 Open Access
Open Access
ARTICLE
Iron Modified Opuntia ficus-indica Cladode Powder as a Novel Adsorbent for Dyes Molecules
1 Laboratory of Materials and Environment for Sustainable Development, LR18ES10, University of Tunis El Manar, 9, Avenue Dr. Zoheir Safi, Tunis, 1006, Tunisia
2 Faculty of Science, University of Sfax, LMSE BP 802-3018, Sfax, 3000, Tunisia
* Corresponding Author: Mehrzia Krimi. Email:
(This article belongs to the Special Issue: Biobased Materials for Advanced Applications )
Journal of Renewable Materials 2025, 13(8), 1623-1644. https://doi.org/10.32604/jrm.2025.02025-0023
Received 08 February 2025; Accepted 07 April 2025; Issue published 22 August 2025
Abstract
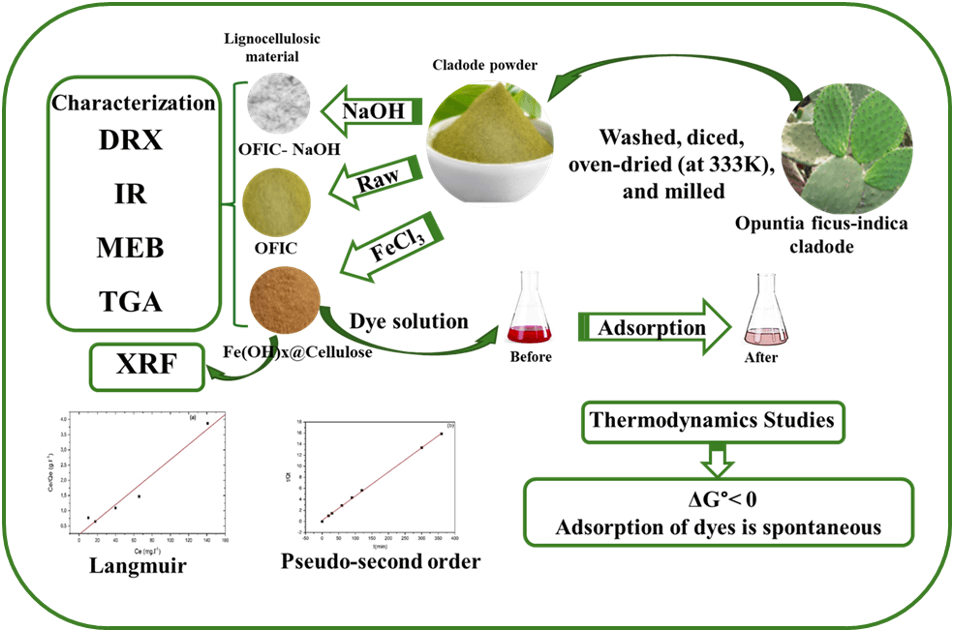
In this study, Opuntia ficus-indica cladode powder (OFIC), locally sourced from Rabta in Tunis, was utilized as a novel, eco-friendly adsorbent in both raw and iron(III) chloride-modified forms. The presence of iron in the modified material was confirmed by X-ray fluorescence spectroscopy (XRF). The neat and modified biomass were characterized by X-ray diffraction (XRD), Fourier Transform Infrared Spectroscopy (FTIR), thermogravimetric analysis (TGA) and scanning electron microscopy (SEM), and their usefulness as adsorbent for cationic Neutral Red (NR) and anionic Congo Red (CR) dyes were explored under batch conditions. Equilibrium studies revealed that the iron-modified Fe(OH)x@Cellulose adsorbent exhibited superior adsorption capabilities for both dyes compared to the raw material. Moreover, CR dye was more effectively adsorbed by Fe(OH)x@Cellulose than NR. The adsorption isotherms for both dyes were fitted. The results demonstrated that the adsorption of both NR and CR dyes onto the biosorbent Fe(OH)x@Cellulose was closely followed by the Langmuir model, with R2 values of 0.980 and 0.973 for NR and CR, respectively, and the pseudo-second-order kinetic model better depicted the adsorption kinetic. Thermodynamic analysis revealed a negative enthalpy value (−67.15 kJ/mol) for NR adsorption, suggesting an exothermic process, while a positive enthalpy value (3.99 kJ/mol) was observed for CR adsorption, indicating an endothermic process.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools