 Open Access
Open Access
ARTICLE
Cervical Cancer Prediction Empowered with Federated Machine Learning
1 Department of Computer Sciences, Bahria University Lahore Campus, Lahore, 54000, Pakistan
2 Faculty of Information Technology, Liwa College, Abu Dhabi, 20009, UAE
3 Department of Computing, Skyline University College, Sharjah, 999041, UAE
4 Department of Computer Science, Taibah University, Medina, 42315, Saudi Arabia
5 Riphah School of Computing and Innovation, Faculty of Computing, Riphah International University, Lahore, 54000, Pakistan
6 Department of Software, Faculty of Artificial Intelligence and Software, Gachon University, Seongnam, 13120, Korea
* Corresponding Author: Khan Muhammad Adnan. Email:
Computers, Materials & Continua 2024, 79(1), 963-981. https://doi.org/10.32604/cmc.2024.047874
Received 20 November 2023; Accepted 01 March 2024; Issue published 25 April 2024
Abstract
Cervical cancer is an intrusive cancer that imitates various women around the world. Cervical cancer ranks in the fourth position because of the leading death cause in its premature stages. The cervix which is the lower end of the vagina that connects the uterus and vagina forms a cancerous tumor very slowly. This pre-mature cancerous tumor in the cervix is deadly if it cannot be detected in the early stages. So, in this delineated study, the proposed approach uses federated machine learning with numerous machine learning solvers for the prediction of cervical cancer to train the weights with varying neurons empowered fuzzed techniques to align the neurons, Internet of Medical Things (IoMT) to fetch data and blockchain technology for data privacy and models protection from hazardous attacks. The proposed approach achieves the highest cervical cancer prediction accuracy of 99.26% and a 0.74% misprediction rate. So, the proposed approach shows the best prediction results of cervical cancer in its early stages with the help of patient clinical records, and all medical professionals will get beneficial diagnosing approaches from this study and detect cervical cancer in its early stages which reduce the overall death ratio of women due to cervical cancer.Keywords
Invasive cervical cancer affects the innate tissues of the cervix in women. Cervical cancer has the potential to spread to other regions of the body, such as the lungs, liver, bladder, vagina, and rectum. Healthy cells in the cervix accumulate changes in their Deoxyribonucleic acid (DNA) that cause them to grow, reproduce at a specific rate, and eventually die at a given period. Furthermore, sick cells’ aberrant mutational processes cause them to proliferate and replicate out of control, amassing abnormal cells that create a deadly tumor. Bad cells in the cervix affect the initial stages of pregnancy and have a direct effect on womb health. Tumor in the cervix causes bad intercourse results and puts a man’s life in danger too because cervical tumor cells directly affect a man’s genital organ.
Unusual discomfort after sex, vaginal hemorrhage after coitus, between menstruation, after puberty, or after a gynecological exam, and vaginal bleeding are the most commonly reported indications of cervical cancer. Many sexual partners, early sexual interaction, sexually transmitted diseases, a weak immune system, smoking, exposure to anti-miscarriage medicines, and other risk factors are among the most prominent [1].
The sexually disseminated human papillomavirus (HPV) is important in the genesis of cervix cancer and venereal herpes. Among the 100 different HPV traumata, HPV-16 and HPV-18 are the most famous cancer-causing types. Papillary cancer, pelvic cancer, testicular cancer, rectal cancer, colon cancer, and oral cancer [2] are all caused by the oncogenic strain of HPV [3]. Cervical cancer is classified into four stages based on its level of spread: Stage 1 only propagates to the lymph nodes, stage 2 propagates outside the vagina and vulva or to the lymph glands, stage 3 propagates to the bottom section of the vagina or femur along it inhibits the renal pelvis, the tube that connects from bladder to kidney for urine, and stage 4 propagates outside the femur to organs such as the liver, bones, or spleen [4]. The HPV vaccination is the simplest way to prevent cervical cancer, contrary to having regular Pap checks, performing safe sex, evading smoking, and so on [5,6].
Machine learning and deep learning are used for detecting brain tumors, cervical cancer, breast cancer, COVID-19, identifying physical activity, detecting wind chill, and assessing the cognitive health of dementia patients [7–11]. It is more productive than conventional detection approaches due to advancements in the healthcare industry [12–15]. According to medical data issued by Global Cancer Statistics each year, 493,000 cervical cancer patients have been added, with cervical cancer accounting for 15% of the total. This illness is most prevalent in impoverished nations, where it has an 83 percent fatality rate. In African nations, for example, Uganda ranks fifteenth in terms of the greatest frequency of cervical cancers, with 65 percent of confirmed cases.
Cervical cancer, on either hand, is the most treatable kind if detected early and treated effectively. The above-mentioned approach takes more effort to analyze the input, and the low-level characteristics acquired do not provide ideal categorization effectiveness, underlining the shortcomings of learning algorithms. In terms of generating an enhanced computer-aided testing framework, a machine learning-based feature-based strategy has significant benefits over all previous cancer detection algorithms. A machine learning-based method produces cutting-edge results in complex computer imaging problems [16].
According to present research, most cervical precancerous illness categorization study relies on individual colposcopy images during acetic acid testing, making cervical cancer difficult to diagnose. This article focuses on a variety of federated machine learning solvers that may reliably detect the development of cervical cancer using a given number of factors of potential risk for each woman. However, when working with an analytic suite to construct a predictor, recall consistency precision, and accuracy might be a challenge. The primary goals of this study are to improve classification performance using federated machine learning classification techniques and provide performance, and analysis of the results based on the main parameters stated in the rapid prediction of cervical cancer risk factors in early stages.
These are the major objectives that are going to be covered in this study:
• State the implications of previous studies.
• State the research methodology clearly to predict cervical cancer.
• The proposed methodology uses federated learning and is explained in detail.
• The proposed methodology uses numerous statistical metrics to check the feasibility of the prediction of the model.
Amazing machine learning offers a wide range of applications for cancer diagnosis in all types of flora and fauna. Currently, a huge variety of machine learning models are presented and used for certain application fields to accelerate and enhance research.
In [17], the authors employed four dependent variables with 32 independent features for analyzing and predicting cervical cancer which is the leading cause of death. To incorporate the major symptoms of cervical cancer they developed a machine learning model including numerous machine learning algorithms i-e logistic regression, decision tree, ensembled method, and random forest. Their model achieved the cervical prediction area under the curve with a classification accuracy of 98.56% and their ensembled method achieved the prediction area under the curve with the help of a synthetic minority over-sampling technique achieved 98.49%.
The authors [18] studied that the grim spread of cervical cancer is a very solemn matter in privileged and underprivileged countries due to the lack of health education, health awareness, and cervical cancer diagnostic facilities. Post-prediction of cervical cancer is very dangerous and leads to death. So, they used the University of California Irvine (UCI) private cervical cancer dataset for machine learning algorithms i-e linear regression, decision tree, support vector machine multi-layer perceptron, and k-nearest neighbors training and prediction diligence. Their model used an adaptive and improved approach to predict cervical cancer and achieved 83.16% cervical cancer prediction accuracy. In [19], authors employed the model to explore the different conditions of cervical cancer in Indonesia. They used clinical data from 38 cervical cancer patients. Their proposed model used random forest with a combination of training and testing sampling to predict cervical cancer. Their employees achieved 50% cervical cancer prediction accuracy.
Over the last decade, the most frequent cancer in women’s bodies has received a lot of focus from research organizations in terms of early identification and treatment. Machine Learning (ML) utilizing classification methods including Multilayer Perceptron (MLP), Decision Trees (DT), Random Forest (RF), K-Nearest Neighbor (KNN), and Nave-Bayes (NB) is used in this study to diagnose early-stage cervical cancer using a dataset derived from ICU risk variables [20]. Using train and test processes, combining various machine learning algorithms in one model exhibited 87.21% cervical cancer prediction accuracy.
The data set is introduced in this study [21] by questioning 858 patients with a total of 33 variables to do a prediction analysis on cervical cancer eradication. This data set was separated into two parts: Training and testing. For the right prediction, multiple machine learning approaches with segmentation such as Multilayer Perceptron, Nave-Bayes, and k-Nearest Neighbor were used. This algorithm’s performance is evaluated using a confusion matrix and accurately identified cases and their employed model achieved 95.89% cervical cancer prediction accuracy.
The study [22] classified cervical cancer material from UCI using three types of machine learning algorithms. To deal with the data set’s imbalance, the suggested model uses the clustering technique of border rows. According to this study, the XG-Boost and Random Forest techniques perform exceptionally well in terms of cancer prediction accuracy rates. Because these cancer data contain numerous missing and partial features, missing attributes must be used with caution. This study employed a model that achieved accuracy area under the curve using 5-fold cross-validation 0.97, 0.82, 0.84, 0.79 of Resnet, XG-boost, support vector machine, and random forest, respectively.
Providing a universal model for the prediction of cervical cancer is very problematic due to computationally and feature-based problems. So, in this study [23], authors proposed a machine learning technique empowered with a supervised optimization model and classification models. Their proposed approach achieved an area under a curve of 0.6875.
Prediction of cervical cancer tumors at the pre-mature stage is very complicated. Early screening does not provide as much better results. So, in this delineated study [24], authors proposed an approach that used machine learning algorithms to predict the risk factors of cervical cancer. Their proposed approach used the random forest technique to predict cervical cancer and their model achieved 93.6% cervical prediction accuracy.
Diagnosing the slow growth of cervix cancer tumors is a problematic and time-consuming process. Delaying in the diagnosis of cervical cancer leads to death. Authors [25] proposed a machine learning-based model that used MLP, RF, KNN, DT, Support vector machine (SVM), Logistic regression (LR), Gradient boosting (GB), and Ada boost algorithms. Their proposed approach of MLP achieved the best cervical cancer prediction accuracy of 97.4%.
Pap smear testing is a primary screening model for cervical cancer prediction in the early stages. So, in the study [26], the authors employed a machine learning technique empowered with the Boruta analysis method which improved RF and SVM challenges. In this study, the main focus is to classify the risk factors in the early stages so, their proposed approach of SVM achieved 0.8456 precision, 0.812 recall, and Boruta analysis achieved 0.912 precision and 0.891 recall. Ozbay et al. [27] predicted cervical cancer at a premature level using transfer learning and their proposed model CRI-ResNet-V1 achieved 0.6894 R2. Fan et al. [28] detected cervical cancer using image classification with the help of conjugated attention mechanism and visual transfer and they achieved a 96.84% positive probability.
Compiling the electronic health records of cervical cancer patients is complicated and in parallel, it is very challenging to diagnose the risk dynamics of cervical cancer using cervical cancer patients’ electronic health records. So, in this delineated study [29], authors proposed approach used a deep learning approach of Long short-term memory (LSTM) neural network and RF to classify the risk factors of the cervical cancer patient with the help of electronic records. So, their proposed model of RF achieved an area under the curve is 0.70 a year before diagnosing cervical cancer and an area under the curve of 0.97 a day before.
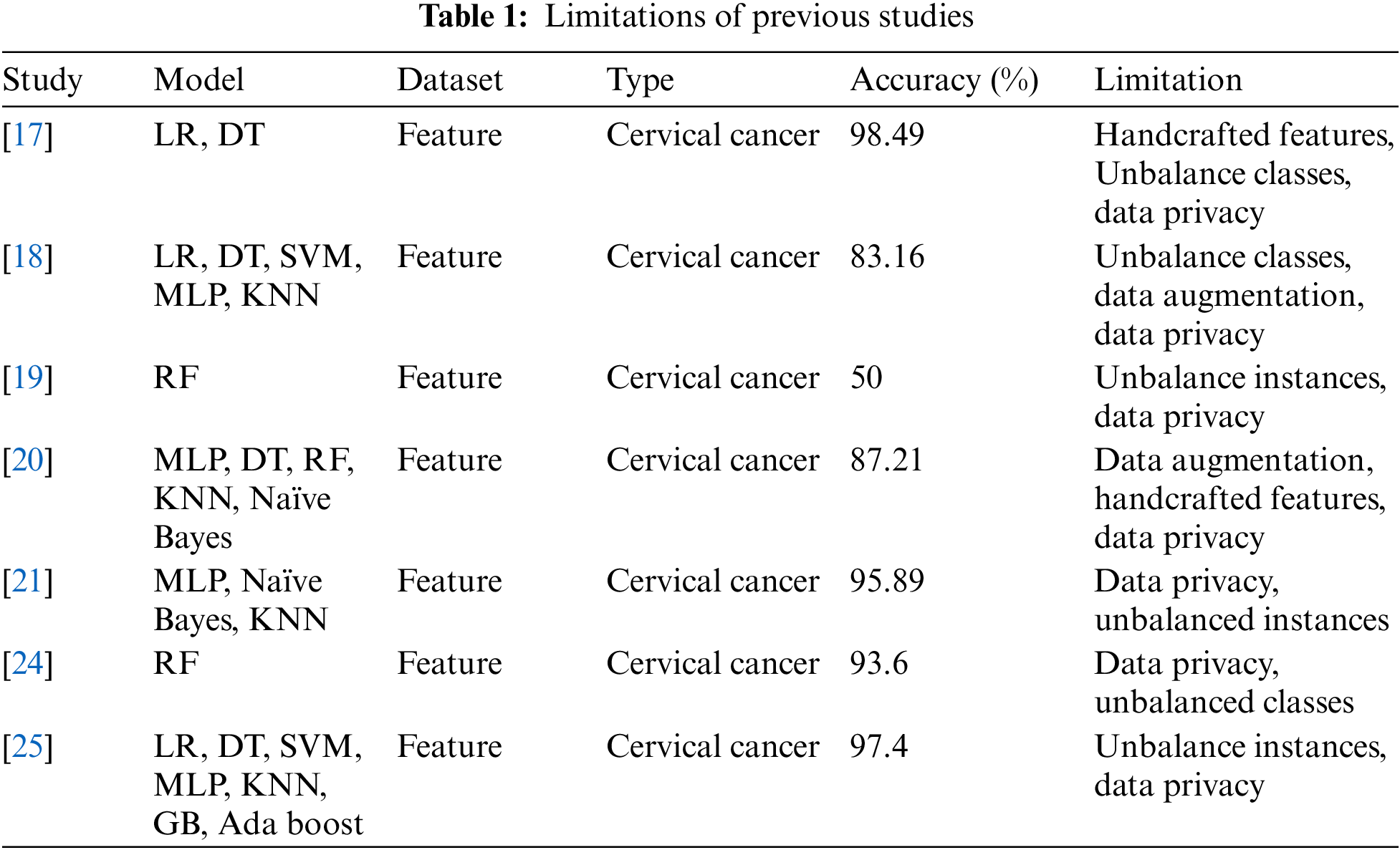
Table 1 shows the details about previous studies and their limitations so, it depicts that reference [17] used LR, DT machine learning techniques applied on feature dataset and achieved 98.49% cervical cancer prediction accuracy and handcrafted features, unbalance classes and data privacy as study limitation, reference [18] used LR, DT, SVM, MLP and KNN machine learning techniques applied on feature dataset and achieved 83.16% cervical cancer prediction accuracy and data augmentation, unbalance classes and data privacy as study limitation, reference [19] used RF machine learning technique applied on feature dataset and achieved 50% cervical cancer prediction accuracy and unbalance classes and data privacy as study limitation, reference [20] used MLP, DT, RF, KNN, NB machine learning techniques applied on feature dataset and achieved 87.21% cervical cancer prediction accuracy and data augmentation, handcrafted features and data privacy as study limitation, reference [21] used MLP, KNN, Naïve Bayes machine learning techniques applied on feature dataset and achieved 95.89% cervical cancer prediction accuracy and unbalance classes and data privacy as study limitation, reference [24] used RF machine learning technique applied on feature dataset and achieved 93.6% cervical cancer prediction accuracy and unbalance classes and data privacy as study limitation and reference [25] used LR, DT, SVM, MLP, KNN, GB, Ada boost machine learning techniques applied on feature dataset and achieved 97.4% cervical cancer prediction accuracy and unbalance classes and data privacy as study limitation. As far as the proposed study highlights the limitations of previous studies but in parallel, there are minor advantages of these studies these studies help the proposed model in finding out the best features for cervical cancer prediction and also help to find the best statistical parameters.
So, the major contributions of this study are given below to overcome the previous studies’ limitations:
• Applied federated machine learning techniques to get precise cervical cancer prediction results.
• The proposed approach uses IoMT to gather data from numerous patients.
• The proposed approach uses data augmentation techniques to overcome the unbalanced instance limitations.
• In this delineated study, the proposed approach uses a blockchain technology layer and access management approach to protect the patient’s data from hazardous attacks and trained model privacy, to check the accuracy the proposed study applied numerous statistical formulas to calculate the model accuracy.
In this study, the primary goal is to develop the federated machine learning technique using fuzzed neurons empowered with IoMT for patient data collection and the proposed study ensures the protection and privacy of patient data and the federated machine learning model. So, with the expansion of technology artificial intelligence plays a foremost role in medical analysis to predict cancer risk factors. To predict cervical cancer, the proposed model uses a federated machine learning technique with numerous solvers to predict the cervical cancer risk factors in the early stages. Fig. 1 shows the proposed model of cervical cancer prediction in its early stages. It depicts that the proposed model consists of five major sections (i) the data collection section empowered with IoMT (ii) the data pre-processing section (iii) the blockchain-empowered security section (iv) the training section (v) the testing section. In the first section, the proposed model uses IoMT to collect clinical data of numerous cervical cancerous patients and non-cancerous patients including their all-cervical cancer diagnosing reports. In the second section, the proposed model applies numerous data augmentation techniques to overcome the unbalanced instances limitation and make balanced data for further processing, right after data augmentation the proposed model applies data cleaning techniques including mean, median, and mode to remove the null values from data and also the proposed model remove the duplicate instances to make patient’s data better for training, at the end of data pre-processing section the proposed model stores pre-processed data in blockchain empowered security for data privacy and protection.
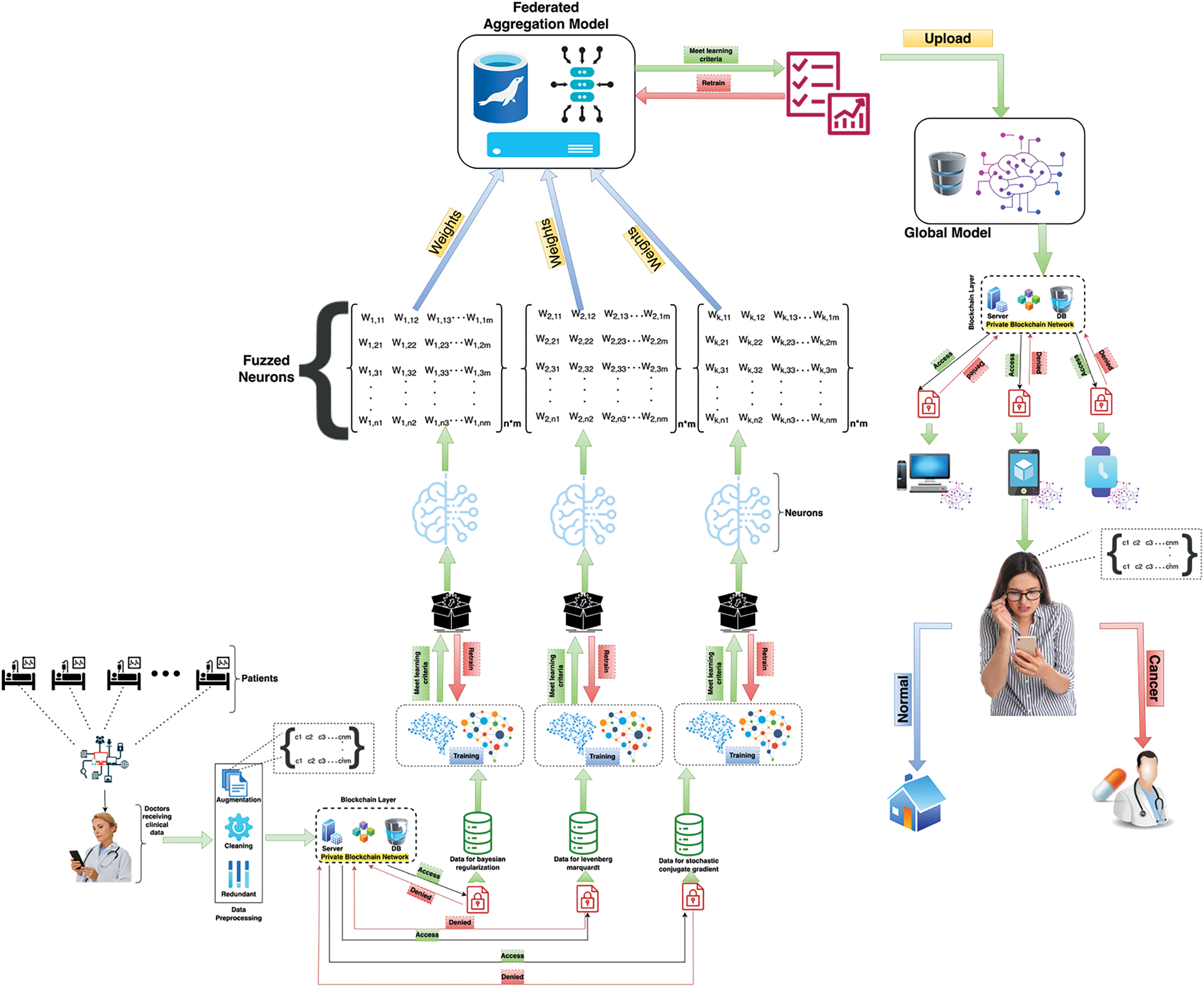
Figure 1: The proposed model for the prediction of cervical cancer using federated machine learning empowered with IoMT and Blockchain technology
In the fourth section, the proposed model divides the data into 80% for training sessions and 20% for testing sessions and feature extraction, the proposed model uses regression technique and then starts the model training process using machine learning solvers including Bayesian Regularization (BR), Scaled Conjugate Gradient (SCG), and Levenberg Marquardt (LM). All machine learning models import data from a private blockchain network and start training their weights with the varying number of neurons of each solver, after training checks the models, and performance using learning criteria, and if the learning criteria match the threshold, then move to tune the neurons and tune the neurons at the max of all models using matrix techniques which are described below to train models.
Eqs. (1)–(4) show the addition of weights of input hidden layers at the kth number of weights. All the above equations represent the weighted inputs and aggregation of weights.
Eqs. (5) and (6) calculate the maximum number of weights from all three models to start the process of addition.
Eqs. (7) to (9) put zeros in blank columns to perform a smooth addition process without any minimal error.
Eqs. (10a)–(10c) concatenate the weights of the input hidden layer horizontally of all models.
Eqs. (11) to (14)show the addition of weights of hidden output layers at the kth number of weights.
Eqs. (15) and (16) calculate the maximum number of weights from all three models from the output is the hidden layer to start the process of addition.
Eqs. (17) and (18) put zeros in blank columns to perform a smooth addition process without minimal error.
Eqs. (20) to (22) concatenate the weights of the output hidden layer horizontally of all models.
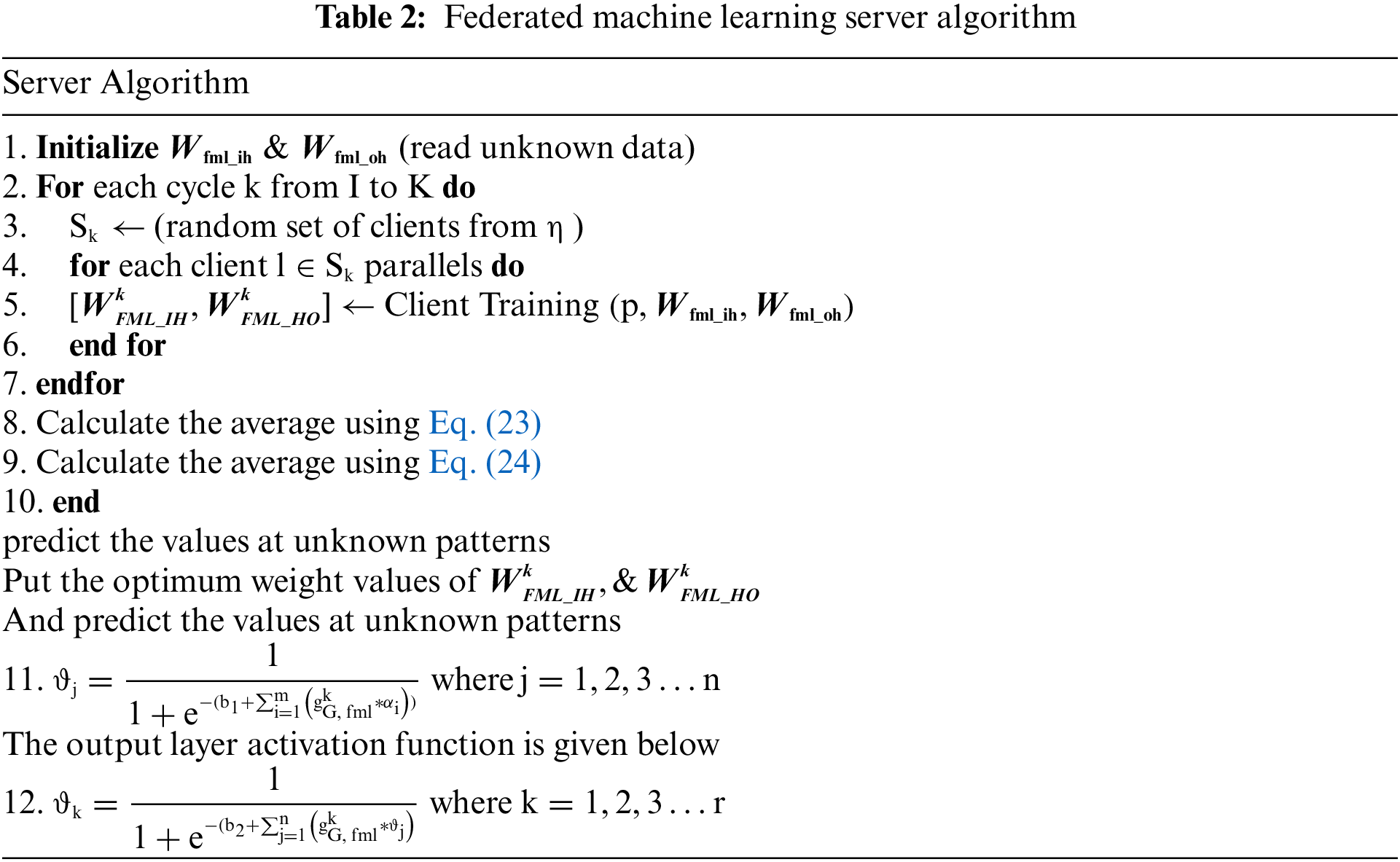
After the neuron tuning trained weights of models are sent to the federated machine learning section and get the federated average learning model pseudocode which is mentioned below, after the federated average check the performance of the federated machine learning model, if the model meets the output threshold, then stores in private blockchain network otherwise retrain all weights including neurons. Table 2 represents the federated machine learning server-side algorithm and Table 3 represents the federated machine learning client-side algorithm.
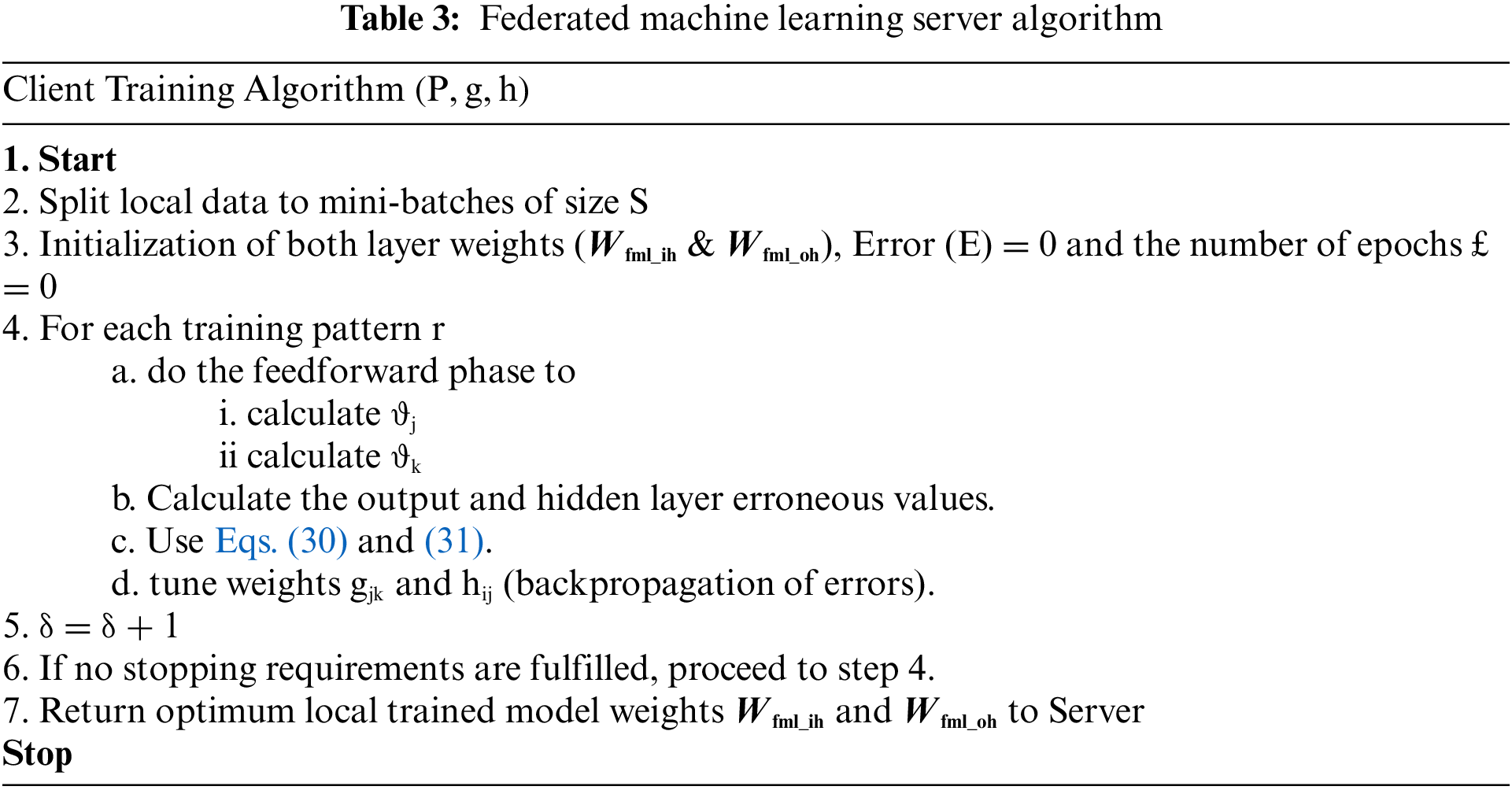
Each client in the proposed model has input, output, and hidden layers. As shown algorithm, the first step in the backpropagation algorithm is to initialize the weights, forward propagation, backpropagation of error, and update of weights and bias. A Sigmoid activation function is present in every neuron in the hidden layer. The proposed study can be written as
Backpropagation error is represented by the above equation, where
The variation in weight for the output layer is stated in Eq. (26) as
After applying the Chain rule method, above equation can be written as
The value of weight altered can be derived by swapping the values in Eq. (18), as shown in Eq. (28)
For updating the weights between the input and hidden layers, use the chain rule.
In the above equation,
After simplifying the equation, it may be expressed as follows:
where,
The weights between the output and hidden layers are adjusted using the above equation. The weights between the input and hidden layers are updated using the equation below:
In the fifth section, the proposed model collects testing data from patients using IoMT for smooth processing and applies data preprocessing techniques including data augmentation, removing null values, excluding data redundancy, and then storing in a private blockchain layer network or model can directly access data from the patient by their mobile phone or smartwatch using the global trained model. For the testing layer, put an access management lock, if the testing section wants to import the trained model and patient testing data, it has to pass the access management lock and then start the testing process. In the testing process, after the import of federated machine learning trained model by the patient and testing data, it applies testing if the proposed model found any cervical cancer risk factor, then it will take immediate steps and call the hospital or physician and if not then patient free to go home. Server training and client training algorithms are described as empowered with the fuzzed neurons technique.
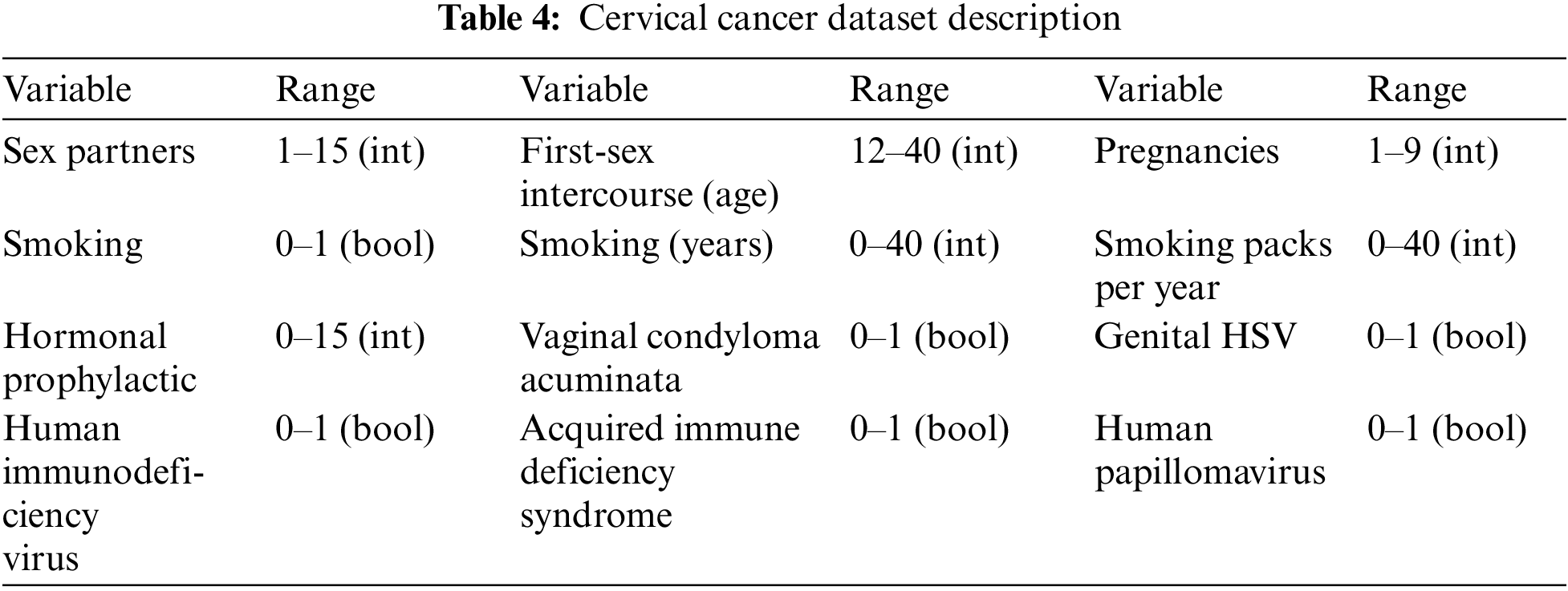
The proposed model uses UCI [30] cervical cancer risk factors data including a total of 858 samples of cervical cancer and normal patients. The proposed model applied different preprocessing techniques to remove redundant entries and check the correlation between the variables. All independent variables have ranges in the form of integers and Boolean (0 for No and 1 for Yes). The dataset includes female patients’ histories with 32 variables. Table 4 shows some samples of cervical cancer data.
For simulation purposes, the proposed approach used MacBook Pro (Macintosh) 2017, MATLAB 2022, 16 GB random access memory, and a 512 GB solid-state drive with an internal graphics processing unit. The proposed model employed a federated machine learning technique using Bayesian regularization, stochastic descent moment, and Levenberg Marquardt. The proposed model uses 1350 cervical cancer patient samples and divides these samples 80% into training and 20% samples for testing purposes. To measure the performance of cervical cancer prediction using federated machine learning the proposed model employed numerous statistical parameters [31] including, Misclassification rate (MCR), Cervical cancer prediction accuracy (CCPA), Cervical cancer prediction sensitivity (CCPSen), Cervical cancer prediction specificity (CCPSpe), Cervical cancer prediction F1-score (CCPF1), Cervical cancer positive predicted value (CCPPV), Cervical cancer negative predicted value (CCNPV), Cervical cancer false positive rate (CCFPR), Cervical cancer false-negative rate (CCFNR), Cervical cancer likelihood positive ratio (CCLPR), Cervical cancer likelihood negative ratio (CCLNR) and Cervical cancer Fowlkes-Mallows index (CCFMI). The proposed approach of cervical cancer prediction using federated machine learning obtained results
Fig. 2 shows the training progress of all machine learning models so, Fig. 2a shows the training progress of Bayesian regularization and it achieves 100% training accuracy with 1 * target + 6.4e-08 output. As training shows Bayesian regularization performs a very smooth training process with no outlier. Fig. 2b shows the training progress of Levenberg Marquardt and it achieves 97.41% training accuracy with 0.83 * target + 0.012 output but this proposed model training is not smooth as Bayesian regularization shows some outliers in both predicted classes. Fig. 2c shows the training progress of the Scaled conjugate gradient and it achieves 98.60% training accuracy with 0.84 * target + 0.0073 output and this model shows miner outliers of both predicted classes of cervical risk factors and normal samples.
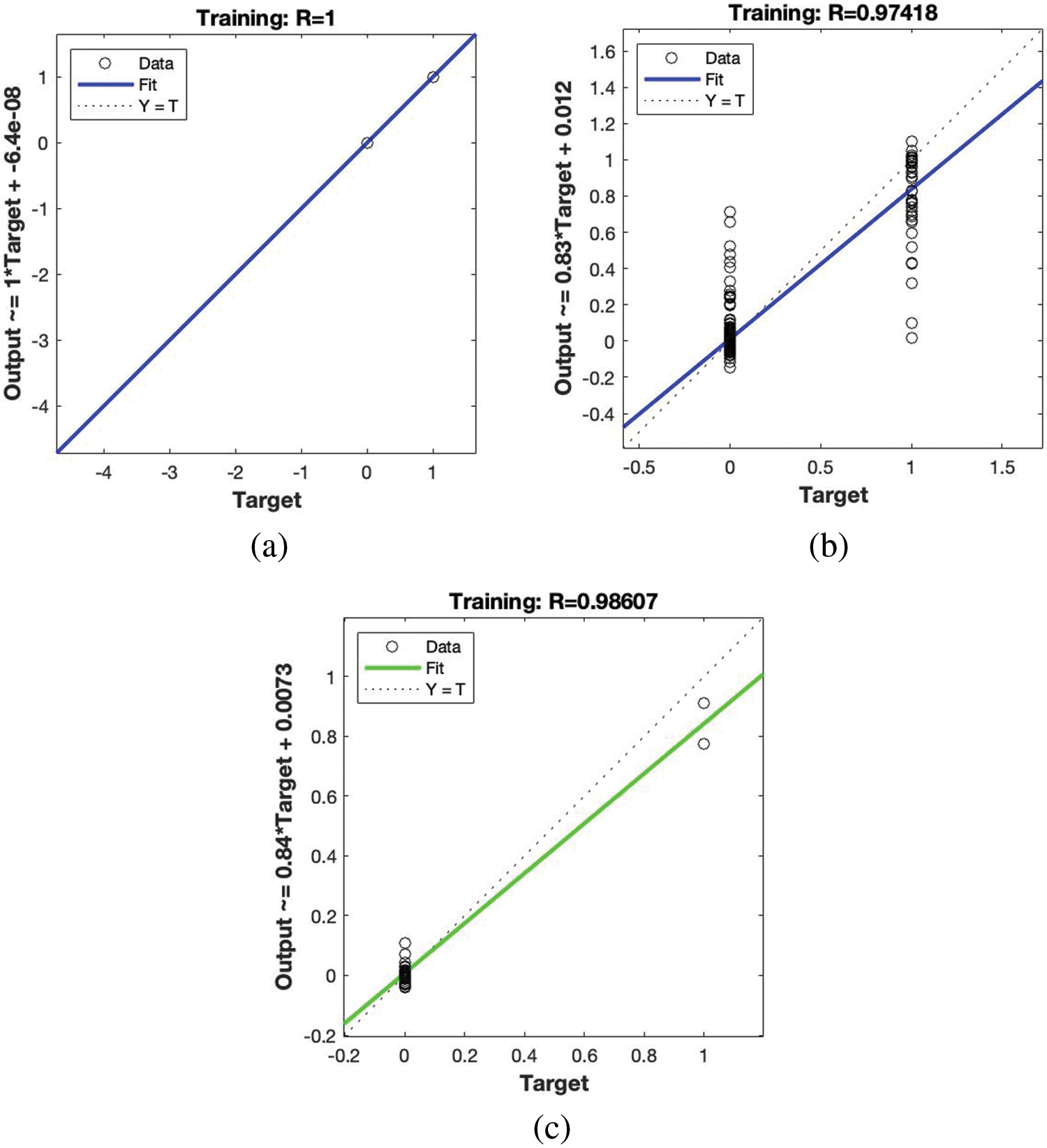
Figure 2: (a) shows the training progress of Bayesian regularization (b) shows the training progress of Levenberg Marquardt (c) shows the training progress of scaled conjugate gradient
Table 5 shows the comparative training progress of all machine learning solvers it depicts that Bayesian regularization trains the model at 42 neurons and achieves 100% CCPA with 3.47137e-11 mean square error, Levenberg Marquardt trains the model at 42 neurons and achieves 97.41% CCPA with 1.11552e-2 mean square error and scaled conjugate gradient train the model at 30 neurons and achieves 98.60% CCPA with 1.79423e-2 mean square error. So, the comparative training study shows that Bayesian regularization achieves the highest training accuracy as compared with all other models.
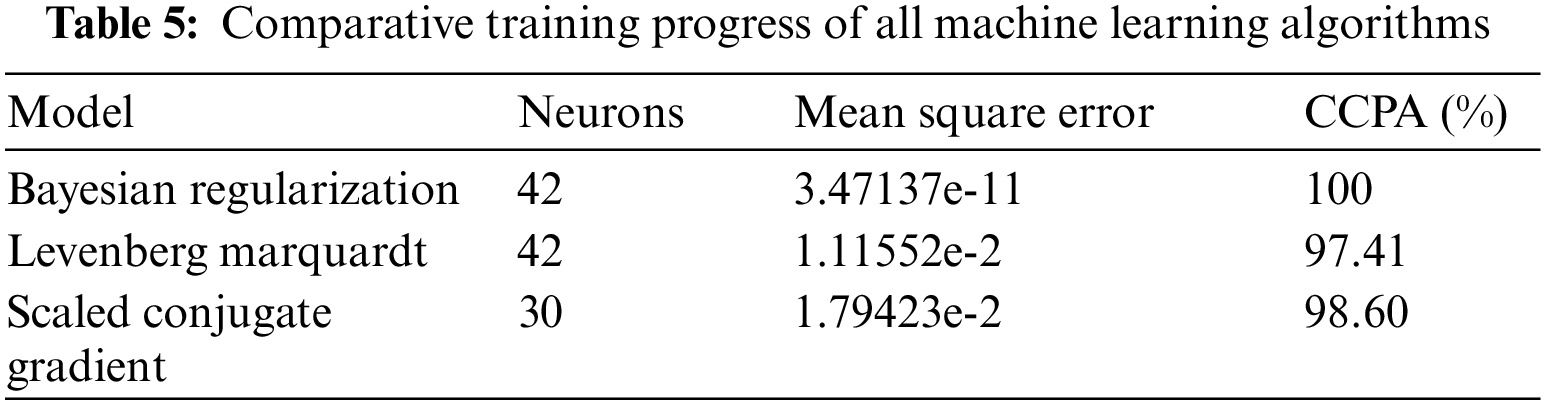
Table 6 shows the training results of federated machine learning, it depicts that the proposed model for cervical cancer prediction achieves 99.72% CCPA with fuzzed neurons at max 42% and 0.35% MCR. Federated machine learning fuzzed Bayesian regularization, Levenberg Marquardt, and scaled conjugate gradient for training and federated machine learning performed very well with fuzzed neurons technique.
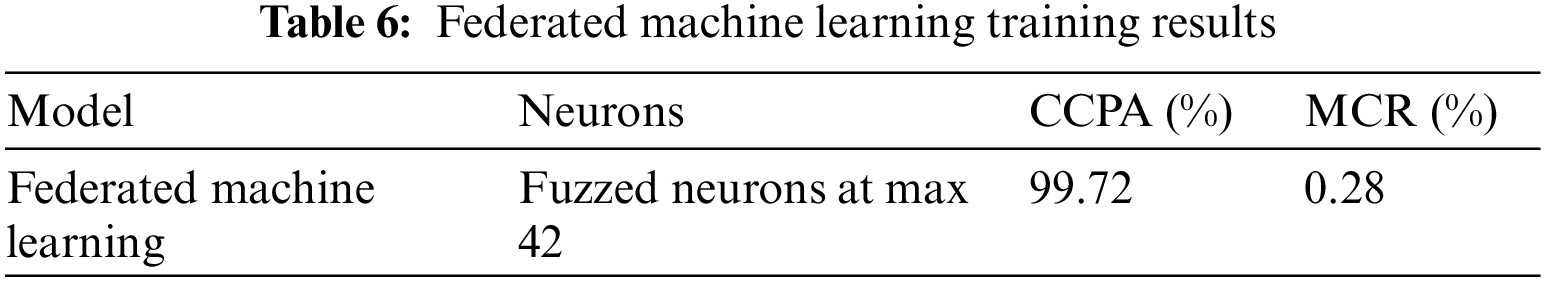
Table 7 demonstrates the training confusion matrix of the proposed approach using federated machine learning, it depicts that the proposed model predicts 1000 cervix cancer females and 77 normal females. Also, the proposed model predicts 3 females’ wrong classes of cervical cancer and normal.
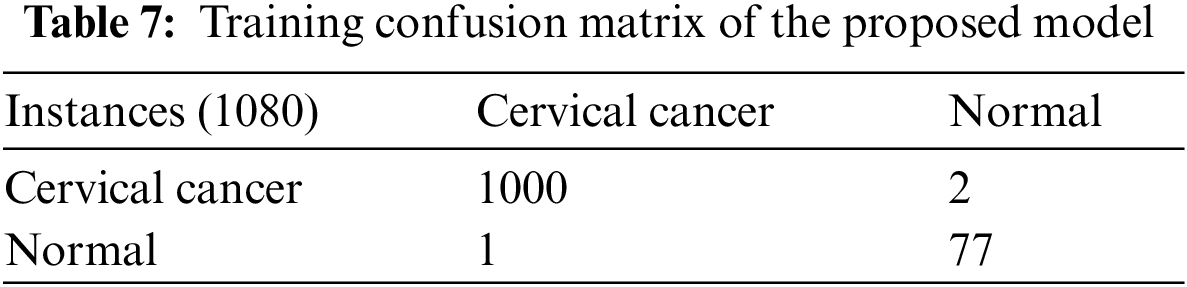
Table 8 shows the testing confusion matrix of the proposed model using federated machine learning, it depicts that the proposed model predicts 250 cervix cancer females and 18 normal females. Also, the proposed model predicts 2 females’ wrong classes of cervical cancer and normal. Table 9 shows all parametric cervical cancer testing results of federated machine learning. So, the proposed model of federated machine learning during cervical cancer testing achieves 99.26%, 0.74%, 99.60%, 99.74%, 99.60%, 99.60%, 94.74%, 5.26%, 0.40%, 18.92%, 0.00%, 99.60% of CCPA, MCR, CCPSen, CCPSpe, CCPF1, CCPPV, CCNPV, CCFPR, CCFNR, CCLPR, CCLNR, and CCFMI, respectively.
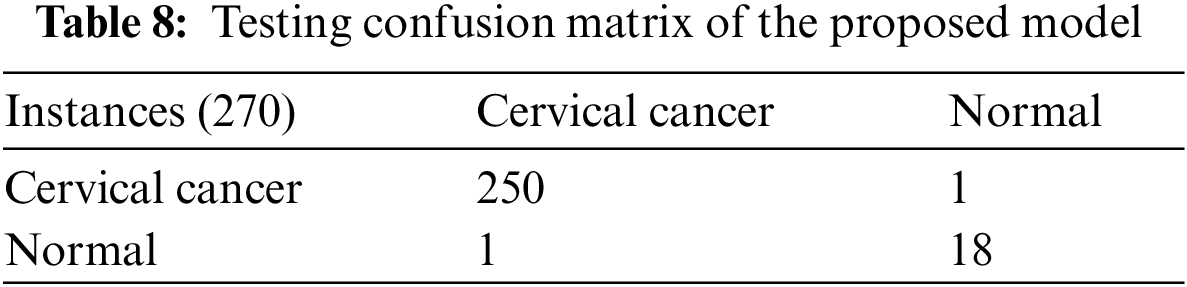
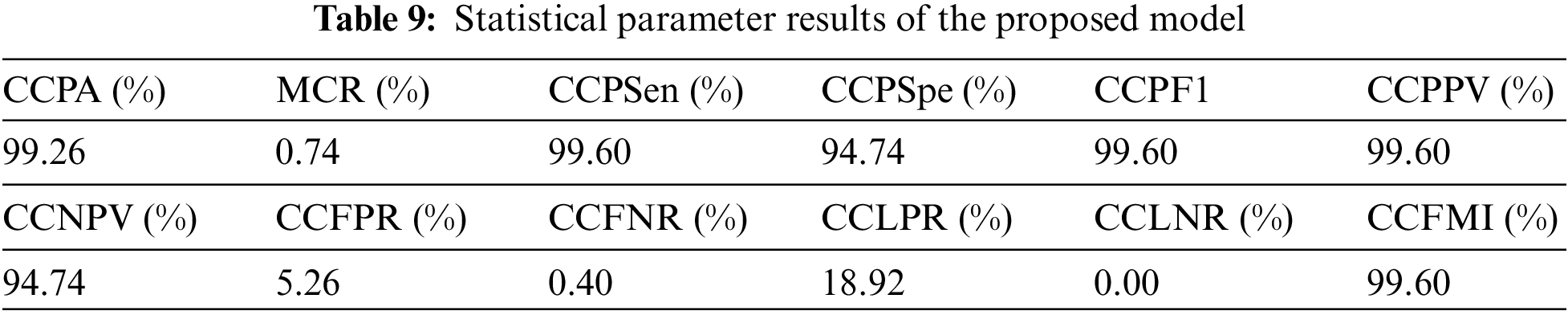
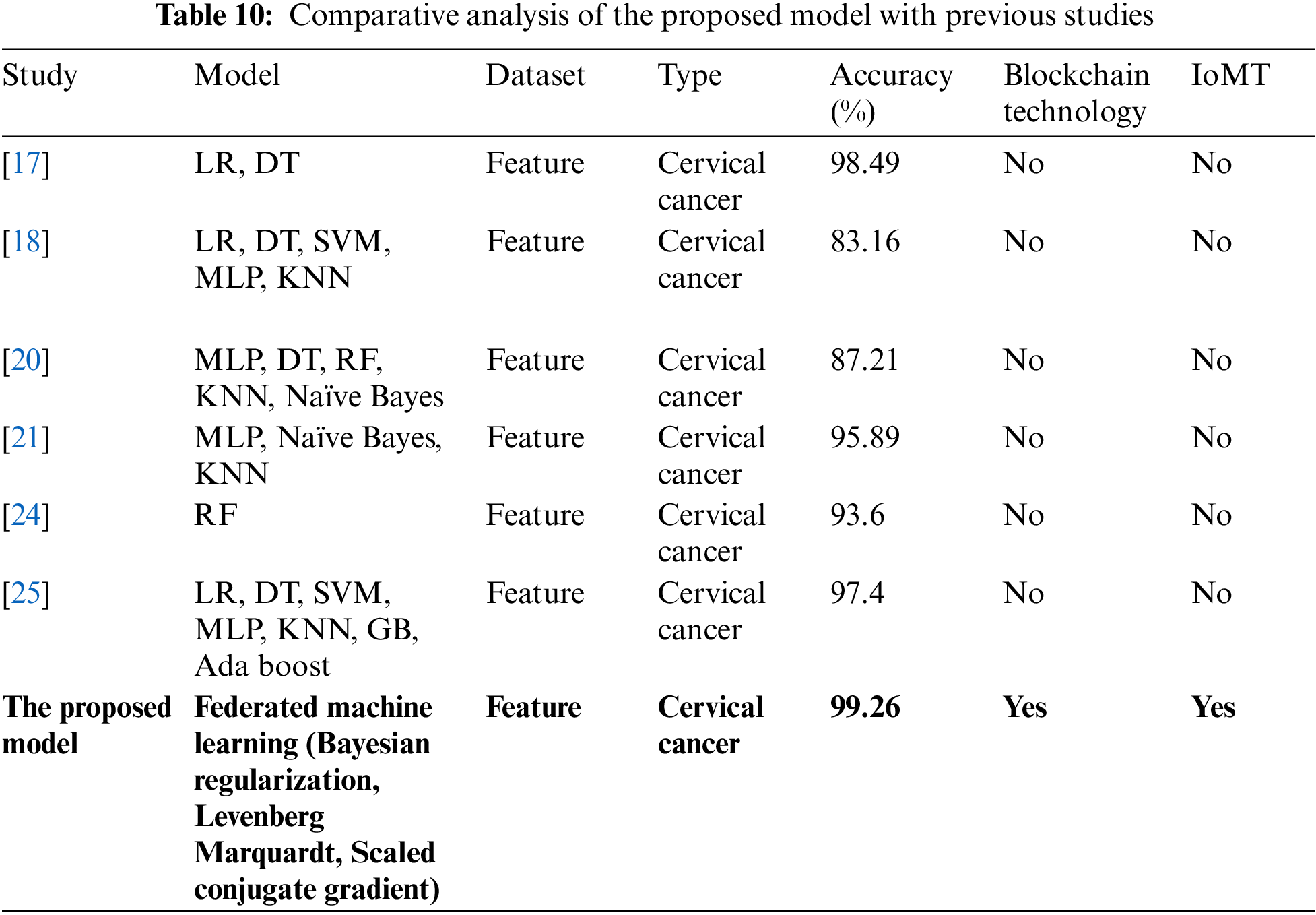
Table 10 demonstrations the comparative study of the proposed research with previous researches, it depicts that [17] used LR, DT machine learning techniques applied on feature dataset and achieved 98.49% cervical cancer prediction accuracy and they did not used IoMT and blockchain technology, [18] used LR, DT, SVM, MLP and KNN machine learning techniques applied on feature dataset and achieved 83.16% cervical cancer prediction accuracy and they did not used IoMT and blockchain technology, reference [20] used MLP, DT, RF, KNN, Naïve Bayes machine learning techniques applied on feature dataset and achieved 87.21% cervical cancer prediction accuracy and they did not used IoMT and blockchain technology, reference [21] used MLP, KNN, Naïve Bayes machine learning techniques applied on feature dataset and achieved 95.89% cervical cancer prediction accuracy and they did not used IoMT and blockchain technology, reference [24] used RF machine learning technique applied on feature dataset and achieved 93.6% cervical cancer prediction accuracy and they did not used IoMT and blockchain technology, reference [25] used LR, DT, SVM, MLP, KNN, GB, Ada boost machine learning techniques applied on feature dataset and achieved 97.4% cervical cancer prediction accuracy and they did not used IoMT and blockchain technology and the proposed mode uses federated machine learning technique with Bayesian regularization, Levenberg Marquardt and scaled conjugate gradient apply on feature dataset and achieves 99.26% CCPA which is higher than all previous researches and the proposed model uses IoMT technology for better and fast collection of patient samples, private blockchain technology network for patient data privacy and trained model protection from hazardous attacks. The primary role played in CCPA improvement has federated machine learning techniques with numerous data preprocessing techniques like data augmentation, etc.
The proposed model surpasses existing models by delivering superior prediction accuracy at both the prototype level and demonstrating ethical considerations such as data security. In the case of federated learning, it ensures patient training data remains confidential, sharing only the trained weights for subsequent predictions. The proposed prototype is used by professional doctors to get more precise results and apply medication on time.
In the current period, cervical cancer is a deadly disease that causes numerous deaths in its early stages. Diagnosing cervical cancer using female clinical records manually or by the physician is a very lengthy and entail process. In artificial intelligence, machine learning and deep learning techniques have colossal competence to develop the diagnostic and prognostic cancer risk factors applications that can do proper early cervical cancer detection and give proper initial treatment by expert physicians. So, in this delineated study, the proposed approach uses a federated machine learning technique with Bayesian regularization, Levenberg Marquardt, and scaled conjugate gradient empowered with IoMT for patient sample collection and blockchain technology to provides a private security layer for data security and trained model security. Also, the proposed model has numerous data augmentation, data cleaning, and data redundant techniques which cause the model to better performance. The proposed model uses numerous statistical parameters to measure the cervical cancer risk factors prediction performance and it achieves 99.26% CCPA and 0.74% MCR with fuzzed neurons technique which is far better results as compared with previous studies. The proposed model provides an optimal approach to predicting cervical cancer risk dynamics in the early stages. The regression technique helps to choose the best features and the augment technique helps to balance the classes which is beneficial to get accurate results. The proposed model prototype will be used by professional doctors to get precise and fast results with the ethical consent of the concerned patient. So, this study will be expanded by using fuzzed transfer learning techniques and fuzzed instances techniques to get more precise results to predict cervical cancer risk dynamics. This study will use fussed data techniques to gather and combine them to get more accurate cancer prediction results. Furthermore, this study will apply to big datasets that will be collected from Pakistan hospitals and use ensemble federated learning techniques. Furthermore, this study helps other medical diagnoses like breast cancer in terms of detection and data privacy due to federated machine learning.
Acknowledgement: Thanks to our families & colleagues who supported us morally.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Study conception and design: M.U. Nasir, O.K. Khalil, M.A. Khan; data collection: K. Ateeq; analysis and interpretation of results: M.U. Nasir, M.A. Khan, B.S. Almogadwy and K.M. Adnan; draft manuscript preparation: M.U. Nasir. K. Ateeq, O.K. Khalil, B.S. Almogadwy and K.M. Adnan. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data used in this paper can be requested from the corresponding author upon request.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. E. Nashar, M. Ahmed, R. Y. Bamjboor, A. Mansour, and B. A. Althobaity, “Awareness of the women about vaginal infection as a risk factor for cervical cancer in Taif city,” Int. J. Med. Dev. Countries, vol. 4, pp. 2108–2114, 2020. [Google Scholar]
2. A. Rahman et al., “Histopathologic oral cancer prediction using oral squamous cell carcinoma biopsy empowered with transfer learning,” Sens., vol. 22, pp. 3833–3854, 2022. doi: 10.3390/s22103833 [Google Scholar] [PubMed] [CrossRef]
3. S. D. Sanjosé et al., “Burden of Human Papillomavirus (HPV)-related cancers attributable to HPVs 6/11/16/18/31/33/45/52 and 58,” JNCI Cancer Spectr., vol. 2, pp. 45–56, 2018. [Google Scholar]
4. P. Sachdev, “Cervical cancer resource center,” Sep. 30, 2021. Accessed: July 1, 2022. [Online]. Available: https://www.webmd.com/cancer/cervical-cancer. [Google Scholar]
5. T. H. Thomas, “Everything you need to know about cervical cancer,” Feb. 14, 2022. Accessed: July 1, 2022. [Online]. Available: https://www.healthline.com/health/cervical-cancer#symptoms. [Google Scholar]
6. W. H. Organization, “WHO supports early detection and control of cervical and breast cancer in Bangladesh,” Nov. 10, 2020. Accessed: July 1, 2022. [Online]. Available: https://www.who.int/bangladesh/news/detail/10-11-2020-who-supports-early-detection-and-control-of-cervical-and-breast-cancer-in-bangladesh. [Google Scholar]
7. A. Khamparia, D. Gupta, J. J. Rodrigues, and V. H. D. Albuquerque, “DCAVN: Cervical cancer prediction and classification using deep convolutional and variational autoencoder network,” Multimed. Tools Appl., vol. 80, pp. 30399–30415, 2021. doi: 10.1007/s11042-020-09607-w. [Google Scholar] [CrossRef]
8. O. K. Kalil et al., “BCD-WERT A novel approach for breast cancer detection using whale optimization based efficient features and extremely randomized tree algorithm,” PeerJ Comput. Sci., vol. 7, pp. 390–403, 2021. doi: 10.7717/peerj-cs.390 [Google Scholar] [PubMed] [CrossRef]
9. A. Ayoub et al., “Classification and categorization of COVID-19 outbreak in Pakistan,” Comput. Mater. Contin., vol. 69, no. 3, pp. 1253–1269, 2021. doi: 10.32604/cmc.2021.015655. [Google Scholar] [CrossRef]
10. C. Iwendi et al., “COVID-19 patient health prediction using boosted random forest algorithm,” Front. Public Health, vol. 8, pp. 357, 2020. doi: 10.3389/fpubh.2020.00357 [Google Scholar] [PubMed] [CrossRef]
11. M. Glučina, A. Lorencin, N. Anđelić, and I. Lorencin, “Cervical cancer diagnostics using machine learning algorithms and class balancing techniques,” Appl. Sci., vol. 13, no. 2, pp. 1–18, 2023. doi: 10.3390/app13021061. [Google Scholar] [CrossRef]
12. B. J. Priyanka, “Machine learning approach for prediction of cervical cancer,” Turkish J. Comp. Math. Edu., vol. 12, no. 8, pp. 3050–3058, 2021. [Google Scholar]
13. H. Chen et al., “CytoBrain: Cervical cancer screening system based on deep learning technology,” J. Comput. Sci. Technol., vol. 36, pp. 347–360, 2021. doi: 10.1007/s11390-021-0849-3. [Google Scholar] [CrossRef]
14. J. Shim et al., “Automated segmentation and diagnostic measurement for the evaluation of cervical spine injuries using X-rays,” J. Imaging Inform. Med., vol. 37, no. 2, pp. 1–11, 2024. doi: 10.1007/s10278-024-01006-z [Google Scholar] [PubMed] [CrossRef]
15. N. Taleb et al., “Ovary cancer diagnosing empowered with machine learning,” in Proc. Int. Conf. Bus. Anal. Technol. Secur., UAE, 2022, pp. 1–6. [Google Scholar]
16. P. Charoenkwan et al., “IPMI: Machine learning-aided identification of parametrial invasion in women with early-stage cervical cancer,” Diagn., vol. 11, no. 8, pp. 1454–1454, 2021. doi: 10.3390/diagnostics11081454 [Google Scholar] [PubMed] [CrossRef]
17. R. Alsmariy, G. Healy, and H. Abdelhafez, “Predicting cervical cancer using machine learning methods,” Int. J. Adv. Comput. Sci. Appl., vol. 11, no. 7, pp. 1–8, 2020. doi: 10.14569/issn.2156-5570. [Google Scholar] [CrossRef]
18. J. Lu, E. Song, A. Ghoneim, and M. Alrashoud, “Machine learning for assisting cervical cancer diagnosis: An ensemble approach,” Future Generation Comput. Syst., vol. 106, pp. 199–205, 2021. doi: 10.1016/j.future.2019.12.033. [Google Scholar] [CrossRef]
19. Y. R. Park et al., “Comparison of machine and deep learning for the classification of cervical cancer based on cervicography images,” Sci. Rep., vol. 11, no. 1, pp. 1–21, 2021. doi: 10.1038/s41598-021-95748-3 [Google Scholar] [PubMed] [CrossRef]
20. E. Ahishakiye, R. Wario, W. Mwangi, and D. Taremwa, “Prediction of cervical cancer basing on risk factors using ensemble learning,” in Proc. 2020 IST-Africa Conf., Africa, 2020, pp. 1–12. [Google Scholar]
21. M. F. Unlersen, K. Sabanci, and M. Özcan, “Determining cervical cancer possibility by using machine learning methods,” Int. J. Latest Res. Eng. Technol., vol. 3, no. 12, pp. 65–71, 2017. [Google Scholar]
22. Y. Park et al., “Classification of cervical cancer using deep learning and machine learning approach,” Sci. Rep., vol. 2021, pp. 1–12, 2021. doi: 10.21203/rs.3.rs-254234/v1. [Google Scholar] [CrossRef]
23. K. Fernandes, D. Chicco, J. S. Cardoso, and J. Fernandes, “Supervised deep learning embeddings for the prediction of cervical cancer diagnosis,” PeerJ. Comput. Sci., vol. 2018, no. 5, pp. 154–166, 2018. doi: 10.7717/peerj-cs.154 [Google Scholar] [PubMed] [CrossRef]
24. M. Mehmood, M. Rizwan, M. Gregus, and O. K. Kalil, “Machine learning assisted cervical cancer detection,” Front. Public Health, vol. 9, pp. 788376–788286, 2021. doi: 10.3389/fpubh.2021.788376 [Google Scholar] [PubMed] [CrossRef]
25. S. Jahan et al., “Automated invasive cervical cancer disease detection at early stage through suitable machine learning model,” SN Appl. Sci., vol. 3, no. 806, pp. 1–17, 2021. [Google Scholar]
26. U. K. Lilhore et al., “Hybrid model for detection of cervical cancer using causal analysis and machine learning techniques,” Comput. Math. Method Med, vol. 2022, pp. 17, 2022. doi: 10.1155/2022/4688327 [Google Scholar] [PubMed] [CrossRef]
27. E. Ozbay and F. A. Ozbay, “Interpretable pap-smear image retrieval for cervical cancer detection with rotation invariance mask generation deep hashing,” Comput. Biol. Med., vol. 154, pp. 10654, 2023. [Google Scholar]
28. Z. Fan et al., “CAM-VT: A weekly supervised cervical cancer nest image identification approach using conjugated attention mechanism and visual transformer,” Comput. Biol. Med., vol. 162, pp. 107070, 2023. doi: 10.1016/j.compbiomed.2023.107070 [Google Scholar] [PubMed] [CrossRef]
29. R. Weegar and K. Sundström, “Using machine learning for predicting cervical cancer from Swedish electronic health records by mining hierarchical representations,” PLoS One, vol. 15, no. 8, pp. 237911–237922, 2020. doi: 10.1371/journal.pone.0237911 [Google Scholar] [PubMed] [CrossRef]
30. K. Fernandes, J. S. Cardoso, and J. Fernandes, “Cervical cancer risk factor dataset,” May 12, 2017. Accessed: June 15, 2022 [Online]. Available: https://archive.ics.uci.edu/ml/datasets/Cervical+cancer+%28Risk+Factors%29. [Google Scholar]
31. A. Giannini et al., “Minimally invasive surgery for cervical cancer: Should we look beyond squamous cell carcinoma?,” J. Invest. Surg., vol. 35, pp. 1602–1603, 2022. doi: 10.1080/08941939.2022.2075495 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools