 Open Access
Open Access
REVIEW
Conjugated Polymer Hydrogel: A Highly Efficient Material of Solar Water Purification
1 Jiangxi Provincial Key Laboratory of Flexible Electronics, Flexible Electronics Innovation Institute, Jiangxi Science and Technology Normal University, Nanchang, 330013, China
2 Jiangxi University of Technology High School, Nanchang, 330096, China
3 Electronic Materials Research Laboratory, Key Laboratory of the Ministry of Education, International Center for Dielectric Research, Shaanxi Engineering Research Center of Advanced Energy Materials and Devices, School of Electronic Science and Engineering, Xi’an Jiaotong University, Xi’an, 710049, China
* Corresponding Author: Baoyang Lu. Email:
# These authors contributed equally to this work
(This article belongs to the Special Issue: Multifunctional Conductive Hydrogels and Their Applications)
Journal of Polymer Materials 2025, 42(2), 339-358. https://doi.org/10.32604/jpm.2024.057955
Received 31 August 2024; Accepted 12 November 2024; Issue published 14 July 2025
Abstract
The global scarcity of clean water is an escalating issue due to climate change, population growth, and pollution. Traditional water purification technologies, while effective, often require significant energy input and complex infrastructure, limiting their accessibility. This review explores the use of conjugated polymer hydrogels as a promising solution for solar water purification. Conjugated polymer hydrogels offer unique advantages, including high photothermal conversion efficiency, effective heat management, and rapid water transport, which are crucial for efficient solar-driven water evaporation. By leveraging the properties of these hydrogels, it is possible to significantly reduce the energy required for water evaporation, making them a cost-effective and scalable option for producing potable water from seawater or wastewater. This review discusses the principles of solar water purification using conjugated polymer hydrogels, strategies to enhance their performance through material and structural design, and their applications in pollutant removal and desalination. Additionally, it addresses the advantages and limitations of these materials, providing insights into their potential future development and applications in sustainable water purification technologies.Keywords
Water is widely recognized as a fundamental element for sustaining life. However, the scarcity of clean water has become a pressing issue in many regions due to global warming, population growth, and severe water pollution problems [1]. According to the World Health Organization (WHO), approximately 2.1 billion people worldwide are currently facing a shortage of safe drinking water, with 8.44 million individuals living below the basic standards for potable water [2]. By 2025, it is predicted that about 4 billion people globally will experience severe water scarcity [3]. Over the past decades, various water purification technologies have made notable advancements [4]. Membrane-based methods, like reverse osmosis and nanofiltration [5–7], effectively remove contaminants but require high energy inputs and are prone to membrane fouling, increasing operational costs and maintenance challenges. Thermal methods, such as multi-stage flash and multi-effect distillation [8,9], produce high-quality water but demand significant electricity and complex infrastructure, limiting their use in remote or resource-limited regions. Adsorption techniques necessitate frequent regeneration of adsorbents and may not eliminate all pollutants [10–12]. Chemical disinfection methods, like chlorination and ozonation [13,14], can introduce harmful by-products and do not remove physical impurities. Advanced oxidation processes are effective but involve complex operations and high costs [15–17]. Given these limitations, it is imperative to explore alternative pathways for freshwater acquisition that offer low cost, environmental friendliness, high efficiency, and sustainability. Using an inexhaustible source of clean energy, solar thermal-driven water purification technology based on solar water distillation stands out from other methods. This method primarily employs materials capable of photothermal conversion and water absorption, integrated with simple vaporization-condensation techniques, to obtain potable water from seawater or wastewater [18–20]. However, in traditional solar water distillation devices, photothermal materials are typically placed at the bottom of the water body. When positioned this way, these materials cannot effectively manage heat loss because not only is the water at the interface heated, but a large volume of water beneath the photothermal material is also heated. Simultaneously, a significant amount of energy is radiated into the environment, resulting in a low solar energy utilization efficiency of approximately 30%–55%. To address this challenge, solar-driven interfacial evaporation technology, based on the localized solar thermal energy conversion of photothermal materials at the air/liquid interface, has been significantly developed. Hogan et al. [21] concentrated gold nanoparticles on the water surface for localized heating. Ghasemi et al. [19] were the first to use porous insulating foam to support a floating photothermal conversion structure on the water surface, effectively preventing the dissipation of solar-generated heat throughout the entire water body. This approach can be regarded as a prototype of interfacial solar vapor generation systems, significantly improving photothermal evaporation efficiency. Furthermore, the development of micro/nanostructured materials has further enhanced light absorption and heat localization, potentially achieving evaporation rates of up to, or even exceeding 100% [3,22].
Materials used for interfacial water purification should possess excellent light absorption, efficient photothermal conversion, effective heat management, the ability to float on water, and rapid water transport and diffusion properties [23]. To date, common photothermal materials including metal nanoparticles [24–27], inorganic semiconductors [28–31], carbon-based materials [32–35], and polymers/gels [36–38] have demonstrated high photothermal conversion efficiencies for solar water purification [39–41]. However, most of these materials face high costs, susceptibility to aggregation, complex fabrication, low evaporation efficiency, and limited long-term stability. Emerging materials like metal-organic frameworks (MOFs) [42–44], advanced membranes [45–47] and MXenes [48–50] have also been explored for their unique properties, such as high porosity, selective filtration, and excellent light absorption. Despite these advancements, they encounter issues related to complex synthesis, high production costs, energy-intensive operations, and environmental stability, particularly for MXenes, which are prone to oxidation in aqueous environments. Furthermore, the vaporization process faces the challenge of overcoming the substantial latent heat of water evaporation (
A novel conjugated polymer hydrogel solar evaporator has been developed to address these challenges. Hydrogels, networks of physically or chemically cross-linked polymers that can absorb water molecules, have tunable physicochemical properties that control the behavior of water molecules, effectively reducing the energy required for evaporation and thereby meeting the stringent demands of efficient solar water purification [52–54]. Traditional hydrogels provide a porous structure for water transport but often exhibit limited photothermal conversion properties. Therefore, by incorporating conjugated polymers with high photothermal conversion efficiency, adjustable optical properties, low cost, and scalability into the hydrogel network, the hydrogel acquires the ability to harness solar energy and promote water evaporation [55–58]. This conjugated polymer hydrogel-based solar evaporator not only achieves efficient solar energy absorption and photothermal conversion but also features excellent thermal energy localization, rapid water transport, and water activation, thereby enabling efficient, cost-effective, and scalable solar water purification under natural sunlight.
In the subsequent discussion, we will explore the concepts of solar water purification using conjugated polymer hydrogels. Additionally, strategies for improving light absorption, managing heat distribution, tuning water transport, and reducing water evaporation enthalpy are delved, focusing on the structural and surface design of conjugated polymer hydrogels to enhance evaporation efficiency. Furthermore, the practical applications of conjugated polymer hydrogel evaporators, effective pollutant removal, and high-efficiency desalination will be examined. Finally, the advantages and limitations of conjugated polymer hydrogel evaporators will be summarized, focusing on the challenges currently hindering their development, and offering insights and projections for their future trajectory.
2 Important Concepts of Solar Water Purification Using Conjugated Polymer Hydrogel
Conjugated polymers are a class of macromolecules characterized by the presence of continuous conjugated structures within their molecular backbone, typically polymerized from monomers containing multiple π-π conjugated aromatic rings or olefins. As depicted in Fig. 1, common examples of conjugated polymers include poly (3,4-ethylenedioxythiophene) (PEDOT) [56–58], polypropylene (PP) [59–61], polyaniline (PANI) [62,63], polydopamine (PDA) [45], polyacrylonitrile (PAN) [64–66], and polypyrrole (PPy) [67,68]. These conjugated polymers exhibit broad light absorption across the solar spectrum, encompassing visible and near-infrared wavelengths, thus enabling efficient solar energy utilization. When illuminated, the π-electrons within the conjugated structure absorb the energy of photons, transitioning from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO), forming excited-state π-electrons. Under excitation, these π-electrons undergo electron-phonon coupling relaxation, inducing intramolecular vibrations and thus converting the absorbed photon energy into internal molecular thermal energy [18,52]. Furthermore, the structure and properties of conjugated polymers can be tailored through synthetic methods and structural design. With their high light absorption, efficient photothermal conversion, and tunable conjugated structures, these polymers represent ideal candidates for photothermal materials.
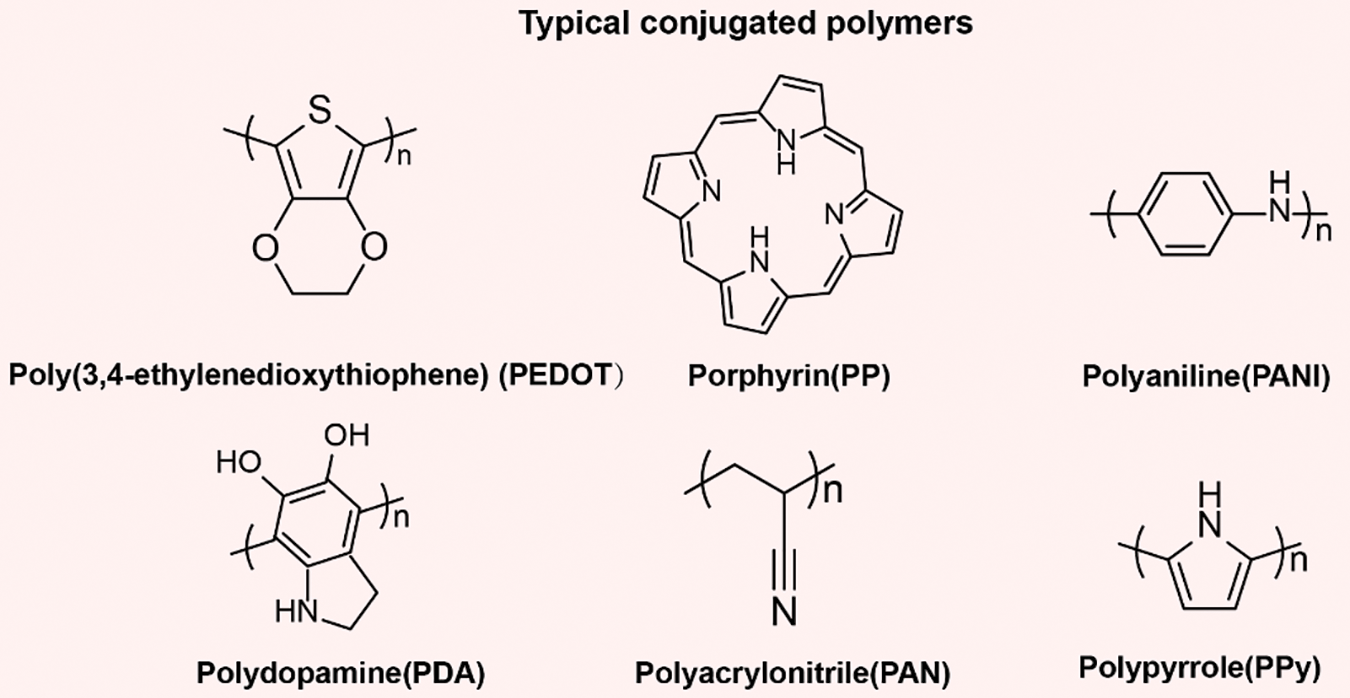
Figure 1: The chemical structures of common conjugated polymers
Hydrogel is a material with a three-dimensional network structure, characterized by its ability to absorb water highly and form a gel state in aqueous environments [52,69]. The chemical structures of common polymers capable of forming hydrogels are depicted in Fig. 2. Typically composed of water-soluble polymer chains, hydrogels consist of hydrophilic groups capable of forming hydrogen bonds or electrostatic interactions with water molecules, thereby exhibiting excellent water absorption properties. As water molecules are adsorbed into the hydrogel, the intermolecular structure of the hydrogel changes, forming a network structure wherein water molecules are encapsulated, thus resulting in gel formation. Hydrogels possess tunable pore structures that enable efficient water transport performance. In the hydrogel, water molecules have three states: free, intermediate, and bound water. Free water behaves as regular water, while bound water is formed when polar functional groups on a polymetric network capture water molecules by the stronger hydrogen bond interacting with adjacent water molecules, making those water molecules become intermediate water, which forms less hydrogen bonds with others, so less energy is needed to evaporate intermediate water [70,71]. There is a large amount of intermediate water inside the structure. Thus, the hydrogel can activate polar water molecules to alter their states and phase-transition behavior, reducing the enthalpy change of water vaporization.
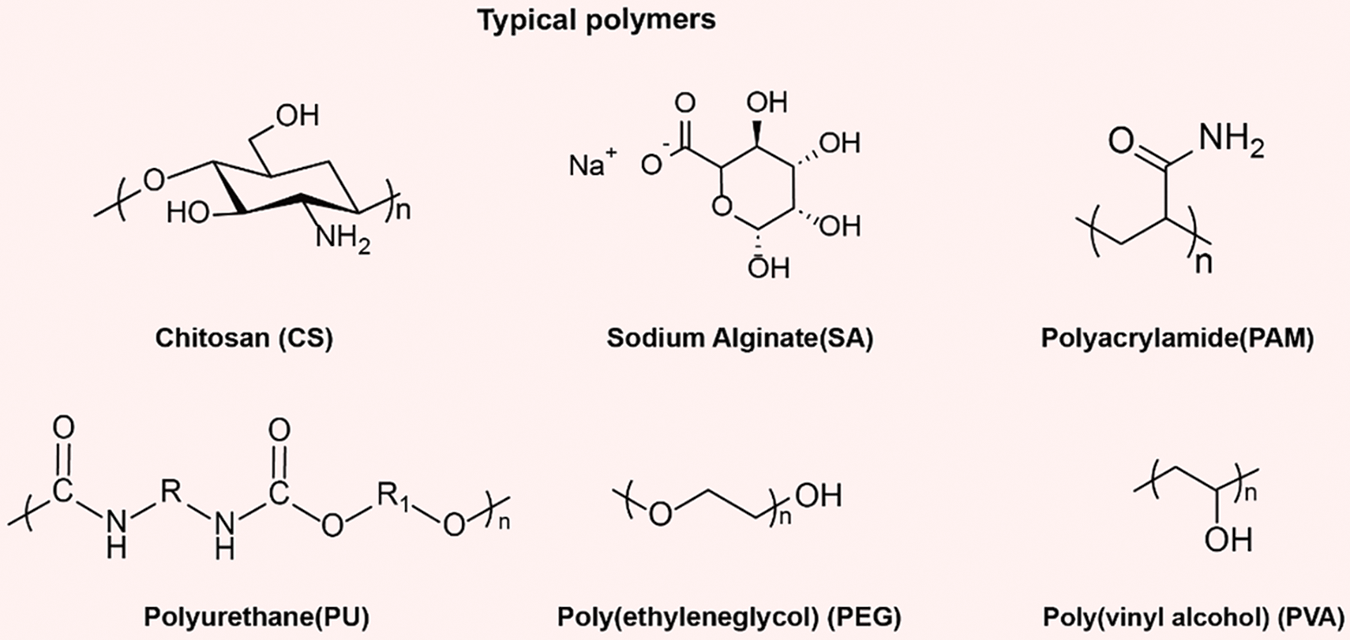
Figure 2: The chemical structures of common polymers capable of forming hydrogels
2.3 Fundamental Principles of Designing Solar Water Purification Systems
The solar-driven interfacial evaporation process occurs at the liquid-gas interface, primarily focusing on enhancing localized heating at the liquid surface. As illustrated in Fig. 3, the typical structure of this system mainly consists of a photothermal layer and a water transport substrate. The top layer, made of photothermal materials, absorbs incident sunlight and converts it into heat, thereby heating the water on the evaporation surface to generate water vapor. The bottom layer continuously supplies water to the top layer through the water transport substrate to replenish the lost water. Hydrogel substrates are particularly effective, as they can simultaneously provide rapid water transport and good thermal insulation. The enhanced photothermal efficiency of conjugated polymer hydrogels arises from several key mechanisms: (1) High light absorption: The extended π-conjugated system in conjugated polymers enables strong absorption across the solar spectrum, efficiently converting sunlight into heat. (2) Localized heating and thermal management: By positioning the photothermal layer at the air-water interface, heat is concentrated on the evaporation surface rather than dissipating into the bulk water. The hydrogel’s porous structure also serves as a thermal insulator, minimizing heat loss and enhancing solar-to-vapor conversion. (3) Rapid water transport: The hydrogel matrix contains interconnected channels that rapidly supply water to the heated surface. Its hydrophilic nature ensures continuous water replenishment, stabilizing evaporation rates and preventing overheating, which could otherwise degrade performance. (4) Reduced water evaporation enthalpy: By confining water within the polymer matrix, the hydrogel alters the interactions between water molecules, effectively lowering the enthalpy of evaporation, thereby enhancing overall efficiency. In addition to these mechanisms, material selection, compositional adjustment, and surface engineering design are strategically implemented to optimize light absorption and minimize systemic heat losses due to conduction, convection, or radiation. Furthermore, the speed of water transport within the hydrogel is a critical factor, as it directly impacts the rate of evaporation and overall system efficiency. By combining these mechanisms, conjugated polymer hydrogels achieve superior solar-to-vapor conversion rates, often approaching or even exceeding 100% under ideal conditions.
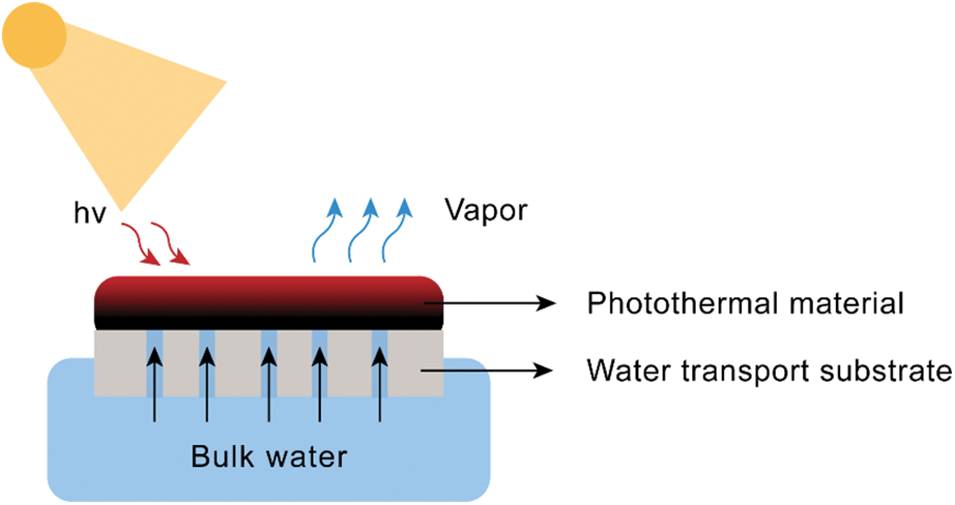
Figure 3: Schematic diagram of a solar water evaporator
3 Advanced Designs of Conjugated Polymer Hydrogels toward High Evaporation Performance
3.1 Improve the Light Absorption
When sunlight reaches the earth, its wavelength varies from 290 to 2500 nm, including ultraviolet light (290–400 nm), visible light (400–760 nm), and infrared light (760–2500 nm). Over half of the total electromagnetic radiation power is in the infrared range [18]. However, most current photothermal materials struggle to absorb infrared wavelengths. For conjugated polymers, narrowing the energy gap between the LUMO and HOMO orbitals can significantly broaden their light absorption range to cover the entire spectrum. Simultaneously, minimizing light reflection and transmission to enhance light absorption and photothermal conversion is essential for achieving optimal photothermal materials. Different conjugated polymers can absorb and emit light at different wavelengths. For instance, PANI, owing to its conjugated aromatic ring structure, has found widespread application in photothermal conversion processes. Yang et al. utilized nanocellulose and PVA hydrogel extracted from marine biomass as water transport matrices and PANI as light absorbers to construct an efficient interfacial vapor generator, achieving a light absorption rate of 94% (Fig. 4a) [72]. Similarly, Wei et al. fabricated a PANI nanofiber array evaporator using interfacial polymerization, which demonstrated excellent sunlight absorption, particularly in the UV and visible light ranges, reaching up to 95% [73]. Furthermore, adjusting the electronic structure can also control the length of the band gap and enhance the range of light that can be absorbed. By introducing electron-withdrawing groups, such as halogen groups, the LUMO energy level will fall. Vice versa, by introducing electron-donating groups such as alkoxyl groups, the HOMO energy level will rise.
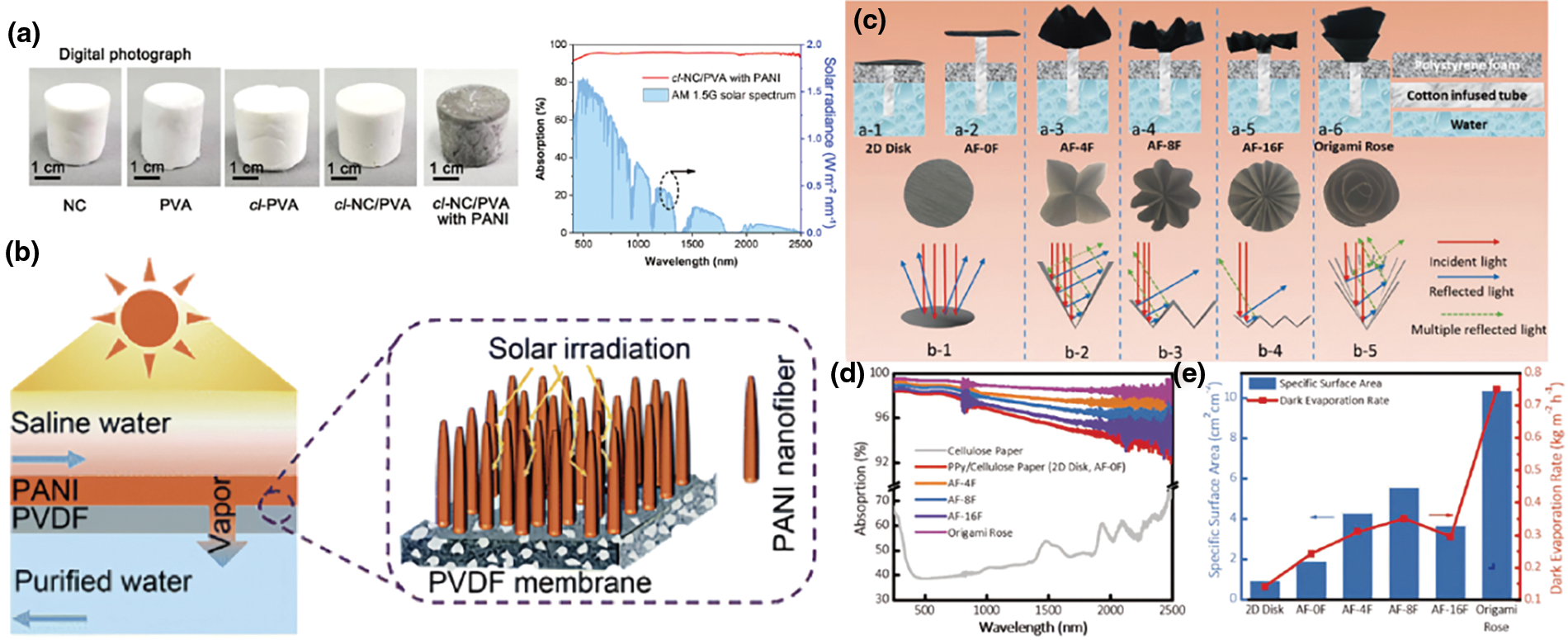
Figure 4: Improving the light absorption of conjugated polymer hydrogels. (a) Digital photographs and absorption spectrum of aerogels. Reproduced with permission [72]. Copyright 2021, Elsevier. (b) Schematic illustration of an SDMD module where light is trapped by the vertically aligned PANI nanofiber layer on the surface of PVDF-m. Reproduced with permission [74]. Copyright 2021, Royal Society of Chemistry. (c–e) Digital photos and simplified reflection scheme of parallel light beams (c), optical absorption (d), and estimated specific surface area (e) of a series of designed PPy origamis. The multiple reflections are shown inside the folded petals with different numbers of folds. Reproduced with permission [75]. Copyright 2019, Wiley
Microstructure and nanostructure of the topography of the surface are important basic strategies to enhance light absorption. The reflection of light at the surface can limit the emissivity of the conjugated polymers. To fully utilize the light, it is important to increase the length of the optical path to produce internal scattering. For instance, in vertically arranged nanotubes, when the wavelength of incident light matches the structure, the refractive properties change, and internal reflection is caused. As the light reflects times inside the material, it is absorbed more completely. Peng et al. developed a vertically aligned PANI nanofiber layer on the surface of a hydrophobic polyvinylidene fluoride (PVDF) microfiltration membrane. This layer features numerous nanoscale conical protuberances, resulting in a gradient refractive index from air to PVDF. As a result, it effectively reduces light reflection and optimizes light utilization. This evaporator with multiple structures can absorb more than 95.0% of light which is in the range of 250~800 nm (Fig. 4b) [74]. The other kind of structure of the surface of the light absorber is an origami-based solar evaporator to traps solar energy via its periodic concavity patterned surface. This structure with wrinkles and ridges on the surface is tiny and achieves microns. It enables the light to be absorbed at a wider angle and broadband, thus reflection is reduced. For example, Li et al. proposed a PPy origami-based photothermal material. Compared with the planar 2D absorber, the 3D origami absorber can increase the light absorption rate from 48.9% to 99% (Fig. 4c–e) [75].
Managing heat distribution is critical in enhancing the efficiency of interfacial evaporation. In theory, all the heat produced by the evaporator can be used in evaporation. However, during the actual operation of the evaporator, unavoidable heat losses occur through various pathways. Firstly, there are radiation heat losses from the evaporating surface itself. Secondly, heat convection losses result from temperature disparities near the liquid surface. Additionally, heat losses occur through thermal conduction between the evaporating interface and the bulk water, between different evaporator components, and between these components and the surrounding air during the evaporation process. Therefore, maximizing the utilization of thermal energy and minimizing heat losses to the greatest extent possible are crucial principles for enhancing the efficiency of solar-driven evaporation.
The radiative energy loss can be reduced by using low radiative material or decreasing the temperature of the evaporator. For example, photothermal materials with low thermal conductivity, such as polyacrylonitrile-conjugated polymer, can also be an optimal choice. Gao et al. have designed a porous hydrophilic carbon black/polyacrylonitrile (CB/PAN) composite nanofiber evaporator, which exhibits thermal conductivity coefficients of 0.075 W m−1 K−1 under wet conditions and 0.046 W m−1 K−1 under dry conditions, significantly reducing downward heat conduction losses (Fig. 5a) [76]. Moreover, the design of solar evaporator structures can also mitigate heat losses. For instance, double-layer structures, cylindrical three-dimensional configurations, and one-dimensional water channel structures are examples of such designs. In a double-layer structure, the whole evaporator is divided into two parts—the photothermal conversion material as the upper layer and the insulated foam as the lower layer. Because of the insulator, heat can gather together at the top layer where vaporization occurs. So far, foam, wood, hydrogels, and so on have been widely used as insulators. Xu et al. have developed a stable bilayered wood-poly(3,4-ethylenedioxythiophene): polystyrene sulfonate (PEDOT:PSS) hydrogel evaporator using a simple method of drop-casting followed by thermal annealing. This evaporator not only achieves efficient broadband light absorption but also exhibits excellent evaporation performance, thanks to the inherent thermal insulation properties of wood used as a water transport substrate (Fig. 5b) [77]. Additionally, the design of three-dimensional (3D) structure evaporators, such as simple cylindrical macroscopic structures, is frequently employed in managing heat design. Due to the elevated position of the top of the extending structure relative to the evaporative water surface, the temperature at the bottom of the structure is lower than the temperature of the surrounding environment. As a result, this structure is not only able to utilize the energy from sunlight but is also able to absorb the energy from the surroundings to improve its performance. Similarly, a 3D origami-based solar evaporator not only enhances light absorption but also optimizes thermal management by improving surface area, thereby increasing the water evaporation rate. Zhen et al. used a sheet of PPy-compounded cellulose paper to make a portable solar evaporator based on the tower-like structure. It absorbs light by facilitating multiple reflections within its framework, resulting in an evaporation rate of 2.67 kg m−2 h−1 under one sun illumination (Fig. 5c) [78]. 1D water pathway is another structure commonly used to reduce heat loss and fully utilize heat energy. It only has one narrow path to pump water upwards, so the heat loss from radiation, conduction, and convection can be suppressed.
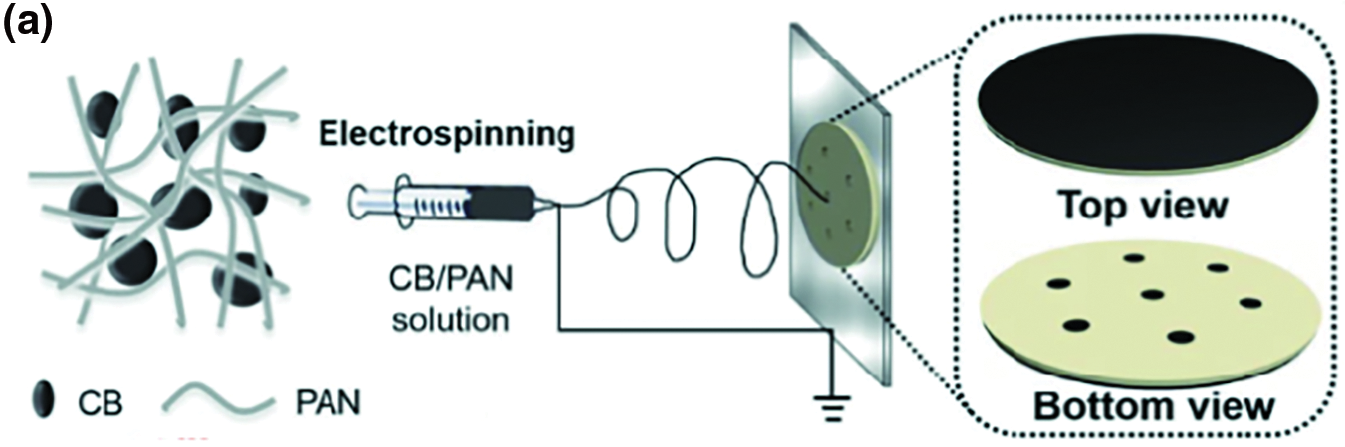
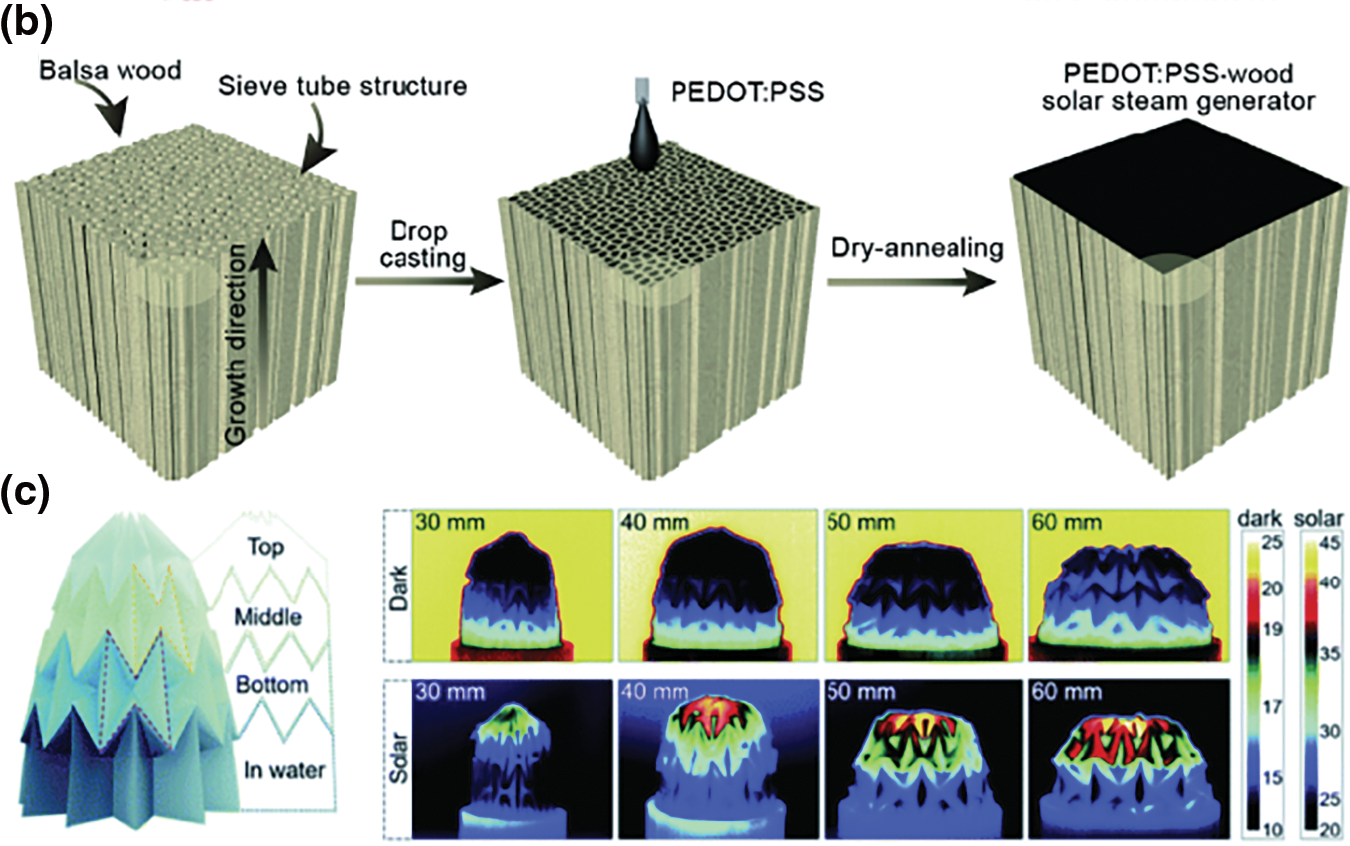
Figure 5: Effective thermal management of conjugated polymer hydrogels. (a) Schematic illustration of the construction of the bilayer device using an electrospinning method. Reproduced with permission [76]. Copyright 2019, Wiley. (b) Schematic illustration for the preparation of the bilayered wood-PEDOT:PSS hydrogel evaporator. Reproduced with permission [77]. Copyright 2023, Multidisciplinary Digital Publishing Institute. (c) Schematic illustrating that the origami tower is divided into top, middle, bottom, and in-water parts. Infrared thermal images of the origami tower with different diameters in the dark environment (the first row) and under solar irradiation (the second row) after one hour of illumination. Reproduced with permission [78]. Copyright 2022, Royal Society of Chemistry
In the solar-driven interfacial evaporation process, water needs to be replenished rapidly and promptly to the evaporation surface to achieve high evaporation rates. Water transport capacity is contingent on the wettability at the top and bottom of the evaporator, as well as the structures and sizes of the water pathway. Firstly, the wettability at the surface of the evaporator is influenced by the hydrophilicity of materials, pores, and topography of materials. Hydrophilic groups (e.g., −OH, −COOH, −NH2) are one of the key factors in controlling wettability, making the evaporator hydrophilic and achieving a better capillary effect, thereby enhancing water transport. The presence of these groups on the conjugated polymer hydrogel surface makes it highly hydrophilic, facilitating water absorption and rapid transport to the evaporation interface. Moreover, porous materials such as polyacrylonitrile, foam, and wood, are commonly used as supports to create effective water pathways in solar evaporators. The size and distribution of these pores can be controlled by adjusting the concentration of gel precursors, cross-linking density, and post-freeze-drying conditions (e.g., temperature or solvents). For instance, Shi et al. [79] obtained a novel porphyrin/aniline-based conjugated microporous polymers (PACMPs) covering sponges and using polyurethane (PU) sponges as skeletons via a simple dip-coating technique. Under 1 sun irradiation, the evaporator can achieve an evaporation rate of 1.31 kg m−2 h−1 in seawater. For conjugated polymer hydrogels, controlling the hierarchical 3D water transport channels is crucial. Internal gaps transport large water volumes, micron channels aid diffusion, and the molecular network limits evaporation. Techniques like directional freezing can regulate this network, shortening the water transport path and improving efficiency. Optimizing its surface morphology is crucial for achieving rapid water transport because it directly enhances capillary action, wettability, surface area, and flow maintenance. A surface with micro- and nano-sized pores facilitates capillary pathways, drawing water efficiently to the evaporation zone. Improved surface roughness and porosity increase hydrophilicity, promoting better water absorption and uniform distribution across the interface. Additionally, a larger surface area allows more water to be transported simultaneously, boosting evaporation rates. Properly designed pore structures also prevent clogging, ensuring continuous water flow, which is essential for maintaining high evaporation efficiency. For example, Zhao et al. [36] developed a solar evaporator based on polyvinyl alcohol (PVA) and PPy through an efficient in-situ energy utilization strategy. This hydrogel solar evaporator (HNG) features hierarchical nano water transport channels composed of internal voids, micron-scale channels, and molecular grids, which accelerate water uptake and facilitate efficient photothermal interfacial evaporation, achieving an evaporation rate of 3.2 kg m−2 h−1 (Fig. 6a). Li et al. [62] fabricated Ti3C2Tx MXene/graphene oxide (GO)/PANI (MGP) hybrids using directional freeze casting, which endowed the hybrids with oriented, rapid water transport channels. They demonstrated that due to the Marangoni effect, the water tension field on the concave pyramid structure exhibits a gradient distribution, leading to a higher local water flow rate compared to a flat evaporator surface. This effect further enhances the water evaporation rate (Fig. 6b–f).
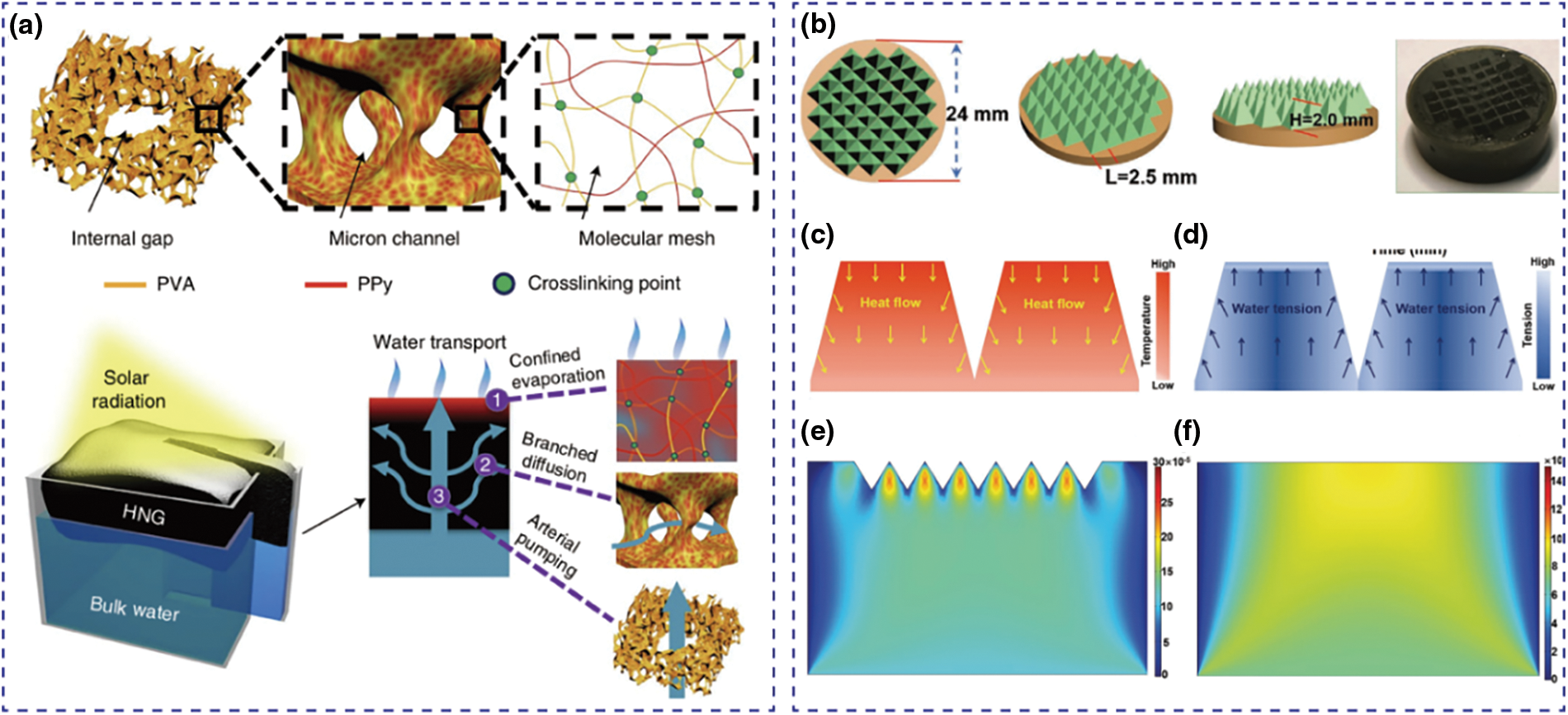
Figure 6: Tunable water transport in conjugated polymer hydrogels. (a) Schematic of highly efficient solar vapour generation based on tailored water transport in HNGs. Reproduced with permission [36]. Copyright 2018, Nature. (b–f) Schematic of concave pyramid patterns models (b). Schematic illustrations of heat flow (c) and water tension (d) (water flow) promoted by the Marangoni effect in the concave pyramid structure. Steady-state simulated velocities of water flow (m s−1) in the MGP evaporators with concave pyramid patterns (e) and flat surface (f) Reproduced with permission [62]. Copyright 2022, Wiley
Meanwhile, different water pathways have different dimensionality (1D, 2D, or 3D) to optimize the water supply and balance thermal insulation. 3D water paths with abundant and open pores are the most common interconnected porous structures that achieve a better performance of rapid water transport by improving the capillary effect. However, because many pores directly connect with the bulk water, heat can be lost through conduction and convection, potentially weakening the heat localization effect. To minimize thermal dissipation, a 2D water pathway is engineered to strategically separate the insulating layer from the water-bearing channels. Materials with capillary-wicking ability, such as polystyrene foam, are wrapped around a thermal insulator to transport water to the top evaporation surface. This arrangement reduces heat loss through conductive transfer, maintaining efficient photothermal conversion. For further reduction of heat loss, 1D pathways restrict water transport to a single direction, thereby enhancing thermal insulation. This design focuses on balancing the rate of water transportation with water content at the evaporating surface. The choice of water pathway (1D, 2D, or 3D) depends on the intrinsic structure and surface wettability of the conjugated polymer hydrogel. The primary goal is to strike a balance between water transport rate, water content at the evaporation surface, and thermal insulation.
3.4 Reduce Water Evaporation Enthalpy
Water vaporization enthalpy refers to the energy change when converting water from liquid to gas at the same temperature and pressure. In liquid water, water molecules are mainly bonded by strong hydrogen bonds, making evaporation energy-intensive. When using photothermal material to carry out interfacial evaporation, the high enthalpy of this phase change reduces the efficiency of the evaporator significantly. Therefore, it is important to use new materials and enhance designs to improve the efficiency of steam production. Among all the materials, hydrogel is widely used in solar water vaporization due to its significant capacity to reduce the required enthalpy. In hydrogels with hydrophilic functional groups, there are free water, intermediate water, and bound water. Bound water forms strong hydrogen bonds with the hydrogel’s hydrophilic groups, hindering evaporation due to the high energy required to break these bonds. Intermediate water, on the other hand, interacts with fewer hydrogen bonds because of the polymer chains inside the hydrogel, requiring less energy to escape. By increasing the amount of intermediate water through altering polymer concentration, cross-linking density, functional additives, and the porous structure of the polymer network, the evaporation enthalpy can be reduced, enhancing energy efficiency. For instance, attaching hydrophilic functional groups, such as hydroxyl, amidic, amino, sulfonic acid, and carboxylic acid into the polymer backbone inside the hydrogels can produce more intermediate water because they can bond water molecules through noncovalent interaction. Zhou et al. developed a highly hydratable water-absorbing hydrogel (h-LAH) composed of chitosan as a hydratable framework and polypyrrole as a light absorber. They demonstrated that enhancing the hydration capacity of h-LAH can alter the state of water and partially activate it, thereby promoting water evaporation. The h-LAH achieved a record solar steam generation rate of approximately 3.6 kg m−2 h−1 under one sun illumination (Fig. 7a) [80]. Interpenetrating hydrogel network (IPNG) formed with PSS and PVA can decrease the energy needed for water vaporization. PSS has a stronger electrostatic attraction to water molecules, it enhances polymer-water interaction and increases the number of bound water. Nevertheless, the incorporation of PSS also leads to the accumulation of free water within the system, determining that the ideal ratio between PSS and PVA stands at 1.5:1. The enthalpy change of water evaporation will be reduced by more than 50%, and the evaporation rate was boosted to 3.9 kg m−2 h−1 under one sun irradiation (Fig. 7b) [81].
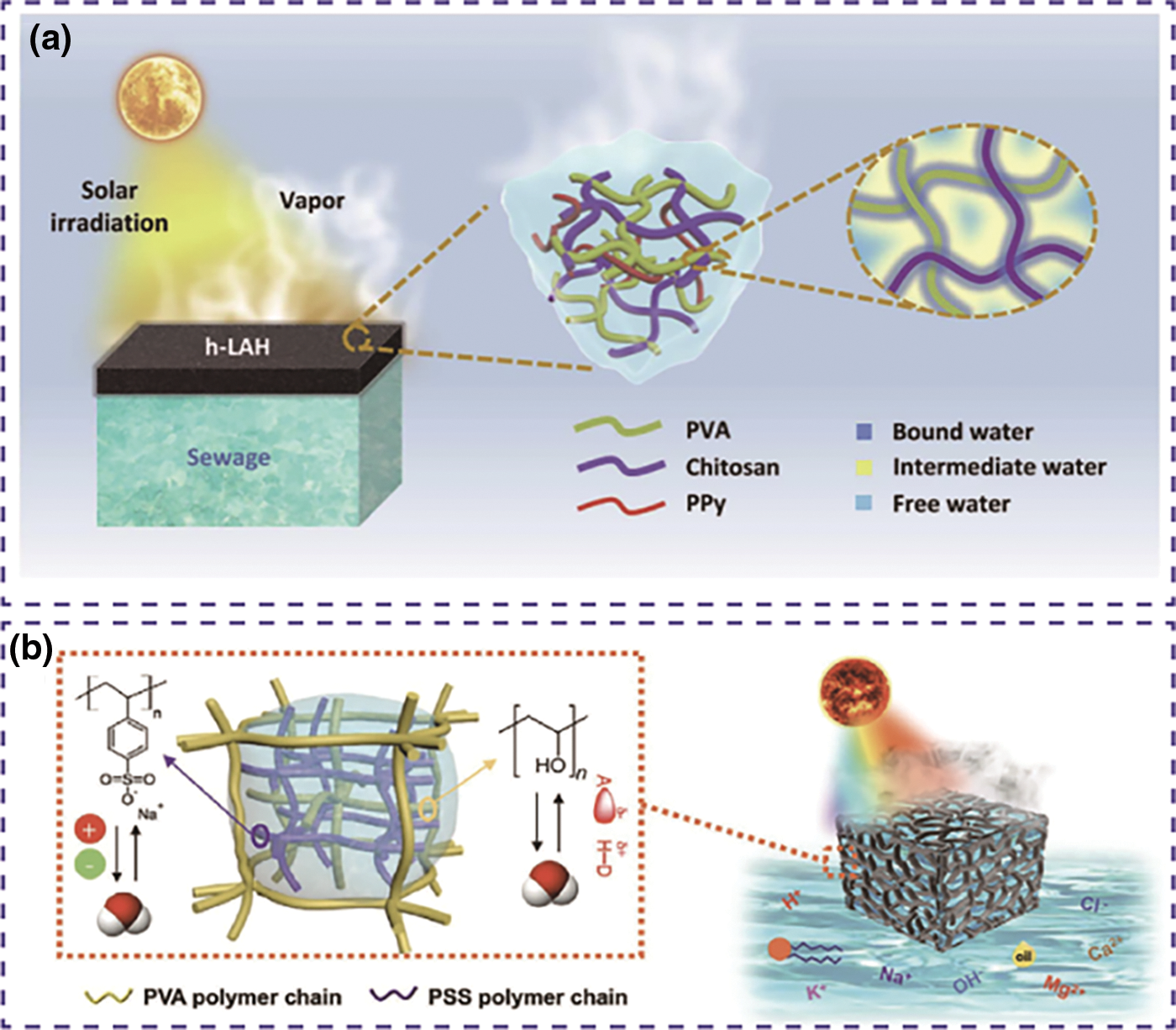
Figure 7: Reducing the evaporation enthalpy of conjugated polymer hydrogels. (a) Schematic illustration of SVG based on the h-LAH. The containing water has three different water types—bound water, IW, and FW. Wherein, the IW can be effectively evaporated with significantly reduced energy demand. Reproduced with permission [80]. Copyright 2019, Science. (b) Schematic of IPNG-based solar water purification with controlled hydration. Reproduced with permission [81]. Copyright 2020, Wiley
4 Practical Application of Water Purification
Desalination is a process of obtaining fresh water from saline water sources, which can be brackish water, seawater, or high-salt effluent. Saline water in Oceans, seas, and saltwater lakes occupy a large portion of the total water resource on the earth, about 97.5% [82]. Practical application of water purification for meeting the escalating water demand in daily life and industrial processes. The utilization of conjugated polymer hydrogel evaporators, known for their exceptional efficacy in treating saltwater, holds significant value in practice. Enhancing salt resistance is the key to improving the performance of saline water desalination. Conjugated polymer hydrogels with tunable light absorption and water transport capabilities have been proven to exhibit excellent desalination performance in interfacial evaporation technologies [83–86]. For instance, Zhao et al. [56] developed a mechanically robust nanocomposite hydrogel with outstanding evaporation performance by simply mixing and crosslinking PEDOT:PSS nanofibers with a viscous PVA solution. They further demonstrated the effectiveness of large-scale solar desalination in simulated seawater environments, including global average seawater, the Bohai Sea, and the Dead Sea. The salinity of the purified water was significantly reduced by more than three orders of magnitude to approximately 0.02 wt‰, which is well below the drinking water standards set by the World Health Organization (1 wt‰) [87] and the U.S. Environmental Protection Agency (0.5 wt‰) [88]. Additionally, the concentrations of major metal ions were also significantly reduced by about 2 to 3 orders of magnitude after purification, indicating the hydrogel’s excellent desalination capabilities. The PEDOT:PSS hydrogel (PPH) with the interconnected and microporous structure is employed by Cai et al. [57] in solar evaporation. As SO3− in the PPHs has electrostatic repulsion, Na+ in the water is separated from Cl−, and thus no salt forms on the evaporating surface. According to the 88-h continuous experiment, The PPH2 evaporates 35 wt‰ NaCl solution under continuous 1 kW m−2 irradiation at the rate of ∼2.32 kg m−2 h−1 without forming crystals on the surface, and the water purified only has a low concentration of salt ions (below 1 mg L−1).
Water, in addition to being found in natural resources, is also generated in significant quantities as polluted effluent by industrial and agricultural sectors daily. Removing organic and inorganic contaminants inside is necessary to purify those waters and utilize them again. The removal of microbes, including bacteria, protozoa, viruses, fungi, algae, and helminths, that are usually found in polluted water is essential for disease prevention. Filtering heavy metals, characterized by a density exceeding 5 g cm−3, is crucial to safeguard both human health and plant well-being, mitigating risks of malnutrition and malformations.
Luckily, most conjugated polymer hydrogels can remove these hazards directly and produce water that achieves WHO’s standards when they purify water without extra process. Conjugated polymer hydrogel can use their negative charge on the surface to attract metal cations, and then use their porous structure to capture the cations successfully. For organic compounds, they can be removed when hydrogen bonds capture nitrogen atoms from tertiary amine groups and oxygen atoms of sulfonic groups. Simultaneously, by employing molecular engineering methods to introduce specific functional groups into conjugated polymer hydrogels, effective resistance against bacterial contamination can be achieved. For instance, the introduction of sulfonic acid groups inhibits bacterial adhesion, while phenolic hydroxyl groups effectively suppress bacterial growth. All these properties of conjugated polymer hydrogel above have been proven to be effective in practice. In their study, Zhao et al. [89] utilized PEDOT:PSS and polyacrylamide to fabricate a dual-network hydrogel evaporator. This evaporator can remove both organic pollutants and metal cations such as Cu²+, Zn²+, Pb²+, and Ni²+. By comparing concentrations of metal cations in water before and after purification, this evaporator proved that it can lower the concentration of all the cations for a range of 2~4 orders of magnitude down and finally to below 1 mg L-1. Dyes such as rhodamine-B (RB) and methylene blue (MB) are also added into the polluted water at a concentration of 20 mg L-1 to examine the ability of the evaporator to remove organic compounds. Analysis through observation and UV-vis spectroscopy indicated complete removal of organic pollutants and dyes (Fig. 8a,b). Inspired by nature, Xu et al. [90] developed a solar-absorbing gel (SAG) composed of an elastic thermoresponsive poly(N-isopropylacrylamide) (PNIPAm) hydrogel, a photothermal polydopamine (PDA) layer, and a sodium alginate (SA) network. The SAG is capable of not only desalinating seawater using sunlight alone but also purifying water from various contaminated sources containing small molecules, oil, and pathogens (Fig. 8c).
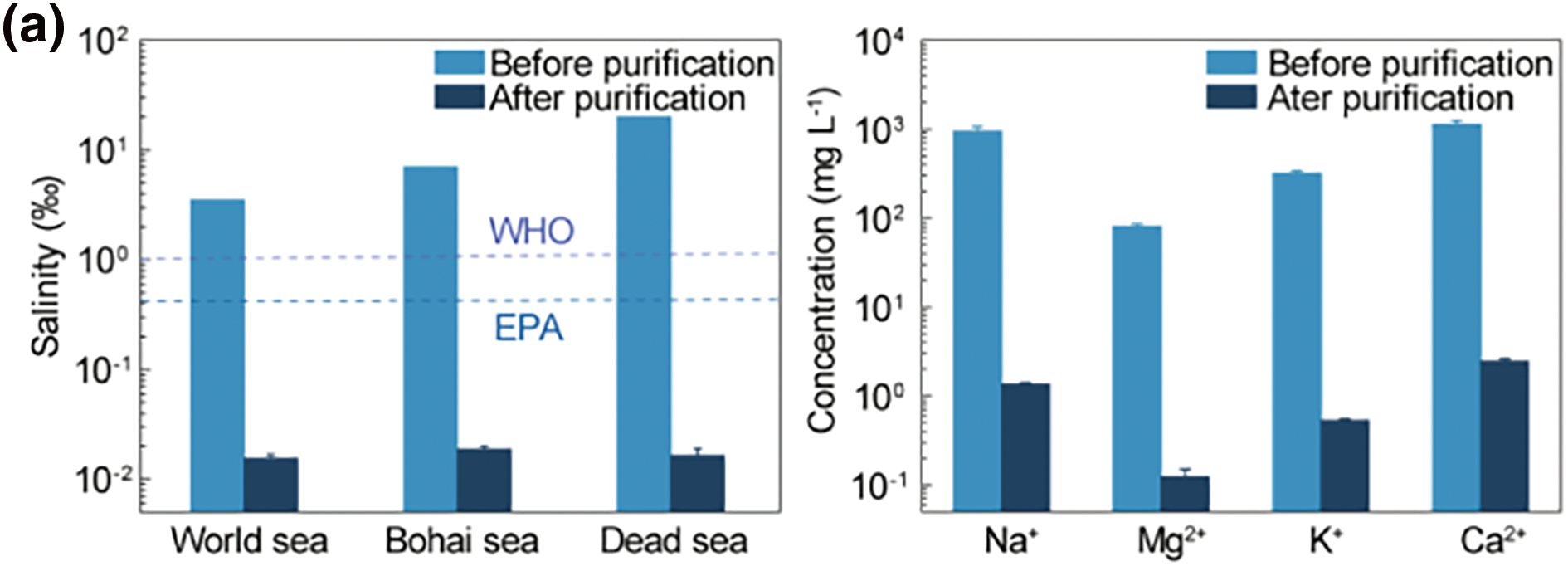
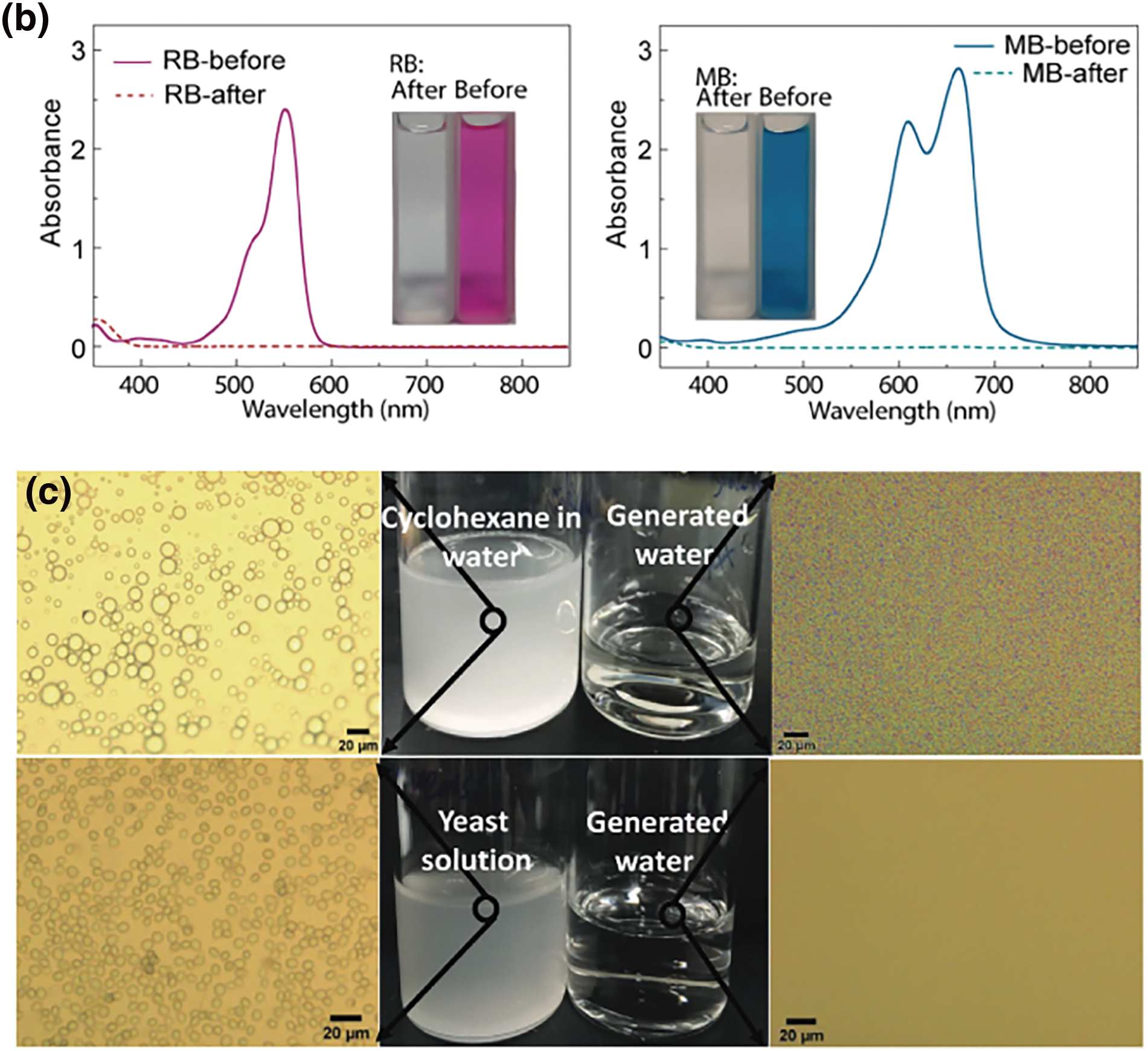
Figure 8: Conjugated polymer hydrogels for seawater desalination and sewage treatment. (a) Salinity measurement of simulated seawater samples and primary ion concentration in different seawater samples before and after desalination. Reproduced with permission [57]. Copyright 2023, American Chemical Society. (b) UV-vis absorption spectra of the sewage sample added with RhB and MB (20 mg L-1) before/after purification. Reproduced with permission [89]. Copyright 2022, Elsevier. (c) Digital and microscopy photographs of SDS-stabilized cyclohexane-in-water emulsion and yeast solution before and after treatment with SAG. Reproduced with permission [90]. Copyright 2021, Wiley
A conjugated polymer hydrogel evaporator uses conjugated polymers as photothermal material to convert solar power into heat. It applies hydrogels to pump water into an evaporating surface where heat vaporizes water, and then gaseous water is condensed and collected as purified water. The evaporator utilizes renewable solar power to purify water without the need for extra significant optical devices. These characteristics make it potentially more cost-effective to manufacture on a large scale, highly portable, and environmentally friendly. In addition, as the whole system is highly tunable, the evaporator can achieve a high photothermal conversion efficiency, lower enthalpy of vaporization, and minimize heat loss. Highly efficient water purification can be obtained if the evaporator can be further enhanced by unique designs of photothermal materials, topography, water pathways, and insulated structures; synthesis techniques, knowledge of polymer-water interaction, and gelation chemistry are also applied to achieve this. In practice, conjugated polymer hydrogel evaporator has a perfect performance of removing heavy metal cations, microbes, dyes, and other organic pollutants due to their polar polymer chains, porous structures, and well-designed water pathways. It can sustain high-efficiency water purification without being worn out or blocked by heavy metal cations or other salts. Thus, it is durable and costs little to maintain after installation.
Although significant progress has been made in the development of conjugated hydrogel evaporators, there still exists a certain gap between their practical application and the current state of research, necessitating further exploration in the following aspects:
(1) Theoretical investigations: (a) Understanding evaporation mechanisms: A deeper exploration of the mechanisms involved in the evaporation process of conjugated polymer hydrogels is essential. This includes investigating how polymer chains interact with water molecules to maximize the formation of intermediate water molecules, which require less energy to evaporate. Elucidating the molecular diffusion kinetics during evaporation can enhance control over the process. Furthermore, enhancing solar-to-thermal energy conversion by precisely controlling water molecules will contribute to improving overall efficiency. Furthermore, understanding degradation mechanisms under various environmental conditions will contribute to improving overall efficiency and longevity. (b) Optimizing performance: A comprehensive theoretical understanding is crucial for optimizing various performance aspects, such as evaporation efficiency, energy utilization, and material longevity.
(2) Material innovation: (a) Developing novel materials: New conjugated polymer hydrogel photothermal materials need to be developed to enhance their solar absorption efficiency, photothermal conversion efficiency, and stability. Materials should be resistant to photobleaching, chemical degradation, and mechanical wear over extended periods. (b) Structural modulation and functionalization for environmental robustness: Achieving enhanced durability can be facilitated through structural modulation of conjugated polymers, incorporation of functional nanomaterials, surface modifications, and appropriate interface engineering. Incorporating antifouling and antibacterial properties can minimize fouling and maintain efficiency under various environmental conditions.
(3) Functional expansion: (a) Multifunctional integration with enhanced durability: Integration of conjugated hydrogel materials with other functional materials is necessary to achieve multifunctional integration. For instance, incorporating photocatalytic nanomaterials into conjugated hydrogels can enable simultaneous solar evaporation and wastewater purification, or combining materials with anti-fouling and antibacterial properties to enhance the lifespan and stability of solar evaporators under different environmental conditions. (b) Addressing volatile pollutants: Tackling issues such as the removal of volatile pollutants from conjugated polymer hydrogel evaporators is essential. Developing strategies to effectively address this challenge will broaden the applicability of the technology. (c) Balancing functionalities: Careful consideration of the interplay between different functionalities is required when developing multifunctional applications to ensure sustained performance over time without compromising efficiency.
(4) Application deepening: (a) Scaling up production: To realize practical applications, developing low-cost raw materials is crucial to facilitate large-scale production. Adopting more efficient and universal manufacturing technologies such as 3D printing [91–94] can be used to scale up production, which is particularly important for making these purification systems accessible in remote, off-grid locations. (b) Sustainability and environmental impact: Selecting renewable, degradable, or environmentally friendly materials will help reduce energy consumption and minimize environmental pollution during production. This aligns with global sustainability goals and enhances the technology’s appeal, particularly for applications in rural or disaster-stricken regions where eco-friendly solutions are highly desirable. (c) System optimization and scalability: Building more scalable system devices is essential to improve the efficiency of condensate water production. This includes optimizing device design for better heat management and water transport mechanisms. (d) Overcoming practical challenges: Addressing challenges such as material degradation over time, environmental exposure effects, and user accessibility will be vital for successful real-world implementation.
Acknowledgement: None.
Funding Statement: This research was funded by the National Natural Science Foundation of China, grant numbers 52373184 & 52473179; the Key Research and Development Program of Jiangxi Province, grant number 20223BBE51023; the Natural Science Foundation of Jiangxi Province, grant numbers 20232ACB204002 & 20232BAB202044; the Jiangxi Provincial Key Laboratory of Flexible Electronics, grant numbers 20212BCD42004 & 20242BCC32010.
Author Contributions: Hanyi Zou: Conceptualization; Formal analysis; Investigation; Writing—manuscript draft; Editing. Xinye Xu: Conceptualization; Writing—review & editing; Supervision; Visualization. Mutian Yao: Investigation; Data curation; Validation; Writing—manuscript draft. Baoyang Lu: Conceptualization; Funding acquisition; Writing—review & editing; Supervision; Visualization. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Mishra BK, Kumar P, Saraswat C, Chakraborty S, Gautam A. Water security in a changing environment: concept, challenges and solutions. Water. 2021;13(4):490. doi:10.3390/w13040490. [Google Scholar] [CrossRef]
2. Salehi M. Global water shortage and potable water safety; today’s concern and tomorrow’s crisis. Environ Int. 2022;158:106936. doi:10.1016/j.envint.2021.106936. [Google Scholar] [PubMed] [CrossRef]
3. Mekonnen MM, Hoekstra AY. Sustainability: four billion people facing severe water scarcity. Sci Adv. 2016;2(2):e1500323. doi:10.1126/sciadv.1500323. [Google Scholar] [PubMed] [CrossRef]
4. Kabeel AE. Review of researches and developments on solar stills. Desalination. 2011;276:1–12. doi:10.1016/j.desal.2011.03.042. [Google Scholar] [CrossRef]
5. Yao Y, Zhang P, Jiang C, DuChanois RM, Zhang X, Elimelech M. High performance polyester reverse osmosis desalination membrane with chlorine resistance. Nat Sustain. 2021;4:138–46. doi:10.1038/s41893-020-00619-w. [Google Scholar] [CrossRef]
6. Kim J, Park K, Yang DR, Hong S. A comprehensive review of energy consumption of seawater reverse osmosis desalination plants. Appl Energy. 2019;254:113652. doi:10.1016/j.apenergy.2019.113652 [Google Scholar] [CrossRef]
7. Gupta N, Kalla S, Murthy Z. Effect of tetramethylurea (TMU) on polysulfone membrane performance for atrazine-containingwastewater treatment. J Polym Mater. 2023;40(3–4):317–28. doi:10.32381/JPM.2023.40.3-4.12. [Google Scholar] [CrossRef]
8. Dashputre A, Kaushik A, Pal A, Jariwala D, Yadav K, Shah M. Geothermal energy integrated multi-effect evaporator (MEE) and multi-effect distillation (MED)-based desalination systems: an ecofriendly and sustainable solutions. Environ Sci Pollut R. 2023;30(26):67941–52. doi:10.1007/s11356-023-26858-w. [Google Scholar] [PubMed] [CrossRef]
9. Prajapati M, Shah M, Soni B. A comprehensive review of the geothermal integrated multi-effect distillation (MED) desalination and its advancements. Groundw Sustain Dev. 2022;19(2):100808. doi:10.1016/j.gsd.2022.100808. [Google Scholar] [CrossRef]
10. Guo X, Wang J. A novel monolayer adsorption kinetic model based on adsorbates infect adsorbents inspired by epidemiological model. Water Res. 2024;253:121313. doi:10.1016/j.watres.2024.121313. [Google Scholar] [PubMed] [CrossRef]
11. Nguyen MN, Jue ML, Buchsbaum SF, Park SJ, Vollnhals F, Christiansen S, et al. Interplay of the forces governing steroid hormone micropollutant adsorption in vertically-aligned carbon nanotube membrane nanopores. Nat Commun. 2024;15(1):1114. doi:10.1038/s41467-024-44883-2. [Google Scholar] [PubMed] [CrossRef]
12. Zhao S, Zhang X, Zhu D, Zhang K, Wei G, Su Z. Regulating ordered structure and multi-functions of zeolite aerogels for solar steam generation and heavy metal ion adsorption. Sep Purif Technol. 2023;324(1):124588. doi:10.1016/j.seppur.2023.124588. [Google Scholar] [CrossRef]
13. Powers LC, Conway A, Mitchelmore CL, Fleischacker SJ, Harir M, Westerman DC, et al. Tracking the formation of new brominated disinfection by-products during the seawater desalination process. Environ Sci. 2020;6(9):2521–41. doi:10.1039/D0EW00426J. [Google Scholar] [CrossRef]
14. Lei X, Xie Z, Sun Y, Qiu J, Yang X. Recent progress in identification of water disinfection byproducts and opportunities for future research. Environ Pollu. 2023;337:122601. doi:10.1016/j.envpol.2023.122601. [Google Scholar] [PubMed] [CrossRef]
15. Yu M, Yang C, Chen M, Li Y, Kang K, Wang C, et al. Multi-chamber membrane capacitive deionization coupled with peroxymonosulfate to achieve simultaneous removal of tetracycline and peroxymonosulfate reaction byproducts. J Hazard Mater. 2024;476(1):135036. doi:10.1016/j.jhazmat.2024.135036. [Google Scholar] [PubMed] [CrossRef]
16. Yi M, Xia Q, Tan J, Shang J, Cheng X. Catalytic-separation technology for highly efficient removal of emerging pollutants, desalination, and antimicrobials: a new strategy for complex wastewater treatment. Chem Eng J. 2024;493:152568. doi:10.1016/j.cej.2024.152568. [Google Scholar] [CrossRef]
17. Kim NH, Kim B, Kim YS, Mule AR, Chung CH. A hybrid electrochemical system for spontaneous green hydrogen production with simultaneous desalination using catechol oxidation. Desalination. 2024;580:117541. doi:10.1016/j.desal.2024.117541. [Google Scholar] [CrossRef]
18. Zhao F, Guo Y, Zhou X, Shi W, Yu G. Materials for solar-powered water evaporation. Nat Rev Mater. 2020;5:388–401. doi:10.1038/s41578-020-0182-4. [Google Scholar] [CrossRef]
19. Ghasemi H, Ni G, Marconnet AM, Loomis J, Yerci S, Miljkovic N, et al. Solar steam generation by heat localization. Nat Commun. 2014;5:4449. doi:10.1038/ncomms5449. [Google Scholar] [PubMed] [CrossRef]
20. Tao P, Ni G, Song C, Shang W, Wu J, Zhu J, et al. Solar-driven interfacial evaporation. Nat Energy. 2018;3:1031–41. doi:10.1038/s41560-018-0260-7. [Google Scholar] [CrossRef]
21. Hogan NJ, Urban A, Orozco A, Pimpinelli A, Nordlander P, Halas NJ. Nanoparticles heat through light localization. Nano Lett. 2014;14(8):4640–5. doi:10.1021/nl5016975. [Google Scholar] [PubMed] [CrossRef]
22. Nawaz F, Yang Y, Zhao Q, Mo Y, Jiang Z, Wu J, et al. Can the interfacial solar vapor generation performance be really “beyond” theoretical limit? Adv Energy Mater. 2024;14:2400135. doi:10.1002/aenm.v14.22. [Google Scholar] [CrossRef]
23. Wu X, Lu Y, Ren X, Wu P, Chu D, Yang X, et al. Interfacial solar evaporation: from fundamental research to applications. Adv Mater. 2024;36:2313090. doi:10.1002/adma.v36.23. [Google Scholar] [CrossRef]
24. Kumar A, Choudhary P, Kumar A, Camargo PHC, Krishnan V. Recent advances in plasmonic photocatalysis based on TiO2 and noble metal nanoparticles for energy conversion, environmental remediation, and organic synthesis. Small. 2022;18(1):2101638. doi:10.1002/smll.v18.1. [Google Scholar] [CrossRef]
25. Chen S, Sun Z, Xiang W, Shen C, Wang Z, Jia X, et al. Plasmonic wooden flower for highly efficient solar vapor generation. Nano Energy. 2020;76:104998. doi:10.1016/j.nanoen.2020.104998. [Google Scholar] [CrossRef]
26. Zhu M, Li Y, Chen F, Zhu X, Dai J, Li Y, et al. Plasmonic wood for high-efficiency solar steam generation. Adv Energy Mater. 2018;8(4):1701028. doi:10.1002/aenm.v8.4. [Google Scholar] [CrossRef]
27. Zhou L, Tan Y, Wang J, Xu W, Yuan Y, Cai W, et al. 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat Photonics. 2016;10(6):393–8. doi:10.1038/nphoton.2016.75. [Google Scholar] [CrossRef]
28. Liu P, Bo HY, Li XY, Xu L, Chen C, Yuan B, et al. Enhanced solar evaporation using a scalable MoS2-based hydrogel for highly efficient solar desalination. Angew Chem Int Ed. 2022;134(37):e202208587. doi:10.1002/ange.202208587. [Google Scholar] [CrossRef]
29. Ghim D, Jiang Q, Cao SS, Singamaneni S, Jun YS. Mechanically interlocked 1T/2H phases of MoS2 nanosheets for solar thermal water purification. Nano Energy. 2018;53:949–57. doi:10.1016/j.nanoen.2018.09.038. [Google Scholar] [CrossRef]
30. Pan J, Yu X, Dong J, Zhao L, Liu L, Liu J, et al. Diatom-inspired TiO2-PANi-decorated bilayer photothermal foam for solar-driven clean water generation. ACS Appl Mater Interfaces. 2021;13(48):58124–33. doi:10.1021/acsami.1c16603. [Google Scholar] [PubMed] [CrossRef]
31. Xu X, Zhao Q, Liu Q, Qiu J, Li J, Zheng W, et al. Full-spectrum-responsive Ti4O7-PVA nanocomposite hydrogel with ultrahigh evaporation rate for efficient solar steam generation. Desalination. 2024;577:117400. doi:10.1016/j.desal.2024.117400. [Google Scholar] [CrossRef]
32. Luo S, Liu Z, Yin X, Lin Z, Zhang S, Chen J, et al. A sandwich structure Ag/MgFe2O4-deposited surface carbonized wood for integrated solar steam generation and photoreduction of Cr(VI). Small. 2024;20:2309087. doi:10.1002/smll.v20.26. [Google Scholar] [CrossRef]
33. Guan W, Guo Y, Yu G. Carbon materials for solar water evaporation and desalination. Small. 2021;17(48):2007176. doi:10.1002/smll.v17.48. [Google Scholar] [CrossRef]
34. Li W, Chen Z, Yu H, Li J, Liu S. Wood-derived carbon materials and light-emitting materials. Adv Mater. 2021;33(28):2000596. doi:10.1002/adma.v33.28. [Google Scholar] [CrossRef]
35. Qiu J, Xu X, Li Z, Hu Y, Liu G, Lv X, et al. A solar-electric dual-driven microporous hydrogel evaporator for all-weather highly efficient water purification. Nano Energy. 2024;130:110057. doi:10.1016/j.nanoen.2024.110057. [Google Scholar] [CrossRef]
36. Zhao F, Zhou X, Shi Y, Qian X, Alexander M, Zhao X, et al. Highly efficient solar vapour generation via hierarchically nanostructured gels. Nat Nanotechnol. 2018;13(6):489–95. doi:10.1038/s41565-018-0097-z. [Google Scholar] [PubMed] [CrossRef]
37. Gao M, Peh CK, Phan HT, Zhu L, Ho GW. Solar absorber gel: localized macro-nano heat channeling for efficient plasmonic Au nanoflowers photothermic vaporization and triboelectric generation. Adv Energy Mater. 2018;8(25):1800711. doi:10.1002/aenm.v8.25. [Google Scholar] [CrossRef]
38. Li Z, Qiu J, Xu X, Wan R, Yao M, Wang H, et al. Solar-driven kaolin-based hydrogels for efficient interfacial evaporation and heavy metal ion adsorption from wastewater. Sep Purif Technol. 2025;354:129243. doi:10.1016/j.seppur.2024.129243. [Google Scholar] [CrossRef]
39. Sun X, Zhang F, Zhang L, Liu G, Wang Y, Wang Y, et al. Enhanced electromechanical conversion via in situ grown CsPbBr3 nanoparticle/poly(vinylidene fluoride) fiber composites for physiological signal monitoring. Soft Sci. 2022;2:1. doi:10.20517/ss.2021.21. [Google Scholar] [CrossRef]
40. Zhang X, Li X, Wang X, Yuan L, Ye J, Wang X, et al. Direct fabrication of high-performance multi-response e-skin based on a graphene nanosheet film. Soft Sci. 2022;2:18. doi:10.20517/ss. [Google Scholar] [CrossRef]
41. Gu X, Fan C, Sun Y. Multilevel design strategies of high-performance interfacial solar vapor generation: a state of the art review. Chem Eng J. 2023;460:141716. doi:10.1016/j.cej.2023.141716. [Google Scholar] [CrossRef]
42. Han X, Besteiro LV, Koh CSL, Lee HK, Phang IY, Phan-Quang GC, et al. Intensifying heat using MOF-isolated graphene for solar-driven seawater desalination at 98% solar-to-thermal efficiency. Adv Funct Mater. 2021;31:2008904. doi:10.1002/adfm.202008904. [Google Scholar] [CrossRef]
43. Li Z, Ma X, Chen D, Wan X, Wang X, Fang Z, et al. Polyaniline-coated MOFs nanorod arrays for efficient evaporation-driven electricity generation and solar steam desalination. Adv Sci. 2021;8(7):2004552. doi:10.1002/advs.v8.7. [Google Scholar] [CrossRef]
44. Ma C, Wang W, Chen Q, Jia Z, Zhang X, Shi J, et al. Flexible hierarchical polypyrrole-coated Cu-BTC MOFs photothermal textile for efficiently solar water evaporation and wastewater purification. Chem Eng J. 2024;480:148248. doi:10.1016/j.cej.2023.148248. [Google Scholar] [CrossRef]
45. Nayak K, Kumar A, Das P, Tripathi BP. Amphiphilic antifouling membranes by polydopamine mediated molecular grafting for water purification and oil/water separation. J Memb Sci. 2021;630:119306. doi:10.1016/j.memsci.2021.119306. [Google Scholar] [CrossRef]
46. Shi Y, Ilic O, Atwater HA, Greer JR. All-day fresh water harvesting by microstructured hydrogel membranes. Nat Commun. 2021;12(1):2797. doi:10.1038/s41467-021-23174-0. [Google Scholar] [PubMed] [CrossRef]
47. Li Y, Gao S, Cui J, Qu Q, Wang Y, Huang C, et al. Self-healing and superwettable nano fibrous membranes with excellent stability toward multifunctional applications in water purification. ACS Appl Mater Interfaces. 2020;12(20):23644–54. doi:10.1021/acsami.0c05701. [Google Scholar] [PubMed] [CrossRef]
48. Lei Z, Sun X, Zhu S, Dong K, Liu X, Wang L, et al. Nature inspired MXene-decorated 3D honeycomb-fabric architectures toward efficient water desalination and salt harvesting. Nanomicro Lett. 2022;14(1):10. [Google Scholar]
49. Wang J, Shen M, Liu Z, Wang W. MXene materials for advanced thermal management and thermal energy utilization. Nano Energy. 2022;97:107177. doi:10.1016/j.nanoen.2022.107177. [Google Scholar] [CrossRef]
50. Li R, Zhang L, Shi L, Wang P. MXene Ti3C2: an effective 2D light-to-heat conversion material. ACS Nano. 2017;11:3752–9. doi:10.1021/acsnano.6b08415. [Google Scholar] [PubMed] [CrossRef]
51. Xu N, Li J, Finnerty C, Song Y, Zhou L, Zhu B, et al. Going beyond efficiency for solar evaporation. Nat Water. 2023;1(6):494–501. doi:10.1038/s44221-023-00086-5. [Google Scholar] [CrossRef]
52. Guo Y, Bae J, Fang Z, Li P, Zhao F, Yu G. Hydrogels and hydrogel-derived materials for energy and water sustainability. Chem Rev. 2020;120(15):7642–707. doi:10.1021/acs.chemrev.0c00345. [Google Scholar] [PubMed] [CrossRef]
53. Zhou X, Zhao F, Guo Y, Zhang Y, Yu G. A hydrogel-based antifouling solar evaporator for highly efficient water desalination. Energy Environ Sci. 2018;11(8):1985–92. doi:10.1039/C8EE00567B. [Google Scholar] [CrossRef]
54. Lu H, Shi W, Zhao F, Zhang W, Zhang P, Zhao C, et al. High-yield and low-cost solar water purification via hydrogel-based membrane distillation. Adv Funct Mater. 2021;31(19):2101036. doi:10.1002/adfm.202101036. [Google Scholar] [CrossRef]
55. Yu MQ, Liao YZ, Zhu MF. Progress in preparation and applications of conjugated polymer hydrogels. Acta Polym Sin. 2021;52(20):113–23. [Google Scholar]
56. Zhao Q, Liu J, Wu Z, Xu X, Ma H, Hou J, et al. Robust PEDOT: PSS-based hydrogel for highly efficient interfacial solar water purification. Chem Eng J. 2022;442:136284. doi:10.1016/j.cej.2022.136284. [Google Scholar] [CrossRef]
57. Cai W, Zhao S, Zhang K, Guo K, Wang Y, Chen Q, et al. Synergy of light trapping and water management in interconnected porous PEDOT: PSS hydrogels for efficient solar-driven water purification. Ind Eng Chem Res. 2023;62(26):10175–83. doi:10.1021/acs.iecr.3c01170. [Google Scholar] [CrossRef]
58. Lu B, Yuk H, Lin S, Jian N, Qu K, Xu J, et al. Pure PEDOT: PSS hydrogels. Nat Commun. 2019;10(1):1043. doi:10.1038/s41467-019-09003-5. [Google Scholar] [PubMed] [CrossRef]
59. Zhang B, Gu Q, Wang C, Gao Q, Guo J, Wong PW, et al. Self-assembled hydrophobic/hydrophilic porphyrin-Ti3C2T xMXene janus membrane for dual-functional enabled photothermal desalination. ACS Appl Mater Interfaces. 2021;13(3):3762–70. doi:10.1021/acsami.0c16054. [Google Scholar] [PubMed] [CrossRef]
60. Zhao K, Zhao X, Lu Q, Jiang Y, Pan J. Porphyrin-based COF nanosheet arrays with donor-acceptor structure for solar-driven water purification. Desalination. 2024;588:117956. doi:10.1016/j.desal.2024.117956. [Google Scholar] [CrossRef]
61. Zhou Y, Lu Q, Liu Q, Yang H, Liu J, Zhuang J, et al. Architecting hybrid donor-acceptor dendritic nanosheets based on polyoxometalate and porphyrin for high-yield solar water purification. Adv Funct Mater. 2022;32(15):2112159. doi:10.1002/adfm.202112159. [Google Scholar] [CrossRef]
62. Li XP, Li X, Li H, Zhao Y, Wu J, Yan S, et al. Reshapable MXene/graphene oxide/polyaniline plastic hybrids with patternable surfaces for highly efficient solar-driven water purification. Adv Funct Mater. 2022;32(15):2110636. doi:10.1002/adfm.202110636. [Google Scholar] [CrossRef]
63. Elessawy NA, Gouda MH, Elnouby M, Ali SM, Salerno M, Youssef ME. Sustainable microbial and heavy metal reduction in water purification systems based on PVA/IC nanofiber membrane doped with PANI/GO. Polymers. 2022;14(8):1558. doi:10.3390/polym14081558. [Google Scholar] [PubMed] [CrossRef]
64. Lv B, Li S, Wang W, Xu Y, Zhao B, Song C, et al. A lotus leaf-inspired janus dual-functional nanofiber evaporator for efficient water purification. J Clean Prod. 2024;438:140880. doi:10.1016/j.jclepro.2024.140880. [Google Scholar] [CrossRef]
65. Liu Z, Zhou Z, Wu N, Zhang R, Zhu B, Jin H, et al. Hierarchical photothermal fabrics with low evaporation enthalpy as heliotropic evaporators for efficient, continuous, salt-free desalination. ACS Nano. 2021;15(8):13007–18. doi:10.1021/acsnano.1c01900. [Google Scholar] [PubMed] [CrossRef]
66. Xiong J, Li A, Liu Y, Wang L, Qin X, Yu J. Scalable and hierarchically designed MOF fabrics by netting MOFs into nanofiber networks for high-performance solar-driven water purification. J Mater Chem A. 2021;9(37):21005–12. doi:10.1039/D1TA05589E. [Google Scholar] [CrossRef]
67. Hanif Z, Tariq MZ, Khan ZA, La M, Choi D, Park SJ. Polypyrrole-coated nanocellulose for solar steam generation: a multi-surface photothermal ink with antibacterial and antifouling properties. Carbohydr Polym. 2022;292:119701. doi:10.1016/j.carbpol.2022.119701. [Google Scholar] [PubMed] [CrossRef]
68. Xie Z, Zhu J, Zhang L. Three-dimensionally structured polypyrrole-coated setaria viridis spike composites for efficient solar steam generation. ACS Appl Mater Interfaces. 2021;13:9027–35. doi:10.1021/acsami.0c22917. [Google Scholar] [PubMed] [CrossRef]
69. Zhou X, Guo Y, Zhao F, Yu G. Hydrogels as an emerging material platform for solar water purification. Acc Chem Res. 2019;52:3244–53. doi:10.1021/acs.accounts.9b00455. [Google Scholar] [PubMed] [CrossRef]
70. Chen L, Ding Y, Gong J, Xie H, Qu J, Niu R. Molecular engineering of biomass-derived hybrid hydrogels for solar water purification. J Colloid Interface Sci. 2022;626:231–40. doi:10.1016/j.jcis.2022.06.145. [Google Scholar] [PubMed] [CrossRef]
71. Zhao Q, Yang Y, Zhu B, Sha Z, Zhu H, Wu Z, et al. Low vaporization enthalpy hydrogels for highly efficient solar-driven interfacial evaporation. Desalination. 2023;568:116999. doi:10.1016/j.desal.2023.116999. [Google Scholar] [CrossRef]
72. Yang L, Li N, Guo C, He J, Wang S, Qiao L, et al. Marine biomass-derived composite aerogels for efficient and durable solar-driven interfacial evaporation and desalination. Chem Eng J. 2021;417(2):128051. doi:10.1016/j.cej.2020.128051. [Google Scholar] [CrossRef]
73. Wei X, Peng Y, Fang W, Hu Z, Li W, Zhang S, et al. A polyaniline nanofiber array supported ultrathin polyamide membrane for solar-driven volatile organic compound removal. J Mater Chem A. 2022;10(38):20424–30. doi:10.1039/D2TA04909K. [Google Scholar] [CrossRef]
74. Peng Y, Wang Y, Li W, Jin J. Bio-inspired vertically aligned polyaniline nanofiber layers enabling extremely high-efficiency solar membrane distillation for water purification. J Mater Chem A. 2021;9(17):10678–84. doi:10.1039/D1TA01336J. [Google Scholar] [CrossRef]
75. Li W, Li Z, Bertelsmann K, Fan DE. Portable low-pressure solar steaming-collection unisystem with polypyrrole origamis. Adv Mater. 2019;31(29):1900720. doi:10.1002/adma.201900720. [Google Scholar] [PubMed] [CrossRef]
76. Gao T, Li Y, Chen C, Yang Z, Kuang Y, Jia C, et al. Architecting a floatable, durable, and scalable steam generator: hydrophobic/hydrophilic bifunctional structure for solar evaporation enhancement. Small Methods. 2019;3(2):1800176. doi:10.1002/smtd.201800176. [Google Scholar] [CrossRef]
77. Xu X, Zhao Q, Liu Q, Qiu J, Yuan S, Wu Z, et al. A bilayered wood-poly(3,4-ethylenedioxythiophenepolystyrene sulfonate hydrogel interfacial evaporator for sustainable solar-driven sewage purification and desalination. Nanomaterials. 2023;13(16):2321. doi:10.3390/nano13162321. [Google Scholar] [PubMed] [CrossRef]
78. Liu X, Tian Y, Chen F, Mu Y, Caratenuto A, Minus ML, et al. A waterbomb origami tower for convertible photothermal evaporation. J Mater Chem A. 2022;109360(36):18657–70. doi:10.1039/D2TA04365C. [Google Scholar] [CrossRef]
79. Shi Y, Meng N, Wang Y, Cheng Z, Zhang W, Liao Y. Scalable fabrication of conjugated microporous polymer sponges for efficient solar steam generation. ACS Appl Mater Interfaces. 2022;14(3):4522–31. doi:10.1021/acsami.1c21693. [Google Scholar] [PubMed] [CrossRef]
80. Zhou X, Zhao F, Guo Y, Rosenberger B, Yu G. Architecting highly hydratable polymer networks to tune the water state for solar water purification. Sci Adv. 2019;5(6):eaaw5484. doi:10.1126/sciadv.aaw5484. [Google Scholar] [PubMed] [CrossRef]
81. Zhou X, Guo Y, Zhao F, Shi W, Yu G. Topology-controlled hydration of polymer network in hydrogels for solar-driven wastewater treatment. Adv Mater. 2020;32(52):2007012. doi:10.1002/adma.202007012. [Google Scholar] [PubMed] [CrossRef]
82. Guo Y, Yu G. Engineering hydrogels for efficient solar desalination and water purification. Acc Mater Res. 2021;2(5):374–84. doi:10.1021/accountsmr.1c00057. [Google Scholar] [CrossRef]
83. Lei AC, Guan W, Guo Y, Shi W, Johnston KP, Yu G. Polyzwitterionic hydrogels for highly efficient high salinity solar desalination. Angew Chem Int Ed. 2022;134(36):e202208487. doi:10.1002/ange.202208487. [Google Scholar] [CrossRef]
84. Yao W, Li X, Zhu X, Pei L, Liu G, Cheng Y, et al. Thermal-localized and salt-resistant polyacrylonitrile/polyvinylidene fluoride aerogel for efficient solar desalination. Desalination. 2022;532:115751. doi:10.1016/j.desal.2022.115751. [Google Scholar] [CrossRef]
85. Xiao P, Gu J, Zhang C, Ni F, Liang Y, He J, et al. A scalable, low-cost and robust photo-thermal fabric with tunable and programmable 2D/3D structures towards environmentally adaptable liquid/solid-medium water extraction. Nano Energy. 2019;65:104002. doi:10.1016/j.nanoen.2019.104002. [Google Scholar] [CrossRef]
86. Liu C, Peng Y, Cai C, Zhang J, Zhao X. Enhancing solar desalination performance based on restricted salt ions transport. J Environ Chem Eng. 2021;9(4):105272. doi:10.1016/j.jece.2021.105272. [Google Scholar] [CrossRef]
87. Organization WH. Safe drinking-water from desalination. Geneva: World Health Organization; 2011. WHO/HSE/WSH/11.03. [Google Scholar]
88. Smith AH, Lopipero PA, Bates MN, Steinmaus CM. Arsenic epidemiology and drinking water standards. In: Proceedings-Annual Public Water Supply Engineers’ Conference, 1979; USA; vol. 296, p. 2145–6. [Google Scholar]
89. Zhao Q, Wu Z, Xu X, Yang R, Ma H, Xu Q. Design of poly(3,4-ethylenedioxythiophenepolystyrene sulfonate-polyacrylamide dual network hydrogel for long-term stable, highly efficient solar steam generation. Sep Purif Technol. 2022;300:121889. doi:10.1016/j.seppur.2022.121889. [Google Scholar] [CrossRef]
90. Xu X, Ozden S, Bizmark N, Arnold CB, Datta SS, Priestley RD. A bioinspired elastic hydrogel for solar-driven water purification. Adv Mater. 2021;33(18):2007833. doi:10.1002/adma.202007833. [Google Scholar] [PubMed] [CrossRef]
91. Dai L, Wang L, Chen B, Xu Z, Wang Z, Xiao R. Shape memory behaviors of 3D printed liquid crystal elastomers. Soft Sci. 2023;3:5. doi:10.20517/ss.2022.28. [Google Scholar] [CrossRef]
92. Luo X, Wan R, Zhang Z, Song M, Yan L, Xu J, et al. 3D-printed hydrogel-based flexible electrochromic device for wearable displays. Adv Sci. 2024;11(38):2404679. doi:10.1002/advs.202404679. [Google Scholar] [PubMed] [CrossRef]
93. Yu J, Tian F, Wang W, Wan R, Cao J, Chen C, et al. Design of highly conductive, intrinsically stretchable, and 3D printable PEDOT: PSS hydrogels via PSS-chain engineering for bioelectronics. Chem Mater. 2023;35:5936–44. doi:10.1021/acs.chemmater.3c00844. [Google Scholar] [CrossRef]
94. Li J, Cao J, Lu B, Gu G. 3D-printed PEDOT: PSS for soft robotics. Nat Rev Mater. 2023;8:604–22. doi:10.1038/s41578-023-00587-5. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools