 Open Access
Open Access
REVIEW
Synthesis of Biomass Polyurethane and Its Properties
1 School of Light Industry Science and Engineering, Tianjin University of Science and Technology, Tianjin, 300222, China
2 Department of Chemistry, Tsinghua University, Beijing, 100084, China
* Corresponding Author: Gui-Chang Jiang. Email:
(This article belongs to the Special Issue: Development of Polyurethane and Composites)
Journal of Polymer Materials 2025, 42(2), 359-377. https://doi.org/10.32604/jpm.2025.063342
Received 12 January 2025; Accepted 13 March 2025; Issue published 14 July 2025
Abstract
PU, or polyurethane, features a repeating urethane group (-NH-COO-) in its molecular structure. Traditionally, PUs are synthesized from isocyanate and polyol compounds derived from fossil resources through polymerization reactions. The depletion of fossil fuels and the increasing climate problems call for the expansion of more renewable sources of chemicals, such as modern biomass. However, the conversion of biomass into chemicals is challenging due to the inherent molecular complexity of its composition. In recent years, advances in green chemistry have led researchers to focus on developing bio-based polyurethanes by sourcing polyols, isocyanates, and chain extender precursors from biological materials. This paper focuses on the preparation of polyols, non-isocyanates and bio-based chain extenders from bio-based materials such as vegetable oils, lignin, sugars, and rosin. The synthetic routes and properties of several bio-based polyurethane materials are analyzed. Additionally, it discusses the current status, future challenges, and potential applications of bio-based polyurethane materials across various fields.Keywords
Polyurethane is one of the most versatile specialty polymers, used in applications such as fibers, coatings, adhesives, insulation, construction, furniture, and medical devices. It ranks as the sixth most widely used polymer, comprising about 8% of the global polymer market [1,2]. Traditionally, polyurethanes are synthesized from diisocyanates or polyisocyanates and diols or polyols, featuring a repeating carbamate group (-NHCOO-). The properties of polyurethanes—whether rigid foams, flexible foams, adhesives, coatings, fibers, elastomers, or thermoplastics—vary depending on the specific isocyanate and polyol used. However, as fossil fuel reserves are projected to deplete within the next 40–50 years, there is a growing emphasis on developing renewable energy sources as sustainable alternatives [3].
Bio-based polyurethane encompasses variants synthesized from renewable biomass resources, with research primarily concentrating on developing bio-based polyols, isocyanates, and chain extenders. Recent advancements in green chemistry have allowed researchers to extract precursors for polyols and isocyanates from biological sources [4]. Bio-based polyols can be classified into several categories, such as vegetable oil-based, wood fiber-based, rosin-based, natural phenolic, and sugar-based polyols, with research in this area being well-developed and showing great application potential. In contrast, traditional isocyanate raw materials are toxic and water-sensitive, and often use hazardous substances like phosgene in their industrial production, posing risks to environmental and human health [5]. This concern has fueled interest in non-isocyanate polyurethanes (NIPUs), which are created through the reaction of cyclic carbonate esters and polyamines. Compared to conventional polyurethanes, the synthesis of NIPU is less sensitive to moisture and avoids the use of highly toxic isocyanates, making it a safer and more sustainable option. This innovation paves the way for new developments in bio-based polyurethanes. However, NIPUs also have some drawbacks, such as limited physical properties. The mechanical properties and temperature resistance of NIPUs are typically inferior to those of conventional isocyanate-based polyurethanes. This can lead to limitations in their suitability for demanding applications. Also, the production processes and raw materials for non-isocyanates may result in higher costs than some conventional polyurethanes. Therefore, future development of NIPIs will require improvements in performance and production processes.
Bio-based polyurethanes have a wide range of applications in many fields due to their greening, sustainability, and many other advantages. By modifying bio-based polyurethanes, it is possible to endow them with specific properties that enable them to be used in a wide range of applications, such as flame retardant materials, food packaging, self-repairing materials, sensors, medical materials, etc. [6–10].
The preparation of traditional polyols primarily depends on petroleum resources, which are non-renewable and limited. Concurrently, increasing environmental concerns have made the exploration of sustainable, renewable bio-based polyols to replace petroleum-based options an irreversible trend (Table 1). Bio-based polyols offer similar functionality to their petroleum-based counterparts, but they are less toxic, more abundant, and more cost-effective, making them favorable for large-scale industrial production [11]. The sustainable production of polyols and polyurethanes from biomass is gaining significant attention due to their potential as renewable resources. Fig. 1 illustrates the production of biomass-based polyols, including vegetable oil-based, lignin-based, microalgae-based, tannins-based, and carbohydrate-based polyols [12]. Recently Ugis Cabulis [13] has discovered these bio-based polyol feedstock can be divided into four generations. The first is mainly edible oils, such as palm oil, which can only be obtained as materials with a low density. The second generation consists mainly of inedible oil seed crops, wood biomass, and food industry waste. The third generation of feedstocks is algae. The fourth generation of feedstocks are genetically modified oilseed plants or algae. To produce bio-based polyols and polyurethanes from biomass, the biomass can be chemically modified or bioconverted, depending on the type of biomass and the desired properties of the resulting polyols and polyurethanes.

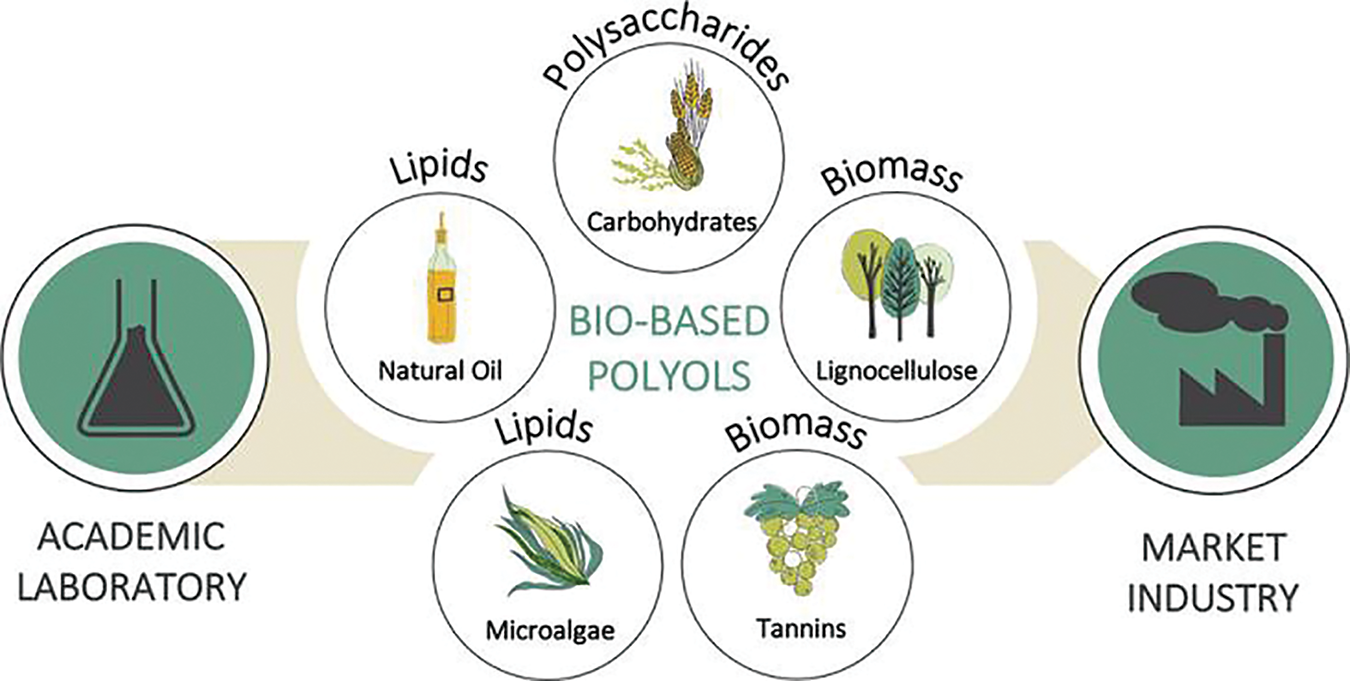
Figure 1: Five bio-based polyols
Vegetable oils from different plants (soya, palm, rapeseed, etc.) are inherently biodegradable and inexpensive due to their abundant renewable resources [14]. Vegetable oils are chemically composed of triglycerides and three different compositions of long-chain fatty acids, the reactivity of which depends on the carbon-carbon double bond (C=C) and the ester active site.
In addition, some vegetable oils can be used directly as reactive natural hydroxy polyols. The most commonly used is castor oil. The structural formula of castor oil is shown in Fig. 2. For example, Zhong et al. [15] prepared a new type of composite film with quaternary ammonium groups grafted onto castor oil-based aqueous polyurethane by directly using castor oil as a polyol. The composite film showed good inhibition of E. coli and S. aureus due to the antimicrobial ability of the quaternary ammonium compounds. The shelf life of strawberries packed in the composite film was extended to more than 6 days. At present, the commercialised castor oil polyols include Polycins from Ventrus (New York, USA), Lupranol Balance 50 from BASF (Frankfurt am Main, Germany) and Ulfcar Polem A from Nivapol (Nørre Aaby, Denmark), which can effectively achieve the purpose of energy saving and emission reduction while improving the bio-based content of polyurethane [16].
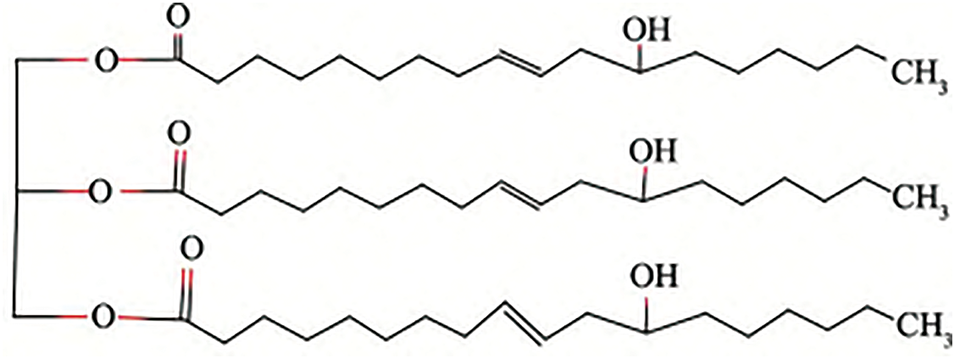
Figure 2: Castor oil structural formula
However, most oils do not have hydroxyl groups, which need to be introduced into the molecular structure by modification of reactive groups such as double bonds and ester groups [17]. Thus, the unsaturated structure of vegetable oils can be converted into hydroxyl-containing molecules by epoxidation, hydrogenation, ester exchange or ozonolysis (Fig. 3). Therefore, the abundance of unsaturated structures makes vegetable oils promising candidates for chemical modification into desired intermediates [18].
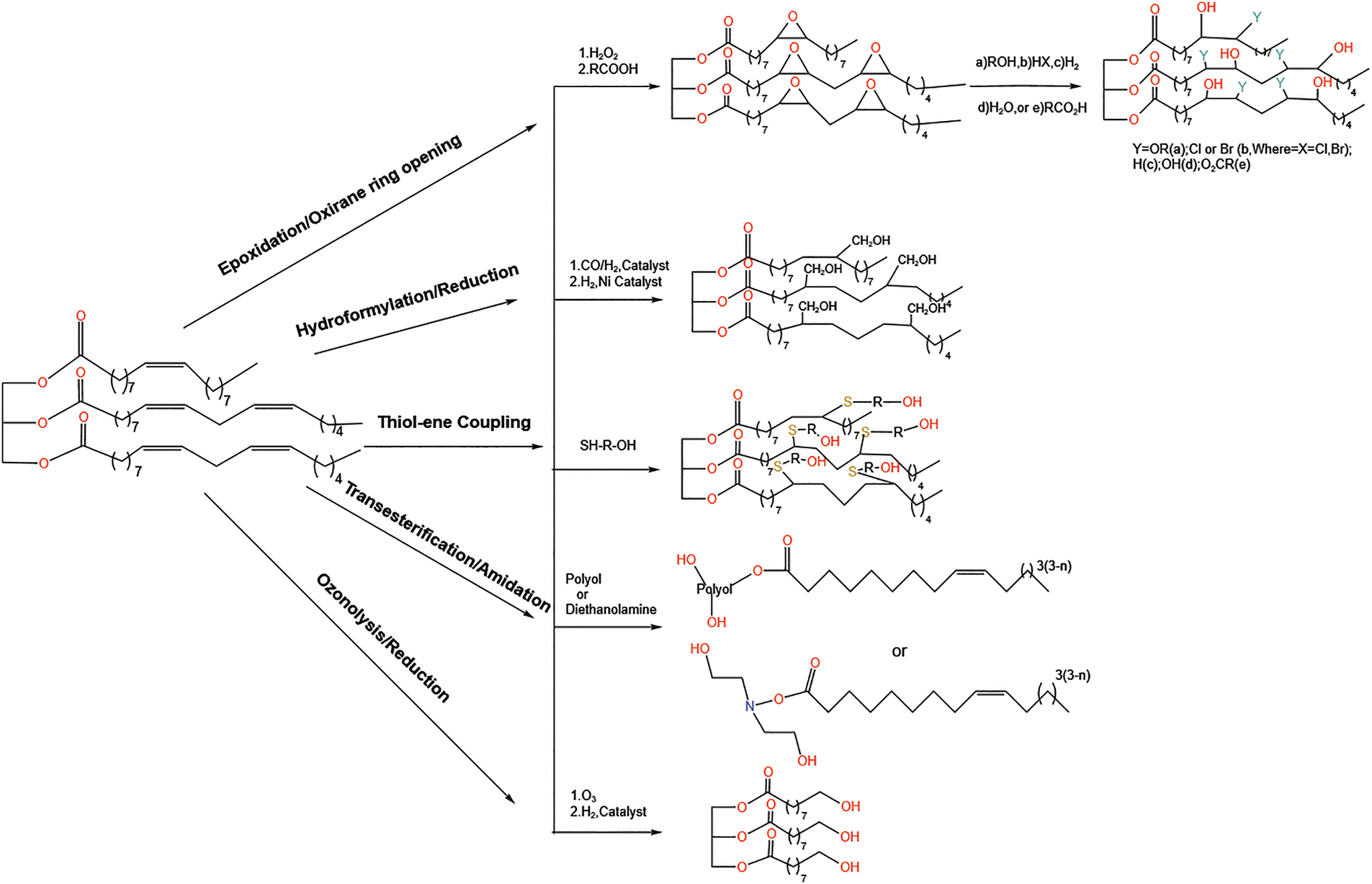
Figure 3: The unsaturated structures of vegetable oils can be converted to hydroxyl-containing molecules by epoxidation, hydroformylation, transesterification, thiol-ene coupling and ozonolysis
The carbon-carbon double bonds in soybean oil, corn oil, peanut oil, and linseed oil—vegetable oils with varying fatty acid compositions—were epoxidized using the strong oxidant H2O2/HCOOH [19]. These epoxidized oils were then subjected to ring-opening reactions with small molecules like methanol, butanol, and hydrochloric acid, resulting in vegetable oil-based polyols with consistent hydroxyl functionality. For instance, Xu et al. [20] utilized epoxidized soybean oil (ESO) as a raw material, conducting ring-opening with polyethylene glycol (PEG). The synthesis of the polyhydroxylated bio-based polyol (ESO-polyol) was confirmed through FT-IR and 1H-NMR analyses, revealing a hydroxyl value of 127 mg KOH/g. Subsequently, bio-based polyols (ESO-polyols) are reacted with isocyanates (IPDIs) to synthesize bio-based polyurethanes (BPU). This contributes to energy saving and environmental protection. Polaczek et al. [21] optimized solvent-free epoxidation processes for hemp seed oil, oilseed radish oil, rapeseed oil, and used rapeseed cooking oil. Bio-polyols were synthesized through a ring-opening reaction with diethylene glycol and tetrafluoroboric acid as catalysts. The resulting polyurethane foams were analyzed for apparent density, thermal conductivity, mechanical strength, closed cell content, short-term water absorption, and water vapor permeability, while their morphology was examined via scanning electron microscopy. The study found that bio-polyols with similar properties could be produced regardless of the oil’s characteristics, especially the unsaturated bond content.
2.1.2 Hydroformylation/Reduction Method
The process of polyol preparation by hydroformylation/reduction involves the use of a synthesis gas (a mixture of H2 and CO) [22]. The hydroformylation of the carbon-carbon double bonds in the molecular structure of vegetable oils to aldehydes is carried out in the presence of a noble metal catalyst such as Rh or Co. The aldehyde is then reduced to primary alcohol catalyzed by catalysts H2 and Raney-Ni, resulting in a bio-based polyol. Hong et al. [23] used Rh(CO)2 as a catalyst to prepare bio-based polyols from rubber seed oil in a two-step process. Firstly, the aldehyde was obtained by reacting with the unsaturated double bond in vegetable oil for 6 h at 90°C and then heated at 110°C for 5 h. Bio-based polyols with a hydroxyl group of 240 mg KOH/g and an acid value of 21 mg KOH/g were obtained. On this basis, a high strength and toughness polyurethane adhesive with a high cross-linking property was produced by polycondensation of MDI with the produced polyol. It was also compared with a commercially available Loctite epoxy resin adhesive, and the results showed that the product had better adhesion. The hydroxyl group of the polyol obtained by this process is the primary hydroxyl group, and its reaction performance is better than that of the epoxy ring-opening method.
The esterification method involves the esterification of the ester-bonded structure of vegetable oils with multifunctional low molecular weight alcohols in the presence of a strong acid or base catalyst [24]. New hydroxyl groups are introduced into the molecular chain to produce new esters and glycerides. Methanol, glycerol, pentaerythritol and trimethylolpropane are the main alcohols suitable for this method. Malewska et al. [25] obtained five biopolyols from watermelon, cherry, blackcurrant, grape and pomegranate fruit seeds using the transesterification reaction of oil with triethanolamine. Insulating polyurethane foams were then obtained by replacing 75 percent of petrochemical polyols with bio-polyols in a polyurethane system.
2.1.4 Some Other Methods of Preparing Polyols
The thiol-alkene reaction is a new process that is characterized by process simplicity, high efficiency, and controllable yield [26]. It has been widely used in the synthesis of vegetable oil-based polyols in recent years. In this process, it is under the condition of UV irradiation that the sulfhydryl group of alcohol sulfhydryl group can react with unsaturated carbon-carbon double bonds in the thiol-alkene reaction. The resulting hydroxyl group is thus successfully introduced into the molecule of the vegetable oil to obtain a bio-based polyol. However, the excessive use of mercaptans and the low carbon-carbon double bond (C=C) conversion will severely limit the performance of polyurethanes (PUs). Hu et al. [27]. Synthesized a series of eugenol-based polyols via thiol-ene click reaction. The eugenol-based polyols were prepared by thiophene click reaction at room temperature. More importantly, it can be used directly without purification. The results showed that the catalyst had good catalytic activity and the conversion of C=C was close to 100%. The prepared polyol was then reacted with diphenylmethane diisocyanate (MDI) to obtain a series of thermosetting polyurethane networks with tunable structures. The networks were colorless and transparent with high glass transition temperature (Tg) and good mechanical properties. The tensile strength was as high as 54.88 MPa, and the glass transition temperature could be adjusted in the range of 36.45°C–78.21°C. In addition, the compounds with the allylic structure were revealed to be favorable for effective click reactions, and their applications in polyurethanes will be greatly expanded.
The ozone redox method for polyol preparation involves ozonolysis, a chemical process that incorporates hydroxyl groups into vegetable oils by cleaving the double bonds in the fatty acid chains [28]. Ozone breaks the unsaturated carbon-carbon double bonds, creating unstable ozonated rings that decompose, ultimately producing aldehydes and hydroxyls.
As one of the three main components of lignocellulosic biomass [29]. Apart from cellulose and hemicellulose, lignin is the richest source of aroma in nature. As shown in Fig. 4, the lignin structure is rich in alcohol hydroxyls and phenolic hydroxyls, etc. [30]. Long regarded as a by-product waste of the pulp and paper industry, the exploitation of lignin is important to alleviate the resource problem. Lignin contains a variety of functional groups, carboxyl, hydroxyl, carbonyl, and methoxy, which can participate in many chemical reactions [31,32]. The utilization of lignin is too low by physical means to allow lignin to be blended with other materials, and the resulting composites have a rough structure and poor properties. However, it is not practical to use lignin directly in chemical synthesis reactions. Because of the complex structure of lignin, the hydroxyl content is low, and the reactivity is low, so it is necessary to modify lignin to increase the content of specific functional groups to improve its reactivity. Common modification methods for lignin include hydroxymethylation modification, demethylation modification, amine methylation modification, phenolisation modification, esterification modification, hydrothermal treatment modification, and graft copolymerization modification.
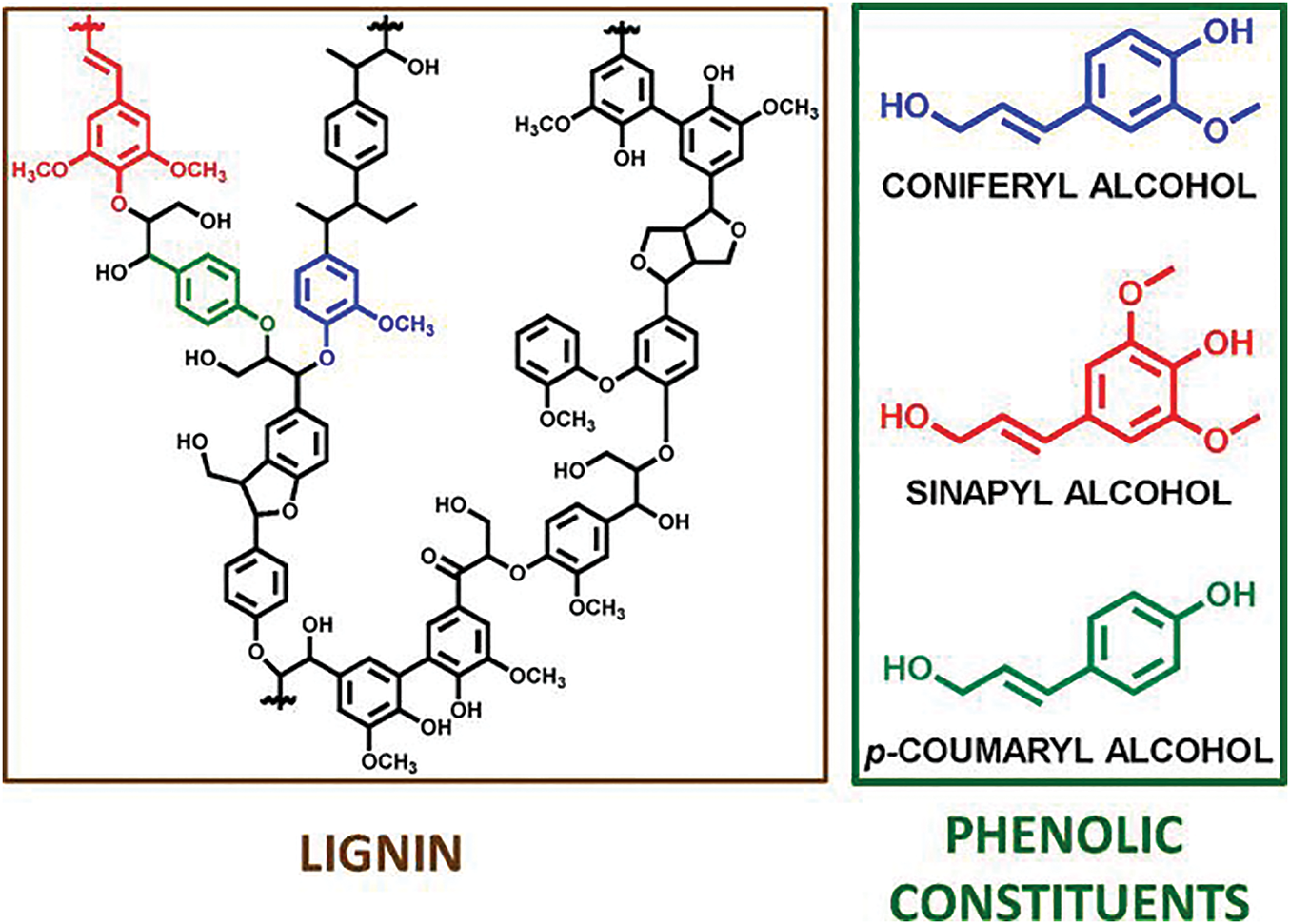
Figure 4: Lignin structure and its phenolic components
2.2.1 Hydroxymethylation Modification
In alkaline environments, lignin contains free phenolic hydroxyl groups on the benzene ring that undergo ionization. The hydrogen activity in the neighboring position of the phenolic hydroxyl group increases and reacts with formaldehyde to form hydroxymethyl. This reaction is also known as ‘Lederer Manasse’ and is shown in Fig. 5.
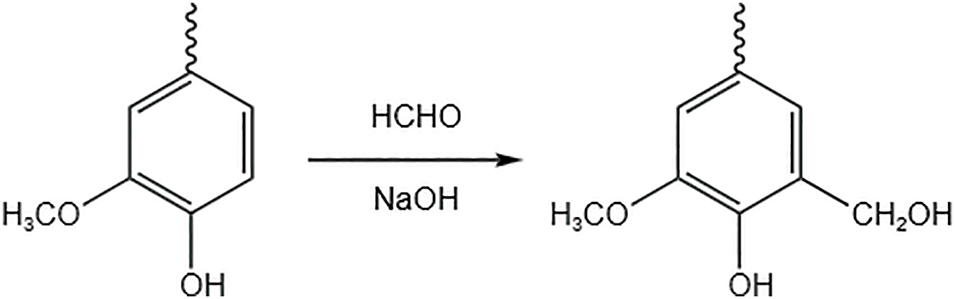
Figure 5: Lignin hydroxymethylation modification
For example, Chen et al. [33] dissolved lignin acetate in aqueous NaOH solution. Then formaldehyde (lignin acetate/NaOH/HCHO molar ratio of 1:1:2.4) was added for hydroxymethylation and then copolymerized with isocyanate to prepare lignin-based polyurethane adhesives. The experimental results showed that the hydroxymethylated lignin and isocyanate formed a close three-dimensional polyurethane crosslinked network. The mechanical properties and thermal stability of the adhesive were improved by15%–30%. It indicates that hydroxymethylation is an effective way to increase the hydroxyl group in lignin, and the modified lignin is sufficient to partially replace petroleum-based polyol to prepare PU adhesives with excellent performance. Similarly, Xue et al. [34] used a simple and effective means to obtain alkali lignin by modification using hydrogen peroxide and sodium hydroxide. Isophosphofluorone diisocyanate (IPDI) and polycarbonate diol (PCDL) were mixed and reacted. Lignin-based polyurethane films were prepared in the presence of dibutyltin bis(formate) (DBTDL) catalyst.
2.2.2 Demethylation Modification
Methoxy in lignin occupies the active site on the benzene ring. Thus, it has an inhibitory effect on the activity of lignin. Methoxy is capable of reacting with divalent sulfur ions, sulfite, and thihydrogen ions in a nucleophilic substitution reaction. Substitution of methoxy by phenolic hydroxyl group, the reaction process is shown in Fig. 6.
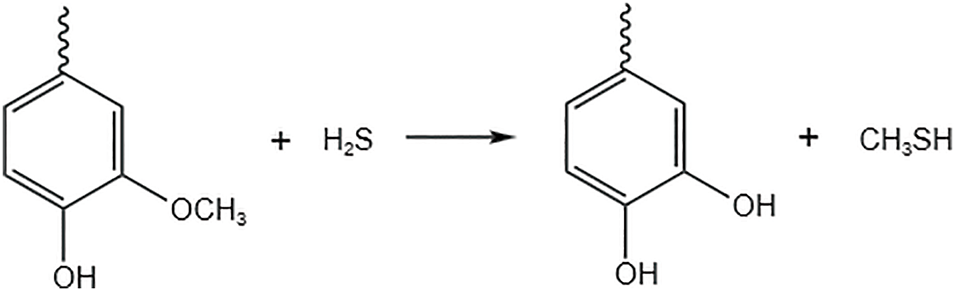
Figure 6: Lignin demethylation reaction
Lignin acetate (AAL) was extracted from sugarcane bagasse by Chen et al. [35] Due to the presence of reactive sites, such as aliphatic and aromatic hydroxyl groups, it was used as a partial substitute for polyols in polyurethane (PU) adhesives. To enhance its reactivity, lignin was partially demethylated before copolymerization with polyisocyanates. The demethylation reaction, catalyzed by hydrobromic acid under heating, resulted in a reduction of methoxy groups and an increase in phenolic hydroxyl groups on the benzene ring. The optimal temperature for demethylation was found to be 120°C, leading to a 36.5% increase in lignin hydroxyl content, up to 5.25 mmol/g. The lignin, enriched with hydroxyl groups via partial demethylation, was better integrated into the three-dimensional crosslinked network of the covalent polymer. Compared to PU adhesives based on virgin AAL, those made from demethylated lignin exhibited higher tensile strength and thermal stability. This indicates that demethylation is an effective strategy for enhancing the mechanical properties of lignin-based PU adhesives.
Phenolic modification is the reaction between lignin and phenol to graft phenol groups onto the lignin molecule to improve the reactivity of lignin [36]. The reaction process is shown in Fig. 7. Phenolisation is used as a simple and straightforward chemical modification method to improve the reactivity of lignin and the compatibility of synthetic polyurethane (PU) materials. Falireas et al. [37] synthesized a series of phenolised lignin-based polyurethane (PhLPU) films using phenolised lignin (PhL) and poly(tetrahydrofuran) as a polyol mixture with aromatic or aliphatic isocyanates. The effects of PhL concentration and the chemical structure of the isocyanate on the thermal stability and mechanical properties of PhLPU films were systematically investigated. The results showed that PhLPU films formulated with small amounts of PhL exhibited significantly improved thermo-mechanical properties compared to lignin-free networks.
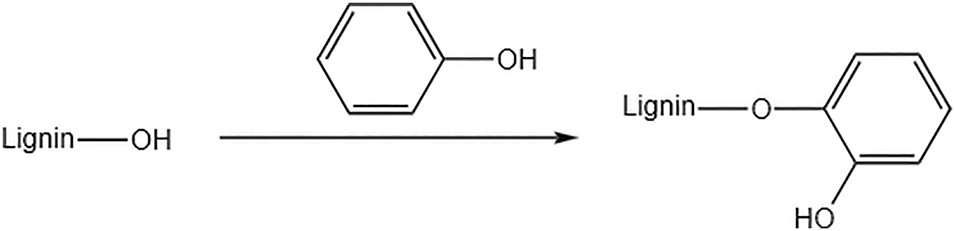
Figure 7: Lignin phenolization reaction
Since sugar constitutes 70–80 percent of lignocellulosic biomass, it offers great potential for conversion into a variety of valuable bio-based platform chemicals and products [38]. Sugar-based polyols have received much attention due to their non-toxic, renewable and biodegradable nature. In this regard, isosorbide is a promising candidate. Because of its unique characteristics, sugar molecules have one or more hydroxyl groups attached to the carbon chain in addition to aldehyde and carbonyl groups. These hydroxyl groups can be involved in the synthesis of bio-based polyols through modification reactions. For example, Kaikade et al. [39] synthesized polyurethane by reacting isophorone diisocyanate (IPDI) with different ratios of sorbitol and polyethylene glycol (PEG200). The synthesized resins were characterized by FTIR, 1HNMR and analytical techniques such as hydroxyl, iodine and acid values. Various formulations were prepared using the developed resins, reactive diluents and photoinitiators. The coatings were applied to silane-treated glass substrates and then cured under UV light. The hydrophilicity of the coatings was studied by water contact angle measurements. Anti-fog, anti-frost and self-cleaning tests were carried out and it was found that coatings with a contact angle of less than 33° showed anti-fog properties, but coatings with a contact angle of less than 7° showed anti-frost effects. Due to the excellent hydrophilicity, black oil stains were easily washed off under water flow, thus also showing excellent oil repellency/self-cleaning performance.
Sugar molecules such as sucrose and glucose generally undergo a two-step reaction to convert them into polyols. Firstly, the sugars are converted to carboxylic acids and then the carboxyl groups are converted to hydroxyl groups using a catalyst. Lakatos et al. [40] synthesized sucrose-1,6-hexamethylene diisocyanate (HDI) co-oligomers and used them as novel polyols for poly(ε-caprolactone) (PCL)-based polyurethanes. The Polymerization reaction of sucrose and HDI was monitored by MALDI-TOF MS. The results showed that most of the linear oligomer chains containing 16 sucrose units could be obtained by selecting suitable reaction conditions.
Rosin is a mixture of tricyclic diterpene resinous acids with a hydrophilic structure, consisting mainly of rosin and caprylic acids, with a small proportion of fatty acids and neutrals [41]. Since the typical hydrofi ring structure of rosin acids is similar to that of some petroleum-based alicyclic and aromatic groups. They have a great potential for replacing petroleum-based compounds in the synthesis of polyurethanes. In addition, the carboxyl group and the double bond are the two main reaction sites in the rosin acid structure. Typical reactions in the carboxyl group include esterification, amination and salt formation. The conjugated double bonds of the rosin resin acid molecular backbone are modified by oxidation, hydrogenation, disproportionation and Diels-Alder reaction. The modified rosin derivatives have increased functionality, and after reacting with polyisocyanate, the rosin cyclo-skeleton structure can be introduced into PU to improve the properties of PU. Such as thermal stability, dimensional stability, shrinkage, adhesion, UV resistance, water resistance and hardness. For example, Li et al. [42] constructed a novel rosin-based polyurethane-based glass polymer network (VPUOH) through the monomer reaction of isocyanate (HDI) as a curing agent with rosin-modified alcohol groups. The dynamic rosin-based polyurethane glass polymer was characterized by FTIR and dynamic mechanical analysis. The resulting rosin-based polyurethane-based glass polymer has superior mechanical properties. Due to the dynamic polyurethane linkages, the network topology of rosin-based polyurethane-based glass polymers can be altered, thus contributing to self-healing and reprocessing capabilities. In addition, crushed samples of 70% VPUOH can be remolded several times by a hot press, and the mechanical properties of the recycled samples are restored with tensile strengths even higher than those of the original samples.
3 Biomass Isocyanates and Non-Isocyanates
Isocyanates are key chemical intermediates in the synthesis of polyurethanes and are mainly produced by the phosgene method [43]. It currently occupies an important position in industrial isocyanate production. However, the synthesis process of isocyanates often uses extremely toxic phosgene, which can have serious adverse effects on the human respiratory system. The isocyanate itself is also a toxic and highly reactive chemical substance, which has strict requirements for storage and use conditions. The search for green and sustainable development of the polyurethane industry is a major issue. O’Dea et al. [44] utilized relatively inexpensive organoboron Lewis acids as depolymerizing agents to directly produce isocyanates under mild reaction conditions (below 100°C and ambient pressure) with near-quantitative conversion rates. The recovered isocyanates are then used to synthesize second-generation polyurethanes (PUs) that exhibit molecular weights and thermal properties comparable to those of virgin PUs. This advanced chemical recovery method not only facilitates the recycling of conventional PUs but also reduces the reliance on the production of new isocyanates from environmentally hazardous and highly toxic petrochemical feedstocks.
Today, the biggest challenge in the synthesis of bio-based polyurethanes is the replacement of petrochemical isocyanate components. Although scientific advances have been made, challenges remain in terms of availability, production costs, and scaling up to industrial scale. The use of bio-based isocyanates is important for sustainable development. This is because it will create products that are less reliant on petrochemical feedstocks, reduce greenhouse gas emissions, and help to achieve more environmentally friendly production. However, a good way to optimize the use of available raw materials is to modify them according to product requirements. Brzoska et al. [45] utilized a portion of a bio-based product of Desmodur eco N 7300 supplied by Costron (Leverkusen, Germany) to be used as an isocyanate. In addition, Cardolite® NX-2026 from Cardolite Corporation (Bristol, PA, USA) was used as a modifier for isocyanate trimers. It was synthesized via a two-step process with bio-based polyols and bio-glycols via a solvent-free synthesis method. The use of bio-based monomers, as shown in Fig. 8, demonstrates significant advances in the production of green polyurethanes, with properties that are not inferior to those of conventional polyurethanes, and with the additional advantage of high transparency.
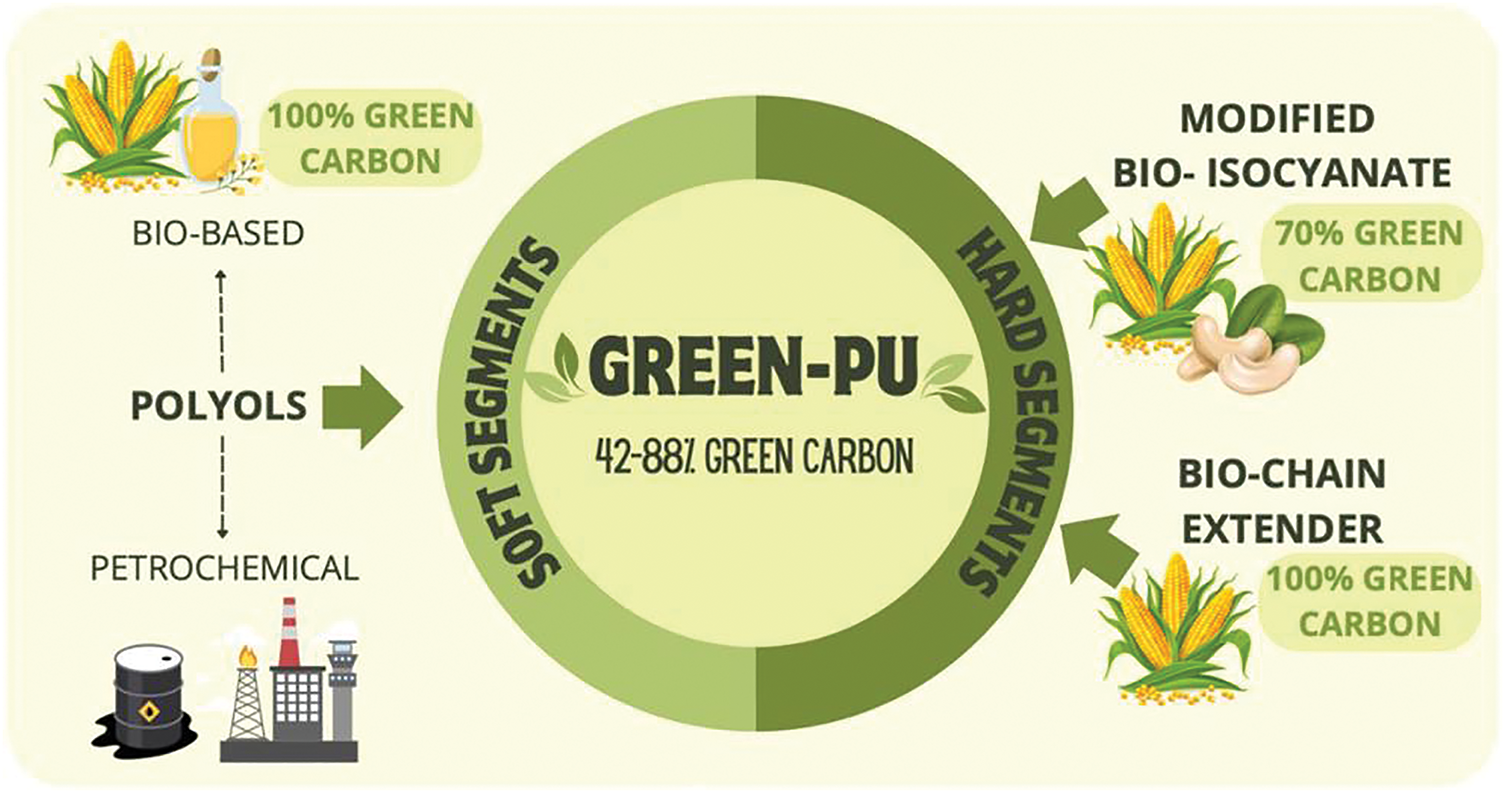
Figure 8: Preparation of PU using bio-based raw materials
In addition to this, there is an urgent need to find an alternative route to synthesizing polyurethane that involves minimal or no toxic reagents. The resulting product is called non-isocyanate polyurethane (NIPU). As shown in Fig. 9 there are four main ways to obtain NIPU: polycondensation, rearrangement, ring-opening polymerization, and polyaddition [46]. The first three routes suffer from a series of disadvantages that are quite similar in nature. These issues are closely related to the toxicity associated with using various phosgene derivatives, acylzines, or carboxamides, making the removal of isocyanates from the production process insufficient or irrelevant. In addition, unwanted by-products such as alcohols and hydrochloric acid are produced, as well as the need to react under extreme temperature conditions [47].
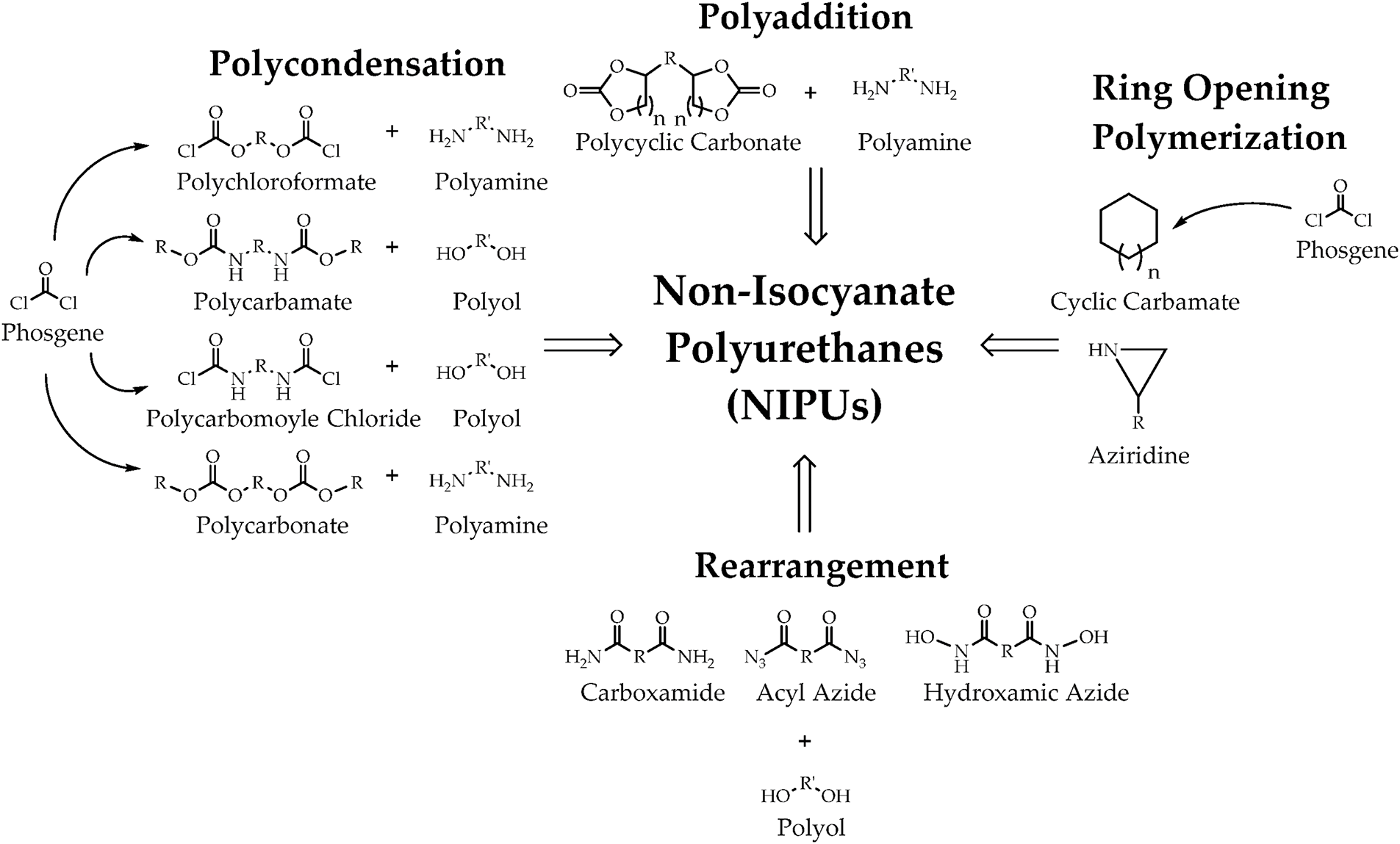
Figure 9: There are four main ways to obtain NIPU: polycondensation, rearrangement, ring-opening polymerization, and adducts. Reprinted with permission from Ref. [46]. Copyright © 2023 Catalá J et al.
Therefore, the preferred route is usually the 4th method. The polyaddition reaction of polycyclic carbonates with polyamines is shown in Fig. 10. is considered to be the most promising method for the green preparation of NIPU [48]. The method does not involve phosgene in the reaction and there is no need to avoid interfering factors such as moisture. In addition, the method generates urethane groups along with a large number of hydroxyl groups. The newly generated hydroxyl groups can form intramolecular or intermolecular hydrogen bonds with the urethane groups in the polymerization system, further enhancing the mechanical properties, chemical resistance, adhesion, and hydrolytic stability of the resulting polyhydroxy polyurethanes (PHUs). Garipov et al. [49] investigated the kinetic features of the reaction of cyclic carbonates with amines and proposed a three-step reaction mechanism. In the first step, the amino group nucleophilically attacks the carbonyl group in the cyclic carbonate to produce a tetrahedral-shaped intermediate. In the second step, the intermediate is attacked by another amino group to remove the hydrogen ion thereby undergoing deprotonation. In the third step, the carbon-oxygen bond of the cyclic carbonate is broken by the strong electron attraction of the nitrogen atom. The resulting alkoxide ion rapidly recombines with the proton to produce the final product NIPU. In this step of the reaction, the carbon-oxygen bond of the cyclic carbonate is broken in two ways. Two isomers, primary and secondary alcohols, are eventually formed.
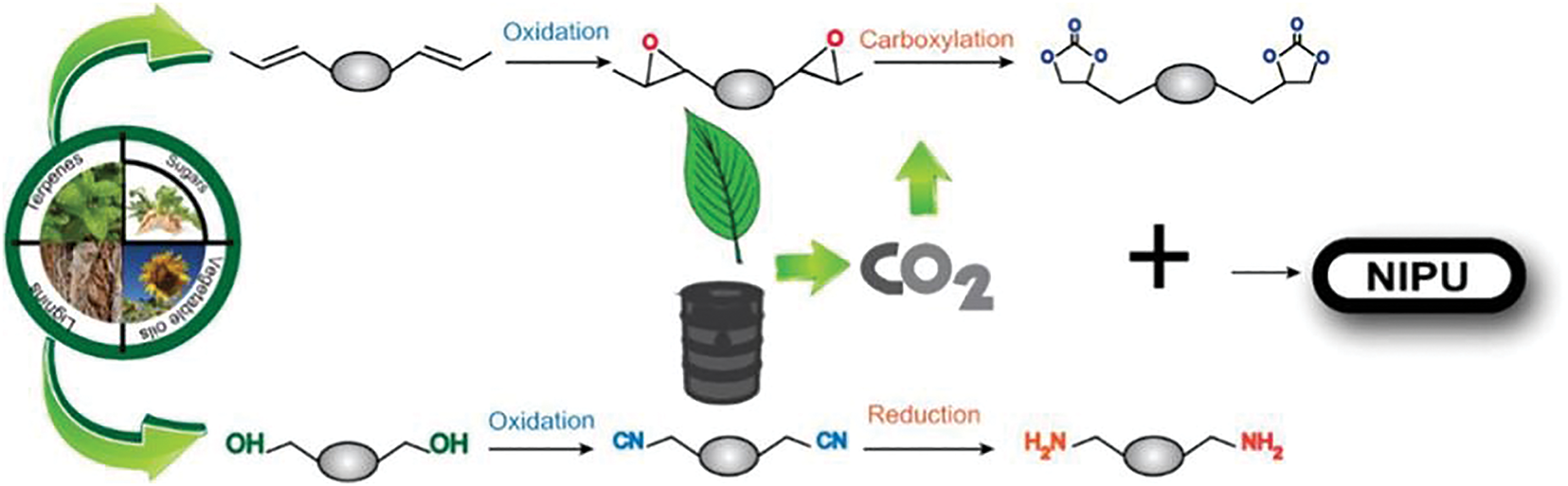
Figure 10: Polyaddition reactions of polycyclic carbonates with polyamines. Reprinted with permission from Ref. [49]. Copyright © 2019 Garipov RM et al.
3.1 Preparation of Vegetable Oil-Based Non-Isocyanate Polyurethanes
Vegetable oils (triglycerides) are promising renewable feedstocks that can be effectively used as a reliable platform for developing non-isocyanate polyurethanes (NIPUs) with diverse structural and functional properties. Their non-toxicity, inherent biodegradability, eco-friendliness, low cost, and functional groups (such as esters and unsaturations) make them ideal alternatives to fossil-based feedstocks for polyurethane synthesis. As a result, there is a growing effort to enhance the efficacy of vegetable oils as chemical platforms for producing thermoset and thermoplastic polyhydroxy polyurethanes (PHUs) [50]. Studies have demonstrated that vegetable oils contain several active fractions suitable for chemical modification, allowing for efficient conversion into new precursors for NIPU synthesis.
3.1.1 Vegetable Oil-Based Polycyclic Carbonates
The polyaddition between carbonated vegetable oils and diamines to form non-isocyanate polyurethanes (NIPUs) has been highlighted as a safer and more sustainable alternative to traditional polyurethanes (PUs) synthesis [51]. Vegetable oil is one of the typical representatives of renewable resources. The carbon-carbon double bond in its molecular structure can be used as a reaction site to prepare epoxidized vegetable oil, which provides a good basis for the synthesis of vegetable oil-based cyclic carbonates [52]. In addition, vegetable oils and their derivatives have the characteristics of low volatility and the ability to react with CO2 at high temperature and high pressure. Zhang et al. [53] used epoxidized linseed oil as the raw material and CO2 addition to produce polycyclic carbonates. Polymerization with different diamines and hydrophilic groups allowed the preparation of a series of cationic, anionic and nonionic vegetable oil-based non-isocyanate aqueous polyurethanes (NIPUs). Haniffa et al. [54] prepared cyclic carbonates by coupling jatropha oil and its alkyl resins with CO2. NIPU films were also prepared by the polyaddition of diamines with different molecular structures (1,3 propylenediamine and isophorone diamine). The NIPU films prepared with isophorone diamine when the ratio of jatropha oil-based cyclic carbonate to alkyl resin cyclic carbonate substance was 1:3 had a high glass transition temperature (44°C) and Young’s modulus up to 680 MPa. It was demonstrated that all the NIPU samples exhibited excellent stability and customizability. It opens a new path for the preparation of high-performance and multifunctional NIPUs from vegetable oils. In addition, researchers can achieve control over the mechanical properties of target polymer materials by treating epoxidized vegetable oils with different degrees of CO2 addition, in anticipation of further expanding their application areas.
3.1.2 Vegetable Oil-Based Polyamines
Vegetable oil-based polyamines are synthesized from triglyceride derivatives by reacting with nitriles, amides, acyl azides or bromo functional groups [55]. Two main pathways exist. Firstly, the cyclic structure of epoxidized triglycerides is first reacted with diols. The product undergoes a series of reactions such as bromination, azide substitution, and reduction in the presence of a catalytic system to produce vegetable oil-based polyamines. The second is the introduction of amine functional groups into vegetable oils through thiol-alkene coupling reactions to synthesize polyamines. Stemmelen et al. [56] synthesized vegetable oil-based polyamines from unsaturated grape seed oil by UV-induced thiol-alkene coupling reactions with cysteine hydrochloride.
3.2 Preparation of Lignin Non-Isocyanate Polyurethane
Lignin has an extensively cross-linked three-dimensional network structure. As well as an abundance of active groups that can be functionalized and modified. Such as phenolic hydroxyl, alcohol hydroxyl, methoxy, and so on. In order to improve the compatibility and reactivity of lignin in the NIPU preparation process. Researchers have developed many strategies to increase the lignin hydroxyl content and its reactivity through lignin functionalization modification and macromolecular dePolymerization [57]. Currently, the synthetic methods of lignin-based NIPU can also be divided into 2 categories: (1) synthesis of lignin-based NIPU precursor cyclic carbonate; (2) synthesis of lignin-based NIPU precursor polyamine.
3.2.1 Lignin-Based Cyclic Carbonates
Lignin is an aromatic compound with groups such as hydroxyl and alkene bonds at different positions. Similar to vegetable oils, lignin can undergo epoxidation to obtain highly reactive epoxide lignin, which can undergo an addition reaction with CO2 to produce lignin-containing cyclic carbonate groups. Currently, there are 2 main synthetic methods used to prepare the precursor lignin-based cyclic carbonates for NIPU.
The first is the ester exchange reaction. For example, Sarazin et al. [58] prepared an organic solvent lignin-based non-isocyanate polyurethane (NIPU) by mixing organic solvent lignin with dimethyl carbonate and hexamethylene diamine, achieving an intra-drying adhesive strength of 0.77 MPa. While the ester-exchange method offers advantages such as low chemical reagent dosage, simplicity in operation, and ease of monitoring and control, it also has drawbacks, including long reaction times and incomplete reactions, which limit its application in synthesizing lignin-based cyclic carbonate esters.
The second is the use of CO2-curing epoxy resin. For example, Quinsaat et al. [59] proposed a method for preparing NIPU/epoxy hybrid thermosets with a high bio-based content (mass fraction of 85%–90%) from depolymerized natural lignin. Lignin hydrolysis oil (LHO) was functionalized with epichlorohydrin to create an epoxide structure (LHO-GE), which was then reacted with CO2 to form lactone (LHO-CC). The LHO-CC was subsequently reacted with glycerol diglycidyl ether to produce thermosets. These blends were cured to obtain thermosets with a flexural modulus of 4.5 GPa and a flexural strength of 160 MPa, demonstrating superior mechanical properties compared to pure epoxy thermosets created from similar compositions.
Beyond its application in cyclic carbonate synthesis, lignin shows potential as a precursor for polyamine production through redox-mediated amine functionalization [60]. Specifically, amine groups can bestrategically incorporated into lignin’s molecular architecture to create reactive intermediates for non-isocyanate polyurethane (NIPU) synthesis. Current modification strategies, however, predominantly yield tertiary amines (-NH-) rather than the more nucleophilic primary amines (-NH₂). This structural limitation significantly reduces the reactivity of lignin-derived amines toward cyclic carbonate partners, ultimately impeding the crucial carbamate formation step in NIPU production. Dou et al. [61] present an efficient, catalyst- and solvent-free method for the synthesis of lignin-based non-isocyanate polyurethanes (LNIPUs). A series of novel LNIPUs were successfully prepared by one-pot, catalyst-free and solvent-free Polymerization reactions involving aminated fractionated lignin (ALFE) and bis(6-membered cyclic carbonate) (6CC). The properties of the resulting LNIPUs were then systematically investigated, with particular attention to the adhesion properties. The doping of ALFE significantly enhanced the adhesion of the resulting LNIPUs on aluminum, wood, and plastic substrates. Maximum bond strength of 3.09 MPa was achieved on aluminum. In addition to that. As shown in Fig. 11, Meng et al. [62] also demonstrate a novel strategy that uses highly pure lignin isolated from co-solvent enhanced lignocellulosic fractionation (CELF) pretreatment of poplar wood to produce biobased NIPUs. In this strategy, hardwood poplar is first fractionated via a CELF pretreatment to produce a clean lignin stream that is rich in phenolics. The CELF lignin was then aminated by a Mannich reaction, and the aminated CELF lignin was finally reacted with bicyclic carbonates to yield an advanced NIPU.
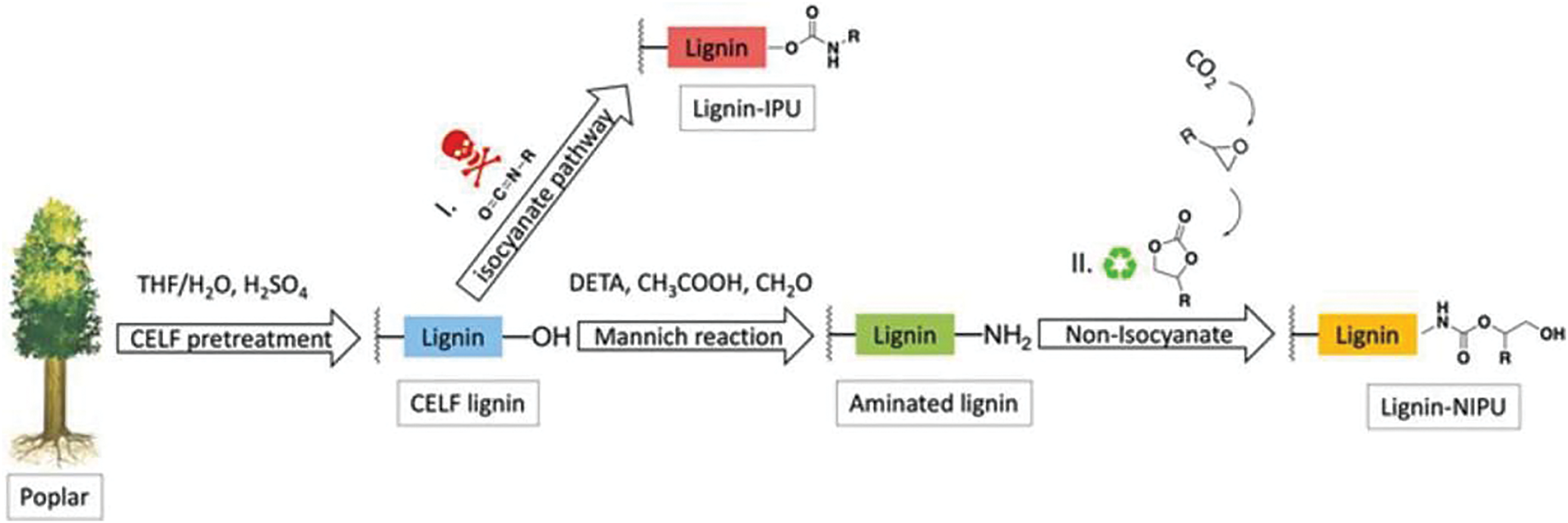
Figure 11: Synthesis of lignin-based PU and NIPU. Reprinted with permission from Ref. [62]. Copyright © 2022 Meng et al.
In the raw material of synthetic polyurethane, the amount of chain extenders is very small, but it plays an important role. Chain extenders can influence the properties of PU by adjusting the molecular chain structure and the ratio of hard and soft segments [63]. The use of bio-based chain extenders in the preparation of polyurethanes has a limited effect on the bio-based content of polyurethanes and is therefore not discussed in this paper. The following is a list of some of the recent research work on bio-based chain extenders. Das et al. [64] prepared antioxidant polyurethane gels by using gallic acid as a chain extender. They were further coated with decellularised extracellular matrix (dECM) to improve absorbability, biocompatibility, and haemocompatibility, and the biomaterials thus prepared are promising for use as patches for cardiac regeneration. Zhao et al. [65] explores the advantages of bio-based multifunctional malonates in the preparation of closed-cell polyurethane foams using them as chain extenders. It was found that the presence of malonates could change the reaction kinetics, chain segment mobility, and thus the relative kinetics of polyurethane and polyurea formation. The ability to manipulate the morphology of polyurethane foams using malonates as chain extenders and their potential application in polyurethane closed-cell foam systems is illustrated. Gnanasekar et al. [66] synthesized a new family of fully bio-based shape memory polyurethanes (Bio-SMPUs). The Bio-SMPU prepolymers were derived from rosinacid-based diisocyanate (AADI), while the novel chemical structure of the chain extender (CE) was derived from vanillin and alanine amino acids (DVA). The microphase separation as well as the physical and mechanical properties of these novel Bio-SMPUs were investigated to determine the effect of different molar ratios of AADI/polycaprolactone (PCL)/DVA. In vitro, hydrolytic biodegradation of Bio-SMPU was investigated. The maximum biodegradation weight loss reached about 71% within eight weeks. The physical and mechanical properties of these new Bio-SMPUs were comparable to those previously reported for SMPUs synthesized with conventional petroleum-derived diisocyanates and chain extenders. Guo et al. [67] synthesized PU elastomers using liquefied waste banana pseudostem as a chain extender. The resulting materials exhibit good tensile strength (up to 30 MPa) and excellent toughness (389.7 MJ m−3). It is shown that the extraordinary mechanical properties of PUs mainly result from strong energy dissipation of hydrogen bonds, microphase separation, and strain-induced crystallisation.
Although biomass-derived polyols and polyurethanes possess significant potential, their production still faces several challenges, primarily due to their inherent heterogeneous structures with varying compositions, which complicates the manufacturing of biomass-based polyols and polyurethanes with controllable and consistent properties. To ensure high performance of bio-based polyurethanes, biomass-derived polyols are frequently blended with commercial petroleum-based polyols and additives. Another existing issue is that these biomass-based products generally incur higher costs compared to their conventional petroleum-based counterparts, resulting in reduced market competitiveness and consumer appeal. To overcome these barriers, concerted efforts should be devoted to developing advanced biomass conversion technologies with enhanced efficiency through exploration of novel renewable feedstocks, innovation in reactor systems, optimization of reaction conditions, design of superior catalysts, and integration with established biomass pretreatment and conversion processes to produce diverse bio-based products. Comprehensive evaluations of product quality, technical performance, environmental impact, and economic feasibility are crucial for the sustainable production of polyols and polyurethanes. A thorough investigation of their technical characteristics, economic benefits, and environmental implications is essential to assess the commercial viability of biomass-derived polyols and polyurethanes [68].
Bio-based non-isocyanate polyurethanes (NIPUs) are emerging as a promising alternative to traditional polyurethanes (PUs) due to their non-toxic synthesis process and superior processing performance, hydrolytic stability, impermeability, and chemical resistance. However, the development of bio-based NIPUs faces several challenges: (1) Mechanical Properties: NIPUs generally exhibit inferior mechanical properties compared to traditional PUs, and there is a lack of data and performance feedback from consumer products. (2) Variability of Raw Materials: The composition of renewable bioresources varies by source, increasing complexity, while the use of synthetic bio-based precursors raises production costs, limiting the commercial scalability of bio-based NIPUs. (3) Reactivity Issues: Low reactivity and a lack of specific catalysts for bio-based polyols and cyclic carbonates hinder the industrial application of bio-based NIPUs. (4) Downstream enterprises have insufficient knowledge of NIPU’s performance advantages, and the mature application ecology of traditional polyurethane (e.g., coating formulation system) is difficult to be completely replaced in the short term, and promotion takes time. The future development of non-isocyanate polyurethane is promising, especially driven by the demand for environmental protection, its technology iteration and market expansion will be accelerated. However, bottlenecks such as cost, performance and market education need to be overcome to realise large-scale application through technological innovation and policy support. In the short term, NIPU may complement traditional polyurethane; in the long term, with the deepening of green transformation, it is expected to become the mainstream direction of the polyurethane industry [69].
The synthesis and application of biomass-derived polyurethanes (PUs) represent a pivotal shift toward sustainable polymer materials, addressing the environmental and resource limitations of traditional petroleum-based counterparts. This review highlights significant advancements in utilizing renewable feedstocks such as vegetable oils, lignin, sugars, and rosin to produce bio-based polyols, non-isocyanates, and chain extenders. These innovations not only reduce reliance on fossil fuels but also enhance material properties, including thermal stability, mechanical strength, hydrophobicity, and biodegradability.
Key progress has been made in synthesizing bio-based polyols through epoxidation, hydroformylation, and thiol-ene reactions, enabling tailored functionalities for diverse applications. Non-isocyanate polyurethanes (NIPUs), derived from cyclic carbonates and polyamines, offer a safer and more sustainable alternative by eliminating toxic isocyanates, though challenges in mechanical performance and costefficiency remain. Lignin, with its aromatic complexity, has emerged as a promising precursor for high-value PU materials through functionalization strategies like hydroxymethylation and demethylation.
Despite these advancements, barriers such as feedstock heterogeneity, high production costs, and the performance gap between bio-based and conventional PUs hinder large-scale adoption. Future efforts must focus on optimizing biomass conversion technologies, developing cost-effective catalysts, and improving the compatibility of bio-based components. Additionally, advancing NIPU formulations to match the mechanical robustness of traditional PUs and fostering industry-academia collaboration will accelerate market penetration.
In conclusion, bio-based polyurethanes hold immense potential to drive the green transition in polymer industries. By addressing current limitations through innovation and policy support, these materials can evolve from niche alternatives to mainstream solutions, contributing to a circular economy and a sustainable future.
Acknowledgement: Not applicable.
Funding Statement: This work was supported by the China Postdoctoral Science Foundation (No. 200902090) and Tianjin Enterprise Science and Technology Commissioner Project (No. 21YDTPJC00570).
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization, Zhen-Yu Chen and Gui-Chang Jiang; methodology, Zhen-Yu Chen; software, Zhen-Yu Chen; validation, Gui-Chang Jiang, Zhen-Yu Chen and Yue-Ru Wang; formal analysis, Gui-Chang Jiang; investigation, Zhen-Yu Chen; resources, Gui-Chang Jiang; data curation, Yue-Ru Wang; writing—original draft preparation, Zhen-Yu Chen; writing—review and editing, Zhen-Yu Chen; visualization, De-Yi Teng; supervision, Yan-Fang Xue; project administration, Gui-Chang Jiang; funding acquisition, Gui-Chang Jiang. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Skoczinski P, Krause L, Raschka A, Dammer L, Carus M. Current status and future development of plastics: solutions for a circular economy and limitations of environmental degradation. Methods Enzym. 2020;11(1):1–26. doi:10.1016/BS.MIE.2020.11.001. [Google Scholar] [PubMed] [CrossRef]
2. Kemona A, Piotrowska M. Polyurethane recycling and disposal: methods and prospects. Polymers. 2020;12(8):1752. doi:10.3390/polym12081752. [Google Scholar] [PubMed] [CrossRef]
3. Tran MH, Phan DP, Lee EY. Review on lignin modifications toward natural UV protection ingredient for lignin-based sunscreens. Green Chem. 2021;23(11):4633–46. doi:10.1039/D1GC01139A. [Google Scholar] [CrossRef]
4. Alinejad M, Henry C, Nikafshar S, Gondaliya A, Nejad M. Lignin-based polyurethanes: opportunities for bio-based foams, elastomers, coatings and adhesives. Polymers. 2019;11(7):1202. doi:10.3390/polym11071202. [Google Scholar] [PubMed] [CrossRef]
5. Word LJ, McAden EP, Poole C, Nylander French LA. The genetics of occupational asthma development among workers exposed to diisocyanates: a systematic literature review with meta-analysis. Front Genet. 2022;9:44197. doi:10.3389/fgene.2022.944197. [Google Scholar] [PubMed] [CrossRef]
6. Mo Y, Huang X, Hu C. Recent advances in the preparation and application of bio-based polyurethanes. Polymers. 2024;16(15):2155. doi:10.3390/polym16152155. [Google Scholar] [PubMed] [CrossRef]
7. Azarmgin S, Torabinejad B, Kalantarzadeh R, Garcia H, Velazquez CA, Lopez G, et al. Polyurethanes and their biomedical applications. ACS Biomater Sci Eng. 2024;10(11):6828–59. doi:10.1021/acsbiomaterials.4c01352. [Google Scholar] [PubMed] [CrossRef]
8. Zhang Z, Qiu J, Sha Y, He Y, Ma X, Li G, et al. Molecularly engineered tough and room-temperature self-healing polyurethanes for resistive strain sensors. ACS Appl Polym Mater. 2024;6(7):3721–31. doi:10.1021/acsapm.3c02867. [Google Scholar] [CrossRef]
9. Li N, Zhang X, Zhang Y, Wang G, Wang R, Zhang C, et al. Preparation of multi-band photothermal responsive bio-based superhydrophobic self-healing poly(siloxane-urethane) coating. J Adhes Sci Technol. 2024;38(1):1–17. doi:10.1080/01694243.2023.2219360. [Google Scholar] [CrossRef]
10. Tang JS, Kuo CT, Liao YC. Transparent biodegradable composite plastic packaging film from TEMPO-oxidized cellulose nanofibers. Int J Biol Macromol. 2024;260(Part 1):129502. doi:10.1016/j.ijbiomac.2024.129502. [Google Scholar] [PubMed] [CrossRef]
11. Tran MH, Lee EY. Production of polyols and polyurethane from biomass: a review. Environ Chem Lett. 2023;21(4):2199–223. doi:10.1007/s10311-023-01592-4. [Google Scholar] [CrossRef]
12. Sardon H, Mecerreyes D, Basterretxea A, Averous L, Jehanno C. From lab to market: current strategies for the production of biobased polyols. ACS Sustain Chem Eng. 2021;9(32):10664–77. doi:10.1021/acssuschemeng.1c02361. [Google Scholar] [CrossRef]
13. Cabulis U, Ivdre A. Recent developments in the sustainability of the production of polyurethane foams from polyols based on the first-to the fourth-generation of biomass feedstock. Curr Opin Green Sustain Chem. 2023;44:100866. doi:10.1016/j.cogsc.2023.100866. [Google Scholar] [CrossRef]
14. Danov SM, Kazantsev OA, Esipovich AL, Belousov AS, Rogozhin AE, Kanakov EA. Recent advances in the field of selective epoxidation of vegetable oils and their derivatives: a review and perspective. Catal Sci Technol. 2017;7(17):3659–75. doi:10.1039/C7CY00988G. [Google Scholar] [CrossRef]
15. Zhong Y, Zhang T, Zhang W, Wang G, Zhang Z, Zhao P, et al. Antibacterial castor oil-based waterborne polyurethane/gelatin films for packaging of strawberries. Food Packag Shelf Life. 2023;36(1):101055. doi:10.1016/j.fpsl.2023.101055. [Google Scholar] [CrossRef]
16. Parcheta PDJ. Environmental impact and industrial development of biorenewable resources for polyurethanes. Crit Rev Environ Sci Technol. 2017;47(20):1986–2016. doi:10.1080/10643389.2017.1400861. [Google Scholar] [CrossRef]
17. Ghasemlou M, Daver F, Ivanova EP, Adhikari B. Polyurethanes from seed oil-based polyols: a review of synthesis, mechanical and thermal properties. Ind Crops Prod. 2019;142(115):111841. doi:10.1016/j.indcrop.2019.111841. [Google Scholar] [CrossRef]
18. Kaikade DS, Sabnis AS. Polyurethane foams from vegetable oil-based polyols: a review. Polym Bull. 2022;80(3):2239–61. doi:10.1007/s00289-022-04155-9. [Google Scholar] [PubMed] [CrossRef]
19. Garrison TF, Kessler MR, Larock RC. Effects of unsaturation and different ring-opening methods on the properties of vegetable oil-based polyurethane coatings. Polymer. 2014;55(4):1004–11. doi:10.1016/j.polymer.2014.01.014. [Google Scholar] [CrossRef]
20. Xu Q, Lin J, Jiang G. Synthesis, characterization and properties of soybean oil-based polyurethane. Polymers. 2022;14(11):2201. doi:10.3390/polym14112201. [Google Scholar] [PubMed] [CrossRef]
21. Polaczek K, Kurańska M. Hemp seed oil and oilseed radish oil as new sources of raw materials for the synthesis of bio-polyols for open-cell polyurethane foams. Materials. 2022;15(24):8891. doi:10.3390/ma15248891. [Google Scholar] [PubMed] [CrossRef]
22. Liang H, Feng Y, Lu J, Liu L, Yang Z, Luo Y, et al. Bio-based cationic waterborne polyurethanes dispersions prepared from different vegetable oils. Ind Crops Prod. 2018;122(6):448–55. doi:10.1016/j.indcrop.2018.06.006. [Google Scholar] [CrossRef]
23. Hong J, Radojčić D, Yang XQ, Wan X, Petrović ZS. Tough thermosetting polyurethanes and adhesives from rubber seed oil by hydroformylation. J Appl Polym Sci. 2020;137(13):48509. doi:10.1002/app.48509. [Google Scholar] [CrossRef]
24. Kurańska M, Benes H, Kockova O, Kucała M, Malewska E, Schmidt B, et al. Rebiopolyols—new components for the synthesis of polyurethane biofoams in line with the circular economy concept. Chem Eng J. 2024;490:151504. doi:10.1016/j.cej.2024.151504. [Google Scholar] [CrossRef]
25. Malewska E, Kurańska M, Tenczyńska M, Prociak A. Application of modified seed oils of selected fruits in the synthesis of polyurethane thermal insulating materials. Materials. 2023;17(1):158. doi:10.3390/ma17010158. [Google Scholar] [PubMed] [CrossRef]
26. Furtwengler P, Avérous L. Renewable polyols for advanced polyurethane foams from diverse biomass resources. Polym Chem. 2018;9(32):4258–87. doi:10.1039/C8PY00827B. [Google Scholar] [CrossRef]
27. Hu Y, Tian Y, Cheng J, Zhang J. Synthesis of eugenol-based polyols via Thiol-Ene click reaction and high-performance thermosetting polyurethane therefrom. ACS Sustain Chem Eng. 2020;8(10):4158–66. doi:10.1021/acssuschemeng.9b06867. [Google Scholar] [CrossRef]
28. Malani RS, Malshe VC, Thorat BN. Polyols and polyurethanes from renewable sources: past, present and future—part 1: vegetable oils and lignocellulosic biomass. J Coat Technol Res. 2021;19(1):1–22. doi:10.1007/s11998-021-00490-0. [Google Scholar] [CrossRef]
29. Pattnaik F, Tripathi S, Patra BR, Nanda S, Naik S. Catalytic conversion of lignocellulosic polysaccharides to commodity biochemicals: a review. Environ Chem Lett. 2021;19(6):4119–36. doi:10.1007/s10311-021-01284-x. [Google Scholar] [CrossRef]
30. Campana F, Brufani G, Mauriello F, Luque R, Vaccaro L. Green polyurethanes from bio-based building blocks: recent advances and applications. Green Synth Catal. 2024;3:1557. doi:10.1016/j.gresc.2024.08.001. [Google Scholar] [CrossRef]
31. Li C, An X, Ren Q, Liu L, Long Y, Zhang H, et al. Nanogrinding/ethanol activation facilitating lignin fractionation for preparation of monodispersed lignin nanoparticles. Int J Biol Macromol. 2022;227(8):608–18. doi:10.1016/j.ijbiomac.2022.12.051. [Google Scholar] [PubMed] [CrossRef]
32. Zhang Z, Chen Y, Wang D, Yu D, Wu C. Lignin-based adsorbents for heavy metals. Ind Crops Prod. 2023;193:116119. doi:10.1016/j.indcrop.2022.116119. [Google Scholar] [CrossRef]
33. Chen Y, Zhang H, Zhu Z, Fu S. High-value utilization of hydroxymethylated lignin in polyurethane adhesives. Int J Biol Macromol. 2020;152(4):775–85. doi:10.1016/j.ijbiomac.2020.02.321. [Google Scholar] [PubMed] [CrossRef]
34. Xue Y, Xu Q, Jiang G, Teng D. Preparation and properties of lignin-based polyurethane materials. Arab J Chem. 2024;17(1):105471. doi:10.1016/j.arabjc.2023.105471. [Google Scholar] [CrossRef]
35. Chen Y, Fu S, Zhang H. Signally improvement of polyurethane adhesive with hydroxy-enriched lignin from bagasse. Colloids Surf A Physicochem Eng Asp. 2020;585(7):124164. doi:10.1016/j.colsurfa.2019.124164. [Google Scholar] [CrossRef]
36. Zhen X, Li H, Xu Z, Wang Q, Xu J, Zhu S, et al. Demethylation, phenolation, and depolymerization of lignin for the synthesis of lignin-based epoxy resin via a one-pot strategy. Ind Crops Prod. 2021;173(8):114135. doi:10.1016/j.indcrop.2021.114135. [Google Scholar] [CrossRef]
37. Falireas PG, Gracia-Vitoria J, Hensen A, Vanbroekhoven K, Vendamme R. Incorporating phenolated lignin into polyurethane materials: impact on mechanical, thermal, and adhesion performance. Ind Eng Chem Res. 2024;63(9):3921–35. doi:10.1021/acs.iecr.3c03453. [Google Scholar] [CrossRef]
38. Brandi F, Khalil I, Antonietti M, Al-Naji M. Continuous-flow production of isosorbide from aqueous-cellulosic derivable feed over sustainable heterogeneous catalysts. ACS Sustain Chem Eng. 2021;9(2):927–35. doi:10.1021/acssuschemeng.0c08167. [Google Scholar] [CrossRef]
39. Kaikade DS, Sabnis AS. A durable and multifunctional antifog, antifrost, and self-cleaning/oil repellant UV curable coating based on poly (sorbitol-co-isophorone-co-ethyleneglycol) acrylate. J Coat Technol Res. 2024;21(3):955–67. doi:10.1007/s11998-023-00863-7. [Google Scholar] [CrossRef]
40. Lakatos C, Kordován MÁ, Czifrák K, Nagy L, Vadkerti B, Daróczi L, et al. Synthesis of sucrose-HDI cooligomers: new polyols for novel polyurethane networks. Int J Mol Sci. 2022;23(3):1444. doi:10.3390/ijms23031444. [Google Scholar] [PubMed] [CrossRef]
41. Ladero M, de Gracia M, Trujillo F, Garcia-Ochoa F. Phenomenological kinetic modelling of the esterification of rosin and polyols. Chem Eng J. 2012;197(1):387–97. doi:10.1016/j.cej.2012.05.053. [Google Scholar] [CrossRef]
42. Li J, Yang W, Ning Z, Yang B, Zeng Y. Sustainable polyurethane networks based on rosin with reprocessing performance. Polymers. 2021;13(20):3538. doi:10.3390/polym13203538. [Google Scholar] [PubMed] [CrossRef]
43. Guo Z, Ding X, Wang Y. How to get isocyanate? ACS Omega. 2024;9(10):11168–80. doi:10.1021/acsomega.3c10069. [Google Scholar] [PubMed] [CrossRef]
44. O’Dea RM, Nandi M, Kroll G, Arnold JR, Korley LT, Epps IIITH, et al. Toward circular recycling of polyurethanes: depolymerization and recovery of isocyanates. JACS Au. 2024;4(4):1471–9. doi:10.1021/jacsau.4c00013. [Google Scholar] [PubMed] [CrossRef]
45. Brzoska J, Smorawska J, Głowińska E, Datta J. A green route for high-performance bio-based polyurethanes synthesized from modified bio-based isocyanates. Ind Crops Prod. 2024;P1:119542. doi:10.1016/j.indcrop.2024.119542. [Google Scholar] [CrossRef]
46. Catalá J, Guerra I, García-Vargas JM, Ramos MJ, García MT, Rodríguez JF, et al. Tailor-made bio-based non-isocyanate polyurethanes (NIPUs). Polymers. 2023;15(6):1589. doi:10.3390/polym15061589. [Google Scholar] [PubMed] [CrossRef]
47. Ghasemlou M, Daver F, Ivanova EP, Adhikari B. Bio-based routes to synthesize cyclic carbonates and polyamines precursors of non-isocyanate polyurethanes: a review. Eur Polym J. 2019;118(1):668–84. doi:10.1016/j.eurpolymj.2019.06.032. [Google Scholar] [CrossRef]
48. Gomez-Lopez A, Elizalde F, Calvo I, Sardon H. Trends in non-isocyanate polyurethane (NIPU) development. Chem Commun. 2021;57(92):12236–53. doi:10.1039/D1CC05009E. [Google Scholar] [PubMed] [CrossRef]
49. Garipov RM, Sysoev VA, Mikheev VV, Zagidullin AI, Deberdeev RY, Irzhak VI, Berlin AA. Reactivity of cyclocarbonate groups in modified epoxy-amine compositions. Dokl Phys Chem. 2003;393:61–4. doi:10.1023/B:DOPC.0000003463.07883.c9. [Google Scholar] [CrossRef]
50. Błażek K, Datta J. Renewable natural resources as green alternative substrates to obtain bio-based non-isocyanate polyurethanes-review. Crit Rev Environ Sci Technol. 2019;49(3):173–211. doi:10.1080/10643389.2018.1537741. [Google Scholar] [CrossRef]
51. Centeno-Pedrazo A, Perez-Arce J, Freixa Z, Ortiz P, Garcia-Suarez EJ. Non-isocyanate polyurethanes derived from carbonated soybean oil: synthesis, characterization and comparison with traditional vegetable oil-based polyurethanes. Prog Org Coatings. 2024;197(1):108830. doi:10.1016/j.porgcoat.2024.108830. [Google Scholar] [CrossRef]
52. Rayung M, Abd Ghani N, Hasanudin N. A review on vegetable oil-based non isocyanate polyurethane: towards a greener and sustainable production route. RSC Adv. 2024;14(13):9273–99. doi:10.1039/D3RA08684D. [Google Scholar] [PubMed] [CrossRef]
53. Zhang W, Wang T, Zheng Z, Quirino RL, Xie F, Li Y, et al. Plant oil-based non-isocyanate waterborne poly(hydroxyl urethane)s. Chem Eng J. 2023;452(1):138965. doi:10.1016/j.cej.2022.138965. [Google Scholar] [CrossRef]
54. Haniffa MACM, Ching YC, Chuah CH, Kuan YC, Liu DS, Liou NS. Synthesis, characterization and the solvent effects on interfacial phenomena of Jatropha Curcas oil based non-isocyanate polyurethane. Polymers. 2017;9(5):162. doi:10.3390/polym9050162. [Google Scholar] [PubMed] [CrossRef]
55. Haniffa MACM, Munawar K, Ching YC, Hazlee AI, Chuah CH. Bio-based poly(hydroxy urethane)s: synthesis and pre/post-functionalization. Chem Asian J. 2021;16(11):1281–97. doi:10.1002/asia.202100226. [Google Scholar] [PubMed] [CrossRef]
56. Stemmelen M, Pessel F, Lapinte V, Caillol S, Habas JP, Robin JJ. A fully biobased epoxy resin from vegetable oils: from the synthesis of the precursors by thiol-ene reaction to the study of the final material. J Polym Sci A Polym Chem. 2011;49(11):2434–44. doi:10.1002/pola.24674. [Google Scholar] [CrossRef]
57. Pei Z, Liu X, Chen J, Wang H, Li H. Research progress on lignin depolymerization strategies: a review. Polymers. 2024;16(17):2388. doi:10.3390/polym16172388. [Google Scholar] [PubMed] [CrossRef]
58. Saražin J, Pizzi A, Amirou S, Schmiedl D, Šernek M. Organosolv lignin for non-isocyanate based polyurethanes (NIPU) as wood adhesive. J Renew Mater. 2021;9(5):881–907. doi:10.32604/jrm.2021.015047. [Google Scholar] [CrossRef]
59. Quinsaat JEQ, Feghali E, Pas DJVD. Preparation of biobased nonisocyanate polyurethane/epoxy thermoset materials using depolymerized native lignin. Biomacromolecules. 2022;23(11):4562–73. doi:10.1021/acs.biomac.2c00706. [Google Scholar] [PubMed] [CrossRef]
60. Froidevaux V, Negrell C, Caillol S, Pascault JP, Boutevin B. Biobased amines: from synthesis to polymers; present and future. Chem Rev. 2016;116(22):14181–224. doi:10.1021/acs.chemrev.6b00486. [Google Scholar] [PubMed] [CrossRef]
61. Dou L, Xue B, Zhao Q, Wang W, Li X, Wen J, et al. Green synthesis of lignin-based non-isocyanate polyurethanes as reusable, self-healable and removable adhesives. Eur Polym J. 2024;221(1):113553. doi:10.1016/j.eurpolymj.2024.113553. [Google Scholar] [CrossRef]
62. Meng X, Zhang S, Scheidemantle B, Wang YY, Pu Y, Wyman CE, et al. Preparation and characterization of aminated co-solvent enhanced lignocellulosic fractionation lignin as a renewable building block for the synthesis of non-isocyanate polyurethanes. Ind Crops Prod. 2022;178(1):114579. doi:10.1016/j.indcrop.2022.114579. [Google Scholar] [CrossRef]
63. Sun J, Huang A, Luo S, Shi M, Song J, Luo H. Effect of chain extender on morphologies and properties of PBAT/PLA composites. J Thermoplast Compos Mater. 2023;36(3):1175–86. doi:10.1177/08927057211051415. [Google Scholar] [CrossRef]
64. Das A, Aman N, Ahmad SP, Yadav B, Jagavelu K, Kumar A. Ameliorating impaired cardiac function in myocardial infarction using exosome-loaded gallic-acid-containing polyurethane scaffolds. Bioact Mater. 2024;33(1):324–40. doi:10.1016/j.bioactmat.2023.11.009. [Google Scholar] [PubMed] [CrossRef]
65. Zhao W, Nolan B, Bermudez H, Hsu SL, Choudhary U, van Walsem J. Spectroscopic study of the morphology development of closed-cell polyurethane foam using bio-based malonic acid as chain extender. Polymer. 2020;197(1):122344. doi:10.1016/j.polymer.2020.122344. [Google Scholar] [CrossRef]
66. Gnanasekar P, Chen H, Luo Q, Tanguy N, Li LC, Chen J, et al. Mechanically robust, degradable, catalyst-free fully bio-based shape memory polyurethane: influence of a novel vanillin-alaninol chain extender. ACS Sustain Chem Eng. 2022;10(16):5203–11. doi:10.1021/acssuschemeng.2c00053. [Google Scholar] [CrossRef]
67. Guo X, Zhang K, Dong Y, Qin J, Xiang Y, Zhu H. Lignocellulosic biomass-derived polyurethane elastomer with high toughness and excellent crack tolerance. J Mater Chem A. 2024;12(32):20967–74. doi:10.1039/D4TA02029D. [Google Scholar] [CrossRef]
68. Kim H, Lee S, Ahn Y, Lee J, Won W. Sustainable production of bioplastics from lignocellulosic biomass: technoeconomic analysis and life-cycle assessment. ACS Sustain Chem Eng. 2020;8(33):12419–29. doi:10.1021/acssuschemeng.0c02872. [Google Scholar] [CrossRef]
69. Mangal M, Supriya H, Bose S, Banerjee T. Innovations in applications and prospects of non-isocyanate polyurethane bioplastics. Biopolymers. 2023;114(12):e23568. doi:10.1002/bip.23568. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools