 Open Access
Open Access
ARTICLE
Microwave-Assisted Synthesis, Characterization, and Performance Assessment of Lemongrass-Derived Activated Carbon for Removal of Fe and Mn from Acid Mine Drainage
1 Chemical Engineering Department, Engineering Faculty, Lambung Mangkurat University, Banjarbaru, 70714, Indonesia
2 Department of Chemical Engineering, Institut Teknologi Sepuluh Nopember, Surabaya, 60111, Indonesia
* Corresponding Author: Lailan Ni`mah. Email:
Journal of Renewable Materials 2025, 13(11), 2169-2190. https://doi.org/10.32604/jrm.2025.02025-0044
Received 21 February 2025; Accepted 21 April 2025; Issue published 24 November 2025
Abstract
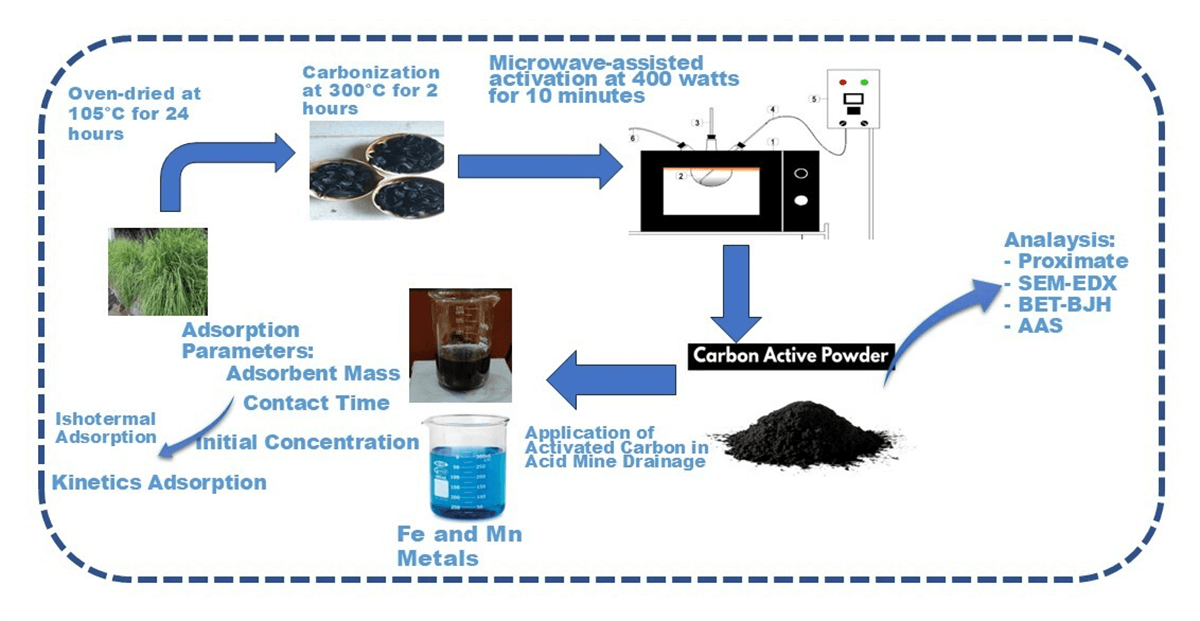
This study evaluates the effectiveness of microwave technology in producing activated carbon from lemongrass waste, an underutilized agricultural byproduct. Microwave-assisted production offers faster heating, lower energy consumption, and better process control compared to conventional methods. It also enhances pore development, resulting in larger, cleaner, and more uniform pores, making the activated carbon more effective for adsorption. The microwave-assisted process significantly accelerates production, reducing the required time to just 10 min at a power of 400 W. Activated carbon derived from lemongrass waste at 400 W exhibits a water absorption capacity of 7.88%, ash content of 5.51%, volatile matter of 6.96%, fixed carbon of 75.79%, and an iodine number of 790.97 g iodine/100 g. Scanning Electron Microscopy (SEM) analysis confirms the formation of larger, cleaner, and smoother pores, contributing to increased porosity and pore size. Additionally, Energy Dispersive X-ray (EDX) analysis identifies key elements in the lemongrass waste, with carbon being the dominant component at 75.57%. The Brunauer-Emmett-Teller (BET) surface area is measured at 818 m2/g, with an average pore diameter of 1.91 nm, classifying the material as microporous. The activated carbon, meeting quality standards, is applied as an adsorbent in acid mine drainage (AMD) treatment, with varying mass concentrations introduced into wastewater samples. Adsorption tests confirm that the microparticle carbon adsorption profile follows the Langmuir model, indicating a monolayer adsorption process. Furthermore, adsorption kinetics were analyzed over different time intervals, revealing that the process aligns with both pseudo-first-order (PFO) and pseudo-second-order (PSO) models, with all cases predominantly following the PFO rate equation.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools