 Open Access
Open Access
CASE REPORT
Delayed Umbilical Venous Access for Stenting of Complex Obstructed TAPVR in a Premature Low Birth Weight Infant: A Case Report
1 Division of Pediatric Cardiology, Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, WI 53792, USA
2 Division of Neonatology, Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, WI 53792, USA
* Corresponding Author: Jesse Boyett Anderson. Email:
(This article belongs to the Special Issue: Novel Methods and Techniques for the Management of Congenital Heart Disease)
Congenital Heart Disease 2025, 20(6), 729-735. https://doi.org/10.32604/chd.2025.071937
Received 16 August 2025; Accepted 15 December 2025; Issue published 10 February 2026
Abstract
Obstructed infradiaphragmatic total anomalous pulmonary venous return (TAPVR) in premature infants presents significant management challenges due to the high surgical risk in low-birth-weight, preterm neonates. We present strategies for managing this condition in a 10-day old 1.3 kg ex-32-week premature infant including late umbilical venous access, use of wire-snare rail for stable stent deployment, and monitoring for progressive multi-level obstruction. Long-term follow-up demonstrated spontaneous stent fracture and occlusion. This approach successfully bridged to definitive repair with excellent outcomes.Keywords
Supplementary Material
Supplementary Material FileTotal anomalous pulmonary venous return (TAPVR) is a rare form of complex congenital heart disease characterized by return of oxygenated blood from the lungs to the right, instead of left, atrium. The blood may follow one of four anomalous routes to the right atrium. Vascular routes that traverse the diaphragm en route from the lungs to the right atrium have the highest risk of becoming obstructed. Obstructed TAPVR is a surgical emergency, often requiring repair in the first days of life to prevent mortality.
The repair of TAPVR in preterm infants presents significant management challenges due to the high surgical risk in low-birth-weight neonates [1]. While previous reports have described ductus venosus (DV) stenting via transumbilical approach as a palliative strategy in preterm, low birth weight infants [2,3,4,5], this case illustrates the feasibility of late access of the umbilical vein, demonstrates the procedural advantages of creating a wire rail for stability during intervention, and highlights the challenges of identifying and managing multiple levels of progressive obstruction and determining optimal timing for intervention(s).
A 1.3 kg ex-32-week gestation twin with intrauterine growth restriction developed progressive respiratory distress on day of life (DOL) nine. Chest X-ray showed significant pulmonary edema. Echocardiography confirmed obstructed infradiaphragmatic TAPVR with a large secundum atrial septal defect. The patient was not a surgical candidate due to technical limitations and known complications of cardiopulmonary bypass in low birth-weight, premature neonates.
On DOL 10, in preparation for catheterization, the fibrotic and desiccated umbilical stump, which had not previously been accessed, was prepared for placement of an umbilical venous catheter (UVC). The cord was softened by wrapping it in saline-soaked gauze for approximately 30 min to facilitate vessel access. A transverse cut was then made with an 11-blade scalpel to expose the umbilical vein, which was identified by the presence of a dark intraluminal clot. The clot was gently grasped and removed, and the vein was probed and dilated using straight and curved iris forceps. The 5 Fr UVC was then advanced into the right portal vein under direct visualization.
In the catheterization laboratory, her right neck, bilateral groins and the entire umbilical area including the line were prepped and draped in the normal sterile fashion. Using ultrasound guidance, the right internal jugular (IJ) vein was accessed and a 4 Fr sheath was placed. After 50 units of heparin and 30 mg/kg of cefalexin were given, a 4 French Berman angiographic catheter was advanced into the pulmonary arteries (PA). Limited hemodynamics demonstrated PA systolic pressures 70% systemic and angiography confirmed infradiaphragmatic TAPVR with mild narrowing at the connection of the vertical vein (VV) to the right portal vein (PV) and no obvious flow through the ductus venosus (DV) back to the right atrium (Fig. 1A, Supplemental Video S1).
Due to concern for severe obstruction at the level of the DV, this structure was further investigated. After pulling the UVC back slightly, a 0.014 Cougar exchange length wire was advanced toward the DV. With some probing and manipulation, the wire was eventually advanced through the DV into the right atrium. Given the anticipated challenges of maintaining wire stability in this small infant, a wire-snare rail was created using a 4-French angled glide catheter advanced into the IJ sheath and a 10 mm gooseneck snare advanced through the glide catheter to the SVC-RA junction. The Cougar exchange length wire was snared and externalized through the right IJ to create a stable rail over which the subsequent intervention was performed.
The UVC was removed and a 4-French Prelude sheath was advanced through the stenotic DV into the right atrium. Angiography through the sheath confirmed DV and PV anatomy demonstrating a 15 mm long DV narrowing to ~1.2 mm along its entire length (Fig. 1B).
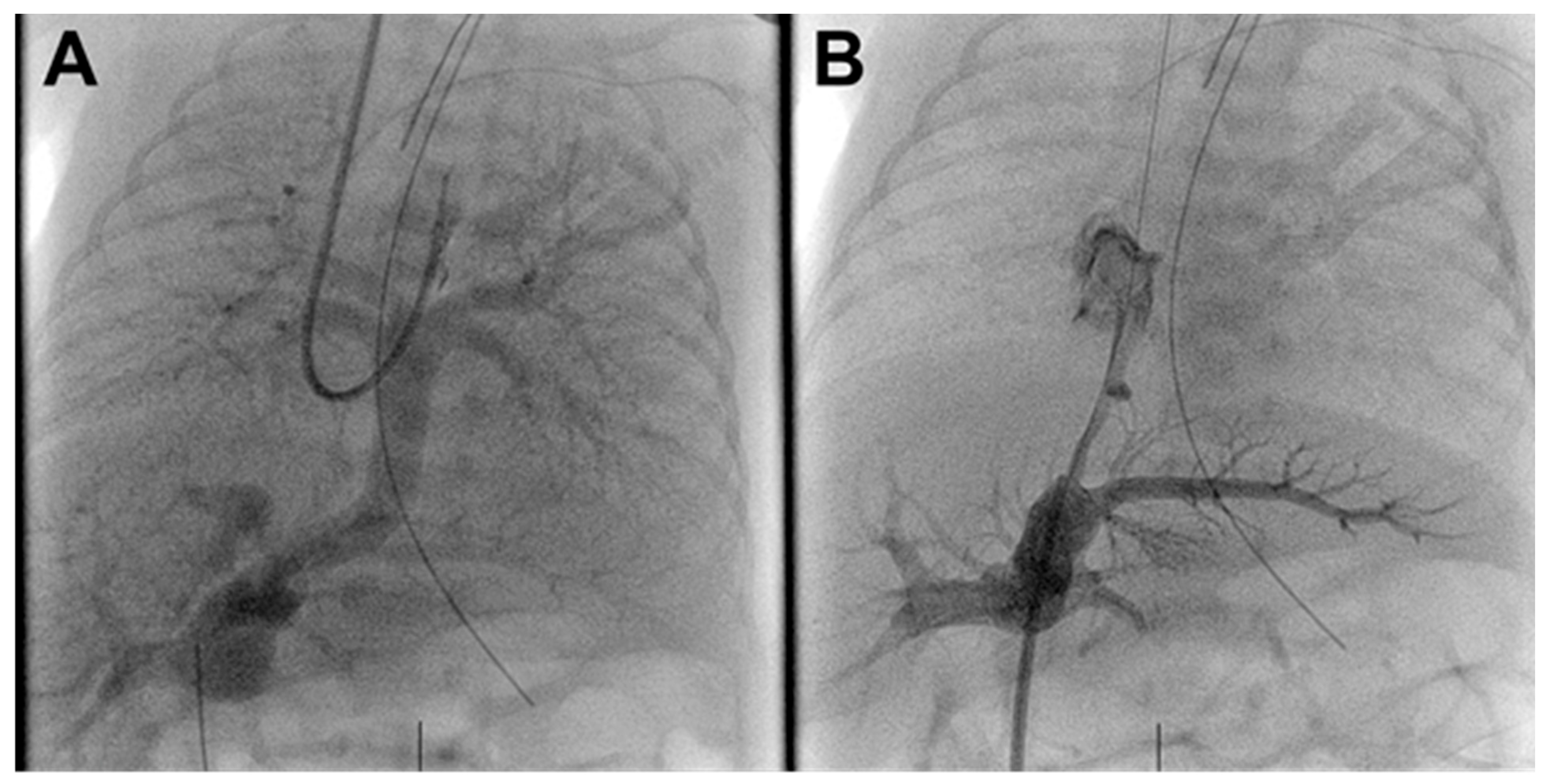
Figure 1: Pre-intervention angiograms. (A). Venous phase pulmonary angiogram demonstrating infradiaphragmatic TAPVR to the portal venous system without vertical vein stenosis. (B). Severe ductus venosus stenosis with filling of the portal venous system.
A 15 × 4 mm Onyx drug-eluting stent (Medtronic Inc., Santa Rosa, CA, USA) was used for intervention. This stent was selected due to its low-profile delivery system, fair radial strength, and drug-eluting properties to potentially reduce the risk of in-stent restenosis. The stent was easily deployed in the DV (Fig. 2A). Post deployment angiography showed improved flow through the TAPVR with no residual DV stenosis (Fig. 2B, Supplemental Video S1).
The infant’s lung compliance and oxygenation improved immediately. Preprocedural blood gas was 7.43/48/104 with SpO2 96% on 43% FiO2. Post procedural blood gas was 7.54/34/110 with SpO2 97% on FiO2 28%. A final PA injection showed no residual stenosis. Given improvement in respiratory status and angiographic evidence of relief of obstruction, the decision was made to end the case. Total contrast 10.8 mL/kg. Fluoroscopy time 11 min. Radiation dose 11 mGy.
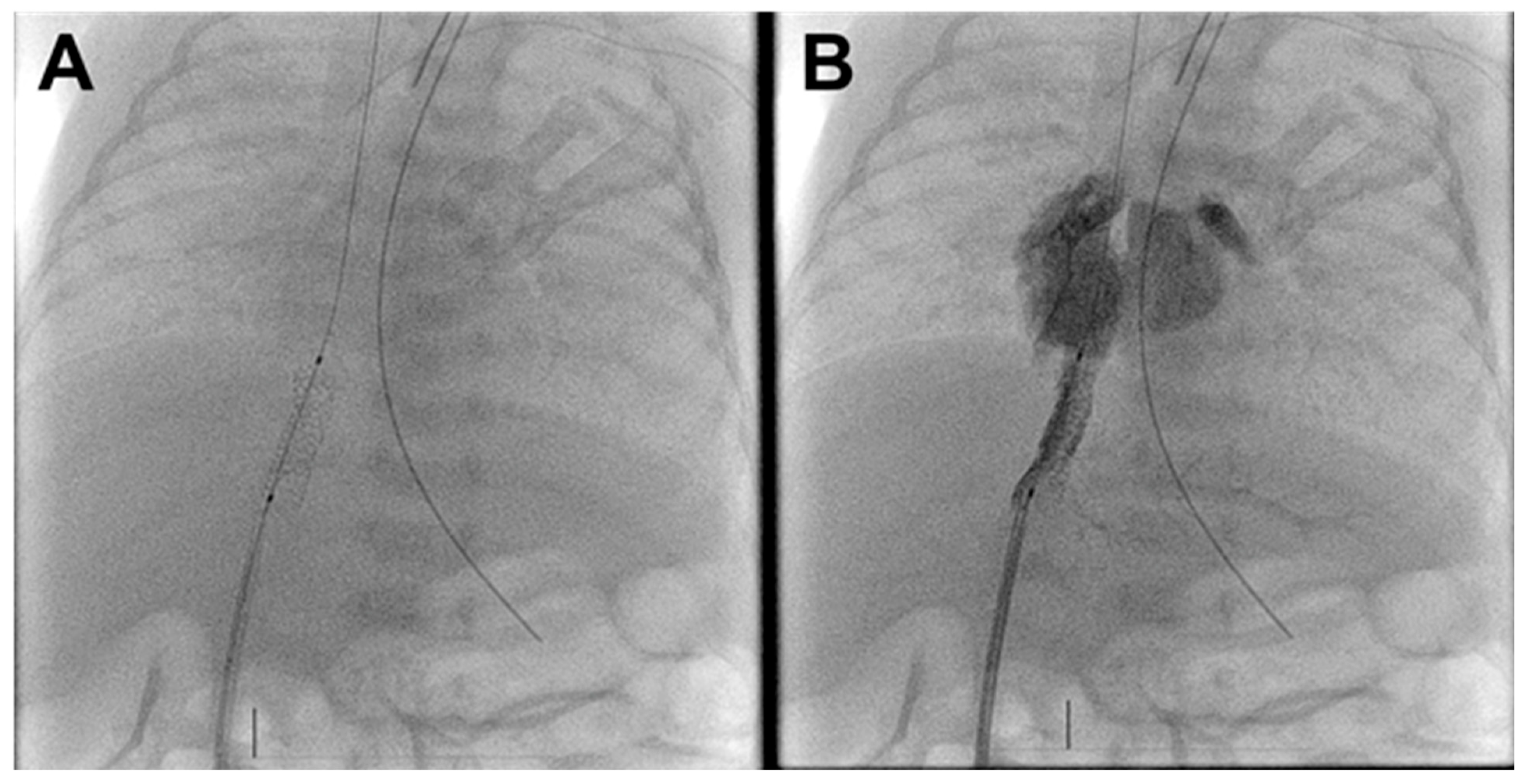
Figure 2: Post-intervention fluoroscopy and angiogram. (A). Well-positioned ductus venosus stent. (B). Umbilical vein injection demonstrates unobstructed ductus venosus flow to the right atrium with limited portal venous filling.
The infant was extubated within 24 h. She remained hospitalized due to gestational age, size, and respiratory status on a heparin drip to prevent stent thrombosis (goal anti-Xa 0.3–0.7).
The hope was that stenting would temporize her cardiac status and allow her to grow until she was out of the highest risk for intraventricular hemorrhage with cardiopulmonary bypass (>36 weeks corrected gestational age). Despite initial improvement, the infant required increasing respiratory support over the next three weeks, eventually requiring re-intubation. Serial echocardiography demonstrated stent patency with no consistent changes in the gradient across her stent (Supplemental Video S3). There was no laboratory evidence of hepatic dysfunction.
Planned CT imaging at 37 weeks corrected gestational age demonstrated moderate VV obstruction at the level of the diaphragm and at the connection of the VV to the right portal vein (Fig. 3 and Fig. 4, Supplemental Video S4).
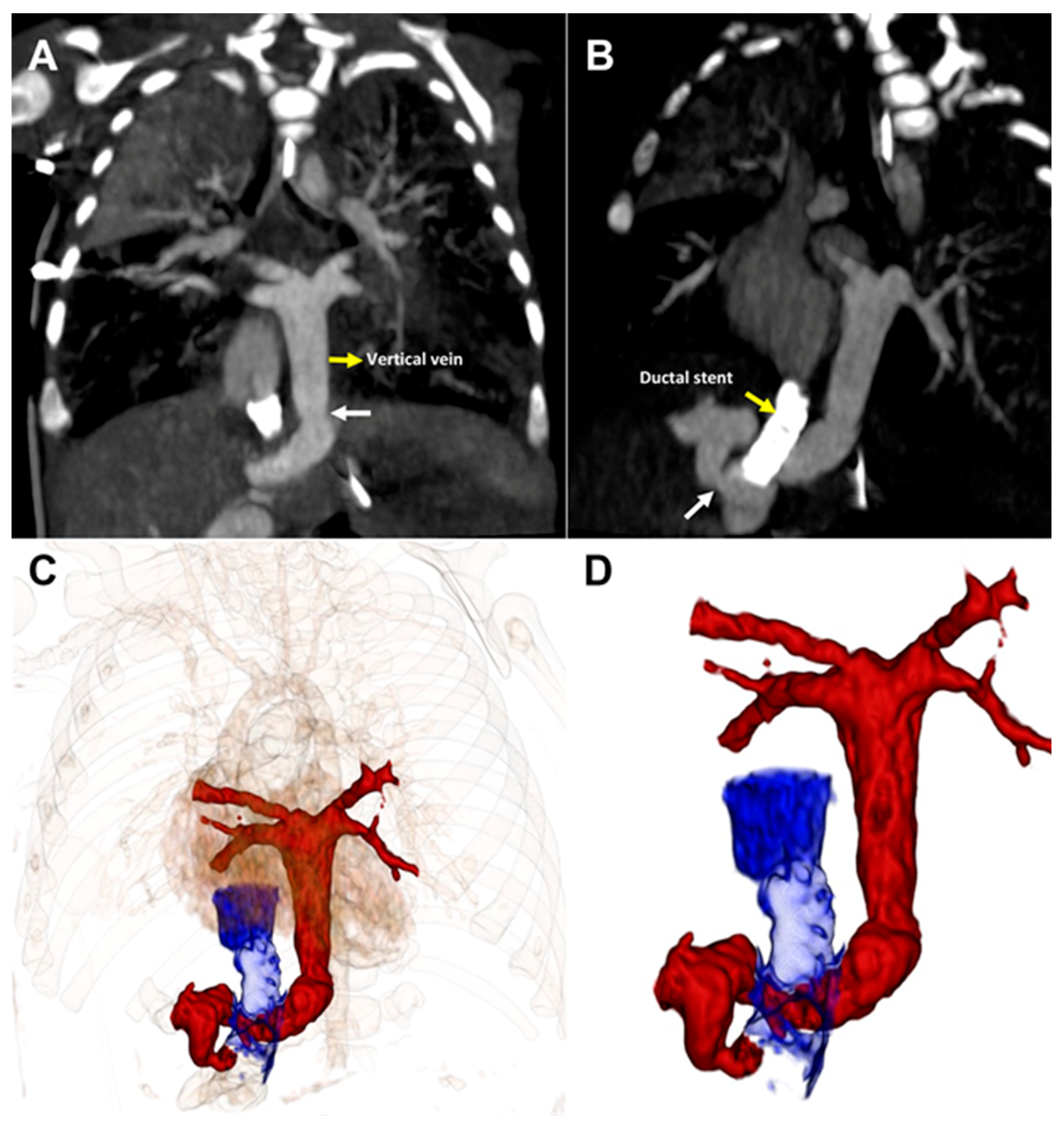
Figure 3: CT imaging (A). Coronal and (B). Sagittal views demonstrate vertical vein stenosis (white arrow) at both the diaphragm and the portal vein connection. (C,D). 3-D reconstructions demonstrate the relationships of the ductus venosus stent to the areas of stenosis.
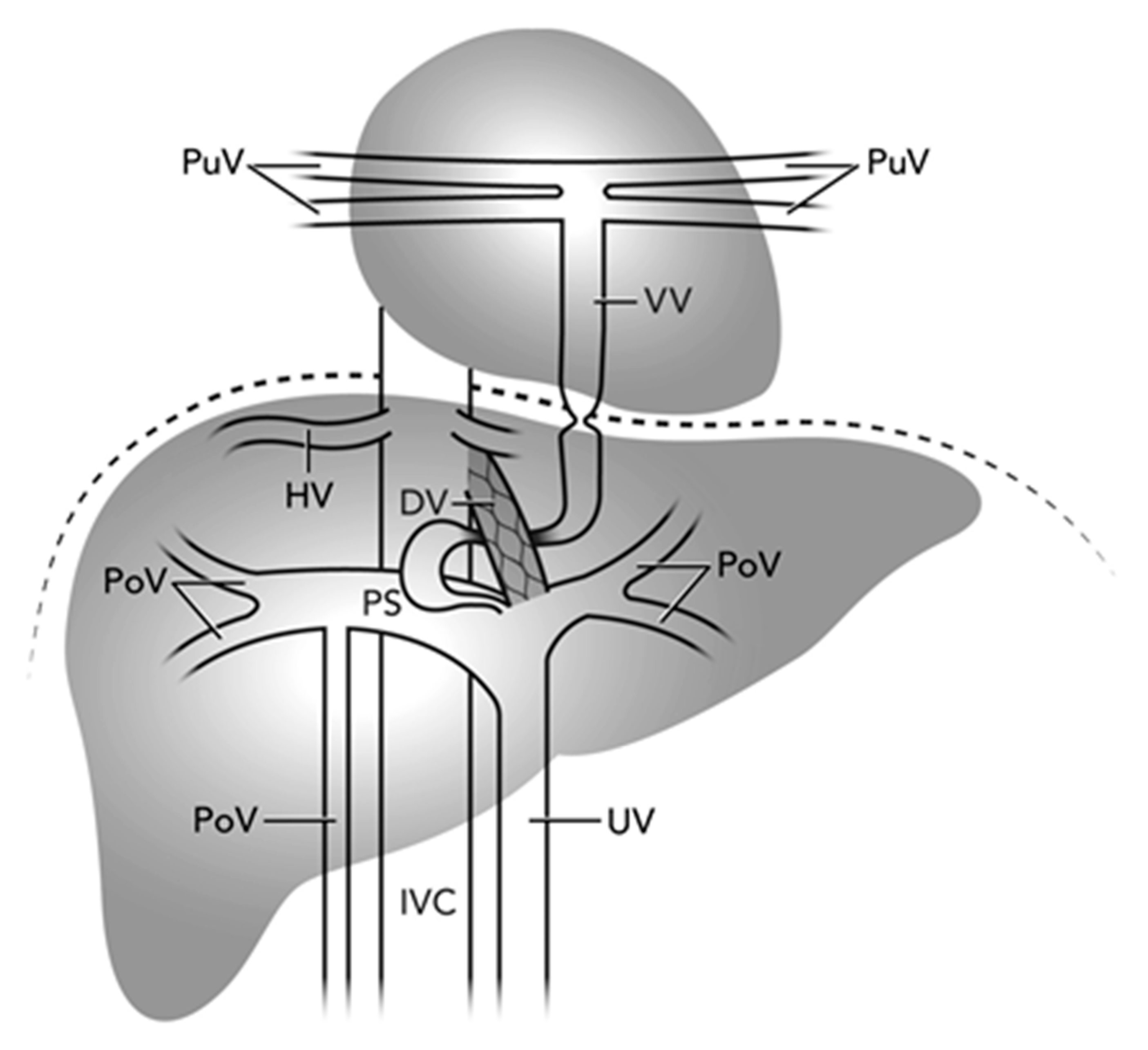
Figure 4: Illustration of the complex course and multilevel TAPVR narrowing one month after ductus venosus stenting. Pulmonary Veins (PuV), Portal Veins (PoV), Hepatic Veins (HV), Inferior Vena Cava (IVC), Vertical Veins (VV), Ductus Venosus (DV), Umbilical Vein (UV), Portal Sinus (PS).
Following multidisciplinary discussion and given the patient’s interval growth to 1.7 kg, the decision was made to proceed with surgical repair. TAPVR repair was performed via direct anastomosis with subtotal closure of the atrial septal defect. The VV and DV stent were intentionally left open to temporarily provide decompression of the pulmonary venous system in case of any anastomotic stenosis and to reduce risk of pulmonary hypertensive crisis, allowing for potential subsequent spontaneous closure [6]. Heparin was discontinued to allow for eventual spontaneous stent thrombosis.
The infant received prophylactic pulmonary hypertension therapy and underwent delayed sternal closure on post-operative day six. Post-operative complications included pneumothorax and chylous effusions. The infant was discharged on DOL 82 on room air, low-dose diuretics, and without anticoagulation. She had serial laboratory evaluations for liver function given the risk of systemic venous congestion due to patent stented ductus venosus.
At six months, echocardiography showed widely patent pulmonary venous return to the left atrium and residual DV stent flow, with normal liver function and ammonia levels. By 15 months, elevated alkaline phosphatase and ammonia levels raised concern for systemic venous congestion in the setting of echocardiographically-confirmed patent ductus venosus. Cardiac catheterization performed at 16 months with the intent to occlude the stent [6] revealed normal right heart pressures and output, no significant pulmonary venous obstruction, and fracture with occlusion of the lower three-quarters of the DV stent; ammonia levels had normalized. These findings confirmed the durability of surgical repair and illustrated the natural course of the DV stent, which fractured and occluded spontaneously without requiring planned closure.
This case illustrates key strategies and considerations for managing infradiaphragmatic TAPVR in premature infants. First, successful late UVC placement on DOL 10 through an umbilical stump reinforces the extended window for umbilical access. Rothman et al. described transumbilical stenting in three patients, two were two days old and one was 13 days old. Our case adds to this limited literature by detailing successful access through a previously unused, scarred umbilical stump, providing additional evidence that late UVC placement remains viable for intervention beyond the traditional immediate post-delivery period.
Second, our wire-snare rail technique provided exceptional stability for stent deployment, particularly valuable in extremely small infants where catheter backlash can compromise positioning. This technical advancement has not been previously reported for DV stenting.
Third, our case uniquely documents the variable and dynamic nature of TAPVR obstruction and the importance of comprehensive imaging and ongoing surveillance after intervention. Prolonged DV patency in prematurity likely contributed to the delayed presentation in this case [7]. Autopsy studies describe variable anomalous connections in infradiaphragmatic TAPVR, with connections to the portal venous system being most common, followed by connections to the inferior vena cava or directly to the DV [8]. Obstruction can develop at multiple levels with reports of successful palliation of both DV and VV obstructions via stenting [2,3,4]. Improper stent positioning within the portal venous system may worsen TAPVR obstruction if the VV connection is inadvertently jailed [2]. To precisely define this variable anatomy and avoid this complication, pre-procedure CT with 3D reconstruction is recommended when feasible; otherwise, detailed angiography to define TAPVR anatomy is essential before stenting. Post-stenting, close monitoring is essential, as repeat catheterizations may be needed for in-stent stenosis, thrombosis, or atrial septal restriction [2,3,4,5]. This case illustrates progressive VV stenosis at the diaphragm and within the liver parenchyma, both initially mild or absent (Fig. 4).
The long-term follow-up provides valuable insights into the natural history of DV stents in this setting. The spontaneous fracture and occlusion at 16 months with normalization of liver function suggests that planned closure intervention may be unnecessary with careful monitoring.
Infradiaphragmatic TAPVR in premature infants is challenging, but this case highlights how catheter-based palliation—using late umbilical access and a wire-snare rail technique—can effectively bridge to surgical repair. Recognizing multi-level obstruction, ensuring close surveillance, and observing spontaneous stent occlusion on follow-up offer important insights for ongoing management.
Acknowledgement:
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: conceptualization, Jesse Boyett Anderson and Luke Lamers; methodology, Jesse Boyett Anderson, Suhaib Kazmouz, Ryan M. McAdams and Luke Lamers; writing—original draft preparation, Jesse Boyett Anderson; writing—review and editing, Jesse Boyett Anderson, Suhaib Kazmouz, Ryan M. McAdams and Luke Lamers. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Ethics Approval: This case report describes a single patient and does not meet the definition of human subjects research. IRB exemption has been confirmed, written informed consent for publication has been obtained from the patient’s legal guardians and both are available upon request.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://www.techscience.com/doi/10.32604/chd.2025.071937/s1. Four supplementary videos are available.
Abbreviations
| CHD | Congenital heart disease |
| CT | Computed tomography |
| DOL | Day of life |
| DV | Ductus venosus |
| IJ | Internal jugular |
| IV | Intravenous |
| IVC | Inferior vena cava |
| PA | Pulmonary artery |
| PoV | Portal vein |
| PS | Portal sinus |
| PuV | Pulmonary vein |
| TAPVR | Total anomalous pulmonary venous return |
| UV | Umbilical vein |
| UVC | Umbilical venous catheter |
| VV | Vertical vein |
References
1. Costello JM , Pasquali SK , Jacobs JP , He X , Hill KD , Cooper DS , et al. Gestational age at birth and outcomes after neonatal cardiac surgery: an analysis of the society of thoracic surgeons congenital heart surgery database. Circulation. 2014; 129( 24): 2511– 7. doi:10.1161/CIRCULATIONAHA.113.005864. [Google Scholar] [CrossRef]
2. Cameron JE , Badran S , Cleveland J , Patel ND . Transjugular ductus venosus stent placement in an extremely premature, low birth weight neonate with infradiaphragmatic total anomalous pulmonary venous connection. Prog Pediatr Cardiol. 2022; 67: 101571. doi:10.1016/j.ppedcard.2022.101571. [Google Scholar] [CrossRef]
3. Chamberlain RC , Hill KD , Fleming GA . Palliating premature infants with obstructed total anomalous pulmonary venous connection via catheterization. World J Pediatr Congenit Heart Surg. 2020; 11( 4): NP164– 7. doi:10.1177/2150135118782191. [Google Scholar] [CrossRef]
4. Meadows J , Marshall AC , Lock JE , Scheurer M , Laussen PC , Bacha EA . A hybrid approach to stabilization and repair of obstructed total anomalous pulmonary venous connection in a critically ill newborn infant. J Thorac Cardiovasc Surg. 2006; 131( 4): e1– 2. doi:10.1016/j.jtcvs.2005.12.009. [Google Scholar] [CrossRef]
5. George RS , Lozier JS , Bocks ML . Palliative stenting of the venous duct in a premature neonate with obstructed infradiaphragmatic total anomalous pulmonary venous connection. Cardiol Young. 2023; 33( 4): 633– 6. doi:10.1017/S1047951122002219. [Google Scholar] [CrossRef]
6. Gumustas M , Donmez YN , Aykan HH , Demircin M , Karagoz T . Transcatheter closure of patent vertical vein after repair of total anomalous pulmonary venous connection: a case series. Cardiol Young. 2021; 31( 11): 1853– 7. doi:10.1017/S1047951121001517. [Google Scholar] [CrossRef]
7. Fugelseth D , Lindemann R , Liestøl K , Kiserud T , Langslet A . Postnatal closure of ductus venosus in preterm infants ≤32 weeks: an ultrasonographic study. Early Hum Dev. 1998; 53( 2): 163– 9. doi:10.1016/S0378-3782(98)00051-6. [Google Scholar] [CrossRef]
8. Duff DF , Nihill MR , McNamara DG . Infradiaphragmatic total anomalous pulmonary venous return. Review of clinical and pathological findings and results of operation in 28 cases. Heart. 1977; 39( 6): 619– 26. doi:10.1136/hrt.39.6.619. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools