 Open Access
Open Access
REVIEW
Climate Change and Congenital Heart Disease: A Narrative Review
1 Weill Cornell Medicine, Greenberg Division of Cardiology, New York Presbyterian Hospital-Weill Cornell Medicine, New York, 10065, USA
2 Department of Medicine, New York Presbyterian Hospital-Weill Cornell Medicine, New York, 10065, USA
3 Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology, Beth Israel Deaconess Medical Center, Boston, 02215, USA
* Corresponding Author: Harsimran S. Singh. Email:
Congenital Heart Disease 2024, 19(6), 627-634. https://doi.org/10.32604/chd.2025.062309
Received 16 December 2024; Accepted 16 January 2025; Issue published 27 January 2025
Abstract
Congenital Heart Disease (CHD) is the most common birth defect and a leading cause of infant morbidity and mortality worldwide. While genetic factors play a significant role in its development, up to 30% of CHD is associated with modifiable risk factors and external maternal exposures. Climate change, driven by increased atmospheric pollutants from fossil fuel combustion, leads to rising global temperatures and worsening air quality, which pose emerging threats to maternal and fetal health. This review explores the mechanisms by which environmental factors associated with climate change, specifically extreme heat and air pollution, may influence CHD incidence. Maternal exposure to extreme heat during the first trimester is linked to an increased risk of atrial and ventricular septal defects in offspring, with risk correlating to the intensity and duration of heat exposure. Air pollution—particularly fine particulate matter and gases like ozone, nitrogen dioxide, and sulfur dioxide—is associated with a broader spectrum of CHD, including tetralogy of Fallot, pulmonary stenosis, and coarctation of the aorta. These effects are present even when the exposure occurred prior to conception. Synergistic effects between air pollution and other exposures, such as tobacco use, may further amplify CHD risk. Clinicians should be aware of the potential risks associated with environmental exposures and counsel prospective mothers accordingly to mitigate CHD risk in their offspring.Keywords
Glossary/Nomenclature/Abbreviations
| CHD | Congenital Heart Disease |
| ToF | Tetralogy of Fallot |
| ASD | Atrial Septal Defect |
| VSD | Ventricular Septal Defect |
| PM2.5 | Particulate Matter less then 2.5 µM in size |
| NO2 | Nitrogen Dioxide |
| SO2 | Sulfur Dioxide |
| CO | Carbon Monoxide |
| O3 | Ozone |
Congenital heart disease (CHD) is the most common birth defect, affecting approximately 1% of all live births [1]. Despite significant advances in early detection and management over the past five decades, CHD remains a leading cause of infant morbidity and mortality, both in the United States and globally [2,3]. In 2017, CHD was the underlying cause of 261,247 deaths globally (95% confidence interval (CI) 216,567–308,159), with 72% of these fatalities occurring in infants under one year old [4]. While the proportion of CHD associated with external exposures and modifiable risk factors is unknown, prior epidemiologic studies attribute modifiable risk factors with up to 30% of certain congenital heart defects [5]. External maternal exposures such as infection, medications, alcohol, and illicit drugs are traditionally associated with CHD, especially during the first trimester when cardiac embryogenesis occurs [6,7].
Climate change and its associated exposures pose an emerging threat to worldwide health gains achieved over the past century. Over 150 years of fossil fuel combustion elevate atmospheric concentrations of pollutants, including greenhouse gases, and lead to increasing global temperature, long-term shifts in weather patterns, ecosystem disruptions, and rising sea levels. An expanding body of research suggests that air pollution, global warming, and associated environmental stressors contribute to cardiovascular morbidity and mortality [8–10]. Polygenic abnormalities are linked to many forms of CHD development, but the environmental contribution and interaction with genetics is less well understood [6]. This review explores the mechanisms—both direct and indirect—through which CHD may be influenced by a changing climate (Fig. 1).
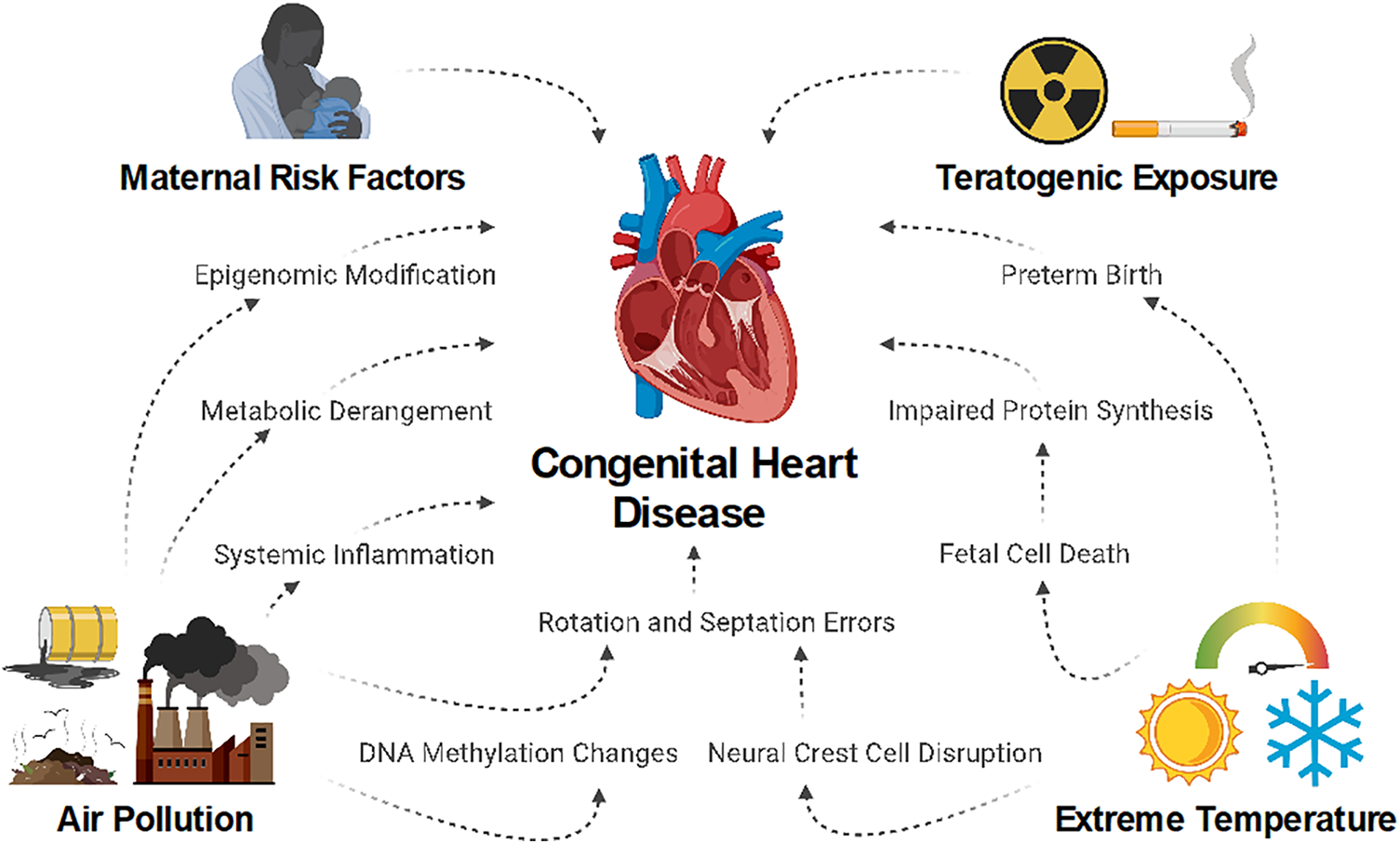
Figure 1: Climate change may lead to an increased risk of congenital heart disease through multiple potential pathways in which maternal health and fetal cellular differentiation and maturation are impacted. Worsening air pollution and extreme temperature fluctuations may exacerbate these mechanisms, with more pronounced as the effects of climate change continue to intensify [11]
As global temperatures continue to rise, heatwaves—defined as prolonged periods of extreme heat—will become more frequent and intense [12]. Although the health implications are undeniable, the thresholds and definitions of extreme heat and heatwaves differ by location. In a study assessing 107 communities in the United States over a fourteen-year period, adult cardiovascular hospitalizations increased at thresholds 20°F higher in the southwestern U.S. compared to the traditionally cooler Pacific Northwest [13].
Maternal exposure to extreme heat and heatwaves during pregnancy is linked to negative effects on birth outcomes, with increased incidence of preterm birth, low birth weight, and stillbirths [14]. However, the relationship between extreme heat and CHD development is less clear. Animal studies suggest that extreme heat exposure leads to fetal cell death and heat shock responses with subsequent disruptions in normal protein synthesis [15,16]. Cardiac neural crest cells express temperature activated ion channels. Disruption of these channels is associated with errors in rotation and septation that lead to a constellation of conotruncal abnormalities such as double outlet right ventricle and Tetralogy of Fallot (ToF) [17]. Despite the pathophysiologic pathway noted above, no existing research in humans has shown a definitive increase in these birth defects after maternal extreme heat exposure [16].
Retrospective studies find an association between maternal heat exposure during first trimester and overall incidence of fetal septal defects. Auger et al. [16] examined extreme heat exposure during weeks 2–8 of pregnancy and found that 15 or more days of extreme heat exposure compared to 0 days of exposure was associated with increased risk of atrial septal defects (ASD) (prevalence ratio 1.32; 95% CI: 1.10 to 1.70). There was also an increase in non-critical CHD, defined as endocardial cushion defects, septal defects, valvular defects, aortic/pulmonic defects, and heterotaxy (prevalence ratio 1.54; 95% CI: 1.20 to 1.97). The researchers noted that higher temperature thresholds (i.e., 95th percentile vs. 90th percentile for extreme heat) were associated with higher odds of ventricular septal defect (VSD) development. Lin et al. [18] assessed extreme heat exposure during weeks 3–8 of pregnancy and noted a similar trend of increasing prevalence of CHD with increasing extreme heat exposure and intensity. Three to five days of cumulative extreme heat exposure with temperatures >90th percentile was associated with increased odds of VSD development, with odds ratio (OR) ranging 2.17 to 2.57 (all p < 0.05), while higher cumulative exposure was associated with even higher odds (OR 3.24; 95% CI: 1.01 to 10.40) [18].
Using models based on the data from Lin et al. [18], Zhang et al. [19] projected an increase in early pregnancy maternal heat exposure by 2035 and consequently significant increases in CHD incidence. Their modelling suggests different increases in CHD subset incidence in different regions of the United States, with projected increase in conotruncal CHD in the south and increased ASDs in the Northeast. While it is unclear what drives this geographic heterogeneity, differences in ambient temperature/temperature ranges along with differences in heat acclimatization may contribute.
Air pollution is the leading cause of reversible premature morbidity and mortality worldwide, with 99% of the global population breathing levels of air pollution above World Health Organization guideline limits [20,21]. Air pollution consists of myriad particles, from anthropogenic sources such as cars or factories, to natural phenomena like wildfires. These anthropogenic particles include particulate matter less than 2.5 µM (PM2.5), as well as the greenhouse gasses nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO), and ozone (O3). These pollutants are inhaled and can enter the bloodstream, where they can exert systemic effects. Wildfires, which are increasing in frequency and intensity, lead to acute heat and air pollution exposure [22]. However, there are no currently published studies addressing the impact of wildfires on CHD.
In adults, inhalation of air pollution leads to increased morbidity and mortality through increases in systemic inflammation, prothrombotic pathways, autonomic imbalance, metabolic derangements, and epigenomic changes [23–27]. It appears that active pregnancy modifies the effect of air pollution on the body at a cellular level. In one study PM2.5 exposure during pregnancy was associated with differential modulation of histone post-translational modifications compared to non-pregnant, age-matched controls [28]. While impacts on maternal health may secondarily affect the fetus, there is additional evidence that the fetus itself is directly affected. Studies associate air pollution with changes in DNA methylation patterns of placental genes involved in fetal development, with implications before and after delivery [29].
As with extreme heat, evidence connects air pollution exposure and cardiac septal defects [30,31]. In a Canadian cohort of approximately 1.3 million newborns, maternal exposure to PM2.5 and NO2 during the first trimester was associated with increased risk of ASD development. These risks increased in mothers with pre-existing comorbidities [32]. In a Chinese case-control study of 1.4 million newborns, each 10 μg/m3 increase in maternal PM2.5 exposure during the first trimester was associated with a 2% increase in risk of CHD (OR 1.02; 95% CI 1.00–1.04). This effect was most evident with septal defects (OR 1.04; 95% CI 1.01–1.08) [33]. Notably, these effects were also evident during the three months prior to conception, implying a long-term effect of air pollution beyond the acute exposure.
Unlike with extreme heat, air pollution has a stronger association with fetal prevalence of more complex, wide-ranging forms of CHD in humans. Zhang et al. [30] found that O3 exposure was associated with increased risk of total CHD, VSD, and ToF; with the greatest risk for ToF at exposure during the third month of pregnancy (OR 1.31; 95% CI 1.13–1.51). A meta-analysis of 10 studies also noted an association between air pollution and ToF, this time with exposure to NO2 (OR 1.20; 95% CI, 1.02–1.42) and SO2 (OR 1.03; 95% CI, 1.01–1.05) [34]. This same meta-analysis found that NO2 and SO2 exposure during early pregnancy was associated with coarctation of the aorta (CoA), a finding corroborated in a separate study of children born via in vitro fertilization [35]. A meta-analysis of 26 epidemiologic studies noted an association between PM2.5 exposure and ToF (OR 1.52; 95% CI 1.44–1.60) [36]. Additionally, both PM2.5 and NO2 were associated with pulmonary stenosis (OR 1.42; 95% CI 1.36–1.48 and OR 1.74; 95% CI, 1.68–1.81, respectively). Although other, more recent meta-analyses confirmed these findings, an analysis of 32 studies by Wan et al. [37,38] noted a small but statistically significant negative association between SO2 and transposition of the great arteries, ToF, VSD, and pulmonary artery and valve defects. There may also be synergy between air pollution and other exposures, such as tobacco use. In a retrospective study of almost 28,000 women at high risk for CHD according to the American Heart Association, paternal tobacco amplified the effect of O3 exposure on CHD incidence [39,40].
Extreme heat and air pollution do not exist in isolation, and appear to have compounding effects on cardiovascular health [10,41]. Limited evidence suggests persistence of this effect in regard to maternal exposure and CHD, but further research is needed better clarify this interaction [42,43].
Environmental air pollution and extreme heat are linked to increased prevalence of extreme weather events, such as hurricanes and tropical storms. Studies show these major events lead to increased mortality rates that persist for over a decade after a storm makes landfall [44]. Storms additionally cause physical damage, disruption of health care and economic systems, and population displacement. An increased incidence of CHD associated with these events may be due to maternal stress and the healthcare disruptions from these events, rather than direct maternal/fetal exposure to heat and/or air pollution. However, there is currently no published data assessing CHD development with extreme weather events.
Exposure to extreme temperatures and air pollution is associated with an increased incidence of CHD. However, there are significant limitations in the largely retrospective and population-based literature that must be considered before confirming causality.
There remain areas for further research to better assess the intersection between climate exposure and CHD. First, as noted above, there are no studies assessing the effects of extreme weather events on CHD incidence. Second, wildfire seasons are also becoming longer and more intense, leading to smoke inhalation by pregnant women hundreds of miles from the source. We found no studies assessing this interaction. Third, there is no data on paternal pre-conception heat and air pollution exposure on CHD incidence. Evaluating the role of paternal exposure will be important for optimal pre-conception counseling. Fourth, there is no research on the impact of climate change related stressors on adults with CHD. Ninety-seven percent of all children born with CHD will survive into adulthood and will require specialized care that is individualized to their condition [45]. This is a diverse population in which close follow-up is critical, but often challenging [46]. There is evidence that short disruptions of care have long term impacts on the general population, but data on adults with CHD is lacking [44,47]. Data indicate that these patients had increased difficulty accessing necessary healthcare during the COVID-19 pandemic, but the long-term health implications of these disruptions is unknown [48]. Finally, 90% of CHD occurs in low- and middle-income countries [49]. There is a paucity of data assessing environmental exposures to CHD in these regions despite the high burden and greater expected impacts from climate change [50]. Further research in these countries will necessetate investing in surveillance systems and data collection. As an example, only 24 of the 54 countries in Africa currently have the capacity to monitor air pollution [51].
Clinicians should be aware that maternal exposure to extreme temperature and air pollution in the weeks preceding and following conception carries an increased risk for the development of CHD. Prospective mothers should be counseled on the risks of extreme heat and air pollution exposure and should be aware that air pollution prior to conception may be relevant as well. Further research on environmental exposures in pregnant women and adult CHD patients will be critical to help inform practice and policy.
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Ethan Katznelson and Harsimran S. Singh; data collection: Ethan Katznelson; analysis and interpretation of results: Ethan Katznelson, Matthew J. Navarro, Su Yuan, Dhruv S. Kazi, Harsimran S. Singh; draft manuscript preparation: Ethan Katznelson, Matthew J. Navarro, Su Yuan, Dhruv S. Kazi, Harsimran S. Singh. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: This study relies on published data that are already in the public domain and can be obtained from the primary publications cited in the references.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Liu Y, Chen S, Zühlke L, Black GC, Choy M-K, Li N, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019;48:455–63. doi:10.1093/ije/dyz009. [Google Scholar] [CrossRef]
2. Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122:2254–63. doi:10.1161/CIRCULATIONAHA.110.947002. [Google Scholar] [CrossRef]
3. Mandalenakis Z, Giang KW, Eriksson P, Liden H, Synnergren M, Wåhlander H, et al. Survival in children with Congenital Heart Disease: have we reached a peak at 97%? J Am Heart Assoc. 2020;9:e017704. doi:10.1161/JAHA.120.017704. [Google Scholar] [CrossRef]
4. Zimmerman MS, Smith AGC, Sable CA, Echko MM, Wilner LB, Olsen HE, et al. Global regional, and national burden of congenital heart disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc Health. 2020;4:185–200. doi:10.1016/S2352-4642(19)30402-X. [Google Scholar] [CrossRef]
5. Wilson PD, Loffredo CA, Correa-Villaseñor A, Ferencz C. Attributable fraction for cardiac malformations. Am J Epidemiol. 1998;148:414–23. doi:10.1093/oxfordjournals.aje.a009666. [Google Scholar] [CrossRef]
6. Baldacci S, Gorini F, Santoro M, Pierini A, Minichilli F, Bianchi F. Environmental and individual exposure and the risk of congenital anomalies: a review of recent epidemiological evidence. Epidemiol Prev. 2018;42:1–34. doi:10.19191/EP18.3-4.S1.P001.057. [Google Scholar] [CrossRef]
7. Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge. Circulation. 2007;115:2995–3014. doi:10.1161/CIRCULATIONAHA.106.183216. [Google Scholar] [CrossRef]
8. Sagheer U, Al-Kindi S, Abohashem S, Phillips CT, Rana JS, Bhatnagar A, et al. Environmental pollution and Cardiovascular disease. JACC Adv. 2024;3:100805. doi:10.1016/j.jacadv.2023.100805. [Google Scholar] [CrossRef]
9. Katznelson E, Malkani K, Zhang R, Patel S. Impact of climate change on Cardiovascular health. Curr Atheroscler Rep. 2024;27:13. doi:10.1007/s11883-024-01261-z. [Google Scholar] [CrossRef]
10. Kazi DS, Katznelson E, Liu C-L, Al-Roub NM, Chaudhary RS, Young DE, et al. Climate change and Cardiovascular health: a systematic review. JAMA Cardiol. 2024;9:748–57. doi:10.1001/jamacardio.2024.1321. [Google Scholar] [CrossRef]
11. Navarro M. Fig. 1. [Figure] Created in BioRender. 2025 [cited 2025 Jan 15]. Available from: https://BioRender.com/e56s708. [Google Scholar]
12. Bell ML, Gasparrini A, Benjamin GC. Climate change, extreme heat, and health. N Engl J Med. 2024;390:1793–801. doi:10.1056/NEJMra2210769. [Google Scholar] [CrossRef]
13. Anderson BG, Bell ML. Weather-related mortality: how heat, cold, and heat waves affect mortality in the United States. Epidemiol Camb Mass. 2009;20:205–13. doi:10.1097/EDE.0b013e318190ee08. [Google Scholar] [CrossRef]
14. Chersich MF, Pham MD, Areal A, Haghighi MM, Manyuchi A, Swift CP, et al. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. BMJ. 2020;371:m3811. doi:10.1136/bmj.m3811. [Google Scholar] [CrossRef]
15. Bennett GD. Hyperthermia: malformations to chaperones. Birth Defects Res B Dev Reprod Toxicol. 2010;89:279–88. doi:10.1002/bdrb.v89:4. [Google Scholar] [CrossRef]
16. Auger N, Fraser WD, Sauve R, Bilodeau-Bertrand M, Kosatsky T. Risk of congenital heart defects after ambient heat exposure early in pregnancy. Environ Health Perspect. 2017;125:8–14. doi:10.1289/EHP171. [Google Scholar] [CrossRef]
17. Hutson MR, Keyte AL, Hernández-Morales M, Gibbs E, Kupchinsky ZA, Argyridis I, et al. Temperature-activated ion channels in neural crest cells confer maternal fever-associated birth defects. Sci Signal. 2017;10:eaal4055. doi:10.1126/scisignal.aal4055. [Google Scholar] [CrossRef]
18. Lin S, Lin Z, Ou Y, Soim A, Shrestha S, Lu Y, et al. Maternal ambient heat exposure during early pregnancy in summer and spring and congenital heart defects—a large US population-based, case-control study. Environ Int. 2018;118:211–21. doi:10.1016/j.envint.2018.04.043. [Google Scholar] [CrossRef]
19. Zhang W, Spero TL, Nolte CG, Garcia VC, Lin Z, Romitti PA, et al. Projected changes in maternal heat exposure during early pregnancy and the associated Congenital Heart Defect burden in the United States. J Am Heart Assoc. 2019;8:e010995. doi:10.1161/JAHA.118.010995. [Google Scholar] [CrossRef]
20. Air Pollution [Internet]. World Health Organiation. [cited 2024 Jul 22]. Available from: https://www.who.int/health-topics/air-pollution#tab=tab_1. [Google Scholar]
21. Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu N (Nilet al. The Lancet commission on pollution and health. The Lancet. 2018;391:462–512. doi:10.1016/S0140-6736(17)32345-0. [Google Scholar] [CrossRef]
22. Cunningham CX, Williamson GJ, Bowman DMJS. Increasing frequency and intensity of the most extreme wildfires on Earth. Nat Ecol Evol. 2024;8:1420–5. doi:10.1038/s41559-024-02452-2. [Google Scholar] [CrossRef]
23. Bevan GH, Al-Kindi SG, Brook RD, Münzel T, Rajagopalan S. Ambient air pollution and atherosclerosis: insights into dose, time, and mechanisms. Arterioscler Thromb Vasc Biol. 2021;41:628–37. doi:10.1161/ATVBAHA.120.315219. [Google Scholar] [CrossRef]
24. Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2054–70. doi:10.1016/j.jacc.2018.07.099. [Google Scholar] [CrossRef]
25. Lederer AM, Fredriksen PM, Nkeh-Chungag BN, Everson F, Strijdom H, de Boever P, et al. Cardiovascular effects of air pollution: current evidence from animal and human studies. Am J Physiol Heart Circ Physiol. 2021;320:H1417–39. doi:10.1152/ajpheart.00706.2020. [Google Scholar] [CrossRef]
26. Snow SJ, Cheng W, Wolberg AS, Carraway MS. Air pollution upregulates endothelial cell procoagulant activity via ultrafine particle-induced oxidant signaling and tissue factor expression. Toxicol Sci off J Soc Toxicol. 2014;140:83–93. doi:10.1093/toxsci/kfu071. [Google Scholar] [CrossRef]
27. Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, et al. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect. 2014;122:79–86. doi:10.1289/ehp.1307151. [Google Scholar] [CrossRef]
28. Jung YS, Aguilera J, Kaushik A, Ha JW, Cansdale S, Yang E, et al. Impact of air pollution exposure on cytokines and histone modification profiles at single-cell levels during pregnancy. Sci Adv. 2024;10:eadp5227. doi:10.1126/sciadv.adp5227. [Google Scholar] [CrossRef]
29. Broséus L, Guilbert A, Hough I, Kloog I, Chauvaud A, Seyve E, et al. Placental DNA methylation signatures of prenatal air pollution exposure and potential effects on birth outcomes: an analysis of three prospective cohorts. Lancet Planet Health. 2024;8:e297–308. doi:10.1016/S2542-5196(24)00045-7. [Google Scholar] [CrossRef]
30. Zhang B, Zhao J, Yang R, Qian Z, Liang S, Bassig BA, et al. Ozone and other air pollutants and the risk of congenital heart defects. Sci Rep. 2016;6:34852. doi:10.1038/srep34852. [Google Scholar] [CrossRef]
31. Jin S, Yoon SZ, Choi YJ, Kang G, Choi SU. Prenatal exposure to air pollutants and the risk of congenital heart disease: a Korean national health insurance database-based study. Sci Rep. 2024;14:16940. doi:10.1038/s41598-024-63150-4. [Google Scholar] [CrossRef]
32. Buteau S, Veira P, Bilodeau-Bertrand M, Auger N. Association between first trimester exposure to ambient PM2.5 and NO2 and congenital heart defects: a population-based cohort study of 1,342,198 live births in Canada. Environ Health Perspect. 2023;131:067009. doi:10.1289/EHP11120. [Google Scholar] [CrossRef]
33. Yuan X, Liang F, Zhu J, Huang K, Dai L, Li X, et al. Maternal exposure to PM2.5 and the risk of congenital heart defects in 1.4 million births: a nationwide surveillance-based study. Circulation. 2023;147:565–74. doi:10.1161/CIRCULATIONAHA.122.061245. [Google Scholar] [CrossRef]
34. Vrijheid M, Martinez D, Manzanares S, Dadvand P, Schembari A, Rankin J, et al. Ambient air pollution and risk of congenital anomalies: a systematic review and meta-analysis. Environ Health Perspect. 2011;119:598–606. doi:10.1289/ehp.1002946. [Google Scholar] [CrossRef]
35. Li L, Zhang N, Wu X, Feng T, Zhao Z, Pang Y, et al. Exposure to air pollution is associated with congenital anomalies in the population born by in vitro fertilization. Environ Res. 2022;207:112161. doi:10.1016/j.envres.2021.112161. [Google Scholar] [CrossRef]
36. Ravindra K, Chanana N, Mor S. Exposure to air pollutants and risk of congenital anomalies: a systematic review and metaanalysis. Sci Total Environ. 2021;765:142772. doi:10.1016/j.scitotenv.2020.142772. [Google Scholar] [CrossRef]
37. Wan X, Wei S, Wang Y, Jiang J, Lian X, Zou Z, et al. The association between maternal air pollution exposure and the incidence of congenital heart diseases in children: a systematic review and meta-analysis. Sci Total Environ. 2023;892:164431. doi:10.1016/j.scitotenv.2023.164431. [Google Scholar] [CrossRef]
38. Hu C-Y, Huang K, Fang Y, Yang X-J, Ding K, Jiang W, et al. Maternal air pollution exposure and congenital heart defects in offspring: a systematic review and meta-analysis. Chemosphere. 2020;253:126668. doi:10.1016/j.chemosphere.2020.126668. [Google Scholar] [CrossRef]
39. Wang H, Ruan Y-P, Ma S, Wang Y-Q, Wan X-Y, He Y-H, et al. Interaction between ozone and paternal smoking on fetal congenital heart defects among pregnant women at high risk: a multicenter maternal-fetal medicine study. World J Pediatr. 2024;20:621–32. doi:10.1007/s12519-023-00755-1. [Google Scholar] [CrossRef]
40. Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American heart association. Circulation. 2014;129:2183–242. doi:10.1161/01.cir.0000437597.44550.5d. [Google Scholar] [CrossRef]
41. Xu R, Huang S, Shi C, Wang R, Liu T, Li Y, et al. Extreme temperature events, fine particulate matter, and myocardial infarction mortality. Circulation. 2023;148:312–23. doi:10.1161/CIRCULATIONAHA.122.063504. [Google Scholar] [CrossRef]
42. Simmons W, Lin S, Luben TJ, Sheridan SC, Langlois PH, Shaw GM, et al. Modeling complex effects of exposure to particulate matter and extreme heat during pregnancy on congenital heart defects: a U.S. population-based case-control study in the national birth defects prevention study. Sci Total Environ. 2022;808:152150. doi:10.1016/j.scitotenv.2021.152150. [Google Scholar] [CrossRef]
43. Stingone JA, Luben TJ, Sheridan SC, Langlois PH, Shaw GM, Reefhuis J, et al. Associations between fine particulate matter, extreme heat events, and congenital heart defects. Environ Epidemiol. 2019;3:e071. doi:10.1097/EE9.0000000000000071. [Google Scholar] [CrossRef]
44. Young R, Hsiang S. Mortality caused by tropical cyclones in the United States. Nature. 2024;635:121–8. doi:10.1038/s41586-024-07945-5. [Google Scholar] [CrossRef]
45. Dellborg M, Giang KW, Eriksson P, Liden H, Fedchenko M, Ahnfelt A, et al. Adults with congenital heart disease: trends in event-free survival past middle age. Circulation. 2023;147:930–8. doi:10.1161/CIRCULATIONAHA.122.060834. [Google Scholar] [CrossRef]
46. Gurvitz M, Valente AM, Broberg C, Cook S, Stout K, Kay J, et al. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART-ACHD (The Health, Education, and Access Research Trial). J Am Coll Cardiol. 2013;61:2180–4. doi:10.1016/j.jacc.2013.02.048. [Google Scholar] [CrossRef]
47. Baum A, Barnett ML, Wisnivesky J, Schwartz MD. Association between a temporary reduction in access to health care and long-term changes in hypertension control among veterans after a natural disaster. JAMA Netw Open. 2019;2:e1915111. doi:10.1001/jamanetworkopen.2019.15111. [Google Scholar] [CrossRef]
48. Akkermann S, Halling T, Löffler F, Silber-Peest AS, Krüger T, Bleich S, et al. Impact of COVID-19 on medical supply in adults with congenital heart disease. Front Psychiatry. 2023;13:812611. doi:10.3389/fpsyt.2022.812611. [Google Scholar] [CrossRef]
49. IPCC, Pörtner, HO, Roberts DC, Poloczanska ES, Mintenbeck K, Tignor M, et al. Climate change 2022: impacts, adaptation, and vulnerability. Contribution of working group II to the sixth assessment report of the intergovernmental panel on climate change. In: Pörtner HO, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, et al., editors. Summary for policymakers. UK: Cambridge University Press; 2022. [Google Scholar]
50. Cheng SPS, Heo K, Joos E, Vervoort D, Joharifard S. Barriers to accessing congenital heart surgery in low-and middle-income countries: a systematic review. World J Pediatr Congenit Heart Surg. 2024;15:94–103. doi:10.1177/21501351231204328. [Google Scholar] [CrossRef]
51. Adeoye M, Rahimzadeh S, Taylor S, Shrikhande S, Perel P, Shah A, et al. The impact of air pollution on cardiovascular health outcomes in African populations. JACC Adv. 2024;3:101371. doi:10.1016/j.jacadv.2024.101371. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools