 Open Access
Open Access
ARTICLE
Influence of Intermolecular Forces and Spatial Effects on the Mechanical Properties of Silicone Sealant by Molecular Dynamics Simulation
1 School of Chemistry and Chemical Engineering, Guangxi Minzu University, Key Laboratory of Chemistry and Engineering of Forest Products, State Ethnic Affairs Commission; Engineering Research Center of Low-Carbon and High-Quality Utilization of Forest Biomass, University of Guangxi, Nanning, 530006, China
2 Library, Dalian University of Technology, Dalian, 116024, China
3 Business Transfer Center, Rizhao Shihua Crude Oil Terminal Co. Ltd., Rizhao, 276817, China
4 School of Materials and Environmental Engineering, Guangxi Minzu University, Nanning, 530006, China
* Corresponding Authors: Gui-Lei An. Email: ; Chun Lu. Email:
(This article belongs to the Special Issue: Molecular Simulations of Polymer Materials)
Computers, Materials & Continua 2025, 85(2), 2763-2780. https://doi.org/10.32604/cmc.2025.069505
Received 25 June 2025; Accepted 18 August 2025; Issue published 23 September 2025
Abstract
In the production process of silicone sealant, mineral oil is used to replace methyl silicone oil plasticizer in silicone sealant to reduce costs and increase efficiency. However, the silicone sealant content in mineral oil is prone to premature aging, which significantly reduces the mechanical properties of the silicone sealant and severely affects its service life. At the same time, there are few reports on the simulation research of the performance of silicone sealant. In this study, three mixed system models of crosslinking silicone sealant/plasticizer are constructed by the molecular dynamics simulation method, and the effect of three influencing factors, namely, crosslinking degree of silicone sealant, plasticizer content and external temperature on the mechanical properties of silicone sealant system is analyzed. The results show that at room temperature, the mechanical properties of the silicone sealant system are enhanced with the increase of its crosslinking degree; At a high crosslinking degree, with the increase of plasticizer content, the mechanical properties of the silicone sealant system show an overall decreasing trend. When the methyl silicone oil in the range of 20%, the mechanical properties of the silicone sealant appeared to be a small degree of enhancement; As the temperature increases, the doped mineral oil mechanical properties of silicone sealant declined significantly, while doped with methyl silicone oil silicone sealant and doped with double-ended vinyl silicone oil silicone sealant mechanical properties have better heat resistance. It will provide scientific theoretical guidance for improving and predicting the mechanical properties of silicone sealant.Keywords
Silicone sealant is a kind of room temperature-curing organosilicon polymer materials. It has good mechanical properties and excellent oxygen resistance, ozone resistance, light aging and weather aging resistance and is widely used in glass curtain walls, insulating glass, and a variety of assembled building envelopes. However, in the production process of silicone sealant, some unscrupulous merchants add mineral oil to replace methyl silicone oil plasticizer in silicone sealant in order to reduce costs and increase efficiency. This mineral oil added silicone sealant is prone to premature aging such as cracking and powdering over time, which greatly reduces the mechanical properties of silicone sealant and seriously affects its service life [1]. The plasticizer is one of the important factors affecting the mechanical properties of silicone sealant.
Studies have shown [2] that the accelerated deterioration of the mechanical properties of this mineral oil silicone sealant may be due to the poor compatibility between mineral oil and the silicone sealant system, which makes it easier for mineral oil to migrate out of the silicone sealant and lead to a decrease in its performance. Therefore, domestic scholars have conducted relevant research on the impact of plasticizers on the performance of silicone sealants. In the study of the effect of white mineral oil on the performance of neutral silicone sealant, Ou et al. [3] investigated the effect of different amounts of mineral oil on the performance of dealcoholized silicone sealant. Kuang et al. [4] studied the effect of different contents of dimethyl silicone oil on the performance of silicone building sealant. Feng et al. [5] selected four commonly used plasticizers in industry, namely methyl silicone oil, hydrogen containing silicone oil, vinyl silicone oil, and white oil, and studied the effects of plasticizer addition amount and type on the surface drying time, hardness, tensile bonding strength, elongation at break, and other parameters of ketoxime type silicone sealant. They further explored the influence of the ratio of methyl silicone oil to hydrogen-containing silicone oil on the performance of silicone sealant, and prepared a silicone sealant with good comprehensive performance.
However, the research on the influence of silicone sealant performance is mostly characterized by measuring the macroscopic performance parameters of silicone sealant through experimental methods, such as surface drying time, Shore hardness, tensile strength, etc. It is difficult to quantitatively analyze the effect of changes in crosslinking degree on the mechanical properties of silicone sealant, and it is difficult to study the relationship between the microstructure and macroscopic properties of silicone sealant under the influence of plasticizers. In recent years, with the continuous development of computer technology, a large number of researchers have used molecular simulation methods to study the mechanical properties of materials [6–10]. Compared with experimental methods, molecular simulation methods can study the molecular structure and intermolecular interactions of silicone sealant at the microscopic level and obtain deeper microscopic mechanisms. Therefore, it is necessary to use molecular dynamics simulation technology to explore the relationship between the molecular structure and mechanical properties of silicone sealant from a microscopic perspective.
This article uses the molecular dynamics (MD) simulation method to establish a series of mixed system models of silicone sealant/white oil, silicone sealant/methyl silicone oil, and silicone sealant/double-ended vinyl silicone oil with different degrees of crosslinking. By calculating the mechanical performance parameters such as Young’s modulus, shear modulus, and bulk modulus of the system, the influence of silicone sealant crosslinking degree, plasticizer content, and external temperature factors on the mechanical properties of silicone sealant is explored. The micro mechanism of plasticizer migration accelerating the decline of mechanical properties of silicone sealant is proposed from the molecular level, to provide scientific theoretical guidance for improving and predicting the mechanical properties of silicone sealant.
2 Model Construction and Simulation Methods
2.1 Crosslinking Reaction Principle of Silicone Sealant
In this paper, the silicone sealant is of the ketoxime type. During the condensation reaction of the silicone sealant, it undergoes cross-linking on its own, and plasticizers do not participate in the cross-linking reaction. The cross-linking and curing mechanism of the silicone sealant is shown in Fig. 1, and briefly described as follows: (1) The silicone sealant binds with H+ in H2O molecules, removing the ketoxime group X1 connected to the Si atom at the end of the molecular chain, forming a silicone sealant containing terminal hydroxyl group 2; (2) The hydroxyl group in hydroxyl terminated silicone sealant reacts with the ketoxime group X3 at the end of another molecular chain, removing the ketoxime group and crosslinking to form a Si-O chemical bond; (3) Repeat the processes of (1) and (2), and finally condense to form a bulk polymer structure.

Figure 1: Crosslinking and curing mechanism of single-component silicone sealant
In addition, to clearly understand the principle and process of the crosslinking reaction, Fig. 2 shows a schematic diagram of the simulated molecular structure of crosslinking. For ease of viewing, the polymerization degree of the silicone sealant molecular chain is temporarily set to 10 (the actual simulated polymerization degree is 80), where the red circle represents the crosslinking reaction site R1/R2, which is the removed molecular structure, and the blue circle represents the new bond generated by the crosslinking reaction, which is the crosslinking bond.

Figure 2: Schematic diagram of simulated cross-linked molecular structure
2.2 Model Construction before Cross-Linking
Using the Visualizer module in Materials Studio (MS) simulation software, molecular models were constructed based on the molecular structure formulas of silicone sealant (as shown in Fig. 1) and plasticizers such as white oil, methyl silicone oil, and double-ended vinyl silicone oil (as shown in Fig. 3). The plasticizers had a molecular polymerization degree of 40 and relative molecular weights of 562, 3048, and 3072.

Figure 3: Simplified molecular structure of plasticizer. (a) Mineral oil; (b) Methyl silicone oil; (c) Double-ended vinyl silicone oil
Then, using the Amorphous Cell module, three mixed system models of silicone sealant before crosslinking were constructed under the conditions of 298 K and 0. 0001 GPa. Based on commonly used component allocation ratios and considering the accuracy of calculation results and computer performance, six molecular chains of silicone sealant and 44 mineral oil molecules were placed in periodic boxes to construct a silicone sealant/mineral oil mixed system; Place 6 chains of silicone sealant molecules and 8 molecules of methyl silicone oil into a periodic box to construct a silicone sealant/methyl silicone oil hybrid system; Place 6 silicone sealant molecular chains and 8 double ended vinyl silicone oil molecules into a periodic box to construct a silicone sealant/double ended vinyl silicone oil hybrid system. In the three mixed systems, the reaction atoms of silicone sealant were labeled as R1/R2, and the mass ratio of silicone sealant and plasticizer was 60:40. The models of each mixed system are shown in Fig. 4. In addition, to investigate the effect of plasticizer content on the mechanical properties of silicone sealant, a mixed system model of silicone sealant and plasticizer with mass ratios of 90:10, 80:20, 70:30, and 60:40 was constructed using the same method. Due to space limitations, the silicone sealant/mineral oil hybrid system model is taken as an example, as shown in Fig. 5. Finally, based on the principle of crosslinking reaction, MD simulation was conducted in the Forcite module to construct a mixed system model of the crosslinking process of silicone sealant.

Figure 4: Model of the pre-crosslinking mixed system. (a) Silicone sealant/mineral oil; (b) Silicone sealant/methyl silicone oil; (c) Silicone sealant/double-ended vinyl silicone oil

Figure 5: Model of mixed system of silicone sealant/mineral oil with different mass ratios before crosslinking. (a) 90:10; (b) 80:20; (c) 70:30; (d) 60:40
2.3 MD Simulation of the Crosslinking Process
Due to the longer molecular chain and fewer cross-linking reaction sites of silicone sealant itself. Therefore, based on computer computing resources and the ability to reflect the real situation, silicone sealant and plasticizer molecules are randomly placed into periodic boxes. Through a series of geometric optimizations and dynamic processes, the dynamic crosslinking process is completed manually by dynamically forming crosslinking bonds according to the selected reaction truncation radius (4–10 Å). The crosslinking process conforms to the actual polymer model, which is also a method adopted by most researchers [8–10]. The specific process is as follows:
(1) Firstly, the established model of the pre-crosslinking mixed system of silicone sealant will be subjected to geometric optimization and annealing treatment to obtain a more reasonable structure; (2) Perform dynamic relaxation of the annealed model using 100 ps canonical ensemble NVT and 500 ps constant temperature and pressure ensemble NPT to achieve equilibrium structure; (3) Dynamically form cross-linking bonds manually based on the selected reaction truncation radius; (4) After reaching the first target crosslinking degree, continue to perform geometric optimization, annealing treatment, and short-term dynamic relaxation to bring the structure back to equilibrium; (5) Based on the reaction truncation radius, dynamically terrain the cross-linking key again to achieve the next target cross-linking degree; (6) Repeat the above process until the final target cross-linking degree is achieved. The definition of crosslinking degree is: the actual number of bonds formed divided by the theoretical number of bonds formed.
Finally, after the completion of the dynamic crosslinking process, the crosslinking structures of silicone sealant with different target crosslinking degrees were formed. Then, dynamic calculations were performed on the canonical ensemble NVT at 100 ps and the constant temperature and pressure ensemble NPT at 600 ps, with a time step of 1 fs, and the full motion trajectory was saved. The mechanical performance parameters, intermolecular interactions, free volume, and other parameters of the mixed system during the crosslinking process of silicone sealant were calculated and analyzed to further demonstrate the rationality of the established crosslinking model and dynamic simulation. During the entire simulation process, the COMPASS II force field was used, with a temperature of 298 K and a pressure of 0. 0001 GPa. Andersen method temperature controller and Berendsen method pressure controller were used, and Ewald method and Atom-based method were used to sum electrostatic and van der Waals effects.
Due to space limitations, taking the mixed system of silicone sealant with a cross-linking degree of 100% and a two-component mass ratio of 60:40 as an example, the three mixed system models of silicone sealant after cross-linking are shown in Fig. 6. Among them, the mixed system model with different plasticizer contents takes the silicone sealant/mineral oil system with a crosslinking degree of 100% as an example, as shown in Fig. 7.

Figure 6: Model of crosslinked silicone sealant/plasticizer hybrid system. (a) Silicone sealant/mineral oil; (b) Silicone sealant/methyl silicone oil; (c) Silicone sealant/double-ended vinyl silicone oil

Figure 7: Model of different mass ratios of cross-linked silicone sealant/mineral oil mixed system. (a) 90:10; (b) 80:20; (c) 70:30; (d) 60:40
2.4 Mechanical Performance Calculation
This article uses the Constant Strain method to apply a small strain of 1% to the cross-linked equilibrium mixed system model, causing three sets of uniaxial tensile deformation and three sets of uniaxial compressive deformation along the x, y and z axes, and six sets of shear deformation in the xy, xz and yz planes, for a total of 12 sets of deformation. The stress of the simulated system is extracted and calculated. According to Hooke’s Law, the stiffness matrix and two Lame constants of the corresponding mixed system are calculated. The Young’s modulus, bulk modulus, and shear modulus of the system are calculated from the Lame coefficients, and the mechanical properties of the silicone sealant mixed system are further analyzed.
Among them, in the mechanical performance simulation of this article, the mixed system of silicone sealant and plasticizer is an amorphous model, which can be regarded as an isotropic material. For isotropic materials, their stiffness matrix can be simplified [11], as shown in Eq. (1).
In the formula, λ and μ are the Lame coefficients (GPa), which can be obtained through the stiffness matrix. The calculation formulas are shown in Eqs. (2) and (3).
Meanwhile, for a mixed system that reaches thermodynamic equilibrium, the mechanical performance parameters such as Young’s modulus (E), shear modulus (G), and bulk modulus (K) of the mixed system can be obtained based on the Lame coefficient [12], as shown in Eq. (4).
3.1 Mechanical Performance Analysis
3.1.1 The Influence of Crosslinking Degree on the Mechanical Properties of Silicone Sealant
To study the mechanical properties of silicone sealant during crosslinking reaction under the influence of different plasticizers, this paper selects the last 30 frames of the NPT ensemble dynamic trajectory file after equilibrium, and analyzes the mechanical properties of the mixed system model of silicone sealant with different crosslinking degrees at 298 K temperature.
According to Eqs. (4)–(6), the variation curves of the mechanical properties of three mixed systems with the crosslinking degree of silicone sealant were calculated, as shown in Fig. 8. As shown in the figure, the mechanical properties of the three mixed systems show a significant upward trend with the increase of the crosslinking degree of silicone sealant. The change in mechanical properties shows a great dependence on the crosslinking degree of silicone sealant, indicating that the crosslinking reaction significantly improves the mechanical properties of silicone sealant.
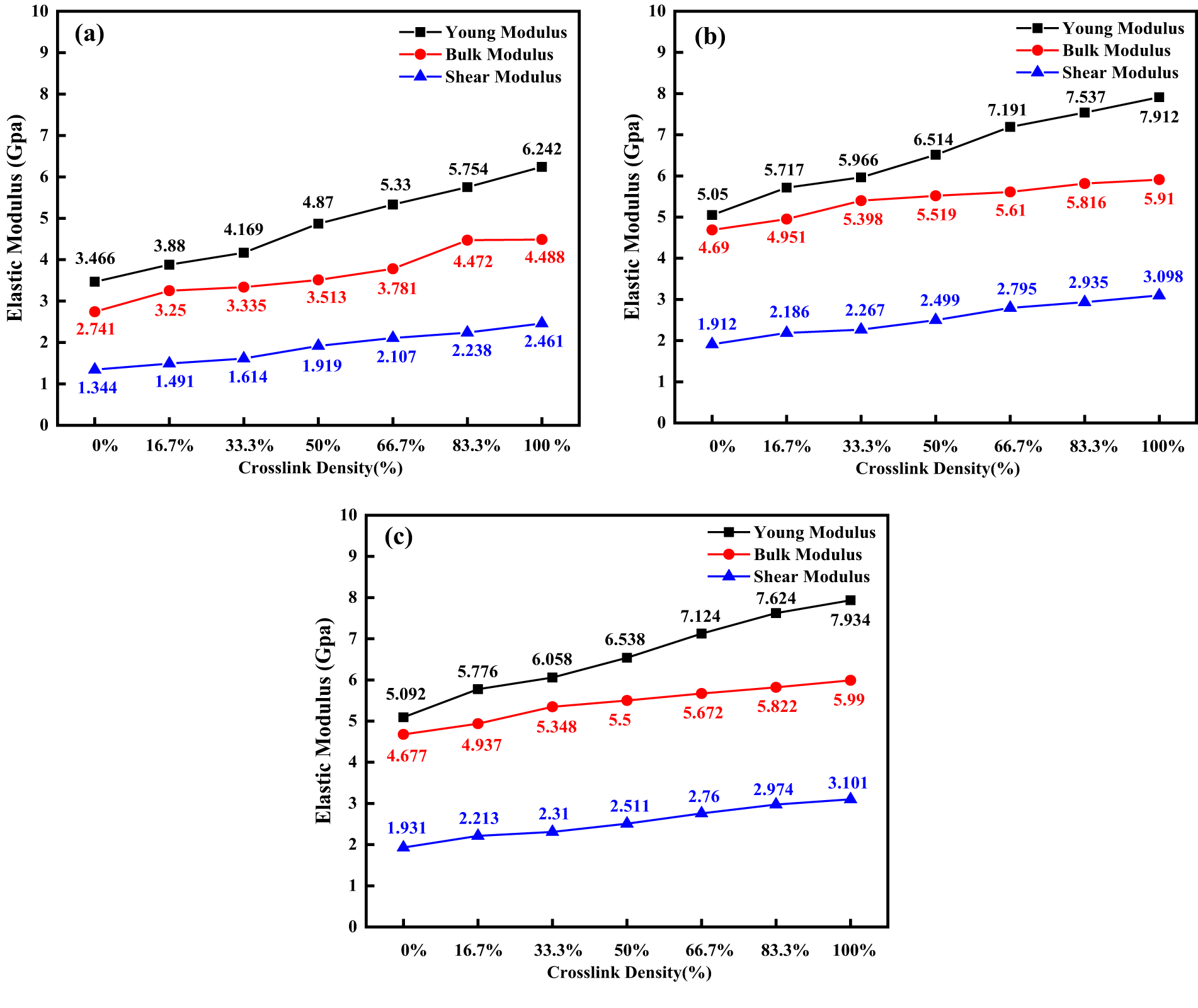
Figure 8: The variation curve of the mechanical properties of the system with the crosslinking degree of silicone sealant, (a) Silicone sealant/mineral oil; (b) Silicone sealant/methyl silicone oil; (c) Silicone sealant/double-ended vinyl silicone oil
3.1.2 Effect of Plasticizer Content on the Mechanical Properties of Silicone Sealant
The content of plasticizers is closely related to the mechanical properties of silicone sealant. In this paper, mechanical properties analysis was conducted on three mixed systems of silicone sealant/white oil, silicone sealant/methyl silicone oil, and silicone sealant/double-ended vinyl silicone oil after crosslinking of silicone sealant (with a proposed crosslinking degree of 100%) at 298 K temperature. The variation curve of the system’s mechanical properties with the content of plasticizers was obtained, as shown in Fig. 9.

Figure 9: The variation curve of the mechanical properties of the system with the content of plasticizers, (a) Silicone sealant/mineral oil; (b) Silicone sealant/methyl silicone oil; (c) Silicone sealant/double-ended vinyl silicone oil
As shown in Fig. 9, the Young’s modulus, shear modulus, and bulk modulus of the three mixed systems all decrease to varying degrees with the increase of plasticizer content. In the silicone sealant/mineral oil mixed system, as the mineral oil content increases, the elastic moduli decrease, and the mechanical properties of the silicone sealant system significantly decrease. This is consistent with the experimental results obtained by Ou et al. [3] in their study on the effect of mineral oil addition on the performance of silicone sealant.
In the mixed system of silicone sealant/methyl silicone oil, as the content of methyl silicone oil increases, the overall mechanical properties of the system show a downward trend. When the content of methyl silicone oil is 20%, the elastic modulus values slightly increase, and the mechanical properties of the system improve; When the elastic modulus exceeds 20%, the mechanical properties gradually decrease. In the mixed system of silicone sealant/double-ended vinyl silicone oil, the mechanical properties of the system show a similar trend to that of the mixed system of silicone sealant/methyl silicone oil. Overall, as the content of double-ended vinyl silicone oil increases, the mechanical properties of the system gradually decrease, but are relatively stable. This is also consistent with the experimental results obtained by Feng et al. [5] and Kuang et al. [4] on the influence of plasticizers on the performance of silicone sealants. In addition, under the same content of plasticizers, the elastic modulus of the silicone sealant/mineral oil mixed system is the lowest. The order of mechanical properties of the three mixed systems is: silicone sealant/double-ended vinyl silicone oil > silicone sealant/methyl silicone oil > silicone sealant/white oil.
3.1.3 Effect of Temperature on the Mechanical Properties of Silicone Sealant
This article analyzes the mechanical properties of three mixed systems of silicone sealant crosslinked (with a proposed crosslinking degree of 100%) at temperatures of 298, 318, 338, 358, and 378 K. The calculation results are shown in Fig. 10.

Figure 10: The variation curve of the mechanical properties of the system with temperature. (a) Silicone sealant/mineral oil; (b) Silicone sealant/methyl silicone oil; (c) Silicone sealant/double-ended vinyl silicone oil
As can be seen from the Fig. 10, the Young’s modulus, shear modulus, and bulk modulus of the three mixed systems all decrease with increasing temperature, but the decreasing trend of the mechanical properties of the three mixed systems with increasing temperature is not completely the same. Among them, the mechanical properties of the silicone sealant/mineral oil hybrid system decrease the fastest. From the perspective of Young’s modulus, the silicone sealant/mineral oil mixture system shows a significant downward trend with increasing temperature, reaching a minimum value of 3.36 GPa at 378 K; The silicone sealant/methyl silicone oil hybrid system and the silicone sealant/double-ended vinyl silicone oil hybrid system have a relatively small decreasing trend with increasing temperature, and still maintain high Young’s modulus values at 378 K, which are 5. 707 and 5.792 GPa, respectively; Obviously, the silicone sealant/mineral oil hybrid system is greatly affected by temperature. Similar to Young’s modulus, as the temperature increases, the bulk modulus of the three mixed systems shows a decreasing trend. At temperatures ranging from 298 to 378 K, the bulk modulus of the three mixed systems follows the order: silicone sealant/double-ended vinyl silicone oil > silicone sealant/methyl silicone oil > silicone sealant/white oil. Compared with the silicone sealant/double-ended vinyl silicone oil system and the silicone sealant/methyl silicone oil mixed system, the decrease rate of the silicone sealant/mineral oil mixed system is greater. At 378 K, the bulk modulus decreases to below 3 GPa, while the bulk modulus of the silicone sealant/double-ended vinyl silicone oil system and the silicone sealant/methyl silicone oil system still remains good, at 4.166 and 4.728 GPa, respectively. Meanwhile, the shear modulus of the three mixed systems also showed a decreasing trend with increasing temperature. When the temperature reached 378 K, the shear modulus of the silicone sealant/mineral oil system decreased to 1.289 GPa, a decrease of 48% compared to 298 K. At 378 K, the shear moduli of silicone sealant/methyl silicone oil and silicone sealant/double-ended vinyl silicone oil mixed systems were 2.244 and 2.135 GPa, respectively, which decreased by 26% and 31% compared to 298 K. Therefore, based on the above, among the three mixed systems, silicone sealant/mineral oil is most affected by temperature, while silicone sealant/double-ended vinyl silicone oil and silicone sealant/methyl silicone oil systems have better heat resistance and exhibit better mechanical properties.
Related studies have shown [12–15] that compatibility between system components, interactions between polymer chains, and free volume of the system are important factors affecting the mechanical properties of materials. Therefore, this article mainly explores the effects of crosslinking degree, plasticizer content, and external temperature on the mechanical properties of silicone sealant from two aspects: intermolecular interactions and free volume.
3.2 Analysis of Intermolecular Interactions
3.2.1 Radial Distribution Function Analysis
In order to better reveal the essence of the interactions between the molecules of silicone sealant and plasticizer blends, the radial distribution functions between the molecules of silicone sealant and three mixed systems of plasticizer white oil, methyl silicone oil, and double-ended vinyl silicone oil were calculated, as shown in Fig. 11. The radial distribution function can reflect the type and magnitude of intermolecular interactions, typically ranging from 2.6 to 3.1 Å for hydrogen bonding and from 3.1 to 5.0 Å for van der Waals interactions [16–19]. As shown in Fig. 11, the radial distribution function of the blend system composed of silicone sealant and plasticizer exhibits peaks between 3.1 and 5.0 Å, indicating that the main mode of intermolecular interaction is van der Waals interaction. In addition, the compatibility between components of blends is a complex intermolecular interaction force. It has been documented in literature [20,21] that in the radial distribution function between molecules in a mixed system, when the g (r) values between molecules of the same component are all smaller than the g (r) values of the blend of two components, compatibility will occur between the components. It can be seen that in the three mixed systems, the intermolecular interactions between mineral oil molecules are strong, and the intermolecular interactions with silicone sealant molecules are weak.

Figure 11: Radial distribution function between molecules in the blend system. (a) Silicone sealant/mineral oil; (b) Silicone sealant/methyl silicone oil; (c) Silicone sealant/double-ended vinyl silicone oil
However, methyl silicone oil and double-ended vinyl silicone oil both have strong interactions with silicone sealant molecules [22], indicating that silicone sealant has good compatibility with methyl silicone oil and double-ended vinyl silicone oil, but poor compatibility with white oil. Therefore, under the same conditions, the mechanical properties of silicone sealant with mineral oil will be lower than those with methyl silicone oil and double-ended vinyl silicone oil.
3.2.2 Influence of Crosslinking Degree of Silicone Sealant on Intermolecular Interactions
From Fig. 8, it can be seen that the mechanical properties of silicone sealant increase with the increase of its crosslinking degree. This is because the network structure generated by the self-crosslinking of silicone sealant is more stable than the previous linear molecular structure, with tighter molecular chains and stronger resistance to external forces. According to the analysis of the radial distribution function, the main interaction mode between molecules in the system is the van der Waals force. Therefore, further calculation of the van der Waals interaction energy of the system can characterize the influence of the crosslinking degree of silicone sealant on the strength of intermolecular interaction. The calculation results are shown in Fig. 12. As shown in Fig. 12, with the increase of crosslinking degree of silicone sealant, the van der Waals interaction energy in the system also increases (in van der Waals interaction energy, when the intermolecular distance is less than a certain value, van der Waals is attractive, and its value is negative [23]). This also indicates that with the increase of crosslinking degree of silicone sealant, the formation of the crosslinking network shortens the intermolecular distance and enhances intermolecular interactions.

Figure 12: van der Waals interaction energy between molecules in a mixed system
3.2.3 Influence of Plasticizer Content on Intermolecular Interactions
To further investigate the micro mechanism of the influence of plasticizer content on the mechanical properties of the three mixed systems, molecular interactions were simulated and calculated in the three mixed systems of silicone sealant/white oil, silicone sealant/methyl silicone oil, and silicone sealant/double-ended vinyl silicone oil with different plasticizer contents during the simulation process. The results are shown in Fig. 13.
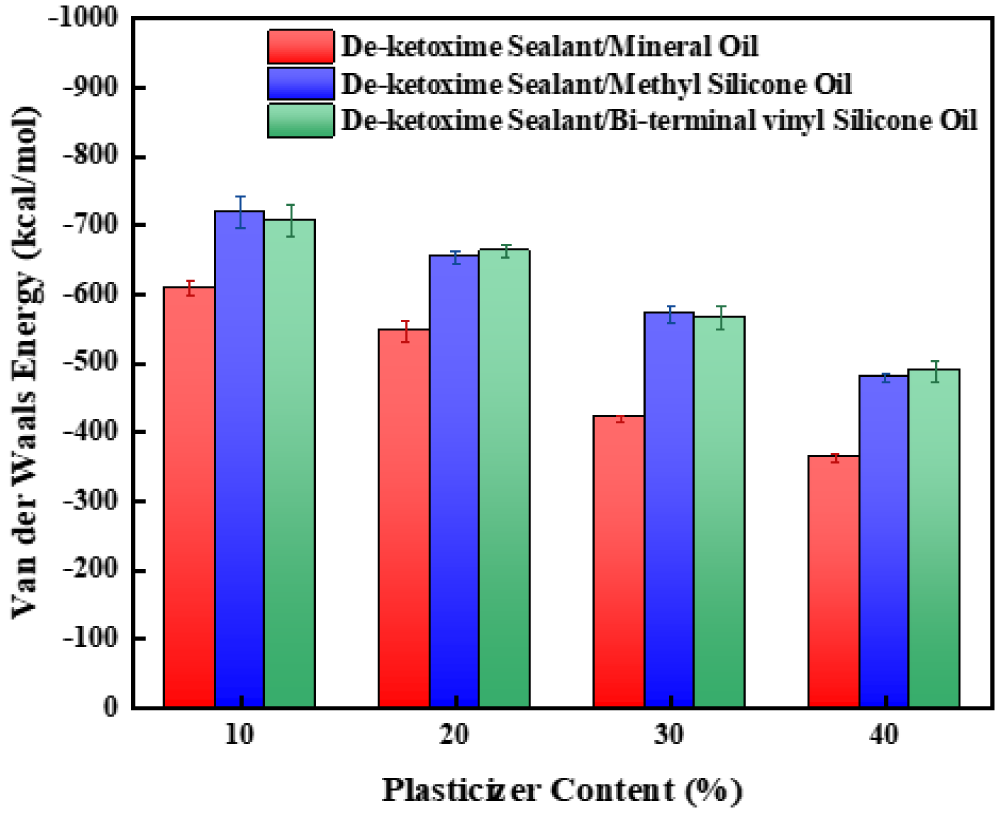
Figure 13: van der Waals interaction energy between silicone sealant molecules at different plasticizer contents
As shown in Fig. 13, with the increase of plasticizer content, the interaction between silicone sealant molecules in the three mixed systems decreases. Moreover, under the same plasticizer content, the interaction between silicone sealant molecules in the silicone sealant/mineral oil mixed system is the smallest, while the interaction between silicone sealant molecules in the silicone sealant/methyl silicone oil and silicone sealant/double-ended vinyl silicone oil mixed systems is not significantly different. This indicates that the increase of plasticizer content increases the intermolecular distance of silicone sealant molecules and weakens their intermolecular interaction. Meanwhile, due to the small molar mass of mineral oil molecules, more molecules are required at the same plasticizer content, resulting in a larger distance between the molecular chains of silicone sealant and lower intermolecular interactions.
In addition, the strength of the interaction between silicone sealant and plasticizer molecules was further calculated, and the results are shown in Fig. 14. As shown in Fig. 14, with the increase of plasticizer content, the interaction between silicone sealant and plasticizer molecules continues to strengthen. Therefore, comprehensive analysis shows that in the silicone sealant/mineral oil mixed system, as the mineral oil content increases, the reducing effect between silicone sealant molecules is always stronger than the increasing interaction between silicone sealant and plasticizer molecules, resulting in a decrease in mechanical properties. In the mixed system of silicone sealant/methyl silicone oil and silicone sealant/double-ended vinyl silicone oil, due to the presence of polysiloxane in the molecular structure of methyl silicone oil and double-ended vinyl silicone oil, the tight binding effect with the organosilicon component in silicone sealant is significant in small amounts. Therefore, when the content of methyl silicone oil does not exceed 20%, the increase in interaction between silicone sealant and plasticizer molecules is greater than the decrease in interaction between silicone sealant molecules, thus improving the mechanical properties. When its content exceeds 20%, the decrease in interaction between silicone sealant molecules is stronger than the increase in interaction between silicone sealant and plasticizer molecules, ultimately leading to a gradual decline in the mechanical properties of silicone sealant.

Figure 14: The binding energy between silicone sealant and plasticizer molecules at different levels of plasticizer content
3.2.4 Effect of Temperature on Intermolecular Interactions
To further investigate the microscopic mechanism of the influence of temperature on the mechanical properties of three mixed systems, the intermolecular interactions of silicone sealant/white oil, silicone sealant/methyl silicone oil, and silicone sealant/double-ended vinyl silicone oil mixed systems at temperatures of 298–378 K during the simulation process were calculated. The results are shown in Fig. 15. As shown in Fig. 15, with the increase of temperature, the intermolecular interactions of silicone sealant in the three mixed systems of silicone sealant/white oil, silicone sealant/methyl silicone oil, and silicone sealant/double-ended vinyl silicone oil all decrease. Moreover, at the same temperature, the van der Waals interaction energy between silicone sealant molecules in the silicone sealant/mineral oil mixed system is the lowest.

Figure 15: van der Waals interaction energy between silicone sealant molecules at different temperatures
3.3.1 The Influence of the Crosslinking Degree of Silicone Sealant on the Free Volume of the System
Studies have shown [15] that the free volume of the system can not only reflect the molecular stacking ability in cross-linked silicone sealant systems, but also predict the mechanical properties of silicone sealant systems. Therefore, the mechanical properties of cross-linked silicone sealant systems are highly dependent on their free volume. The reduction of the free volume of the system will hinder the movement ability of molecular chains during the cross-linking process of silicone sealant, thereby improving the mechanical properties of its cross-linked hybrid system [14]. Table 1 shows the free volume fraction of three mixed systems of silicone sealant/white oil, silicone sealant/methyl silicone oil, and silicone sealant/double-ended vinyl silicone oil with different cross-linking degrees. As can be seen from the table, the free volume fraction of all three mixed systems decreases with the increase of the cross-linking degree.
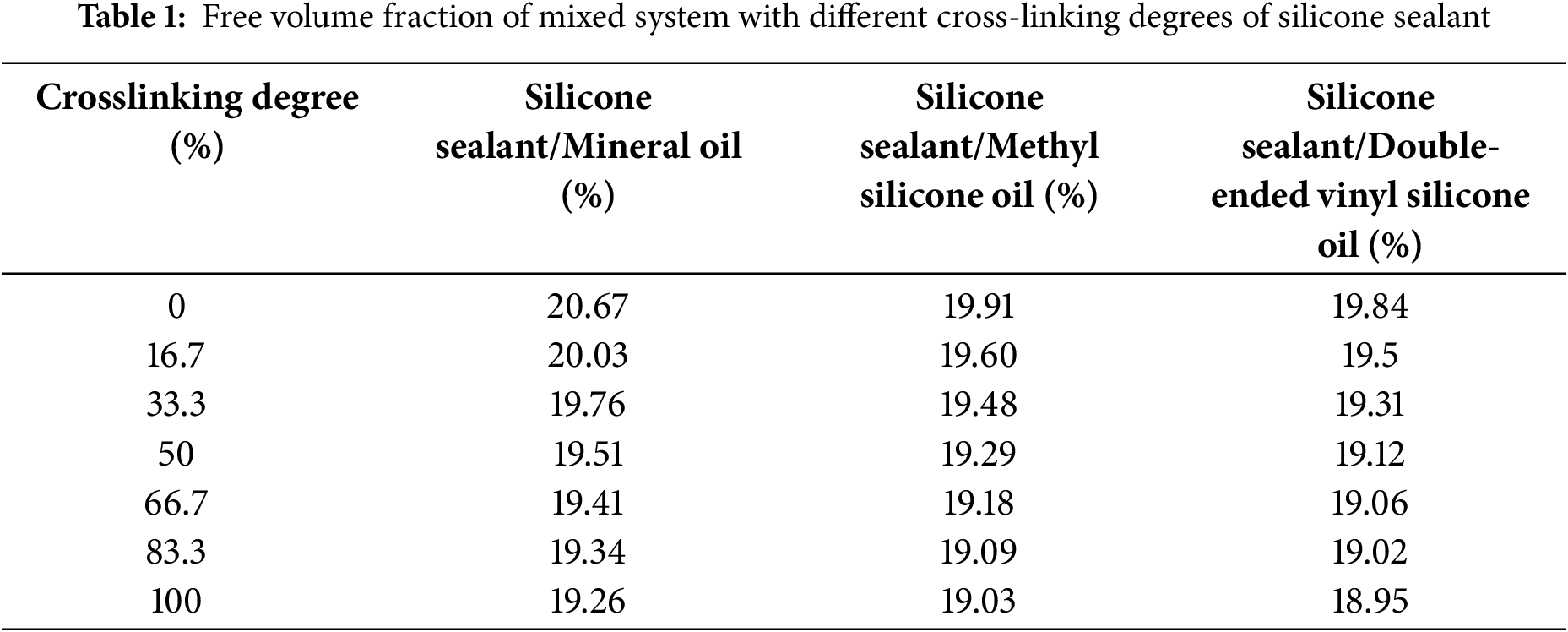
Therefore, based on the analysis of the above two aspects, with the increase of cross-linking degree of silicone sealant, the formation of cross-linking network reduces the distance between molecules, and at the same time causes the free volume of the system to continuously decrease, enhancing the inter-molecular interactions, thus improving the mechanical properties of silicone sealant.
3.3.2 Influence of Plasticizer Content on the Free Volume of the System
Table 2 shows the free volume of three mixed systems with different plasticizer contents. As shown in Table 2, with the increase of plasticizer content, the free volume of the system also increases. The order of free volume of the three mixed systems is: silicone sealant/white oil > silicone sealant/methyl silicone oil > silicone sealant/double-ended vinyl silicone oil.

Based on the analysis of the above two aspects, as the content of plasticizers increases, the interaction between silicone sealant and plasticizer molecules strengthens. However, at the same time, the free volume of the system increases, and the interaction between silicone sealant molecular chains weakens. Therefore, the increase in free volume of the system and the decrease in intermolecular interactions of silicone sealant molecules work together to cause a decrease in the overall mechanical properties of silicone sealant with increasing plasticizer content. Meanwhile, according to the radial distribution function analysis in Section 3.2.1, due to the differences in the molecular structure of plasticizers, the mechanical properties of silicone sealants with mineral oil are the worst compared to those with methyl silicone oil and double-ended vinyl silicone oil under the same conditions.
3.3.3 The Effect of Temperature on the Free Volume of the System
The increase in temperature leads to changes in intermolecular interactions, and at the same time, the change in free volume in the system is bound to be affected. Table 3 shows the free volume of three mixed systems at different temperatures. As shown in Table 3, with the increase in temperature, the free volume of each mixed system increases. Under the same temperature conditions, the order of free volume size of the three mixed systems is silicone sealant/white oil > silicone sealant/methyl silicone oil > silicone sealant/double-ended vinyl silicone oil.

Based on the analysis of the above two aspects, with the increase of temperature, the Brownian motion between molecules intensifies, and molecules obtain higher kinetic energy, which enhances the activity of plasticizer molecules and silicone sealant molecular chains, weakens intermolecular interactions, and increases the distance between molecules. This increases the free volume of the system, which is more conducive to the extension of molecular chains and accelerates the relaxation process, resulting in a decrease in the modulus and rigidity of silicone sealant. Meanwhile, due to the different intermolecular interactions and free volumes of polymer chains in the three mixed systems, there are significant differences in the degree of molecular chain extension and system relaxation, resulting in a significant decrease in the mechanical properties of the silicone sealant/mineral oil mixed system with temperature. The mechanical properties of the silicone sealant/methyl silicone oil mixed system and the silicone sealant/double-ended vinyl silicone oil mixed system have good heat resistance.
A molecular dynamics model of a cross-linked silicone sealant/plasticizer mixture system was constructed, and the mechanical performance parameters of the cross-linked silicone sealant mixture system, and explored. The effects of silicone sealant crosslinking degree, plasticizer content, and external temperature on the mechanical properties of silicone sealant were studied. It is indicated that during the crosslinking process, as the crosslinking degree of silicone sealant increases, the Young’s modulus, bulk modulus, and shear modulus of the three mixed systems gradually increase. The trend of elastic modulus changes reflects the strengthening effect of silicone sealant crosslinking reaction on its stiffness. In the cross-linking process of silicone sealant, the mechanical properties of the three mixed systems are in the following order: silicone sealant/double-ended vinyl silicone oil > silicone sealant/methyl silicone oil > silicone sealant/white oil. With the increase of plasticizer content, the overall Young’s modulus, bulk modulus, and shear modulus of the silicone sealant system show a decreasing trend. However, when the content of methyl silicone oil is 20%, the interaction between the components is slightly enhanced, and the mechanical properties of the silicone sealant are slightly improved. As the temperature increases, the elastic modulus values of the three mixed systems all show a decreasing trend, with the mineral oil silicone sealant system showing the largest decrease. When the temperature reaches 378 K, the methyl silicone oil silicone sealant and double-ended vinyl silicone oil silicone sealant colloidal system still maintain high elastic modulus values and have good heat resistance. It will provide scientific theoretical guidance for improving and predicting the mechanical properties of silicone sealant. The computer simulation method provides theoretical data support for the study of material structure performance relationships and saves experimental economic costs, labor costs, and time costs, and provides a convenient and efficient method for predicting and improving the performance of silicone sealants.
Acknowledgement: We thank Ping Chen (College of Chemical Engineering, Dalian University of Technology) for his assistance with technical support. We also acknowledge Juying Zhou (School of Chemistry and Chemical Engineering, Guangxi Minzu University) for her helpful comments on the manuscript.
Funding Statement: This work was supported by The Guangxi Scholarship Fund of Guangxi Education Department (GED), Guangxi Key Research and Development Project (Grant No. Guike AB24010217), the Major Special Project of Guangxi Science and Technology (Grant No. Guike AA23062020), the Guangxi Science and Technology Base and Talent Project (Grant No. Guike AD20297016), and the Guangxi Minzu University Startup Project for Talent Introduction in 2019 (Grant No. 2019KJQD11).
Author Contributions: Every author played a role in conceptualizing and designing the study. All authors have reviewed and endorsed the final version of the manuscript. Specific contributions were as follows: Wen Qi: Conceptualization, Methodology, Writing—original draft, Funding acquisition. Yu-Fei Du: Data curation, Writing—original draft. Bo-Han Chen: Investigation, Data curation. Gui-Lei An: Formal analysis, Writing—review & editing. Chun Lu: Writing—review & editing, Supervision. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Research data supporting the conclusions of this article will be made available by the authors.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Karambar S, Tenbohlen S. Compatibility study of silicone rubber and mineral oil. Energies. 2021;14(18):5899. doi:10.3390/en14185899. [Google Scholar] [CrossRef]
2. Qi W, Qi DW. A green detection method of silicone sealant mixed with white mineral oil. China Adhes. 2018;27(7):25–8. (In Chinese). doi:10.13416/j.ca.2018.07.007. [Google Scholar] [CrossRef]
3. Ou JL, Chen BY, Quan WG, Yang YQ, Chen YY. Effect of white oil content on the properties of dealcoholized silicone sealant. Chem Adhes. 2023;45(1):90–2. (In Chinese). doi:10.3969/j.issn.1001-0017.2023.01.020. [Google Scholar] [CrossRef]
4. Kuang M, Zeng R, Zhu YH, Jiang JB. The influence of dimethyl silicone oil on the performance of silicone building sealant. Chin Build Waterproofing. 2018;17:16–9. doi:10.15901/j.cnki.1007-497x.2018.17.005. [Google Scholar] [CrossRef]
5. Feng Q, Ding YF, Kang JH, Zhao YQ, Li H, Chen X, et al. Effect of plasticizer on properties of silicone sealant for building. China Adhes. 2019;28(12):15–9. (In Chinese). doi:10.13416/j.ca.2019.12.005. [Google Scholar] [CrossRef]
6. Fan J, Anastassiou A, Macosko CW, Tadmor EB. Molecular dynamics predictions of thermomechanical properties of an epoxy thermosetting polymer. Polymer. 2020;196(1–2):122477. doi:10.1016/j.polymer.2020.122477. [Google Scholar] [CrossRef]
7. Zhang H, Deng C, Shang Y, Zhao H, Wang X, Han B, et al. Theoretical study on the hydrogen addition reactions to bismaleimide in the ultra-violet radiation cross-linking process of polyethylene. J Mol Graph Model. 2020;100:107679. doi:10.1016/j.jmgm.2020.107679. [Google Scholar] [PubMed] [CrossRef]
8. Kim B, Shin H, Choi J, Cho M. Multiscale modeling of load transfer characteristics in crosslinked epoxy nanocomposites. Mech Adv Mater Struct. 2022;29(26):4768–78. doi:10.1080/15376494.2021.1937759. [Google Scholar] [CrossRef]
9. Tack JL, Ford DM. Thermodynamic and mechanical properties of epoxy resin DGEBF crosslinked with DETDA by molecular dynamics. J Mol Graph Model. 2008;26(8):1269–75. doi:10.1016/j.jmgm.2007.12.001. [Google Scholar] [PubMed] [CrossRef]
10. Lv G, Li K, Shi Y, Zhang R, Tang H, Tang C. Effect of aminosilane coupling agent-modified nano-SiO2 particles on thermodynamic properties of epoxy resin composites. Processes. 2021;9(5):771. doi:10.3390/pr9050771. [Google Scholar] [CrossRef]
11. Jeyranpour F, Alahyarizadeh G, Arab B. Comparative investigation of thermal and mechanical properties of cross-linked epoxy polymers with different curing agents by molecular dynamics simulation. J Mol Graph Model. 2015;62(9):157–64. doi:10.1016/j.jmgm.2015.09.012. [Google Scholar] [PubMed] [CrossRef]
12. Xiong J, Li K, Lin C, Shen H, Lu C. Molecular interaction mechanism and mechanical performance of lignin/gelatin composites by molecular dynamics simulation study. Langmuir. 2025;41(12):8369–79. doi:10.1021/acs.langmuir.5c00274. [Google Scholar] [PubMed] [CrossRef]
13. Violano G, Chateauminois A, Afferrante L. Rate-dependent adhesion of viscoelastic contacts, part I: contact area and contact line velocity within model randomly rough surfaces. Mech Mater. 2021;160(2):103926. doi:10.1016/j.mechmat.2021.103926. [Google Scholar] [CrossRef]
14. Tong Z, Qiu R, Cai S, Lin G, Xie H, Jiang T, et al. Molecular dynamics study of polyurethane and KH550/SiO2 in enhancing epoxy resin (EP) mechanical properties. Langmuir. 2025;41(27):17749–63. doi:10.1021/acs.langmuir.5c01077. [Google Scholar] [PubMed] [CrossRef]
15. Jagarlapudi SS, Cross HS, Das T, Goddard WAIII. Thermomechanical properties of nontoxic plasticizers for polyvinyl chloride predicted from molecular dynamics simulations. ACS Appl Mater Interfaces. 2023;15(20):24858–67. doi:10.1021/acsami.3c02354. [Google Scholar] [PubMed] [CrossRef]
16. Xu P, Wang Z, Zhao Q, Liu M, Xue J, Pan Y, et al. Solid-liquid equilibrium of molnupiravir in 15 monosolvents: an experimental and molecular simulation study. J Chem Eng Data. 2024;69(9):3167–78. doi:10.1021/acs.jced.4c00256. [Google Scholar] [CrossRef]
17. Klemm A, Vicchio SP, Bhattacharjee S, Cagli E, Park Y, Zeeshan M, et al. Impact of hydrogen bonds on CO2 binding in eutectic solvents: an experimental and computational study toward sorbent design for CO2 capture. ACS Sustainable Chem Eng. 2023;11(9):3740–9. doi:10.1021/acssuschemeng.2c06767. [Google Scholar] [CrossRef]
18. Nandi R, Paul N, Banerjee T. Molecular dynamics insights of CO2 capture through phosphonium-based deep eutectic solvents for direct air capture. ACS Sustainable Chem Eng. 2025;13(22):8234–48. doi:10.1021/acssuschemeng.4c10594. [Google Scholar] [CrossRef]
19. Clancy TC, Pütz M, Weinhold JD, Curro JG, Mattice WL. Mixing of isotactic and syndiotactic polypropylenes in the melt. Macromolecules. 2000;33(25):9452–63. doi:10.1021/ma0011035. [Google Scholar] [CrossRef]
20. Akten ED, Mattice WL. Monte Carlo simulation of head-to-head, tail-to-tail polypropylene and its mixing with polyethylene in the melt. Macromolecules. 2001;34(10):3389–95. doi:10.1021/ma0020739. [Google Scholar] [CrossRef]
21. Gestoso P, Brisson J. Towards the simulation of poly(vinyl phenol)/poly(vinyl methyl ether) blends by atomistic molecular modelling. Polymer. 2003;44(8):2321–9. doi:10.1016/S0032-3861(03)00098-3. [Google Scholar] [CrossRef]
22. Maguire SM, Krook NM, Kulshreshtha A, Bilchak CR, Brosnan R, Pana AM, et al. Interfacial compatibilization in ternary polymer nanocomposites: comparing theory and experiments. Macromolecules. 2021;54(2):797–811. doi:10.1021/acs.macromol.0c02345. [Google Scholar] [CrossRef]
23. Zhou GY. Quantum chemical study of the role of the van der Waals interaction (1). J Adv Phys Chem. 2013;2(2):21–6. doi:10.12677/japc.2013.22004. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools