 Open Access
Open Access
ARTICLE
Engineering Amorphous Solid Dispersions of Abiraterone Acetate via HPMC HME: A Polymer-Centric Hot-Melt Extrusion Strategy for Formulation-Driven Bioavailability Improvement
1 Department of Pharmacy, Birla Institute of Technology and Science, Pilani Campus, Pilani, 333031, Rajasthan, India
2 Colorcon Asia Pvt. Ltd. Verna Industrial Estate, Verna, 403722, Goa, India
3 Biophore Group of Companies, Hyderabad, 500033, Telangana, India
4 R&D Healthcare Division Emami Ltd., Kolkata, 700056, West Bengal, India
* Corresponding Author: Gautam Singhvi. Email:
(This article belongs to the Special Issue: Polymer Materials in Controlled Drug Delivery)
Journal of Polymer Materials 2025, 42(4), 1199-1229. https://doi.org/10.32604/jpm.2025.072987
Received 08 September 2025; Accepted 11 December 2025; Issue published 26 December 2025
Abstract
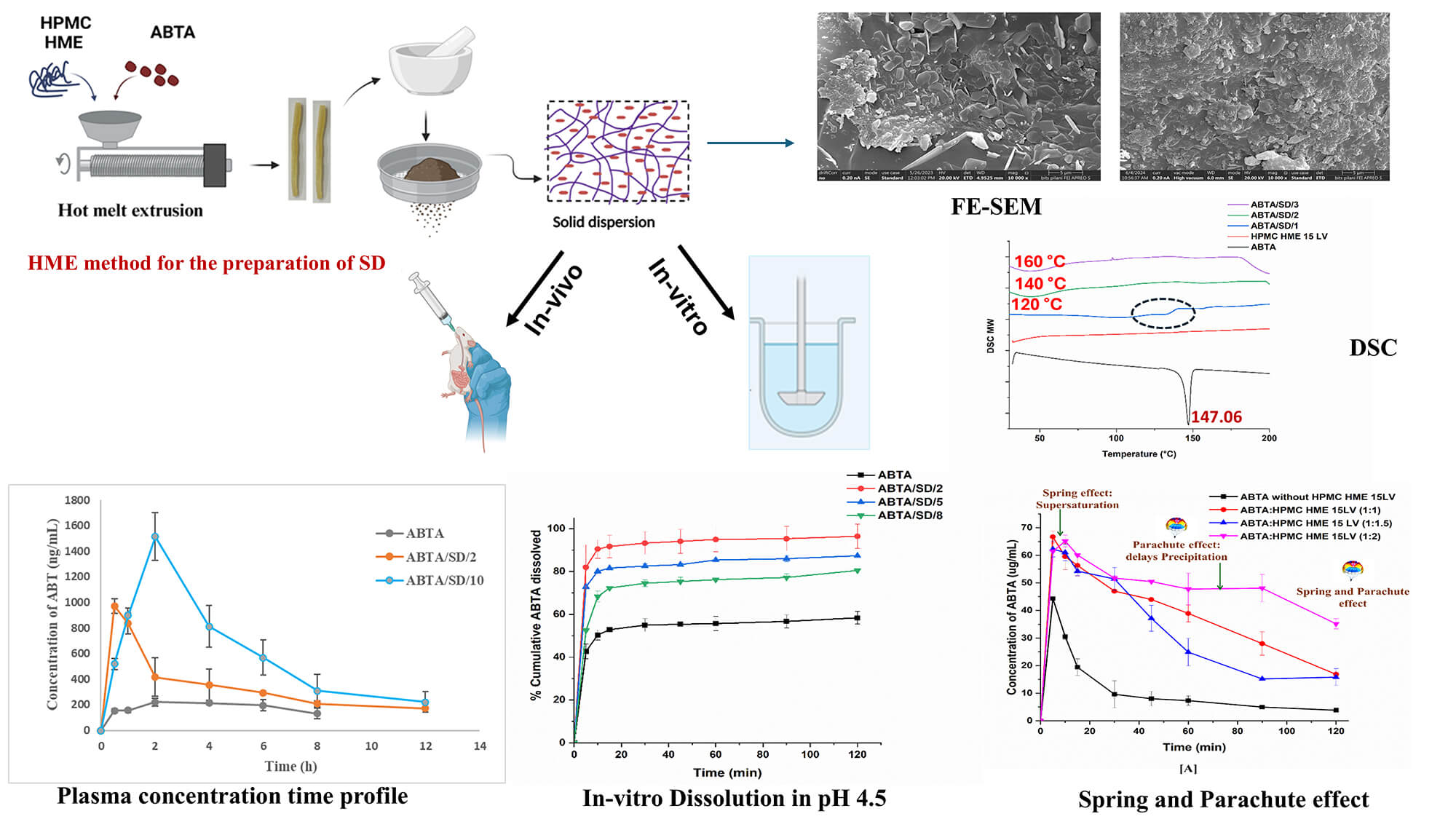
Abiraterone acetate (ABTA) was approved by the USFDA in 2011 for treating metastatic castration-resistant prostate cancer (mCRPC). ABTA exhibits poor aqueous solubility, inadequate dissolution, low oral bioavailability (<10%), and significant positive food effects. To overcome these limitations, in the present work, ABTA solid dispersions (SDs) were developed by using hot melt extrusion technology (HME) with various grades of hydroxypropyl methylcellulose HME (HPMC HME 15LV and 100LV) at different extrusion temperatures. HPMC HME demonstrated the ability to prevent drug precipitation for up to 120 min compared to the free drug (10 min), sustaining the supersaturation state of the drug in the solution phase and demonstrating the spring and parachute effect. The physical interactions of the ABTA SD’s were evaluated by Fourier transform infrared spectroscopy, powder X-ray diffraction, and differential scanning calorimetry confirming the conversion of ABTA into the amorphous state and the molecular interaction between HPMC and ABTA. The bio-relevant dissolution study of ABTA SD showed 2–5 times higher dissolution in fasted (FaSSIF) and fed (FeSSIF) conditions compared to free ABTA. Pharmacokinetic studies in Wistar rats revealed a 6.22 and 4.94-fold increase in Cmax and AUC0–t for the optimized ABTA SD formulation compared to free ABTA. Accelerated stability testing (40 ± 2°C/75 ± 5% RH, 90 days) confirmed retained amorphous state, unchanged drug content and dissolution performance for the optimized formulations. The dissolution and bioavailability studies reflected that the prepared SD of ABTA may improve the therapeutic efficacy of ABTA in prostate cancer. The manufacturing technology is scalable and easy to commercialize, revealing the hope of a better treatment strategy for prostate cancer.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools