 Open Access
Open Access
ARTICLE
Synthesis of Cyclohexyl Acrylate Derivative and Its Evaluation as a Plasticizer for PVC
1 Guangdong Provincial Key Laboratory of Silviculture, Protection and Utilization, Guangdong Academy of Forestry, Guangzhou, 510520, China
2 College of Chemistry and Chemical Engineering, Zhongkai University of Agriculture and Engineering, Guangzhou, 510225, China
* Corresponding Author: Lei Zeng. Email:
# These authors contributed equally to this work
Journal of Polymer Materials 2025, 42(2), 449-462. https://doi.org/10.32604/jpm.2025.066668
Received 14 April 2025; Accepted 23 June 2025; Issue published 14 July 2025
Abstract
In this study, 3,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate (ECC) and refined Camellia oleifera seed oil (RCOSO) obtained from saponification and acidified hydrolysis has been used as raw materials to perform ring-opening reactions for the preparation of cyclohexyl acrylate derivative (CAR). The structure of the synthesized product was characterized using infrared spectroscopy, Raman spectroscopy, and nuclear magnetic resonance spectroscopy. CAR was employed as a plasticizer to produce modified polyvinyl chloride (PVC) films, dioctyl phthalate plasticizer (DOP) used in reference samples to investigate the influence of different plasticizer molecular structures on the properties of PVC films. Observation suggested that (1) CAR plasticizer was successfully synthesized; (2) CAR can interact with PVC, exhibiting good compatibility; (3) PVC films contain CAR showed improved thermal stability, hydrophilicity, and tensile strength. Therefore, CAR has the potential to replace DOP as a plasticizer for PVC.Keywords
PVC is a versatile thermoplastic material. Its production has favorable features such as wide availability of raw materials, mature technology, and affordability [1]. PVC is one of the most widely consumed polymers globally, but pure PVC is glassy at room temperature, lacking in processability and performance [2]. The addition of plasticizers can impart excellent flexibility and other unmatched properties, making PVC suitable for a wide range of applications in various fields [3].
Traditional plasticizers refer to chemicals produced from petroleum-based raw materials [4]. The most common traditional plasticizers are phthalates, of which dioctyl phthalate (DOP) and dibutyl phthalate (DBP), accounting for 80% of the plasticizer market [5]. However, these plasticizers have inherent drawbacks and pose potential risks to human health and the environment [6]. Phthalates are endocrine disruptors that influence human development, affecting fetuses, newborns, and children [7,8]. The development of non-toxic and environmentally friendly bio-based plasticizers has become a trend. The epoxidized or esterified products of triglycerides (such as epoxidized soybean oil and acetylated monoglycerides), featuring tunable polar groups, have become a research focus. By combining the sustainability of biomonomers with the performance-tunability of synthetic monomers [9–12].
Camellia oleifera Abel. is a woody oil species found worldwide and represents a perennial evergreen small tree of the Theacea [13,14]. As an oil-rich seed tree, Camellia oleifera has great economic value, nutritional and medicinal value, and its seed oil contains rich natural bioactive ingredients such as unsaturated fatty acids [15]. Camellia oleifera seed oil has good stability, high nutritional value, good antioxidant properties, and is easily digested and absorbed by the human body. Its fatty acid composition is very similar to olive oil, mainly oleic acid, which accounts for 75% to 80% of the fatty acid residence present (oleic acid: 72%–80%, linoleic acid: 8%–12%, and palmitic acid: 7%–10%) [16–18].
3,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate (ECC), refined Camellia oleifera seed oil (RCOSO) obtained by saponification and acidified hydrolysis have been added as raw materials for the preparation cyclohexyl acrylate derivative (CAR) plasticizer. The structure of CAR was characterized, and the synthesized target product was confirmed. CAR was then used as a plasticizer to prepare modified polyvinyl chloride (PVC) films. As a representative of phthalate plasticizers, dioctyl phthalate (DOP) was used for the preparation of films for comparison. The promise of using Camellia oleifera seed oil for the preparation of bio-based plasticizers has been demonstrated. The potential value of byproducts generated during the production process of Camellia oleifera seed oil was illustrated. It represents technology for recycling and further processing of these byproducts. The differences in various properties of PVC films plasticized with either CAR or DOP have been examined. The potential of CAR as a replacement for DOP has been established.
Organic Camellia oleifera seed oil (edible oil, Guangdong Huabao Agricultural Technology Co., Ltd., Guangzhou, China); Ethanol anhydrous (AR, Shanghai Titan Technology Co., Ltd., Shanghai, China); Potassium hydroxide (AR, Shanghai Maclin Biochemical Technology Co., Ltd., Shanghai, China); Hydrochloric acid (AR, Guangzhou Chemical Reagent Factory, Guangzhou, China); 3,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate (97%, Aladdin Reagent Co., Ltd., Shanghai, China); Benzyltriethylammonium chloride (AR, Shanghai Maclin Biochemical Technology Co., Ltd., Shanghai, China); Polyvinyl chloride (K-value 68-65, Aladdin Reagent Co., Ltd., Shanghai, China); Tetrahydrofuran (AR, China National Pharmaceutical Group Chemical Reagent Co., Ltd., Shanghai, China); Dioctyl phthalate (AR, Aladdin Reagent Co., Ltd., Shanghai, China).
2.2 Preparation of Refined Camellia Oleifera Seed Oil (RCOSO)
26.4 g of Potassium hydroxide was dissolved in 100 g of Ethanol anhydrous. To this solution, 100 g of organic Camellia oleifera seed oil was added, and the reaction was carried out at 60°C for 2 h. Then, 140 mL of 10% Hydrochloric acid solution was added, and the mixture was allowed to separate into layers. The upper clear liquid was collected and subjected to rotary evaporation to remove alcohol and water. The resulting material was dried at 110°C for 2 h to obtain refined Camellia oleifera seed oil (RCOSO). The product was sealed and stored away from light for later use.
2.3 Preparation of Cyclohexyl Acrylate Derivative (CAR)
5 g of 3,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate (ECC), 8.96 g of RCOSO, and 0.045 g of the catalyst benzyltriethylammonium chloride were added to a three-necked flask with a thermometer. The mixture was reacted at 115°C for 2 h. This resulted in the formation of cyclohexyl acrylate derivative (CAR) product. The obtained product was sealed and stored away from light for later use. The preparation reaction is illustrated in Fig. 1. Under the catalysis of TEBAC, the epoxy groups on ECC undergo ring-opening esterification reaction with the carboxyl groups on ROSOCO at 115°C, generating a CAR plasticizer with hydroxyl and ester groups.

Figure 1: Preparation of CAR
2.4 Formulation of Cyclohexyl Acrylate Derivative (CAR) Plasticizer for PVC Modification
In this experiment, different ratios of CAR were used to modify PVC films according to the experimental formulations listed in Table 1. For comparison, plastic films were also prepared with Dioctyl phthalate (DOP), a commonly used plasticizer for PVC. Accurately measured amounts of Polyvinyl chloride powder, CAR, and DOP were added to a beaker along with 50 mL of Tetrahydrofuran (THF) solution. The reaction mixture was stirred and reacted at 38°C for 22 min. The solution from the beaker was poured into a glass petri dish and placed in a forced air-drying oven at 40°C for 24 h. This allowed the solvent to evaporate completely, resulting in the formation of plasticized PVC film.

2.5.1 Fourier Transform Infrared Spectroscopy Analysis (FT-IR)
The structural analysis of the synthesized plasticizer CAR was conducted within the infrared wavenumber range of 4000–500 cm−1.
2.5.2 Raman Spectroscopy Analysis (Raman)
Raman spectroscopy analysis was performed on the CAR and various plasticized PVC films samples, covering the range of 400–3600 cm−1.
2.5.3 Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis
Using deuterated chloroform (CDCl3) as a solvent, the 1H NMR and 13C NMR spectra of CAR were obtained using 300 MHz and 100.61 MHz nuclear magnetic resonance spectrometers, respectively.
2.5.4 Scanning Electron Microscopy (SEM) Analysis
The cross-sectional surface of PVC film samples was fixed onto the electron microscope stage and coated with a thin layer of gold using an ion sputter coater. Scanning electron microscopy utilized an electron beam (approximately 50 μm) to bombard the sample, collecting backscattered electrons and secondary electrons to observe and analyze the surface morphology of the sample.
2.5.5 Energy Dispersive X-Ray Spectroscopy (EDS) Analysis
The elemental composition and content of the cross-sectional surface of PVC film samples were tested using X-ray energy dispersive spectroscopy (EDS) with the X-ACT system.
2.5.6 Atomic Force Microscopy (AFM) Analysis
Clean PVC film samples were cut to an appropriate size and adhered to the sample stage. Atomic force microscopy was employed to scan the samples in tapping mode, observing the surface roughness of the samples.
2.5.7 Differential Scanning Calorimetry (DSC) Analysis
Using nitrogen as the carrier gas at a flow rate of 20 mL/min, the DSC analysis covered a temperature range of −15°C to 100°C with a heating rate of 10°C/min. The sample mass was 5–8 mg, and the analysis aimed to determine the thermal behavior of the PVC film samples.
2.5.8 Thermogravimetric (TG) Analysis
Using nitrogen as the carrier gas at a flow rate of 20 mL/min, the thermogravimetric analysis covered a temperature range of 30°C to 600°C with a heating rate of 10°C/min. The sample mass was 1–3 mg, and the analysis aimed to study the thermal degradation behavior of the PVC film samples.
2.5.9 Contact Angle Measurement
The test liquid was ultra-pure water, measuring the contact Angle of PVC film samples.
2.5.10 Mechanical Property Testing
Test samples were cut from PVC film using a 4 mm × 50 mm dumbbell-shaped cutting tool. The samples were subjected to a stretching rate of 10 mm/min, and the test length and thickness were measured and recorded for each sample in triplicate.
From Fig. 2, it can be observed that both CAR plasticizer and DOP plasticizer exhibit structurally similar features, including cyclic groups, ester groups, and long carbon chains. It is precisely these functional groups that enable them to possess good compatibility with PVC [19,20]. The CAR molecule contains two hydroxyl groups, which can form hydrogen bonds with the chlorine atoms in the PVC molecule, and the higher mass—specific carbonyl density can also establish hydrogen bonds with PVC. The plasticization mode of action of CAR can be summarized as follows: After the addition of cyclohexyl ester to PVC, its polar functional groups, including ester and hydroxyl groups, interact hydrogen bond with the chlorine atoms in PVC, facilitating good miscibility. This interaction disrupts the strong intermolecular forces between PVC molecules, weakening the forces along the polymer chains, thus promoting chain mobility and enhancing the plasticization effect.
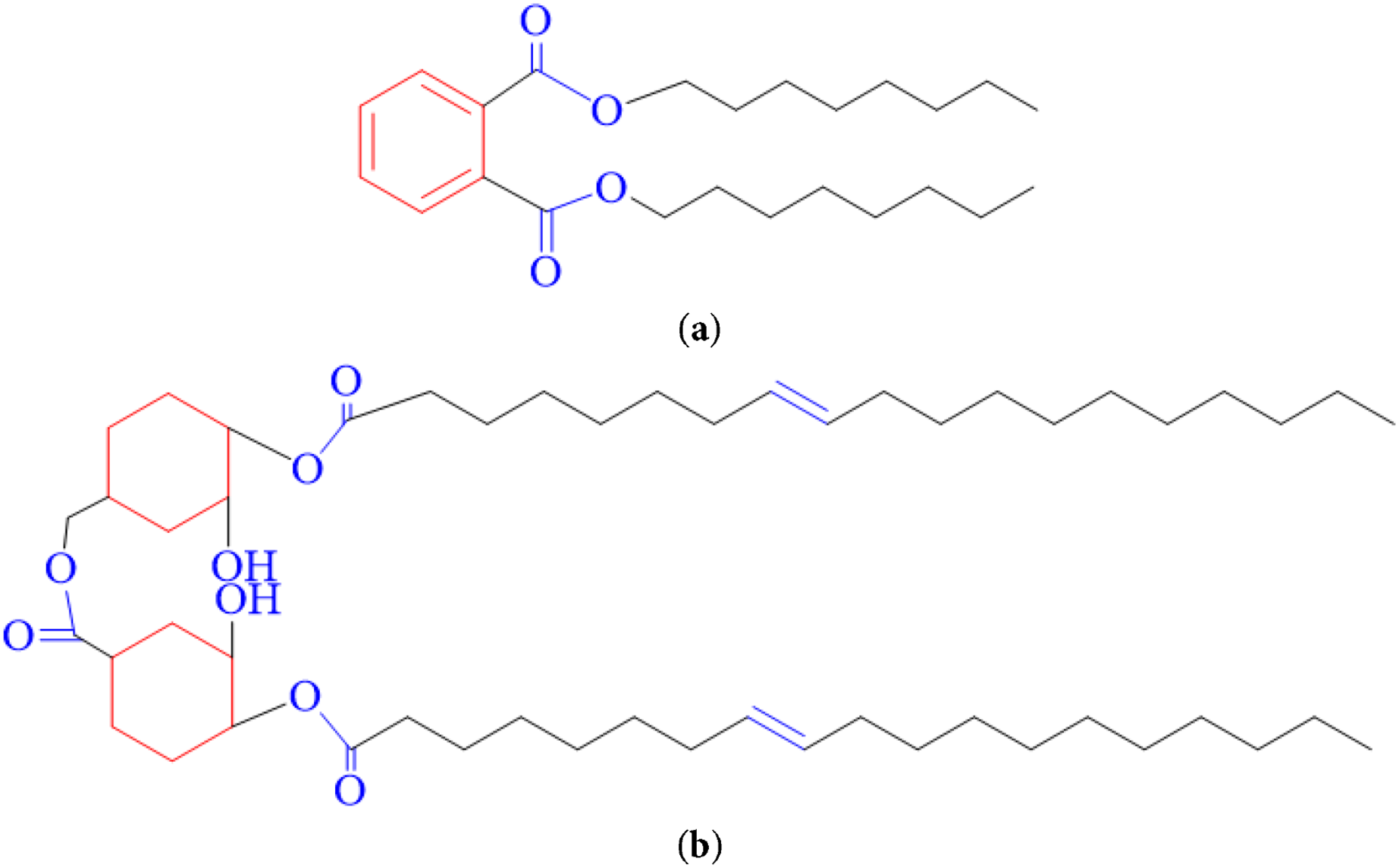
Figure 2: Structural formula for DOP and CAR: (a) DOP; (b) CAR
3.1 Structural Characterization of CAR
Fig. 3I presents the infrared spectra of RCOSO, and CAR. From the infrared curves, it can be observed that the structure of ECC contains epoxy groups, as evidenced by the strong stretching vibration peak at 1170 cm−1. However, this peak is absent in CAR, implying the participation of its epoxy groups in the ring-opening reaction, resulting in the formation of new functional groups. The hydroxyl group stretching vibration absorption peak at 3460 cm−1 and the ester C=O stretching vibration peak at 1730 cm−1 in CAR indicate the presence of ester and hydroxyl groups. RCOSO exhibits a distinct carboxyl characteristic absorption peak at 1700 cm−1, whereas in CAR, the carboxyl C=O absorption peak disappears, and a new ester C=O absorption peak emerges at 1730 cm−1. Based on the above analysis, it can be inferred that the carboxyl group of RCOSO has undergone a ring-opening reaction with the epoxy group of ECC, resulting in the formation of CAR.

Figure 3: (I) FT-IR spectra of CAR, RCOSO, ECC; (II) Raman spectra of CAR, RCOSO, ECC; (III) 13C NMR of ECC, CAR; (IV) 1H NMR of ECC, CAR
In Fig. 3II, the Raman spectra of ECC, RCOSO, and CAR are shown. It can be seen from the Raman curve of ECC that the signal peak at 787 cm−1 belongs to the characteristic peak of the epoxy group. while Raman curve of CAR lacks this signal peak. This indicates that the epoxy groups in ECC have undergone a ring-opening reaction, resulting in the appearance of the C=O stretching Raman peak at 2914 cm−1 in the synthesized product. Raman curve of RCOSO features a signal peak at 1656 cm−1 attributed to C=C bonds, and the same peak is observed in the Raman curve of CAR, indicating the reaction between refined tea seed oil and ECC to generate CAR.
Fig. 3III,IV represents the 13C NMR and 1H NMR spectra of ECC and CAR, respectively. The 0 ppm peak is the solvent peak.
In the 13C NMR spectrum of ECC, the peaks at 66.00 ppm and 51.70 ppm correspond to the carbon atoms of the epoxy group’s 1st and 2nd positions, respectively. These signals shift to 77.50 ppm and 65.90 ppm in CAR. Furthermore, characteristic peaks of ester groups, double bonds, and methyl groups appear at 173.10 ppm, 130.60 ppm, and 14.00 ppm, respectively.
In the 1H NMR spectrum of ECC, the peak at 3.24 ppm corresponds to the proton signal of the 1st carbon atom in the epoxy group. In the 1H NMR spectrum of CAR, this proton signal disappears, and in its place, the proton signal of the 1st carbon atom, adjacent to the methyl group (-CH-), appears. This change indicates the disruption of the epoxy bond in ECC. In CAR’s 1H NMR spectrum, the peak at 5.34 ppm corresponds to the proton signal of the carbon-carbon double bond (-CH=CH-), while the peak at 3.95 ppm corresponds to the proton signal of the secondary methyl group (≡CH) linked to the hydroxyl group in the six-membered ring. Peaks at 2.35 ppm, 1.99 ppm, and 1.80 ppm are attributed to the proton signals of carbon atoms 4, 6, and 5, respectively. This confirms the successful synthesis of CAR.
3.2 Structure Analysis of Plasticized PVC Samples
Fig. 4I displays the Raman spectra of plasticized PVC film H1, H2, H3, and H4. From Fig. 4, it can be observed that all films exhibit the ester characteristic peak at 2940 cm−1. Additionally, the signals of PVC samples plasticized with various levels of CAR are stronger than those of PVC films plasticized with DOP. In the curves of H2, H3, and H4, a signal peak at 1653 cm−1 corresponds to the C=C bond, which is absent in the DOP structure, hence no such signal peak is seen. In summary, these Raman spectroscopy results align with expectations and are consistent with the CAR Raman test outcomes.
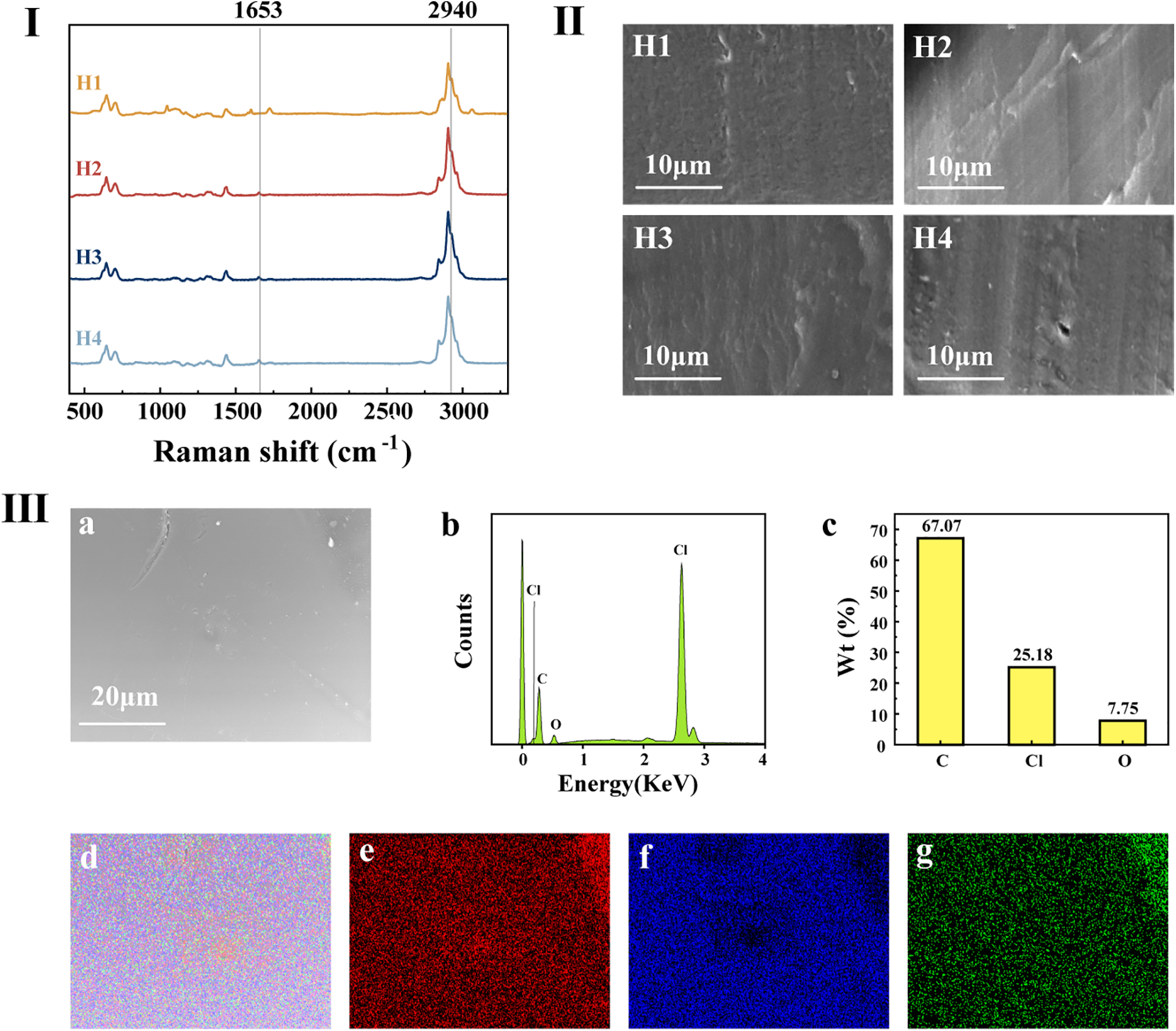
Figure 4: (I) Raman spectra of H1, H2, H3, H4; (II) SEM photographs of H1, H2, H3, H4; (III) EDS photographs of H4, (a) Surface micrograph of H4, (b) EDS spectra of H4, (c) Content histogram of C, Cl and O elements, (d) Distribution of C, Cl and O elements of H4, (e) Distribution of C element of H4, (f) Distribution of Cl element of H4, (g) Distribution of O element of H4
Fig. 4II illustrates the scanning electron microscope (SEM) images of the cross-sections of plasticized PVC films H1, H2, H3, and H4. All of them exhibit rough surface morphology. As the amount of CAR added gradually increases, the roughness becomes more pronounced. The distribution and compatibility of CAR with the PVC matrix appear favorable.
Fig. 4III presents the energy-dispersive X-ray spectroscopy (EDS) results of plasticized PVC film H4. From panels (b) and (c), it can be observed that the mass percentages of the three elements in CAR—C, Cl, and O, are 67.07%, 25.18%, and 7.75%, respectively. Both PVC and CAR contain a substantial amount of carbon (C) elements, while chlorine (Cl) is found in PVC, and oxygen (O) is present in CAR. As CAR is added in lower quantities compared to PVC, the EDS analysis results align with expectations.
From panels (e) and (g), it is evident that the carbon and oxygen element distribution maps of the plasticized PVC film are brighter in the upper-right region, indicating higher carbon and oxygen concentrations in that area, resulting in stronger signals. Panel (f) demonstrates that chlorine elements are uniformly distributed around the membrane, with a minor concentration at the center. The distribution of carbon, chlorine, and oxygen elements appears relatively uniform, indicating good dispersion and even distribution of components in the PVC film plasticized with the CAR, with only minor instances of aggregation.
Fig. 5 depicts the atomic force microscopy images of plasticized PVC films H1, H2, H3, and H4. The roughness of CAR plasticized PVC films increases with the higher content of CAR, and the roughness coefficient (Rq) and average surface roughness (Ra) of CAR-plasticized PVC films are larger than those of DOP-plasticized films. The order of Ra and Rq values for CAR plasticized PVC films are as follows: H2 (Rq = 6.783 nm, Ra = 4.022 nm) < H3 (Rq = 7.484 nm, Ra = 5.254 nm) < H4 (Rq = 8.703 nm, Ra = 5.852 nm). The average surface roughness of H2 is close to that of H1 (Rq = 5.970 nm, Ra = 4.050 nm).
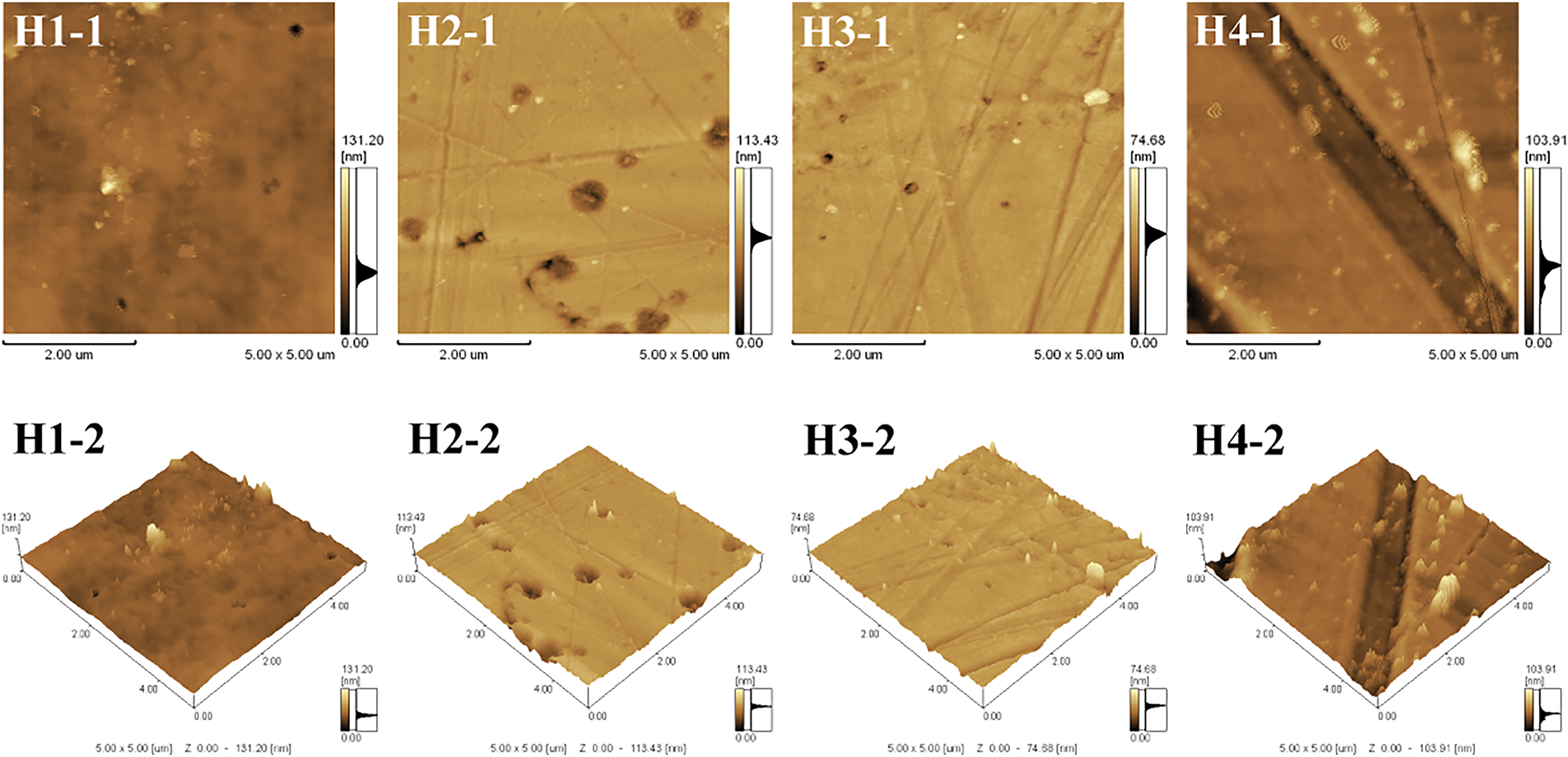
Figure 5: 2D and 3D AFM of plasticized PVC samples. (H1-1), (H2-1), (H3-1), (H4-1) are 2D figures; (H1-2), (H2-2), (H3-2), (H4-2) are 3D images
3.3 Performance Analysis of Plasticized PVC Samples
Fig. 6I presents the DSC curves of plasticized PVC films H1, H2, H3, and H4. Within the temperature range of −15°C to 100°C, all the four formulations of plasticized PVC films exhibited closely similar glass transition temperatures: 63.90°C (H1), 63.93°C (H2), 63.96°C (H3), 63.87°C (H4). This result indicates that CAR-plasticized PVC film possess stability and can be used as a long-term substitute for DOP up to ≤100°C. When cross-referencing DMA testing data from the literature, the glass transition temperature of DOP-based plasticized PVC reported in previous studies ranges from 59°C–69°C. This literature-derived Tg interval aligns well with the closely clustered glass transition temperatures observed in our DSC curves for formulations H1–H4, further validating the thermal consistency between CAR-plasticized systems and traditional DOP counterparts within the tested temperature regime. The congruence between our DSC results and the literature-reported DMA data strengthens the reliability of using CAR as a viable DOP substitute, as both methodologies converge on consistent thermal behavioral characteristics for plasticized PVC matrices [10].
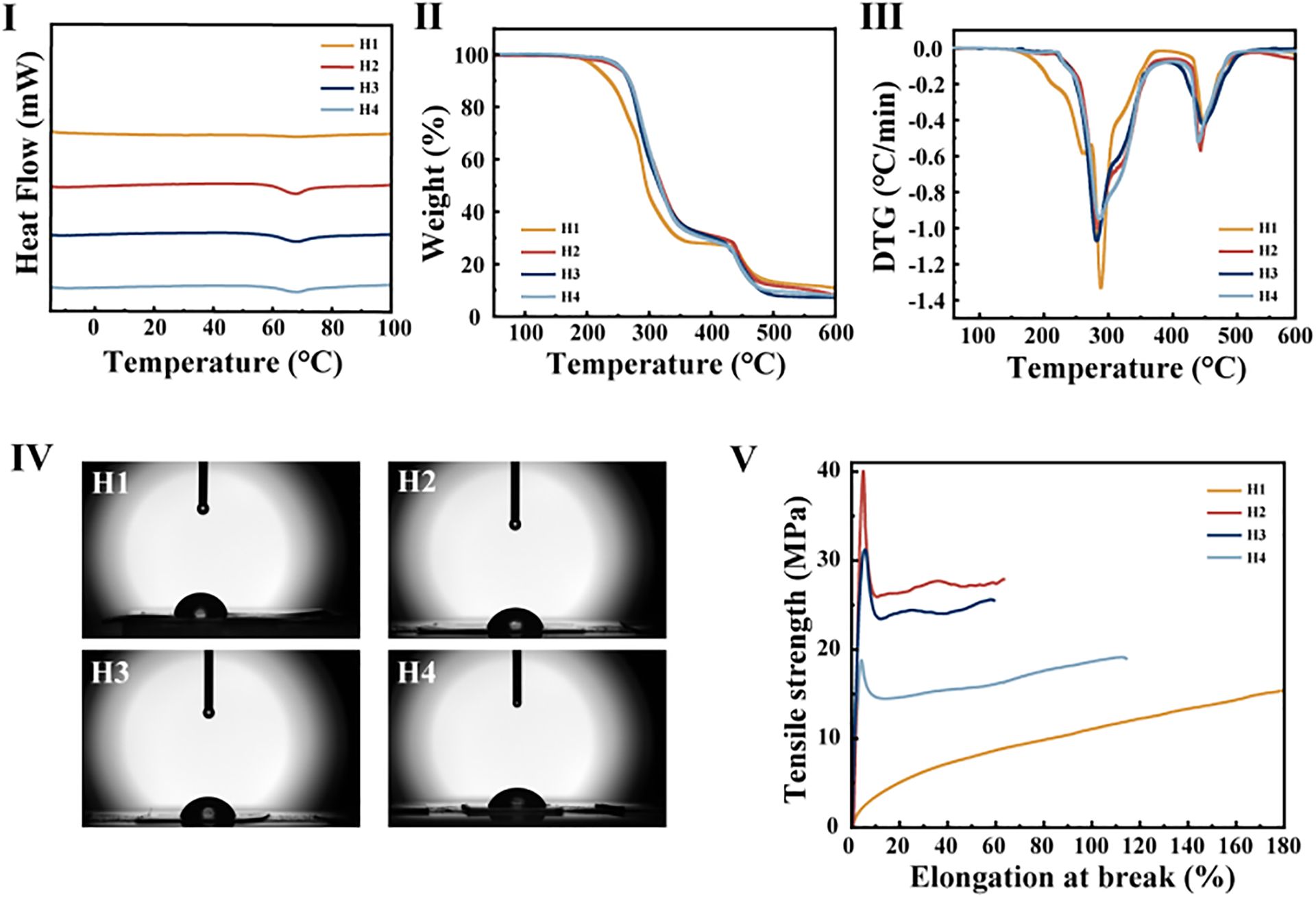
Figure 6: (I) DSC curves of plasticized PVC samples; (II) TGA curves of plasticized PVC samples; (III) DTG curves of plasticized PVC samples; (IV) Contact angle of plasticized PVC samples; (V) Stress-strain curves of plasticized PVC samples
Fig. 6II,III shows the TGA and DTG curves of plasticized PVC films H1, H2, H3, and H4, respectively. Table 2 provides the thermal weight loss data for H1, H2, H3, and H4. According to the TGA and DTG curves, all four PVC samples experienced negligible mass loss until approximately 170°C, which is consistent with the DSC results. The thermal decomposition process of PVC samples mainly comprises three stages. In the first stage, occurring from 190°C to 350°C, mass loss primarily occurs due to the continuous release of HCl gas resulting from the thermal energy imposed on the PVC samples, thereby forming compounds with multiple conjugated double bonds. The second stage, from 350°C to 420°C, involves minimal weight loss and mainly corresponds to the cyclization, benzene ring formation, and polymerization reactions of the conjugated polyenes generated in the first stage. The third stage, with weight loss concentrated between 420°C and 550°C, is predominantly attributed to the decomposition of crosslinked structures of conjugated polyenes and residual char in the PVC samples [21–23]. The TGA curve indicates that, during the second stage, the relative mass of CAR-plasticized PVC films is notably higher than those plasticized with DOP, demonstrating better thermal stability of CAR.

Based on the thermal weight loss data presented in Table 2, it can be concluded that, for the same degree of mass loss, the Ti and T10 values of CAR-plasticized PVC films are higher than those of the DOP comparative samples. The Ti value of CAR-plasticized PVC films is elevated by 35.64°C to 38.99°C compared to DOP-plasticized PVC films. Between 200°C and 350°C, the weight loss rate of CAR-plasticized PVC films is reduced by 3.488% to 6.537% compared to DOP-plasticized PVC films. The DTG curve indicates that, at 288°C, the weight loss rate of DOP plasticized samples exceeds that of all CAR plasticized PVC samples, suggesting that CAR is more effective than DOP in improving the thermal stability of PVC materials [24–27].
In comparison to DOP, CAR-plasticized PVC films exhibit better thermal stability, demonstrating potential as a replacement for DOP in industrial applications. This may be attributed to the presence of carbon-carbon double bonds in CAR, allowing it to undergo addition reactions with HCl produced during the thermal degradation of PVC. Furthermore, due to the abundance of carbonyl and hydroxyl groups in the molecular structure of CAR, its interactions with PVC chains are stronger, collectively inhibiting the continuous degradation of PVC and effectively enhancing the thermal stability of PVC [28,29].
Fig. 6IV illustrates the contact angle schematic of plasticized PVC films H1, H2, H3, and H4. The contact angle test results are as follows: H1 (80.01°), H2 (73.87°), H3 (73.54°), H4 (64.89°). The contact angles of all films are less than 90°, indicating that H1, H2, H3, and H4 are all hydrophilic materials. This is because both CAR and DOP contain hydrophilic functional groups—carbonyl groups [30]. Among them, the water contact angles of CAR-plasticized PVC films are all lower than those of DOP-plasticized PVC films, demonstrating better hydrophilicity. This is due to CAR having two additional hydroxyl groups, and the contact angles follow the trend: H4 < H3 < H2. This indicates that the contact angle of plasticized PVC films is influenced by the addition of CAR and decreases with increasing CAR content.
Fig. 6V presents the stress-strain curves of plasticized PVC films H1, H2, H3, and H4. Table 3 presented are the tensile property test results and standard deviations of four samples (H1–H4). From the stress-strain curves of H2, H3, and H4, it can be observed that with an increase in CAR content, the tensile strength of PVC samples decreases while the fracture elongation increases, indicating CAR’s effective plasticizing ability. This suggests that increasing the CAR content to a certain extent enhances the flexibility of the plasticized PVC. When the CAR content is 30%, PVC film exhibits the highest tensile strength of 38.88 MPa, while the tensile strength of DOP-plasticized PVC films is relatively lower, with a difference of 16.56 MPa between the two. DOP-plasticized PVC films showed the highest elongation at break, reaching 225.89%. The addition of plasticizers interacts with PVC molecular chains on one hand and increases the distance between PVC molecules on the other, weakening intermolecular forces and reducing friction between the PVC chains. This facilitates chain movement, making the PVC material more flexible. CAR, due to its higher molecular weight compared to DOP, and its higher content of ester carbonyl groups, can form numerous hydrogen bonds that impede chain movement. This results in lower elongation at break but better tensile strength in PVC samples plasticized with CAR compared to DOP-plasticized PVC samples [30]. As a biobased plasticizer, CAR is environmentally friendly. It holds potential application value when PVC requires low elongation at break but high tensile strength.

Using Camellia oleifera seed oil as the primary raw material for synthesizing biobased plasticizers highlights the advantages of renewability and non-toxicity while also reducing environmental pollution, embodying the concept of environmental friendliness. By utilizing 3,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate (ECC) and refined Camellia oleifera seed oil (RCOSO) as the main starting materials, a biobased plasticizer called cyclohexyl acrylate derivative (CAR) was synthesized through ring-opening reactions. Characterization tests were employed to identify its structure and confirm the successful synthesis of the target product. To evaluate the performance of the synthesized plasticizer, PVC films were prepared and compared to samples plasticized with DOP. The results of the experiments indicate that PVC films plasticized with CAR exhibit superior thermal stability and hydrophilicity, suggesting the potential for biobased plasticizers to replace traditional ones. Developing a novel, non-toxic, environmentally friendly biobased plasticizer with excellent comprehensive properties based on renewable resources provides a new direction.
Acknowledgement: We sincerely appreciate the financial support from the Scarce and Quality Economic Forest Engineering Technology Research Center (2022GCZX002), the Key Lab. of Biomass Energy and Material, Jiangsu Province (Grant No. JSBEM-S-202305), and the Forestry Scientific Research Monitoring Project of Guangdong Province (2025KJXM01).
Funding Statement: This work was funded by the Scarce and Quality Economic Forest Engineering Technology Research Center (2022GCZX002) and the Key Lab. of Biomass Energy and Material, Jiangsu Province (Grant No. JSBEM-S-202305) and Forestry Scientific Research Monitoring Project of Guangdong Province (2025KJXM01).
Author Contributions: Zhihong Wang: experiment, data analysis, manuscript review and editing; Wei Wei: methodology, manuscript writing; Song Wang: supervision, data analysis; Bingli Zhou: methodology, experiment; Yingle Chen: supervision; Liu Yang: data analysis; Qiaoguang Li: funding acquisition, methodology; Lei Zeng: supervision, funding acquisition. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data will be made available on request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Zhu H, Yang J, Wu M, Wu Q, Liu J, Zhang J. Synthesis of a bio-based plasticizer from vanillic acid and its effects on poly(vinyl chloride). J Appl Polym Sci. 2023;140(2):e53288. doi:10.1002/app.53288. [Google Scholar] [CrossRef]
2. Feng S, Jiang P, Zhang P, Cui Z, Lu M, Chen S, et al. Ricinoleic acid-based plasticizer with excellent optical properties for PVC polymers. Ind Crops Prod. 2023;199(37):116699. doi:10.1016/j.indcrop.2023.116699. [Google Scholar] [CrossRef]
3. Chu HY, Li HB, Sun XY, Zhang YW. Synthesis of biomass based plasticizer from linoleic acid and cardanol for preparing durable PVC material. J Polym Environ. 2022;30(8):3270–8. doi:10.1007/s10924-022-02423-3. [Google Scholar] [CrossRef]
4. Liu D, Jiang P, Nie Z, Wang H, Dai Z, Deng J, et al. Synthesis of an efficient bio-based plasticizer derived from waste cooking oil and its performance testing in PVC. Polym Test. 2020;90:106625. doi:10.1016/j.polymertesting.2020.106625. [Google Scholar] [CrossRef]
5. Ma Y, Song F, Yu J, Wang N, Jia P, Zhou Y. Combining renewable eleostearic acid and eugenol to fabricate sustainable plasticizer and its effect of plasticizing on PVC. J Polym Environ. 2022;30(5):2099–108. doi:10.1007/s10924-021-02341-w. [Google Scholar] [CrossRef]
6. Ledniowska K, Nosal-Kovalenko H, Janik W, Krasuska A, Stańczyk D, Sabura E, et al. Effective, environmentally friendly PVC plasticizers based on succinic acid. Polymers. 2022;14(7):1295. doi:10.3390/polym14071295. [Google Scholar] [PubMed] [CrossRef]
7. Bailey-Hytholt CM, Puranik T, Tripathi A, Shukla A. Investigating interactions of phthalate environmental toxicants with lipid structures. Colloids Surf B Biointerfaces. 2020;190:110923. doi:10.1016/j.colsurfb.2020.110923. [Google Scholar] [PubMed] [CrossRef]
8. Grindler NM, Vanderlinden L, Karthikraj R, Kannan K, Teal S, Polotsky AJ, et al. Exposure to phthalate, an endocrine disrupting chemical, alters the first trimester placental methylome and transcriptome in women. Sci Rep. 2018;8(1):6086. doi:10.1038/s41598-018-24505-w. [Google Scholar] [PubMed] [CrossRef]
9. Xu YJ, Zhang KT, Wang JR, Wang YZ. Biopolymer-based flame retardants and flame-retardant materials. Adv Mater. 2025;37(22):e2414880. doi:10.1002/adma.202414880. [Google Scholar] [PubMed] [CrossRef]
10. Zhang Y, Liu Y, Dong C, Li R, Zhang X, Wang T, et al. Transparent, thermal stable, water resistant and high gas barrier films from cellulose nanocrystals prepared by reactive deep eutectic solvents. Int J Biol Macromol. 2024;276(Pt 2):134107. doi:10.1016/j.ijbiomac.2024.134107. [Google Scholar] [PubMed] [CrossRef]
11. Zhang Y, Tao L, Zhao L, Dong C, Liu Y, Zhang K, et al. Fabrication of flame-retardant and water-resistant nanopapers through electrostatic complexation of phosphorylated cellulose nanofibers and chitin nanocrystals. J Colloid Interface Sci. 2024;676(28):61–71. doi:10.1016/j.jcis.2024.07.111. [Google Scholar] [PubMed] [CrossRef]
12. Chen T, Tang M, Zhao XR, Feng SL, Liu L, Zhou LJ, et al. Antioxidant potential evaluation of polysaccharides from Camellia oleifera Abel in vitro and in vivo. Int J Biol Macromol. 2023;248:125726. doi:10.1016/j.ijbiomac.2023.125726. [Google Scholar] [PubMed] [CrossRef]
13. Zhang M, Wang A, Qin M, Qin X, Yang S, Su S, et al. Direct and indirect somatic embryogenesis induction in Camellia oleifera Abel. Front Plant Sci. 2021;12:644389. doi:10.3389/fpls.2021.644389. [Google Scholar] [PubMed] [CrossRef]
14. Li J, Xiong C, Ruan D, Du W, Li H, Ruan C. Identification of Camellia oleifera WRKY transcription factor genes and functional characterization of CoWRKY78. Front Plant Sci. 2023;14:1110366. doi:10.3389/fpls.2023.1110366. [Google Scholar] [PubMed] [CrossRef]
15. Huang S, Hu Y, Li F, Jin W, Godara V, Wu B. Optimization of mechanical oil extraction process from Camellia oleifera seeds regarding oil yield and energy consumption. J Food Process Eng. 2019;42(6):e13157. doi:10.1111/jfpe.13157. [Google Scholar] [CrossRef]
16. Yao GL, He W, Wu YG, Chen J, Hu XW, Yu J. Purification of angiotensin-I-converting enzyme inhibitory peptides derived from Camellia oleifera Abel seed meal hydrolysate. J Food Qual. 2019;2019(1):7364213. doi:10.1155/2019/7364213. [Google Scholar] [CrossRef]
17. Zhang F, Zhu F, Chen B, Su E, Chen Y, Cao F. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: a review. Food Res Int. 2022;156(2021):111159. doi:10.1016/j.foodres.2022.111159. [Google Scholar] [PubMed] [CrossRef]
18. Jia P, Zhang M, Hu L, Wang R, Sun C, Zhou Y. Cardanol groups grafted on poly(vinyl chloride)—synthesis, performance and plasticization mechanism. Polymers. 2017;9(11):621. doi:10.3390/polym9110621. [Google Scholar] [PubMed] [CrossRef]
19. Jia P, Hu L, Shang Q, Wang R, Zhang M, Zhou Y. Self-plasticization of PVC materials via chemical modification of mannich base of cardanol butyl ether. ACS Sustainable Chem Eng. 2017;5(8):6665–73. doi:10.1021/acssuschemeng.7b00900. [Google Scholar] [CrossRef]
20. Huang J, Li X, Zeng G, Cheng X, Tong H, Wang D. Thermal decomposition mechanisms of poly(vinyl chloridea computational study. Waste Manag. 2018;76:483–96. doi:10.1016/j.wasman.2018.03.033. [Google Scholar] [PubMed] [CrossRef]
21. Ye L, Li T, Hong L. Understanding enhanced char formation in the thermal decomposition of PVC resin: role of intermolecular chlorine loss. Mater Today Commun. 2021;26(10):102186. doi:10.1016/j.mtcomm.2021.102186. [Google Scholar] [CrossRef]
22. Li D, Lei S, Wang P, Zhong L, Ma W, Chen G. Study on the pyrolysis behaviors of mixed waste plastics. Renew Energy. 2021;173:662–74. doi:10.1016/j.renene.2021.04.035. [Google Scholar] [CrossRef]
23. Qian B, Cui B, Wu Z, Wang Y, Tong Q, Yang P, et al. Synthesis, performance evaluation, and plasticization dynamics of a dual-green components flame-retardant plasticizer for poly(vinyl chloride). ACS Appl Polym Mater. 2024;6(19):12007–17. doi:10.1021/acsapm.4c02169. [Google Scholar] [CrossRef]
24. Qian B, Zhu H, Wang P, Peng P, Zhang J, Wu M, et al. Synthesis, characterization and performance evaluation of different alkyl chain lengths flame-retardant plasticizers for poly(vinyl chloride) derived from sustainable vanillic acid. Eur Polym J. 2024;214(1):113154. doi:10.1016/j.eurpolymj.2024.113154. [Google Scholar] [CrossRef]
25. Qian B, Peng P, Wang C, Wang L, Zhang J, Wu M, et al. Synthesis, characterization and performance evaluation of a nontoxic functional plasticizer for poly(vinyl chloride) derived from sustainable lactic acid. J Clean Prod. 2024;450:141895. doi:10.1016/j.jclepro.2024.141895. [Google Scholar] [CrossRef]
26. Qian B, Wang W, Zhu H, Zhang J, Wu M, Liu J, et al. Synthesis, characterization and performance evaluation of a flame retardant plasticizer for poly(vinyl chloride) derived from biobased vanillic acid. Chem Eng J. 2023;476(10):146859. doi:10.1016/j.cej.2023.146859. [Google Scholar] [CrossRef]
27. Amer AR, Shapiro JS. Hydrogen halide-catalyzed thermal decomposition of poly(vinyl chloride). J Macromol Sci Part A Chem. 1980;14(2):185–200. doi:10.1080/00222338008066631. [Google Scholar] [CrossRef]
28. Bei Y, Hu Y, Jia P, Ma Y, Hu F, Xiao T, et al. Bio-based hyperbranched ester plasticizers from woody oil based on cohesive energy via a one-pot synthesis reaction. J Polym Environ. 2024;32(3):1028–38. doi:10.1007/s10924-023-03023-5. [Google Scholar] [CrossRef]
29. Feng G, Hu L, Ma Y, Jia P, Hu Y, Zhang M, et al. An efficient bio-based plasticizer for poly (vinyl chloride) from waste cooking oil and citric acid: synthesis and evaluation in PVC films. J Clean Prod. 2018;189(6):334–43. doi:10.1016/j.jclepro.2018.04.085. [Google Scholar] [CrossRef]
30. Ma Y, Song F, Hu Y, Kong Q, Liu C, Rahman MA, et al. Highly branched and nontoxic plasticizers based on natural cashew shell oil by a facile and sustainable way. J Clean Prod. 2020;252(2):119597. doi:10.1016/j.jclepro.2019.119597. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools