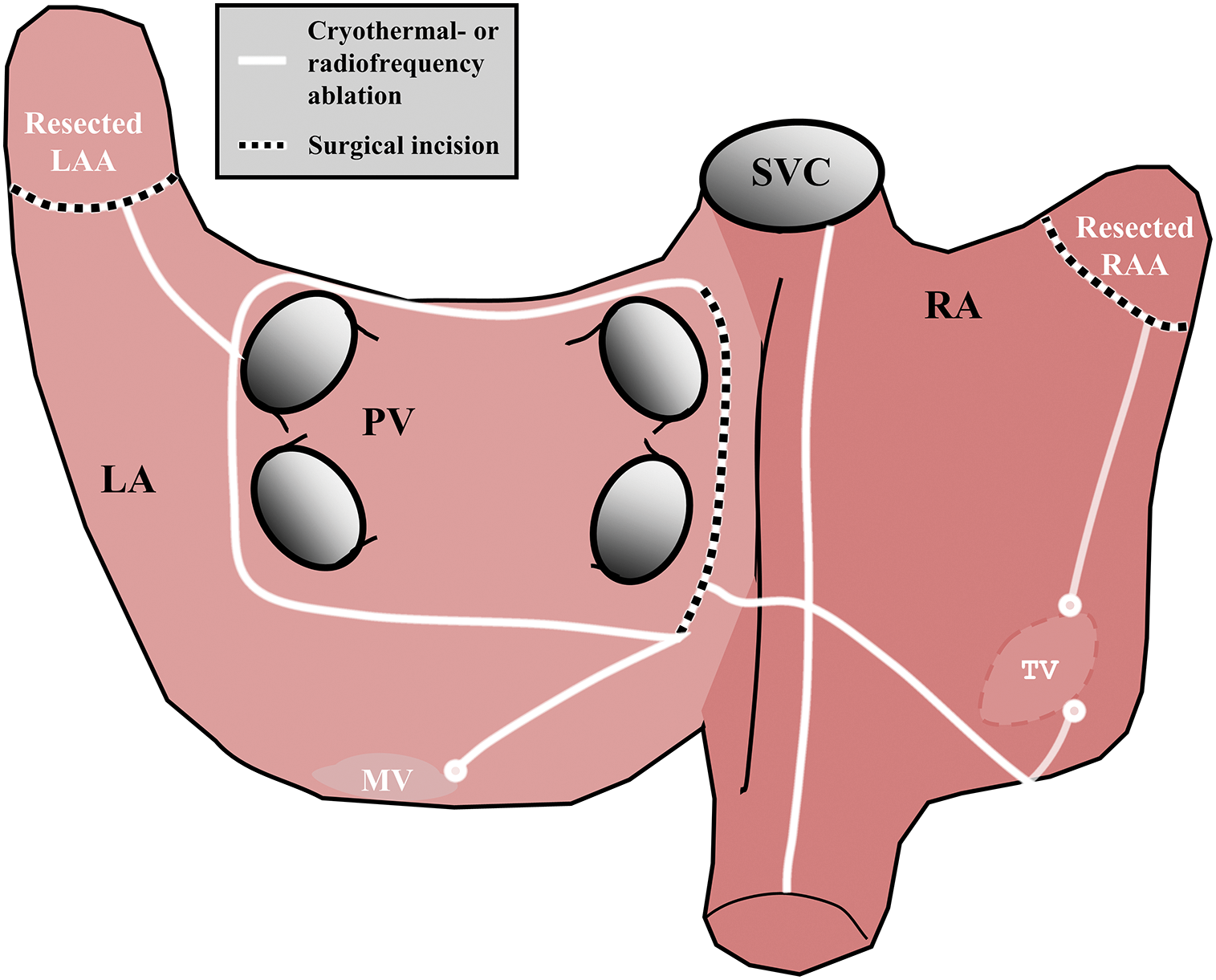
Figure 4: Cox-Maze IV procedure. LA = left atrium, RA = right atrium, SVC = superior vena cava, IVC = inferior vena cava, MV = mitral valve, TV = tricuspid valve, LAA = left atrial appendage, RAA = right atrial appendage, PV = pulmonary veins
In the Cox-Maze IV procedure, radiofrequency current or cryothermal energy is used to reduce the morbidity associated with the original “cut-and-sew” method. This procedure effectively treats AF in CHD patients by targeting both atria to restore rhythm and preserve atrial function. With success rates of AF termination in patients with adult CHD exceeding 95% in the study from Boersma and colleagues, freedom from AF-recurrences after a Cox-Maze procedure has shown to be superior to CA [21,22].
5.3 Accessory Pathways and Focal Atrial Tachycardia
Focal atrial tachycardia is managed by resecting or targeting the arrhythmogenic focus by ablation therapy. The success rate of this procedure highly dependents on precise mapping. Solitary foci, especially in the right atrial appendage, can be effectively treated with surgical resection, while multiple foci require extensive ablation. Reported recurrence rates are as high as 33%, and in some cases, the only remaining treatment is His bundle ablation preceded by implantation of a permanent pacemaker [17].
APs found during initial electrophysiological studies, particularly in complex CHD like transposition of the great arteries or double outlet right ventricle, are often located at variable and challenging locations, such as the coronary sinus or epicardial surface. In case of a subepicardial course of the APs, CA has shown to be less effective [23]. In these patients, particularly concomitant SA is effective.
In patients with EA, in whom APs are common and technically challenging for CA, SA during tricuspid valve repair has a high success rate. Studies suggest that a combined approach of tricuspid valve repair with SA is more effective than sequential CA and surgery, though the latter is associated with reduced cardiopulmonary bypass times and complication rates [24–26].
The Wolf-Parkinson-White syndrome, characterized by pre-excitation due to antegrade conduction via an AP, poses a significant tachyarrhythmia risk when the refractory period of the AP is short. When APs are not accessible via an endovascular approach, SA remains as a viable options for tachyarrhythmia elimination [13,23].
6 Ablation Strategies for Ventricular Tachyarrhythmias
VTs in CHD patients commonly occur in those who have undergone surgical repairs, especially in ToF patients in whom prior ventriculotomies or ventricular septal defect closures induces arrhythmogenic substrates. In addition, the substrate in CHD patients is often increased by structural remodeling, such as scars or lesions induced by the high pressure and flow of echocardiographic jets [7].
Preoperative electrophysiological studies are crucial to map zones of conduction slowing, typically found between the tricuspid valve annulus and right ventricular outflow tract scar, or between the pulmonary valve annulus and ventricular septal defect patch. Ablation, using radiofrequency- or cryothermal energy is indicated primarily for monomorphic VT. Success rates vary widely, ranging from 50% to 90%, with long-term arrhythmia-free survivals. Postoperative electrophysiological studies in children and adults with CHD are recommended to assess VT inducibility, supporting decision making for implantable cardioverter-defibrillators [7,16].
According to Nevvazhay and collegues, ablation of VT is particularly beneficial in patients with ToF [27]. Monomorphic macroreentrant VT in ToF patients often arises from areas of myocardial fibrosis, creating anatomical isthmuses bordering reentrant circuits. Notably, the isthmus that is located between the pulmonary valve and the ventricular septal defect patch, is a critical region which is involved in most macroreentrant VTs. Given that pulmonary valve replacement can obscure this isthmus, preoperative electrophysiological studies and electroanatomical mapping are essential for identifying patients at high risk for inducible macroreentrant VTs. Intraoperative cryoablation of the critical isthmus can effectively disrupt potential reentrant wavelet propagation, thereby preventing recurrent VT episodes, reducing the need for future interventions, and ultimately lowering the risk of VT-related sudden cardiac death [27]. This highlights the importance of individualized ablation strategies in managing VTs in CHD patients, tailored to the specific anatomical and etiological factors.
Prophylactic SA during cardiac repairs targets potential arrhythmogenic substrates before they give rise to clinical tachyarrhythmias, thereby improving long-term outcomes and reducing the need for future interventions. However, prophylactic ablation is not without risks, and is associated with prolonged procedure time, increased cardiopulmonary bypass duration, and potential damage to conduction tissues that can result in complications like heart block or sinus node dysfunction [5,8].
Prophylactic ablation for VT is generally not recommended due to the risk of creating pro-arrhythmogenic lesions. However, in patients with ToF undergoing pulmonary valve replacement, intraoperative cryothermal ablation of specific isthmuses can be considered. These critical isthmuses are rendered inaccessible after surgical repair, making preemptive SA a potential strategy to mitigate future arrhythmia risk. ToF patients therefore represent a specific subgroup of CHD patients who might benefit from a prophylactic approach [27].
Furthermore, while prophylactic modified RA maze procedures are recommended for Fontan revisions, their role in primary Fontan procedures is less definitive, as trials have not shown a significant reduction in tachyarrhythmia risk with additional RA lesions during the initial lateral tunnel Fontan procedures.
Despite the lack of a standardized approach due to variability in lesion sets and patient populations, prophylactic arrhythmia surgery is gaining attention. Individualized strategies are critical, taking specific risks into account, such as atrial dilation, mitral valve disease, and previous surgical repairs, which are all associated with increased lifetime risk of tachyarrhythmias. Observational data suggest that atrial tachyarrhythmias, including AF, increase with age and time since surgery, underscoring the need for proactive, prophylactic treatment [2].
ASDs are linked to a significant risk of late-onset atrial tachyarrhythmias, such as AFL and AF, affecting 20% to 35% of patients post-repair [2]. The risk of developing these tachyarrhythmias increases with age, highlighting the importance of addressing both ASD and AF concurrently, especially in adults. Age at surgery plays a crucial role in long-term outcomes, as patients who undergo repair early in life have higher AF-free survival rates compared to older patients [2,9].
ASD closure alone reduces the occurrence of atrial tachyarrhythmias, but it may not be sufficient to prevent atrial tachyarrhythmias in patients with substantial atrial remodeling prior to surgery, necessitating additional interventions [9,20,28]. In 2020, Houck and colleagues performed intra-operative epicardial mapping in ASD patients to quantify characteristics of atrial conduction disorders during sinus rhythm. Conduction block was present in the RA, particularly in the intercaval region, and in Bachmann’s bundle. This arrhythmogenic substrate is believed to be the result of the high volume overload and right atrial dilatation caused by the interatrial shunt [20]. The Cox-Maze IV procedure, which indeed not only targets the LA but also the RA may be particularly effective in these cases. For older patients undergoing ASD repair, a prophylactic left-sided maze procedure together with cavotricuspid isthmus ablation is recommended, especially when atrial dilation is present [17].
EA is characterized by apical displacement of the tricuspid valve, leading to significant right atrial enlargement and a high prevalence of tachyarrhythmias, including atrial tachyarrhythmias and atrioventricular reentrant tachycardias. Up to 80% of patients with EA experience tachyarrhythmias, making tailored surgical interventions critical [3,6]. The modified RA maze procedure, which includes cavotricuspid isthmus ablation, has proven especially effective in EA patients, significantly reducing tachyarrhythmia recurrence compared to isolated cavotricuspid isthmus ablation [17,26].
Reports on the success of atrial tachyarrhythmia management in EA patients are variable. Bockeria and colleagues compared a 1-stage combined approach (tricuspid valve repair with SA) with a 2-stage approach (CA followed by tricuspid valve repair) [29]. The 1-stage approach was more effective at eliminating tachyarrhythmias, despite being associated with longer cardiopulmonary bypass times and increased surgical complexity [17,28]. Khositseth and colleagues demonstrated high success rates with concomitant SA without increased mortality, supporting the addition of SA to the tricuspid valve repair for patients with EA and atrial tachyarrhythmias [25]. However, it is important to note that surgical management can complicate future CAs, as additions like a bidirectional Glenn can limit access to the right atrium, and an annuloplasty ring can impede access to the tricuspid valve annulus [24,25].
ToF is a complex cyanotic CHD characterized by a ventricular septal defect, pulmonary stenosis, right ventricular hypertrophy, and an overriding aorta. Patients with repaired ToF are at risk for both atrial and ventricular tachyarrhythmias, often due to surgical scars from previous repairs that contribute to formation of reentrant circuits. Critical isthmuses are commonly found between the tricuspid valve annulus and right ventricular outflow tract scar, or between the pulmonary valve annulus and the ventricular septal defect patch [2,5,30].
Prophylactic tachyarrhythmia management in ToF patients may include cavotricuspid isthmus ablation during reoperations, such as pulmonary valve replacement, to mitigate the risk of development of late AFL. Extending the atrial incisions towards the inferior caval vein combined with SA of the cavo-tricuspid isthmus during ToF repairs, can further reduce the risk of development of atrial reentrant tachycardias [31]. For patients with monomorphic VT, targeted ablation during cardiac repair, guided by pre- or intra-operative mapping, may be beneficial, though success rates vary from 50% to 90% [5]. However, postoperative electrophysiological studies will remain necessary in most cases, and an implantable cardioverter-defibrillator placement may be required for patients with inducible VT [5,13].
SA in CHD patients generally results in notable improvements in tachyarrhythmia-free survival and quality of life. However, the efficacy of these procedures vary, depending on the type of tachyarrhythmia, the specific congenital defect, and the timing of intervention.
9.1 Short-Term vs. Long-Term Outcomes of Surgical Ablation
SA has a high short-term efficacy, particularly in achieving conduction block and reducing the recurrence of tachyarrhythmias in the first months after the procedure. Procedures like the Cox-Maze IV, most commonly used concomitant to surgical repair of residual defects in CHD patients with AF, have demonstrated high conversion rates to sinus rhythm (95%) and low perioperative mortality [7,16,32]. Gonzalez Corcia MC and colleagues reported freedom from atrial tachyarrhythmias in 82% and 67% of patients at one and five years post-procedure, respectively, highlighting the procedure’s effectiveness in reducing tachyarrhythmia burden [8].
Long-term outcomes, however, are influenced by ongoing atrial remodeling, progression of the underlying heart disease, and the patient’s age at the time of surgery. Studies indicate that younger patients generally have better long-term outcomes, with lower rates of atrial tachyarrhythmia recurrences and improved overall survival, suggesting that earlier intervention may mitigate the adverse effects of chronic atrial dilation and fibrosis [2,5,8].
Additionally, Shivapour and colleagues found that patients with EA who underwent an initial electrophysiological study followed by SA, when indicated, had fewer atrial and ventricular tachyarrhythmias during long-term follow-up compared to those who did not have an initial electrophysiological study, emphasizing the importance of thorough preoperative assessment in improving long-term outcomes [24].
9.2 Surgical Ablation Compared to Catheter Ablation
SA generally offers superior long-term efficacy compared to CA, particularly in patients with complex CHD. While CA is less invasive and highly effective in structurally normal hearts, its efficacy diminishes in the presence of significant anatomical abnormalities, such as those found in EA or transposition of the great arteries [25]. Meta-analyses in patients without CHD have shown that SA has a significantly higher one-year success rate, especially in patients with persistent or long-standing AF, with approximately 3.5 times higher odds of achieving arrhythmia-free survival compared to CA [6].
CA tends to be less effective in patients with residual hemodynamic issues and thickened atria wall, as for instance in the Fontan population. In these patients, SA is a potential effective treatment modality. The high recurrence rates of tachyarrhythmias in Fontan patients after CA are associated with chronic atrial hypertension, complex anatomy, and limited catheter access, particularly after lateral tunnel-type repairs. Furthermore, the inability to deliver sufficiently deep radiofrequency lesions further compromises the effectiveness of CA in patients with markedly thickened atria [15]. In patients with other types of complex CHD, like EA or ToF effectiveness of CA is also low. For example, the acute success rates for CA of AP termination in EA patients with Wolf-Parkinson-White syndrome are approximately 81% [25].
Likewise, to reduce the risk of recurrent VT in ToF patients post repair, concomitant SA with a surgical repair of residual defects may be more advantageous than preoperative CA [30]. Restoration of right ventricular structure and function after surgery may play a key role in reducing the risk of recurrent VT [30].
Both CA and SA present with technical challenges, especially in small patients, where risks such as heart block, and coronary damage are significant. Given these complexities, the choice between CA and SA should be guided by the specific anatomical and arrhythmic characteristics of the patient, with a tailored approach to optimize outcomes [16,33,34].
10 Risks and Complications of Surgical Ablation
While SA is effective in managing tachyarrhythmias in CHD patients, it is not without risks. Potential complications include stroke, renal failure and the need for pacemaker implantation. These risks are generally similar to those associated with CA, and the long-term benefits of SA often outweigh these potential complications [6].
One notable complication of the maze procedure is sinus node dysfunction, which can be mitigated by limiting prophylactic maze lines to areas away from the sinus node [8]. Ablation targeting APs in the septal region also carries an elevated risk of atrioventricular conduction block, particularly in young children, with an incidence of about 3% [14]. These potential complication highlights the need for close monitoring of the patient postoperatively as they may necessitate implantation of a pacemaker.
Most procedural risks are either related to adhesions from prior cardiac surgeries or ancillary techniques, such as general anesthesia, median sternotomy, and cardiopulmonary bypass, rather than the ablation itself, which is considered relatively low risk [35]. The advancements in minimally invasive surgery, which often eliminate the need for a sternotomy, significantly improve therapy outcomes, reducing recovery time and procedural risks [6,35]. These minimally invasive procedures may have substantial benefits for patient outcomes.
Long-term risks of SA include tachyarrhythmia recurrences, especially in patients with complex CHD or extensive surgical scarring. Patients may develop reentrant tachyarrhythmias due to incomplete lesion sets or the progression of their underlying heart disease [8]. Although recurrence rates for AF and VT are generally lower with SA compared to CA, they are not negligible, and frequent post-operative monitoring is crucial [6].
Overall, while SA involves certain risks, its long-term efficacy in patients with complex anatomy or those who have failed CA makes it a valuable treatment option. The decision between SA and CA should be based on the patient’s specific congenital defect, type of tachyarrhythmia, and overall risk profile.
11 Challenges in the Pediatric Population
The management of tachyarrhythmias in pediatric patients with CHD poses unique challenges due to the smaller size and ongoing development of the heart in the post-operative period. One major challenge is the technical difficulty of performing SA in small children, where the limited size of cardiac structures increases the risk of collateral damage to critical areas. This risk is particularly pronounced in neonates and infants, in whom achieving effective lesion sets without compromising vital structures is challenging [16,33,34].
Additionally, the presentation and progression of tachyarrhythmias in pediatric CHD patients varies considerably. Children with complex CHD, such as single ventricle physiology or ToF, may develop tachyarrhythmias at different stages of life. Surgical scars from previous operations can serve as substrates for reentrant tachyarrhythmias, further complicating rhythm control [2,8,15,31]. Moreover, recurrent tachyarrhythmias and multiple surgeries can have a significant psychological and emotional impact on both the child and their family members, often leading to considerable stress [7,8].
Overall, these factors underscore the complexity of managing tachyarrhythmias in the pediatric population and the importance of individualized care plans that address both the technical challenges and the broader impacts on patient and family well-being.
The field of SA for tachyarrhythmias in CHD is rapidly advancing, with several promising developments on the horizon. One key development is the refinement of hybrid procedures that combine the strengths of both surgical and catheter-based approaches. These hybrid techniques enable simultaneous access to both the epicardium and endocardium thereby maximizing the effectiveness of ablation. Especially in CHD patients with complex anatomies, the combination of simultaneous endocardial and epicardial access improves the accuracy of ablation [6]. A second key development is the shift towards more minimally surgical invasive techniques which offer new opportunities for incorporating SA to a larger patient population. Yet, open-heart surgery, while more invasive, provides direct access to areas of the heart that may be difficult to reach by less- or non-invasive approaches, enabling more comprehensive and potentially more effective ablation. As both the minimally invasive and open procedures continue to evolve, the balance between the two will play a critical role in optimizing treatment strategies for CHD patients.
Cardiac sympathetic denervation is a surgical option for VT, primarily reserved for patients with structurally normal hearts and drug refractory VTs. This procedure, involving the left sympathetic chain, reduces arrhythmia burden by targeting norepinephrine-containing vesicles in the stellate ganglion and associated nerves. Modifications to the procedure, such as sparing parts of the stellate ganglion or performing bilateral sympathectomy, have improved outcomes and minimized complications like Horner syndrome [36]. This technique may prove beneficial in the treatment of VT in CHD, however this still requires further investigation.
In summary, ongoing technical innovations improving (less invasive) SA procedures fuel optimalization of SA for CHD patients, addressing the challenges posed by complex anatomies and variable arrhythmogenic substrates, and hold the potential to significantly improve patient outcomes in the future.
13 Recommendations for Clinical Practice
In CHD patients in whom CA is unsuccessful or is deemed unfeasible, SA may play a pivotal role in optimizing clinical outcomes. Based on the current literature discussed in this review, an overview of the SA strategies for tachyarrhythmias associated with specific congenital heart defects is provided in Table 2.
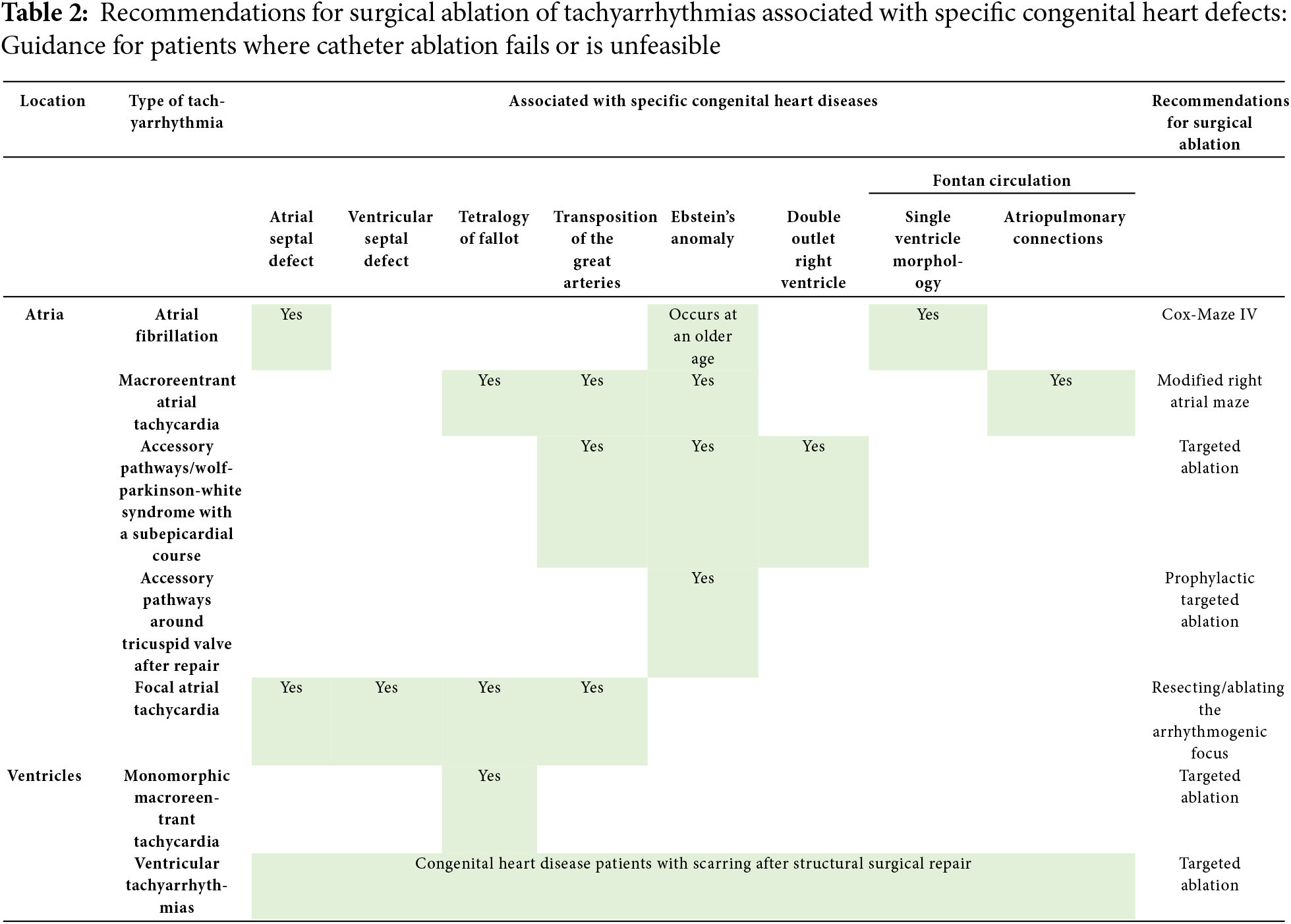
SA for tachyarrhythmias in patients with CHD offers significant benefits, including the potential for long-term tachyarrhythmia control and improved quality of life. SA is indicated when CA fails, CA is unfeasible due to complex anatomy, multiple arrhythmia substrates, or small patient size, and when SA can be performed concomitantly to the corrective surgery to reduce the procedural burden. SA is currently underutilized in clinical practice, despite the potential it offers as a more comprehensive treatment in specific CHD populations. With approximately 3.5 times higher odds of achieving arrhythmia-free survival compared to CA, SA has been proven to be effective in treating tachyarrhythmias in CHD patients. To optimize outcomes, a patient-tailored approach, guided by the patient-specific anatomical and arrhythmic characteristics, is needed. As our understanding of arrhythmogenesis in CHD continues to grow, and as new mapping and ablation technologies emerge, the role of SA in treating arrhythmias in CHD patients is likely to expand, offering hope to a growing number of patients with challenging tachyarrhythmias.
Acknowledgement: Not applicable.
Informed Consent: Not applicable.
Funding Statement: N. M. S. de Groot, MD, PhD is supported by funding grants from NWO-Vidi [grant number 91717339], Medical Delta and CIRCULAR NWO (NWA.1389.20.157)/NWO.
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization, Manouk H. C. Linderhof, Hoang H. Nguyen, Natasja M. S. de Groot; methodology, Manouk H. C. Linderhof, Hoang H. Nguyen; formal analysis, Manouk H. C. Linderhof, Hoang H. Nguyen; investigation, Manouk H. C. Linderhof, Hoang H. Nguyen; writing—original draft preparation, Manouk H. C. Linderhof, Hoang H. Nguyen; writing—review and editing, Annemien E. van den Bosch, Mathijs S. van Schie, Vehpi Yildirim, Yannick J. H. J. Taverne, Natasja M. S. de Groot; visualization, Manouk H. C. Linderhof; supervision, Natasja M. S. de Groot. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data underlying this article will be shared on reasonable request to the corresponding author.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Pelosi C, Kauling RM, Cuypers JAAE, Utens EMWJ, van den Bosch AE, van der Heide A, et al. Life expectancy and end-of-life communication in adult patients with congenital heart disease, 40–53 years after surgery. Eur Heart J Open. 2023 Jul 13;3(4):oead067. doi:10.1093/ehjopen/oead067. [Google Scholar] [CrossRef]
2. Mavroudis C, Deal BJ. Prophylactic arrhythmia surgery in association with congenital heartdisease. Transl Pediatr. 2016;5(3):148–59. doi:10.21037/tp.2016.06.04. [Google Scholar] [CrossRef]
3. Stulak JM, Sharma V, Cannon BC, Ammash N, Schaff HV, Dearani JA. Optimal surgical ablation of atrial tachyarrhythmias during correction of Ebstein anomaly. Ann Thorac Surg. 2015;99(5):1700–5. doi:10.1016/j.athoracsur.2015.01.037. discussion 1705. [Google Scholar] [CrossRef]
4. Teuwen CP, Ramdjan TTTK, Götte M, Brundel BJJM, Evertz R, Vriend JWJ, et al. Time course of atrial fibrillation in patients with congenital heart defects. Circ Arrhythm Electrophysiol. 2015 Oct;8(5):1065–72. doi:10.1161/CIRCEP.115.003272. [Google Scholar] [CrossRef]
5. Caldaroni F, Lo Rito M, Chessa M, Varrica A, Micheletti A, Pappone C, et al. Surgical ablation of ventricular tachycardia in patients with repaired tetralogy of Fallot. Eur J Cardiothorac Surg. 2019;55(5):845–50. doi:10.1093/ejcts/ezy407. [Google Scholar] [CrossRef]
6. Huang H, Wang Q, Xu J, Wu Y, Xu C. Comparison of catheter and surgical ablation of atrial fibrillation: a systemic review and meta-analysis of randomized trials. J Thorac Cardiovasc Surg. 2022;163(3):980–93. doi:10.1016/j.jtcvs.2020.04.154. [Google Scholar] [CrossRef]
7. Mavroudis C, Deal BJ, Backer CL, Tsao S. Arrhythmia surgery in patients with and without congenital heart disease. Ann Thorac Surg. 2008 Sep;86(3):857–68. doi:10.1016/j.athoracsur.2008.04.087. [Google Scholar] [CrossRef]
8. Gonzalez Corcia MC, Walsh EP, Emani S. Long-term results of atrial maze surgery in patients with congenital heart disease. Europace. 2019;21(9):1345–52. doi:10.1093/europace/euz056. [Google Scholar] [CrossRef]
9. Wu SJ, Fan YF, Chien CY. Surgical or interventional treatment for adult patients with atrial septal defect and atrial fibrillation: a systemic review and meta-analysis. Asian J Surg. 2022;45(1):62–7. doi:10.1016/j.asjsur.2021.06.021. [Google Scholar] [CrossRef]
10. Khairy P, Van Hare GF, Balaji S, Berul CI, Cecchin F, Cohen MI, et al. PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease. Can J Cardiol. 2014 Oct;30(10):e1–63. doi:10.1016/j.cjca.2014.09.002. [Google Scholar] [CrossRef]
11. Cobb FR, Blumenschein SD, Sealy WC, Boineau JP, Wagner GS, Wallace AG. Successful surgical interruption of the bundle of kent in a patient with wolff-parkinson-white syndrome. Circulation. 1968 Dec;38(6):1018–29. doi:10.1161/01.CIR.38.6.1018. [Google Scholar] [CrossRef]
12. Cox JL, Schuessler RB, D’Agostino HJ, Stone CM, Chang BC, Cain ME, et al. The surgical treatment of atrial fibrillation: III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991 Apr;101(4):569–83. doi:10.1016/S0022-5223(19)36684-X. [Google Scholar] [CrossRef]
13. Vigneshwar M, Vigneshwar NG, Kron J, Koneru J, Ellenbogen KA, Kasirajan V. Surgical treatment of Wolff-Parkinson-White syndrome: resuscitation of aforgotten technique. JTCVS Tech. 2023;19:47–8. doi:10.1016/j.xjtc.2023.02.012. [Google Scholar] [CrossRef]
14. Tsao S, Deal BJ. Management of symptomatic Wolff-Parkinson–White syndrome in childhood. Prog Pediatr Cardiol. 2013;35(1):7–15. doi:10.1016/j.ppedcard.2012.11.002. [Google Scholar] [CrossRef]
15. Mavroudis C, Deal BJ, Backer CL. Surgery for arrhythmias in children. Int J Cardiol. 2004 Dec;97:39–51. doi:10.1016/j.ijcard.2004.08.008. [Google Scholar] [CrossRef]
16. Crawford FA Jr, Gillette PC, Case CL, Zeigler V. Surgical management of dysrhythmias in infants and small children. Ann Surg. 1992;216(3):318–26. doi:10.1097/00000658-199209000-00011. [Google Scholar] [CrossRef]
17. Feins EN, Emani SM. Surgery for arrhythmia management in congenital heart disease: the surgeon’s perspective. In: Catheter ablation of cardiac arrhythmias in children and patients with congenital heart disease. Boca Raton, FL, USA: CRC Press; 2021. p. 301–8. [Google Scholar]
18. Deal BJ, Mavroudis C, Backer CL, Buck SH, Johnsrude C. Comparison of anatomic isthmus block with the modified right atrial maze procedure for late atrial tachycardia in fontan patients. Circulation. 2002 Jul 30;106(5):575–9. doi:10.1161/01.CIR.0000025876.82336.26. [Google Scholar] [CrossRef]
19. Ueda A, Adachi I, McCarthy KP, Li W, Ho SY, Uemura H. Substrates of atrial arrhythmias: histological insights from patients with congenital heart disease. Int J Cardiol. 2013 Oct 3;168(3):2481–6. doi:10.1016/j.ijcard.2013.03.004. [Google Scholar] [CrossRef]
20. Houck CA, Lanters EAH, Heida A, Taverne YJHJ, van de Woestijne PC, Knops P, et al. Distribution of conduction disorders in patients with congenital heart disease and right atrial volume overload. JACC Clin Electrophysiol. 2020 May 1;6(5):537–48. doi:10.1016/j.jacep.2019.12.009. [Google Scholar] [CrossRef]
21. Mavroudis C, Deal BJ, Backer CL, Stewart RD, Franklin WH, Tsao S, et al. 111 Fontan conversions with arrhythmia surgery: surgical lessons and outcomes. Ann Thorac Surg. 2007 Nov 1;84(5):1457–66. doi:10.1016/j.athoracsur.2007.06.079. [Google Scholar] [CrossRef]
22. Backer CL, Tsao S, Deal BJ, Mavroudis C. Maze procedure in single ventricle patients. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2008 Jan;11(1):44–8. [Google Scholar]
23. Marazzato J, Marazzi R, Angeli F, Vilotta M, Bagliani G, Leonelli FM, et al. Ablation of accessory pathways with challenging anatomy. Card Electrophysiol Clin. 2020;12(4):555–66. doi:10.1016/j.ccep.2020.08.003. [Google Scholar] [CrossRef]
24. Shivapour JK, Sherwin ED, Alexander ME, Cecchin F, Mah DY, Triedman JK, et al. Utility of preoperative electrophysiologic studies in patients with Ebstein’s anomaly undergoing the Cone procedure. Heart Rhythm. 2014;11(2):182–6. doi:10.1016/j.hrthm.2013.10.045. [Google Scholar] [CrossRef]
25. Khositseth A, Danielson GK, Dearani JA, Munger TM, Porter CJ. Supraventricular tachyarrhythmias in Ebstein anomaly: management and outcome. J Thorac Cardiovasc Surg. 2004;128(6):826–33. doi:10.1016/j.jtcvs.2004.02.012. [Google Scholar] [CrossRef]
26. Lazorishinets VV, Glagola MD, Stychinsky AS, Rudenko MN, Knyshov GV. Surgical treatment of Wolf-Parkinson-White syndrome during plastic operations in patients with Ebstein’s anomaly. Eur J Cardiothorac Surg. 2000;18(4):487–90. doi:10.1016/S1010-7940(00)00466-8. [Google Scholar] [CrossRef]
27. Nevvazhay T, Zeppenfeld K, Brouwer C, Hazekamp M. Intraoperative cryoablation in late pulmonary valve replacement for tetralogy of Fallot. Interact Cardiovasc Thorac Surg. 2020 May 1;30(5):780–2. doi:10.1093/icvts/ivaa013. [Google Scholar] [CrossRef]
28. Morton JB, Sanders P, Vohra JK, Sparks PB, Morgan JG, Spence SJ, et al. Effect of chronic right atrial stretch on atrial electrical remodeling in patients with an atrial septal defect. Circulation. 2003 Apr 8;107(13):1775–82. doi:10.1161/01.CIR.0000058164.68127.F2. [Google Scholar] [CrossRef]
29. Bockeria L, Golukhova E, Dadasheva M, Revishvili A, Levant A, Bazaev V et al. Advantages and disadvantages of one-stage and two-stage surgery for arrhythmias and Ebstein’s anomaly. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2005 Oct;28(4):536–40. [Google Scholar]
30. Karamlou T, Silber I, Lao R, McCrindle BW, Harris L, Downar E, et al. Outcomes after late reoperation in patients with repaired tetralogy of fallot: the impact of arrhythmia and arrhythmia Surgery. Ann Thorac Surg. 2006;81(5):1786–93. doi:10.1016/j.athoracsur.2005.12.039. [Google Scholar] [CrossRef]
31. de Groot NM, Lukac P, Schalij MJ, Makowski K, Szili-Torok T, Jordaens L, et al. Long-term outcome of ablative therapy of post-operative atrial tachyarrhythmias in patients with tetralogy of Fallot: a European multi-centre study. Europace. 2012;14(4):522–7. doi:10.1093/europace/eur313. [Google Scholar] [CrossRef]
32. Guiraudon GM, Klein GJ, Sharma AD, Yee R. Surgical alternatives for supraventricular tachycardias. Am J Cardiol. 1989;64(20):92J–6J. doi:10.1016/0002-9149(89)91209-5. [Google Scholar] [CrossRef]
33. Perry JC, Bratincsak A, Shepard S, Williams MR, Loslo J, Murthy R, et al. Epicardial intraoperative three-dimensional mapping of Wolff-Parkinson-White syndrome in a child with ebstein’s anomaly. Ann Thorac Surg. 2018;106(4):e179–81. doi:10.1016/j.athoracsur.2018.03.074. [Google Scholar] [CrossRef]
34. Drago F, Tamborrino PP, Cazzoli I. Ablation in pediatric patients and in association with congenital heart disease. Card Electrophysiol Clin. 2020 Dec;12(4):583–90. doi:10.1016/j.ccep.2020.08.006. [Google Scholar] [CrossRef]
35. Guiraudon GM. Surgical treatment of Wolff-Parkinson-White syndrome: a retrospectroscopic view. Ann Thorac Surg. 1994;58(4):1254–61. doi:10.1016/0003-4975(94)90524-X. [Google Scholar] [CrossRef]
36. Vaseghi M, Barwad P, Malavassi Corrales FJ, Tandri H, Mathuria N, Shah R, et al. Cardiac sympathetic denervation for refractory ventricular arrhythmias. J Am Coll Cardiol. 2017 Jun 27;69(25):3070–80. doi:10.1016/j.jacc.2017.04.035. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools