 Open Access
Open Access
ARTICLE
A Novel Fracturing Fluid with High-Temperature Resistance for Ultra-Deep Reservoirs
1 Northwest Oilfield Branch, China Petroleum & Chemical Corporation, Urumqi, 830011, China
2 Sinopec Key Laboratory for Enhanced Recovery in Fractured and Cavernous Reservoir, Urumqi, 830011, China
* Corresponding Author: Kebo Jiao. Email:
Fluid Dynamics & Materials Processing 2024, 20(5), 975-987. https://doi.org/10.32604/fdmp.2023.030109
Received 22 March 2023; Accepted 27 June 2023; Issue published 07 June 2024
Abstract
Ultra-deep reservoirs play an important role at present in fossil energy exploitation. Due to the related high temperature, high pressure, and high formation fracture pressure, however, methods for oil well stimulation do not produce satisfactory results when conventional fracturing fluids with a low pumping rate are used. In response to the above problem, a fracturing fluid with a density of 1.2~1.4 g/cm was developed by using Potassium formatted, hydroxypropyl guanidine gum and zirconium crosslinking agents. The fracturing fluid was tested and its ability to maintain a viscosity of 100 mPa.s over more than 60 min was verified under a shear rate of 170 1/s and at a temperature of 175°C. This fluid has good sand-carrying performances, a low viscosity after breaking the rubber, and the residue content is less than 200 mg/L. Compared with ordinary reconstruction fluid, it can increase the density by 30%~40% and reduce the wellhead pressure of 8000 m level reconstruction wells. Moreover, the new fracturing fluid can significantly mitigate safety risks.Keywords
Fracturing is a crucial technology for stimulating oil and gas production and has been extensively employed in the field [1–5]. The fracturing fluid system and technology used in the petroleum industry are highly developed [6–10]. The main medium of fracturing fluid can be divided into water-based fracturing fluid, oil-based fracturing fluids b, concentrated energy foam fracturing fluid, and alcohol-based fracturing fluid [11–14].
Ultra-deep reservoir resources are crucial for our oil and gas exploration and development. An oilfield block aims to achieve a production capacity of 30 million tons. The target layer depth is becoming increasingly deep, with the physical properties of the reservoir deteriorating. The reservoir is buried at a depth of over 8000 m, and the design well depth exceeds 8500 m. The formation pressure is close to 140 MPa, and the formation temperature is almost 180°C. The matrix is denser, and the heterogeneity of natural fractures is more pronounced. In order to improve single-well production, it is necessary to reform these reservoirs [15].
In 2011, Wu et al. [16] and their team disclosed a hydroxypropyl guanidine gum weight fracturing fluid with a weighting density of 1.5 g/cm3 using sodium bromide and sodium chloride and can resist temperatures up to 180°C. In 2013, Cui et al. [17] and his team revealed an APCF-weighted fracturing fluid with a weighting density of 1.55 g/cm3 using potassium bromide and sodium chloride. They can tolerate temperatures up to 160°C, successfully utilized in field construction. In 2019, Zeng et al. [18] obtained a deep-water oilfield sodium bromide plus heavy fracturing fluid system TPH with a 1.65 g/cm3 density and temperature resistance of 170°C. In 2021, Quan et al. [19] studied a boron-crosslinked guanidine gum-weighted fracturing fluid with a density of 1.38 g/cm3, which maintained a viscosity above 60 mPa.s when continuously sheared for 90 min at 170°C and 170 1/s. Most existing guar gum fracturing fluids have a temperature resistance below 150°C. Therefore, it is necessary to modify them to improve their temperature resistance. Hydroxypropyl guanidine gum and GHPG ultra-high temperature guanidine gum can meet the temperature tolerance requirement of 170°C. Currently, most guar gum thickeners do not have a temperature resistance of 180°C and require modification [20,21]. Inorganic salt is used for weighting, and the fracturing fluid group must contain a salt-resistant monomer. Currently, the weighting agent materials selected for ultra-high temperature weighting fracturing fluid are mostly sodium bromide, nitrate, etc. Their cost and safety must be considered, especially in the northwest region.
Based on the geological conditions of onshore ultra-deep well oilfields, this paper presents a performance evaluation of a potassium formatted heavy fracturing fluid system for onshore ultra-deep well oilfields in the western region. The study conducts laboratory experiments to optimize the weighting agent, thickening agent, crosslinking agent, and gel breaker. The results show that a heavy fracturing fluid system suitable for ultra-high temperature oilfields can be obtained. This system can reduce wellhead pressure and significantly reduce construction safety risks for 8000 m level reconstruction wells. The findings of this study provide valuable technical references for future fracturing construction in onshore ultra-deep well oilfields.
2 Fracturing Fluid System Design
Since the patent of weighting technology for fracturing fluid was first reported in 2002, research on saline fracturing fluid has been started at home and abroad. Inorganic salts or organic salts are used as weighting agents, and thickening agents and crosslinking agents are optimized; the brine fracturing fluid with high density is prepared, which can be used for fracturing construction of different reservoirs to improve production. Organic salt and inorganic salt are commonly used as weighting agents to form organic salt high-density brine fracturing fluid systems and inorganic salt high-density brine fracturing fluid systems. In the development process, it is generally necessary to optimize the weighting agent and study the influence of the weighting agent at different content on the crosslinking system. Select the system with the best performance and determine the high-density brine fracturing fluid formula under different construction requirements based on cost optimization.
Since the fracturing fluid is required for this time, the thickener molecule must show the characteristics of instant solubility, good thickening effect, and good crosslinking effect in the salt solution. Generally speaking, salt-resistant thickeners must meet the following three requirements:
(1) To improve the temperature resistance of the fracturing fluid system, the thickener molecule should be able to crosslink with the crosslinking agent under certain conditions to form a gel so that it still has good sand-carrying capacity under high-temperature shear conditions. Therefore, the synthesized polymer molecular chain must have the crosslinking site of the crosslinking agent;
(2) The fracturing fluid thickener has good compatibility with the metal ions in the additive without precipitation, crosslinking, and other reactions;
(3) The main chain of fracturing fluid thickener must have necessary hydrophilic groups.
Hydroxypropyl guanidine gum (HPG) meets our requirements but must be modified. The process is as follows:
First, small molecular weight guanidine gum was obtained by enzymatic hydrolysis, and then the ammonium salt cationic hydrophilic group was introduced into guanidine gum to obtain modified guanidine gum.
The modified HPG hydroxypropyl guanidine gum has high water solubility, and the insoluble matter content is reduced. The modified guanidine gum reduces the hydroxyl content. It increases the number of branched groups, so it weakens the intermolecular hydrogen bond to a certain extent, thus increasing the water solubility of guanidine gum molecules, thus reducing the content of insoluble substances after fracturing fluid gel breaking, and greatly reducing the damage to the formation and proppant filling layer.
2.2 Selection of a Crosslinking Agent
The zirconium crosslinking agent is selected for a fracturing fluid system with high temperature and salinity. The zirconium atom is a transition metal atom with many empty orbitals in the outer layer, which can form hybrid orbitals with the outer orbitals of the oxygen atom. Therefore, in crosslinking with thickener, the zirconium crosslinking agent mainly uses organic ligands to preferentially occupy the outer space orbitals of the zirconium atom to slow down the release rate of the zirconium ion, thus delaying the reaction of zirconium ion, thus realizing delayed crosslinking.
After the organic zirconium crosslinking agent and the thickener molecule are crosslinked, the gel with a three-dimensional network structure can be formed and has excellent temperature resistance.
2.3 Optimization of Weighted Fracturing Fluid System
The guanidine gel thickener, Potassium formatted, acid gel breaker, temperature stabilizer, and drainage aid are mixed proportionately to obtain the fracturing fluid base fluid (Fig. 1). A different proportion of Potassium formatted changes the density of the base solution. At the same time, the viscosity of the base solution is different with different dosages of guanidine gel thickener.

Figure 1: Base solution configured
The amount of thickener is closely related to the viscosity of the base fluid (Fig. 2). The amount of thickener for base fluid prepared this time is optimized, and the results are as follows.

Figure 2: Relationship between the viscosity of the base solution and guanidine gum content (1.3 g/cm3)
The solubility of the prepared modified guanidine gum was increased in concentrated brine. Compared with conventional guanidine gum, the viscosity increases slightly, and the friction resistance is within the acceptable range. To avoid too high a viscosity of the base liquid or poor hydration effect of the base liquid, the thickener should be between 0.4% and 0.7%.
2.4 The Optimum Dosage of Crosslinking Agent
The crosslinking agent in this weighted fracturing fluid system is the compound of organic boron and organic zirconium.
The crosslinking agent has the following advantages:
① At low temperatures, the primary boron ion is gradually released to realize weak cross-linking;
② When the temperature gradually increased, the secondary complexed boron ions were released, which enhanced the crosslinking ability of the gel;
③ When the temperature rises above 140°C, the complexed zirconium ions are released, further enhancing the temperature resistance of the crosslinked gel.
(1) Amount of crosslinking agent
Select 0.5% guanidine gel thickener to prepare 100 ml of base solution, add a different proportion of crosslinking agent, and select the appropriate amount. The results are shown in Fig. 3 and Table 1.

Figure 3: Crosslinking effect of different proportions of crosslinking agent

Through the optimization experiment of crosslinking agent, we get that when the dosage of crosslinking agent is more than 0.5%, the crosslinking is better, and when the dosage is more than 2%, it is easy to over-crosslink; The best dosage of crosslinking agent is 0.5%–2%.
(2) Crosslinking conditions
Both crosslinking effect and the PH regulator have a great influence on the crosslinking time, as shown in Fig. 4.

Figure 4: Crosslinking effect diagram
According to Fig. 5, the higher the dosage of the PH regulator, the longer the crosslinking time; When the dosage reaches a certain level, the change in crosslinking time is not obvious.
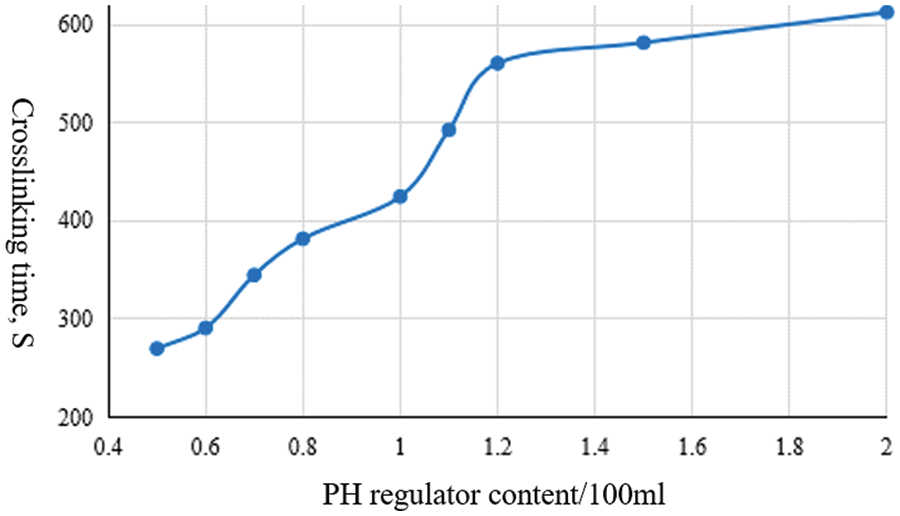
Figure 5: Relationship between PH-regulated dose and crosslinking time
Finally, HPG hydroxypropyl guanidine gum, potassium format weighting agent, organic zirconium crosslinking agent, and the additive system is adopted for the guanidine gum weighted fracturing fluid, and the density can reach 1.4 g/cm3. The coniguration method of the weighted fracturing fluid of the system: first, add potassium format into the water; then, the modified guanidine gum (0.4%~0.7%), temperature stabilizer, and drainage aid are added successively under the emotional state to obtain the weighted fracturing fluid base fluid; Finally, add the crosslinking agent according to the crosslinking ratio (0.5~2.0):100 to obtain the crosslinked gel of fracturing fluid, and evaluate the performance of potassium format weighted guanidine gel fracturing fluid concerning the oil and gas industry standard SY/T 5107-2016 Evaluation Method for Performance of Water-based Fracturing Fluid.
3 Performance Evaluation of Fracturing Fluid
3.1 High-Temperature Rheological Test
According to the formula of 0.5%/0.6% original powder+0.8%/1.0% PH modifier+1.0%/1.5% crosslinking agent, add the weight-cracking liquid, stir it with a glass rod until the crosslinking is hanging, pour it into a beaker, tilt the gel to a tongue shape, and observe its crosslinking state. Fill the viscometer sample cup with fracturing fluid and heat the sample. At the same time, the rotor maintains the temperature unchanged after the shear rate is 170 1/s, and the heating rate is controlled at 3°C/min ± 0.2°C/min to 80°C.
According to Fig. 6, the morphology of the weighted fracturing fluid after crosslinking is visually inspected, and the fracturing fluid system is stable and colorless, without many vesicles, fish eyes, and other conditions, meeting the requirements of fracturing fluid technical indicators. It is preliminarily observed that the gel has certain viscoelasticity.

Figure 6: Morphology of crosslinked fracturing fluid
The rheological test temperature is set at 170°C, and the rotor rotates for 120 min at the shear rate of 170 1/s. The rheological curve is as follows:
It can be seen from Fig. 7 that at the beginning of the experiment, the viscosity of the fracturing fluid system rapidly decreased with the increasing temperature. After heating for 10 min, the temperature reached about 150 C, and a relatively obvious viscosity increase appeared. When the temperature approaches 170°C, the viscosity of the fracturing fluid begins to decrease slowly again. The analysis of four formulations with different proportions concluded that the more PH regulator was, the better the temperature resistance would be. The more crosslinking agent, the better the temperature resistance; in large doses of crosslinking agents, 0.6% of the raw powder temperature resistance effect is poor.

Figure 7: Rheological curve of 170°C guar gum fracturing fluid system
According to Fig. 8, the formula that can maintain viscosity (100 mPa.s) for more than 70 min at 170°C is 0.55% raw powder+1.20% PH regulator+1.30% crosslinking agent and 0.55% raw powder+1.20% PH regulator+1.5% crosslinking agent, respectively (base liquid density is 1.3 g/cm3); With the increasing temperature, the viscosity of the fracturing fluid system decreases rapidly. Because the viscosity of the crosslinked system decreases gradually, this is mainly because the network structure of the crosslinked system is destroyed under the action of shear force; When the viscosity decreases for about 10 min, the fracturing fluid viscosity increases, and the temperature increases, which increases the activity of zirconium ions in the fracturing fluid and the crosslinking group in the solution, and increases the crosslinking strength of the dislocation ions and the crosslinking group, so that the fracturing fluid crosslinks to form a tight network structure, which increases its viscosity. In this process, the crosslinking viscosity increasing effect is stronger than the shear viscosity reducing effect, so the fracturing fluid viscosity generally shows an increase; secondary crosslinking occurs within 20 min; the viscosity of the fracturing fluid in the subsequent continuous heating process generally shows a downward trend. This phenomenon will not occur if the crosslinking agent is excessive, as shown in Fig. 9.
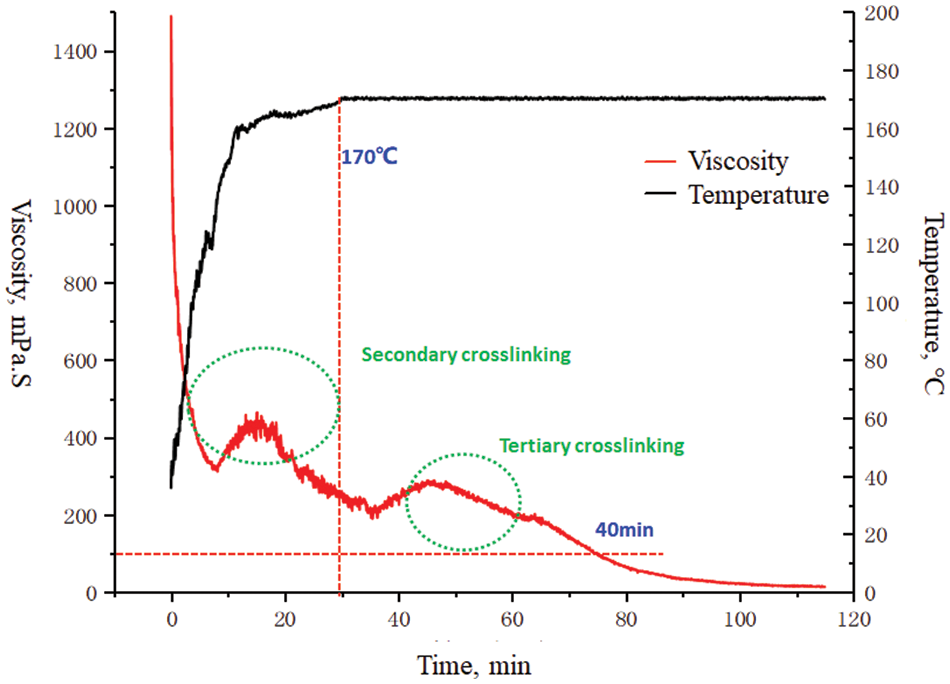
Figure 8: Rheological curve of 170°C guar gum fracturing fluid system (0.55% raw powder+1.20% PH modifier+1.30% cross-linking agent/1.3)
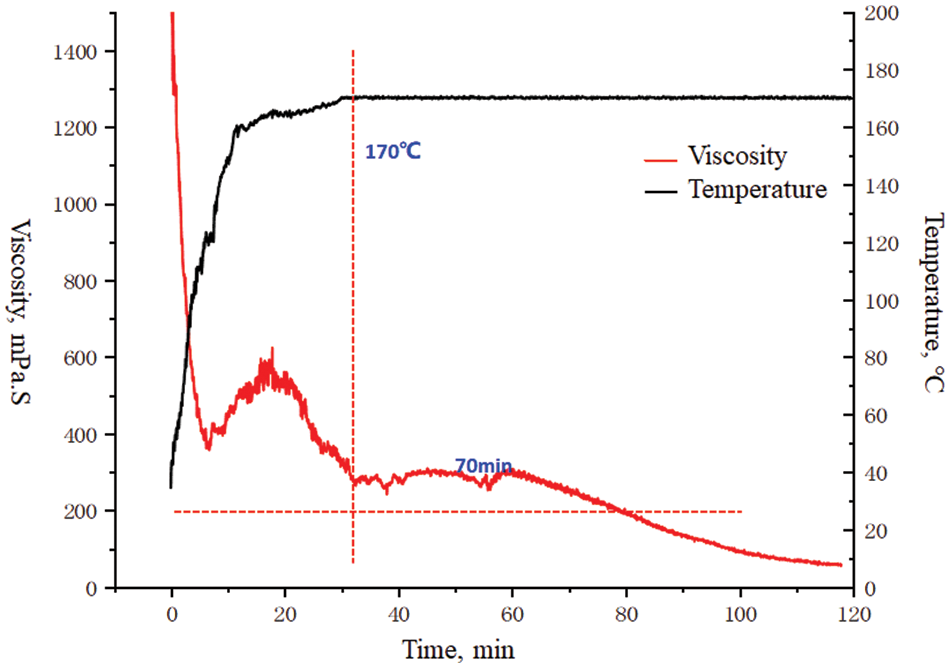
Figure 9: Rheological curve of 170°C guar gum fracturing fluid system (0.55% raw powder+1.20% PH modifier+1.5% cross-linking agent/1.3)
The reason may be that some macromolecular chains in the polymer solution degrade under high temperatures. At the same time, the viscosity of the fracturing fluid will also decrease under high-speed shear; After about 50 min, the viscosity rose again, and crosslinking occurred three times. The viscosity of the crosslinked system of organic boron and organic zirconium can be maintained for about 70 min at 170°C, indicating that the formulation system has a certain temperature and shear resistance.
The formula is improved by using 0.65% raw powder+1.20% PH regulator+1.9% crosslinking agent at the base liquid density of 1.35 g/cm3. The high-temperature rheological diagram is shown in Fig. 10.

Figure 10: Rheological curve of 175°C modified guanidine gum fracturing fluid system (0.65% raw powder+1.20% PH modifier+1.9% cross-linking agent/1.35)
Finally, the formula of potassium format high-temperature resistant weighting fracturing fluid system that can maintain 150 mPa.s viscosity for more than 40 min at 175°C and 170 1/s shear rate was obtained.
3.2 Gel-Breaking Performance Test
At a certain temperature, ammonium persulfate produces oxygen-free radicals and the acetal bond on the guanidine gum molecular chain, which breaks the guanidine gum molecular chain into small molecular fragments and forms a low-viscosity liquid. In this experiment, ammonium persulfate was selected as the gel breaker, As shown in Fig. 11. At constant temperature, the gel breaking time was 1.5 h, and the dosage of the gel breaker was 200, 300, 400, 500, and 600 ppm, respectively. Put the prepared guanidine glue into the beaker container, add the ammonium persulfate solvent at a constant temperature, lift it with a glass rod every 30 min until it cannot be hung, and take the supernatant every 15 min to measure its viscosity until the viscosity is less than 5 mPa.s, the guanidine glue will break. In this experiment, the test of gel breaking performance of the crosslinked system was repeated three times, and the experimental results were taken as the average value. The experimental results are shown in Table 2.

Figure 11: Gel breaker and gel breaking effect

It can be seen from the Fig. 12 that the gel breaking time decreases with the increase of the concentration and increases with the increase of the amount of gel breaker. The gel-breaking time is another 2 h, and the gel-breaking speed is faster. When the amount of gel breaker reaches 600 ppm, the gel breaking can be completed in about 90 min, meeting the site construction requirements. Therefore, the weight fracturing fluid system has good gel-breaking performance.

Figure 12: Gel breaking data of 0.5% guar gum system
A large amount of water-insoluble substances (i.e., fracturing fluid residue) will be produced during the oxidative gel-breaking process, which is mainly related to the oxidative gel-breaking mechanism of polymers. The residue in the fracturing fluid gel breaker will affect the conductivity of the fracture. Too much residue will reduce the fracture’s conductivity, the oil drainage area, and the recovery factor. Therefore, it is necessary to keep the residue content of fracturing fluid as low as possible; Because the residue of gel breaker mainly refers to the insoluble substance after gel breaking. The residue entering the reservoir gap will block the pore roar, reduce the permeability of the reservoir, and also cause damage to the reservoir. After the fracturing fluid gel breaking performance test, dry the beaker to constant weight, then pour a certain volume of fracturing fluid gel breaking filtrate into the centrifuge tube, put the centrifuge tube into the centrifuge in turn, set the speed of the centrifuge to 3000 r/min, and the operation time to 30 min. After centrifugation, pour out the upper clear liquid, put the centrifuge tube into the oven to dry to constant weight, weigh and calculate the mass of the residue, and the formula calculates the residue content of the fracturing fluid:
where is the content of fracturing fluid residue, mg/L; is the residue mass, mg; is the volume of residue, mL.
The experimental results are shown in Fig. 13.
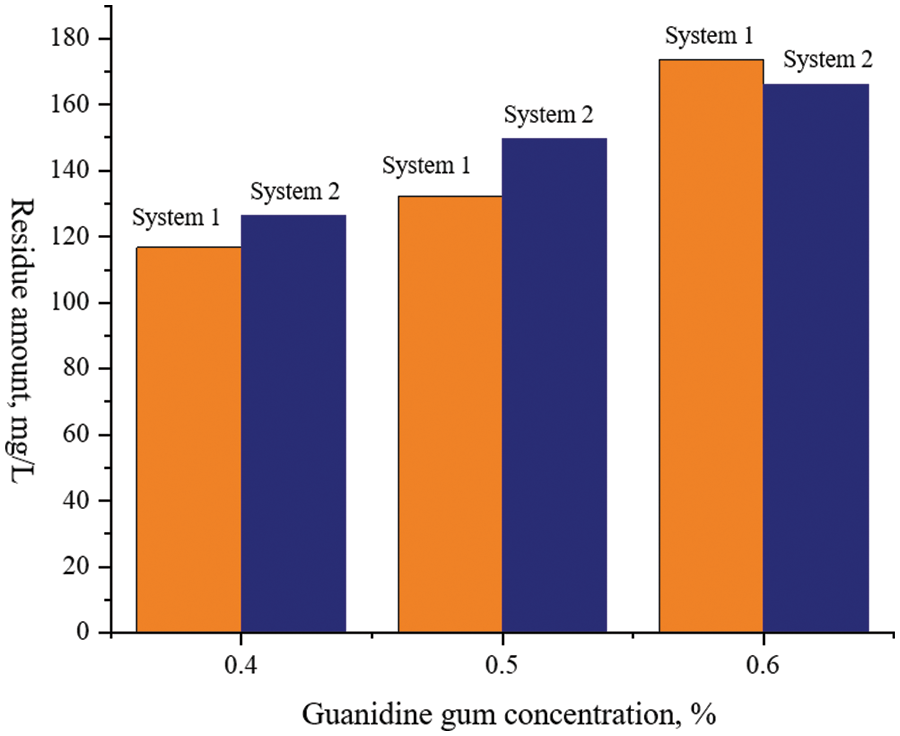
Figure 13: Data of residue content of guanidine gum system
From the test results in the Table 3 above, when the dosage of the guanidine gum system is 0.4%, the residue content of fracturing fluid is as low as 116.7 mg/L-1, which meets the requirements of ≤600 mg/L-1 specified in the oil and gas industry standard SY/T 6376-2008 General Technical Conditions for Fracturing Fluid, and meets the requirements of fracturing fluid construction.

Based on temperature and pressure conditions of ultra-deep reservoirs, a potassium formatted heavy fracturing fluid system with good high-temperature resistance, shear resistance, and suspended sand performance was developed by type and composition of chemical agent optimized. The system has the following advantages:
(1) The newly-developed guanidine gum plus fracturing fluid system can maintain viscosity above 100 mPa.s after shearing at 175°C for 60 min under the weight density of 1.35 g/cm3;
(2) The system is cross-linked several times under high-temperature conditions, which greatly enhances the temperature resistance of the system;
(3) The weighted fracturing fluid has suitable proppant carrying capacity, low viscosity after gel breaking, and residue content is lower than 200 mg/L, far lower than the requirements of the industry standard.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Lian Liu, Liang Li; background research and drawing: Yun Luo; experiment and result analysis: Kebo Jiao, Junwei Fang; draft manuscript preparation: Kebo Jiao. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data in this article are all original.
Conflicts of Interest: The authors declare that they have no conflicts of inter-est to report regarding the present study.
References
1. Jiang, R., Jiang, T., Wang, Y. (2004). Present development and prospecting of hydraulic fracturing technology. Oil Drilling & Production Technology, 4, 52–57. [Google Scholar]
2. Hu, K. X., Wang, X. H. (2015). Situation fracturing fluid technology development research. Petrochemical Industry Applicatio, 2, 13–16. [Google Scholar]
3. Demin, M., Group, Y. P. (2017). Optimization experiments for fracturing fluid. Petrochemical Industry Technology, 24, 124. [Google Scholar]
4. Yang, D., Yang, B., Ren, M., Liu, Y., Cao, H. et al. (2023). Construction of fracturing fluid with excellent proppant transport capacity using low molecular weight hydrophobic association polymer and surfactant. Journal of Molecular Liquids, 377, 121546. https://doi.org/10.1016/j.molliq.2023.121546 [Google Scholar] [CrossRef]
5. Yang, X., Chen, A., Mao, J., Zhang, C., Wang, J. et al. (2023). Synthetic polymer fracturing fluid weighted by sodium formate enables fracture stimulations in ultra-high pressure and high-temperature reservoir. Fuel, 353, 129170. https://doi.org/10.1016/j.fuel.2023.129170 [Google Scholar] [CrossRef]
6. Abdulaziz Alghamdi, O., Mansha, M., Kalanthoden, A. N., Kamal, M. S., Khan, M. (2022). Fracturing fluid applications of carboxylate-terminated low molecular weight PEI and CTAB formulations. Colloid and Interface Science Communications, 49, 100643. https://doi.org/10.1016/j.colcom.2022.100643 [Google Scholar] [CrossRef]
7. Mao, J., Liao, Z., Jiang, J., Yang, X., Zhang, Y. et al. (2022). One practical CaCl2-weighted fracturing fluid for high-temperature and high-pressure reservoir. Petroleum Science and Technology, 40, 2877–2889. https://doi.org/10.1080/10916466.2022.2050388 [Google Scholar] [CrossRef]
8. Liao, Z., Chen, F., Deng, Y., Wang, K., von Gunten, K. et al. (2022). Organic weighting hydraulic fracturing fluid: Complex interactions between formate salts, hydroxy carboxylate acid, and guar. SPE Journal, 27, 2334–2351. https://doi.org/10.2118/209606-PA [Google Scholar] [CrossRef]
9. Xu, H., Zhou, F., Li, Y., Chen, Z., Yao, E. (2022). Development and evaluation of a novel high-density weighted fracturing fluid in ultra-deep reservoirs. IOP Conference Series: Earth and Environmental Science, 984, 012015. [Google Scholar]
10. Maekawa, M., Morita, K., Kaneko, N. (2022). Study on the performance of high temperature resistant composite salt weighted fracturing fluid system. Applied Chemical Industry, 51, 2898–2907. [Google Scholar]
11. Guo, Y., Zhang, M., Yang, H., Wang, D., Ramos, M. A. et al. (2022). Friction challenge in hydraulic fracturing. Lubricants, 10, 14. https://doi.org/10.3390/lubricants10020014 [Google Scholar] [CrossRef]
12. Gaurina-Međimurec, N., Brkić, V., Topolovec, M., Mijić, P. (2021). Fracturing fluids and their application in the Republic of Croatia. Applied Sciences, 11, 2807. https://doi.org/10.3390/app11062807 [Google Scholar] [CrossRef]
13. Liu, Y., Liu, J., Li, Y., Yang, H., Yan, F. et al. (2020). Development and field application of a new ultralow guar gum concentration weighted fracturing fluid in HPHT reservoirs. Journal of Chemistry, 2020, 1–10. [Google Scholar]
14. Fheed, A., Kłodowski, K., Krzyżak, A. (2020). Fracture orientation and fluid flow direction recognition in carbonates using diffusion-weighted nuclear magnetic resonance imaging: An example from Permian. Journal of Applied Geophysics, 174, 103964. https://doi.org/10.1016/j.jappgeo.2020.103964 [Google Scholar] [CrossRef]
15. Yang, X., Mao, J., Zhang, W., Zhang, H., Zhang, Y. et al. (2020). Tertiary cross-linked and weighted fracturing fluid enables fracture stimulations in ultra high pressure and temperature reservoir. Fuel, 268, 117222. https://doi.org/10.1016/j.fuel.2020.117222 [Google Scholar] [CrossRef]
16. Wu, L., Wang, S., Lei, Y. (2011). Weighting performance research of ultra-high temperature guar gum fracturing fluid. Journal of Chongqing University of Science and Technology (Natural Sciences Edition), 11, 98–100. [Google Scholar]
17. Cui, H. J., Yu, D. H., Li, J. P., Che, H., Li, Y. T. et al. (2013). Research and application of high density guar gum fracturing fluid system. Oil Drilling & Production Technology, 35, 64–66. [Google Scholar]
18. Zeng, L. C., Jian, Z., Jie, W. X., Zeng, H. Y., Qing, Y. et al. (2019). Research and performance evaluation of high temperature weighted fracturing fluid system in deep sea oilfield. Ocean Engineering Equipment and Technology, A1, 116–121. [Google Scholar]
19. Quan, S. J., Tong, L. Y., Zi, T. Z., Lan, D. X., Jie, C. G. et al. (2021). Preparation and properties of borate crosslinking weighted fracturing fluid with high temperature resistance and low friction (Preparation and properties of borate crosslinking weighted fracturing fluid with high temperature resistance and low friction). Applied Chemical Industry, 50, 2649–2652+2656. [Google Scholar]
20. Raya, S. A., Saaid, I. M., Ahmed, A. A., Umar, A. A. (2020). A critical review of development and demulsification mechanisms of crude oil emulsion in the petroleum industry. Journal of Petroleum Exploration and Production Technology, 10, 1711–1728. https://doi.org/10.1007/s13202-020-00830-7 [Google Scholar] [CrossRef]
21. Issaka, S. A. (2015). Review on the fundamental aspects of petroleum oil emulsions and techniques of demulsification. Journal of Petroleum & Environmental Biotechnology, 6(2), 214. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools