 Open Access
Open Access
ARTICLE
A Comparative Investigation of the Biodegradation Behaviour of Linseed Oil-Based Cross-Linked Composites Filled with Industrial Waste Materials in Two Different Soils
1
Biodeterioration Research Laboratory, Nature Research Centre, Vilnius, LT-08412, Lithuania
2
Department of Polymer Chemistry and Technology, Kaunas University of Technology, Kaunas, LT-50254, Lithuania
3
Laboratory of Bedrock Geology, Nature Research Centre, Vilnius, LT-08412, Lithuania
* Corresponding Author: Eglė Malachovskienė. Email:
(This article belongs to the Special Issue: Bio-based/Degradable Materials towards A Sustainable Future)
Journal of Renewable Materials 2023, 11(3), 1255-1269. https://doi.org/10.32604/jrm.2022.023574
Received 06 May 2022; Accepted 18 July 2022; Issue published 31 October 2022
Abstract
The biodegradation of polymeric biocomposites formed from epoxidized linseed oil and various types of fillers (pine needles, pine bark, grain mill waste, rapeseed cake) and a control sample without filler was studied during 180 days of exposure to two types of forest soil: deciduous and coniferous. The weight loss, morphological, and structural changes of polymer composites were noticed after 180 days of the soil burial test. The greatest weight loss of all tested samples was observed in coniferous forest soil (41.8%–63.2%), while in deciduous forest soil, it ranged between 37.7% and 42.3%. The most significant changes in the intensities of the signals evaluated by attenuated total reflectance infrared spectroscopy, as well as morphological changes determined by scanning electron microscopy, were assessed for polymer composite with rapeseed cake and specimen without filler in coniferous forest soil and are in a good agreement with weight loss results. Whereas significantly lower changes in weight loss, morphology, and structure of polymeric film with pine bark were noticed in both soils. It was suggested that fungi of Trichoderma, Penicillium, Talaromyces and Clonostachys genera are the possible soil microorganisms that degrade linseed oil-based cross-linked polymer composites. Moreover, the novel polymer composites have the potential to be an environmentally friendly alternative to petroleum-based mulching films.Keywords
In the last decade, environmentally degradable bio-based polymers and their composites with renewable raw materials have gained the attention of scientists due to ecological and economic concerns along with the depletion of crude oil reserves. Such biocomposites could be used in various fields–automobile industry, medical devices, packaging, etc. [1]. Furthermore, due to the agricultural plastic waste disposal problem, the creation of bio-based films and mulches has also received a lot of attention [2]. The creation of biodegradable mulching films might serve as a potential solution to the final disposal of plastic mulch films, as sustainability in agriculture needs to be achieved.
Traditionally, most mulching films used in agriculture are produced from non-renewable petroleum-based plastics, usually polyethylene [3]. Plastic mulch films keep the plants and edible products free of dirt, reduce diseases coming from the soil, control weeds, protect soil from water evaporation, increase soil temperature, maintain a good soil structure, and enhance fertilizer use efficiency [2,4]. Unfortunately, the excessive use of hardly degradable polyethylene has resulted in large quantities of plastic wastes [5]. Consequently, plastic waste is not properly disposed of, which in turn leads to the emission of harmful substances and a negative impact on the environment [6]. On the contrary, biodegradable polymeric composites from renewable recourses filled with industrial waste materials could be applied as sprayable or precured mulching films in agriculture and forestry and disposed of or composted at the end of their life without harming the environment [7].
The process of polymer degradation depends on a combination of abiotic (temperature, moisture, pH, UV radiation, oxygen, mechanical stress, etc.) and biotic factors (fungi, bacteria, and algae) [8]. A key step for microorganisms to decompose long polymers is the use of extracellular enzymes to break down complex compounds into smaller subunits which can be used as carbon and energy sources [9,10]. In addition, the chemical composition, structure, and physical properties of the polymer also play an important role in the degradation process [11]. Polymers with hetero chain undergo hydrolytic degradation and polymers with carbon backbone are oxidized [12].
Vegetable oils are widely used by polymer chemists to produce “green composites” due to their availability, renewable nature, biodegradability, possibility to make a wide range of chemical modifications and broad applicability [13]. The polymer matrix of a linseed oil polymer with bisphosphonate crosslinks can be readily hydrolyzed as described earlier by Kasetaite et al. [14]. Waste of industrial production is not landfilled but is used to produce added value products. The fillers of a natural origin are characterized by ready availability, biodegradability, non-toxicity, low cost, and biocompatibility [15]. To impart strength and stiffness to plant oil-based polymers, such reinforcements from biorenewable resources are often used [16]. Formulation of multiphase materials by introducing reinforcements improves their properties compared to those of every single component, reaching international standards [15]. Works on the creation and characterisation of linseed oil-based polymer composites from renewable raw materials have been widely reported [17–20], though the lack of information on the biodegradability of such biocomposites is still appreciable. Shogren et al. [21] reported the potential biodegradability of several vegetable oil-based polymers under laboratory conditions. The compostability of linseed resin composites reinforced with natural fibers was studied by Ngo et al. [22].
Although the biodegradability of linseed oil-based cross-linked polymer composites filled with industrial waste materials was already estimated by soil burial test, up to date, there is no work done to compare the biodegradation rate of such biocomposites across different soils and to determine the impact of biotic and abiotic factors on their biodegradability. In this study, we aimed to evaluate in more detail the degradation behaviour of linseed oil-based cross-linked biocomposites with different types of industrial waste materials used as fillers (pine needles, pine bark, grain mill waste, rapeseed cake) and a control sample without filler in two different soils: coniferous and deciduous forest soil. This work reports on the weight loss, morphological and structural changes of polymer composites after exposure to the soil for 180 days. In addition, isolation and identification of the soil fungi which colonized the buried samples are also discussed.
The polymer composites composed of epoxidized linseed oil and 1-hydroxyethane-1,1-diphosphonic acid are filled with different types of industrial waste materials: pine needles (PN), pine bark (PB), grain mill waste (GW), rapeseed cake (RC), were used in this study. The polymer composite film samples were prepared by direct mixing of epoxidized linseed oil, an aqueous solution of 1-hydroxyethane-1,1-diphosphonic acid (ratio of P-OH groups to epoxy groups was 2:1), and 5 wt.% of the industrial waste materials, then casting on a plastic sheet and keeping at room temperature for 1 day. The cross-linked polymer with 2:1 ratio of monomer P-OH groups to epoxy groups and without the addition of industrial waste materials was used as control (WF). The composite films were prepared at the Department of Polymer Chemistry and Technology, Kaunas University of Technology. More detailed information on the preparation of film samples, thermal, mechanical, and other properties can be found in Vaicekauskaite et al. [7]. The experiment was performed in coniferous (CF) and deciduous (DF) forest soil with characteristics given in Table 1. The chemical properties and granulometric composition of tested soils were performed in the Agrochemical Research Laboratory, Lithuanian Research Centre for Agriculture and Forestry.

The degradability of polymers was assessed by a soil burial test that was carried out according to the standard EN ISO 846 [23] with slight modifications. The polymer composites and control sample (10 mm × 10 mm) were weighed and placed into poly (vinyl chloride) bags (50 mm × 60 mm) with 2 mm mesh diameter. Three replicates of each sample were buried into preserving jars (5 L) filled with corresponding quality of soil having a moisture content equal to 60 ± 5% of the water holding capacity and kept at 26 ± 2°C for 180 days. At the end of each testing period (60, 120 and 180 days), the samples were removed, washed with sterile water, and dried in a desiccator for 48 h until a constant weight was reached. Change in weight (%) was determined using the following equation:
2.3 Assay of Soil CM-Cellulase Activity
Biological activity of the soil was determined by estimating spectrophotometrically its CM-cellulase activity [24]. A 100 ml Erlenmeyer flask was filled with 10 g moist soil, mixed with 15 ml sodium carboxymethyl cellulose (Roth, Germany) and 15 ml 2 M acetate buffer. A control (without sodium carboxymethyl cellulose) was prepared for each soil and treated like a sample. The flasks were shaken briefly, sealed with a rubber stopper, and incubated at 50°C for 24 h. After incubation, samples were filtered immediately and diluted to 20 ml with distilled water in test tubes. For the spectrophotometric analysis 1 ml of diluted soil filtrate, 1 ml of reagent A (16 g of anhydrous sodium carbonate (Lachema, Czech Republic), 0.9 g of potassium cyanide (Alfa Aesar, Germany) dissolved in 1000 ml of distilled water) and 1 ml of reagent B (0.5 g of potassium hexacyanoferrate (III) (Roth, Germany) dissolved in 1000 ml of distilled water) were added into a test tube, mixed, sealed and boiled in a water bath for 15 min. After cooling, 5 ml of reagent C (2.7 g of ammonium iron (III) sulfate dodecahydrate (Penta, Czech Republic), 1 g of sodium dodecyl sulfate (Sigma-Aldrich, China) and 4.2 ml of concentrated H2SO4 (Honeywell Fluka, Germany) dissolved in 1000 ml of distilled water) was added, mixed, and allowed to stand at 20°C for 60 min. The absorbance was measured at 690 nm within the following 30 min with a spectrophotometer Evolution 60S (ThermoFisher Scientific, USA). The CM-cellulase activity of soil was expressed as µg of glucose equivalents (GE) per gram of dry matter (dm) and incubation time (µg GE g−1 × dm × 24 h−1).
2.4 The Abundance of Cultivable Soil Fungi
The number of cultivable fungi in CF and DF soil was determined by the soil serial plate method [25]. The soil sample (10 g) was added into 95 mL of sterilized water and shaken on an orbital shaker (KS 501 digital, Germany) at 200 rpm for 1 h. Immediately after shaking, a series of 10-fold dilutions of the suspension were carried out, and appropriate dilutions were plated on malt extract agar (MEA) (Blakeslee’s) containing: 20 g l−1 malt extract, 20 g l−1 glucose, 20 g l−1 agar, 1 g l−1 peptone supplemented with chloramphenicol (250 mg L−1). The plates were incubated at 26°C for 5 days. All experiments were repeated in triplicate and the total number of filamentous fungi (as colony forming units (CFUs)) per gram of soil dry weight was calculated.
2.5 Isolation and Identification of Cultivable Fungi from Polymeric Composites
Fungi were isolated from polymeric composites and control samples by the imprint technique. After soil burial test, samples were rinsed under running water and then were washed with sterile distilled water. Cleaned samples were imprinted on MEA (Blakeslee’s) supplemented with chloramphenicol (250 mg L−1) and incubated at 26°C for 5 days. Isolated fungi were identified according to macroscopic and microscopic features [26–29]. The morphology of isolated fungi was examined using a Leica DM5000 microscope with a Leica DFC450 camera mounted on top of cultures grown on oat meal agar (OA) (Difco) or MEA (Blakeslee’s) at 26°C for 5–10 days.
Trichoderma and Clonostachys fungi isolated from CF and DF soil were identified to species level by its macro- and micromorphology. The isolates of Clonostachys spp. were grown on MEA at 25°C for 10 days. The appearance and size of a colony, conidia, verticillium-like and penicillate conidiophores were examined. Observations of Trichoderma spp. colonies were based on isolates grown on PDA and OA. The growth rate was observed on PDA at 25°C, 30°C and 35°C, and measurements of the colony radius were taken. Conidium-bearing structure and conidia were assessed from cultures grown on OA.
The chemical changes of polymer biocomposites and control samples were investigated by attenuated total reflectance infrared spectroscopy (ATR-IR) before and after 180 days of soil burial testing. The IR spectra were recorded on a Bruker Vertex 70 ATR-IR spectrometer over a 4000 to 600 cm−1 range.
2.7 Scanning Electron Microscopy (SEM) Analysis
The surface morphology of the polymer composites and control samples were examined by a scanning electron microscope (QUANTA 250) before and after 180 days of soil burial testing. The specimens were coated with carbon or gold.
The statistical program (GNU PSPP) was used to calculate t values for the determination of variable significance at p ≤ 0.05.
The polymer composites with different types of industrial waste materials used as fillers and a control sample without filler were tested for their degradability in CF and DF soil. The polymer composites changed their colour and became more brittle after the soil burial test (Fig. 1). The weight of tested films decreased gradually over 180 days of exposition period in both soils (Fig. 2). The greater weight loss of all tested samples was noticed in CF, and at the end of the experiment it ranged between 41.8% and 63.2%, while the weight loss of tested samples in DF varied between 37.3%–42.3% after 180 days of soil burial test.

Figure 1: Polymeric biocomposites with pine needles (PN), pine bark (PB), grain mill waste (GW), rapeseed cake (RC), and a control specimen without filler (WF) before (Initial) and after exposition in deciduous (DF) and coniferous (CF) forest soil

Figure 2: The weight loss (%) of polymer composites with pine needles (PN), pine bark (PB), grain mill waste (GW), rapeseed cake (RC), and a control sample without filler (WF) after 60-, 120-, and 180-day of exposition in coniferous (CF) and deciduous (DF) forest soil. Error bars represent standard deviation, and significant differences at = 0.05 are indicated (*)
According to Folino et al. [11], biodegradability depends on the nature of the tested material as well as on the selected soil environment (temperature, pH, moisture, nutrient, and oxygen availability) and microbial population. In this research, the highest weight loss was observed for the control sample in both CF and DF soil, and it reached 63.2% and 42.3%, respectively. Following the previous study, our results confirm that the degradation of the polymer binder was more rapid than that of the filler in these cases [7]. The lowest weight loss after 180 days of exposition was observed for polymer composite with PB in both DF (37.3%) and CF (41.8%) forest soil. On the contrary, samples with PB lost less weight compared to results from the previous study (nearly 60%), and it confirms the importance of biotic and abiotic factors to the degradation of each tested material [22].
Though the experiment was carried out under aerobic conditions and the temperature (26 ± 2°C) and water content (20%–30%) in both tested soils were maintained within the prescribed range, the samples buried in CF lost more weight than in DF soil. These findings can be attributed to differences between abiotic (soil chemical composition, pH) factors in CF and DF soil (Table 1). According to Brodhagen et al. [4], the neutral soil pH, determined in CF during our experiment, is optimal for microorganisms and enzymes in most soils. Moreover, lower organic C (%) and total N (%) values in CF might be related to the fact that under starvation a variety of microorganisms use the polymers as nutrients [30]. In addition, slightly increased background concentrations of heavy metals such as Zn (77.7 mg/kg) and Pb (31.7 mg/kg) were determined in DF, but they did not exceed the permissible heavy metal limits [31]. Heavy metals found in the soil at low concentrations provide essential micronutrients for soil organisms. However, increased levels of heavy metals might bring the adverse effect on plants and microorganisms [32]. Moreover, the main soil characteristics such as pH, organic matter and clay can strongly change the effects of heavy metals on enzyme activities [28]. Wyszkowska et al. [33] estimated that Zn and Pb at a concentration of 50 mg/kg inhibited activities of soil enzymes such as dehydrogenase, urease, acid- and alkaline phosphatase.
Differences in the biodegradation rate among biodegradable polymers are also related to structural and physicochemical peculiarities of their surfaces, determining how strongly microorganisms can adhere to the surface [11]. According to Brebu [8], a rough hydrophilic surface is more biodegradable than a smooth and inert one. In our study, the samples with PB (37.3% (DF), 41.8% (CF)) and GW (40.3% (DF), 49.2% (CF)) having organic filler particles with the largest size showed different weight loss results. The type of filler is no less important, since lignin is known to be recalcitrant to biological degradation due to its highly branched structure, aromatic, and water-insoluble nature.
3.2 The Abundance of Cultivable Soil Fungi and the CM-Cellulase Activity of Soil
The biotic factors such as enzymatic activity, abundance and diversity of soil microorganisms are no less important. During the entire experiment, slightly greater numbers of cultivable fungi were determined in DF, and it ranged between 1.3 × 105 CFUg−1 and 2.6 × 105 CFUg−1 dry soil, while in CF it varied from 1.0 × 105 CFUg−1 to 2.0 × 105 CFUg−1 dry soil (Table 2).

The cellulolytic activity of the soil was measured to evaluate the activity of the entire flora. The greater cellulase activity was observed in DF as well, but in CF it was less variable, which may also have contributed to a greater change in the weight of the samples buried in CF (Fig. 3). Although, the cellulolytic enzymes are among the major enzymes involved in the decomposition of natural polymers, no correlation was found between soil enzymatic activity and the biodegradation rate of polymer composites. However, other enzymes such as laccase, protease, esterase, cutinase, urease, etc. are known to be involved in the biodegradation of man-made polymers [34].

Figure 3: CM-cellulase activity of the coniferous (CF) and deciduous (DF) forest soil after 60, 120 and 180 days
3.3 Isolation and Identification of Cultivable Fungi
Fungi of seven genera from CF soil and three genera of DF soil were isolated from the polymeric composites and control sample (Table 3).

Fungi of Trichoderma, Penicillium and Talaromyces genera were detected almost on all tested specimens buried in CF except for polymer composites with PB and RC. The dominant fungal species belonging to Trichoderma sp. and Clonostachys sp. were isolated from all samples buried in DF soil. The members of the genus Trichoderma and Clonostachys were also isolated from composites buried in CF soil. Fungi of the genus Trichoderma and Clonostochys have been reported for their ability to biodegrade plastic waste [35,36]. The higher weight loss of polymer composites in CF soil might be related to the greater diversity of fungal genera isolated from tested samples. It is important to note that most fungal colonizers of soil-buried biodegradable polymers are plant pathogens and saprophytes or belong to the family Trichocomaceae, which members are known for their ubiquity, oligotrophy, and broad metabolic capacity [6].
The isolates belonging to the genus Trichoderma had fast-growing hyaline colonies, later becoming green due to conidium production, repeatedly branched conidiophores, and flask-shaped phialides (Fig. 4). Trichoderma sp. isolated from the polymer composites buried in DF soil was identified as one of the Trichoderma koningii Oudem. aggregate species. After 72 h in darkness, the colony radius on PDA was 4.5–4.7 cm at 25°C and 3.8–4.0 cm at 30°C. No distinctive odour or diffusing pigment was detected on any medium, only sparingly produced aerial mycelium. Phialides arising in whorls of 3–4 had a size of 9–10 × 3 µm. Conidia oblong, smooth, green, 5–6 × 3–4 m. Trichoderma sp. isolated from polymer composites buried in CF soil was identified as Trichoderma harzianum Rifai. After 72 h in darkness, the colony radius on PDA was 5.8–6.0 cm at 25°C, 6.7–7.0 cm at 30°C and 3–3.2 cm at 35°C. Phialides ampulliform with a size of 3–6 µm. Conidia pale green, globose to subglobose, smooth, 3 × 3 µm.

Figure 4: Macromorphology and micromorphology of Trichoderma fungi on OA after 7 days. (A) Trichoderma koningii isolated from deciduous forest soil and (C) conidiophores and conidia. (B) Trichoderma harzianum isolated from coniferous forest soil and (D) conidiophores and conidia. Magnification × 1000
Colonies of the isolates identified as Clonostachys rosea (Link) Schroers, Samuels, Seifert & W. Gams retrieved from specimens buried in DF and CF soil reached 3.0–3.4 cm and 3.3–3.6 cm diam in 5 days at 25°C, respectively (Fig. 5). The appearances of the colony of both fungi were whitish, reverse colourless, conidial areas turning pale green after 10 days. C. rosea isolated from the samples buried in DF soil had Verticillium-like branches of 100–120 µm tall, phialides in the size of 15–13 × 2 µm, while penicillate conidiophores were of 45–65 µm long, phialides with a measurement of 10 × 2 µm. Conidia from both types of conidiophores were hyaline, elongate, slightly asymmetrical, smooth-walled, 5 × 3 µm. C. rosea isolated from the samples buried in CF soil had Verticillium-like branches of 180–200 µm tall, phialides in the size of 22–23 × 2 µm, and penicillate conidiophores of 85–100 µm long, phialides with a measurement of 17–18 × 2 µm. Conidia from both types of conidiophores were hyaline, elongate, slightly asymmetrical, smooth-walled, 5–6 × 3–4 µm.

Figure 5: Macromorphology and micromorphology of Clonostachys fungi on MEA. Clonostachys rosea isolated from deciduous forest soil after 5 (A) and 10 (B) days. Verticillium-like (C) and penicillate conidiophores and conidia (D). Clonostachys rosea isolated from coniferous forest soil after 5 (E) and 10 days (F). Verticillium-like (G) and penicillate conidiophores and conidia (H). Magnification × 1000
3.4 Structural Characterization of Polymer Composites
The ATR-IR method was used to record IR spectra of polymer composites and the control sample before and after 180 days in DF and CF soil (Fig. 6). After 180 days, the signals of the tested samples assigned to C=O, C-O-C, P=O, and P-O-C bonds at 1739 cm−1, 1158–1166 cm−1, 1054–1063 cm−1, and 1005–1015 cm−1 decreased or disappeared. The signals assigned to CH2, CH3, and CH bonds at 2923–2926 cm−1, 2853–2856 cm−1, 1456–1458 cm−1, and 722–723 cm−1 were also affected. Low molecular weight compounds were formed during the cleavage of these groups.
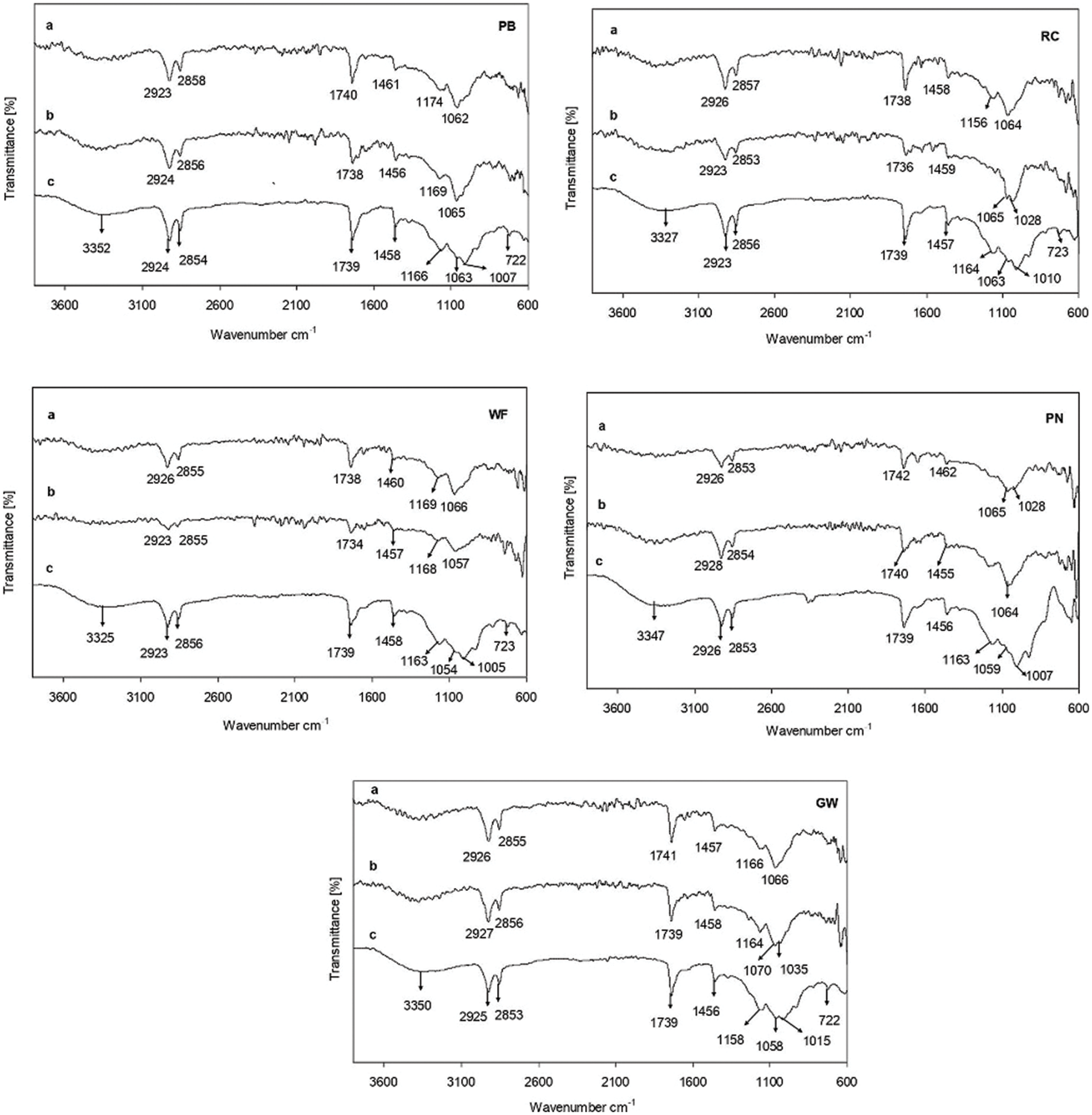
Figure 6: ATR-IR spectra of polymer composites composed with pine needles (PN), pine bark (PB), grain mill waste (GW), rapeseed cake (RC), and a control sample without filler (WF) before (c) and after 180 days of soil burial test in deciduous (a) and coniferous (b) forest soil
The ATR-IR spectra results indicated the dependence on the chemical composition of tested samples and the type of soil environment. The samples with RC and WF, both retrieved from CF soil, showed the most significant changes in the intensities of the signals and were in agreement with the weight loss results. The previous works confirm the importance of the material composition, biodegradation environment and exposure time for the degradation of polymers and their composites [21,22,37]. The cleavage of the cross-linked polymer was confirmed by the reduction or disappearance of the signals at 1739 cm−1, 1163 cm−1, 1054 cm−1, and 1005 cm−1. According to Zykova et al. [38], the extracellular enzyme mediated oxidation or hydrolysis is the primary mechanism of polymer biodegradation which improves the hydrophilicity and leads to further biodegradation and mineralization. In our case, the degradation of the polymer matrix might be explained by the better diffusion of water inside the polymer matrix and access to the hydrolysable functional groups due to its high swelling value. The fewer signals in the ATR-IR spectrum, and those present were smaller than in the control sample, indicated the hydrolysis of polymer composites with different types of fillers. The nature and size of the filler particles resulted in slightly different ATR-IR results, showing that each polymer biocomposite has its own degradation rate.
3.5 Scanning Electron Microscopy Study
The scanning electron microscope images of the polymeric composites and control sample before and after 180 days of soil burial test in CF and DF soil are shown in Fig. 7.

Figure 7: SEM images of polymer composites with pine needles (PN), pine bark (PB), grain mill waste (GW), rapeseed cake (RC), and a control specimen without filler (WF) before (a) and after (b) 180 days of soil burial test in deciduous (DF) and coniferous forest soil (CF). Magnification from the left to right: ×3203, ×4000, ×3000 (RC); ×2622, ×2864, ×2000 (PB); ×3000, ×3000, ×2514 (PN); ×3459, ×6000, ×2600 (GW); ×2278, ×3000, ×2500 (WF)
Before the soil burial test, all tested samples had a relatively smooth appearance. However, after 180 days of soil burial test in both environments, the surfaces of the tested films became rough with clearly visible irregularities, surface erosion, holes, and cracks. Moreover, fungal growth on the surface of almost all tested specimens was noticed, except for samples with PN and WF in CF soil. Tested films buried in CF soil had a more altered appearance compared to the samples buried in DF soil. SEM images of samples with GW, and WF exposed in CF soil are in good agreement with weight loss results because of deep surface erosion and large holes. According to Wahyuningtyas et al. [37], due to such changes, the diffusion of oxygen and enzymes into tested material can increase the speed of degradation. On the other hand, significantly lower changes of polymeric films with PB in both soils were noticed. These results indicate the importance of the filler type on the biodegradation rate. In contrary, numerous holes are seen in SEM images of WF from both soils. It has a strong correlation with gravimetric results, where the highest weight loss was observed. The degradation of the polymer composites in the soil environment depends on many factors, including biotic and abiotic ones. Tested samples in which high porosity and penetrated fungal hyphae were observed allow us to conclude that fungi are involved in the destruction process of tested materials.
The degradability of composites with different types of industrial waste materials used as fillers (pine needles, pine bark, grain mill waste, rapeseed cake) and control sample without filler was higher in CF soil, which was characterized as neutral (pH 7), having a lower organic C (1.54%) and total N (0.117%) values and less variable cellulolytic activity compared to DF soil. The highest weight loss was observed for WF (63.2%) and for polymer composite with RC (52.7%), after 180 days of soil burial test in CF soil, which also was confirmed by ATR-IR and SEM results. The lowest weight loss after 180 days of exposition was observed for polymer composite with PB in both DF (37.3%) and CF (41.8%) soil. The reduction or disappearance of the signals at 1739, 1163, 1054 and 1005 cm−1 in IR spectrum revealed the destruction of the polymer matrix. Dominant fungal isolates of Trichoderma sp., Clonostachys sp., Penicillium sp., and Talaromyces sp. were suggested as possible soil microorganisms that degrade linseed oil-based cross-linked polymer composites. The tested biocomposites demonstrated promising potential for application as mulching films in agriculture or forestry as ecofriendly alternatives to polyethylene films.
Acknowledgement: This research was performed employing the facilities of the Nature Research Centre and the Valley Santaka.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Vasile, C., Pamfil, D., Râpă, M., Darie-Niţă, R. N., Mitelut, A. C. et al. (2018). Study of the soil burial degradation of some PLA/CS biocomposites. Composites Part B: Engineering, 142, 251–262. [Google Scholar]
2. Thompson, A. A., Samuelson, M. B., Kadoma, I., Soto-Cantu, E., Drijber, R. et al. (2019). Degradation rate of bio-based agricultural mulch is influenced by mulch composition and biostimulant application. Journal of Polymers and the Environment, 27, 498–509. [Google Scholar]
3. Zumstein, M. T., Schintlmeister, A., Nelson, T. F., Baumgartner, R., Woebken, D. et al. (2018). Biodegradation of synthetic polymers in soils: Tracking carbon into CO2 and microbial biomass. Science Advances, 4(7), eaas9024. [Google Scholar]
4. Brodhagen, M., Peyron, M., Miles, C., Inglis, D. A. (2014). Biodegradable plastic agricultural mulches and key features of microbial degradation. Applied Microbiology and Biotechnology, 99, 1039–1056. [Google Scholar]
5. Sankhla, I. S., Sharma, G., Tak, A. (2020). Chapter 4–Fungal degradation of bioplastics: An overview. In: Singh, J., Gehlot, P. (Eds.New and future developments in microbial biotechnology and bioengineering, pp. 35–47. Netherlands: Elsevier. [Google Scholar]
6. Thakur, S., Chaudhary, J., Sharma, B., Verma, A., Tamulevicius, S. et al. (2018). Sustainability of bioplastics: Opportunities and challenges. Current Opinion in Green and Sustainable Chemistry, 13, 68–75. [Google Scholar]
7. Vaicekauskaite, J., Ostrauskaite, J., Treinyte, J., Grazuleviciene, V., Bridziuviene, D. et al. (2019). Biodegradable linseed oil-based cross-linked polymer composites filled with industrial waste materials for mulching coatings. Journal of Polymers and the Environment, 27(2), 395–404. DOI 10.1007/s10924-018-1360-y. [Google Scholar] [CrossRef]
8. Brebu, M. (2020). Environmental degradation of plastic composites with natural fillers—A review. Polymers, 12(1), 166. DOI 10.3390/polym12010166. [Google Scholar] [CrossRef]
9. Kliem, S., Kreutzbruck, M., Bonten, C. (2020). Review on the biological degradation of polymers in various environments. Materials, 13(20), 4586. DOI 10.3390/ma13204586. [Google Scholar] [CrossRef]
10. Gomez, E. J., Delgado, J. A., Gonzalez, J. M. (2020). Environmental factors affect the response of microbial extracellular enzyme activity in soils when determined as a function of water availability and temperature. Ecology and Evolution, 10(18), 10105–10115. DOI 10.1002/ece3.6672. [Google Scholar] [CrossRef]
11. Folino, A., Karageorgiou, A., Calabro, P. S., Dimitrios, K. (2020). Biodegradation of wasted bioplastics in natural and industrial environments: A review. Sustainability, 12(15), 6030. DOI 10.3390/su12156030. [Google Scholar] [CrossRef]
12. Muniyasamy, S., John, M. J. (2017). Biodegradability of biobased polymeric materials in natural environments. In: Thakur, V. K., Thakur, M. K., Kessler, M. R. (Eds.Handbook of composites from renewable materials, Volume 5, Biodegradable materials, pp. 625–653. USA: Scrivener Publishing LLC. [Google Scholar]
13. Mosiewicki, M. A., Aranguren, M. I. (2013). A short review on novel bio-composites based on plant oil precursors. European Polymer Journal, 49(6), 1243–1256. DOI 10.1016/j.eurpolymj.2013.02.034. [Google Scholar] [CrossRef]
14. Kasetaite, S., Ostrauskaite, J., Grazuleviciene, V., Svediene, J., Bridziuviene, D. (2014). Camelina oil- and linseed oil-based polymers with bisphosphonate crosslinks. Journal of Applied Polymer Science, 131(17), 40683. DOI 10.1002/app.40683. [Google Scholar] [CrossRef]
15. Menossi, M., Cisneros, M., Alvarez, V. A., Casalongué, C. (2021). Current and emerging biodegradable mulch films based on polysaccharide bio-composites. A review. Agronomy for Sustainable Developement, 41(4), 53. DOI 10.1007/s13593-021-00685-0. [Google Scholar] [CrossRef]
16. Pfister, D. P., Larock, R. C. (2010). Green composites from a conjugated linseed oil-based resin and wheat straw. Composites Part A: Applied Science and Manufacturing, 41(9), 1279–1288. DOI 10.1016/j.compositesa.2010.05.012. [Google Scholar] [CrossRef]
17. Dominici, F., Samper, M. D., Carbonell-Verdu, A., Luzi, F., López-Martínez, J. (2020). Improved toughness in lignin/natural fiber composites plasticized with epoxidized and maleinized linseed oils. Materials, 13(3), 600. DOI 10.3390/ma13030600. [Google Scholar] [CrossRef]
18. Gonzalez, L., Agüero, A., Quiles-Carrillo, L., Lascano, D., Montanes, N. (2019). Optimization of the loading of an environmentally friendly compatibilizer derived from linseed oil in poly(lactic acid)/diatomaceous earth composites. Materials, 12(10), 1627. DOI 10.3390/ma12101627. [Google Scholar] [CrossRef]
19. Liminana, P., Quiles-Carrillo, L., Boronat, T., Balart, R., Montanes, N. (2018). The effect of varying almond shell flour (ASF) loading in composites with poly butylene succinate (PBS) matrix compatibilized with maleinized linseed oil (MLO). Materials, 11(11), 2179. DOI 10.3390/ma11112179. [Google Scholar] [CrossRef]
20. Liminana, P., Garcia-Sanoguera, D., Quiles-Carrillo, L., Balart, R., Montanes, N. (2019). Optimization of maleinized linseed oil loading as a biobased compatibilizer in poly(butylene succinate) composites with almond shell flour. Materials, 12(5), 685. DOI 10.3390/ma12050685. [Google Scholar] [CrossRef]
21. Shogren, R. L., Petrovic, Z., Liu, Z., Erhan, S. Z. (2004). Biodegradation behavior of some vegetable oil-based polymers. Journal of Polymers and the Environment, 12(3), 173–178. DOI 10.1023/B:JOOE.0000038549.73769.7d. [Google Scholar] [CrossRef]
22. Ngo, T. T., Lambert, C. A., Kohl, J. G. (2014). Characterization of compostability and mechanical properties for linseed oil resin composites reinforced with natural fibers. Polymer-Plastics Technology and Engineering, 53(12), 1215–1222. DOI 10.1080/03602559.2014.886121. [Google Scholar] [CrossRef]
23. International Organization for Standardization (1997). Plastics–Evaluation of the action of microorganisms (BS EN ISO 846: 1997). Berlin: Beuth Verlag GmbH. [Google Scholar]
24. Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R. (1996). Methods in soil biology. Heidelberg, Berlin: Springer. [Google Scholar]
25. Germida, J. J., de Freitas, J. R. (2007). Cultural methods for soil and root-associated microorganisms. In: Carter, M. R., Gregorich, E. G. (Eds.Soil sampling and analysis, 2nd edition, pp. 341–354. USA: CRC Press Taylor & Francis Group. [Google Scholar]
26. Domsch, K. H., Gams, W., Anderson, T. (2007). Compendium of soil fungi, 2nd edition. Eching: IHW Verlag. [Google Scholar]
27. Carmichael, J. W., Kendrick, W. B., Conners, I. L., Sigler, L. (1980). Genera of hyphomycetes. Edmonton: University of Alberta Press. [Google Scholar]
28. Chaverri, P., Branco-Rocha, F., Jaklitsch, W., Gazis, R., Degenkolb, T. et al. (2015). Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia, 107(3), 558–590. DOI 10.3852/14-147. [Google Scholar] [CrossRef]
29. Samuels, G. J., Dodd, S. L., Lu, B. S., Petrini, O., Schroers, H. J. et al. (2006). The Trichoderma koningii aggregate species. Studies in Mycology, 56, 67–133. DOI 10.3114/sim.2006.56.03. [Google Scholar] [CrossRef]
30. Al Hosni, A. S., Pittman, J. K., Robson, G. D. (2019). Microbial degradation of four biodegradable polymers in soil and compost demonstrating polycaprolactone as an ideal compostable plastic. Waste Management, 97, 105–114. DOI 10.1016/j.wasman.2019.07.042. [Google Scholar] [CrossRef]
31. Lithuanian Hygiene Norm HN 60 (2004). Ministry of health care of the Republic of Lithuania, |order| |V114|03/08/2004. Valstybes zinios, 41, 1357. [Google Scholar]
32. Aponte, H., Meli, P., Butler, B., Paolini, J., Matus, F. et al. (2020). Meta-analysis of heavy metal effects on soil enzyme activities. Science of the Total Environment, 737, 139744. DOI 10.1016/j.scitotenv.2020.139744. [Google Scholar] [CrossRef]
33. Wyszkowska, J., Kurcharski, J., Lajszner, W. (2006). The effects of copper on soil biochemical properties and its interaction with other heavy metals. Polish Journal of Environmental Studies, 15, 927–934. [Google Scholar]
34. Bhardwaj, H., Gupta, R., Tiwari, A. (2013). Communities of microbial enzymes associated with biodegradation of plastics. Journal of Polymers and the Environment, 21, 575–579. [Google Scholar]
35. Treviño, A. L., Garcia, G. (2011). Polyurethane foam as substrate for fungal strains. Advances in Bioscience and Biotechnology, 2, 52–58. [Google Scholar]
36. Sun, Z. B., Li, S. D., Ren, Q., Xu, J. L., Lu, X. et al. (2020). Biology and applications of Clonostachys rosea. Journal of Applied Microbiology, 129(3), 486–495. [Google Scholar]
37. Wahyuningtyas, N., Suryanto, H. (2017). Analysis of biodegradation of bioplastics made of cassava starch. Journal of Mechanical Engineering Science and Technology, 1(1), 24–31. [Google Scholar]
38. Zykova, A. K., Pantyukhov, P. V., Mastalygina, E. E., Chaverri-Ramos, C., Nikolaeva, S. G. et al. (2021). Biocomposites of low-density polyethylene plus wood flour or flax straw: Biodegradation kinetics across three environments. Polymers, 13(13), 2138. DOI 10.3390/polym13132138. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools