 Open Access
Open Access
REVIEW
Synthesis and Properties of Biomimetic Self-Assembling Structures from Poultry Feather Keratin
School of Science and Technology, Università di Camerino, via Gentile III da Varano 7, Camerino, 62032, Italy
* Corresponding Author: Carlo Santulli. Email:
Journal of Renewable Materials 2025, 13(1), 1-19. https://doi.org/10.32604/jrm.2024.056251
Received 17 July 2024; Accepted 19 September 2024; Issue published 20 January 2025
Abstract
Taking a widely contaminated yet abundant waste, such as poultry feathers, and extracting keratin from this structure appears to be a real challenge whenever the preservation of the secondary structure of the protein is desired. This process would allow exploiting it in ways (e.g., in the biomedical field) that are inspired by a structure that is primarily designed for flight, therefore capable specifically of withstanding flexure and lateral buckling, also with very low thicknesses. The preservation of the structure is based on disulfide crosslinks, and it is offered with preference by some chemical treatments, mainly those based on ionic liquid and on a reduction process. However, the degree of preservation cannot always be precisely assessed; however, beyond chemical characterization, the formation of homogeneous gels can also suggest that the process was successful in this sense. An extraction respectful of nature’s intentions, considering that the secondary structure builds up according to the very function of the feathers in the animal, can be deemed to be biomimetic. In particular, biomimetic extractions comply with the very characteristics the protein was designed for to serve in the specific environmental and mechanical situation in which it is inserted. This review tries to elucidate in which cases this aim is achieved and for which specific applications a chicken feather keratin that has preserved its secondary structure can be suited.Graphic Abstract
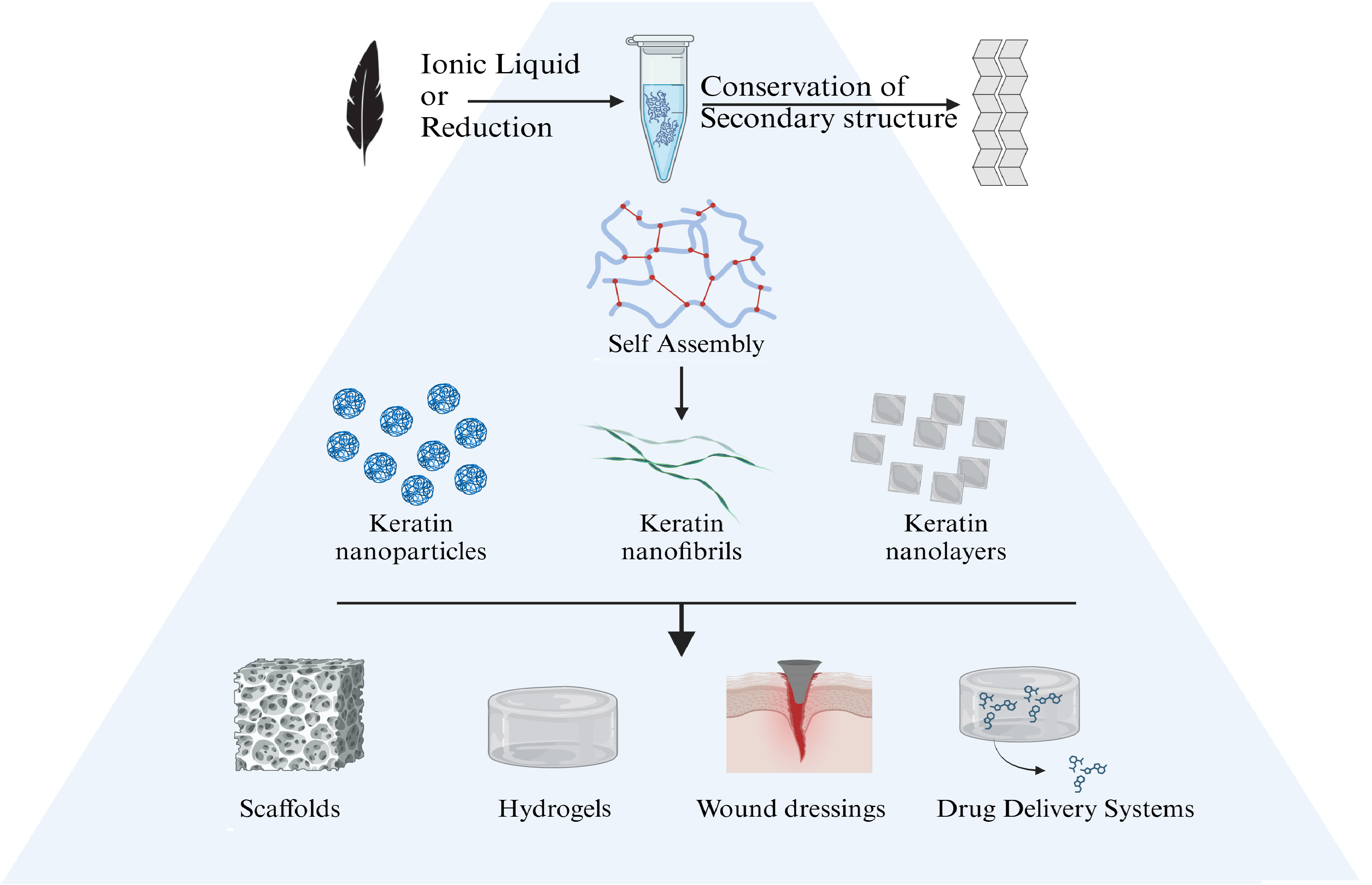
Keywords
Nomenclature
| [Amim]Cl | 1-Allyl-3-methyl-1H-imidazol-3-ium chloride |
| [Bmim]Br | 1-Butyl-3-methylimidazolium bromide |
| [Bmim]Cl | 1-Butyl-3-methylimidazolium chloride |
| [HOEMIm] | 1-hydroxyethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)amide |
| FTIR | Fourier transform infrared spectroscopy |
| ILs | Ionic Liquids |
| [NTf2] | 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| PBAT | Poly(butylene adipate-co-terephtalate) |
| PCL | Polycaprolactone |
| PVA | Poly(vinylalcohol) |
| SAXS | Small angle X-ray scattering |
A biomimetic approach to materials would regard the possibility of promoting their self-assembly in a way that allows the growth of autonomous structures to be used for functional purposes [1,2]: this procedure is particularly sought in the case of peptides and proteins [3]. More specifically, the typical structures of proteins, such as collagen, elastin, and keratin, all include some degree of α-helices arrangements [4]. The most recent novelty, however, is constituted by the possibility of obtaining these structures out of the material extracted from animal waste, which enables the establishment of correlations between their aspect and the route by which self-assembly has been realized [5]. As far as protein extraction is concerned, extracted material takes the form of hydrolysates and small peptides [6]. These nanomaterials can have a role in acquiring a biomimetic function towards appropriate training exerted on non-natural protein backbones [7]. In other words, some extraction methods confer to the material a higher capability to resist proteolysis than others and can be regarded as adapted to its biomimicking [8].
In practice, keratin-based waste from different origins/biological structures can be used for the purpose: these include, among others, sheep wool in the form of hydrolyzed peptides [9], or in more structured form for direct blending with biopolymers [10], where a biomimetic approach might reduce the final risk of generating other hazardous material difficult to dispose of [11]. Other keratin residues in search of sustainable routes for disposal are human hair [12], epidermal waste [13], nails [14] or claws [15], and poultry feathers [16], on which this review specifically focuses.
Chicken feathers can be considered dense shells with a porous core, which provides lightness and is able to be compressed or even locally torqued without being buckled [17]. Their application as fibers (chicken feather fibers, CFF) in composites, purposely separated from the rachis [18], though proposed in many cases, does reduce this structural complexity to the bare tensile support of a polymer resin [19]. On the other hand, keratin extraction may result in the destruction of the composite structure, which is the scope of the biomimetic process to avoid. The main issue is unlocking the protein by extracting it without damaging its secondary structure and, therefore, promoting the self-assembly of the keratin structure in another geometry from the original one [20]. The secondary structure is mainly influenced by the position of the side chains and hence the degree of close packing in the protein, which for keratin is controlled by the presence of a number of different motifs [21], namely α-helical, β sheets, β-turn, and random coil structures [22].
In practice, the organization of chicken feathers includes, as the effect of the judicious combination of α and β forms, the combined presence of crossed-lamellar structure (300–600 nm thick) in lateral walls of rachis and barbs, arranged into alternate layers of crossed-fibers [23]. A significant grade of preservation of this structure out of keratin extraction is particularly beneficial in terms of toughness, which also allows its blending with other proteins isolated, e.g., from soy, for the formation of composite films [24].
The isolation of keratin from poultry feathers has been demonstrated of interest in the last few decades, especially, but not exclusively, in the food-related and the cosmetics sector [25]. Here, the preservation of secondary structure is not always considered essential [26], while rather preventive purification processes, based, e.g., on ethanol, ozone, and sodium chlorite, are given larger significance [27]. Another possibility, which is specific to poultry-originated keratin is the capability to absorb some metals, e.g., in soil treatment, such as cadmium, nickel, chromium, and zinc [28]. In some cases, nonetheless, the use of keratin from poultry feathers can be considered biomimetic, more explicitly whenever the architecture of the folding structure is considered and used to achieve specific functionalities. This has been, e.g., recently performed in the case of carbonized feathers therefore used as biochar [29], where the preservation of keratin structure did result in the possible application to the removal of the residues of drugs, such as amoxicillin, from aqueous solutions [30]. Keratin biochar is also particularly adapted to be possibly blended with other similarly abundant biomass waste, such as is the case for sugarcane bagasse, which suggests synergistic effects to be obtained from adapted doping strategies between the two feedstocks [31]. However, the energy-intensive character of these processes based on carbonization has also suggested that to use chicken feathers with a biorefinery approach, biodegradation/metabolism by the action of bacteria would represent a more suitable route towards the extraction of free amino-acids and soluble proteins [32].
To allow the potential use of keratin extracted, hence solubilized, from chicken feathers, a possible approach is based on the fabrication of autogenous cross-linked gels, e.g., serving as plant growth media [33]. On the one hand, this route can provide some mechanical performance that might ease application and compete, at least as blends, with other categories of gel structures, such as those obtained from polysaccharides, e.g., starch [34], alginates [35], or guar gum [36]. On the other hand, restoring secondary structure, hence protein cross-linking, starting from disulfide links, but not limiting to them, would represent a more natural application of keratin, therefore providing also other characteristics, such as controlled water retention, elongation, and strength [37]. These properties were exploited for some uses, such as wound healing, using a blended film with polysaccharides [38], or in a more general sense, in the biomedical engineering sector, including, e.g., also applications for drug delivery [39].
This review concentrates on chicken feathers since they constitute a very large waste of the food-related production sector, and therefore, their functional use would possibly result in a circular economy approach, offering a larger value to waste. Using poultry feathers in a biomimetic way for the production of engineered structures does involve preserving as much as possible their features during extraction, in the understanding that keratin-based structures do present a number of fundamental biomimetic properties in a thermal insulation and low-density context, which include reversible adhesion, structural coloration and the possibility to offer super-hydrophobic surfaces [40]. To achieve this potential, it is important, though, to preserve as much as possible the secondary structure of feather keratin, which will be described in Section 2. A number of methods exist for the extraction of keratin from feathers, which are reported in Section 3.
Following this, the discussion does particularly concentrate on those works where keratin is extracted with the preservation of its secondary structure, which can be suggested to represent a biomimetic application of poultry feathers’ keratin. With this aim, bio-inspired materials based on poultry feather keratin are discussed, after general considerations of the keratin role in these materials (Section 4.1), being either exclusively based on keratin or as a significant component of a blend with another biopolymer (Section 4.2). Finally, applications with particular reference to the bio-inspired potential, therefore with evidence of self-assembly, are discussed (Section 4.3). Conclusions and potential for future research are offered in Section 5.
2 Structure of Keratin in Feathers
The design of a chicken feather is constituted on hierarchical levels, depicted in Fig. 1 [41], namely the basal calamus, the main structure of the rachis, from which barbs, and then barbules irradiate. Recent studies also emphasized the variable characteristics of feather design between broiler chicken, reared for meat production, and layer chicken, which are intended at egg production instead [42]. This occurs since the feathers have specialized functions, in particular, contour feathers are dedicated to flight, down feathers serve for insulation, and small ornamental ones are aimed at signaling for social activity. The different dimensional levels represented are deemed to constitute the base for the engineering of feathers’ functionality, comprising flight ability and thermal insulation properties [43]. In other words, they are able to transform through a controlled and tailored spiraliform arrangement, a unidimensional appendage into a volumetric body capable of actively sustaining the flight action [44]. In particular, the section of the rachis comprises epicortex, with crossed-fiber architecture, made of β keratin, which offers a trabeculae-like support [45], then cortex and the medullary pith with its foam-like structure [46]. The latter has recently been proposed as to offer some bio-inspired action of thermal insulation due to its cellular geometry [47]. Keratin fibers are arranged in a cross-like architecture being coated with amorphous protein: this disposition is connected to the need to withstand specific forces during flight, mainly by flexural and shear loads, maintaining a sufficient flexibility notwithstanding the required stiffness offered by the protein structure [48]. In particular, an uninterrupted structural connection appears to be formed between the cortex of the rachis and the barbs [49]. The idea is that the rooting of barbs within the rachis is helpful in withstanding the aerodynamic forces during flight [50]. As a matter of fact, the whole structure of the feather optimizes bending stiffness to sustain loading in flight: in that respect, the heavily deformed structure is then recovered by immersion in water, aimed at simulating air moisture effect [51].
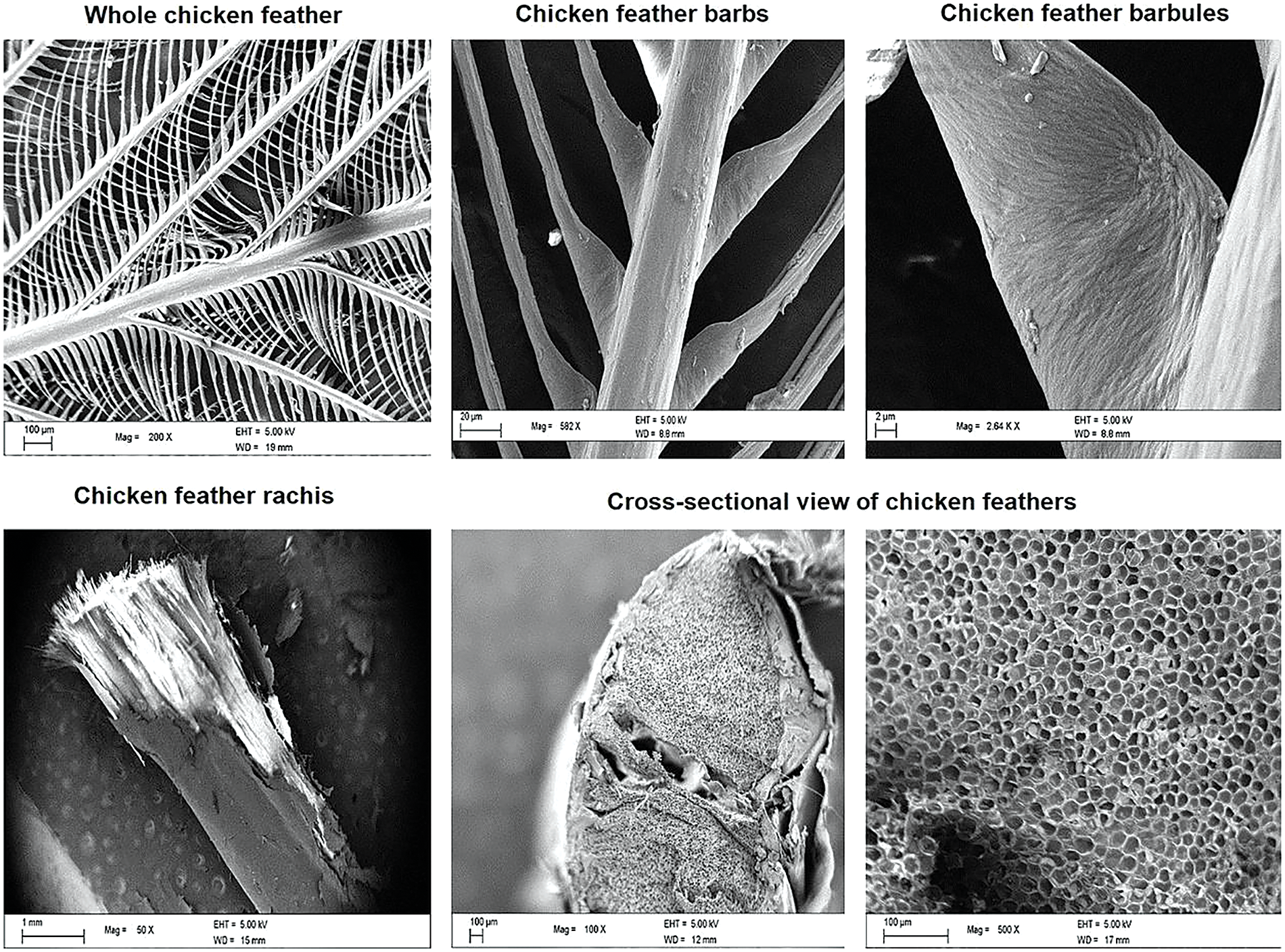
Figure 1: Different parts of keratin feathers, showing hierarchical structure (Reproduced with permission from Reference [43], © Elsevier 2017)
At a microscopical level, though, the structure of feather keratin takes the form of microfibrils, less ordered with respect to alpha-keratin, yet still following a composite arrangement such as fiber + matrix [52]. It has been also suggested that the natural model followed during feather development, and that has therefore to be accounted for in the self-assembly process, is that of a simple twisted β-sheet [53], as indicated in Fig. 2, which details the different dimensional levels. Investigations carried out by small angle X-ray scattering (SAXS) have demonstrated the more compact short-range organization of feather keratins with respect to wool keratin [54].
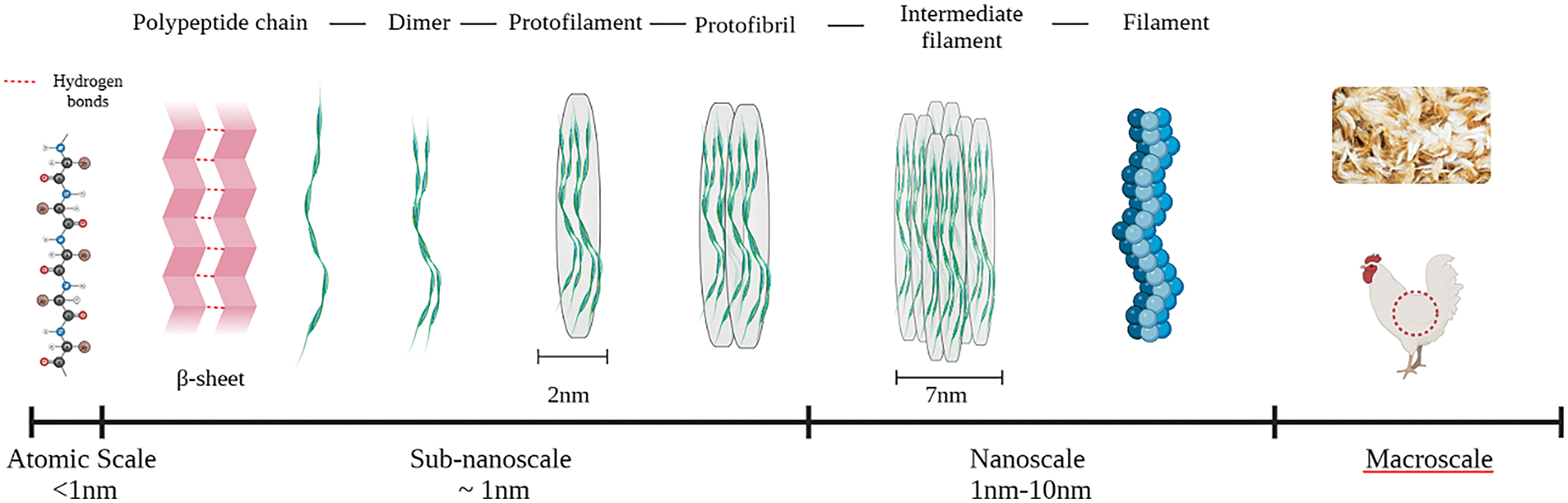
Figure 2: Schematic representation of keratin structure ranging from the atomic scale to the macroscale (Adapted from Reference [20] © Elsevier 2022)
Going more into detail, into the molecular level, keratin is generally rich in cysteine, which creates a strong covalent disulfide bond, which results in crosslinking, promoting therefore an increased hardness of the structures [55]. This ultimately contributes to cornification, hence conversion into a horn structure, of epithelial cells of feathers [56].
3 Methods for the Extraction of Keratin from Feathers and Preservation of the Secondary Structure
Different methods are available to extract keratin from feathers (a summary of the various techniques adopted is offered in Fig. 3), among which this review gives preference to those that are able to preserve its secondary structure, which results also in maintaining its antioxidant potency [57]. A number of chemical methods like reduction [58], ionic liquid [59] and alkaline hydrolysis [60], are used for keratin extraction, but not all of them preserve the secondary structure. The completeness of the secondary structure preservation is difficult to assess, even for recent studies that concentrate on the morphological characteristics of the keratin extracted from feathers the amount of β sheets crosslinked by disulfide bonds was not easily measurable from microscopical observation [61]. More reliable measurements can be offered by quantitative Raman spectroscopy, especially by comparing the intensity of the peaks representing S-S disulfide bridges and β sheets, typically around 521 and 1662 cm−1, respectively [62]. Apart from the extraction process, it is also worth noting that the application of tensile stress on the keratin structure might lead to a more reduced preservation of disulfide bonds, due to their stretching, which might also be ascribed to the agitation process during chemical action [63].
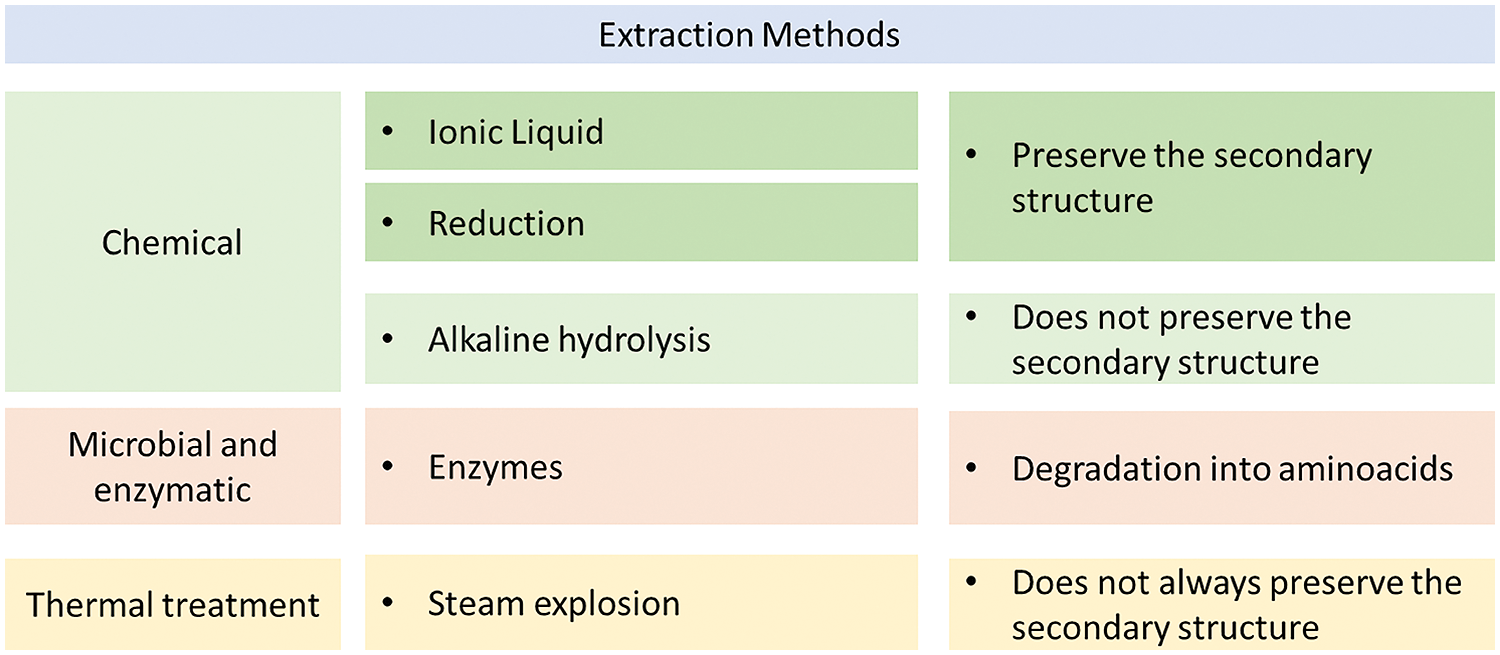
Figure 3: Summary of extraction methods used for feathers keratin
In particular, alkaline hydrolysis, also facilitated by cetrimonium bromide, resulted in a significant disruption of the hydrogen links and in the virtual absence of peptides at 200°C [64]. Often alkaline methods are assisted by microbial and enzymatic ones [65] using keratinases [66]. These can operate autonomously in a biorefinery concept, involving diversification of products and full use of waste resources [67]. This contributes to the complete use of the whole of feather residues, in a circular economy approach, as reported in Fig. 4. Further assistance can be provided by ultrasound irradiation [68], or by microwave treatment [69], where the texture and morphology of extracted material can be controlled by the time and energy of irradiation [70]. In all these cases, the final product yielded are functional protein + hydrolysates of interest for the food industry [71]. Another important factor, which in particular influenced the preservation of the secondary structure, while not directly related with the adopted method, is the retention time in the alkaline solution, since keratin is typically soluble in variable amounts depending on the pH of the relevant environment [72].

Figure 4: Different destinations of poultry feathers by a microbial enzymatic extraction (Reproduced with permission from Reference [67], © Elsevier 2015)
Ionic liquids (ILs) method for the extraction preserves the structure of the keratin, at least partially, with yields as high as 45% in turkey feathers [73]. In particular, on chicken feathers, the use of ILs [Amim]Cl, [Bmim]Cl and [Bmim]Br have been studied by Ji et al. [74], demonstrating that the imidazole liquid [Bmim]Cl can extract keratin from feathers, the solvent of IL is non-volatile and easy recyclable. Other hydrophobic IL have been used, such as [HOEMIm] [NTf2], which offers molecular weights for extracted keratin in the order of 10,000 [75]. Moreover, with extraction using ionic liquids, there is no discharge of pollutants, which goes in agreement with circular economy and eco-friendliness, since the ionic liquid is easily separated from keratin, and it benefits the recycling of the salt, being not detrimental to the quality of extracted keratin [76].
The reduction method that uses mercaptoethanol has a high yield, and preserves the secondary structure of the keratin, yet being harmful for the possible non-preservation of mercaptan in the final product, hence its possible discharge [77]. The reduction with sulfitolysis method, using sodium sulfate or sodium metabisulfite, is the most extensively used for the extraction of keratin due the good yield, low toxicity, and the likely preservation of the secondary structure of the protein [78]. Treatment with thiourea leads also to sufficient self-assembly properties, which are deemed to depend on the preservation of cysteine residues, with possible re-oxidation of free cysteine thiols, specifically responsible for the possible recoiling of the protein: this method has been widely applied on wool keratin [79]. A symptom of the degree of potential self-assembly of extracted keratin is described by the formation of homogeneous gels, normally at rather neutral pH. As a consequence, methods based on sulfitolysis treatment offer larger availability due to the larger presence of sulfato-kerateines, for biomedical applications [80].
Thermal treatments for the extraction of keratin appear to be quicker and are therefore prevalently used in the industrial sector. The steam flash explosion is a process based on a hydrolysis in high temperature and pressure, the exposure of keratin to these conditions allows obtaining the disintegration of the proteins [81], and a significant modification of the secondary structure with an increase of the disorder domains [82]. Furthermore, it can even assist alkaline hydrolysis into increasing the extraction rate of keratin from feathers up to over 65% [83]. However, although β sheets are comparatively more diffuse than α-helical, β-turn, and random coil structures, steam explosion does maintain on the other side a tertiary structure of partially coiled proteins in a generally more polar environment [84]. In this context, the use of supercritical water does also induce an extensive depolymerization of keratin into aliphatic chains [85]: in other contexts, this process appears particularly suitable for the production of bio-hydrogen [86].
4 Bio-Inspired Materials Obtained from Poultry Feathers with Preservation of Secondary Protein Structure
In this section, the structures that have been obtained using keratin extracted from chicken feathers with preservation of protein secondary structure, further down defined as “conservative methods”, therefore with the possibility of self-assembly, are presented and discussed. As from the above considerations, the preservation of secondary structure is allowed by extracting keratin through chemical processes (ionic liquids, and reduction) and all the works that will be mentioned in this section will involve the use of the aforementioned methods. Possibly the most suitable demonstration that the secondary structure has been preserved is linked to Fourier transform infrared spectroscopy (FTIR), finding out that cysteine-S-sulfonated residues are still present in the sample, which are able to promote self-assembly [87]. This is usually correlated with a 1024 cm−1 sharp peak observed in the FTIR results [88].
To recognize the function of keratin and its self-assembly, it is sometimes complicated. This was noticed in early studies on avian feathers, in particular, due to the fact that the fit-for-purpose alternance of α and β structures with specific monomers, defined as φ keratins, leads to a non-obvious relation between the subunits and the tissue morphology [89]. In other words, the macroscopic geometry does not immediately recall the function, such as it occurs instead in other cases, for example ligno-cellulosic fibers, which are clearly designed for structures intended for tension and possibly torque [90]. Provided the preservation of the secondary structure is adequately achieved, feather keratin arranged in the β form suggests the prevalence of the resistance to defect propagation, described as toughness, rather than the bare mechanical strength [91]. This exceptional toughness is achieved by incorporating matrix and filaments into an only protein, whose structural preservation is therefore of paramount importance [92].
It has been elsewhere recognized that the characteristics of crosslinked gels formed from keratin obtained from chicken feathers are different from those from other sources, such as hair and wool, namely as for viscoelastic properties and in particular a higher cell proliferation [93]. In terms of blending, poultry keratin can be associated with a large variety of biodegradable and conventional polymers, as reported in [94,95]. The already mentioned autogenous cross-linking of poultry feather keratin gels has also potential to offer bio-based cross-linking agents in other contexts, such as rubber, avoiding on one hand the use of carbon black as hardener, while on the other side the high nitrogen content of feathers would delay the thermal degradation of rubber up to 400°C [96]. However, this is not the kind of operation that would provide any bio-inspired sense to the keratin structure, which is used as just the replacement of the synthetic counterpart, trying to match as much as possible its performance. The same concept applies when using keratin as a bare filler for lignin and bio-epoxy to increase possibly to 100% the bio-based content of a composite [97].
4.2 Development and Function of Keratin Materials and Blends Extracted with Conservative Methods
The preservation of the secondary structure in chicken keratin can serve to various purposes, which enhance the characteristics of the material beyond its bare properties of hardness and toughness, enabling applications that span from the biomedical field to the production of biodegradable materials to other technical applications, such as water treatment. This potential has particularly been demonstrated in connection with the large availability of keratin waste products to be reprocessed, which offer large amounts of material for study [98]. Typically, to grow beyond the nanometric level, the material needs to be added with some plasticizers/biopolymers, a large variety of which was demonstrated effective to the purpose. The use of keratin feather fibers by bare alkali treatment, therefore at a micrometric dimensional level, taking the example of lignocellulosic fillers, while it allows introducing large amounts of fibers (up to 60 wt.% in [99] using PLA and PBAT), on the other hand it penalizes tensile strength in polymers, suggesting rather their use for acoustic panels, or similar applications, generally as bio-insulation [100]. It is also noteworthy that preservation of secondary structure is not particularly sought for when extracted feather fibers are only exploited in terms of their compressive densification in a sponge-like geometry, such as in [101], where enzymatic extraction was preferred [102]. In this sense, other applications are not in need of any particular efficiency for keratin extraction, such as it is the case for oil spill absorption [103], use as natural flocculants for the treatment of potato starch wastewater [104], or heavy metal ions (e.g., hexavalent chromium) removal from wastewater [105], as an alternative to the use of polysaccharide absorbers, such as chitosan [106]. However, also for this application, an extraction of keratin with reduction by sodium sulfite and sodium hydroxide showed effectiveness over a larger spectrum of metals [107].
The same applies when keratin fibers are supposed to be employed as fillers possibly with considerable tensile elongation and in small tenors in biopolymer blends, such as for PLA/PHB in [108], where strain at break was brought as high as to 140%. Here, it can be suggested that protein hydrolysates would do their job better in terms of low-quantity fillers for tensile elongation, with no need for preservation of the secondary structure. Conversely, the extraction of keratin capable of potential self-assembly opens the field to further sectors, which require smaller film thicknesses and more controllable mechanical properties, especially in shear, as desirable in applications such as biomedical and bioplastics. The quality of self-assembly through preservation of the secondary structure offers keratin with higher shear properties, which enable molding of structure for wound healing and tissue regeneration [109].
To avoid including chicken keratin in other polymers, which is likely to be a suitable approach for keratin with self-assembling properties, a possible solution is its plasticization through polyols, such as glycerol. An amount of glycerol between 2% and 10% was used in [110], processing bioplastics at 60°C, from feathers extracted using sodium sulfide. Another work on sodium sulfide extraction from chicken feathers used glycerol in an amount of 3.5%, adding then a smaller amount of microcrystalline cellulose (0.2%) in a sodium hydroxide solution for 48 h again at 60°C, to offer improved mechanical properties [111]. The idea was to offer a bioplastic film, which could be aimed at various applications, including biomedical, pharmaceutical and generally biopolymer development. Other films with chicken keratin extracted by sulfitolysis, yet with plasticization enhanced by citric acid, did include 25% glycerol [112]. Adding more glycerol gradually affects tensile strength and solubility, whilst increasing elongation at break up to 35% glycerol content, where still swelling is below 17% for a urea-sodium sulfide high yield (73%) extraction [113]. A possible alternative polyol plasticizer for chicken keratin is sorbitol, which offered good performance in a 2-mercaptoethanol extraction with concentrated urea solution using sodium dodecyl sulfate (SDS) [114].
Passing to the blends of conservatively extracted keratin with chicken feathers with biopolymers, the variety of solutions attempted appear considerable and would especially depend on the application that was proposed for the keratin-based structure. It is also noteworthy that some polymers, such as poly(ethylene oxide) (PEO), are able to hinder the self-assembly of cysteine residues, and therefore keratin blending with them might not always be desirable for the production of biomaterials, though it eases electrospinning of fibers [115].
In particular, in the biomedical field, keratin nanoparticles are particularly effective in producing drug delivery systems, where their distinct advantages are their generous surface area, and encapsulation efficiency, which results in a controlled drug release [116]. Successful examples have been provided using poly(vinyl alcohol) (PVA) in crosslinked films with dialdehyde starch [117,118]. In other uses, the objective might also be orienting the specific polymer towards more focused properties through its blending with potentially self-assembling keratin [119]. This occurred for example with polycaprolactone (PCL)-human hair keratin blend coated with hydroxyapatite particles, when the objective is to fabricate scaffolds for human bone regeneration [120].
The capability of keratin to contribute to faster regeneration and to promote hydration in tissue engineering is well recognized [121]. More recently, also the combination of an adapted reduction process and the capability to regenerate natural tissue has received a considerable deal of attention [122–124]. In the case of the use of keratin from chicken feathers, a limited number of studies, summarized in Table 1, do possibly represent combinations with polymers that do not exclude in principle a self-assembly process, because of keratin extraction performed through reduction processes.
A conclusion can be that most studies on keratin from poultry feathers do not effectively preserve the secondary structure of the protein, though in general this would appear to be necessary to improve the application profile of the material, especially in terms of upcycling, whenever this is obtained from industrial waste. As a consequence, the following section does concentrate on those studies where explicitly this characteristic leading to self-assembly is declared or evident and possibly the extraction method for keratin is tailored to obtain this result.
4.3 Self-Assembled Structures: Nanoparticles and Nanofibers
To summarize what has been exposed previously, when maintained in a natural system and at adapted conditions of pH, temperature, etc. Keratin has the tendency to self-assemble into functional structures (e.g., hair, nails, feathers…) due to their specific amino acid sequences and interactions.
A natural example of self-assembly of beta keratin fibers is the photonic system that is created inside the feathers of some birds which generates the structural color of the feather itself [130,131].
In general, self-assembly is an intrinsic property of keratin proteins that is favored by environmental conditions during the experimental process. In particular, the self-assembly potential does depend on the chemical action performed, including nature of the chemical involved pH, temperature, and retention time, yet also may be affected by the mechanical action, such as in the case of vapor pressure for steam explosion, which hinders the preservation of cysteine residues [132]. Cysteine residues present in keratin proteins form the S-S bonds, which are responsible for the structural stability and self-assembly. At the same time the hydrophobic and hydrophilic regions of the proteins allow it to interact with itself and other molecules in aqueous environments, promoting self-assembly [133]. This suggested the potential use of keratin waste-based materials also in the field of biomedical scaffolding [134].
Self-assembling properties are found in the formation of keratin nanoparticles. Keratin nanoparticles have a high tendency to create interparticle bonds, leading to the creation of larger structures such as nanofibrils or nanolayers. The reconstruction of disulfide bonds during dialysis, used during the extraction method, is crucial for the structural integrity and stability of the keratin nanoparticles. These bonds help maintain the folded structure of the proteins and promote aggregation. Hydrogen bonds between the backbone and side chains of keratin molecules stabilize the secondary and tertiary structures, facilitating the formation of beta-sheets and subsequent self-assembly into nanoparticles [135]. Hydrophobic regions of keratin molecules tend to aggregate to minimize exposure to the aqueous environment, driving the self-assembly process. By modifying the incubation time, the temperature, together with pH and concentration of keratin in solution, the dimensions and characteristics of the nanostructures can be controlled, offering various building blocks that allow a tailored and effective design of the nanostructure passing from 1-D to 3-D geometries at the nano-level, as detailed in Fig. 5. This suggests to better orient the envisaged application in the biomedical field offering further advantages are low immunogenicity, colloidal stability and biodegradability [136].

Figure 5: A schematic diagram demonstrating how keratin nanoparticles can be formed and served as the building blocks for higher nanostructures. Letters P, F and L indicate nanoparticles, nanofibrils and nanolayers, respectively (Reproduced with permission from Reference [133], © Elsevier 2020)
Dialysis process can also influence the mechanism of self-assembly creating a gel. The mechanism called “gelation” is due to the intermolecular interactions and the reformation of disulfide bonds. Viscoelastic properties of this kind of gels can be controlled by manipulating disulfide bond reoxidation and cross-link density during the dialysis process. Keratin gels are primarily stabilized by disulfide bonds, though complete dissolution is only possible by disrupting hydrophobic interactions and hydrogen bonds as well. As suggested above, the appearance and viscoelastic properties of the gels are also influenced by pH and temperature.
The self-assembly of keratin fibers plays a crucial role in determining their mechanical properties and performance; this characteristic was also observed in the wet spinning process. To start from an extraction by chemical process, using mercaptoethanol, helps manipulating disulfide bonds, essential for building the keratin fibers. In particular, the self-assembly of keratin fibers via controlled disulfide bond formation involves the gradual recovery of secondary structures and the creation of ordered protein configurations. This process ultimately improves the mechanical properties of the fibers, including their toughness and durability. For instance, the regenerated keratin fibers exhibit breaking strain and toughness that were much higher than those of cotton and linen. Their toughness was nearly equivalent to that of viscose fibers. These results indicate that keratin fibers, which retain their secondary structures through continuous production, are well-suited for practical applications.
The ability to control and utilize this natural self-assembly process opens possibilities for creating advanced materials for biomedical applications, such as wound dressings, drug delivery systems, and tissue engineering scaffolds. In Wang et al. [137], for the production of hydrogel, it was used as an extraction method, a reduction and then an oxidation, that preserve the secondary structure of keratin. The hydrogel formation was favored by the use of H2O2 which promotes the formation of disulfide bonds, finally the hydrogel was used as an effective base for the formation of scaffolds useful for cell proliferation in wound healing. Similar applications have been tested by Polesca et al. [76], starting from the extraction process based on ionic liquids, they create a film of pure keratin. The formation of the film is favored by using temperatures between 50°C and 60°C. They also demonstrated non-toxicity to cells and the in vitro wound healing study demonstrates that this type of film improves the proliferation of keratinocytes and fibroblasts, accelerating wound healing up to 16 h.
Self-assembly of chicken feather extracted keratin, therefore intended as use in biomedical devices, such as for scaffolds and wound dressing, received some degree of attention in recent studies. On the other hand, not many works that resulted in regenerating the disulfide bonds explicitly declared the potential for prospective self-assembly of obtained biomaterials, despite the fact that the attention towards the synthesis of nanoparticles does appear to be gradually increasing. The outcome of this review indicated the reduction extraction processes as the most suitable for the purpose, including those with use of sodium sulfite or mercaptoethanol, or also those with ionic liquids, and even more surprisingly, the extraction of keratin through steam explosion.
A final comment would concern the fact that future developments in this field, also given the very large availability of poultry feather would also be likely to invest in larger value applications for extracted keratin, which will necessarily involve the preservation of secondary structure to enable its self-assembly process in the form of nanoparticles, gels, and blends with biopolymers.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Study conception and design: Carlo Santulli; data collection: Sara Mattiello, and Carlo Santulli; draft manuscript preparation: Sara Mattiello, and Carlo Santulli. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: No new data were generated.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Aliprandi A, Mauro M, De Cola L. Controlling and imaging biomimetic self-assembly. Nat Chem. 2016;8(1):10. doi:10.1038/nchem.2383. [Google Scholar] [PubMed] [CrossRef]
2. Levin A, Hakala TA, Schnaider L, Bernardes GJ, Gazit E, Knowles TP. Biomimetic peptide self-assembly for functional materials. Nat Rev Chem. 2020;4(1):615–34. [Google Scholar]
3. Pizzi A, Pigliacelli C, Bergamaschi G, Gori A, Metrangolo P. Biomimetic engineering of the molecular recognition and self-assembly of peptides and proteins via halogenation. Coord Chem Rev. 2020;411:213242. doi:10.1016/j.ccr.2020.213242. [Google Scholar] [CrossRef]
4. Kumawat TK, Sharma A, Sharma V, Chandra S. Keratin waste: the biodegradable polymers. In: Blumenberg M, editor. Keratin. London: IntechOpen; 2018. doi:10.5772/intechopen.79502. [Google Scholar] [CrossRef]
5. Piras S, Salathia S, Guzzini A, Zovi A, Jackson S, Smirnov A, et al. Biomimetic use of food-waste sources of calcium carbonate and phosphate for sustainable materials—a review. Materials. 2024;17(4):843. doi:10.3390/ma17040843. [Google Scholar] [PubMed] [CrossRef]
6. Ferraro V, Anton M, Santé-Lhoutellier V. The “sisters” α-helices of collagen, elastin and keratin recovered from animal by-products: Functionality, bioactivity and trends of application. Trends Food Sci Technol. 2016;51:65–75. doi:10.1016/j.tifs.2016.03.006. [Google Scholar] [CrossRef]
7. Czyzewski AM, Barron AE. Protein and peptide biomimicry: gold-mining inspiration from nature’s ingenuity. AIChE J. 2008;54:2–8. doi:10.1002/aic.v54:1. [Google Scholar] [CrossRef]
8. Hasan A, Saxena V, Castelletto V, Zimbitas G, Seitsonen J, Ruokolainen J, et al. Chain-end modifications and sequence arrangements of antimicrobial peptoids for mediating activity and nano-assembly. Front Chem. 2020;8:416. doi:10.3389/fchem.2020.00416. [Google Scholar] [PubMed] [CrossRef]
9. Barba C, Méndez S, Roddick-Lanzilotta A, Kelly R, Parra JL, Coderch L. Cosmetic effectiveness of topically applied hydrolysed keratin peptides and lipids derived from wool. Skin Res Technol. 2008;14:243–8. doi:10.1111/srt.2008.14.issue-2. [Google Scholar] [CrossRef]
10. Shavandi A, Bekhit AEDA, Carne A, Bekhit A. Evaluation of keratin extraction from wool by chemical methods for bio-polymer application. J Bioact Compat Polym. 2017;32:163–77. doi:10.1177/0883911516662069. [Google Scholar] [CrossRef]
11. Ali MA, Gould M. Untapped potentials of hazardous nanoarchitectural biopolymers. J Hazard Mater. 2021;411:124740. doi:10.1016/j.jhazmat.2020.124740. [Google Scholar] [PubMed] [CrossRef]
12. Havryliak V, Mykhaliuk V. The comparative analysis of the methods for keratin extraction from sheep wool and human hair. Bìol Tvarin. 2020;22(4):9–12. doi:10.15407/animbiol22.04.009. [Google Scholar] [CrossRef]
13. Holkar CR, Jain SS, Jadhav AJ, Pinjari DV. Valorization of keratin based waste. Proc Saf Environ Protect. 2018;115:85–98. doi:10.1016/j.psep.2017.08.045. [Google Scholar] [CrossRef]
14. Timorshina S, Popova E, Osmolovskiy A. Sustainable applications of animal waste proteins. Polymers. 2022;14(8):1601. doi:10.3390/polym14081601. [Google Scholar] [PubMed] [CrossRef]
15. Petrucci R, Dominici F, Santulli C, Puglia D, Kenny J. Mechanical and thermal characterisation of poly(ethylene) and thermoplastic starch filled with keratin horn powder from bovine claws. Mater Sci Eng Adv Res. 2015;1(1):1–4. doi:10.24218/msear.2015.06. [Google Scholar] [CrossRef]
16. Shih JC. Recent development in poultry waste digestion and feather utilization—a review. Poultry Sci. 1993;72(9):1617–20. doi:10.3382/ps.0721617. [Google Scholar] [CrossRef]
17. McKittrick J, Chen PY, Bodde SG, Yang W, Novitskaya EE, Meyers MA. The structure, functions, and mechanical properties of keratin. JOM. 2012;64(4):449–68. doi:10.1007/s11837-012-0302-8. [Google Scholar] [CrossRef]
18. Paşayev N, Tekoğlu O, Kocatepe S, Erol M, Maraş N. The machine method for processing chicken feathers by splitting them into fibers and rachis. Tekstil ve Mühendis. 2021;28(124):248–60. doi:10.7216/1300759920212812401. [Google Scholar] [CrossRef]
19. Kurien RA, Biju A, Raj KA, Chacko A, Joseph B, Koshy CP. Chicken feather fiber reinforced composites for sustainable applications. Mater Today Proc. 2022;58(1):862–6. doi:10.1016/j.matpr.2021.10.400. [Google Scholar] [CrossRef]
20. Hussain FS, Memon N. Recent developments in extraction of keratin from industrial wastes. In: Bhawani SA, Khan A, Ahmad FB, editors. Extraction of natural Products from agro-industrial wastes. Amsterdam: Elsevier; 2022. p. 281–302. [Google Scholar]
21. Zhu J, Avakyan N, Kakkis A, Hoffnagle AM, Han K, Li Y, et al. Protein assembly by design. Chem Rev. 2021;121(22):13701–96. doi:10.1021/acs.chemrev.1c00308. [Google Scholar] [PubMed] [CrossRef]
22. Mason TO, Shimanovich U. Fibrous protein self-assembly in biomimetic materials. Adv Mater. 2018;30(41):1706462. doi:10.1002/adma.201706462. [Google Scholar] [PubMed] [CrossRef]
23. Wang B, Yang W, McKittrick J, Meyers MA. Keratin: structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Progr Mater Sci. 2016;76:229–318. doi:10.1016/j.pmatsci.2015.06.001. [Google Scholar] [CrossRef]
24. Li X, Wei Y, Jiang S, Zhou Y, Li J, Li K, et al. Full bio-based soy protein isolate film enhanced by chicken feather keratin. Macromol Mater Eng. 2021;306:2100004. doi:10.1002/mame.v306.5. [Google Scholar] [CrossRef]
25. Maurya SD, Singh A. Application and future perspectives of keratin protein extracted from waste chicken feather: a review. Sustain Chem Eng. 2024;5:31–45. [Google Scholar]
26. Cherry JP, Young CT, Shewfelt AL. Characterization of protein isolates from keratinous material of poultry feathers. J Food Sci. 1975;40:331–5. doi:10.1111/jfds.1975.40.issue-2. [Google Scholar] [CrossRef]
27. Li H, Hu J, Wang X, An L. Development of a bio-inspired photo-recyclable feather carbon adsorbent towards removal of amoxicillin residue in aqueous solutions. Chem Eng J. 2019;373:1380–8. doi:10.1016/j.cej.2019.03.160. [Google Scholar] [CrossRef]
28. Pourjavaheri F, Mohaddes F, Shanks RA, Czajka M, Gupta A. Effects of different purification methods on chicken feather keratin. Adv Mater Res. 2014;941:1184–7. [Google Scholar]
29. Adil S, Tariq S. Study of traditional and modern applications of feathers—a review. J Wild Ecol. 2020;4:141–50. [Google Scholar]
30. Chilakamarry CR, Mahmood S, Saffe SNBM, Arifin MAB, Gupta A, Sikkandar MY. Extraction and application of keratin from natural resources: a review. 3 Biotech. 2021;11:1–12. [Google Scholar]
31. Emenike EC, Amusa VT, Iwuozor KO, Ojeyemi T, Micheal TT, Micheal KT, et al. Enhancing biochar properties through doping: a comparative study of sugarcane bagasse and chicken feather. Biofuels. 2024;15:627–34. doi:10.1080/17597269.2023.2274694. [Google Scholar] [CrossRef]
32. Chaturvedi V, Agrawal K, Verma P. Chicken feathers: a treasure cove of useful metabolites and value-added products. Environ Sustain. 2021;4:231–43. doi:10.1007/s42398-021-00160-2. [Google Scholar] [CrossRef]
33. Brenner M, Weichold O. Autogenous cross-linking of recycled keratin from poultry-feather waste to hydrogels for plant-growth media. Polym. 2021;13:3581. doi:10.3390/polym13203581. [Google Scholar] [PubMed] [CrossRef]
34. Oluba OM, Obi CF, Akpor OB, Ojeaburu SI, Ogunrotimi FD, Adediran AA, et al. Fabrication and characterization of keratin starch biocomposite film from chicken feather waste and ginger starch. Sci Rep. 2021;11(1):8768. doi:10.1038/s41598-021-88002-3. [Google Scholar] [PubMed] [CrossRef]
35. Gupta P, Nayak KK. Compatibility study of alginate/keratin blend for biopolymer development. J Appl Biomater Funct Mater. 2015;13(4):332–9. doi:10.5301/jabfm.5000242. [Google Scholar] [PubMed] [CrossRef]
36. Das A, Das A, Basu A, Datta P, Gupta M, Mukherjee A. Newer guar gum ester/chicken feather keratin interact films for tissue engineering. Int J Biol Macromol. 2021;180:339–54. doi:10.1016/j.ijbiomac.2021.03.034. [Google Scholar] [PubMed] [CrossRef]
37. Mi X, Mu B, Li W, Xu H, Yang Y. From poultry wastes to quality protein products via restoration of the secondary structure with extended disulfide linkages. ACS Sustain Chem Eng. 2020;8(3):1396–405. doi:10.1021/acssuschemeng.9b05545. [Google Scholar] [CrossRef]
38. Shanmugasundaram OL, Ahmed KSZ, Sujatha K, Ponnmurugan P, Srivastava A, Ramesh R, et al. Fabrication and characterization of chicken feather keratin/polysaccharides blended polymer coated nonwoven dressing materials for wound healing applications. Mater Sci Eng C. 2018;92(3):26–33. doi:10.1016/j.msec.2018.06.020. [Google Scholar] [PubMed] [CrossRef]
39. Sharma S, Rostamabadi H, Gupta S, Nadda AK, Kharazmi MS, Jafari SM. Nano/micro-formulations of keratin in biocomposites, wound healing and drug delivery systems; recent advances in biomedical applications. Eur Polym J. 2022;180:111614. doi:10.1016/j.eurpolymj.2022.111614. [Google Scholar] [CrossRef]
40. Tesfaye T, Sithole B, Ramjugernath D, Mokhothu T. Valorisation of chicken feathers: characterisation of thermal, mechanical and electrical properties. Sustain Chem Pharm. 2018;9:27–34. doi:10.1016/j.scp.2018.05.003. [Google Scholar] [CrossRef]
41. Lazarus BS, Chadha C, Velasco-Hogan A, Barbosa JD, Jasiuk I, Meyers MA. Engineering with keratin: a functional material and a source of bioinspiration. iScience. 2021;24:102798. doi:10.1016/j.isci.2021.102798. [Google Scholar] [PubMed] [CrossRef]
42. Chitnis S, Gaikwad SA, Lambate SB, Ingole SD, Zende RJ. Comparative morphological study of feathers of broiler and layer chicken. Ind J Vet Anat. 2024;35(2):113–6. [Google Scholar]
43. Tesfaye T, Sithole B, Ramjugernath D, Chunilall V. Valorisation of chicken feathers: characterisation of physical properties and morphological structure. J Clean Prod. 2017;149:349–65. doi:10.1016/j.jclepro.2017.02.112. [Google Scholar] [CrossRef]
44. Chang WL, Wu H, Chiu YK, Wang S, Jiang TX, Luo ZL, et al. The making of a flight feather: bio-architectural principles and adaptation. Cell. 2019;179:1409–23. doi:10.1016/j.cell.2019.11.008. [Google Scholar] [PubMed] [CrossRef]
45. Ritchison G. Integument. In: In a class of their own: a detailed examination of avian forms and functions. Cham: Springer International Publishing; 2023. p. 319–477. [Google Scholar]
46. Schmidt RE, Struthers JD, Phalen DN. Pathology of pet and aviary birds. New York: John Wiley & Sons; 2024. [Google Scholar]
47. Metwally S, Comesaña SM, Zarzyka M, Szewczyk PK, Karbowniczek JE, Stachewicz U. Thermal insulation design bioinspired by microstructure study of penguin feather and polar bear hair. Acta Biomater. 2019;91:270–83. doi:10.1016/j.actbio.2019.04.031. [Google Scholar] [PubMed] [CrossRef]
48. Lingham-Soliar T. Feather structure, biomechanics and biomimetics: the incredible lightness of being. J Ornithol. 2014;155(2):323–36. doi:10.1007/s10336-013-1038-0. [Google Scholar] [CrossRef]
49. Lingham-Soliar T, Murugan N. A new helical crossed-fibre structure of β-keratin in flight feathers and its biomechanical implications. PLoS One. 2013;8(6):e65849. doi:10.1371/journal.pone.0065849. [Google Scholar] [PubMed] [CrossRef]
50. Lingham-Soliar T. Microstructural tissue-engineering in the rachis and barbs of bird feathers. Sci Rep. 2017;7(1):45162. doi:10.1038/srep45162. [Google Scholar] [PubMed] [CrossRef]
51. Sullivan TN, Zhang Y, Zavattieri PD, Meyers MA. Hydration-induced shape and strength recovery of the feather. Adv Funct Mater. 2018;28(30):1801250. doi:10.1002/adfm.201801250. [Google Scholar] [CrossRef]
52. Filshie BK, Rogers GE. An electron microscope study of the fine structure of feather keratin. J Cell Biol. 1962;13(1):1–12. doi:10.1083/jcb.13.1.1. [Google Scholar] [PubMed] [CrossRef]
53. Fraser RB, Parry DA. Molecular packing in the feather keratin filament. J Struct Biol. 2008;162(1):1–13. doi:10.1016/j.jsb.2008.01.011. [Google Scholar] [PubMed] [CrossRef]
54. Mattiello S, Guzzini A, Del Giudice A, Santulli C, Antonini M, Lupidi G, et al. Physico-chemical characterization of keratin from wool and chicken feathers extracted using refined chemical methods. Polymers. 2022;15(1):181. doi:10.3390/polym15010181. [Google Scholar] [PubMed] [CrossRef]
55. Dale BA, Lonsdale-Eccles JD, Holbrook KA. Stratum corneum basic protein: an interfilamentous matrix protein of epidermal keratin. In: Mali JWH, editor. Biochemistry of normal and abnormal epidermal differentiation. Basel: Karger Publishers; 1981. vol. 10, p. 311–25. [Google Scholar]
56. Ehrlich F, Lachner J, Hermann M, Tschachler E, Eckhart L. Convergent evolution of cysteine-rich keratins in hard skin appendages of terrestrial vertebrates. Mol Biol Evol. 2020;37(4):982–93. doi:10.1093/molbev/msz279. [Google Scholar] [PubMed] [CrossRef]
57. Alahyaribeik S, Ullah A. Methods of keratin extraction from poultry feathers and their effects on antioxidant activity of extracted keratin. Int J Biol Macromol. 2020;148(4):449–56. doi:10.1016/j.ijbiomac.2020.01.144. [Google Scholar] [PubMed] [CrossRef]
58. IIsarankura Na Ayutthaya S, Tanpichai S, Wootthikanokkhan J. Keratin extracted from chicken feather waste: extraction, preparation, and structural characterization of the keratin and keratin/biopolymer films and electrospuns. J Polym Environ. 2015;23(4):506–16. doi:10.1007/s10924-015-0725-8. [Google Scholar] [CrossRef]
59. Polesca C, Passos H, Neves BM, Coutinho JA, Freire MG. Valorization of chicken feathers using aqueous solutions of ionic liquids. Green Chem. 2023;25(4):1424–34. doi:10.1039/D2GC04477C. [Google Scholar] [CrossRef]
60. Nagai Y, Nishikawa T. Alkali solubilization of chicken feather keratin. Agric Biol Chem. 1970;34(1):16–22. doi:10.1080/00021369.1970.10859572. [Google Scholar] [CrossRef]
61. Wang Z, Lu B, Xiao N, Guo S, Liu C, Ai M. Structural and functional assessment of keratin extracted from chicken feathers using microwave-assisted l-cysteine method. Food Biosci. 2024;61(5):104712. doi:10.1016/j.fbio.2024.104712. [Google Scholar] [CrossRef]
62. Windt X, Scott EL, Seeger T, Schneider O, Asadi Tashvigh A, Bitter JH. Fourier transform infrared spectroscopy for assessing structural and enzymatic reactivity changes induced during feather hydrolysis. ACS Omega. 2022;7(44):39924–30. doi:10.1021/acsomega.2c04216. [Google Scholar] [PubMed] [CrossRef]
63. Harland DP, Popescu C, Richena M, Deb-Choudhury S, Wichlatz C, Lee E, et al. The susceptibility of disulfide bonds to modification in keratin fibers undergoing tensile stress. Biophys J. 2022;121(11):2168–79. doi:10.1016/j.bpj.2022.04.029. [Google Scholar] [PubMed] [CrossRef]
64. Qiu J, Wilkens C, Barrett K, Meyer AS. Microbial enzymes catalyzing keratin degradation: classification, structure, fuction. Biotechnol Adv. 2020;44:107607. doi:10.1016/j.biotechadv.2020.107607. [Google Scholar] [PubMed] [CrossRef]
65. Faraon VA, Mihăilă EG, Tritean N, Trică B, Capră L, Roman MB, et al. Keratin extraction from chicken feathers in aqueous solutions. Sci Bull Ser F. Biotechnol. 2023;27:106–12. [Google Scholar]
66. Nurkhasanah U, Susanti E, Idris AM, Suharti S. Keratin biofilm from chicken feathers. IOP Conf Ser: Earth Environ Sci. 2020;475:012073. doi:10.1088/1755-1315/475/1/012073. [Google Scholar] [CrossRef]
67. Brandelli A, Sala L, Kalil SJ. Microbial enzymes for bioconversion of poultry waste into added-value products. Food Res Int. 2015;73:3–12. doi:10.1016/j.foodres.2015.01.015. [Google Scholar] [CrossRef]
68. Qin X, Yang C, Guo Y, Liu J, Bitter JH, Scott EL, et al. Effect of ultrasound on keratin valorization from chicken feather waste: process optimization and keratin characterization. Ultrason Sonochem. 2023;93:106297. doi:10.1016/j.ultsonch.2023.106297. [Google Scholar] [PubMed] [CrossRef]
69. Feroz S, Muhammad N, Ratnayake J, Dias G. Keratin-Based materials for biomedical applications. Bioact Mater. 2020;5(3):496–509. doi:10.1016/j.bioactmat.2020.04.007. [Google Scholar] [PubMed] [CrossRef]
70. Rodríguez-Clavel IS, Paredes-Carrera SP, Flores-Valle SO, Paz-García EJ, Sánchez-Ochoa JC, Pérez-Gutiérrez RM. Effect of microwave or ultrasound irradiation in the extraction from feather keratin. J Chem. 2019;2019:1326063. [Google Scholar]
71. Alahyaribeik S, Sharifi SD, Tabandeh F, Honarbakhsh S, Ghazanfari S. Bioconversion of chicken feather wastes by keratinolytic bacteria. Proc Saf Environ Protect. 2020;135(4):171–8. doi:10.1016/j.psep.2020.01.014. [Google Scholar] [CrossRef]
72. Meko OA, Eraga SO, Arhewoh MI. Effect of extraction parameters on some properties of keratin obtained from waste chicken feathers. Trop J Nat Prod Res. 2024;8:7423. [Google Scholar]
73. Idris A, Vijayaraghavan R, Rana UA, Fredericks D, Patti AF, Macfarlane DR. Dissolution of feather keratin in ionic liquids. Green Chem. 2013;15(2):525–34. doi:10.1039/c2gc36556a. [Google Scholar] [CrossRef]
74. Ji Y, Chen J, Lv J, Li Z, Xing L, Ding S. Extraction of keratin with ionic liquids from poultry feather. Separ Purif Technol. 2014;132:577–83. doi:10.1016/j.seppur.2014.05.049. [Google Scholar] [CrossRef]
75. Wang YX, Cao XJ. Extracting keratin from chicken feathers by using a hydrophobic ionic liquid. Process Biochem. 2012;47(5):896–9. doi:10.1016/j.procbio.2012.02.013. [Google Scholar] [CrossRef]
76. Polesca C, Al Ghatta A, Passos H, Coutinho JA, Hallett JP, Freire MG. Sustainable keratin recovery process using a bio-based ionic liquid aqueous solution and its techno-economic assessment. Green Chem. 2023;25(10):3995–4003. doi:10.1039/D3GC00850A. [Google Scholar] [CrossRef]
77. Sinkiewicz I, Śliwińska A, Staroszczyk H, Kołodziejska I. Alternative methods of preparation of soluble keratin from chicken feathers. Waste Biomass Valorization. 2017;8(4):1043–8. doi:10.1007/s12649-016-9678-y. [Google Scholar] [CrossRef]
78. Shavandi A, Silva TH, Bekhit AA, Bekhit AEDA. Keratin: dissolution, extraction and biomedical application. Biomater Sci. 2017;5(9):1699–1735. doi:10.1039/C7BM00411G. [Google Scholar] [PubMed] [CrossRef]
79. Buchacher M, Bechtold T, Pham T. Characterisation of reduction state of cystine linkages on wool fibre surface under heterogeneous reaction conditions. Polym Test. 2022;106:107438. doi:10.1016/j.polymertesting.2021.107438. [Google Scholar] [CrossRef]
80. Reddy CC, Khilji IA, Gupta A, Bhuyar P, Mahmood S, AL-Japairai KAS, et al. Valorization of keratin waste biomass and its potential applications. J Water Proc Eng. 2021;40:101707. doi:10.1016/j.jwpe.2020.101707. [Google Scholar] [CrossRef]
81. Vadillo J, Montes S, Grande HJ, Verstichel S, Almqvist J, Wrześniewska-Tosik K. Enhanced biodegradability in soil of chicken feather by steam explosion for potential application in agricultural biodegradable plastics. Polymers. 2023;15:3701. doi:10.3390/polym15183701. [Google Scholar] [PubMed] [CrossRef]
82. Shen Q, Ma Y, Qin X, Guo Y, Zhang C. Steam explosion as a green method to treat animal waste: a mini-review. Proc Saf Environ Protect. 2024;181:43–52. doi:10.1016/j.psep.2023.11.012. [Google Scholar] [CrossRef]
83. Zhang Y, Zhao W, Yang R. Steam flash explosion assisted dissolution of keratin from feathers. ACS Sustain Chem Eng. 2015;3:2036–42. doi:10.1021/acssuschemeng.5b00310. [Google Scholar] [CrossRef]
84. Rigueto CVT, Rosseto M, Alessandretti I, Krein DDC, Emer CD, Loss RA, et al. Extraction and improvement of protein functionality using steam explosion pretreatment: advances, challenges, and perspectives. J Food Sci Technol. 2024;61:1215–37. doi:10.1007/s13197-023-05817-w. [Google Scholar] [PubMed] [CrossRef]
85. Wei N, Xu D, Hao B, Guo S, Guo Y, Wang S. Chemical reactions of organic compounds in supercritical water gasification and oxidation. Water Res. 2021;190(1):116634. doi:10.1016/j.watres.2020.116634. [Google Scholar] [PubMed] [CrossRef]
86. Škerget M, Čolnik M, Zemljič LF, Gradišnik L, Semren TŽ, Lovaković BT, et al. Efficient and green isolation of keratin from poultry feathers by subcritical water. Polymers. 2023;15(12):2658. doi:10.3390/polym15122658. [Google Scholar] [PubMed] [CrossRef]
87. Poole AJ, Lyons RE, Church JS. Dissolving feather keratin using sodium sulfide for bio-polymer applications. J Polym Environ. 2011;19(4):995–1004. doi:10.1007/s10924-011-0365-6. [Google Scholar] [CrossRef]
88. Erra P, Gomez NDLM, Dolcet LM, Juliá MR, Lewis DM, Willoughby JH. FTIR analysis to study chemical changes in wool following a sulfitolysis treatment. Text Res J. 1997;67(6):397–401. doi:10.1177/004051759706700602. [Google Scholar] [CrossRef]
89. Brush AH. Self-assembly of avian φ-keratins. J Protein Chem. 1983;2(1):63–75. doi:10.1007/BF01025168. [Google Scholar] [CrossRef]
90. Badrulzaman SZS, Aminan AW, Ramli ANM, Che Man R, Wan Azelee NI. Extraction and characterization of keratin from chicken and swiftlet feather. In: Materials science forum. Zurich: Trans Tech Publications Ltd; 2021. vol. 1025, p. 157–62. doi:10.4028/www.scientific.net/MSF. [Google Scholar] [CrossRef]
91. Esparza Y, Ullah A, Wu J. Molecular mechanism and characterization of self-assembly of feather keratin gelation. Int J Biol Macromol. 2018;107:290–6. doi:10.1016/j.ijbiomac.2017.08.168. [Google Scholar] [PubMed] [CrossRef]
92. Perţa-Crişan S, Ursachi CŞ, Gavrilaş S, Oancea F, Munteanu FD. Closing the loop with keratin-rich fibrous materials. Polymers. 2021;13:1896. doi:10.3390/polym13111896. [Google Scholar] [PubMed] [CrossRef]
93. Esparza Y, Bandara N, Ullah A, Wu J. Hydrogels from feather keratin show higher viscoelastic properties and cell proliferation than those from hair and wool keratins. Mater Sci Eng C. 2018;90:446–53. doi:10.1016/j.msec.2018.04.067. [Google Scholar] [PubMed] [CrossRef]
94. Donato RK, Mija A. Keratin associations with synthetic, biosynthetic and natural polymers: an extensive review. Polymers. 2019;12:32. doi:10.3390/polym12010032. [Google Scholar] [PubMed] [CrossRef]
95. Mi X, Li W, Xu H, Mu B, Chang Y, Yang Y. Transferring feather wastes to ductile keratin filaments towards a sustainable poultry industry. Waste Manage. 2020;115:65–73. doi:10.1016/j.wasman.2020.07.022. [Google Scholar] [PubMed] [CrossRef]
96. Brenner M, Weichold O. Poultry feather waste as bio-based cross-linking additive for ethylene propylene diene rubber. Polymers. 2020;13:3908. [Google Scholar]
97. Dinu R, Cantarutti C, Mija A. Design of sustainable materials by cross-linking a biobased epoxide with keratin and lignin. ACS Sustain Chem Eng. 2020;8:6844–52. doi:10.1021/acssuschemeng.0c01759. [Google Scholar] [CrossRef]
98. Banasaz S, Ferraro V. Keratin from animal by-products: structure, characterization, extraction and application—a review. Polymers. 2024;16:1999. doi:10.3390/polym16141999. [Google Scholar] [PubMed] [CrossRef]
99. Aranberri I, Montes S, Azcune I, Rekondo A, Grande HJ. Fully biodegradable biocomposites with high chicken feather content. Polymers. 2017;9:593. doi:10.3390/polym9110593. [Google Scholar] [PubMed] [CrossRef]
100. Fedorik F, Zach J, Lehto M, Kymäläinen HR, Kuisma R, Jallinoja M, et al. Hygrothermal properties of advanced bio-based insulation materials. Energy Build. 2021;253:111528. doi:10.1016/j.enbuild.2021.111528. [Google Scholar] [CrossRef]
101. Ramakrishnan N, Sharma S, Gupta A, Alashwal BY. Keratin based bioplastic film from chicken feathers and its characterization. Int J Biol Macromol. 2018;111:352–8. doi:10.1016/j.ijbiomac.2018.01.037. [Google Scholar] [PubMed] [CrossRef]
102. Sadeghi S, Dadashian F, Eslahi N. Recycling chicken feathers to produce adsorbent porous keratin-based sponge. Int J Environ Sci Technol. 2019;16:1119–28. doi:10.1007/s13762-018-1669-z. [Google Scholar] [CrossRef]
103. Strnad S, Jug A, Peršin Fratnik Z. Composite materials based on waste chicken feather fibers for oil-spill management. J Nat Fib. 2024;21:2346803. doi:10.1080/15440478.2024.2346803. [Google Scholar] [CrossRef]
104. Wang RM, Li FY, Wang XJ, Li QF, He YF, Wang YB. The application of feather keratin and its derivatives in treatment of potato starch wastewater. Funct Mater Lett. 2010;3:213–6. doi:10.1142/S1793604710001275. [Google Scholar] [CrossRef]
105. Chakraborty R, Asthana A, Singh AK, Verma R, Sankarasubramanian S, Yadav S, et al. Chicken feathers derived materials for the removal of chromium from aqueous solutions: kinetics, isotherms, thermodynamics and regeneration studies. J Dispers Sci Technol. 2022;43:446–60. doi:10.1080/01932691.2020.1842760. [Google Scholar] [CrossRef]
106. Saha S, Zubair M, Khosa MA, Song S, Ullah A. Keratin and chitosan biosorbents for wastewater treatment: a review. J Polym Environ. 2019;27(7):1389–403. doi:10.1007/s10924-019-01439-6. [Google Scholar] [CrossRef]
107. Donner MW, Arshad M, Ullah A, Siddique T. Unravelled keratin-derived biopolymers as novel biosorbents for the simultaneous removal of multiple trace metals from industrial wastewater. Sci Total Environ. 2019;647(9):1539–46. doi:10.1016/j.scitotenv.2018.08.085. [Google Scholar] [PubMed] [CrossRef]
108. Mosnáčková K, Opálková Šišková A, Kleinová A, Danko M, Mosnáček J. Properties and degradation of novel fully biodegradable PLA/PHB blends filled with keratin. Int J Molec Sci. 2020;21(24):9678. doi:10.3390/ijms21249678. [Google Scholar] [PubMed] [CrossRef]
109. Yan RR, Gong JS, Su C, Liu YL, Qian JY, Xu ZH, et al. Preparation and applications of keratin biomaterials from natural keratin wastes. Appl Microbiol Biotechnol. 2022;106(7):2349–66. doi:10.1007/s00253-022-11882-6. [Google Scholar] [PubMed] [CrossRef]
110. Sharma S, Gupta A, Kumar A, Kee CG, Kamyab H, Saufi SM. An efficient conversion of waste feather keratin into ecofriendly bioplastic film. Clean Technol Environ Pol. 2018;20(10):2157–67. doi:10.1007/s10098-018-1498-2. [Google Scholar] [CrossRef]
111. Athwal S, Sharma S, Gupta S, Nadda AK, Gupta A, Husain MSB. Sustainable biodegradation and extraction of keratin with its applications. In: Thomas S, Ajitha AR, Chirayil CJ, Thomas B, editors. Handbook of Biopolymers. Singapore: Springer Nature Singapore; 2023. p. 713–47. [Google Scholar]
112. Vanderlei RM, Novo-Mansur MT, Mattoso LH, Moreira FK. Effect of precipitation methods on physicochemical properties of keratin films produced from chicken feather waste. J Appl Polym Sci. 2024;141(34):e55863. doi:10.1002/app.55863. [Google Scholar] [CrossRef]
113. Dou Y, Zhang B, He M, Yin G, Cui Y. The structure, tensile properties and water resistance of hydrolyzed feather keratin-based bioplastics. Chin J Chem Eng. 2016;24(3):415–20. doi:10.1016/j.cjche.2015.11.007. [Google Scholar] [CrossRef]
114. Martelli SM, Moore GRP, Laurindo JB. Mechanical properties, water vapor permeability and water affinity of feather keratin films plasticized with sorbitol. J Polym Environ. 2006;14(3):215–22. doi:10.1007/s10924-006-0017-4. [Google Scholar] [CrossRef]
115. Aluigi A, Varesano A, Montarsolo A, Vineis C, Ferrero F, Mazzuchetti G, et al. Electrospinning of keratin/poly (ethylene oxide) blend nanofibers. J Appl Polym Sci. 2007;104(2):863–70. doi:10.1002/app.25623. [Google Scholar] [CrossRef]
116. Srinivasan V, Palanisamy P. A state-of-the-art review on keratin biomaterial as eminent nanocarriers for drug delivery applications. Lett Drug Des Discov. 2023;20(3):245–63. doi:10.2174/1570180819666220620094943. [Google Scholar] [CrossRef]
117. Khumalo M, Tesfaye T, Sithole B, Ramjugernath D. Possible beneficiation of waste chicken feathers via conversion into biomedical applications. Int J Chem Sci Rev. 2019;17:1–20. [Google Scholar]
118. Dou Y, Zhang B, He M, Yin G, Cui Y. Preparation and physicochemical properties of dialdehyde starch crosslinked feather keratin/PVA composite films. J Macromol Sci A. 2014;51:1009–15. doi:10.1080/10601325.2014.967108. [Google Scholar] [CrossRef]
119. Dou Y, Zhang B, He M, Yin G, Cui Y, Savina IN. Keratin/polyvinyl alcohol blend films crosslinked by dialdehyde starch and their potential application for drug release. Polymers. 2015;7(3):580–91. doi:10.3390/polym7030580. [Google Scholar] [CrossRef]
120. Zhao X, Lui YS, Choo CKC, Sow WT, Huang CL, Ng KW, et al. Calcium phosphate coated Keratin-PCL scaffolds for potential bone tissue regeneration. Mater Sci Eng C. 2015;49:746–53. doi:10.1016/j.msec.2015.01.084. [Google Scholar] [PubMed] [CrossRef]
121. Zamri MFMA, Bahru R, Amin R, Khan MUA, Abd Razak SI, Hassan SA, et al. Waste to health: a review of waste derived materials for tissue engineering. J Clean Prod. 2021;290:125792. doi:10.1016/j.jclepro.2021.125792. [Google Scholar] [CrossRef]
122. Salleh KM, Abd Rashid NF. Keratin-based biomaterials for biomedical applications. In: Sapuan SM, Azhari CH, Nurazzi NM, editors. Polymer composites derived from animal sources. Sawston, UK: Woodhead Publishing; 2024. p. 219–42. [Google Scholar]
123. Abrar S, Kiran S, Ashraf A, Ghaffar A, Farooq T, Rahmat M. Chemical modifications of keratin. In: Handbook of Natural Polymers. Amsterdam: Elsevier; 2024. vol. 2, p. 155–76. [Google Scholar]
124. Soleymani Eil Bakhtiari S, Karbasi S. Keratin-containing scaffolds for tissue engineering applications: a review. J Biomater Sci, Polym Ed. 2024;35:916–65. doi:10.1080/09205063.2024.2311450. [Google Scholar] [PubMed] [CrossRef]
125. Saravanan S, Sameera DK, Moorthi A, Selvamurugan N. Chitosan scaffolds containing chicken feather keratin nanoparticles for bone tissue engineering. Int J Biol Macromol. 2013;62:481–6. doi:10.1016/j.ijbiomac.2013.09.034. [Google Scholar] [PubMed] [CrossRef]
126. Tanase CE, Spiridon I. PLA/chitosan/keratin composites for biomedical applications. Mater Sci Eng C. 2014;40:242–7. doi:10.1016/j.msec.2014.03.054. [Google Scholar] [PubMed] [CrossRef]
127. Husain MSB, Gupta A, Alashwal BY. Development of keratin based hydrogels for biomedical applications. In: IOP Conference Series: Materials Science and Engineering, 2019; Bristol, UK: IOP Publishing, vol. 702, no. 1. [Google Scholar]
128. Zarei M, Tanideh N, Zare S, Aslani FS, Koohi-Hosseinabadi O, Rowshanghias A, et al. Electrospun poly (3-hydroxybutyrate)/chicken feather-derived keratin scaffolds: fabrication, in vitro and in vivo biocompatibility evaluation. J Biomater Appl. 2020;34:741–52. doi:10.1177/0885328219873090. [Google Scholar] [PubMed] [CrossRef]
129. Mahanta B, Mary SA, Bhaduri A, Giridev VR. Electrospun PVA/keratin nanofibrous scaffold and its application in neural repair. Trends Biomater Artif Organs. 2014;28:188–96. [Google Scholar]
130. Dufresne ER, Noh H, Saranathan V, Mochrie SG, Cao H, Prum RO. Self-assembly of amorphous biophotonic nanostructures by phase separation. Soft Matt. 2009;5(9):1792–5. doi:10.1039/b902775k. [Google Scholar] [CrossRef]
131. D’Alba L, Saranathan V, Clarke JA, Vinther JA, Prum RO, Shawkey MD. Colour-producing β-keratin nanofibres in blue penguin (Eudyptula minor) feathers. Biology Lett. 2011;7(4):543–6. doi:10.1098/rsbl.2010.1163. [Google Scholar] [PubMed] [CrossRef]
132. Nepal D, Kang S, Adstedt KM, Kanhaiya K, Bockstaller MR, Brinson LC, et al. Hierarchically structured bioinspired nanocomposites. Nat Mater. 2023;22(1):18–35. doi:10.1038/s41563-022-01384-1. [Google Scholar] [PubMed] [CrossRef]
133. Pakdel M, Moosavi-Nejad Z, Kermanshahi RK, Hosano H. Self-assembled uniform keratin nanoparticles as building blocks for nanofibrils and nanolayers derived from industrial feather waste. J Clean Prod. 2020;335(4):130331. doi:10.1016/j.jclepro.2021.130331. [Google Scholar] [CrossRef]
134. Woodin AM. Molecular size, shape and aggregation of soluble feather keratin. Biochem J. 1954;57(1):99–109. doi:10.1042/bj0570099. [Google Scholar] [PubMed] [CrossRef]
135. Mu B, Hassan F, Yang Y. Controlled assembly of secondary keratin structures for continuous and scalable production of tough fibers from chicken feathers. Green Chem. 2020;22:1726–34. doi:10.1039/C9GC03896E. [Google Scholar] [CrossRef]
136. Diwan H, Sah MK. Exploring the potential of keratin-based biomaterials in orthopedic tissue engineering: a comprehensive review. Emergent Mater. 2023;6(5):1441–60. doi:10.1007/s42247-023-00545-5. [Google Scholar] [CrossRef]
137. Wang J, Hao S, Luo T, Cheng Z, Li W, Gao F, et al. Feather keratin hydrogel for wound repair: preparation, healing effect and biocompatibility evaluation. Coll Surf B: Biointerfaces. 2017;149(6):341–50. doi:10.1016/j.colsurfb.2016.10.038. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF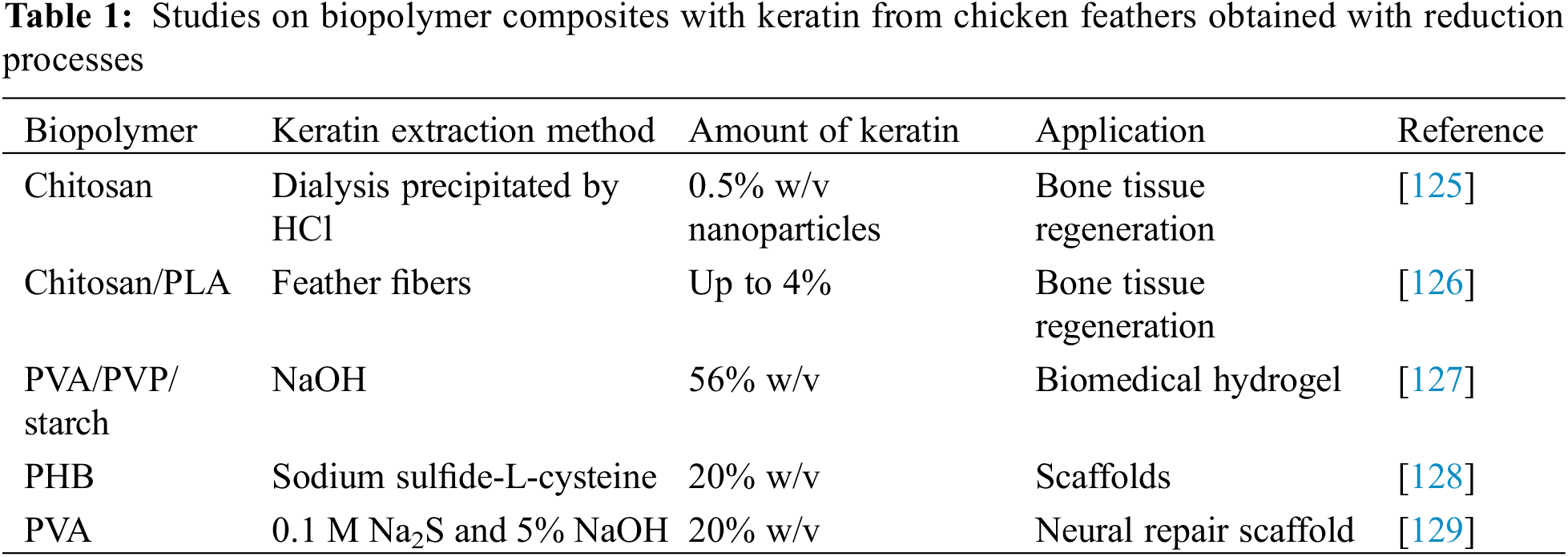
 Downloads
Downloads
 Citation Tools
Citation Tools